Abstract
Anhedonia, marked by deficits in reward processing, is a prominent symptom of several psychiatric conditions and has been shown to influence functional connectivity between reward-related regions. However, the unique influence of anhedonia severity on reward circuit connectivity in posttraumatic stress disorder (PTSD) remains unclear. To address this, we examined resting-state functional connectivity (rsFC) of the ventral striatum as a function of anhedonia for individuals with PTSD. Resting-state functional MRI scans and behavioral assessments were collected for 71 women diagnosed with PTSD. Seed-based voxelwise rsFC analyses for left and right nucleus accumbens (NAcc) seed regions of interest were performed. Voxelwise regression analyses were conducted to examine the relationship between anhedonia severity and rsFC of left and right NAcc. Results indicated that greater anhedonia severity was associated with reduced rsFC between the left NAcc and a cluster in the left caudate extending to the thalamus. This relationship between anhedonia and rsFC remained significant after controlling for PTSD symptom severity or depression severity. Our findings suggest that reward circuit dysfunction at rest is associated with anhedonia in PTSD. These results further contribute to our understanding of the neural correlates of anhedonia in psychiatric conditions.
Keywords: anhedonia, posttraumatic stress disorder, resting-state fMRI, functional connectivity, reward circuit, nucleus accumbens
1. Introduction
Anhedonia, characterized by persistent deficits in reward processing, is a core symptom of multiple psychiatric disorders [1,2]. Previous work has primarily focused on the role of anhedonia in major depressive disorder (MDD) and schizophrenia [3,4]. However, recent research and clinical diagnostic criteria have increasingly recognized anhedonia and deficits in the experience of positive affect as major symptoms of posttraumatic stress disorder [PTSD; 2,5–7]. Regardless of the specific diagnosis, anhedonia can have detrimental effects on mental health and well-being. For instance, increased anhedonic symptom severity has been associated with reduced treatment response, increased suicidality, reduced quality of life, and increased risk of developing mood and anxiety disorders [4,7–10]. Considering the prevalence of anhedonia in psychopathology and its risk factors, it is critical to understand the neural correlates of anhedonia in PTSD to identify potential biomarkers for early intervention and treatment.
Anatomical research in animals and functional neuroimaging studies in humans have implicated a set of cortical and subcortical brain structures in reward processing. This reward circuit is a collection of interconnected regions along the mesocorticolimbic dopaminergic pathway, including the ventral tegmental area, ventral and dorsal striatum, orbitofrontal cortex (OFC), and the medial prefrontal cortex (mPFC), that are active during the anticipation or experience of a rewarding stimuli [11–14]. In particular, the nucleus accumbens (NAcc) of the ventral striatum has been identified as an integral part of the reward circuit, consistently showing strong activation in response to rewarding stimuli [15,16]. During reward processing, the NAcc also exhibits functional connectivity with the OFC, mPFC, posterior cingulate cortex (PCC), thalamus, caudate, and amygdala [12,17].
Given the diffuse connectivity of the NAcc to regions involved in emotion processing and decision making, it is unsurprising that its dysfunction has been associated with reduced hedonic capacity in both healthy and clinical populations [16,18–24]. In response to a monetary reward, healthy individuals with increased anhedonic symptom severity showed decreased volume and reactivity of the NAcc [23]. Similar decreased NAcc activity was found in a sample of depressed individuals when responding to happy and neutral stimuli [19]. Differences in functional connectivity of the NAcc have also been demonstrated across clinical diagnoses in relation to hedonic deficits. In a sample of individuals with different psychiatric disorders, including depression and schizophrenia, decreased connectivity between the NAcc and regions of the default mode network (DMN) was associated with greater reward processing deficits in comparison to controls [22]. Another study similarly showed that adolescents with increased anhedonia have decreased resting-state functional connectivity (rsFC) between the left NAcc, subgenual anterior cingulate cortex, and left caudate [18]. Taken together, this research indicates that activity and connectivity of the NAcc is related to anhedonic symptom severity. More specifically, decreased activity of the NAcc and decreased connectivity between the NAcc and other reward-related regions appears to be a biomarker of anhedonia in healthy individuals as well as those with depression and schizophrenia.
However, fewer studies have investigated whether these same patterns of neural dysfunction underlie anhedonic symptoms in PTSD. Neural dysfunction associated with anhedonia in PTSD has only recently been examined, despite the fact that diagnostic criteria for PTSD include symptoms of reduced motivation and diminished pleasure in previously rewarding experiences [1,2]. Using task-based neuroimaging, individuals with PTSD in comparison to controls tend to report less motivation and satisfaction in response to rewards, and these decreased hedonic responses have been associated with altered activity in NAcc, mPFC, and striatum [21,25]. In a similar study, decreased activity was observed in the left OFC, ventral mPFC, and amygdala for individuals with PTSD in response to imagery of positive social events, and this reduction was exacerbated by higher PTSD symptom cluster severity scores [26].
To our knowledge, only two resting-state neuroimaging studies have investigated connectivity of the reward circuit in relation to PTSD or trauma. One study comparing rsFC between individuals with comorbid PTSD-MDD, PTSD only, or trauma-exposure, found significantly reduced connectivity between the NAcc and thalamus in the comorbid PTSD-MDD group when compared with the PTSD only and trauma-exposed groups [24]. Moreover, in all participants with PTSD, reduced rsFC involving the reward circuit appeared to be driven by depression severity but not PTSD severity. A separate study examined the influence of anhedonic symptom severity and trauma on resting-state NAcc connectivity. Olson and colleagues [20] found that higher anhedonia scores in a sample of trauma-exposed men and women correlated with greater rsFC between the NAcc and dorsal mPFC, though these findings were not moderated by PTSD or depression severity.
Together, these studies implicate altered reward circuit activity and connectivity in PTSD and in trauma-exposed individuals with anhedonic symptoms. However, no studies have directly examined the influence of anhedonia on rsFC within the reward circuit in individuals with PTSD. The present study examined rsFC within the reward circuit as a function of anhedonic symptom severity in a sample of women diagnosed with PTSD resulting from interpersonal trauma. We hypothesized that increased anhedonia would be associated with reduced rsFC between the NAcc and reward-related brain regions. In particular, based on the reviewed literature, we predicted that anhedonia would be associated with reduced rsFC between the NAcc and striatum, thalamus, and ventral mPFC.
2. Materials and Methods
2.1. Participants
Data for the current study were collected as part of a larger NIH funded study investigating the neurobiological changes following cognitive processing therapy in PTSD [e.g., 27,28]. Participants included 71 women between the ages of 18 and 56 meeting DSM-IV-TR criteria [1] for PTSD resulting from interpersonal trauma, such as physical or sexual assault, molestation, or intimate partner violence. All participants reported that their most recent traumatic event had occurred at least 1 month prior to the initial assessment. As long as the primary diagnosis was PTSD, individuals with other current comorbid psychological disorders were included: major depressive disorder (n = 21), panic disorder (n = 5), social phobia (n = 6), specific phobia (n = 8), agoraphobia (n = 1), obsessive compulsive disorder (n = 2). Participants were excluded for the following reasons: active suicidality, homicidal ideation, Axis II disorders, current alcohol or substance abuse disorder, schizophrenia or other psychotic disorder, bipolar disorder, history of head trauma, current use of psychotropic prescription or nonprescription drugs, previous trauma-focused therapy, or MRI contraindications (e.g., metallic implants, implanted medical devices).
Participants were recruited through referrals and advertisements at a Midwestern, multidisciplinary trauma recovery center. All participants provided written informed consent and were paid for their participation. All study procedures were in accordance with the local institutional review board. For participant demographics and clinical symptoms, see Table 1.
Table 1.
Participant Demographics and Study Variables
| Variable | n | Mean | SD | Range |
|---|---|---|---|---|
| Age | 71 | 31.93 | 9.39 | 18–56 |
| Educationa | 14.83 | 2.49 | 6–20 | |
| CAPS total | 69b | 66.51 | 16.83 | 35–104 |
| MASQ-AD Score | 67c | 73.34 | 15.52 | 34–101 |
| BDI-II Score | 68d | 24.82 | 10.34 | 5–46 |
Notes. CAPS total = Clinician-Administered PTSD Scale total severity score; MASQ-AD = Mood and Anxiety Symptoms Questionnaire Anhedonia Subscale.
Education is reported in years.
CAPS total severity scores missing for two participants
MASQ-AD scores missing for four participants
BDI-II scores missing for three participants
2.2. Clinical Assessments
2.2.1. Anhedonia.
Anhedonia symptom severity was measured with the Anhedonic Depression (AD) subscale of the Mood and Anxiety Symptoms Questionnaire [MASQ; 29,30]. The MASQ-AD subscale is comprised of 22 items related to symptoms of anhedonia, with questions such as “Felt like there wasn’t anything interesting or fun to do” and “Felt like nothing was really enjoyable”. For each item, participants rated how frequently they had felt or experienced the symptom in the past week on a scale from 1 (not at all) to 5 (extremely).
2.2.2. PTSD symptom severity.
PTSD symptom severity was assessed using the 30-item Clinician-Administered PTSD Scale [CAPS; 31]. This measure has demonstrated high internal consistency (Cronbach’s αs = .92 – .99) and is an accepted valid measure of PTSD symptoms and diagnosis [31]. This measure provides frequency and severity ratings of DSM-IV-TR PTSD symptoms over the past month. For the current study, total PTSD symptom severity scores were calculated for each participant. The internal consistency of the CAPS in this sample was high (Cronbach’s α = .95).
2.2.3. Depression.
All participants completed the Beck Depression Inventory-II [BDI-II; 32]. On this 21-item, self-report questionnaire, participants rated items such as “feeling sad” and “discouraged about my future” on a scale from 0 (indicating an absence of symptoms) to 3 (indicating the maximum severity). Total depressive symptom severity on the BDI-II was calculated. The internal consistency of the BDI-II in this sample was high (Cronbach’s α = .90).
2.3. Functional MRI Data Acquisition
MRI data were acquired using a Siemens 3.0 T TrioTrim MRI scanner (Siemens, Erlangen, Germany). Two resting-state fMRI (rs-fMRI) scans were collected using an asymmetric spin-echo planar imaging sequence (TR/TE/flip angle (FA): 2.2 s/27 ms/90°, field of view (FOV): 384 cm, slice thickness: 4mm, number of slices: 36 transverse, voxel size: 4×4×4 mm3). Each participant was instructed for the resting-state scan (~8 min) to keep their eyes open and fixated on a cross and to remain still and not to move during the scan. High-resolution T1-weighted structural imaging data were acquired sagittally using a magnetization prepared rapid echo gradient (MPRAGE) sequence (TI: 1000ms, TR/TE/FA: 2.4 s/3.13 ms/8°, FOV: 255×256 mm2, matrix: 256×256, slice thickness: 1mm, number of slices: 1024, voxel size: 1×1×1 mm3). For the present study, the first rs-fMRI scan was used for analysis as this scan was collected for all participants.
2.4. Preprocessing and Motion Analysis for rs-fMRI Data
The rs-fMRI EPI functional and T1 structural images were processed using AFNI, FSL, and ANTs [33 FMRIB Software Library; http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/; http://stnava.github.io/ANTs/]. Initial preprocessing steps for the EPI were conducted as follows: deoblique (3dWarp), removal of the first three volumes (3dcalc), motion correction of time points by rigid body alignment to first EPI image (3dvolreg), despiking to remove time series outliers (3dDespike), bandpass filtering to reflect the low frequency neuronal fluctuations for resting-state BOLD activity (0.01 – 0.10 Hz), and spatial smoothing with a 3D 4-mm full-width half-maximum (FWHM) Gaussian kernel (3dmerge).
T1 images were then skull-stripped, coregistered with the EPI, normalized to Montreal Neurological Institute (MNI)-152 template space, and resampled to 3mm cubic voxels. Next, cerebrospinal fluid (CSF), white matter (WM), and gray matter (GM) masks were segmented from normalized T1 images for later nuisance regression [FAST in FSL; ,34].
Prior to the final preprocessing steps, individual scans were assessed for excessive motion, defined as mean framewise motion displacement > 4mm and/or total scan time < 3 min after motion censoring all time points with framewise motion displacement > .2mm and extreme timeseries outliers (i.e., time points where > 10% of voxels were outliers) [35,36]. These motion censoring criteria were based on previous recommendations and our prior research [35–39], resulting in exclusion of ten participants. Additionally, average root-mean-squared (RMS) displacement was calculated to estimate individual participant motion [40]. Note, RMS did not correlate with anhedonic symptom severity (r(61) = .01, p = .93); thus, it was not included as a covariate in rsFC analyses.
For the final preprocessing steps, a GLM was run in AFNI (3dDeconvolve) to account for motion and other nuisance variables [as in 40], including six motion parameters and their derivatives, the WM time series and its derivative, and the CSF time series and its derivative. The output from these final preprocessing steps was then used in the seed-based rsFC analyses described below.
2.5. Statistical Analyses
2.5.1. rsFC Analysis.
To evaluate the influence of anhedonic symptom severity on rsFC within the reward circuit in PTSD, we first conducted seed-based voxelwise rsFC analyses for both the left and right NAcc [12; Figure 1A]. For each seed region of interest (ROI), a 3-mm radius seed mask was created in MNI space and then applied to the fully preprocessed EPI data of each participant in AFNI. Then, the mean resting-state BOLD time series from each ROI for each participant was entered in a GLM to calculate the correlation between each seed ROIs time series and all other voxels. For each participant, correlation maps were created for each ROI by converting R2 values to correlation coefficients and then applying Fisher’s r-to-z transformation [as in 38]. The z-score correlation maps were then used in the multivariate linear regression analyses detailed below.
Figure 1. Reduced functional connectivity associated with anhedonia severity.
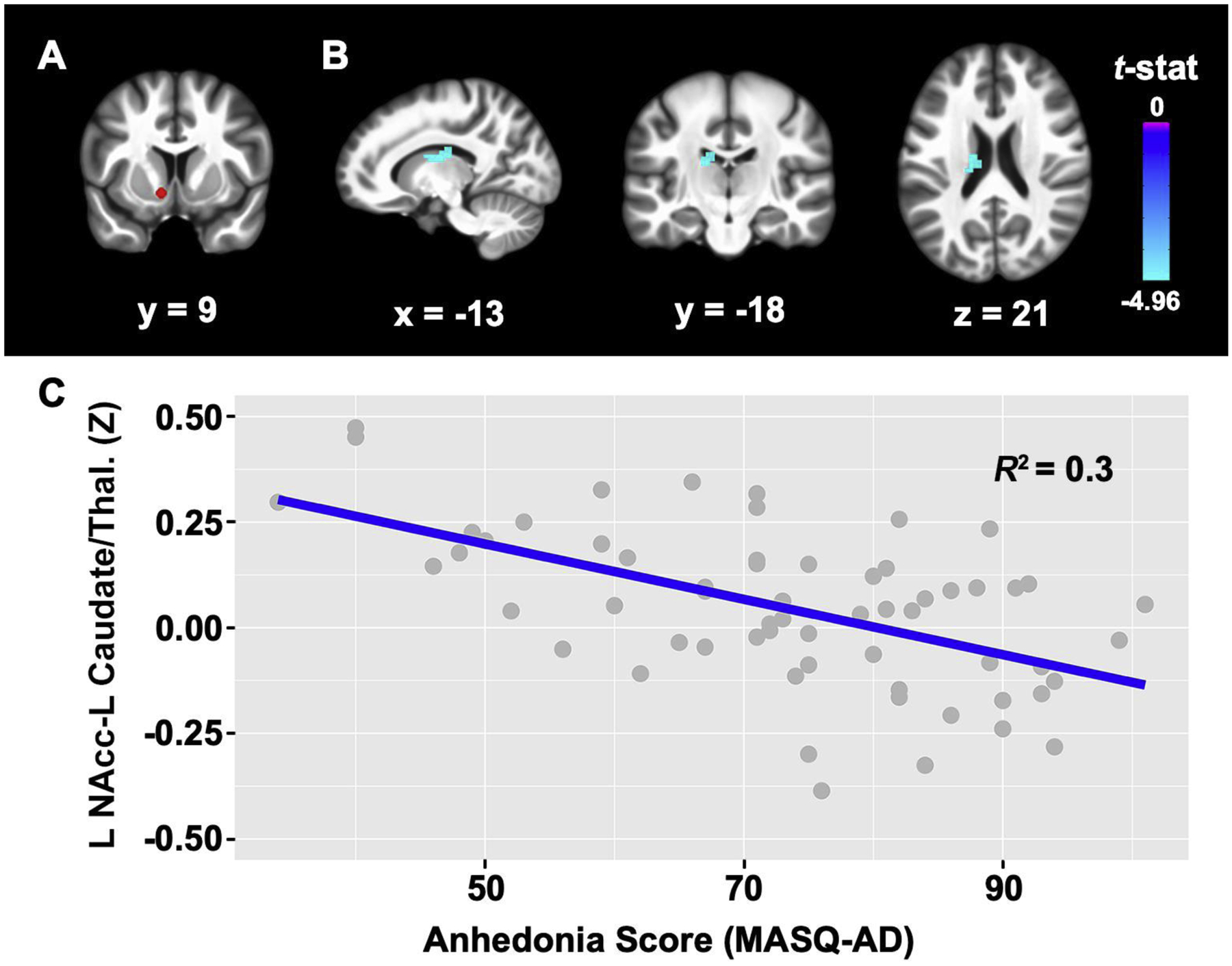
A, Left nucleus accumbens (NAcc) seed (in red). B, Higher MASQ-AD (anhedonia) scores were associated with reduced connectivity between the L NAcc seed and the L caudate extending to the thalamus. Cluster size = 50 voxels; Peak t-value: x = −13, y = −18, z = 21; t = −4.96, C, Scatter plot showing the relationship between MASQ-AD scores and connectivity values between the L NAcc and L caudate extending to the thalamus. Results survived whole-brain cluster correction Bonferroni-corrected for seed ROIs (pFWE < .025, p = .001 uncorrected). Results are displayed on the group average structural MRI in MNI-space. L = left, Thal. = thalamus.
2.5.2. Influence of Anhedonic Symptom Severity.
Two multivariate linear regressions were run for the entire sample after motion exclusion (N = 61) to determine whether anhedonia predicted rsFC of the two NAcc ROIs (3dttest++ in AFNI). Separate models were performed in 3dttest++ with the z-score correlation maps for either left NAcc or right NAcc seed ROIs and anhedonia score entered as a covariate in the model. As fMRI data has a non-Gaussian distribution, the autocorrelation function (-acf) was included to calculate FWHM for each subject [3dFWHMx in AFNI, 41]. Furthermore, we adjusted for multiple comparisons by applying a family-wise error (FWE) cluster-correction at the whole-brain level for all analyses using 3dClustSim with the autocorrelation function output from 3dFWHMx in AFNI [AFNI version updated February 2020, 42,43]. Resulting maps were tested for significance at a predefined voxelwise threshold of p < .001 (uncorrected) and pFWE < .025 (Bonferroni-corrected for two NAcc seed ROIs), with a cluster-corrected size of ≥ 42 voxels.
2.5.3. Influence of PTSD and Depression Symptoms.
To examine whether PTSD or depression symptoms predicted rsFC of NAcc connectivity, we conducted correlation analyses with PTSD or depression symptoms for any significant rsFC findings. We also performed correlation analyses to determine whether NAcc-hippocampal connectivity was associated with PTSD or depression symptoms following the methods reported in a previous study [24].
3. Results
We first examined relationships between anhedonia and PTSD and depression symptoms. Across the full sample, greater anhedonia symptom severity scores were significantly correlated with more severe PTSD (r(65) = .25, p = .048) and depression symptoms (r(64) = .61, p < .001). For the resting-state analyses after motion exclusion, anhedonic symptom severity scores were associated with significantly decreased rsFC between the left NAcc seed and a cluster in the left caudate extending to the left thalamus (Figure 1; pFWE < .025). These results for the left NAcc remained significant after controlling for PTSD and depression symptom severity in multiple linear regression analyses (Table 2). No significant relationships were found between anhedonia severity and rsFC of the right NAcc seed.
Table 2.
Multiple Linear Regressions with Left NAcc Results Controlling for PTSD and Depression Symptoms
| left NAcc-left caudate connectivity | |||
|---|---|---|---|
| Modelsa | B | SE B | β |
| b(Constant) | 0.46 | 0.12 | |
| MASQ-AD | −0.01 | 0.00 | −.51** |
| CAPS total | 0.00 | 0.00 | .03 |
| c(Constant) | 0.46 | 0.10 | |
| MASQ-AD | −0.00 | 0.00 | −.38* |
| BDI-II | −0.00 | −0.18 | −.18 |
Notes. MASQ-AD = Mood and Anxiety Symptoms Questionnaire Anhedonia Subscale; CAPS total = Clinician-Administered PTSD Scale total symptom severity score; BDI-II = Beck Depression Inventory-Version II;
p < .05;
p< .01
Regression model included all participants after motion exclusion (n = 61)
Regression model with PTSD symptoms: R2 = .26, ps < .001
Regression model with depression symptoms: R2 = .26, ps < .001
For our post hoc correlation analyses, rsFC results for the left NAcc seed were significantly associated with depression (r(61) = −.41, p = .001) but not PTSD (r(61) = −.07, p > .05) symptoms. We also examined rsFC between NAcc and hippocampal seeds from a previous study [24]. There were no significant correlations between NAcc-hippocampal connectivity and PTSD (r(61) = −.04, p > .05) or depression (r(61) = .06, p > .05) symptoms.
4. Discussion
To our knowledge, this is the first study to investigate the effect of anhedonic symptom severity on resting-state connectivity within the reward circuit in individuals meeting diagnostic criteria for PTSD. Consistent with our hypothesis, our findings demonstrate that increased anhedonic symptom severity is predictive of reduced connectivity within the reward circuit in PTSD. More specifically, increased anhedonia was associated with reduced rsFC between the left NAcc and the left caudate extending to the thalamus, even after controlling for the severity of PTSD or depression symptoms.
Our main finding is congruent with several previous studies linking the caudate and thalamus with reward processing in healthy and clinical populations. In healthy individuals, the caudate has been implicated in responses to unpredictable rewarding stimuli and stimulus-response-reward learning [15,44,45]. As for the thalamus, the NAcc primarily projects its output to the cerebral cortex through the mediodorsal nucleus of the thalamus [46]. This NAcc-thalamic circuit has further been shown to modulate responses to reward in both animals and humans [24,46,47]. In individuals with depression, disrupted activity and connectivity of the NAcc, caudate, and thalamus have been observed [16,24,48]. When responding to monetary rewards, Pizzagalli and colleagues [16] demonstrated that individuals with depression had significantly reduced activation in the left NAcc and bilateral caudate in comparison to controls. Similarly, reduced thalamic activity was found in relation to anhedonia for a sample of women with unipolar depression [48]. In addition, Zhu et al. [24] reported reduced rsFC between the NAcc-thalamic circuit in individuals with comorbid MDD-PTSD and a correlation with depression symptoms in participants with a PTSD diagnosis. Note, in our study the cluster extending from the caudate into a portion of the thalamus only partially overlapped with the thalamic seed region used in Zhu et al. [24]. In conjunction with our results, this previous work clearly associates aberrant neural activity and connectivity of the NAcc, caudate, and thalamus with reward processing deficits. We did not find evidence for a relationship between anhedonia and reduced connectivity between NAcc and ventral mPFC as reported in a previous study of adolescents with MDD [18]. Given that previous studies have found both increased and decreased connectivity with the mPFC in relation to anhedonia [18,20], additional research is needed.
Considering the risk factors associated with anhedonia in psychopathology [4,8–10], our findings may have important implications for the development of clinical interventions to address anhedonic symptoms in PTSD. For example, deep brain stimulation of the ventral striatum has been promising for alleviating anhedonic symptoms in treatment-resistant depression. Bewernick and colleagues [49,50] demonstrated that stimulation to the ventral striatum including the NAcc yielded reduced depressive symptom severity and increased the number of self-reported pleasant activities. Furthermore, deep brain stimulation of the NAcc decreased the hyperactivity of the PCC, OFC, caudate, and thalamus [49]. Besides brain stimulation methods, certain psychotherapies may also be beneficial for treating anhedonia. For instance, behavioral activation therapy, which involves increasing engagement with rewarding stimuli and decreasing avoidance, has been shown to reduce depressive symptoms and normalize activity in reward-related brain regions in MDD [51]. Given these results in depression and the similar patterns of reward circuit dysfunction observed across diagnostic categories, it is possible that neurostimulation of the NAcc or targeted psychotherapy could alleviate anhedonic symptoms in individuals with PTSD.
Some limitations are worth noting for the current study. First, this sample included only female participants diagnosed with PTSD due to interpersonal trauma. For this reason, it is unclear whether our findings would replicate in males or vary by type of trauma experienced. Thus, future studies could assess whether there are differences in reward circuit connectivity specific to anhedonic symptom severity in PTSD based on sex or type of trauma exposure. Second, anhedonic symptom severity was measured using the MASQ-AD subscale. Though this subscale has demonstrated efficacy for anhedonic symptoms [52], there are several alternative measures of hedonic capacity that may better reflect deficits in each phase of reward processing. For instance, the Snaith-Hamilton Pleasure Scale measures four domains of reward processing: interests, social interaction, sensory experience, food and drink [53]. There is also the Temporal Experience of Pleasure Scale that measures items specific to consummatory and anticipatory anhedonia [54]. As the unique neurobiological correlates of anticipatory and consummatory anhedonia are yet to be determined in PTSD, subsequent studies should intentionally collect measures of hedonic capacity with the ability to distinguish between the different phases of reward processing.
Despite these limitations, we have reported novel findings of functional reward circuit abnormalities related to anhedonia in PTSD that were not driven by PTSD or depression symptom severity. More specifically, we found reduced functional connectivity within pathways that have been linked to reward processing. These findings reflect seminal and recent literature on the neurobiological mechanisms of anhedonia in mood disorders and support emerging literature on the neural correlates of anhedonia in trauma.
Highlights.
Anhedonia is a core symptom of multiple psychiatric disorders, including PTSD
We examined the resting-state neural signatures of anhedonia in PTSD
Greater anhedonia severity was related to reduced connectivity of the NAcc
Findings remained significant after controlling for PTSD and depression symptoms
Results support common neural correlates of anhedonia across psychiatric disorders
Acknowledgements
This work was supported by the National Institutes of Health [K23 MH090366, PI: Steven E. Bruce; RC1 MH089704, PI: Yvette Sheline]. We recognize the essential contributions of all of the participants in making this research possible.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Declarations of Interest
Dr. Bruce reports receiving grants from the National Institute of Mental Health during the conduct of this study. The authors have no other conflicts of interest to disclose.
References
- [1].American Psychiatric Association, Diagnostic and statistical manual of mental disorder, text revision (DSM-IV-TR), American Psychiatric Association, Washington, D.C., 2000. [Google Scholar]
- [2].American Psychiatric Association, Diagnostic and statistical manual of mental disorders: DSM-5, American Psychiatric Association, Washington, D.C., 2013. [Google Scholar]
- [3].Horan WP, Kring AM, Blanchard JJ, Anhedonia in Schizophrenia: A Review of Assessment Strategies, Schizophrenia Bulletin. 32 (2005) 259–273. 10.1093/schbul/sbj009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Pizzagalli DA, Depression, Stress, and Anhedonia: Toward a Synthesis and Integrated Model, Annu. Rev. Clin. Psychol 10 (2014) 393–423. 10.1146/annurev-clinpsy-050212-185606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Liu P, Wang L, Cao C, Wang R, Zhang J, Zhang B, Wu Q, Zhang H, Zhao Z, Fan G, Elhai JD, The underlying dimensions of DSM-5 posttraumatic stress disorder symptoms in an epidemiological sample of Chinese earthquake survivors, Journal of Anxiety Disorders. 28 (2014) 345–351. 10.1016/j.janxdis.2014.03.008. [DOI] [PubMed] [Google Scholar]
- [6].Nawijn L, van Zuiden M, Frijling JL, Koch SBJ, Veltman DJ, Olff M, Reward functioning in PTSD: A systematic review exploring the mechanisms underlying anhedonia, Neuroscience & Biobehavioral Reviews. 51 (2015) 189–204. 10.1016/j.neubiorev.2015.01.019. [DOI] [PubMed] [Google Scholar]
- [7].Pietrzak RH, Johnson DC, Goldstein MB, Malley JC, Southwick SM, Posttraumatic stress disorder mediates the relationship between mild traumatic brain injury and health and psychosocial functioning in veterans of operations Enduring Freedom and Iraqi Freedom., Journal of Nervous and Mental Disease. 197 (2009) 748–753. 10.1097/NMD.0b013e3181b97a75. [DOI] [PubMed] [Google Scholar]
- [8].Risbrough VB, Glynn LM, Davis EP, Sandman CA, Obenaus A, Stern HS, Keator DB, Yassa MA, Baram TZ, Baker DG, Does Anhedonia Presage Increased Risk of Posttraumatic Stress Disorder?, in: Vermetten E, Baker DG, Risbrough VB (Eds.), Behavioral Neurobiology of PTSD, Springer International Publishing, Cham, 2018: pp. 249–265. 10.1007/7854_2018_51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Rizvi SJ, Pizzagalli DA, Sproule BA, Kennedy SH, Assessing anhedonia in depression: Potentials and pitfalls, Neuroscience & Biobehavioral Reviews. 65 (2016) 21–35. 10.1016/j.neubiorev.2016.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Uher R, Farmer A, Maier W, Rietschel M, Hauser J, Marusic A, Mors O, Elkin A, Williamson RJ, Schmael C, Henigsberg N, Perez J, Mendlewicz J, Janzing JGE, Zobel A, Skibinska M, Kozel D, Stamp AS, Bajs M, Placentino A, Barreto M, McGuffin P, Aitchison KJ, Measuring depression: comparison and integration of three scales in the GENDEP study, Psychological Medicine. 38 (2008) 289–300. 10.1017/S0033291707001730. [DOI] [PubMed] [Google Scholar]
- [11].Der-Avakian A, Markou A, The neurobiology of anhedonia and other reward-related deficits, Trends in Neurosciences. 35 (2012) 68–77. 10.1016/j.tins.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Di Martino A, Scheres A, Margulies DS, Kelly AMC, Uddin LQ, Shehzad Z, Biswal B, Walters JR, Castellanos FX, Milham MP, Functional connectivity of human striatum: a resting state fMRI study, Cerebral Cortex. 18 (2008) 2735–2747. 10.1093/cercor/bhn041. [DOI] [PubMed] [Google Scholar]
- [13].Haber SN, Knutson B, The Reward Circuit: Linking Primate Anatomy and Human Imaging, Neuropsychopharmacology. 35 (2010) 4–26. 10.1038/npp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Williams LM, Defining biotypes for depression and anxiety based on large-scale circuit dysfunction: a theoretical review of the evidence and future directions for clinical translation, Depression and Anxiety. 34 (2016) 9–24. 10.1002/da.22556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].O’Doherty JP, Reward representations and reward-related learning in the human brain: insights from neuroimaging, Current Opinion in Neurobiology. 14 (2004) 769–776. 10.1016/j.conb.2004.10.016. [DOI] [PubMed] [Google Scholar]
- [16].Pizzagalli DA, Holmes AJ, Dillon DG, Goetz EL, Birk JL, Bogdan R, Dougherty DD, Iosifescu DV, Rauch SL, Fava M, Reduced Caudate and Nucleus Accumbens Response to Rewards in Unmedicated Individuals With Major Depressive Disorder, AJP. 166 (2009) 702–710. 10.1176/appi.ajp.2008.08081201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Cauda F, Cavanna AE, D’agata F, Sacco K, Duca S, Geminiani GC, Functional Connectivity and Coactivation of the Nucleus Accumbens: A Combined Functional Connectivity and Structure-Based Meta-analysis, Journal of Cognitive Neuroscience. 23 (2011) 2864–2877. 10.1162/jocn.2011.21624. [DOI] [PubMed] [Google Scholar]
- [18].Gabbay V, Ely BA, Li Q, Bangaru SD, Panzer AM, Alonso CM, Castellanos FX, Milham MP, Striatum-Based Circuitry of Adolescent Depression and Anhedonia, Journal of the American Academy of Child & Adolescent Psychiatry. 52 (2013) 628–641.e13. 10.1016/j.jaac.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Keedwell PA, Andrew C, Williams SCR, Brammer MJ, Phillips ML, The Neural Correlates of Anhedonia in Major Depressive Disorder, Biological Psychiatry. 58 (2005) 843–853. 10.1016/j.biopsych.2005.05.019. [DOI] [PubMed] [Google Scholar]
- [20].Olson EA, Kaiser RH, Pizzagalli DA, Rauch SL, Rosso IM, Anhedonia in Trauma-Exposed Individuals: Functional Connectivity and Decision-Making Correlates, Biological Psychiatry: Cognitive Neuroscience and Neuroimaging. 3 (2018) 959–967. 10.1016/j.bpsc.2017.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Sailer U, Robinson S, Fischmeister F.Ph.S., König D, Oppenauer C, Lueger-Schuster B, Moser E, Kryspin-Exner I, Bauer H, Altered reward processing in the nucleus accumbens and mesial prefrontal cortex of patients with posttraumatic stress disorder, Neuropsychologia. 46 (2008) 2836–2844. 10.1016/j.neuropsychologia.2008.05.022. [DOI] [PubMed] [Google Scholar]
- [22].Sharma A, Wolf DH, Ciric R, Kable JW, Moore TM, Vandekar SN, Katchmar N, Daldal A, Ruparel K, Davatzikos C, Elliott MA, Calkins ME, Shinohara RT, Bassett DS, Satterthwaite TD, Common Dimensional Reward Deficits Across Mood and Psychotic Disorders: A Connectome-Wide Association Study, AJP. 174 (2017) 657–666. 10.1176/appi.ajp.2016.16070774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Wacker J, Dillon DG, Pizzagalli DA, The role of the nucleus accumbens and rostral anterior cingulate cortex in anhedonia: Integration of resting EEG, fMRI, and volumetric techniques, NeuroImage. 46 (2009) 327–337. 10.1016/j.neuroimage.2009.01.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Zhu X, Helpman L, Papini S, Schneier F, Markowitz JC, Van Meter PE, Lindquist MA, Wager TD, Neria Y, Altered resting state functional connectivity of fear and reward circuitry in comorbid PTSD and major depression, Depression and Anxiety. 34 (2017) 641–650. 10.1002/da.22594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Elman I, Lowen S, Frederick BB, Chi W, Becerra L, Pitman RK, Functional Neuroimaging of Reward Circuitry Responsivity to Monetary Gains and Losses in Posttraumatic Stress Disorder, Biological Psychiatry. 66 (2009) 1083–1090. 10.1016/j.biopsych.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Frewen PA, Lundberg E, Brimson-Théberge M, Théberge J, Neuroimaging self-esteem: a fMRI study of individual differences in women., Social Cognitive & Affective Neuroscience. 8 (2013) 546–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Buchholz KR, Bruce SE, Koucky EM, Artime TM, Wojtalik JA, Brown WJ, Sheline YI, Neural correlates of trait rumination during an emotion interference task in women with PTSD, Journal of Traumatic Stress. 29 (2016) 317–324. 10.1002/jts.22112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Yang Z, Oathes DJ, Linn KA, Bruce SE, Satterthwaite TD, Cook PA, Satchell EK, Shou H, Sheline YI, Cognitive behavioral therapy Is associated with enhanced cognitive control network activity in major depression and posttraumatic stress disorder, Biological Psychiatry: Cognitive Neuroscience and Neuroimaging. 3 (2018) 311–319. 10.1016/j.bpsc.2017.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Watson D, Clark LA, Weber K, Assenheimer JS, Strauss ME, McCormick RA, Testing a tripartite model: II Exploring the symptom structure of anxiety and depression in student, adult, and patient samples, Journal of Abnormal Psychology. 104 (1995) 15–25. 10.1037/0021-843X.104.1.15. [DOI] [PubMed] [Google Scholar]
- [30].Watson D, Weber K, Assenheimer JS, Clark LA, Strauss ME, McCormick RA, Testing a tripartite model: I Evaluating the convergent and discriminant validity of anxiety and depression symptom scales, Journal of Abnormal Psychology. 104 (1995) 3–14. 10.1037/0021-843X.104.1.3. [DOI] [PubMed] [Google Scholar]
- [31].Blake DD, Weathers FW, Nagy LM, Kaloupek DG, Gusman FD, Charney DS, Keane TM, The development of a Clinician-Administered PTSD Scale, Journal of Traumatic Stress. 8 (1995) 75–90. 10.1007/BF02105408. [DOI] [PubMed] [Google Scholar]
- [32].Beck AT, Steer RA, Brown GK, Beck Depression Inventory-II, Psychological Corporation, San Antonio, TX, 1996. [Google Scholar]
- [33].Cox RW, AFNI: Software for Analysis and Visualization of Functional Magnetic Resonance Neuroimages, Computers and Biomedical Research. 29 (1996) 162–173. 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- [34].Zhang Y, Brady M, Smith S, Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm, IEEE Transactions on Medical Imaging. 20 (2001) 45–57. 10.1109/42.906424. [DOI] [PubMed] [Google Scholar]
- [35].Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE, Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion, NeuroImage. 59 (2012) 2142–2154. 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Yan C-G, Cheung B, Kelly C, Colcombe S, Craddock RC, Di Martino A, Li Q, Zuo X-N, Castellanos FX, Milham MP, A comprehensive assessment of regional variation in the impact of head micromovements on functional connectomics, NeuroImage. 76 (2013) 183–201. 10.1016/j.neuroimage.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Philippi CL, Pujara MS, Motzkin JC, Newman J, Kiehl KA, Koenigs M, Altered resting-state functional connectivity in cortical networks in psychopathy, J. Neurosci 35 (2015) 6068. 10.1523/JNEUROSCI.5010-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Philippi CL, Cornejo MD, Frost CP, Walsh EC, Hoks RM, Birn R, Abercrombie HC, Neural and behavioral correlates of negative self-focused thought associated with depression, Hum. Brain Mapp 39 (2018) 2246–2257. 10.1002/hbm.24003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Philippi CL, Pessin S, Reyna L, Floyd T, Bruce SE, Cortical midline structures associated with rumination in women with PTSD, Journal of Psychiatric Research. 131 (2020) 69–76. 10.1016/j.jpsychires.2020.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Ciric R, Wolf DH, Power JD, Roalf DR, Baum GL, Ruparel K, Shinohara RT, Elliott MA, Eickhoff SB, Davatzikos C, Gur RC, Gur RE, Bassett DS, Satterthwaite TD, Benchmarking of participant-level confound regression strategies for the control of motion artifact in studies of functional connectivity, NeuroImage. 154 (2017) 174–187. 10.1016/j.neuroimage.2017.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Eklund A, Nichols TE, Knutsson H, Cluster failure: Why fMRI inferences for spatial extent have inflated false-positive rates, Proceedings of the National Academy of Sciences. 113 (2016) 7900–7905. 10.1073/pnas.1602413113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Carp J, The secret lives of experiments: Methods reporting in the fMRI literature, NeuroImage. 63 (2012) 289–300. 10.1016/j.neuroimage.2012.07.004. [DOI] [PubMed] [Google Scholar]
- [43].Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC, Improved Assessment of Significant Activation in Functional Magnetic Resonance Imaging (fMRI): Use of a Cluster-Size Threshold, Magn. Reson. Med 33 (1995) 636–647. 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- [44].Haruno M, Kuroda T, Doya K, Toyama K, Kimura M, Samejima K, Imamizu H, Kawato M, A neural correlate of reward-based behavioral learning in caudate nucleus: a functional magnetic resonance imaging study of a stochastic decision task, J. Neurosci 24 (2004) 1660. 10.1523/JNEUROSCI.3417-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Tricomi EM, Delgado MR, Fiez JA, Modulation of caudate activity by action contingency, Neuron. 41 (2004) 281–292. 10.1016/S0896-6273(03)00848-1. [DOI] [PubMed] [Google Scholar]
- [46].Leung BK, Balleine BW, Ventral Pallidal Projections to Mediodorsal Thalamus and ntral Tegmental Area Play Distinct Roles in Outcome-Specific Pavlovian-Instrumental Transfer, J. Neurosci 35 (2015) 4953. 10.1523/JNEUROSCI.4837-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Haber SN, Calzavara R, The cortico-basal ganglia integrative network: The role of the thalamus, Brain Research Bulletin. 78 (2009) 69–74. 10.1016/j.brainresbull.2008.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Mitterschiffthaler MT, Kumari V, Malhi GS, Brown RG, Giampietro VP, Brammer MJ, Suckling J, Poon L, Simmons A, Andrew C, Sharma T, Neural response to pleasant stimuli in anhedonia: an fMRI study, NeuroReport. 14 (2003). https://journals.lww.com/neuroreport/Fulltext/2003/02100/Neural_response_to_pleasant_stimuli_in_anhedonia_.3.aspx. [DOI] [PubMed] [Google Scholar]
- [49].Bewernick BH, Hurlemann R, Matusch A, Kayser S, Grubert C, Hadrysiewicz B, Axmacher N, Lemke M, Cooper-Mahkorn D, Cohen MX, Brockmann H, Lenartz D, Sturm V, Schlaepfer TE, Nucleus Accumbens Deep Brain Stimulation Decreases Ratings of Depression and Anxiety in Treatment-Resistant Depression, Biological Psychiatry. 67 (2010) 110–116. 10.1016/j.biopsych.2009.09.013. [DOI] [PubMed] [Google Scholar]
- [50].Bewernick BH, Kayser S, Sturm V, Schlaepfer TE, Long-Term Effects of Nucleus Accumbens Deep Brain Stimulation in Treatment-Resistant Depression: Evidence for Sustained Efficacy, Neuropsychopharmacology. 37 (2012) 1975–1985. 10.1038/npp.2012.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Dichter GS, Felder JN, Petty C, Bizzell J, Ernst M, Smoski MJ, The Effects of Psychotherapy on Neural Responses to Rewards in Major Depression, Biological Psychiatry. 66 (2009) 886–897. 10.1016/j.biopsych.2009.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Bredemeier K, Spielberg JM, Silton RL, Berenbaum H, Heller W, Miller GA, Screening for depressive disorders using the Mood and Anxiety Symptoms Questionnaire Anhedonic Depression Scale: A receiver-operating characteristic analysis., Psychological Assessment. 22 (2010) 702–710. 10.1037/a0019915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Snaith RP, Hamilton M, Morley S, Humayan A, Hargreaves D, Trigwell P, A Scale for the Assessment of Hedonic Tone the Snaith–Hamilton Pleasure Scale, British Journal of Psychiatry. 167 (1995) 99–103. 10.1192/bjp.167.1.99. [DOI] [PubMed] [Google Scholar]
- [54].Gard DE, Gard MG, Kring AM, John OP, Anticipatory and consummatory components of the experience of pleasure: A scale development study, Journal of Research in Personality. 40 (2006) 1086–1102. 10.1016/j.jrp.2005.11.001. [DOI] [Google Scholar]


