Abstract
Decision makers rely on episodic memory to calculate choice values in everyday life, yet it is unclear how neural mechanisms of valuation differ when value-related information is encoded versus retrieved from episodic memory. The current fMRI study compared neural correlates of value while information was encoded versus retrieved from memory. Scanned tasks were followed by a behavioral episodic memory test for item-attribute associations. Our analyses sought to (i) identify neural correlates of value that were distinct and common across encoding and retrieval, and (ii) determine whether neural mechanisms of valuation and episodic memory interact. The study yielded three primary findings. First, value-related activation in the fronto-striatal reward circuit and posterior parietal cortex was comparable across valuation phases. Second, value-related activation in select fronto-parietal and salience regions was significantly greater at value retrieval than encoding. Third, there was no interaction between neural correlates of valuation and episodic memory. Taken with prior research, the present study indicates that fronto-parietal and salience regions play a key role in retrieval-dependent valuation and context-specific effects likely determine whether neural correlates of value interact with episodic memory.
Keywords: value network, episodic memory, fMRI, decision making, encoding, retrieval
Adaptive decision making involves the representation of choice values, and decision makers often rely on episodic memory to calculate choice values in everyday life. For example, consumers may rely on episodic memory to encode specific value-related information from product reviews, advertisements, or personal experiences, and retrieve this information from long-term storage when making purchasing decisions. As depicted in Figure 1, the neural substrates most commonly implicated in valuation include fronto-striatal “reward circuitry”, particularly the ventromedial prefrontal cortex and ventral striatum (light blue regions; Bartra, McGuire, & Kable, 2013; Brosch & Sander, 2013; Clithero & Rangel, 2014; Gläscher, Hampton, & O’Doherty, 2009; Kable & Glimcher, 2009; Levy & Glimcher, 2012; Liu, Hairston, Schrier, & Fan, 2011; Zhang, Larcher, Misic, & Dagher, 2017). Additionally, more lateral regions of the orbitofrontal cortex support the integration of value information for goal-directed decision making (Nogueira et al., 2017; Wallis, 2007; Walton, Chau, & Kennerley, 2015). Brain regions within the fronto-parietal “control network” have also been implicated in choice valuation. These include the posterior parietal, posterior cingulate, lateral prefrontal, and middle frontal cortices (dark blue regions; Bartra, McGuire, & Kable, 2013; Clithero & Rangel, 2014; Dosenbach, Fair, Cohen, Schlaggar, & Petersen, 2008; Gläscher et al., 2012; Hare, Camerer, & Rangel, 2009; Hsu & Goh, 2016; Liu et al., 2011; Ptak, 2011; Sadaghiani & D’Esposito, 2015). Finally, value processing has been associated with recruitment of the arousal-based “salience network” – particularly the dorsal anterior cingulate cortex, anterior insula, thalamus, and lateral prefrontal cortex (red regions; Bartra, McGuire, & Kable, 2013; Lamichhane & Dhamala, 2015; Ploran et al., 2007; Sadaghiani & D’Esposito, 2015; Seeley et al., 2007; Wallis, Stokes, Cousijn, Woolrich, & Nobre, 2015).
Figure 1. Brain regions implicated in valuation.
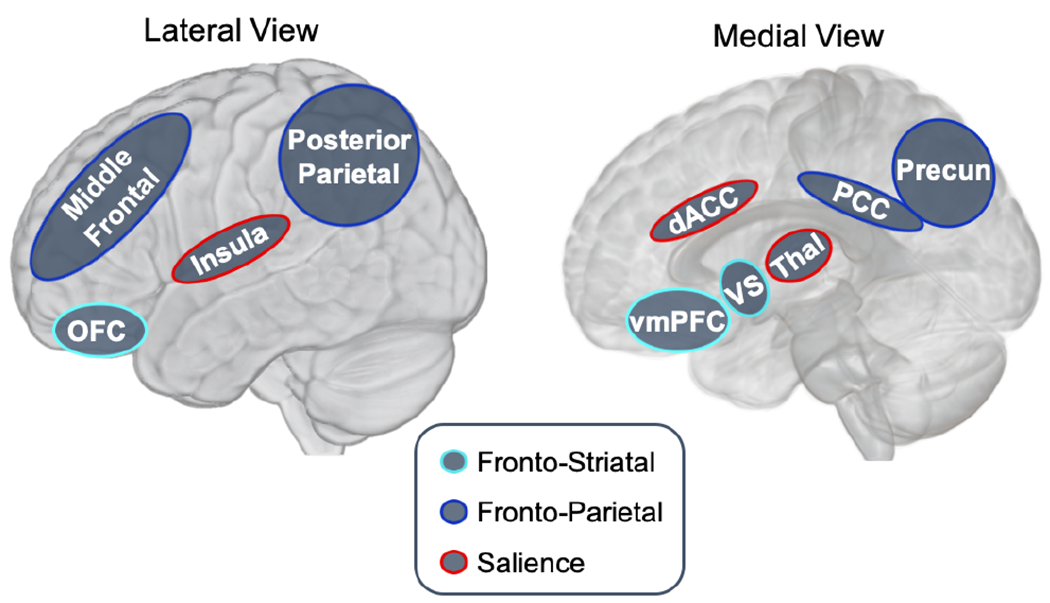
Note. Previous research has implicated a variety of brain areas spanning the fronto-striatal (in light blue), fronto-parietal (in dark blue), and salience networks (in red) in different aspects of value processing.
Abbreviations: OFC – orbitofrontal cortex; vmPFC – ventromedial prefrontal cortex; VS – ventral striatum; dACC – dorsal anterior cingulate cortex; Thal – thalamus; PCC – posterior cingulate cortex; Precun – precuneus.
Despite this wealth of research, and the fact that valued-based decision making frequently relies on episodic memory (Enkavi et al., 2017; Murty, FeldmanHall, Hunter, Phelps, & Davachi, 2016; Shadlen & Shohamy, 2016), it is presently unclear how encoding and retrieval processes affect the recruitment and interplay of regions in the value network. In particular, we are unaware of any studies that have directly examined common and distinct neural mechanisms of value encoding and value retrieval. Because of this gap, it is unclear if brain regions representing the “common currency” of value (Levy & Glimcher, 2012) differ during encoding versus retrieval of value. Further, effective mapping of value mechanisms allow for better insight into the building blocks of optimal decision making. A central reason for this gap in the literature is that separate contributions of encoding and retrieval to value-based processing cannot be assessed by tasks commonly used to elicit neural value signals.
Specifically, studies of value processing often rely on reinforcement learning paradigms, in which stimulus- or action-outcome associations are learned implicitly and gradually from experience (Daw, Gershman, Seymour, Dayan, & Dolan, 2011; Dickerson, Li, & Delgado, 2011; Gershman, Pesaran, & Daw, 2009; Gläscher, Daw, Dayan, & O’Doherty, 2010; Gläscher, Hampton, & O’Doherty, 2009; Knutson, Taylor, Kaufman, Peterson, & Glover, 2005; Montague, King-Casas, & Cohen, 2006; Palminteri, Boraud, Lafargue, Dubois, & Pessiglione, 2009; Rushworth & Behrens, 2008; Rushworth, Noonan, Boorman, Walton, & Behrens, 2011; Valentin, Dickinson, & O’Doherty, 2007; Wimmer, Braun, Daw, & Shohamy, 2014; Wimmer, Li, Gorgolewski, & Poldrack, 2018). One-shot decision tasks are also commonly employed to assess neural correlates of value. In these tasks, decisions are made from information presented simultaneously during, or immediately preceding, choice (e.g., potential rewards, outcome probabilities, reward delays; Carter, Meyer, & Huettel, 2010; Clithero, Carter, & Huettel, 2009; Jimura, Chushak, & Braver, 2013; Kable & Glimcher, 2007; Koscik, Man, Jahn, Lee, & Cunningham, 2020; Levy & Glimcher, 2012; Levy, Snell, Nelson, Rustichini, & Glimcher, 2010; McClure, Laibson, Loewenstein, & Cohen, 2004; Padoa-Schioppa & Assad, 2006; Peters & Büchel, 2009; Shenhav, Rand, & Greene, 2017; Tom, Fox, Trepel, & Poldrack, 2007).
While these paradigms do not explicitly examine the influence of episodic memory processes, it can be argued that both types of valuation tasks include features of encoding – comprising the acquisition and integration of available information for subsequent cognitive processes. Thus, dissociating value encoding and retrieval is not possible in these paradigms. Further, what is currently considered the canonical valuation network may be more reflective of value encoding than value retrieval.
There is also a lack of clarity about whether and how valuation and episodic memory processing may interact. Some studies have suggested competition between value and episodic memory systems, while others suggest cooperation or more independent, non-interactive processing. Resolving these mixed findings is critical for determining situations in which engagement of memory processing will impair or facilitate value-based decision making. With respect to findings of memory and valuation competition, when value learning takes place over many prior experiences, select and incidental episodic memory for past choices can result in unequal weighting of prior experiences and biased decision making (Bhui, 2018; Bornstein, Khaw, Shohamy, & Daw, 2017; Bornstein & Norman, 2017; Wimmer et al., 2014). For example, a gamble option may yield a net gain over many selections – but if one past loss is more available to episodic memory, the value of the choice option is likely to be underestimated. This kind of decision bias is predicted by the degree to which previously experienced learning contexts trigger neural reinstatement (Bornstein & Norman, 2017).
Neural mechanisms of these effects further suggest competition between valuation and memory processes during reward-guided reinforcement learning. Indeed, neural responses to value-learning contexts in the parahippocampal place area predict greater influence of past experiences from that context (Bornstein & Norman, 2017), and greater episodic memory for stimuli presented at choice predict weaker predictor error signals in the functionally connected striatum (Wimmer et al., 2014).
In contrast, evidence of cooperation includes behavioral findings that encoding and retrieval of associated episodic information facilitate value-based decision making in novel situations and when choice valuation is based on limited prior experience. For example, when choice values are encoded via discrete experiences (episodic encoding of value), memory for salient choice-associated stimuli predicts value-based decision performance (Duncan & Shohamy, 2016; Murty et al., 2016). Such findings have led to the proposal that familiar stimuli can trigger a “retrieval state” in which value information from associated episodic memories are more easily recollected (Duncan & Shohamy, 2016).
While these studies did not examine neural mechanisms, a conceptually related line of neuroimaging studies have examined the neural correlates of value inferences from stored representations of relevant associations. Such studies find related recruitment of the orbitofrontal or ventromedial prefrontal cortices alongside functionally connected hippocampal regions (Jones et al., 2012; Petrides, 2007; Richter, Chanales, & Kuhl, 2015; Spalding et al., 2018; Stalnaker, Cooch, & Schoenbaum, 2015; Zeithamova, Dominick, & Preston, 2012). Also relevant are studies of episodic encoding tasks with variation in stimuli values. For example, one study demonstrated that increased engagement of semantic processing regions was associated with selective encoding of high-value words during an episodic memory task, suggesting stimulus value effects on level of processing (Cohen, Rissman, Suthana, Castel, & Knowlton, 2014). Other studies suggest that medial temporal regions are more responsive to novel high-value stimuli, with distinguishable engagement of anterior and posterior hippocampal regions, respectively, for processing novel information versus recognizing and retrieving repeated information (Kuhl, Shah, DuBrow, & Wagner, 2010; Poppenk, McIntosh, Craik, & Moscovitch, 2010; Ritchey, Wing, LaBar, & Cabeza, 2013; Wolosin, Zeithamova, & Preston, 2013).
Thus, a number of regions supporting associative memory appear to be sensitive to stimuli values. It is still unclear, however, whether the neural mechanisms of episodically encoded value have a cooperative relationship with episodic memory mechanisms (e.g., enhanced value signal with greater memory for associates) or whether the two systems function independently (e.g., non-interacting neural correlates). Our study attempts to address this remaining knowledge gap on the relationship between value and memory mechanisms.
The present study contributes to our understanding of valuation-memory intersections by comparing the neural mechanisms of value-based judgments when relevant information is present (during encoding) versus when it is absent and must be retrieved from episodic memory (during retrieval). During the study task, participants made value-related judgments about different products based on “consumer reviews” presented an encoding phase and a retrieval phase, while undergoing functional magnetic resonance imaging (fMRI). This design allowed for identifying the neural mechanisms of valuation unique to, and common across, value encoding and value retrieval (Fig. 2a). Additionally, our study task manipulated encoding experiences by presenting products with either one or two consumer reviews at encoding and assessed memory for product attributes using a behavioral memory recognition test. These task features allowed for examining the relationship between valuation and episodic memory systems (Fig. 2b).
Figure 2. Visualization of study goals.
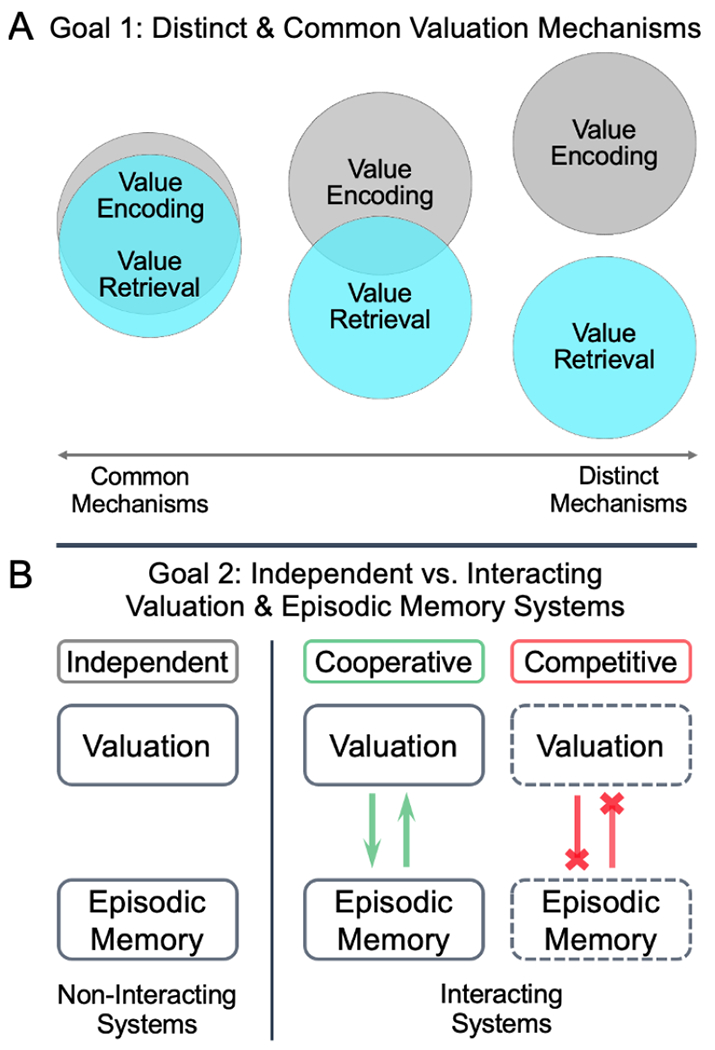
Note. (A)) The primary study goal was to identify distinct and common neural mechanisms of valuation across value encoding and retrieval. To address this goal, brain activation associated with value for consumer products was determined while value-related information was present (value-encoding phase), and later, when value-related information was absent (value-retrieval phase). Resulting phase-subtraction and conjunction-based ROI masks were used to determine whether value-related activity was significantly distinctive during value encoding and retrieval. (B) The secondary study goal was to test for evidence of interaction versus independence between neural mechanisms of valuation and episodic memory. In the case of interaction, we then aimed to determine the nature of the relationship between these systems by probing for competition versus cooperation. To address this goal, demands on memory was manipulated at encoding and retrieval, with corresponding neuroimaging analyses examining effects of encoding experiences on value-related brain activation. Additional analyses were used to determine whether brain activation related to value differed with successful encoding of associated episodic details (product attributes). An absence of memory-dependent effects on the neural correlates of value was taken as evidence for independence. Enhanced value-related activation with more encoding experiences or successful episodic encoding was taken as evidence of cooperation (reciprocal green arrows). Diminished value-related activation with more encoding experiences or successful episodic encoding was taken as evidence of competition (inhibiting red lines).
Based on findings using probabilistic learning and one-shot decision paradigms, we expected that recruitment of regions most commonly implicated in valuation (e.g., fronto-striatal regions) would be more characteristic of valuation at encoding while participants actively acquired and integrated information. As our task involved discrete encoding of value in an episodic context, we expected to find evidence of cooperation or independence, rather than competition, between valuation and episodic memory mechanisms.
Materials and Methods
2.1. Participants
Twenty-seven young adults were recruited from the University of Florida (UF) student population for participation in this fMRI study. Given the novel task and associated lack of prior results for power calculation, the sample size was based on guidelines by Simmons et al. (2011) and Thirion et al. (2007). All participants were healthy, right-handed, fluent in English, free of MRI contraindications, and had normal or corrected-to-normal vision (using MR-compatible eyeglasses). One participant was excluded from data analysis due to excessive head motion across multiple scans, along with four participants for technical errors during scanning or data transfer. Two additional participants were excluded due to failing task performance criteria (i.e., memory false alarm rate ≥ 3 SDs above the mean; responding with single value rating throughout the session). Our final sample included twenty participants (MAge = 22.55, SD = 3.72; 18-31 years old; 9 females).
2.2. Procedure
Participants were recruited from a psychology student database or via flyer, phone call, or email contact from participant registries. Prior to study enrollment, a phone screening was conducted to determine participant interest and eligibility. Data collection took place at the McKnight Brain Institute on the UF Gainesville campus. On the scan day, participants provided written informed consent and completed questionnaires that assessed demographic, health, and psychosocial factors (e.g., affect, motivation, and decision-making style; data not reported here). Participant compensation consisted of either course credit or a $20 gift card depending on participant preference, with an additional $10 bonus paid to all participants (see section 2.3.4. Incentive structure). UF and University of Central Florida (UCF) Institutional Review Boards approved the study.
2.3. Consumer Judgment Task
At session start, participants responded to questionnaires, reviewed task instructions, and completed a set of practice trials on a computer. After entering the scanner, participants reviewed task instructions again and practiced use of a four-button response box. After anatomical and resting-state scans, participants completed three blocks of the value-encoding task (10.02 min, 80 trials each) and two blocks of the value-retrieval task (9.26 min, 75 trials each) during fMRI. Immediately after the value-retrieval task, participants exited the scanner and completed a self-paced behavioral memory test on product attributes from the encoding phase (M = 15.95 min, SD = 2.37 min, 390 trials). Participants then responded to a questionnaire about their experience in the study and task-performance strategies. At the end of the session, participants received their compensation and were debriefed.
2.3.1. Stimuli
The stimuli used in this task were created to resemble common consumer products from a popular online shopping website. Each product was represented by an image and a product name (e.g., “Clock”). During the encoding phase, products were presented with associated “consumer reviews.” Text was derived from real consumer reviews of the product rated as “most helpful” from the same online shopping website. For each product, review excerpts were taken from three comments that focused on different product attributes (e.g., battery life, ticking sound, number size). These attribute-specific comments were edited to fit three valence conditions: positive (e.g., “Quiet, hardly noticeable tick.”), negative (e.g., “Extremely loud ticking noise.”), and neutral (e.g., “Audible tick.”). To confirm that each consumer review reflected its valence category, valence ratings were collected from a separate sample via Amazon Mechanical Turk (MTurk). Individual MTurk raters assessed attribute-specific consumer reviews for only one valence condition. Review stimuli received 10 ratings each. Stimuli in the fMRI study were presented against a white background using E-Prime 2.0 software (Psychology Software Tools, Pittsburgh, PA) on a screen in the scanner room.
2.3.2. Value encoding
To index value at encoding and address our primary study goal, participants were asked to rate the valence of each consumer review while undergoing fMRI (Fig. 3a). Ratings were given on a discrete valence scale from 1 (negative) to 4 (positive) while the product was visible on the screen for 4 seconds. A black fixation cross was shown when the participant responded in time. Participants were informed that, if unable to respond within the allotted time, they could respond for an additional 2 seconds while a red ‘X’ was on the screen. Failure to make any button press resulted in the trial being coded as a non-response; non-response trials were not included in the data analysis (~6%). To address trials with multiple button presses, the last response was used for the review rating. A jittered inter-stimulus interval (ISI) of 2 to 4 seconds (e.g., pseudo-random at discrete intervals of 250 ms) occurred between each product.
Figure 3. Consumer Judgment Task design.
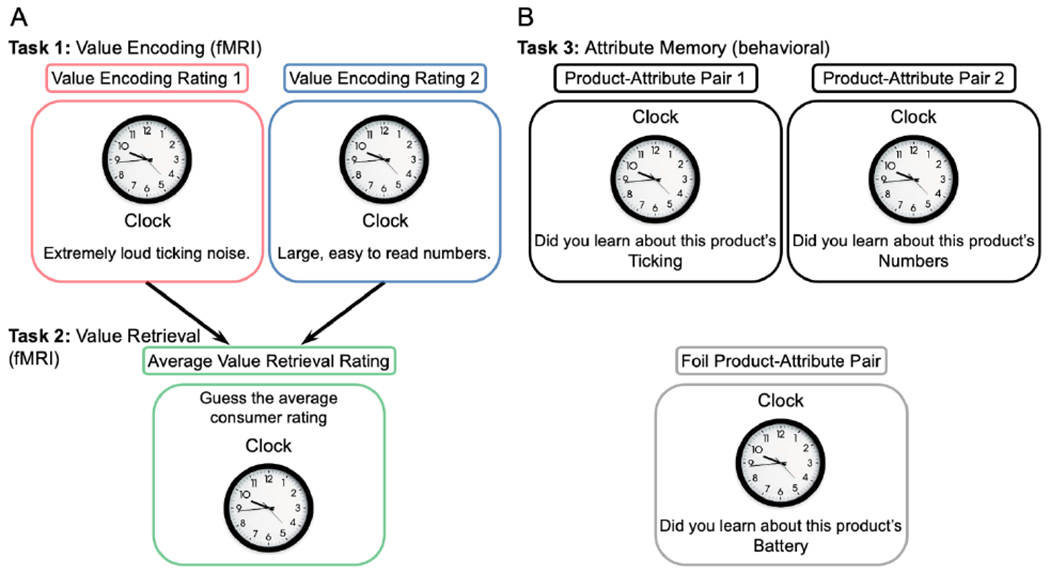
Note. The Consumer Judgment Task was divided into three phases: value encoding, value retrieval, and attribute memory. Value encoding and retrieval phases were completed during fMRI; the attribute memory task followed outside the scanner. (A) During value encoding, reviews of consumer products were rated using a discrete 1 (negative) to 4 (positive) value scale. Products were either presented with one consumer review or two different consumer reviews. During value retrieval, the average rating for each product was estimated based on the previously encountered consumer reviews, using a continuous 1 (negative) to 5 (positive) rating scale. (B) Attribute memory was tested using a 1 (definitely yes) to 4 (definitely not) scale as to whether attribute-product pairs were presented together during encoding.
Toward our secondary study goal, we manipulated the need for integration of value-related information during retrieval to probe demands on the memory system. Correspondingly, products varied in the number of encoding experiences such that products were presented with either one consumer review or two different reviews. In the one-experience condition, products were presented with either a positive or negative review. Products in the two-experience condition were presented twice within 2 to 4 encoding trials and were accompanied by two different attribute-specific reviews. In the two-experience condition, products were presented with either two reviews of opposing valence (negative followed by positive, positive followed by negative) or two neutral reviews. Ten products were presented per valence sequence category in each value-encoding block.
2.3.3. Value retrieval
To index value at retrieval and further address our primary study goal, participants were asked to estimate the average consumer rating of each product from the encoding phase on a continuous 1 (negative) to 5 (positive) scale based on the previously encountered consumer reviews while undergoing fMRI (Fig. 3a). Product ratings from the one-experience condition required the retrieval of only a single consumer review from recent memory. Ratings for products in the two-experience condition required retrieval of information from two different consumer reviews, adding the need to integrate previously encountered value information.
A continuous rating scale was used with a value range that differed from the discrete scale used in the encoding phase, such that rating consistency across phases would require a numerical translation for products in both the one- and two-experience conditions. Consistency in value ratings therefore required more than a simple repeated response across the encoding and retrieval phases. Products from value encoding were presented in random order during value retrieval. Retrieval trials began with the rating marker at the neutral position (‘3’), and participants were given 4 seconds to respond on the slider scale, using their index and middle fingers to indicate increasingly negative or positive ratings, respectively. To make an exact rating of ‘3’, participants pressed a key with their ring finger. Failure to press any button resulted in the trial being coded as a non-response; non-response trials were not included in the data analysis (~3%).
2.3.4. Incentive structure
Participants were told that the amount of their payout bonus would be calculated based on the value of two products randomly selected at the end of the experiment. The task’s incentive structure was tied to valuation in two ways. First, participants were told that trials (products) with more positive consumer reviews had the potential to yield more monetary gain, such that the most valuable products were associated with $5 rewards and least valuable products with $1 rewards. They were told that an external sample had rated all products based on the presented consumer reviews on a 1-5 scale and the average of those ratings determined the value of each product (i.e., $1-5). This incentive structure parallels naturalistic memory-based consumer decision making, such that consumers are incentivized to remember high-value products due to their greater reward potential (utility), but memory for low-value products is also important as it helps consumers avoid poor choices.
Second, participants were told that their final bonus depended on correspondence between their ratings and the external sample’s average ratings. As the participants were told the external sample’s ratings were based on the same consumer reviewer comments they saw in the task, participants were incentivized to give ratings that reflected the product reviews. Specifically, participants were told that any discrepancy between their rating and the external sample’s rating for a given product would be subtracted from the product’s value. For example, if the external sample gave a product an average rating of ‘3’ (worth $3) and the participant guessed that the average rating was a ‘4’ (valuation error of 1 = $1), that product would only add $2 toward their earnings if selected for the bonus. Notably, this incentive structure penalizes over- and under-estimations of value, ensuring that participants had a vested interest in attending to, and correctly retrieving, information from all consumer reviews when making their ratings. As participants were told that all products were equally likely to be selected for the bonus, performance on each trial was incentivized. At the end of the study, trials were not randomly selected and all participants received the maximum possible bonus of $10.
2.3.5. Episodic memory for product attributes
Toward addressing our secondary goal, participants completed a memory test for product attributes outside the scanner, immediately after the value-retrieval phase. Each self-paced memory trial included the picture and name of a product presented during value encoding, along with a non-affective attribute (e.g., battery life, ticking sound, number size; Fig. 3b). Participants provided responses reflecting the degree to which they remembered specific attributes mentioned in the consumer reviews for individual products using a discrete 1 (definitely yes) to 4 (definitely not) scale. Each pairing of product and attribute encountered during the encoding phase was presented during the attribute memory phase, intermixed with foil attributes (one per product) to distinguish between false alarms and correct recognitions.
2.4. Behavioral data analysis
Behavioral data were analyzed using SPSS Statistics 25 (IBM Corp., RRID:SCR_002865). Valence ratings from encoding and retrieval were used to index valuation. To facilitate comparisons across encoding and retrieval ratings, transformations were applied to both measures to put them on 0-1 scales. The transformation for encoding responses converted the original 1-4 interval scale into four equally spaced values ranging from 0-1. Retrieval responses were converted from the original 1-5 continuous scale into z-score values. Because participants did not use the full range of the original retrieval scale, z-scored retrieval responses were based on the highest and lowest responses entered by each participant within a block. Z-scored retrieval ratings were then transformed to a 0-1 scale. Transformed ratings were used to calculate value-retrieval accuracy, which corresponded to the similarity between product-specific ratings at encoding and retrieval (i.e., encoding rating – retrieval rating). For products with two encoding experiences, value-retrieval accuracy calculations used the average of the two product-specific encoding ratings. Finally, episodic memory for product attributes was indexed using corrected recognition scores (% hit rate – % false alarm rate). Ratings of 1 (definitely yes) or 2 (maybe yes) for correct product pairings encountered in the attribute memory task phase were coded as hits, while the same ratings for incorrect (“foil”) product pairs were coded as false alarms.
2.4.1. Value rating behavior
To establish task validity, we used a repeated measures analysis of variance (RM-ANOVA) to assess consistency between participant’s value ratings at encoding and the three preset valence conditions (negative, neutral, and positive) for consumer reviews. Next, we utilized a linear mixed-effects model to verify the correspondence in value ratings at encoding and later retrieval and assess the effects of the number of encoding experiences on value retrieval. The linear mixed-effects model included subject as a random effect, with a random intercept for each subject, a restricted maximum likelihood estimation, and an unstructured covariance type. We conducted additional analyses to confirm that the unique consumer reviews presented with two-experience products during encoding both contributed to later retrieval ratings and determined the relative influence of first and second encoding trials on subsequent value-retrieval ratings (see Supplementary Material, Section I).
2.4.2. Value retrieval and episodic memory performance
Toward our secondary goal of examining interactions between valuation and memory systems, we assessed episodic memory for product attributes. We first conducted a one-sample t-test on participants’ corrected recognition scores to confirm that performance was above chance. We then utilized a trial-level linear mixed-effects model to gauge the potential interaction between value-retrieval accuracy and memory accuracy for each product, using hit rate as a representation of attribute memory performance and modeling subject as a random effect. Additional analyses were conducted to confirm that the number of encoding experiences did not alter the correspondence between value ratings and attribute memory strength (see Supplementary Material, Section II).
2.5. Neuroimaging data acquisition
Brain images were acquired with a Philips Achieva 3T scanner using a 32-channel RF head coil. Participants viewed the experiment screen via a mirror placed inside of the head coil and used their right hand to respond via an MR-compatible four-button response box. Anatomical image acquisition involved a T1-weighted 3D localizer series (MPRAGE sequence), with 176 axial oblique slices (TR: 7.1 ms; TE: 3.2 ms; FOV: 240 mm2; flip angle: 8°; voxel size: 1 mm3; scan time: 4.5 min). After the anatomical scan, functional images were acquired using a single-shot Fast Field Echo-Planar Imaging (FFE-EPI) sequence (sensitivity-encoded [SENSE] for 2-fold acquisition speed) sensitive to the blood oxygenation level-dependent (BOLD) signal (TR: 2000 ms; TE: 30 ms; FOV: 252 mm2; flip angle: 90°; 38 interleaved transverse slices; voxel size: 3.5 mm3; no slice gap; acquisition matrix: 72 x 72 voxels; SPIR fat suppression). Functional scanning included a resting-state functional scan (not reported here), followed by three runs of the encoding task (295 TRs each) and two runs of the retrieval task (277 TRs each). Four acquired volumes from the start of each functional run were discarded prior to data analysis.
2.6. Neuroimaging data analysis
Structural and functional image analyses were conducted using FMRI Expert Analysis Tool (FEAT), from FMRIB’s Software Library (FSL v6.0.1; RRID:SCR_002823; Smith et al., 2004). Image preprocessing steps included: (i) motion correction with MCFLIRT (FMRIB’s Motion Correction Linear Image Registration Tool); (ii) spatial smoothing with a 5-mm full-width half-maximum (FWHM) Gaussian kernel registered to the first image; (iii) high-pass temporal filtering equivalent to 100 sec; and (iv) skull stripping of structural images with BET (FMRIB’s Brain Extract Tool). Registration was performed with FLIRT (FMRIB’s Linear Image Registration Tool) such that each functional image was registered to both the participant’s high-resolution brain-extracted structural image (6 df) and the FSL Montreal Neurological Institute (MNI) template using an affine transformation (12 df). All scans included in the analysis had less than 3 mm relative head motion and were free of severe artifacts (e.g., spiking, ghosting, radio frequency noise, signal inhomogeneity).
All reported Z-statistic maps survived cluster-wise whole-brain correction with a cluster-forming threshold of Z > 2.3 and cluster-correction at p = .05 (Worsley, 2001). To reduce the proportion of false positives identified within our group-level activation clusters and develop spatially constrained regions-of-interest (ROIs) for comparison of regions with robust value-associated activity at encoding and retrieval, we further applied voxel-wise false discovery rate (FDR) multiple comparison corrections at a threshold of q = .05 to group-level contrast images (FSL-FDR; Genovese, Lazar, & Nichols, 2002; Woo, Krishnan, & Wager, 2014). Image coordinates are presented in MNI space and anatomical regions were identified using the Harvard-Oxford cortical and subcortical structural atlases (Makris et al., 2006). For visualization, Z-statistic maps are presented over an MNI-152 standard-space T1-weighted average structural template image with 2 mm resolution using the FSL image viewer (FSLeyes v0.27.3; McCarthy, 2020). ROI masks are rendered over a 3D MNI-152 standard brain using MRIcroGL (v1.2; Rorden, Karnath, & Bonilha, 2007) with partial transparency set to relay depth information.
Three value-encoding and two value-retrieval phase scans (Fig. 3a) were analyzed per participant using general linear models (GLM) with local autocorrelation correction (FMRIB’s Improved Linear Model prewhitening). Trial events were convolved with a double-gamma hemodynamic response function and motion parameters were included as nuisance regressors. Trials with missing rating responses were not modeled (~6% of encoding and 3% of retrieval trials). Subject-level analyses used a fixed-effects model to combine data across runs for each participant. Group-level analyses used a mixed-effects model to combine data across participants, allowing for the examination of group means.
2.6.1. Distinct and common neural correlates of value encoding and retrieval
2.6.1.1. Value encoding network.
We applied a GLM to fMRI data from the encoding phase (Fig. 3a) to identify BOLD responses associated with value at encoding. The encoding analysis included two trial events modeled by time of onset and subject response time (≤ 4 sec) for event duration: (i) encoding trials and (ii) encoding trials with associated value ratings as a parametric modulator orthogonalized to the main encoding trial regressor. The main contrast of interest tested the parametric effect of encoding value rating, representing the value network during encoding.
2.6.1.2. Value retrieval network.
To identify BOLD response associated with value at retrieval, we applied a GLM to fMRI data from the retrieval phase (Fig. 3a) with events modeled by time of trial onset and the full trial duration (~4 sec). The retrieval analysis included four trial events: (i) retrieval trials for one-experience products, (ii) retrieval trials for one-experience products with associated value ratings as a parametric modulator orthogonalized to the main retrieval trial regressor, (iii) retrieval trials for two-experience products, and (iv) retrieval trials for two-experience products with associated value ratings as a parametric modulator orthogonalized to the main retrieval trial regressor. The main contrasts of interest tested (i) the parametric effect of retrieval value rating, (ii) the bidirectional effects of one versus two encoding experiences, and (iii) bidirectional effects of encoding experiences weighted by retrieval value ratings. Together with the above analyses of fMRI data at encoding, these analyses addressed our two central study goals. Full whole-brain value-related activation results are available in the Supplementary Materials under Section III.
2.6.1.3. Distinct and common neural correlates of value encoding and retrieval.
Toward our primary goal, we created binarized masks of group-level FDR-corrected parametric activity related to value ratings at encoding and retrieval. To identify distinct neural correlates of value during encoding and retrieval, we conducted bidirectional contrasts of valuation masks between valuation phases. The resulting phase-subtraction masks (i.e., Encoding > Retrieval; Retrieval > Encoding) represented regions exhibiting significant value-related activation in only one phase – encoding or retrieval. To identify common neural correlates of value across phases, a conjunction analysis was conducted using the aforementioned masks of value-related activity from each phase. The resulting conjunction map represents areas where value-related activations were significant during both encoding and retrieval (i.e., Encoding x Retrieval; Biswal et al., 2010; Friston, Holmes, Price, Büchel, & Worsley, 1999).
To test whether activity differed significantly by value-encoding versus value-retrieval phase within the resulting phase-subtraction and conjunction clusters, we compared mean activity levels between valuation phases in the resulting ROI clusters. Mean percent BOLD signal change values were extracted from subject-level value-weighted contrast images for encoding and retrieval within binarized masks from the phase-subtraction and conjunction clusters using FSL featquery. Included ROIs represented regions previously implicated in valuation (Fig. 1), with regions associated primarily with sensorimotor functions excluded from the ROI analysis. To compare ROI activity levels between value-encoding and value-retrieval phases, separate two-way RM-ANOVAs (valuation ROIs x valuation phases) with Greenhouse-Geisser sphericity correction were conducted for each group of phase-subtraction and conjunction ROIs. Post-hoc pairwise comparisons were conducted with Bonferroni-Holm correction for multiple comparisons to assess significant ROI-phase relationships.
Additional analyses were conducted to examine effects of the encoding experience manipulation. First, a control analysis was conducted to address the possibility that repeated presentation of products in the two-experience condition caused memory interference effects that impacted our observed results for value-related activity. This analysis examined value-related activity at encoding for products in the one-experience condition alone in order to compare them with our main analysis of value correlates across encoding experience conditions. Then, to address our secondary study goal, analyses were conducted to examine the impact of multiple encoding experiences on value-related brain activation (e.g., memory demand from information integration). In brief, the encoding experience manipulation did not yield effects on value-related activation that survived FDR correction, consistent with an independence of valuation and memory systems. More detailed methods and results of these analyses are available in the Supplementary Materials under Sections IV and V.
2.6.2. Relationship between valuation and episodic memory systems
Toward our secondary study goal, we next conducted a GLM analysis to determine if value-related activation depended on whether or not associated episodic details were successfully encoded. The analysis included four trial events: all trials, encoding trials with associated value ratings as a parametric modulator orthogonalized to the main encoding trial regressor, encoding trials with subsequent product attribute memory test responses as a parametric modulator orthogonalized to the main encoding trial regressor, and the interaction of encoding value rating and attribute memory test response. Trials were modeled by time of onset and participant response time for duration. The primary contrasts of interest examined the main effect of encoding trials associated with successful subsequent memory and the interaction of parametric value rating and product attribute memory test regressors. To examine valuation and episodic memory interactions at the retrieval phase, a parallel GLM analysis assessed activation related to successful episodic retrieval, with trials modeled by onset time and full trial duration (~4 sec). The retrieval-oriented analysis design followed the same structure as in the encoding phase, with the purpose of identifying the neural correlates of value-retrieval trials associated with successful memory and the interaction of parametric retrieval value rating and product attribute memory test regressors. These neural analyses were aimed at determining if episodic memory and valuation mechanisms interacted, and if so, whether interactions indicated cooperation (i.e., enhanced engagement in valuation mechanisms with episodic processing) or competition (i.e., reduced engagement in valuation mechanisms with episodic processing).
Results
3.1. Behavioral results
3.1.1. Successful retrieval of value ratings
First, we confirmed that value ratings at encoding scaled linearly with the preset valence condition (F(1.32,25.07) = 513.06, p < .001, ηp2 = .96). Post-hoc pairwise comparisons verified significant differences between rating conditions, such that ratings were higher for product comments in the positive (M = 3.56, SEM = 0.06) compared to negative (M = 1.48, SEM = 0.04; t(19) = −24.01, pholm < .001, d = −5.37) valence condition. Product ratings were also confirmed to be less positive for products in the negative compared to neutral (M = 2.92, SEM = 0.05; t(19) = −24.21, pholm < .001, d = −5.41) condition and more positive for the positive compared to neutral (t(19) = −13.59, pholm < .001, d = −3.04) condition.
We further confirmed that value-related information presented at encoding was remembered during retrieval, as product-specific value ratings from the encoding phase strongly predicted value ratings from the retrieval phase (F(1,2881) = 786.91, b = 1.60, SEb = 0.06, p < .001, ηp2 = .22; Fig. 4). The direction and strength of the relationship between value ratings in the two phases indicates that higher value at encoding corresponded to higher value at later retrieval. We did not find an effect of the number of encoding experiences (F(1,2881) = 0.63, b = 0.03, SEb = 0.03, p = .43, ηp2 < .001), indicating that the valence of value ratings at retrieval was not systematically different for products that were presented with one or two consumer reviews during encoding.
Figure 4. Correspondence between value ratings across value encoding and retrieval.
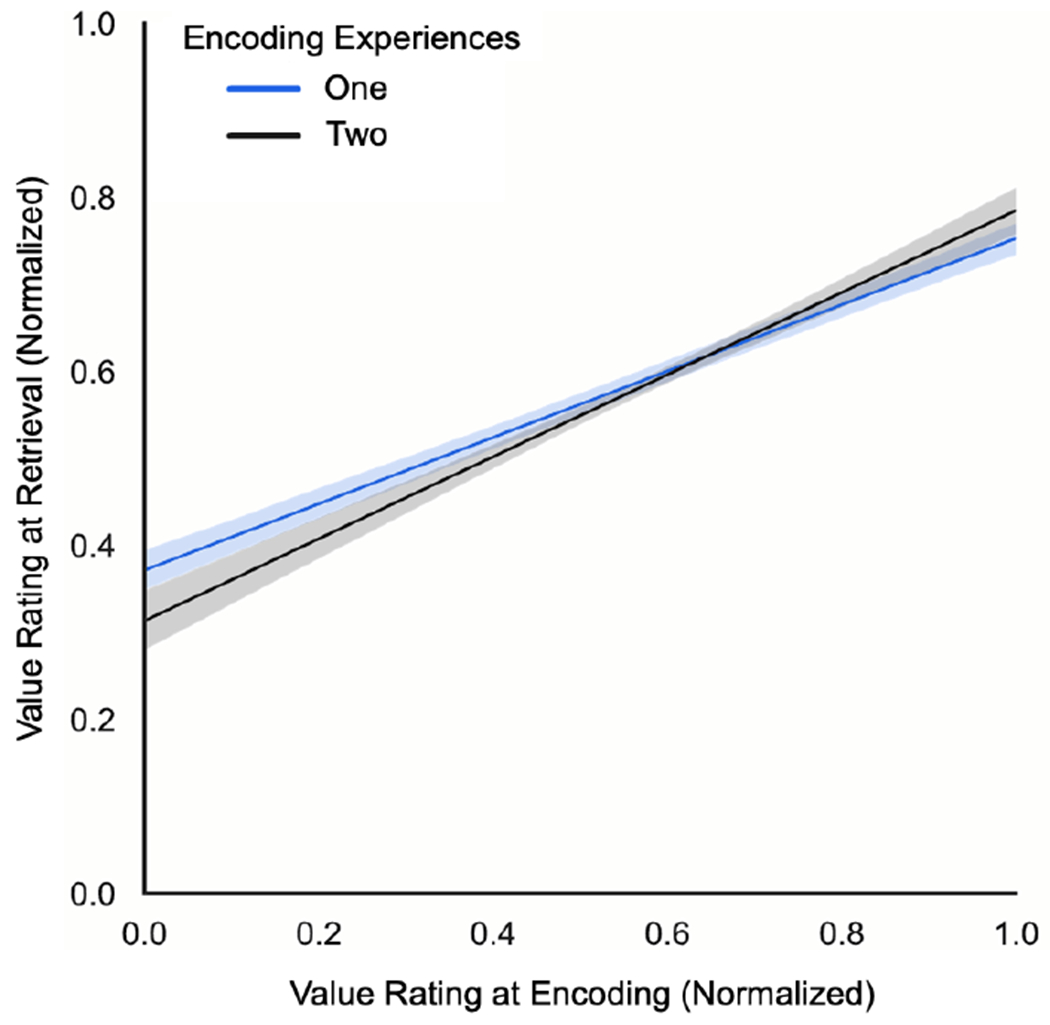
Note. Normalized value ratings during value retrieval were found to scale alongside value encoding ratings (averaged for products with two consumer reviews), regardless of the number of encoding experiences. For visual comparison purposes, discrete value encoding ratings (X-axis) and continuous value retrieval ratings (Y-axis) were normalized to a 0-1 scale, representing negative (0) to positive (1) value ratings. Shading represents 95% confidence intervals for each regression line.
Additional analyses confirmed that the two consumer reviews presented with two-experience products during encoding both contributed to later retrieval ratings (see Supplementary Material, Section I). Results confirmed that value information was integrated across product-specific encoding trials; however, the first product comment appeared to have more influence than the second on subsequently retrieved value ratings. Together, these results signify the successful retrieval of value-related information presented during encoding across encoding experience conditions. Critically, previous research confirms that differences in neural recruitment due to experienced task demands can be observed in the absence of behavioral differences (e.g., Cabeza et al., 2004; Cappell, Gmeindl, & Reuter-Lorenz, 2011; Nagel et al., 2013). As such, we included the number of encoding experiences as a factor in our fMRI analyses to test for differences in neural recruitment when value retrieval required accessing memory for one encoding event versus accessing and integrating two encoding events.
3.1.2. Relationship between value retrieval and episodic memory performance
We next examined performance on the product attribute memory task, confirming successful memory retrieval with an average corrected recognition score of 46% (SEM = 0.04; t(19) = 11.41, p < .001, d = 5.24), corresponding to a hit rate of 79% and a false alarm rate of 34%. Toward our goal of examining interactions between value- and memory-related processing, results of a trial-level linear mixed-effects model examining the relationship between value-retrieval accuracy and attribute memory showed that value-retrieval accuracy (M = 0.80, SEM = 0.01) predicted better subsequent memory for product attributes (F(1,2876.53) = 22.42, b = 0.17, SEb = 0.04, p < .001, ηp2 = .13). Thus, our behavioral results indicate a correspondence between memory for value-related information and episodic details.
3.2. Neuroimaging results
3.2.1. Distinct and common neural correlates of value encoding and retrieval
3.2.1.1. Identification of phase-distinct versus common valuation regions.
To address our primary study goal, we identified BOLD responses associated with value ratings at value encoding and retrieval. FDR-corrected value-encoding and value-retrieval activation maps were then compared to identify phase-distinct and -common valuation mechanisms.
In line with our first hypothesis, subtractions of the FDR-corrected value-weighted encoding and retrieval contrast images (i.e., Encoding > Retrieval) identified non-overlapping clusters of primary fronto-striatal and secondary fronto-parietal valuation network activity that scaled significantly with value ratings at each phase (Fig. 5a; Table 1 for cluster information). Focal clusters of activity were distinctly associated with value at encoding within the striatal left anterior caudate nucleus and right caudal body, along with the left superior parietal lobule (FDR-corrected threshold of p < .0001). Notably, while canonical fronto-striatal reward and salience regions were recruited during our value-encoding task (Supplementary Material, Section III, Table S1), activity within striatal and posterior parietal areas was most robustly connected to value rating behavior at encoding.
Figure 5. Phase-distinct and common neural activity related to value ratings.
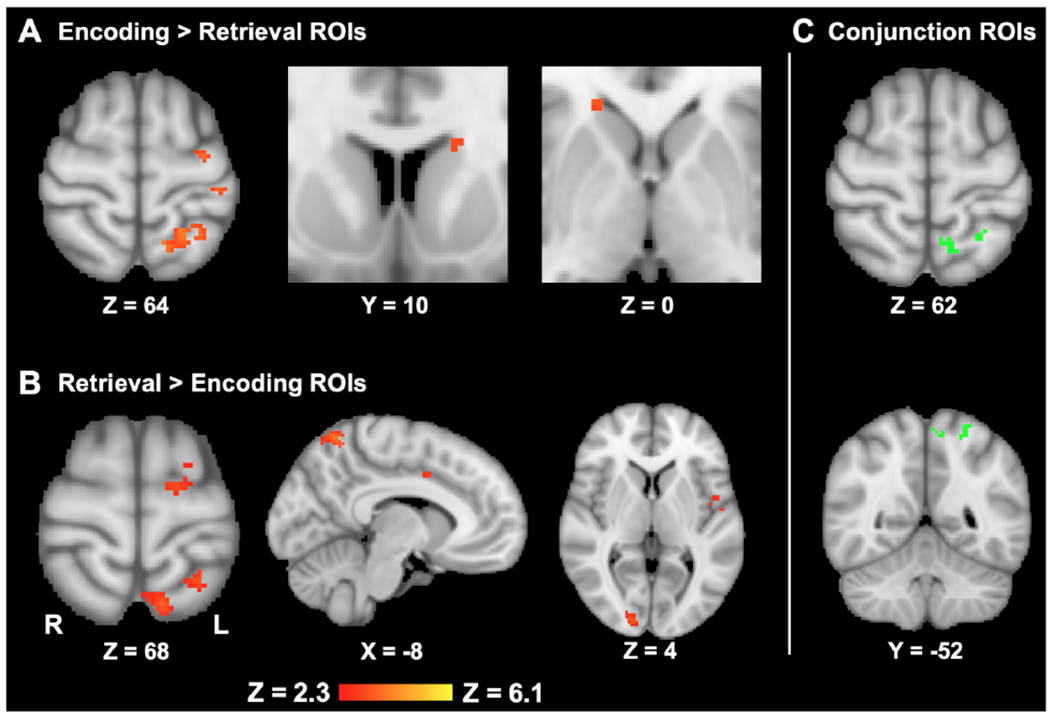
Note. (A) Greater overall recruitment of fronto-striatal valuation and fronto-parietal control network areas was associated with encoding and integrating value information, with areas of the bilateral striatal caudate nucleus and left superior parietal lobule surviving FDR-correction (voxel-wise threshold of q = .05). (B) While retrieving value information from recent memory, robust neural recruitment was primarily observed in salience and fronto-parietal control network areas, including superior frontal, insular, dorsal anterior cingulate, precuneal, and superior parietal clusters. (C) Recruitment of fronto-parietal and sensory processing regions was associated with value processing across valuation phases, with clusters in the left superior parietal lobule, precuneus, and right occipital fusiform areas surviving FDR-correction. Image slices are presented in radiological orientation with MNI slice coordinates.
Table 1.
Value-related activity distinct or common to valuation phases (FDR-corrected at q =.05)
| Cluster | MNI | Peak | |||
|---|---|---|---|---|---|
| Region | Extent | X | Y | Z | Z-stat |
| Value Encoding (Encoding > Retrieval) | |||||
| L Superior Parietal Lobule | 126 | −22 | −50 | 64 | 4.44 |
| R Temporal Occipital Fusiform Cortex / R Occipital Fusiform Gyrus / Lingual Gyrus | 52 | 26 | −56 | −16 | 4.27 |
| L Postcentral Gyrus | 42 | −42 | −28 | 60 | 4.55 |
| L Occipital Fusiform Gyrus | 29 | −38 | −70 | −12 | 4.01 |
| L Inferior Occipital Cortex | 28 | −36 | −80 | 4 | 4.20 |
| R Inferior Occipital Cortex | 22 | 50 | −72 | −10 | 4.00 |
| Cerebellum | 13 | 18 | −66 | −18 | 3.93 |
| L Superior Occipital Cortex | 8 | −22 | −72 | 38 | 3.92 |
| L Precentral Gyrus | 8 | −32 | −12 | 64 | 4.19 |
| R Caudate Nucleus (Anterior) | 7 | 20 | 28 | 0 | 3.96 |
| L Caudate Nucleus (Body) | 5 | −15 | 9 | 20 | 3.94 |
| Value Retrieval (Retrieval > Encoding) | |||||
| Lingual Gyrus / R Occipital Fusiform Gyrus / R Inferior Occipital Cortex | 1457 | 6 | −78 | −8 | 6.06 |
| Precuneus / L Superior Occipital Cortex / L Superior Parietal Lobule | 159 | −10 | −60 | 64 | 4.41 |
| L Superior Parietal Lobule | 36 | −26 | −50 | 68 | 3.88 |
| L Superior Frontal Gyrus | 29 | −16 | −8 | 70 | 3.88 |
| Anterior Cingulate Cortex (Dorsal) | 10 | −8 | 4 | 38 | 3.65 |
| L Insular Cortex / Central Opercular Cortex / Planum Polare | 6 | −50 | 2 | 4 | 3.57 |
| Across phases (Encoding x Retrieval) | |||||
| R Occipital Fusiform Gyrus | 67 | 20 | −77 | −15 | – |
| Precuneus / L Superior Parietal Lobule / L Superior Occipital Cortex | 39 | −11 | −56 | 64 | – |
| L Superior Parietal Lobule | 30 | −26 | −50 | 64 | – |
Note. Cluster peak coordinates represent local maxima and are presented in MNI space. Reported activation clusters survived voxel-wise FDR multiple comparison correction (threshold of q = .05) and are greater than 4 voxels in size. Anatomical labels are presented for the primary regions represented within each cluster based on Harvard-Oxford cortical and subcortical structural atlas labels. L = left lateralized; R = right lateralized.
Also in line with our first hypothesis, the regions identified during value retrieval (i.e., Retrieval > Encoding) matched with secondary valuation and memory mechanisms (fronto-parietal control and salience networks), rather than the canonical fronto-striatal valuation network. During value retrieval, distinctive activity was identified within fronto-parietal and salience network regions, including clusters within the left insular cortex, left superior frontal gyrus, and dorsal anterior cingulate cortex (Fig. 5b; FDR-corrected threshold of p < .0003). Additional clusters spanned posterior parietal regions, encompassing the left superior parietal lobule and superior precuneus cortex (Table 1 for cluster information).
To determine areas recruited during both value encoding and retrieval, we utilized a conjunction comparison of value-weighted activity across valuation phases (i.e., Encoding x Retrieval). In comparing FDR-corrected cluster masks across phases, significant activation overlap was identified within precuneal, left superior parietal, and right occipital fusiform clusters adjacent to those identified in the phase-subtraction contrasts (Fig. 5c; Table 1). As described in the Methods (Section 2.6.1.3), comparable neural correlates of valuation across phases were identified in additional analyses of value-related activity for products with one encoding experience (i.e., representing value-related activity without information integration; Supplementary Material, Section IV, Fig. S1, Table S2).
3.2.1.2. Direct comparison of valuation ROI activity across valuation phases.
ROIs from the value network contrast analysis were used to test for significant differences in value-related activation by encoding and retrieval phase (for full details of ROI cluster information and associated analyses, see Supplementary Material, Section VI). ROIs resulting from the Encoding > Retrieval contrast mask were in the right anterior caudate, left caudate, and left superior parietal areas. RM-ANOVA results indicated that value-related signal did not differ for encoding and retrieval phases across these ROIs (F(1,19) = 0.74, p = .40, ηp2 = .04; Fig. 6a), and there was no significant interaction between valuation phase and ROIs (F(2,38) = 0.44, p = .65, ηp2 = .02).
Figure 6. Comparison of ROI cluster activity levels across valuation phases.
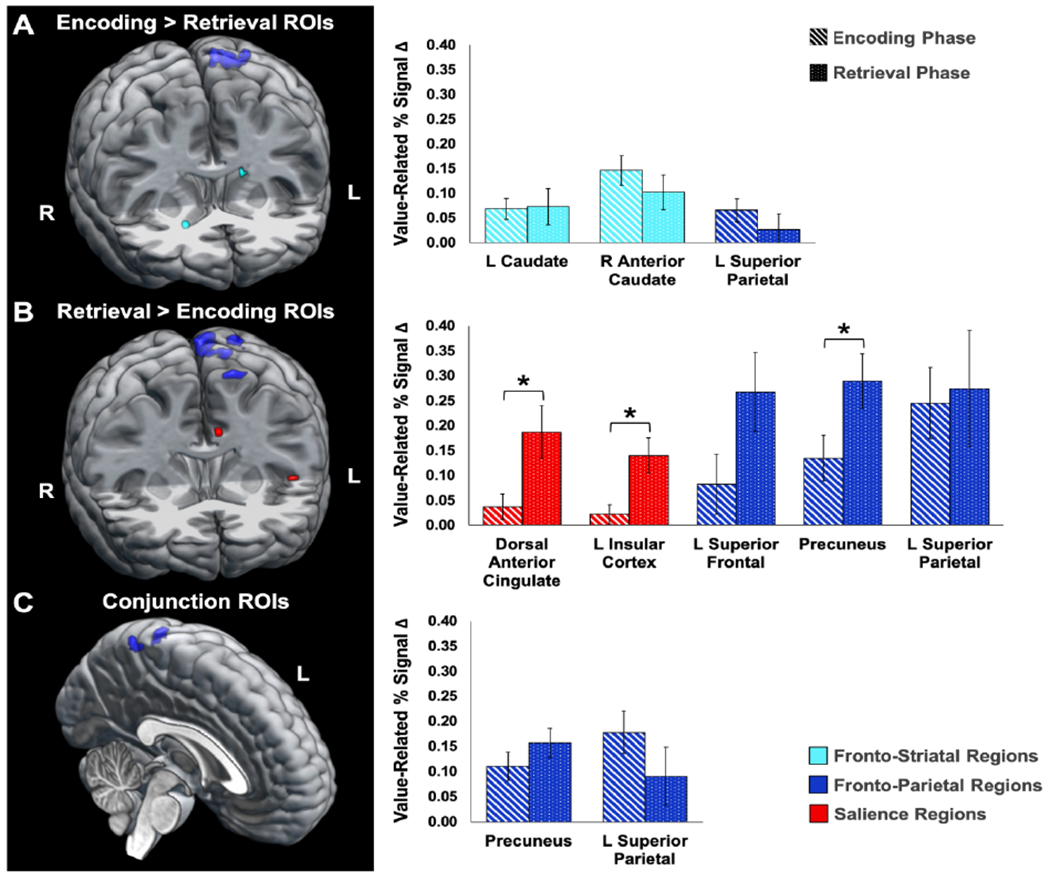
Note. (A) Similar value-related activation levels were observed across valuation phases within the caudal (light blue) and superior parietal (dark blue) clusters identified from the value encoding subtraction contrast, reflecting dependence on striatal and posterior parietal areas for value processing whether the value details were present or retrieved from recent memory. (B) During the value-retrieval phase, however, significantly greater activity was observed for the dorsal anterior cingulate gyrus (red; t(19) = −2.26, p = .04, d = −.51), left lateralized insular cortex (red; t(19) = −2.75, p = .01, d = −.62), and precuneus (dark blue; t(19) = −2.75, p = .01, d = −.61) in comparison to activity at value encoding. (C) Posterior parietal ROIs (dark blue) identified from a conjunction analysis represent regions recruited for value processing across phases and therefore were not expected to have significant activation differences. Error bars represent standard error of the mean; asterisks (*) indicate significant differences in activity between phases at p < .05.
ROIs resulting from the Retrieval > Encoding contrast mask were in the left insula, left superior frontal gyrus, dorsal anterior cingulate cortex, left superior parietal lobule, and superior precuneus. RM-ANOVA results for value-related activity within these ROIs yielded no significant interactions (F(2,29,43,42) = 0.60, p = .58, ηp2 = .03), but did reveal a main effect of valuation phase (F(1,19) = 5.77, p = .03, ηp2 = .23; Fig. 6b). Post-hoc paired-samples t-tests revealed medium significant effects of valuation phase in three ROIs – the left insula (t(19) = −2.75, p = .01, d = −.62), precuneus (t(19) = −2.75, p = .01, d = −.61), and dorsal anterior cingulate gyrus (t(19) = −2.26, p = .04, d = −.51). The direction of the test statistics indicated significantly greater activity within these ROIs during value retrieval compared to value encoding. Phase differences in activation foi the left superior frontal gyrus ROI did not reach significance (t(19) = − 1.89, p = .07, d = −.42).
ROIs resulting from the Encoding x Retrieval conjunction mask were in the precuneus and left superior parietal cortex. Consistent with expectation, RM-ANOVA results for value-related activity within these ROIs showed no significant differences between phases (F(1,19) = 0.16, p =.70, ηp2 = .01; Fig. 6c), nor evidence of an interactive effect of ROI and valuation phase (F(1,19) 3.56, p = .08, ηp2 = .16).
Together, the tests of ROI activity suggest reliance on posterior parietal and fronto-striatal valuation regions for both encoding and retrieval of value information, with significantly greater recruitment of select parietal control and salience regions during the retrieval of value. Further, consistently high recruitment of adjacent clusters within the left superior parietal lobule and precuneus across phases suggests a strong reliance on the posterior parietal cortex across value encoding and retrieval.
3.2.2. Relationship between valuation and episodic memory systems
Toward our secondary goal, we examined brain activation corresponding to episodic memory for product attributes relative to the neural mechanisms of value. GLM analyses examined interactions between regressors representing valuation and successful episodic memory for product attributes. In the encoding and retrieval phases, we observed neural correlates of successful subsequent memory for product attributes across regions commonly associated with episodic memory, but associated brain activation did not survive FDR correction (Supplementary Material, Section VII). Most notably, interaction between valuation and subsequent attribute memory did not yield any significant clusters during either phase. These analyses uniformly support independence, rather than interaction, between neural correlates of valuation and episodic memory.
Discussion
Everyday decision making requires efficient assessment of available options, which often involves active evaluation and retrieval of previous experiences. To determine how encoding and retrieval processes affect the composition of the value network, we used a novel memory-dependent valuation paradigm and fMRI to compare behavior and neural processing during encoding versus retrieval of value. Further, to clarify the nature of the relationship between valuation and memory systems, we examined evidence for interactions between the neural mechanisms of valuation and episodic memory processes. Consistent with our first hypothesis and prior studies of valuation, our whole brain analysis of value-related activation revealed significant engagement of striatal regions in the canonical valuation network during value encoding. Diverging slightly from our prediction, however, direct comparison of valuation-associated activity in functionally determined ROIs indicated similar fronto-striatal network engagement during value encoding and retrieval. Our results also revealed robust value-related activation in posterior parietal regions across phases, while the value-retrieval phase was associated with significantly greater engagement of salience and parietal regions previously implicated in valuation. Finally, our examination of valuation and episodic memory interactions largely supported independence of these neural systems in task contexts where value judgments are based on episodic encoding of value and associates are not salient at value retrieval.
4.1. Distinct and common neural correlates of value encoding and retrieval
This study aimed to identify distinctive and common neural correlates of valuation during encoding and retrieval. Our findings showed that neural engagement of striatal and posterior parietal regions was similar across valuation phases, while highlighting distinctive reliance on the precuneus and salience network regions during value retrieval. In accordance with our first prediction, results from the value-encoding phase indicated recruitment of key fronto-striatal regions commonly implicated in valuation paradigms (see Hsu & Goh, 2016; Kable & Glimcher, 2009; Levy & Glimcher, 2012; Rangel, Camerer, & Montague, 2008; Rushworth et al., 2011 for review), particularly the striatal caudate nucleus, in addition to the left superior parietal lobule.
Notably, however, a direct comparison of value-related activity across encoding and retrieval from functionally determined value-encoding ROIs yielded relative similarity in activation across phases. This result indicates a similar reliance on striatal and posterior parietal regions during encoding and retrieval of value. In addition to fronto-parietal control areas, salience network regions including the anterior insula and dorsal anterior cingulate were uniquely associated with value retrieval. Direct comparison of value-related BOLD activity within functionally determined ROIs across phases further confirmed significantly greater value-related engagement of the dorsal anterior cingulate, left insular, and precuneal cortices during value retrieval in comparison to value encoding.
Recruitment of fronto-striatal circuitry during the assessment and integration of value based on presented product information is consistent with previously identified mechanisms of value encoding and updating from reinforcement learning and one-shot decision tasks (Bartra, McGuire, & Kable, 2013; Delgado, 2007; Foerde & Shohamy, 2011; Jocham et al., 2014; Kable & Glimcher, 2007; Kable & Glimcher, 2009; Knutson et al., 2005; Koscik et al., 2020; Levy et al., 2010; McClure et al., 2004; Peters & Büchel, 2009; Shenhav, Barrett, & Bar, 2013; Strait, Sleezer, & Hayden, 2015). In particular, our analyses highlighted the role of the caudate nucleus in valuation during our task, which involved assigning valence weightings to consumer products based on “consumer reviews” that were present (encoding) or called from memory (retrieval). The specific location of observed value-related activation during our task was within the anterior caudate (head) and posterior caudate (body), rather than the more typically implicated nucleus accumbens. While the ventral striatum has a well-established reward-processing role in representing and updating stimulus value (Daw et al., 2011; Haber, 2011; Knutson, Adams, Fong, & Hommer, 2001; Levy et al., 2010; Morris et al., 2016; O’Doherty et al., 2004; Peters & Büchel, 2010; Strait, Sleezer, & Hayden, 2015), the anterior and posterior caudal regions have more recently been connected to evaluating action outcomes and exerting cognitive control, respectively, toward value-mediated action selection (Choi, Shin, & Kim, 2020; Pauli, O’Reilly, Yarkoni, & Wager, 2016; Tricomi & Lempert, 2015; Watson, van Wingen, & de Wit, 2018).
Our study also yielded robust value-related activation of posterior parietal regions, while the ventromedial prefrontal cortex was not as robustly activated during our experiment. Task-specific effects may explain the relative robustness of fronto-striatal versus fronto-parietal region engagement during valuation. The ventromedial prefrontal cortex has been specifically implicated in deliberation of value differences toward value maximization – particularly in evaluating expected value from choice outcomes or with self-relevant reward or punishment outcomes (Camille, Griffiths, Vo, Fellows, & Kable, 2011; Grabenhorst & Rolls, 2011; Hunt et al., 2012; Kim, Shimojo, & O’Doherty, 2011; Kim & Johnson, 2015; Levy & Glimcher, 2012; Lin, Horner, & Burgess, 2016; Nicolle et al., 2012; Roy, Shohamy, & Wager, 2012; Wunderlich, Rangel, & O’Doherty, 2009).
While our task did include monetary incentives designed to elicit self-relevant value responses, it did not include trial-wise choices or reinforcements, which may have reduced the immediate self-relevance of our stimuli compared with those in previous studies. Beyond self-relevant values, the observation of robust value-related activation in the superior parietal lobule and larger fronto-parietal network is in line with previous connections to choice action planning, trial-by-trial updating, and integration of bottom-up sensory information with top-down task goals (Andersen & Buneo, 2002; Cohen et al., 2014; Cole, Repovs, & Anticevic, 2014; Park & Kayser, 2019; Ptak, 2011; Tosoni et al., 2014; Yeo et al., 2011).
Furthermore, fitting with value encoding in our task, value comparisons have been associated with superior parietal lobule engagement when value choices are made under restricted time limits, with the ventromedial prefrontal cortex keeping track of overall value (Domenech, Redouté, Koechlin, & Dreher, 2018; Jocham et al., 2014). In a recent study, Koscik and colleagues (2020) used a simple 50/50 chance gambling task to examine specific components of choice valuation. Their results suggested that the ventral striatum extracts value information and compares choice options, while the posterior parietal cortex extracts value magnitude information with no need for a mediating role of the ventromedial prefrontal cortex. Future research that compares choices with more immediate self-relevance versus more abstract valuation will help clarify the unique roles that fronto-striatal and fronto-parietal regions play in valuation and value-based decision making.
Our findings of retrieval-distinct neural correlates of value include significantly greater recruitment of dorsal anterior cingulate and insular regions during value retrieval. These results suggest that salience network regions play a stronger role in valuation when value judgments must be made from recently encoded representations in episodic memory. Salience network regions contribute to value processing by selecting, integrating, and utilizing goal-relevant sensory information (Dosenbach et al., 2008; Ham et al., 2013; Hélie, Shamloo, Novak, & Foti, 2017; Kahnt, Park, Haynes, & Tobler, 2014; Koscik et al., 2020; Krebs, Boehler, Roberts, Song, & Woldorff, 2012; Litt, Plassmann, Shiv, & Rangel, 2011; Menon & Uddin, 2010; Seeley et al., 2007). The anterior insula and dorsal anterior cingulate have also been specifically implicated in assessing choice uncertainty and utilizing available value representations to resolve ambiguity (Lamichhane & Dhamala, 2015; Loued-Khenissi, Pfeuffer, Einhäuser, Preuschoff, 2020; Rushworth & Behrens, 2008; Stöttinger, Aichhorn, Anderson, & Danckert, 2018). Our results are congruent with these prior studies, suggesting that salience-based components of value representations are less vulnerable to degradation from encoding to retrieval, and make greater contributions to valuation as dependence on memory causes uncertainty about value.
We also observed greater engagement of select fronto-parietal control regions during value retrieval, particularly in the precuneus. Posterior parietal regions support planning and initiating value-based behaviors (Andersen & Buneo, 2002; Clithero, Carter, & Huettel, 2009; Domenech et al., 2018; Tosoni et al., 2014). Of particular relevance to value retrieval, prior studies have implicated the precuneus in accessing stored mental representations of value and regenerating associated context (Johnson, Mitchell, Raye, D’Esposito, & Johnson, 2007; Lundstrom, Ingvar, & Petersson, 2005; Zhang & Li, 2012). Thus, collectively, our findings add to the growing literature that suggests salience network and posterior parietal regions play a critical role in the retrieval of value representations from memory.
While select subregions of the posterior parietal cortex may be recruited to a greater extent during the value retrieval phase in our task, our results support the conclusion that posterior parietal regions are robustly associated with value rating behavior whether value-based information is being encoded or retrieved from memory. Recruitment of posterior parietal regions across value encoding and retrieval is consistent with research showing these regions support the accumulation of choice-relevant information and the transformation of integrated representations into behavioral responses (Andersen & Buneo, 2002; Clithero, Carter, & Huettel, 2009; Domenech et al., 2018; Kable & Glimcher, 2009; Park & Kayser, 2019). Further, superior subregions of the posterior parietal cortex have been associated with goal-directed valuation beyond bottom-up salience-driven responses (Kahnt et al., 2014; Koscik et al., 2020; Uncapher & Wagner, 2009). In the current study, the posterior parietal regions were paramount to both actively encoding and integrating value information while product comments were present, and may represent accumulated value information to initiate a rating response.
4.2. Relationship between valuation and episodic memory systems
Our secondary study goal was to examine potential interactions between valuation and episodic memory systems. Behaviorally, we found an association between retrieval of value and attribute details, indicating a correspondence in memory for product values and those products’ features. Our neural data, however, did not yield evidence of an interaction between valuation and memory processing; nor did we observe any effects of the number of encoding experiences on value-related brain activation. In accordance with our second hypothesis, these results indicate independence of neural mechanisms of value and memory-related processing.
This conclusion conflicts with previous reports suggesting competition between valuation and memory systems, but differing results are likely due to task conditions. For example, Wimmer and colleagues (2014) previously found that incidental encoding of value-irrelevant episodic details (i.e., overlaid object images) during a reinforcement-learning task was associated with decreased influence of recent reward outcomes on subsequent choice. In contrast, product values in the present experiment were based on limited prior experience, allowing for effective episodic encoding of value. In addition, episodic information encountered in our valuation task was relevant to information about the product value, as attribute details were embedded in our consumer review stimuli. Our results suggest that in such contexts, stimuli values and episodic associates can be learned in parallel – without competition for processing resources. Thus, our findings are more consistent with behavioral research showing a relationship between memory for associates of choice stimuli and value-based decision performance when choice values can be effectively encoded and retrieved via episodic memory (Duncan & Shohamy, 2016; Murty et al., 2016).
Notably, results from these behavioral paradigms indicated facilitation of value-based decisions with better memory for choice associates. While our behavioral results are generally congruent with these studies, our functional brain imaging results indicate independent neural mechanisms of valuation and episodic memory. An important and distinctive feature of the present study’s paradigm is that memory-associates (product attribute cue words) were not made salient during the valuation phases. Indeed, cue words were not present during value retrieval. In behavioral studies indicating cooperation between value and memory systems, stimuli previously paired with choice options were present in value-based decision context (Duncan & Shohamy, 2016), or they were the choice stimulus itself (Murty et al., 2016). When combined with findings from the present study, extant research suggests that manipulating the salience of memory associates during value learning and value-based choice may impact the degree to which neural mechanisms of value and memory show cooperation or facilitation effects.
4.3. Concluding remarks
Naturalistic decision making often involves encoding incoming value information and integrating that information with previous experiences to select the best choice option. Although previous studies have identified regions associated with valuation, we argue that typical valuation paradigms involve the encoding and integration of present value information more than strict retrieval-based valuation. The present study addressed this issue by dissociating valuation in distinct phases of memory using ecologically valid stimuli. By distinguishing between the neural correlates underlying value processing at different phases of memory, we advance understanding of the value network’s composition during value encoding versus retrieval.
Further, the present study provides novel insights into the conditions under which neural mechanisms of value and episodic memory will interact. Considered alongside prior literature, our findings suggest that the number of interactions with choice stimuli and salience of memory associates during value-based choice likely determine the presence and nature of interactions between the neural mechanisms of valuation and episodic memory. These findings hold relevance for much of our everyday decision making, ranging from shopping to selecting between healthcare plan options, and demonstrate how recent exposures with product marketing may affect value-based processing.
Supplementary Material
Highlights.
Using a novel task, we isolate effects of memory processes on value mechanisms.
Neural correlates of value encoding are shared with value retrieval.
Value retrieval involves distinct recruitment of parietal and salience regions.
Number of encoding experiences and associative memory did not affect neural correlates of value.
Salience of episodic associates during valuation may influence system interactions.
Acknowledgements
The authors gratefully acknowledge the contributions of Ian Dalton, Ian Frazier, Desiree Lussier, and Eliany Perez for their assistance with protocol development, participant recruitment, data collection, and data management. We also gratefully acknowledge the contributions of Samuel Naranjo Rincon and Yerika Germosen toward quality control and data analysis procedures. We further thank the staff of the McKnight Brain Institute of the University of Florida for their assistance with MRI data collection and the participants for their time.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. MH was supported by a Pre-Doctoral Fellowship on Physical, Cognitive & Mental Health in Social Context from the National Insitute on Aging (T32AG020499) and a Post-Doctoral Fellowship from the UF Substance Abuse Training Center in Public Health from the National Institute of Drug Abuse of the National Institutes of Health (T32DA035167). The content is solely the responsibility of the author(s) and does not necessarily represent the official views of the National Institutes of Health. A portion of this work was performed in the McKnight Brain Institute at the National High Magnetic Field Laboratory’s AMRIS Facility, which is supported by National Science Foundation Cooperative Agreement No. DMR-1644779 and the State of Florida.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of competing interest
The authors declare no competing financial interests.
References
- Andersen RA & Buneo CA (2002). Intentional maps in posterior parietal cortex. Annual Review of Neuroscience, 25(1), 189–220. doi: 10.1146/annurev.neuro.25.112701.142922 [DOI] [PubMed] [Google Scholar]
- Bartra O, McGuire JT, & Kable JW (2013). The valuation system: A coordinate-based meta-analysis of BOLD fMRI experiments examining neural correlates of subjective value. NeuroImage, 76, 412–427. doi: 10.1016/j.neuroimage.2013.02.063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhui R (2018). Case-based decision neuroscience: Economic judgment by similarity. In Morris RW, Bornstein AM, & Shenhav A (Eds.), Goal-directed decision making: Computations and neural circuits (pp. 67–103). Elsevier Academic Press. doi: 10.1016/b978-0-12-812098-9.00004-8 [DOI] [Google Scholar]
- Biswal BB, Mennes M, Zuo XN, Gohel S, Kelly C, Smith SM, … & Castellanos FX. (2010). Toward discovery science of human brain function. Proc. Natl. Acad. Sci. USA, 107(10), 4734–4739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornstein AM, Khaw MW, Shohamy D, & Daw ND (2017). Reminders of past choices bias decisions for reward in humans. Nature Communications, 8, 15958. doi: 10.1038/ncomms15958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornstein AM & Norman KA (2017). Reinstated episodic context guides sampling-based decisions for reward. Nature Neuroscience, 20(7), 997–1003. [DOI] [PubMed] [Google Scholar]
- Brosch T & Sander D (2013). Neurocognitive mechanisms underlying value-based decision-making: From core values to economic value. Frontiers in Human Neuroscience, 7, 398. doi: 10.3389/fnhum.2013.00398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza R, Prince SE, Daselaar SM, Greenberg DL, Budde M, Dolcos F, LaBar KS, & Rubin DC (2004). Brain activity during episodic retrieval of autobiographical and laboratory events: an fMRI study using a novel photo paradigm. Journal of Cognitive Neuroscience, 16(9), 1583–1594. doi: 10.1162/0898929042568578 [DOI] [PubMed] [Google Scholar]
- Camille N, Griffiths CA, Vo K, Fellows LK, & Kable JW (2011). Ventromedial frontal lobe damage disrupts value maximization in humans. Journal of Neuroscience, 31(20), 7527–7532. doi: 10.1523/jneurosci.6527-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cappell KA, Gmeindl L, & Reuter-Lorenz PA (2010). Age differences in prefrontal recruitment during verbal working memory maintenance depend on memory load. Cortex, 46(4), 462–473. doi: 10.1016/j.cortex.2009.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter RM, Meyer JR, & Huettel SA (2010). Functional neuroimaging of intertemporal choice models: a review. J. Neurosci. Psychol. Econ, 3, 27–45. [Google Scholar]
- Choi Y, Shin EY, & Kim S (2020). Spatiotemporal dissociation of fMRI activity in the caudate nucleus underlies human de novo motor skill learning. Proc. Natl. Acad. Sci. USA, 202003963. doi: 10.1073/pnas.2003963117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clithero JA, Carter RM, & Huettel SA (2009). Local pattern classification differentiates processes of economic valuation. NeuroImage, 45(4), 1329–1338. doi: 10.1016/j.neuroimage.2008.12.074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clithero JA & Rangel A (2014). Informatic parcellation of the network involved in the computation of subjective value. Social Cognitive and Affective Neuroscience, 9(9), 1289–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen M, Rissman J, Suthana NA, Castel AD, & Knowlton BJ (2014). Value-based modulation of memory encoding involves strategic engagement of fronto-temporal semantic processing regions. Cognitive, Affective & Behavioral Neuroscience, 14, 578–592. doi: 10.3758/s13415-014-0275-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole MW, Repovs G, & Anticevic A (2014). The frontoparietal control system: A central role in mental health. The Neuroscientist, 20(6), 652–664. doi: 10.1177/1073858414525995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daw ND, Gershman SJ, Seymour B, Dayan P, & Dolan RJ (2011). Model-based influences on humans’ choices and striatal prediction errors. Neuron, 69(6), 1204–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado MR (2007). Reward-related responses in the human striatum. Annals of the New York Academy of Sciences, 1104(1), 70–88. doi: 10.1196/annals.1390.002 [DOI] [PubMed] [Google Scholar]
- Dickerson KC, Li J, & Delgado MR (2011). Parallel contributions of distinct human memory systems during probabilistic learning. NeuroImage, 55(1), 266–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domenech P, Redouté J, Koechlin E, & Dreher J-C (2018). The neuro-computational architecture of value-based selection in the human brain. Cerebral Cortex, 8, 585–601. doi: 10.1093/cercor/bhw396 [DOI] [PubMed] [Google Scholar]
- Dosenbach NU, Fair DA, Cohen AL, Schlaggar BL, & Petersen SE (2008). A dual-networks architecture of top-down control. Trends in Cognitive Sciences, 12(3), 99–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan KD & Shohamy D (2016). Memory states influence value-based decisions. Journal of Experimental Psychology, 145(11), 1420–1426. doi: 10.1037/xge0000231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enkavi AZ, Weber B, Zweyer I, Wagner J, Elger CE, Weber EU, & Johnson EJ (2017), Evidence for hippocampal dependence of value-based decisions. Scientific Reports, 7, 17738. doi: 10.1038/s41598-017-18015-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foerde K & Shohamy D (2011). The role of the basal ganglia in learning and memory: Insight from Parkinson’s disease. Neurobiology of Learning and Memory, 96(4), 624–636. doi: 10.1016/j.nlm.2011.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Price CJ, Büchel C, Worsley KJ (1999). Multisubject FMRI studies and conjunction analyses. NeuroImage, 10, 385–396. [DOI] [PubMed] [Google Scholar]
- Genovese C, Lazar N, & Nichols T (2002). Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage, 15, 870–878. [DOI] [PubMed] [Google Scholar]
- Gershman SJ, Pesaran B, & Daw ND (2009). Human reinforcement learning subdivides structured action spaces by learning effector-specific values. Journal of Neuroscience, 29(43), 13524–13531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gläscher J, Adolphs R, Damasio H, Bechara A, Rudrauf D, Calamia M, Paul LK, & Tranel D (2012). Lesion mapping of cognitive control and value-based decision making in the prefrontal cortex. Proc. Natl. Acad. Sci. USA, 109, 14681–14686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gläscher J, Daw N, Dayan P, & O’Doherty JP (2010). States versus rewards: Dissociable neural prediction error signals underlying model-based and model-free reinforcement learning. Neuron, 66(4), 585–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gläscher J, Hampton AN, & O’Doherty JP (2009). Determining a role for ventromedial prefrontal cortex in encoding action-based value signals during reward-related decision making. Cerebral Cortex, 19(2), 483–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabenhorst F & Rolls ET (2011). Value, pleasure and choice in the ventral prefrontal cortex. Trends Cogn. Sci, 15, 56–67. doi: 10.1016/j.tics.2010.12.004 [DOI] [PubMed] [Google Scholar]
- Haber SN (2011). Neuroanatomy of reward: A view from the ventral striatum. In Gottfried JA (Ed.), Neurobiology of sensation and reward (pp. 235–261). CRC Press/Taylor & Francis. doi: 10.1201/b10776-15 [DOI] [PubMed] [Google Scholar]
- Ham T, Leff A, de Boissezon X, Joffe A, & Sharp DJ (2013). Cognitive control and the salience network: An investigation of error processing and effective connectivity. The Journal of Neuroscience, 33(16), 7091–7098. doi: 10.1523/JNEUROSCI.4692-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare T, Camerer C, & Rangel A (2009). Self-control in decision-making involves modulation of the vmPFC valuation system. NeuroImage, 47, 646–648. doi: 10.1016/s1053-8119(09)70776-1 [DOI] [PubMed] [Google Scholar]
- Hélie S, Shamloo F, Novak K, & Foti D (2017). The roles of valuation and reward processing in cognitive function and psychiatric disorders. Annals of the New York Academy of Sciences, 1395, 33–48. doi: 10.1111/nyas.13327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt LT, Kolling N, Soltani A, Woolrich MW, Rushworth MFS, & Behrens TEJ (2012). Mechanisms underlying cortical activity during value-guided choice. Nature Neuroscience, 15(3), 470–476. doi: 10.1038/nn.3017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu CW & Goh JO. (2016). Distinct and overlapping brain areas engaged during value-based, mathematical, and emotional decision processing. Frontiers in Human Neuroscience, 10, 275. doi: 10.3389/fnhum.2016.00275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimura K, Chushak MS, & Braver TS (2013). Impulsivity and self-control during intertemporal decision making linked to the neural dynamics of reward value representation. Journal of Neuroscience, 33(1), 344–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jocham G, Furlong PM, Kröger IL, Kahn MC, Hunt LT, & Behrens TE (2014). Dissociable contributions of ventromedial prefrontal and posterior parietal cortex to value-guided choice. NeuroImage, 100, 498–506. doi: 10.1016/j.neuroimage.2014.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MR, Mitchell KJ, Raye CL, D’Esposito M, & Johnson MK (2007). A brief thought can modulate activity in extrastriate visual areas: Topdown effects of refreshing just-seen visual stimuli. Neuroimage, 37(1), 290–299. doi: 10.1016/j.neuroimage.2007.05.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones JL, Esber GR, Mcdannald MA, Gruber AJ, Hernandez A, Mirenzi A, & Schoenbaum G (2012). Orbitofrontal cortex supports behavior and learning using inferred but not cached values. Science, 335(6109), 953–956. doi: 10.1126/science.1227489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kable JW & Glimcher PW (2007). The neural correlates of subjective value during intertemporal choice. Nature Neuroscience, 10(12), 1625–1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kable JW & Glimcher PW (2009). The neurobiology of decision: Consensus and controversy. Neuron, 63(6), 733–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahnt T, Park SQ, Haynes J-D, & Tobler PN (2014). Disentangling neural representations of value and salience in the human brain. Proc. Natl. Acad. Sci. USA, 111(13), 5000–5005. doi: 10.1073/pnas.1320189111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K & Johnson MK (2015). Activity in ventromedial prefrontal cortex during self-related processing: Positive subjective value or personal significance? Social Cognitive and Affective Neuroscience, 10(4), 494–500. doi: 10.1093/scan/nsu078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Shimojo S, & O’Doherty JP (2011). Overlapping responses for the expectation of juice and money rewards in human ventromedial prefrontal cortex. Cerebral Cortex, 21(4), 769–776. doi: 10.1093/cercor/bhq145 [DOI] [PubMed] [Google Scholar]
- Knutson B, Adams CM, Fong GW, & Hommer D (2001). Anticipation of increasing monetary reward selectively recruits nucleus accumbens. J. Neurosci, 21, RC159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B, Taylor J, Kaufman M, Peterson R, & Glover G (2005). Distributed neural representation of expected value. The Journal of Neuroscience, 25, 4806–4812. doi: 10.1523/JNEUROSCI.0642-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koscik TR, Man V, Jahn A, Lee CH, & Cunningham WA (2020). Decomposing the neural pathways in a simple, value-based choice. NeuroImage, 214, 116764. doi: 10.1016/j.neuroimage.2020.116764 [DOI] [PubMed] [Google Scholar]
- Krebs RM, Boehler CN, Roberts KC, Song AW, & Woldorff MG (2012). The involvement of the dopaminergic midbrain and cortico-striatal-thalamic circuits in the integration of reward prospect and attentional task demands. Cerebral Cortex, 22(3), 607–615. doi: 10.1093/cercor/bhr134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhl BA, Shah AT, DuBrow S, & Wagner AD (2010). Resistance to forgetting associated with hippocampus-mediated reactivation during new learning. Nature Neuroscience, 13(4), 501–506. doi: 10.1038/nn.2498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamichhane B & Dhamala M (2015). The salience network and its functional architecture in a perceptual decision: An effective connectivity study. Brain Connectivity, 5(6), 362–370. doi: 10.1089/brain.2014.0282 [DOI] [PubMed] [Google Scholar]
- Levy DJ & Glimcher PW (2012). The root of all value: A neural common currency for choice. Current Opinion in Neurobiology, 22(6), 1027–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy I, Snell J, Nelson AJ, Rustichini A, & Glimcher PW (2010). Neural representation of subjective value under risk and ambiguity. Journal of Neurophysiology, 103(2), 1036–1047. doi: 10.1152/jn.00853.2009 [DOI] [PubMed] [Google Scholar]
- Lin W, Horner A & Burgess N (2016). Ventromedial prefrontal cortex, adding value to autobiographical memories. Scientific Reports, 6, 28630. doi: 10.1038/srep28630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litt A, Plassmann H, Shiv B, & Rangel A (2011). Dissociating valuation and saliency signals during decision-making. Cerebral Cortex, 21(1), 95–102. doi: 10.1093/cercor/bhq065 [DOI] [PubMed] [Google Scholar]
- Liu X, Hairston J, Schrier M, & Fan J (2011). Common and distinct networks underlying reward valence and processing stages: A meta-analysis of functional neuroimaging studies. Neuroscience & Biobehavioral Reviews, 35(5), 1219–1236. doi: 10.1016/j.neubiorev.2010.12.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loued-Khenissi L, Pfeuffer A, Einhauser W, & Preuschoff K (2020). Anterior insula reflects surprise in value-based decision-making and perception. NeuroImage, 210, 116549. doi: 10.1016/j.neuroimage.2020.116549 [DOI] [PubMed] [Google Scholar]
- Lundstrom BN, Ingvar M, & Petersson KM (2005). The role of precuneus and left inferior frontal cortex during source memory episodic retrieval. Neuroimage, 27, 824–834. doi: 10.1016/j.neuroimage.2005.05.008 [DOI] [PubMed] [Google Scholar]
- Makris N, Goldstein JM, Kennedy D, Hodge SM, Caviness VS, Faraone SV, Tsuang MT, Seidman LJ (2006). Decreased volume of left and total anterior insular lobule in schizophrenia. Schizophr. Res 83(2-3), 155–171. [DOI] [PubMed] [Google Scholar]
- McCarthy P (2020). FSLeyes (Version 0.27.3). Zenodo. doi: 10.5281/zenodo.1470761 [DOI] [Google Scholar]
- McClure SM, Laibson DI, Loewenstein G, & Cohen JD (2004). Separate neural systems value immediate and delayed monetary rewards. Science, 306, 503–507. [DOI] [PubMed] [Google Scholar]
- Menon V & Uddin LQ (2010). Saliency, switching, attention and control: A network model of insula function. Brain Structure & Function, 214(5-6), 655–667. doi: 10.1007/s00429-010-0262-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montague PR, King-Casas B, & Cohen JD (2006). Imaging valuation models in human choice. Annu. Rev. Neurosci, 29, 417–448. [DOI] [PubMed] [Google Scholar]
- Morris LS, Kundu P, Dowell N, Mechelmans DJ, Favre P, Irvine MA, Robbins TW, Daw N, Bullmore ET, Harrison NA, & Voon V (2016). Fronto-striatal organization: Defining functional and microstructural substrates of behavioural flexibility. Cortex, 74, 118–133. doi: 10.1016/j.cortex.2015.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murty VP, FeldmanHall O, Hunter LE, Phelps EA, & Davachi L (2016). Episodic memories predict adaptive value-based decision-making. Journal of Experimental Psychology, 145(5), 548–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel BJ, Herting MM, Maxwell EC, Bruno R, & Fair D (2013). Hemispheric lateralization of verbal and spatial working memory during adolescence. Brain and Cognition, 82(1), 58–68. doi: 10.1016/j.bandc.2013.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolle A, Klein-Flügge MC, Hunt LT, Vlaev I, Dolan RJ, & Behrens TE (2012). An agent independent axis for executed and modeled choice in medial prefrontal cortex. Neuron, 79(3), 607. doi: 10.1016/j.neuron.2013.07.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nogueira R, Abolafia JM, Drugowitsch J, Balaguer-Ballester E, Sanchez-Vives MV, & Moreno-Bote R (2017). Lateral orbitofrontal cortex anticipates choices and integrates prior with current information. Nature Communications, 8, 14823. doi: 10.1038/ncomms14823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Doherty J, Dayan P, Schultz J, Deichmann R, Friston K, & Dolan RJ (2004). Dissociable roles of ventral and dorsal striatum in instrumental conditioning. Science, 304, 452–454. [DOI] [PubMed] [Google Scholar]
- Padoa-Schioppa C & Assad JA (2006). Neurons in the orbitofrontal cortex encode economic value. Nature, 441(7090), 223–226. doi: 10.1038/nature04676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palminteri S, Boraud T, Lafargue G, Dubois B, & Pessiglione M (2009). Brain hemispheres selectively track the expected value of contralateral options. Journal of Neuroscience, 29(43), 13465–13472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H & Kayser C (2019). Shared neural underpinnings of multisensory integration and trial-by-trial perceptual recalibration in humans. eLife, 8, e47001. doi: 10.7554/eLife.47001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauli WM, O’Reilly RC, Yarkoni T, & Wager TD (2016). Regional specialization within the human striatum for diverse psychological functions. Proc. Natl. Acad. Sci. USA, 113(7), 1907–1912. doi: 10.1073/pnas.1507610113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters J & Büchel C (2009). Overlapping and distinct neural systems code for subjective value during intertemporal and risky decision making. Journal of Neuroscience, 29(50), 15727–15734. doi: 10.1523/jneurosci.3489-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters J & Büchel C (2010). Neural representation of subjective reward value. Behav. Brain. Res, 213, 135–141. [DOI] [PubMed] [Google Scholar]
- Petrides M (2007). The orbitofrontal cortex: Novelty, deviation from expectation, and memory. Annals of the New York Academy of Sciences, 1121, 33–53. [DOI] [PubMed] [Google Scholar]
- Ploran EJ, Nelson SM, Velanova K, Donaldson DI, Petersen SE, & Wheeler ME (2007). Evidence accumulation and the moment of recognition: Dissociating perceptual recognition processes using fMRI. Journal of Neuroscience, 27(44), 11912–11924. doi: 10.1523/jneurosci.3522-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poppenk J, McIntosh AR, Craik FI, & Moscovitch M (2010). Past experience modulates the neural mechanisms of episodic memory formation. The Journal of Neuroscience, 30(13), 4707–4716. doi: 10.1523/JNEUROSCI.5466-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ptak R (2011). The frontoparietal attention network of the human brain. The Neuroscientist, 18(5), 502–515. doi: 10.1177/1073858411409051 [DOI] [PubMed] [Google Scholar]
- Rangel A, Camerer C, & Montague PR (2008). A framework for studying the neurobiology of value-based decision making. Nature Reviews Neuroscience, 9(7), 545–556. doi: 10.1038/nrn2357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter FR, Chanales AJ, & Kuhl BA (2015). Predicting the integration of overlapping memories by decoding mnemonic processing states during learning. NeuroImage, 124, 323–335. doi: 10.1016/j.neuroimage.2015.08.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchey M, Wing EA, LaBar KS, & Cabeza R (2013). Neural similarity between encoding and retrieval is related to memory via hippocampal interactions. Cerebral Cortex, 23(12), 2818–2828. doi: 10.1093/cercor/bhs258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rorden C, Karnath H, & Bonilha L (2007). Improving lesion-symptom mapping. Journal of Cognitive Neuroscience, 19, 1081–1088. doi: 10.1162/jocn.2007.19.7.1081 [DOI] [PubMed] [Google Scholar]
- Roy M, Shohamy D, & Wager TD (2012). Ventromedial prefrontal-subcortical systems and the generation of affective meaning. Trends in Cognitive Sciences, 16(3), 147–156. doi: 10.1016/j.tics.2012.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushworth MFS & Behrens TEJ (2008). Choice, uncertainty and value in prefrontal and cingulate cortex. Nature Neuroscience, 11, 389–397. doi: 10.1038/nn2066 [DOI] [PubMed] [Google Scholar]
- Rushworth M, Noonan M, Boorman E, Walton M, & Behrens T (2011). Frontal cortex and reward-guided learning and decision-making. Neuron, 70(6), 1054–1069. doi: 10.1016/j.neuron.2011.05.014 [DOI] [PubMed] [Google Scholar]
- Sadaghiani S & D’Esposito M (2015). Functional characterization of the cingulo-opercular network in the maintenance of tonic alertness. Cerebral Cortex, 25(9), 2763–2773. doi: 10.1093/cercor/bhu072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, & Greicius MD (2007). Dissociable intrinsic connectivity networks for salience processing and executive control. The Journal of Neuroscience, 27(9), 2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shadlen MN, & Shohamy D (2016). Decision making and sequential sampling from memory. Neuron, 90(5), 927–939. doi: 10.1016/j.neuron.2016.04.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenhav A, Barrett LF, & Bar M (2013). Affective value and associative processing share a cortical substrate. Cognitive, Affective, & Behavioral Neuroscience, 13(1), 46–59. doi: 10.3758/s13415-012-0128-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenhav A, Rand DG, & Greene JD (2017). The relationship between intertemporal choice and following the path of least resistance across choices, preferences, and beliefs. Judgment and Decision Making, 12(1), 1–18. [Google Scholar]
- Simmons J, Nelson L, & Simonsohn U (2011). False-positive psychology: Undisclosed flexibility in data collection and analysis allows presenting anything as significant. Psychological Science, 22(11), 1359–1366. doi: 10.1037/e519702015-014 [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, ∖ & Matthews PM (2004). Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage, 23. doi: 10.1016/j.neuroimage.2004.07.051 [DOI] [PubMed] [Google Scholar]
- Spalding KN, Schlichting ML, Zeithamova D, Preston AR, Tranel D, Duff MC, & Warren DE (2018). Ventromedial prefrontal cortex is necessary for normal associative inference and memory integration. The Journal of Neuroscience, 38(15), 3767–3775. doi: 10.1523/JNEUROSCI.2501-17.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stalnaker TA, Cooch NK, & Schoenbaum G (2015). What the orbitofrontal cortex does not do. Nature Neuroscience, 15(5), 620–627. doi: 10.1038/nn.3982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stöttinger E, Aichhorn M, Anderson B, & Danckert J (2018). The neural systems for perceptual updating. Neuropsychologia, 112, 86–94. doi: 10.1016/j.neuropsychologia.2018.03.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strait CE, Sleezer BJ, & Hayden BY (2015). Signatures of value comparison in ventral striatum neurons. PLoS Biol, 13(6), e1002173. doi: 10.1371/journal.pbio.1002173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thirion B, Pinel P, Mériaux S, Roche A, Dehaene S, & Poline J-B (2007). Analysis of a large fMRI cohort: Statistical and methodological issues for group analyses. NeuroImage, 35(1), 105–120. doi: 10.1016/j.neuroimage.2006.11.054 [DOI] [PubMed] [Google Scholar]
- Tom SM, Fox CR, Trepel C, & Poldrack RA (2007). The neural basis of loss aversion in decision-making under risk. Science, 315(5811), 515–518. doi: 10.1126/science.1134239 [DOI] [PubMed] [Google Scholar]
- Tosoni A, Corbetta M, Calluso C, Committeri G, Pezzulo G, Romani GL, & Galati G (2014). Decision and action planning signals in human posterior parietal cortex during delayed perceptual choices. European Journal of Neuroscience, 39(8), 1370–1383. doi: 10.1111/ejn.12511 [DOI] [PubMed] [Google Scholar]
- Tricomi E, & Lempert KM (2015). Value and probability coding in a feedback-based learning task utilizing food rewards. Journal of Neurophysiology, 113(1), 4–13. doi: 10.1152/jn.00086.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uncapher MR & Wagner AD (2009). Posterior parietal cortex and episodic encoding: Insights from fMRI subsequent memory effects and dual-attention theory. Neurobiology of Learning and Memory, 91(2), 139–154. doi: 10.1016/j.nlm.2008.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentin VV, Dickinson A, & O’Doherty JP (2007). Determining the neural substrates of goal-directed learning in the human brain. Journal of Neuroscience, 27(15), 4019–4026. doi: 10.1523/jneurosci.0564-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallis JD (2007). Orbitofrontal cortex and its contribution to decision-making. Annual Review of Neuroscience, 30(1), 31–56. doi: 10.1146/annurev.neuro.30.051606.094334 [DOI] [PubMed] [Google Scholar]
- Wallis G, Stokes M, Cousijn H, Woolrich M, & Nobre AC (2015). Frontoparietal and cingulo-opercular networks play dissociable roles in control of working memory. Journal of Cognitive Neuroscience, 27(10), 2019–2034. doi: 10.1162/jocn_a_00838 [DOI] [PubMed] [Google Scholar]
- Walton ME, Chau BK, & Kennerley SW (2015). Prioritising the relevant information for learning and decision making within orbital and ventromedial prefrontal cortex. Current Opinion in Behavioral Sciences, 1, 78–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson P, van Wingen G, & de Wit S (2018). Conflicted between goal-directed and habitual control, an fMRI investigation. eNeuro, 5(4), ENEURO.0240-18.2018. doi: 10.1523/ENEURO.0240-18.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wimmer GE, Braun EK, Daw ND, & Shohamy D (2014). Episodic memory encoding interferes with reward learning and decreases striatal prediction errors. The Journal of Neuroscience, 34(45), 14901–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wimmer GE, Li JK, Gorgolewski KJ, & Poldrack RA (2018). Reward learning over weeks versus minutes increases the neural representation of value in the human brain. The Journal of Neuroscience, 38(35), 7649–7666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolosin SM, Zeithamova D, & Preston AR (2013). Distributed hippocampal patterns that discriminate reward context are associated with enhanced associative binding. Journal of Experimental Psychology, 142(4), 1264–1276. doi: 10.1037/a0033609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo C-W, Krishnan A, & Wager TD (2014). Cluster-extent based thresholding in fMRI analyses: Pitfalls and recommendations. NeuroImage, 91, 412–419. doi: 10.1016/j.neuroimage.2013.12.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worsley KJ (2001). Statistical analysis of activation images. In Jezzard P, Matthews PM, Smith SM (Eds.), Functional MRI: An introduction to methods. Oxford University Press; (pp. 251–270). [Google Scholar]
- Wunderlich K, Rangel A, & O’Doherty JP (2009). Neural computations underlying action-based decision making in the human brain. Proc. Natl Acad. Sci. USA, 106, 17199–17204. doi: 10.1073/pnas.0901077106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo BT, Krienen FM, Sepulcre J, Sabuncu MR, Lashkari D, Hollinshead M, ∖ & Buckner RL (2011). The organization of the human cerebral cortex estimated by intrinsic functional connectivity. Journal of Neurophysiology, 106(3), 1125–1165. doi: 10.1152/jn.00338.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeithamova D, Dominick AL, & Preston AR (2012). Hippocampal and ventral medial prefrontal activation during retrieval-mediated learning supports novel inference. Neuron, 75(1), 168–179. doi: 10.1016/j.neuron.2012.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, & Li CS (2012). Functional connectivity mapping of the human precuneus by resting state fMRI. NeuroImage, 59(4), 3548–3562. doi: 10.1016/j.neuroimage.2011.11.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Larcher KM, Misic Β, & Dagher A (2017). Anatomical and functional organization of the human substantia nigra and its connections. eLife, 6, e26653. doi: 10.7554/eLife.26653 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


