Introduction
The nucleus is a highly structured organelle with many chromatin and protein compartments that partition the genome into regulatory domains. One such compartment within the mammalian nucleus is the microenvironment underlying the nuclear envelope (NE) where intermediate filament proteins, lamins, act as a link between cytoskeletal and inner nuclear membrane (INM) proteins, chromatin binders and modifiers, and heterochromatin. These dynamic interactions regulate many cellular processes and, when they are perturbed, can lead to genome dysregulation and disease.
3D Genome Organization and Lamina Associated Domains
The mammalian nucleus is highly functionally organized, with regions of peripheral heterochromatin called Lamina Associated Domains (LADs) positioned at the nuclear periphery and more euchromatic regions positioned in the nuclear interior or interacting with nuclear pore complexes (NPC). It is important to note that not all heterochromatin is proximal to the nuclear lamina, for example centromeric and telomeric heterochromatin (and their adjacent regions) are not preferentially positioned at the nuclear periphery in mammalian cells and are visible as large heterochromatin domains within the nuclear interior. While both heterochromatin and euchromatin compartments have long been identified by cytological measures using DNA stains or electron microscopy,, modern descriptions and analyses of these chromatin domains and compartments has been obtained using mainly three different molecular methods and their derivatives: Chromatin Immunoprecipitation (ChIP), DNA Adenine Methyltransferase Identification (DamID), and Chromatin Conformation Capture (3C) methods such as HiC. ChIP is used to identify chromatin domains and binding sites of chromatin interactors, while DamID and HiC measure 3D genome organization. DamID is most often used to detect LADs,1,2 while HiC is used to identify both local and long-range chromatin interactions.3–7 In particular, HiC identifies local self-interacting regions called Topologically Associated Domains (TADs) and, in active regions of the genome, promoter-enhancer interactions. In addition, HiC also identifies longer-range chromosome and genome-wide self-interacting domains: the A (active) and B (inactive) compartments, with activity state of the compartments being operationally defined via intersections with transcriptome or, more often, with chromatin state as determined by ChIP to specific post translational modifications to histone proteins.3–6
LAD regions are mostly heterochromatic and largely correlate with the inactive B compartment as determined by HiC, sharing the same domain structures and boundaries.8 In contrast to the A compartment, which is comprised self-interacting domains and regulatory loops (TADs, sub-TADs) anchored by CTCF and cohesin, LADs are not organized in the same way (Figure 1). Although CTCF is found at the boundaries between LADs and non-LAD regions, demarcating the transition between B and A compartments, it is depleted within LADs, suggesting that LADs (and the B-compartment) have a fundamentally different organization than the CTCF and cohesin mediated looping structures found in the active non-LAD regions (A-compartment). Indeed, depletion of either CTCF or cohesin leads to loss of observable TAD organization, but A/B compartmentalization is largely maintained, with some changes, including movement of some inactive regions in the A-compartment to the B-compartment. B-compartment organization (and other heterochromatic regions such as telomeric and centromeric regions) are likely not affected by the loss, and thus the genome remains partitioned, even if reconfigured.9,10 It remains unclear, however, whether LADs remain at the nuclear lamina in the absence of CTCF or cohesin.
Figure 1: Chromatin in the nucleus is arranged geographically.
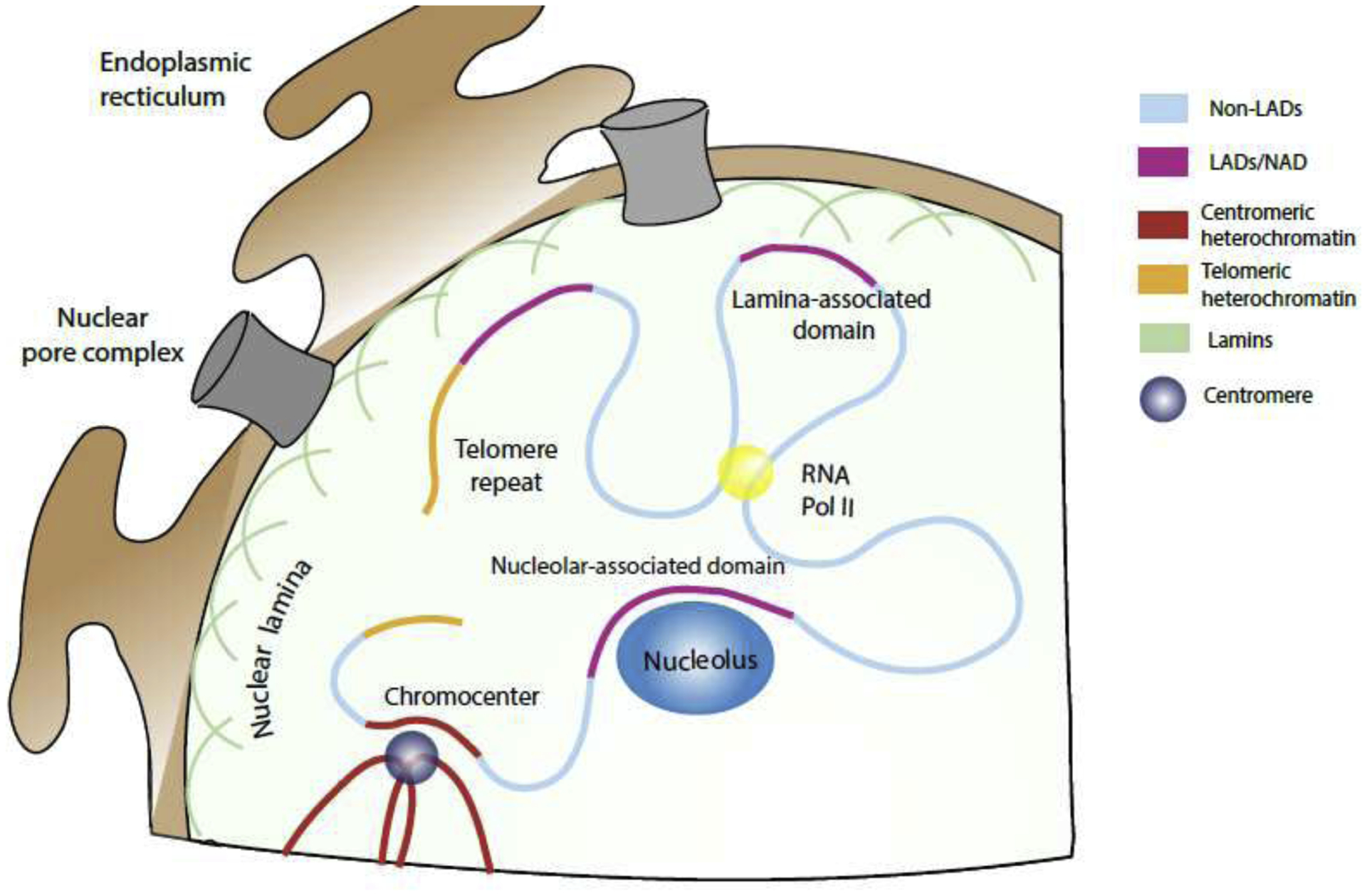
A schematic representing the geographical disposition of chromatin with a nucleus. LAD regions (pink, A-compartment) are anchored along the nuclear lamina while non-LAD regions (cyan, A-compartment) are more central. Additional nonLAD compartments include centromeric (red), telomeric (), and nucleolar associated (NADs, pink) heterochromatin domains. There is substantive overlap between NADs and LADs. Euchromatic nonLAD (A-compartment) regions display looping organization mediated by CTCF and cohesin and participate in hubs of activity hubs (such as polII transcription factories and nuclear speckles). In yeast telomeres localize to the nuclear periphery, but in mammalian cells it is less clear how tightly or regularly telomeres associate with the lamina. In both mouse and human cells, there is no apparent preference by centromeric heterochromatin for the nuclear lamina, but in mouse cells these regions coalesce to form chromocenters, visible by DAPI or hoechst stain.
While LADs and the B-compartment do not seem to be dependent on loop formation via CTCF and cohesin, some regulators and organizers of these regions are known, such histone post-translational modifications. Methylation of histone H3 lysine 9, particularly di- and trimethylation (H3K9me2/3), is required for LAD recruitment to and maintenance at the nuclear lamina.11–14 In C. elegans, the INM-bound chromodomain protein CEC-4 tethers peripheral heterochromatin through interactions with H3K9me3, while in mammals Proline Rich Protein 14 (PRR14) has been shown to anchor these regions to the lamina through its interactions with Heterochromatin Protein 1 (HP1), a chromodomain protein that binds H3K9me315,16;17–21,14–16. Beyond driving LAD anchoring these histone modifications are also important for LAD compaction and aggregation.8,12 Heterochromatic regions have been shown accrete due to phase-separation directed by HP1α and LADs cluster within a single chromosome after exit from mitosis22,23,24 In particular, biophysical models predict that, in the absence of an active constraint at the nuclear lamina, LADs would form large clusters in the nucleoplasm. This is supported by studies in cells lacking both lamin A/C and Lamin B Receptor (LBR), which display an inverted heterochromatin configuration. Thus, the heterochromatic nature of LADs leads to their separation from euchromatic regions, but other forces and interactions are likely at play in organizing these regions to the lamina. For example, in murine cells, there is a less clear role for histone H3 lysine 27 trimethylation (H3K27me3), since this modification is found mostly outside of LADs, but is enriched at LAD borders and necessary to target an inserted test locus to the lamina in coordination with H3K9me2/3. In a clever screen in cec-4 null embryos in C. elegans, where H3K27me3 modifications seem to be dispensable for driving peripheral localization, acetylation of these same histone H3 residues prevents proper organization to the lamina. In this case, the de-localization of CBP/p300 causes aberrant H3K27Ac to replace H3K27me3, suggesting that sequestration of active chromatin modifiers is also a driving force for tethering of heterochromatin to the nuclear periphery. Indeed, in a recent study in mouse cells in which a LAD border region was deleted, H3K27ac was found to drive a region away from the nuclear lamina.25 Taken together, these studies suggest a complex interplay between chromatin state and chromatin binding proteins in the separation and organization of LADs to the periphery.
LAD regions contain relatively few genes, but are, conversely, enriched in developmental and lineage specific genes, leading to the hypothesis that the epigenetic state of these regions is tied to both organization and developmental control of gene expression.11,13,26,27 Single cell DamID uncovered some cell to cell heterogeneity of LAD organization between individual cells, with more gene-dense LADs displaying greater fluctuations in their association with the lamina, implying that these disruptions may be due to differential gene usage between cells.28 In addition, it has recently been shown that different promoters respond differently to integration into a LAD. Certain promoters are sensitive to integration into LADs and are almost universally repressed, while other promoters can “escape” silencing within LADed regions. These escapers were generally less sensitive to H3K27me3 and repressive domains generally. Interestingly, all promoters showed some variation in regulation by LADs and less repressed promoters were found to reside in more weakly (and perhaps transiently) bound regions.29 These data suggest that LADs and the regulation of genes within them are heterogeneous, even if generally repressive. One such heterogeneous region of LADs are the LAD boundaries, which are enriched in H3K27me3 and are delimited by CTCF and active promoters in the adjacent inter-LAD (iLAD; A-compartment) chromatin. Previous studies had implicated LAD boundaries as important for LAD organization suggesting that sites within LAD boundaries are sufficient to target an ectopic site to the nuclear lamina through accumulation of histone PTMs (H3K9me2/3 and H3K27me3). But what happens when a LAD boundary is removed altogether? A recent study in developing murine T cells found that removing a LAD boundary at the T-cell Receptor locus (TCR) led to loss of lamina interaction concomitant with the spread of euchromatin, aberrant enhancer-promoter interactions and upregulation of TCR gene segments proximal to the boundary.25
Lamins dynamically regulate chromatin and the INM
Lamins play a crucial role in genome organization by constraining LADs and through dynamic interactions with other proteins at the INM-chromatin interface. Lamins are intermediate filament proteins that underlie the nuclear envelope (NE) acting as an interface between the inner nuclear membrane (INM) and chromatin, providing both structural and regulatory support to both. There are two main subgroups of lamins: 1) the A-type lamins, which include lamin A and lamin C, are mostly expressed in more differentiated cell types and 2) the B-type lamins, which include lamin B1 and lamin B2 and are expressed throughout development
Lamins A and C are alternatively spliced isoforms transcribed from the LMNA gene, while lamin B1 and B2 are transcribed from separate genes. The majority of lamin A and lamin C protein sequence is identical, with lamin A harboring a longer C-terminal tail. This tail undergoes specific processing steps that, when perturbed, leads to numerous and varied disease phenotypes.30 The different lamin isoforms form distinct irregular networks extending 14 ± 2nm below the NE.31 Each lamin isotype has been shown to form its own network, but conversely perturbations to one network affects the arrangement of the others, suggesting cross-talk between the different meshworks.32 The lamin isotypes are also expressed in different ratios over developmental time and across tissue types, with B type lamins expressed throughout development and lamins A and C increasing expression levels as cells differentiate.33 This increase in lamin A/C expression is, largely, accompanied by a decrease in LBR expression and these two proteins have both been implicated in sequestering LADs to the nuclear lamina. Lamins are decorated with numerous post-translational modifications that attenuate their functions, including regulation by cyclin dependent kinase 1 (Cdk1) which phosphorylates lamins and is vital for lamin network disassembly as cells enter mitosis.34 Lamins, while expressed pleiotropically, also have unique cell type specific roles and interactions, including with cell type specific Nuclear Envelope Transmembrane proteins (NETs), which have been implicated in LAD organization.35–37
The role of B types lamins in genome organization remains unclear. While several studies show that lamin B1 is dispensable for genome organization,38,39one recent study found that disrupted LAD organization in mouse embryonic stem cells depleted of lamins A, B, and C (triple knockout or TKO) can be rescued by reintroducing lamin B1.40 Another intriguing study found that lamin B1 associates with TAD boundaries dynamically during epithelial to mesenchymal transition (EMT) in development, potentially alluding to a role for lamin B in the establishment of new LADs during differentiation.41 One intriguing possibility is that cells at different stages of differentiation may exhibit a different dependence on lamin B1 for LAD organization, with earlier developmental stages and interactions of LADs with LBR perhaps more reliant on the expression of this isotype.
In contrast, A-type lamins are crucial to the maintenance of heterochromatic LADs at the nuclear periphery, as well as for LAD and overall chromosome compaction in more differentiated cell types.11,42 It remains unclear if these LAD/lamina interactions are direct, but a recent proteomic study investigating the overlap between the proteome of LADs and the INM, found that some chromatin binding proteins, such as PRR14 (proline rich 14) and MECP2 (methyl CpG binding protein 2), are enriched on both LADs and with lamins, suggesting that such proteins might contribute to the apparent scaffolding of LADs at the INM [Wong et al., in press, Life Science Alliance, Figure 2]. Furthermore, lamin A/C interacts with histone modifying proteins which contribute to gene repression, such as histone deacetylases (HDAC). For example HDAC2 interacts with lamin A/C during oxidative stress to allow for the upregulation of response factors.43 Recently, lamin A/C has also been shown to dynamically recruit a regulator of HDAC2, PCAF, during differentiation of myoblasts leading to gene silencing ot target genes.44,38,45–47 In addition, previous studies found that HDAC3 interacts with lamin A/C and lap2β (lamina associated polypeptide 2β) and is important for recruiting test loci to the nuclear lamina. More recently this modification was found to be required for LAD organization in cardiomyocytes48. Like HDAC2, HDAC3 has also been shown to interact with PCAF.
Figure 2: The interface at the nuclear lamina.
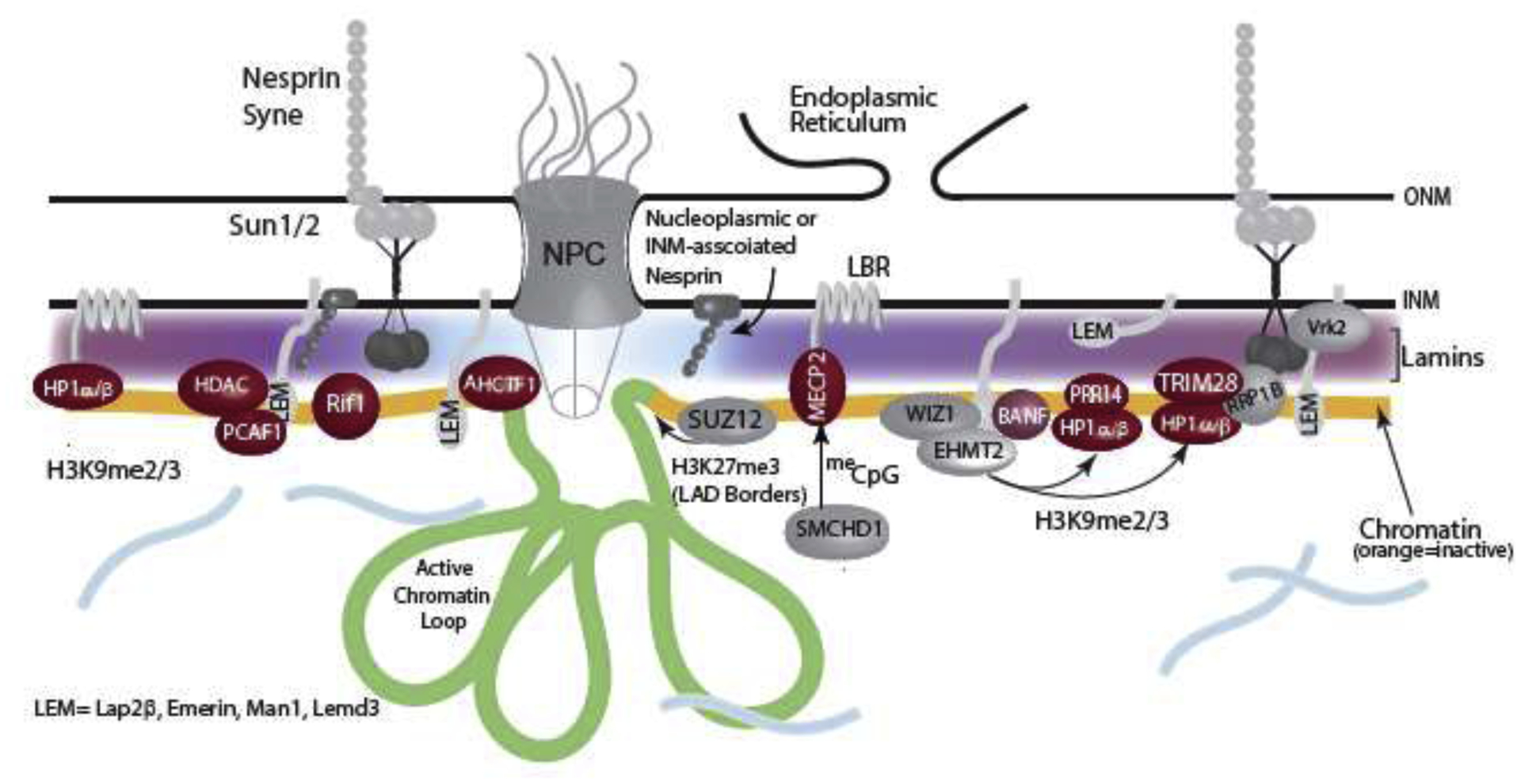
A schematic representing the complex protein-DNA interface at the nuclear lamina where lamins mediate interactions between nuclear envelope proteins and chromatin. Here lamins A and B are represented in red and lamin C is shown in cyan. Lamins have different dispositions across the nuclear periphery, with lamin C displaying a higher concentration near NPCs than other lamin variants. Lamins interact with and help anchor many INM proteins, including LEM domain containing proteins such as lap2β, emerin, man1 and Lemd3. Lamins also interact with cytoskeletal proteins, such as nesprins and SUN and KASH domain proteins (LINC) complexes. Lamins are necessary to constrain heterochromatic LADs to the nuclear lamina. LADs are enriched in epigenetic machinery involved in maintaining a repressive state, including the enzymes involved in H3K27 methylation, H3K9 methylation and DNA methylation. Non-INM proteins identified as interacting with LADs and lamins are shown in red and these potentially serve as ‘middlemen’ in mediating LAD association with the lamina. Many of the proteins in this peripheral zone facilitate and help maintain a repressive chromatin state. It is important to note that the regions at the nuclear periphery are heterogeneous, with active regions juxtaposed to the nuclear pore complexes (NPC). This heterogeneity could lead to differential regulation of lamina-proximal sequences and differential activity of promoters within LADs.
Conversely, lamin A/C has also been shown to play roles in regulating and organizing euchromatic regions of the genome, including splicing, DNA damage response, and transcriptional regulation. Some recent studies have focused on more direct interactions of lamin A/C with euchromatin. For instance, a recent study found that lamin A/C binds euchromatin early in development preceding the transition of these regions to heterochromatin, process facilitated by GlcNAcylated histone H2B.49 While this study implicated lamin binding as a precursor to repression and LAD status, other studies have shown persistent interaction of non-polymerized lamins with euchromatin. One recent study found that phosphorylated (S22P) nucleoplasmic lamin A/C binds enhancer regions and affects transcription, possibly through interactions with other proteins such as Lap2α (lamina associated polypeptide 2a), which had previously been implicated in enhancing lamin A/C binding to euchromatin.50,51 Intriguingly, the S22P modification was found at higher levels on lamin C than lamin A, implicating a strong role for lamin C in interactions on euchromatic regions of chromatin. Strikingly, a distinct role has also been recently found for lamin C in organizing compartmentalization by promoting proper self-aggregation of LADs and for LAD tethering at the nuclear periphery.38 Taken together, These results suggest that there may be a link between the apparent euchromatic roles of lamins and their presumed roles in organization of LADs and higher order chromatin structures.
Beyond potential direct chromatin regulation and organization, lamins also interact with INM proteins, which also interact with chromatin binders and modifiers, thus indirectly affecting chromatin organization and regulation. Numerous studies have investigated the protein:protein interactions of the nuclear lamina (the ‘laminome’). More recently, we identified the LAD proteome in mouse embryonic fibroblasts and integrated these data with previous proteomic analyses of lamin interactions to develop a preliminary map of the NE/lamina/chromatin interface.52 A-type lamins in particular seem to display many important interactions with INM proteins, including sequestering resident INM proteins to the INM . While this function of lamins has been well-studied, some recent studies highlight the roles lamins play in spatio-temporal regulation of the NE. For example, in agreement with previous findings, a recent study found that emerin is unable to properly localize to the INM in the absence of A-type lamins.46 The interaction of these two proteins is important for regulating cytoskeleton and chromatin mobility in concert, again stressing the interconnectedness of this compartment in regulation of chromatin.53 A-type lamins also play a role in regulating NPC distribution. In the absence of both lamin A and C NPCs cluster together rather than dispersing across the NE. Intriguingly, using super-resolution microscopy, lamin C fibers are found to co-localize with NPCs, indicating lamin C may be responsible for the spacing of NPCs. Intriguingly, TPR (Translocated Promoter Region), a protein found in the nucleoplasm and in the basket of NPCs, also interacts with lamins and regulates NPC spacing, although it does not bind A-type lamins exclusively.54,55 Adding to the complexity of these interactions, the lamins and proteins of the INM also interact with the cytoskeleton through LINC complexes, which are more fully discussed_[wong et al in this issue]. The dynamic and spatio-temporally heterogeneous interactions of the INM and lamina (and LINC complexes) with the underlying heterochromatin remain poorly understood. The role of post-translational modifications, for example, is likely to modulate these interactions in response to stress or through the cell cycle.
Other heterochromatin domains
LADs are far from the only heterochromatic domains in the nucleus. Of special note are nucleolar associated domains (NADs) is and recent work has focused on how they relate to LADs. It has been noted for some time that some LADs and NADs overlap.56 This is not surprising in some ways, since lamina invaginations often associate with nucleoli. More recent studies have shown there are two types of NADs, those that overlap with LADs and those that are unique to NADs57–59. It has been proposed that LAD/NAD regions associate with the lamina or nucleolus is stochastically, but it remains unclear whether these regions are scaffolded by the nucleolus alone, or whether they still interact with lamina invaginations56. Interestingly, Centromeric and telomeric heterochromatin do not preferentially interact with the nuclear lamina in mammalian cells. This is particularly intriguing since they harbor H3K9me2/3 modifications. One difference in these regions is an enrichment of histone H3 variants: CENP-A for centromeric heterochromatin, and histone H3.3, instead of the predominant H3.2, in telomeres. However, it remains unclear why different “types” of heterochromatin segregate independently into different genome compartments (Figure 1). It is, however, clear that lamin association is but one pathway to organize, sequester, and regulate heterochromatin.
The lamina interface through cell division
The complex interplay of chromatin, INM proteins, and lamins is perhaps most evident during and after mitosis. As cells enter mitosis, the complex set of interaction between lamins, INM and chromatin are disrupted and, after the segregation of chromosomes into daughter cells, this network must be functionally re-established. At the onset of mitosis there is large scale reorganization of genome organization to form the mitotic chromosome structure, including dissociation of LADs from lamin proteins, which become dispersed into the cytoplasm (Figure 3).12,60–62 While TAD and loop organization, TAD insulation, and compartment organization are all governed by cell cycle dynamics, with the most dramatic reorganization occurring in mitosis and as cells exit mitosis.61 Studies using a chicken B cell line, DT40, harboring a regulatable CDK1 to tightly control entry into mitosis, found that the mitotic chromatin organization is formed by a nested looping structure determined by length rather than by any other content (LAD/nonLAD identity, histone modifications, sequence identity, gene content etc.) and is anchored by condensins, with condensin I forming the outer scaffold and condensin II the inner scaffold.60,62,12,60–62 Additional studies have revealed the sequence of events and some mechanisms in re-establishing genome organization after mitosis. As cells enter into anaphase and sister chromatids begin to segregate, LADs of individual chromosomes start to agglomerate, independent of NE anchoring and prior to complete NE reformation (Figure 3).12,61 As cell transit out of mitosis, there is a transition from condensin driven organization (mitotic) to cohesin driven organization (interphase) where chromosomes initially appear to exist in untangled loops without much structure or TAD organization.63,64 Intriguingly, it appears that CTCF remains partially loaded on mitotic chromosomes and resumes full binding at anaphase/telophase, with cohesin more gradually loading onto chromosomes starting in telophase and into G1.63,64
Figure 3: Broad view of genome organization through mitosis.
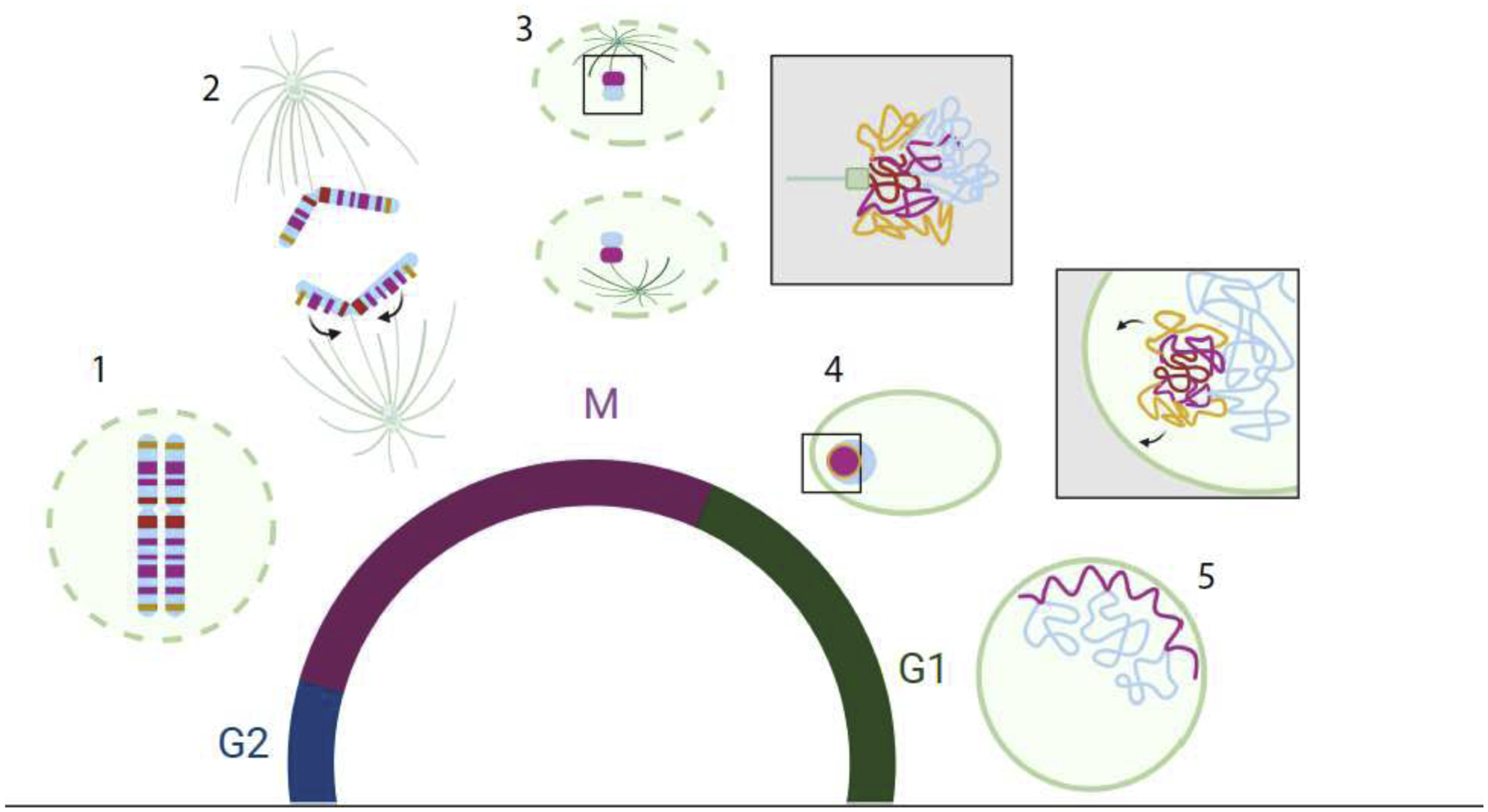
A schematic representing the progression of genome organization through mitosis and re-establishment. Lamins and spindles (green) centromere (dark blue) Centromere proximal LADS (red) telomere proximal LADs (Orange) LADs (pink) nonLADs (light blue). Clockwise from bottom left: 1. (M) As cells enter mitosis the nuclear envelope breaks down (green dashed line) and nuclear lamins become phosphorylated and disperse into the cytoplasm. At this time interphase genome organization is also dismantled and chromosomes become configured into a linearly arranged looping structure. 2. As cells proceed into anaphase and sister chromosomes begin to segregate the LAD regions begin to coalesce into an intrachromosomal cluster towards the centromere (arrows), excluding nonLAD regions, thus separating LADs/nonLADs (A/B compartment). Telomeric proximal LADs are shown in yellow. 3. As the nuclear envelope and lamina reform the LAD regions remain in tight agglomerates and separated from nonLAD regions. 4. (G1) As cells enter early G1 the nonLAD regions (A-comaprtment) begin to form looping structures and the LAD agglomerates increasingly associate with the nuclear lamina where they eventually become constrained. Possibly due to the orientation of the chromosomes when LAD coalesce, the telomere proximal LADs make contact with the nuclear envelope first followed later by centromere proximal LADs. 5. As G1 continues, A/B compartmentalization strengthens, with increased inter-chromosomal contact within the A-compartment, and increased interaction (flattening) of LADs with the nuclear lamina. Black boxes indicate magnified views.
This transition, anaphase/telophase, is also when the NE and nuclear lamina must begin to reform. In more differentiated cell types, lamin B is incorporated first into the reforming nuclear lamina at the transition from anaphase to telophase, followed quickly by lamin A.38,65,66. At this time lamin C, likely retaining its S22P modification, remains nucleoplasmic. Intriguingly, chromatin regions destined to be LADs are not yet preferentially positioned at the nuclear lamina, but instead appear by microscopy as intrachromosomal clusters in the nucleoplasm surrounded by lamin C and active regions of the genome.38,51 In agreement with this finding, early separation of LADs/nonLADs (A/B chromatin), analyses of A/B compartment and TAD organization post mitosis by Hi-C in synchronized erythroid cells found that A/B compartmentalization occurred rapidly and strengthened over G1, while TAD organization lagged, with smaller sub-TAD organization slowly yielding to larger multi-domain/multi-loop TADs over G1. While the A-compartment greatly expands in early G1, concomitant with elevated levels of transcription, the B-compartment intensifies, with LADs increasing their interactions at the NE.12,67,68. As LADs contact the nuclear lamina, the LADs at the distal portion of the chromosome near the telomere make contact first in early G1 and, over the course of several hours, the more central portions become anchored, coinciding with the strengthening of compartmentalization and the flattening of domains against the lamina.12,69 Furthermore, across this same time course lamin C becomes belatedly incorporated at the nuclear lamina. In the absence of lamin C during this reorganization, LAD clusters fail to become established at the periphery.38 As cells proceed through G1 and into S phase, compartmentalization and LAD contacts at the lamina continue to strengthen.61,69 Intriguingly, a study pre-implantation embryos suggests that LAD organization is established de novo almost immediately after fertilization and is blocked by over-expression of KDM5 (Lysine Demethylase 5b, JARID5B), a histone demethylase of histone H3 lysine 4, suggesting that euchromatin contributes to LAD organization in these early stages of development, reminiscent of the findings for a role for H3K27Ac in LAD organization in C. elegans70. While these studies in disparate cell types collectively reveal some of the dynamic forces of 3D genome and lamina organization, either through the cell cycle or de novo organization in the embryo, there is not yet much insight into the interplay of these transitions in genome structure and the rebuilding of the INM or a clear understanding of how other types of heterochromatin, such as telomeres and centromeres, may factor into this dynamic reorganization.
Impacts of Laminopathies on Genome Organization and Gene Regulation
Because A-type lamins are foundational for this complex dynamic interface between the INM and chromatin, when they are perturbed they lead to severe and varied phenotypes. The diseases that result from mutations in LMNA, or other INM proteins that heavily interact with lamin A/C, are collectively termed “laminopathies”. Due to the complex interactions of LMNA and varied expression levels during development and in different cells, laminopathies display a wide breadth of phenotypes. Broadly, laminopathies fall into five general categories: lipodystrophies, skeletal muscle disorders, peripheral neuropathies, systemic, and premature aging. Most laminopathies are autosomal dominant and generally cause late-onset degeneration of mesenchymal derived cells, such as muscle, heart, adipocytes, peripheral neurons, skin and bones.71 Nearly 500 disease causing mutations have mapped to the LMNA gene, each with its own specific phenotype, and many of these mutations are dominant. A variety of models have been suggested to explain the variety of cell and tissue specific phenotypes. Indeed, it appears that disruption of lamins or lamin binding proteins affect numerous cellular functions including perturbations in mechano-sensation and resilience, DNA repair, signaling pathways, interactions with specific transcription factors (such as SREBP1 and E2F1), altered interactions with NETs, and altered genome organization, none of which are mutually exclusive37,72–74;37,75–78 (and reviewed in71). Because this review focuses on lamins and LADs and genome organization, we will highlight a few studies that suggest genome dysregulation plays a role in disease pathology.
Hutchinson’s-Gilford Progeria Syndrome (HGPS), the most well-known laminopathy, is a rare mendelian disorder caused by a dominant mutation leading to aberrant splicing and subsequent protein processing defects that fail to cleave a C-terminal farnesyl group.79–82 Patients expressing progerin, the name for the aberrant and permanently farnesylated protein, experience severe symptoms that are consistent with hallmarks of premature aging, including hair loss, loss of subcutaneous fat, vascular disease and early death. Molecular effects of these mutations include morphological anomalies of cell nuclei, disruption and loss of heterochromatin organization, aberrant mitosis, problems in DNA replication and repair, and altered gene transcription profiles (Reviewed in83). The first observable phenotypes of HGPS were the altered nuclear morphology (“blebby” nuclei) and lamin organization as well as a loss of peripheral heterochromatin. These early findings were supported by HiC mapping of patient fibroblasts, where widespread genome dysregulation and disorganization can be observed (after extended culture) when compared to control fibroblasts.84 This is thought to be caused by changes in H3K27me3 deposition, which show new enrichment in some gene rich regions and losses in gene poor regions, similar to what is observed in cellular senescence models85.84 A more recent study suggests these observed epigenetic changes could also be linked to loss of lamin A/C interactions with HDAC2 under oxidative stress when progerin is expressed, leading to global changes in heterochromatin state.43 Interestingly, other types of heterochromatin are also dysregulated in this syndrome. HGPS stemming from the lamin A E145K mutation results in abnormal clustering of centromeres along with the mislocalization of telomeres.60 The diffusion of centromeric and telomeric heterochromatin are altered in the presence of lamin A depletion as well.61
Genome disorganization has also been observed Emery-Dreifuss Muscular Dystrophy (EDMD), another laminopathic condition caused by any one of numerous mutations in either lamin A or Emerin, an inner nuclear membrane protein. Because laminopathies result in late-onset and tissue-specific defects, the use of model organisms and cell culture models to elucidate disease mechanisms are providing much needed mechanistic insights. In humans, one such mutation that causes EDMD is LMNA-Y45C. In C. elegans, which has only one lamin isoform, the corresponding mutation is LMN-1 Y59C. In a recent elegant study, C. elegans harboring the LMN-1 Y59C mutation showed an increased anchoring of heterochromatin to the nuclear lamina and EDMD like phenotypes18. This hyper-tethering of heterochromatin was ablated when CEC-4, the chromodomain protein that mediated heterochromatin anchoring to the nuclear periphery, was ablated. The hyper-tethered regions were enriched in E2F1 binding sites, in agreement with previous reports of disruption of E2F1 regulation in some laminopathic mutations. Another recent study showed changes in LAD organization during muscle differentiation is disrupted with a different EDMD-causing mutation (LMNA H222P). In the LMNA-H22P mutation, some LADs are not recruited to the lamina and some nonLADs become aberrantly anchored at the periphery. This also leads to downstream gene expression changes, especially of the Sox2 transcription factor, which inhibits differentiation.76 Similar results were obtained with the loss of Emerin. This mechanism of transcriptional disruption, whether by sequestering transcription factor binding sites or through regulation of transcription factors, is not unique to EDMD. Impacts on genome organization often cause downstream transcriptional changes such as in Familial Partial Lipodystrophy (FPLD, lamin A R482W), which results from a mutation in the IG tail region of lamin A that results in an upregulation of a miRNA (MIR335) which is normally in a LAD and silenced, but becomes aberrantly upregulated.86 This mutation disrupts the local chromatin environment, shifting H3K27me3 domains to H3K27Ac, allowing enhancer promoter coupling and expression of this microRNA.86 Furthermore, this mutation has recently been shown to have no effect on the structural properties of lamin A, suggesting and uncoupling of mechano- and signaling properties of lamin A from its role in regulating this locus.87
However, these few examples of genome disorganization in laminopathic mutations are far from the rule. Indeed, the extent to which genome organization is affected in most laminopathies remains a mystery. Complicating this picture are recurrent findings that A-type lamins also have roles in the nucleoplasm and euchromatin is likely to play a role in LAD organization18,70,88. A recent study linked nucleoplasmic, presumably unpolymerized lamins, directly to transcription by showing that lamin A/ C binds to enhancers when phosphorylated at Serine 22 (S22P). In cells expressing progerin, S22P lamin A and C were shown to bind novel sights, causing an upregulation of clinically relevant HGPS genes.51
Conclusion
The nuclear peripheral zone is a unique environment where nuclear lamins act as a bridge between the cytoskeleton, nuclear envelope, and chromatin. These interactions are highly dynamic and serve important regulatory functions that lead to often severe disease phenotypes when perturbed. A recent and intriguing focus of the field has been cell division, during which both lamins and LADs are dynamically reorganized. Recent findings have shed light on the spatio-temporal regulation of genome organizer proteins (e.g. CTCF, cohesins, lamins), transcription factors, and their interactions with chromatin domains. As we focus on such dynamic interactions, it will be important to determine how LADs and other heterochromatin compartments, such as telomeres and centromeres, assemble and contribute to global genome organization and function.
Acknowledgements
K.L.R. And V.E.H. were funded from NIH grant R01GM132427 and V.E.H. partly funded from NIH training grant T32GM07814. K.S, was funded through the SARE (Johns Hopkins University) and Ingenuity (Baltimore Polytechnic Institute) programs.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Greil F, Moorman C & van Steensel B DamID: mapping of in vivo protein-genome interactions using tethered DNA adenine methyltransferase. Methods Enzymol. 410, 342–359 (2006). [DOI] [PubMed] [Google Scholar]
- 2.Vogel MJ, Peric-Hupkes D & van Steensel B Detection of in vivo protein–DNA interactions using DamID in mammalian cells. Nature Protocols vol. 2 1467–1478 (2007). [DOI] [PubMed] [Google Scholar]
- 3.Dekker J Capturing Chromosome Conformation. Science vol. 295 1306–1311 (2002). [DOI] [PubMed] [Google Scholar]
- 4.Dixon JR et al. Topological domains in mammalian genomes identified by analysis of chromatin interactions. Nature 485, 376–380 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lieberman-Aiden E et al. Comprehensive mapping of long-range interactions reveals folding principles of the human genome. Science 326, 289–293 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rao SSP et al. A 3D map of the human genome at kilobase resolution reveals principles of chromatin looping. Cell 159, 1665–1680 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Phillips-Cremins JE et al. Architectural Protein Subclasses Shape 3D Organization of Genomes during Lineage Commitment. Cell vol. 153 1281–1295 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Luperchio TR et al. Chromosome Conformation Paints Reveal the Role of Lamina Association in Genome Organization and Regulation. doi: 10.1101/122226. [DOI] [Google Scholar]
- 9.Falk M et al. Heterochromatin drives compartmentalization of inverted and conventional nuclei. Nature vol. 570 395–399 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schwarzer W et al. Two independent modes of chromatin organization revealed by cohesin removal. Nature 551, 51–56 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harr JC et al. Directed targeting of chromatin to the nuclear lamina is mediated by chromatin state and A-type lamins. J. Cell Biol 208, 33–52 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- *12.Luperchio TR et al. The repressive genome compartment is established early in the cell cycle before forming the lamina associated domains. doi: 10.1101/481598. [DOI] [Google Scholar]; LAD formation is established in 3 steps; 1) LADs aggregate together as early as anaphase prior to nuclear lamina re-formations 2)LADs slowly make their way to the nuclear periphery over the course of several hours into G1 3)LADs spread out along the lamina strengthening their contact with the lamin and other LAD regions into S phase.
- 13.Guelen L et al. Domain organization of human chromosomes revealed by mapping of nuclear lamina interactions. Nature 453, 948–951 (2008). [DOI] [PubMed] [Google Scholar]
- 14.Towbin BD et al. Step-wise methylation of histone H3K9 positions heterochromatin at the nuclear periphery. Cell 150, 934–947 (2012). [DOI] [PubMed] [Google Scholar]
- 15.Dunlevy KL et al. The PRR14 heterochromatin tether encodes modular domains that mediate and regulate nuclear lamina targeting. Journal of Cell Science vol. 133 jcs240416 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Poleshko A & Katz RA Specifying peripheral heterochromatin during nuclear lamina reassembly. Nucleus 5, 32–39 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bian Q, Anderson EC, Yang Q & Meyer BJ Histone H3K9 methylation promotes formation of genome compartments in via chromosome compaction and perinuclear anchoring. Proc. Natl. Acad. Sci. U. S. A 117, 11459–11470 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- *18.Harr JC et al. Loss of an H3K9me anchor rescues laminopathy-linked changes in nuclear organization and muscle function in an Emery-Dreifuss muscular dystrophy model. Genes Dev. 34, 560–579 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]; This study provides an important mechanistic insight into an EDMD-causing mutation in lamin A (LMNA-Y45C). This mutation leads to muscular dystrophy phenotypes in C. elegans and leads to a hyper-tethering of heterochromatin to the nuclear lamina. This mutation can be suppressed by removing the chromodomain protein CEC-4, which is required to anchor heterochromatin to the nuclear lamina. The dysregulated regions of the genome are enriched for E2F1 binding sites.
- 19.Gonzalez-Sandoval A & Gasser SM Mechanism of chromatin segregation to the nuclear periphery in embryos. Worm vol. 5 e1190900 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harr JC, Gonzalez-Sandoval A & Gasser SM Histones and histone modifications in perinuclear chromatin anchoring: from yeast to man. EMBO Rep. 17, 139–155 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gonzalez-Sandoval A et al. Perinuclear Anchoring of H3K9-Methylated Chromatin Stabilizes Induced Cell Fate in C. elegans Embryos. Cell 163, 1333–1347 (2015). [DOI] [PubMed] [Google Scholar]
- 22.Falk M et al. Heterochromatin drives compartmentalization of inverted and conventional nuclei. Nature vol. 570 395–399 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Larson AG et al. Liquid droplet formation by HP1α suggests a role for phase separation in heterochromatin. Nature 547, 236–240 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- *24.Strom AR et al. Phase separation drives heterochromatin domain formation. Nature 547, 241–245 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]; Heterochromatin domains show the properties of liquid-liquid phase separated compartments and this phase separation may be driven by HP1α, a heterochromatin binding protein, that forms liquid phase droplets in vitro and in vivo.
- *25.Chen S et al. A Lamina-Associated Domain Border Governs Nuclear Lamina Interactions, Transcription, and Recombination of the Tcrb Locus. Cell Rep. 25, 1729–1740.e6 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]; Deletion of a LAD border at the Tcrb locus causes spreading of H3K27ac into the previously LADed region and causes mis-expression and V(D)J recombination of normally repressed regions.
- 26.Bian Q, Khanna N, Alvikas J & Belmont AS β-Globin cis-elements determine differential nuclear targeting through epigenetic modifications. J. Cell Biol 203, 767–783 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peric-Hupkes D et al. Molecular maps of the reorganization of genome-nuclear lamina interactions during differentiation. Mol. Cell 38, 603–613 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kind J et al. Genome-wide maps of nuclear lamina interactions in single human cells. Cell 163, 134–147 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leemans C et al. Promoter-Intrinsic and Local Chromatin Features Determine Gene Repression in LADs. Cell 177, 852–864.e14 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.González-Cruz RD, Dahl KN & Darling EM The Emerging Role of Lamin C as an Important LMNA Isoform in Mechanophenotype. Frontiers in Cell and Developmental Biology vol. 6 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Turgay Y et al. The molecular architecture of lamins in somatic cells. Nature 543, 261–264 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shimi T et al. Structural organization of nuclear lamins A, C, B1, and B2 revealed by superresolution microscopy. Mol. Biol. Cell 26, 4075–4086 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Solovei I et al. LBR and lamin A/C sequentially tether peripheral heterochromatin and inversely regulate differentiation. Cell 152, 584–598 (2013). [DOI] [PubMed] [Google Scholar]
- 34.Simon DN & Wilson KL Partners and post-translational modifications of nuclear lamins. Chromosoma 122, 13–31 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- *35.de Las Heras JI et al. Tissue-specific NETs alter genome organization and regulation even in a heterologous system. Nucleus 8, 81–97 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]; Tissue specific NETs expressed in their non-native cell type can induce gene expression and LAD re-positioning suggesting a role for NETs in genome organization leading to potential cell identity changes.
- 36.Robson MI et al. Tissue-Specific Gene Repositioning by Muscle Nuclear Membrane Proteins Enhances Repression of Critical Developmental Genes during Myogenesis. Mol. Cell 62, 834–847 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Worman HJ & Schirmer EC Nuclear membrane diversity: underlying tissue-specific pathologies in disease? Curr. Opin. Cell Biol 34, 101–112 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- *38.Wong X, Hoskins VE, Harr JC, Gordon M & Reddy KL Lamin C regulates genome organization after mitosis. doi: 10.1101/2020.07.28.213884. [DOI] [PMC free article] [PubMed] [Google Scholar]; Lamin C is the specific lamin isoform implicated in LAD self-aggregation and association with the lamina in MEF cells through its potential role in stabilizing LAD domains, prior to nuclear envelope association, as cells exit mitosis and enter G1 phase.
- 39.Amendola M & Steensel B Nuclear lamins are not required for lamina‐ associated domain organization in mouse embryonic stem cells. EMBO reports vol. 16 610–617 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zheng X et al. Lamins Organize the Global Three-Dimensional Genome from the Nuclear Periphery. Mol. Cell 71, 802–815.e7 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pascual-Reguant L et al. Lamin B1 mapping reveals the existence of dynamic and functional euchromatin lamin B1 domains. Nat. Commun 9, 3420 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- *42.Falk M et al. Heterochromatin drives compartmentalization of inverted and conventional nuclei. Nature vol. 570 395–399 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]; Using the inverted genome organization of rod cells as a model to uncouple lamina interactions from heterochromatin domain formation, this group found that the self-attraction of heterochromatin, but not euchromatin, is crucial for genome compartmentalization. Using a polymer model to further simulate the interactions they found that a peripheral attraction force can cause recapitulation on non-inverted genome organization
- 43.Mattioli E et al. Altered modulation of lamin A/C-HDAC2 interaction and p21 expression during oxidative stress response in HGPS. Aging Cell 17, e12824 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Santi S, Cenni V, Capanni C, Lattanzi G & Mattioli E PCAF Involvement in Lamin A/C-HDAC2 Interplay during the Early Phase of Muscle Differentiation. Cells 9, (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fong LG et al. Prelamin A and lamin A appear to be dispensable in the nuclear lamina. J. Clin. Invest 116, 743–752 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xie W et al. A-type Lamins Form Distinct Filamentous Networks with Differential Nuclear Pore Complex Associations. Curr. Biol 26, 2651–2658 (2016). [DOI] [PubMed] [Google Scholar]
- 47.de Toledo M et al. Lamin C Counteracts Glucose Intolerance in Aging, Obesity, and Diabetes Through β-Cell Adaptation. Diabetes 69, 647–660 (2020). [DOI] [PubMed] [Google Scholar]
- 48.Poleshko A et al. Genome-Nuclear Lamina Interactions Regulate Cardiac Stem Cell Lineage Restriction. Cell 171, 573–587.e14 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rønningen T et al. Prepatterning of differentiation-driven nuclear lamin A/C-associated chromatin domains by GlcNAcylated histone H2B. Genome Res. 25, 1825–1835 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gesson K et al. A-type lamins bind both hetero- and euchromatin, the latter being regulated by lamina-associated polypeptide 2 alpha. Genome Res. 26, 462–473 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- *51.Ikegami K, Secchia S, Almakki O, Lieb JD & Moskowitz IP Phosphorylated Lamin A/C in the Nuclear Interior Binds Active Enhancers Associated with Abnormal Transcription in Progeria. Dev. Cell 52, 699–713.e11 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]; A-type lamins are retained in the nucleoplasm during interphase when S22P modified and bind to euchromatic regions through a facilitator protein. This modification is found preferentially on lamin C. Disruption of S22P binding by progerin lamin A expression might contribute to aberrant gene regulation in progeria.
- *52.Cutler JA et al. Mapping the micro-proteome of the nuclear lamina and lamin associated domains. doi: 10.1101/828210. [DOI] [PMC free article] [PubMed] [Google Scholar]; Identification of the complex network of proteins at the nuclear lamina via mapping the inner nuclear envelope proteome, the LAD proteome and cross-referencing these proteomes to publicly available lamin proteomes to find 3 zones of interactions: 1)nuclear lamina and inner nuclear envelope specific, LAD specific, and mediators between chromatin and the nuclear lamina.
- *53.Santi S, Cenni V, Capanni C, Lattanzi G & Mattioli E PCAF Involvement in Lamin A/C-HDAC2 Interplay during the Early Phase of Muscle Differentiation. Cells 9, (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]; LaminA recruits PCAF to the nuclear lamina and the association of PCAF with lamin A is high in myoblasts and decreases in differentiating myotubes. In cells expressing Emery-dreifuss mutated lamin A, PCAF import into the nucleus and lamin-PCAF interactions are downregulated.
- 54.Xie W et al. A-type Lamins Form Distinct Filamentous Networks with Differential Nuclear Pore Complex Associations. Curr. Biol 26, 2651–2658 (2016). [DOI] [PubMed] [Google Scholar]
- 55.Fišerová J et al. Nuclear pore protein TPR associates with lamin B1 and affects nuclear lamina organization and nuclear pore distribution. Cell. Mol. Life Sci 76, 2199–2216 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cremer T & Cremer C Chromosome territories, nuclear architecture and gene regulation in mammalian cells. Nature Reviews Genetics vol. 2 292–301 (2001). [DOI] [PubMed] [Google Scholar]
- *57.Vertii A et al. Two contrasting classes of nucleolus-associated domains in mouse fibroblast heterochromatin. Genome Res. 29, 1235–1249 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]; There are two distinct categories of Nucleolar Associated Domains (NADs) found in MEFs; type I) those that are found both at the lamina and nucleolus and type II) those that are uniquely nucleolar domains. Type I NADs display the hallmark characteristics of LADs where as type II NADs are more highly transcribed and enriched for H3K27me3.
- *58.Bizhanova A, Yan A, Yu J, Zhu LJ & Kaufman PD Distinct features of nucleolus-associated domains in mouse embryonic stem cells. Chromosoma 129, 121–139 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]; mESCs also display the two types of NADs found in MEFS; however, type II NADS were less abundant and contained less H3K27me3 than those found in MEF cells. This study also found that cell type specific NADs were enriched for developmentally regulated genes.
- 59.Bersaglieri C & Santoro R Genome Organization in and around the Nucleolus. Cells 8, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- *60.Gibcus JH et al. A pathway for mitotic chromosome formation. Science 359, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]; The mitotic chromosome is formed by nested looping structure determined by length rather than by any other content (histone modifications, sequence identity, gene content etc) which is anchored by condensin I and condensinII. Where condensin I forms the outer scaffold and condensin II forms this inner scaffold.
- *61.Nagano T et al. Cell-cycle dynamics of chromosomal organization at single-cell resolution. Nature 547, 61–67 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]; Genome organization is dynamic during the cell cycle. Compartmentalization disappears at the beginning of mitosis and gradually re-strengthens during G. It continues to strengthen even into S phase.
- 62.Naumova N et al. Organization of the mitotic chromosome. Science 342, 948–953 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- *63.Abramo K et al. A chromosome folding intermediate at the condensin-to-cohesin transition during telophase. Nat. Cell Biol 21, 1393–1402 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]; During telophase chromosome architecture transitions from being condensin driven to being cohesin dirven and chromosomes exhibit a lack of looping structure by HiC which could possibly represent untangled loops. At the onset of cytokinesis CTCF and cohesin begin to load and form TADs and loops.
- *64.Zhang H et al. Chromatin structure dynamics during the mitosis-to-G1 phase transition. Nature 576, 158–162 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]; Compartments are formed quickly after mitosis and TAD structures in the A compartment are formed more slowly starting with smaller looping structures followed by multi-domain TADs. Further CTCF is retained on chromosomes through mitosis and cohesin is loaded post-mitosis when loops gradually grow consistent with a loop extrusion mechanism.
- 65.Oneill C Evidence for two distinct mechanisms of anchorage stimulation in freshly explanted and 3T3 Swiss mouse fibroblasts. Cell vol. 44 489–496 (1986). [DOI] [PubMed] [Google Scholar]
- 66.Moir RD, Yoon M, Khuon S & Goldman RD Nuclear lamins A and B1: different pathways of assembly during nuclear envelope formation in living cells. J. Cell Biol 151, 1155–1168 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nagano T et al. Cell-cycle dynamics of chromosomal organization at single-cell resolution. Nature 547, 61–67 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hsiung CC-S et al. A hyperactive transcriptional state marks genome reactivation at the mitosis-G1 transition. Genes Dev. 30, 1423–1439 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.van Schaik T, Vos M, Peric-Hupkes D & van Steensel B Cell cycle dynamics of lamina associated DNA. bioRxiv 2019.12.19.881979 (2019) doi: 10.1101/2019.12.19.881979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Borsos M et al. Genome-lamina interactions are established de novo in the early mouse embryo. Nature 569, 729–733 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wong X & Stewart CL The Laminopathies and the Insights They Provide into the Structural and Functional Organization of the Nucleus. Annu. Rev. Genomics Hum. Genet 21, 263–288 (2020). [DOI] [PubMed] [Google Scholar]
- 72.Osmanagic-Myers S, Dechat T & Foisner R Lamins at the crossroads of mechanosignaling. Genes Dev. 29, 225–237 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Le Dour C et al. Extracellular matrix remodeling and transforming growth factor-β signaling abnormalities induced by lamin A/C variants that cause lipodystrophy. J. Lipid Res 58, 151–163 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Vadrot N et al. The p.R482W substitution in A-type lamins deregulates SREBP1 activity in Dunnigan-type familial partial lipodystrophy. Hum. Mol. Genet 24, 2096–2109 (2015). [DOI] [PubMed] [Google Scholar]
- 75.Briand N & Collas P Laminopathy-causing lamin A mutations reconfigure lamina-associated domains and local spatial chromatin conformation. Nucleus 9, 216–226 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Perovanovic J et al. Laminopathies disrupt epigenomic developmental programs and cell fate. Sci. Transl. Med 8, 335ra58 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bianchi A, Manti PG, Lucini F & Lanzuolo C Mechanotransduction, nuclear architecture and epigenetics in Emery Dreifuss Muscular Dystrophy: tous pour un, un pour tous. Nucleus 9, 276–290 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mewborn SK et al. Altered chromosomal positioning, compaction, and gene expression with a lamin A/C gene mutation. PLoS One 5, e14342 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Eriksson M et al. Recurrent de novo point mutations in lamin A cause Hutchinson-Gilford progeria syndrome. Nature 423, 293–8 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Goldman RD et al. Accumulation of mutant lamin A causes progressive changes in nuclear architecture in Hutchinson-Gilford progeria syndrome. Proc. Natl. Acad. Sci. U. S. A 101, 8963–8968 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.De Sandre-Giovannoli A et al. Lamin A Truncation in Hutchinson-Gilford Progeria. Science 300, 2055 (2003). [DOI] [PubMed] [Google Scholar]
- 82.Dechat T et al. Alterations in mitosis and cell cycle progression caused by a mutant lamin A known to accelerate human aging. Proc. Natl. Acad. Sci. U. S. A 104, 4955–4960 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Vidak S & Foisner R Molecular insights into the premature aging disease progeria. Histochem. Cell Biol 145, 401–417 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.McCord RP et al. Correlated alterations in genome organization, histone methylation, and DNA-lamin A/C interactions in Hutchinson-Gilford progeria syndrome. Genome Res. 23, 260–269 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Shah PP et al. Lamin B1 depletion in senescent cells triggers large-scale changes in gene expression and the chromatin landscape. Genes Dev. 27, 1787–1799 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Oldenburg A et al. A lipodystrophy-causing lamin A mutant alters conformation and epigenetic regulation of the anti-adipogenic MIR335 locus. Journal of Cell Biology vol. 216 2731–2743 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mio M et al. Structural instability of lamin A tail domain modulates its assembly and higher order function in Emery-Dreifuss muscular dystrophy. Biochem. Biophys. Res. Commun 512, 22–28 (2019). [DOI] [PubMed] [Google Scholar]
- 88.Cabianca DS et al. Active chromatin marks drive spatial sequestration of heterochromatin in C. elegans nuclei. Nature 569, 734–739 (2019). [DOI] [PubMed] [Google Scholar]


