Abstract
Low income and low educational attainment are among the strongest predictors of both smoking prevalence and lapse (i.e., return) to smoking following cessation attempts. Treatment refinement is limited by inadequate knowledge of the specific lapse/relapse-relevant vulnerabilities characteristic of populations that should be the target of treatment. In the context of a randomized clinical trial design, we describe an experimental medicine approach for evaluating the role of two specific lapse-relevant targets relative to the higher stress characteristic of low-socioeconomic contexts: low distress tolerance and low working memory capacity. Furthermore, we use an innovative approach for understanding risk of smoking lapse in smokers undergoing a quit attempt to examine candidate mechanistic targets assessed not only during nicotine use, but also during the conditions smokers will face upon a cessation attempt – during stressful nicotine-deprivation windows. This study is designed to show the incremental value of assessments during deprivation windows, in part due to the way in which specific vulnerabilities are modified by, and interact with, the heightened stress and withdrawal symptoms inherent to nicotine-deprivation states. Specifically, the study is designed to evaluate whether a novel mindfulness intervention (mindfulness combined with interoceptive exposure) can improve upon existing mindfulness interventions and extend therapeutic gains to the modification of mechanistic targets assessed in high-stress/negative affectivity contexts. The overall goal is to validate mechanistic targets and associated interventions for the purpose of expanding treatment options for at-risk smokers.
Keywords: science of behavior change, smoking cessation, distress tolerance, anxiety sensitivity, working memory, mindfulness
Inoculating Individuals for Stressful Contexts: Engaging Distress Tolerance and Working Memory to Aid Smoking Cessation
The task of identifying and modifying behavioral or social processes relevant to a range of negative health behaviors is at the heart of the Science of Behavior Change (SOBC) Common Fund (Nielsen et al., 2018; Riddle & SOBC Working Group, 2015). The SOBC was established to identify common processes and to build a more unified science of behavior change that focuses on causal mechanisms relevant to multiple health conditions. A central feature of the SOBC initiative is the utilization of an experimental medicine approach with four core steps: (1) identification of an intervention target, (2) development/utilization of measures (assays) to allow verification of the target, (3) demonstration of target engagement through intervention, and (4) evaluation of the degree to which target engagement produced the desired behavior change (Riddle et al., 2015).
In this manuscript, we provide evidence for the role of distress tolerance (DT; a perceived or behavioral tendency to tolerate affective and physical distress) and working memory capacity (WMC) as important mechanistic targets for a wide range of negative health behaviors. We further detail the role of stressful contexts relative to these factors. We then describe the particular relevance of DT and WM for smoking in individuals from low SES backgrounds, and apply the experimental medicine approach in the design of a novel intervention strategy to engage these vulnerability factors (mechanistic targets) under conditions of heightened stress.
Central to our approach is an appreciation of the importance of contexts to behavioral persistence and the enactment of treatment-related skills. Context plays an important role in occasioning both adaptive and maladaptive behaviors; indeed, for both operant and extinction research demonstrates that a change in context after learning may result in a decrement in performance of the new learning (Bouton & Todd, 2014; Todd, Winterbauer & Bouton, 2012). Context changes can include both external and internal cues (Bouton, 2002; Mystkowski, Craske, & Echiverri, 2002; Mystkowski, Mineka, Vernon, & Zinbarg, 2003), and have been identified as a potentially important source of relapse across both avoidance-related and appetitive disorders (M. W. Otto, O’Cleirigh, & Pollack, 2007; Thomas, Larsen, & Ayres, 2003). In short, behavioral skills learned under one emotional context may not extend to a different emotional context.
These considerations are particularly apt for the vulnerability factors of DT and WM. M.W. Otto and associates (2016) provided a model for the way in which low DT and low WMC represent separate liabilities for health-behavior and self-control lapses, particularly under conditions of stress. DT can be assessed by self-report or performance measures across any number of domains of emotional or somatic distress (McHugh & Otto, 2011). A particularly well-performing marker of low DT is elevated anxiety sensitivity - the fear of anxiety and related sensations. M.W. Otto and associates (2016) conceptualize anxiety sensitivity (and the intolerance of distress more generally) as a negative emotion/somatic sensation amplification factor, enhancing the need to escape/avoid negative affective or somatic experiences. Consistent with this accounting, anxiety sensitivity predicts engagement in a wide range of negative health behaviors, including tobacco, alcohol, and other drug use as well as exercise avoidance, overeating, and other cardiac risk behaviors (for review see Horenstein et al., 2018; M. W. Otto et al., 2016). Further, we believe the predictive power of DT (assessed by anxiety sensitivity or other indices of DT) relies on the fact that “it is not just negative affect/sensations that drives maladaptive or impulsive behavior, but the relative intolerance of these experiences” (p. 67; M. W. Otto et al., 2016). Hence, low DT both predicts negative affective or somatic sensations and interacts with these to predict negative outcomes (Leventhal & Zvolensky, 2015; M. W. Otto et al., 2016).
WMC, reflecting the ability to maintain attention on task-relevant information, is a crucial ability for problem solving and planning (Baddeley, 2012). Relative to negative health behaviors, higher WMC is linked to the successful use of self-control strategies to resist maladaptive urges. As stated by Grenard et al. (2008):
For those higher in WMC, more top-down, goal-directed attentional resources are available to (a) suppress the influence of associative tendencies when they interfere with other active goal-states, (b) maintain conflicting goals in active memory, (c) draw on more knowledge concerning potential short vs. long-term outcomes, and (d) apply one of several cognitive processing strategies to resolve the goal conflict” (p. 427).
Consistent with this model, low WMC is strongly linked to delay discounting and a range of negative health behaviors and addictive disorders (Bickel, Quisenberry, Moody, & Wilson, 2015; M. W. Otto et al., 2016; Sheffer et al, 2013). Furthermore, WMC moderates the effects of stress, such that high WMC may help individuals better withstand the derailing and demotivating effects of stress, and help them apply adaptive behaviors or pursue adaptive goals relative to their low WMC counterparts (A.R. Otto, Raio, Chiang, Phelps, & Daw, 2013; M. W. Otto et al., 2016).
Accordingly, DT and WMC appear to be prototypical examples of the sort of mechanistic targets relevant to a wide range of health behaviors that the SOBC program seeks to elucidate and validate. We next illuminate their potential role in smoking and the high rates of failure of smoking cessation efforts (i.e., lapses), particularly under conditions of heightened stress (i.e., nicotine deprivation).
Attending to Smoking Vulnerabilities in Low SES Individuals
Although almost half of smokers report making a quit attempt each year, less than 10% do so successfully (Babb, Malarcher, Schauer, Asman, & Jamal, 2017), and large disparities in tobacco use remain across a number of groups, particularly in disadvantaged populations (Green, Beckham, Youssef, & Elbogen, 2014). Studies show that youth from low socioeconomic status (SES) families have greater rates of smoking initiation, duration, and persistence, resulting in rates of smoking initiation and maintenance twice that for individuals who are well above the poverty level (Mathur, Erickson, Stigler, Forster & Finnegan, 2013; Mercken et al., 2012; Patnode et al., 2013; Siahpush, Singh, Jones, & Timsina, 2009; Wang et al., 2018). Thus, it is a priority to increase effective smoking cessation resources for population subsets with low education attainment and low income (i.e., low SES smokers).
Negative affect and stress are consequences of economic vulnerability and persistent financial pressure (Lantz, House, Mero, & Williams, 2005; McLeod & Kessler, 1990). Moreover, a major contributing factor to smoking among low SES persons appears be the increased exposure to multiple stressors associated with low SES environments, which in turn, contribute to cognitive and emotional vulnerabilities (Doan, Fuller-Rowell, & Evans, 2012; Kendzor et al., 2010; Ludman et al., 2002; Reitzel et al., 2011). For example, SES is a significant predictor of individual differences in WMC, and WMC deficits have been found in children between low and middle SES and among those living in poverty (Evans & Schamberg, 2009; Noble, Norman, & Farah, 2005). With respect to addictive behaviors, high WMC is hypothesized to allow use of cognitive and other self-control strategies to resist maladaptive urges, and low WMC is linked to poorer response to smoking cessation treatment in a low-SES sample (Bickel, Jarmolowicz, Mueller, Gatchaljan, &McClure, 2012; Sheffer et al, 2012). Similarly, low SES is associated with the use of negative health behaviors to regulate stress and negative affect/stress mediates the relation between low SES and difficulties with smoking cessation (Doan et al., 2012; Kendzor et al., 2009; Reitzel et al, 2012; Zvolensky et al., 2017). Further, low DT indeed predicts problems in quitting smoking (Brown, Lejuez, Kahler, Strong, & Zvolensky, 2005; Brown et al., 2009; Hajek, Belcher, & Stapleton, 1987).
DT and WMC as Mechanistic Targets in High Stress/Negative Affectivity Contexts
Evidence suggests that both DT and WMC are modifiable risk factors, and they appear to have at least one intervention in common – mindfulness training (M. W. Otto et al., 2016). Mindfulness-based interventions have reliable effects on multiple indices of stress (Goyal et al., 2014), and also appear to have beneficial effects on DT and WMC (e.g., Chiesa, Calati & Serretti, 2011; Jha, Stanley, Kiyonaga, Wong, & Gelfand, 2010; McCracken & Keogh, 2009). Furthermore, mindfulness interventions can enhance smoking cessation efforts (Oikonomou, Arvanitis, & Sokolove, 2016). However, a combination of low WMC, low DT, and high stress may overwhelm the efficacy of mindfulness interventions. Specifically, low DT (assessed by anxiety sensitivity) has been found to moderate the benefits of mindfulness interventions, appearing to impair the success of mindfulness strategies for coping with negative affect (anxiety) and substance cravings (Arch & Ayers, 2013; Rogojanski, Vettese, & Antony, 2011; see also Raines et al., 2018; Zvolensky et al., 2015). Likewise, low WMC may impair the application of recently learned therapeutic skills under high stress conditions (A. R. Otto, Raio, Chiang, Phelps, & Daw, 2013). The result is that without additional targeting of WMC or DT vulnerability factors, mindfulness training alone may be inadequate to reliably overcome the stressful conditions of nicotine withdrawal (e.g., Murphy & MacKillop, 2014). Accordingly, assessment of mechanistic targets under standard smoking conditions may offer only imprecise assessment of intervention success compared to assessments during the high-stress contexts in which learned interventions need to be applied.
There is evidence that treatments specifically targeting DT can help “pre-train” individuals for coping with high stress and withdrawal sensations. For example, M. W. Otto and associates have repeatedly documented the value of interoceptive exposure (IE) interventions targeting anxiety sensitivity for helping prepare patients to persist through the stress, negative affect, and somatic symptoms inherent to benzodiazepine withdrawal, with intervention effects reflecting large effect sizes (M. W. Otto et al., 1993; M. W. Otto et al., 2010; for additional IE effects on anxiety sensitivity see Smits, Berry, Tart, & Powers, 2008). Use of IE or exercise interventions to aid smoking cessation have also been validated by members of our study team, with evidence that the mechanism of action for successful cessation relied on reduction in anxiety sensitivity and dysphoria (Feldner, Zvolensky, Babson, Leen-Feldner, & Schmidt, 2008; Zvolensky, Yartz, Gregor, Gonzalez, & Bernstein, 2008; Zvolesnky et al., 2018). Brief training with IE has been found valuable for enhancing DT as assessed by reductions in anxiety sensitivity, and for facilitating reductions in smoking (Feldner et al., 2008). Accordingly, the available evidence suggests that IE procedures have the potential to help individuals increase DT, thereby offering a strategy to bridge the gap between the successful application of therapeutic interventions under low stress conditions and the success of these interventions in the context of the high stress, negative affect, and physiological challenges inherent to nicotine withdrawal.
In sum, given evidence that: (1) stress acts to impair self-regulation, particularly in interaction with lower WMC, and (2) low DT acts to amplify stress and negative affectivity to impair self-control and motivate negative health behaviors, we propose that interventions that inadequately target these vulnerabilities will leave smokers at risk for cessation failure. In contrast, interventions that can comprehensively engage these mechanistic targets across low and high-stress contexts should offer stronger benefits to at-risk subsamples, such as low SES smokers. Accordingly, mindfulness interventions have some effects on these mechanistic targets (negative affectivity, nicotine withdrawal symptoms, DT, and WM), but treatment effects should be be enhanced by conducting mindfulness training in the context of induced stress symptoms (using IE) to increase resilience to subsequent exposure to high stress conditions (i.e., reduced negative affectivity, withdrawal symptoms, and WM due to enhanced DT). Specifically, the goal of mindfulness training in conjunction with IE is twofold: (1) to allow patients to learn therapeutic skills under conditions of induced stress-related sensations so that these skills better transfer to high-stress contexts, and (2) to reduce distress in response to stressful contexts by directly enhancing DT.
Study Approach and Aims
Early lapse to smoking is both common and predictive of subsequent return to regular smoking (i.e., relapse; Brown, Lejuez, Kahler, Strong, & Zvolensky, 2005; Shiffman et al., 1997; Zvolensky, Stewart, Vujanovic, Gavric, & Steeves, 2009). McKee and associates have established a protocol for assessing lapse behavior. This protocol uses empirically-derived monetary rewards so that smokers, on average, are able to delay smoking for effective assessment during a 50-minute assessment window, and provides measures of two critical features of lapse behavior: (a) the ability to resist the first cigarette and (b) subsequent smoking if a participant decides to “give in” and starts to smoke (McKee, 2009; McKee et al., 2011; McKee, Weinberger, Shi, Tetrault, & Coppola, 2012). The McKee procedure has been shown to reflect non-laboratory predictors of smoking behavior and to be sensitive to the effects of stress on attenuating the latency and increasing the amount of smoking during the protocol (Kahler et al., 2014; McKee et al., 2006; McKee et al., 2011; Roche et al., 2014). As such, the protocol provides an ideal format for quickly and efficiently assessing the relationship between putative mechanistic variables and lapse behaviors.
In addition, we sought to further integrate work on the McKee paradigm with assessments of smoking topography; smoking topography measures puffing volume CO (plus a complex combination of particles and vapors). This advancement is clinically important, as smokers can control the timing and to a large extent, the amount/dose of nicotine they consume by altering the depth, speed, and/or frequency of each cigarette puff (Perkins et al., 2012). Initial work indicates that stress alters the intensity of smoking (increased puffs, shorter inter-puff interval, and greater peak puff velocity) in the McKee paradigm (McKee et al., 2011; see also Borges, Leyro, Rosen, Zvolensky, & Farris, 2019). Using these design features (see Figure 1), the specific aims for this randomized clinical trial are:
Figure 1.
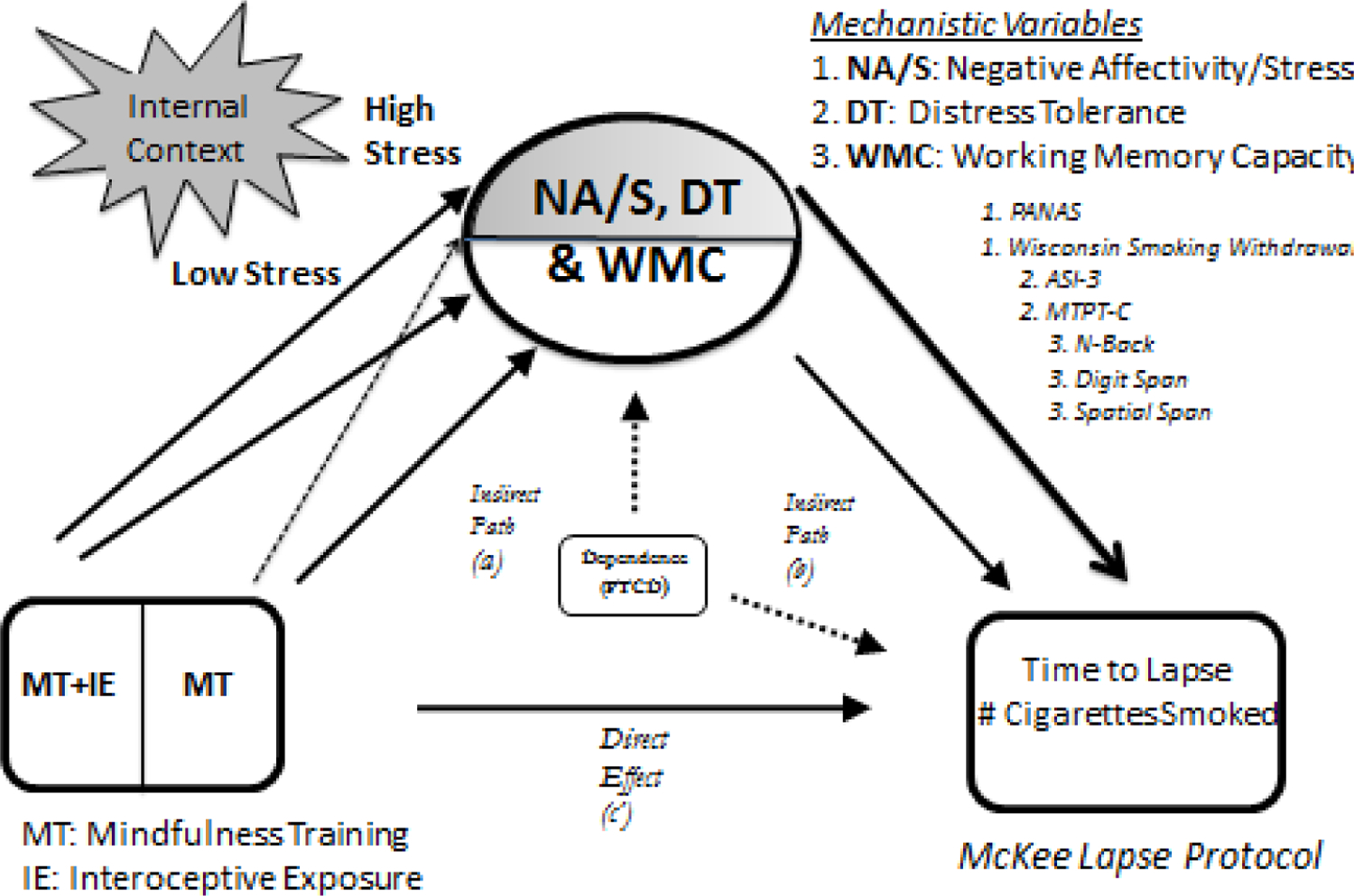
Conceptual model for treatment effects in this study of mindfulness treatment with and without interoceptive exposure on mechanistic variables (negative affectivity/stress, distress tolerance, and working memory capacity), and the effect of these variables on smoking outcomes, with lines indicating potential differential effects for low and high stress contexts (with dashed lines indicating weaker effects of MT relative to MT + IE under high stress/negative affectivity contexts, and the bolded line to the clinical outcomes indicating a stronger relationship between mechanistic outcomes that are evaluated in the high- relative to the low-stress/negative affectivity context).
- To evaluate the ability of two forms of mindfulness training (mindfulness with and without IE interventions), relative to a control intervention, to engage specific mechanistic targets--negative affectivity and withdrawal sensations for the stress/negative affectivity domain; anxiety sensitivity and behavioral persistence for the DT domain; and N-back, digit span, and spatial span for the domain of WMC--relevant to low SES smokers.
- We hypothesize that both versions of the mindfulness training will show greater target engagement than the control treatment for mechanistic targets assessed under standard smoking conditions.
- We hypothesize that the enhanced mindfulness training (mindfulness training plus interoceptive exposure) will show greater target engagement than each of the other two conditions for mechanistic targets assessed during the nicotine deprivation window (the high stress/negative affectivity context).
To show that the hypothesized differential target engagement results in differential smoking self-control as evaluated by greater time to lapse, fewer cigarettes smoked, and less severe smoking topography in the McKee Lapse protocol.
To expand and show the interrelationship between currently identified SOBC assays (negative affectivity/stress, and WM; see methods) and our measures of DT (anxiety sensitivity and mirror tracing; see methods), and clinical outcome variables, with attention to separate evaluation in low and high-stress contexts.
Design Overview
The design for this randomized clinical trial included all four steps of an experimental medicine approach: (1) identification of an intervention target, (2) development/utilization of measures (assays) to allow verification of the target, (3) target engagement through intervention, and (4) testing the degree to which target engagement produced the desired behavior change (Riddle et al., 2015). A unique feature of this study is evaluation of the success of target engagement in two specific contexts: under standard smoking conditions and under nicotine deprivation (stress) conditions.
After screening, consent, and baseline assessment, participants selected on the basis of inclusion/exclusion criteria will be randomized to one of three intervention conditions: (1) a health-education control condition (CC), (2) a mindfulness training condition (MT), or (3) a mindfulness training condition combined with training applying mindfulness skills in the context of interoceptive exposure (MT+IE). Following 6 sessions of these interventions, scheduled to be delivered over 3 weeks, participants will undergo the standard smoking assessment of mechanistic targets. Two days later (and matching the progression to quit-attempt challenges following treatment), participants are scheduled to undergo the deprivation window assessment (16 hours of smoking abstinence) of these same mechanistic targets, followed by evaluation of lapse behavior and smoking topography in the McKee Lapse protocol to yield: (1) latency to initiate smoking during a monetarily reinforced delay period, (2) the number of cigarettes smoked during a subsequent 60-minute self-administration period, and (3) exploratory analysis of smoking topography: puff volume, puff duration, and inter-puff interval. Participants will be recruited through study advertisements targeted to low SES neighborhoods, with attention to health service centers, religious institutions, food pantries, and other establishments serving low SES individuals. Study procedures take place in a Department of Psychology clinic setting. All procedures have been approved by the relevant Institutional Review Board (IRB). Any important protocol modifications will be approved by study consultants, the IRB, and the funding agency; and will be reflected in the trial registry.
Participant Selection
We anticipate consenting and randomizing up to 107 participants in order to reach an assessed sample (defined as providing post-treatment assessments, including the McKee lapse protocol) of 75. For inclusion in the study, participants must (1) be between 18 and 65 years of age; (2) have reported household income of less than or equal to 1.5 times the 2018 federal poverty level; (3) be a regular smoker for at least one year; (4) report daily smoking (minimum of 5 cigarettes per day and biochemically confirmed via Carbon Monoxide [CO] analysis; > 10ppm CO); and (5) not be presently engaged in a quit attempt. Exclusion criteria include history of psychosis (as determined by a brief psychotic screen), pregnancy, nursing mothers, medical conditions that would contraindicate smoking (e.g., current diagnoses of chronic medical diseases including heart disease, chronic obstructive pulmonary disease, or seizure disorders - assessed during telephone prescreen and initial assessment via medical checklist), current use of any pharmacotherapy for smoking cessation, or insufficient command of the English language (i.e., cannot carry on a conversation with an interviewer in the English language or read associated text).
Procedures
Following telephone screening for basic eligibility, participants will be scheduled for an informed consent interview, an in person screening of inclusion and exclusion criteria, and a subsequent baseline assessment session. Written consent is obtained by trained research staff. Telephone screening information is destroyed for individuals who do not choose to enter or who are not eligible to enter the trial. Following the baseline assessment, eligible participants are allocated to an intervention condition using computer-generated random numbers in block sizes of 6, transmitted by the PI to the research coordinator. Interventionists and participants learn of the allocation at the first intervention visit. Interventions are scheduled for the 3 weeks following baseline evaluation, and post-treatment assessments of the mechanistic targets are scheduled for the week following the last intervention session (standard smoking assessment) and again 2 days later (deprivation-window assessment). For the standard smoking window assessments, participants are asked to smoke 0.5 hour before the scheduled assessment session, to standardize the time from the last cigarette. The deprivation-window assessment is scheduled two days later (prototypically scheduled for 2PM, with smoking to cease at 10PM the night before). Upon arrival to the lab, nicotine abstinence will be verified by expired CO levels, as determined by a criterion of half of the participant’s screening session CO concentration or <10 PPM (those failing will be rescheduled). After verification of abstinence, participants will complete assessment of the mechanistic target variables. These assessments will be followed by the McKee Lapse protocol: participants will be instructed that over the next 50 min, they will have the option to initiate a cigarette self-administration (smoking) session at any point or to delay initiation in exchange for monetary reinforcement. If participants choose to delay, they will be awarded one dollar for each 5-minute increment they are able to resist smoking. Once participants choose to end the delay period in order to smoke (or resist smoking for the entire 50-minute delay period), they will then participate in a 60-minute cigarette self-administration session, in which they will be given the option to smoke at any point during the 60 minutes. The first time a participant smokes in this protocol, we will use the Clinical Research Support System (CReSS; Plowshare Technologies, Borgwaldt KC, Inc) to measure smoking topography. Topography data from the first cigarette smoked will include puff CO volume, puff duration, and inter-puff interval. Recruitment/adherence is aided by financial compensation for all assessments and intervention sessions, with compensation of up to $180 per participant, depending on McKee Lapse protocol results. Self-report measures are checked for completion at the time of assessment. Research assistants are trained in all assessment procedures with regular review of fidelity to procedures.
Baseline Assessments
Demographics. Participants will be asked to provide standard demographic information (age, sex/gender, race/ethnicity) as well as history of medical problems and current treatments.
Smoking History Questionnaire. Smoking history and pattern will be assessed with a semi-structured interview with items targeting smoking frequency, age of smoking initiation, years of being a regular smoker, etc. The Timeline follow-back method will be used to establish degree of cigarette use in the last 30 days (a baseline predictor). Additional questionnaires will assess attitudes toward smoking as well as e-cigarette use.
Fagerström Test for Nicotine Dependence (FTND). The FTND is a six-item scale designed to assess gradations in nicotine dependence (Heatherton, Kozlowski, Frecker, & Fagerström, 1991). The FTND will be used as a baseline covariate in the analyses.
Questionnaire of Smoking Urges-Brief (QSU-Brief). The QSU-Brief measures urge to smoke and craving (Cox, Tiffany & Christen, 2001; Toll, Katulak, & McKee, 2006). The baseline and deprivation window Total Craving Scores will be used as covariates.
Executive Function – Behavioral Regulation. Baseline levels of behavioral regulation will be assessed with the Behavioral Regulation Index from the from the SOBC assay, Behavioral Rating Inventory of Executive Function – Adults (BRIEF-A, http://scienceofbehaviorchange.org/measures/).
Psychological Distress. Baseline levels of psychological distress will be assessed with the SOBC assay, Kessler Psychological Distress Scale (K6+, http://scienceofbehaviorchange.org/measures/).
Mechanistic Targets
Each of the mechanistic targets will be assessed at the post-intervention standard-smoking and deprivation-window assessments.
Negative Affectivity/Stress: Negative affect will be assessed with the SOBC assay, PANAS (PANAS-state negative (http://scienceofbehaviorchange.org/measures/), and withdrawal symptoms will be assessed with the Wisconsin Smoking Withdrawal Scale (WSWS; Welsch et al., 1999). Each of these mechanistic targets will be assessed at the standard smoking and deprivation-window assessments.
WMC: To provide multi-domain assessment of WMC, we will adopt 3 specific SOBC assays: Adaptive N-Back, Digit Span, and Spatial Span (http://scienceofbehaviorchange.org/measures/).
Distress Tolerance: Because self-report and behavioral measures of DT are only partially correlated, we will utilize both assessment strategies (McHugh & Otto, 2011; M.W. Otto et al., in press). For self-report we will use the ASI-3, an 18-item measure of anxiety sensitivity (Taylor et al., 2007). The ASI-3 has sound psychometric properties, including excellent internal consistency, predictive validity, and reliability among treatment-seeking smokers (Farris et al., 2015). Behavioral Distress Tolerance will be assessed with the computerized Mirror-Tracing Persistence Task (MTPT-C), with evidence this measure captures the inability to tolerate distress and not just the experience of distress itself (Daughters et al., 2005; Strong et al., 2003).
Interventions
All interventions are delivered in six, 90-minute individual sessions over 3 weeks. There are no specific a priori contingencies for discontinuing an allocated intervention other than participant request or an alarming pattern of severe adverse events. Given the nature of the interventions under study, no adverse event screen is used in this study; reported adverse events (other than the anticipated nicotine withdrawal symptoms assessed by the WSWS) are recorded in study files, are regularly reviewed by the study team, and will be summarized at trial endpoint.
CC:
The wellness education control condition (CC) will be modeled after that used in our studies of exercise for smoking cessation, but delivered in an individual format (Smits et al., 2016). Content focuses on discussions of a variety of healthy lifestyle topics, such as healthy eating, regular exercise, time management, recommended health screenings, stress, and sleep. Content is delivered using a combination of presentations, handouts, and discussions while working with participants to set their own realistic wellness goals in the content areas, which they can gradually incorporate into their lives. Emphasis is placed on development and implementation plans for these health-related goals.
MT:
Mindfulness training was adapted for 6 individual sessions from previous MT manuals for substance dependence (Bowen et al., 2009; Witkiewitz, Marlatt, & Walker, 2005). The length of treatment in the current protocol corresponds well to the mean number of sessions [5.2] attended by smokers in treatment, even when eight sessions are offered (Bowen et al., 2009). Formal intervention elements include: (1) a body scan designed to teach participants to pay attention/shift attention to specific parts of their bodies; (2) non-judgmental awareness of the present moment, and (3) awareness of breath meditation, with an additional focus on helping participants become more aware of the present moment and refrain from habitually engaging in self-related pre-occupations concerning the future or the past, (4) and urge surfing, which incorporates imaginal exposure of stressful situations that often elicit reactivity with instructions to ride out the urges to react using focused awareness of breath and body sensations. Informal daily practices include performing daily activities mindfully, with emphasis on recognizing and accepting mind-states, emotions, and body sensations from moment-to-moment. Participant progress with at-home practice is discussed with the interventionist at the start of each intervention session with special regard to normalizing and trouble-shooting any barriers to practice.
MT+IE.
This condition will mirror the MT condition for the first 3 individual sessions, then for the final 3 individual sessions, MT will be rehearsed under conditions of sensations induced by interoceptive exposure procedures (IE). Core procedures include the induction of bothersome sensations (e.g., hyperventilating to produce dizziness, lightheaded, numbness, or tingling; full body muscular contractions to produce feelings of tension and fatigue; see Otto & Pollack, 2009) and then rehearsal of mindful attention and acceptance in this context. The goal is to both reduce reactivity to the somatic sensations induced by IE and to help ensure that mindfulness skills can be applied in the context of high levels of emotional/somatic symptoms.
Data Analytic Plan
All data will be double entered to help ensure accuracy; only de-identified data are entered in the statistical analysis database. Data monitoring is conducted by study staff in this single-site trial, with weekly evaluation of study progress and procedures and monthly evaluation of data completeness and participant retention. Analyses for outliers, non-normal distributions, nonlinear relations, and influence statistics will be conducted; data transformations will be considered where appropriate. We will analyze data using multilevel models because they allow inclusion of all participants regardless of missing data (which improves power and generalizability) and are the recommended method for longitudinal data analysis (Hamer & Simpson, 2009). For each analysis, the distribution of the outcome measures will be evaluated and appropriate linking functions and outcome distributions will be used if the outcome measures are not normally distributed. For all analyses, the following baseline assessments will be assessed as meaningful covariates in the analyses: FTND score, QSU score, BRIEF-A scores for the Behavioral Regulation Index, and psychological distress from the K6+ symptoms. No interim analyses are planned.
Aim 1.
We will perform repeated-measures multilevel models with multiple outcomes. Independent variables will be intervention condition (3 levels) and assessment context (smoking vs. deprivation; 2 levels). Multilevel models will be performed for each of the three risk categories (negative affectivity/stress, WMC, DT) with the multiple measures of each risk factor being the multivariate outcomes. For Aim 1a, we will use contrasts to test whether MT and MT+IE will each significantly improve negative affectivity/stress, WMC, and DT relative to CC, during the standard smoking assessment. For Aim 1b, we hypothesize that MT+IE will significantly improve negative affectivity/stress, WMC, and DT relative to both MT and CC, during the smoking deprivation assessment.
Aim 2:
For Aim 2, we will examine the bivariate associations between the risk variables (mechanistic targets) and McKee Lapse outcomes. We will attempt to balance the need to determine which individual variables at which assessment context are related to which outcomes, with the need to minimize the number of tests. Thus, multivariate multilevel models will be used with the 2 McKee Lapse outcomes as the multiple outcomes. One multilevel model will be performed for each individual risk variable, with specific evaluation of differential prediction between the 2 assessments (smoking context and deprivation context). This multilevel models will be compared to the same multilevel models that constrains the coefficients of each independent variable to be equal. If the likelihood ratio test indicates that the constrained model does not fit the data worse than the unconstrained model, then the relation between the risk factor and the outcomes does not differ by assessment context. If the test is significant, assessment context would be considered a moderator of the relation between the risk factor and outcome (context effects will be interpreted with attention to the role of proximity as well nicotine deprivation/stress). Further, we will include a dummy variable to differentiate the 2 outcomes (0=latency, 1=number of offers of cigarettes resisted). If an interaction between that dummy variable and a regression coefficient is significant, the relation between the risk factor and outcome is different for the 2 outcomes.
Aim 3:
To determine which of the individual risk assays are related to outcomes over and above the other factors, including consideration of the assessment context of each risk factor, we will perform analyses similar to the multilevel models in Aim 2 with the following differences: 1) if Aim 2 shows that the relation between a risk assay and outcomes did not differ by context, the 2 measures of the risk factor will be averaged across the 2 contexts and used as a single predictor, and 2) all the risk factors will be entered as simultaneous predictors of outcomes. Non-significant predictors will be dropped and the analysis rerun.
Exploratory Analyses:
1) In consideration of sex as a biological variable, sex and the interactions between sex and all predictors will be considered in exploratory analyses for each Aim. 2) We will examine whether anxiety sensitivity moderates the effects of treatment condition and context (stress) on the risk categories in Aim 1, by adding anxiety sensitivity and its interaction with the treatment condition and context to the multilevel models in Aim 1. 3) We will examine smoking topography as a predictor of outcomes in Aims 2 and 3. 4) We will examine number of intervention sessions attended and dropouts to aid data interpretation and future study planning.
Power Analysis.
We used PinT 2.12 (Power in Two-Level models) to calculate the smallest effect size detectable given our N=75 at the deprivation assessment. Since no power analysis programs are available for multivariate multilevel models, we performed power analyses for the univariate multilevel models knowing that power for the multivariate multilevel models will be greater (Hox, 2010). PinT indicated that we had greater than .80 power to detect an effect size of d =.40 for Aim 1, d =.39 for Aim 2, and d =.48 for Aim 3. Preliminary evidence shows that our interventions have the ability to activate mechanistic targets, and for mechanistic targets to be related to clinical outcomes, at effect sizes beyond these powered values (Ashare et al., 2014; M. W. Otto et al., 2010; McKee, 2009; Patterson et al., 2010; Tang et al., 2013).
Dissemination and Open Science Plans.
At the conclusion of this study, we plan to make the de-identified study data and associated analytic syntax available on the Open Science Framework (https://osf.io/), and to provide summary data through the ClinicalTrials.gov registry (NCT03565497), consistent with an open-science approach (Toelch & Ostwald, 2018). All investigators and consultants will be invited to serve as authors on manuscripts to be submitted to relevant journals, with final authorship determined by contributions relative to collaborative discussions and the American Psychological Association ethical guidelines. There are no contractual limitations for access to data. As noted in the methods, assessments used in this study are drawn from SOBC Measure Repository (http://scienceofbehaviorchange.org/measures/), and likewise assessment strategies not included in the repository will be submitted for inclusion by trial completion.
Discussion
Consistent with an experimental medicine approach (Riddle & SOBC Working Group, 2015; Nielsen et al., 2018), we designed the current study to examine the degree to which engaging DT and WMC can produce significant changes in a health behavior by focusing on markers of early smoking lapse in a vulnerable low-income sample. Our methods are organized around the potential influence of a high stress context: specifically, the stress and negative affectivity induced by acute nicotine deprivation. Our goal is not to simply improve DT and WMC, but to ensure these improvements are resilient to changes in context, to realize more powerful effects on smoking cessation. This work is based on a comprehensive accounting of the role of low DT and WMC in placing individuals at risk for negative health behaviors, and the potential improvements in health behaviors provided by ameliorating these deficits (Bickel et al., 2015; M.W. Otto et al., 2016).
Achievement of Aim 1a and 2 would provide specific evidence for the value of standard mindfulness training for smoking cessation in low SES smokers, a high-risk for relapse group. Achievement of Aim 1b and 2 would introduce to the field a novel intervention (mindfulness plus interoceptive exposure) for enhancing target engagement in relevant high-stress (nicotine deprivation/negative affectivity) contexts, with particular attention to its ability to rescue potential limitations of standard mindfulness interventions. In short, this study has the potential to validate a strategy to jump-the-gap between low stress (e.g., during a therapy session) and high stress (when skills are particularly needed) contexts by training therapeutic skills under the stress sensations brought by IE. Specifically, IE procedures provide two opportunities for additional therapeutic gain. First, they allow patients to learn therapeutic skills under conditions of stress-related sensations. Second, they hold the potential to reduce the impact of stress by reducing distress in response to stress (by directly enhancing DT).
Relative to the goal of expanding and refining SOBC measures (https://scienceofbehaviorchange.org/measures/), as per Aim 3, this study is designed to show the value of engaging WMC and DT as mechanistic targets, with specific attention to multimodal assessment of these constructs. Also, this study is designed to show the incremental value of assessments during deprivation windows, in part due to the way in which behavior change mechanisms are modified by and interact with the heightened stress and additional withdrawal symptoms inherent to nicotine deprivation states in smokers. Finally, this study utilizes an enhanced laboratory lapse-behavior assessment (supplemented by smoking topography assessments), permitting an evaluation of the influence of behavioral mechanisms in a clinically important manner, while implementing a rigorous laboratory-based assessment protocol.
Achievement of the aims for this study will advance attention to the mechanistic targets of DT and WM relevant to a wide range of negative health behaviors, including the health behaviors linked to the top three preventable causes of disease and death in the United States: tobacco use, alcohol use, and poor diet/physical inactivity (obesity) (Bickel et al., 2015; Horenstein et al., 2018; M. W. Otto et al., 2016). As such, there are important transdiagnostic implications for the methods described herein. At a measurement level, this includes underscoring the importance of assessments during high stress/negative affectivity assessment windows, demonstrating how specific vulnerabilities are modified by, and interact with, heightened stress and negative affect. At a clinical level, transdiagnostic implications most prominently include validation of interventions for increasing health goal persistence under high stress/negative affectivity contexts.
Acknowledgments
Role of Funding Sources;
This study was supported by the National Institutes of Health (NIH) Science of Behavior Change Common Fund Program through an award administered by the National Institute on Drug Abuse (R21DA DA046963).
Footnotes
Conflict of Interest Statement:
Although the following activities/relationships do not create a conflict of interest pertaining to this manuscript, in the interest of full disclosure, the authors would like to report the following. Dr. Bickel would like to report the following: Dr. Otto receives support as a speaker and Chair of the Scientific Advisory Board for Big Health. Dr. Bickel is a principal of HealthSim, LLC; Notifius, LLC; BEAM Diagnostics, Inc.; and Red 5 Group, LLC. In addition, he serves on the scientific advisory board for Sober Grid, Inc.; Ria Health; US WorldMeds, LLC; and is a consultant for Alkermes, Inc. Sandoz, and Nektar Therapeutics. Dr. Smits is a paid clinical advisor to Big Health. No other authors have industry relationships to report.
Trial Registration: ClinicalTrials.gov identifier: NCT03565497
References
- Arch JJ & Ayers CR (2013). Which treatment worked better for whom? Moderators of group cognitive behavioral therapy versus adapted mindfulness based stress reduction for anxiety disorders. Behaviour Research and Therapy, 51(8), 434–442. [DOI] [PubMed] [Google Scholar]
- Ashare RL, Falcone M, & Lerman C (2014). Cognitive function during nicotine withdrawal: Implications for nicotine dependence treatment. Neuropharmacology, 76(0 0), 581–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babb S, Malarcher A, Schauer G, Asman K, & Jamal A (2017). Quitting smoking among adults – United States, 2000–2015. MMWR Morbidity and Mortality Weekly Report, 65, 1457–1464 [DOI] [PubMed] [Google Scholar]
- Baddeley A (2012). Working memory: theories, models, and controversies. Annual Review of Psychology, 63, 1–29. [DOI] [PubMed] [Google Scholar]
- Bickel WK, Quisenberry AJ, Moody L, & Wilson AG (2015). Therapeutic opportunities for self-control repair in addiction and related disorders: change and the limits of change in trans-disease processes. Clinical Psychological Science, 3(1), 140–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickel WK, Jarmolowicz DP, Mueller ET, Gatchalian KM, & McClure SM (2012). Are executive function and impulsivity antipodes? A conceptual reconstruction with special reference to addiction. Psychopharmacology (Berl), 221(3), 361–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borges AM, Leyro TM, Rosen RL, Zvolensky MJ, & Farris SG (2019). Negative urgency and ad-libitum smoking topography. Drug and Alcohol Dependence, 201, 220–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouton ME (2002). Context, ambiguity, and unlearning: Sources of relapse after behavioral extinction. Biological Psychiatry, 52, 976–986. [DOI] [PubMed] [Google Scholar]
- Bouton ME & Todd TP (2014). A fundamental role for context in instrumental learning and extinction. Behavioural Processes, 104, 13–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowen S, Chawla N, Collins SE, Witkiewitz K, Hsu S, Grow J, … Marlatt A (2009). Mindfulness-Based Relapse Prevention for Substance Use Disorders: A Pilot Efficacy Trial. Substance Abuse, 30(4), 295–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RA, Lejuez CW, Kahler CW, Strong DR, & Zvolensky MJ (2005). Distress tolerance and early smoking lapse. Clinical Psychology Review, 25(6), 713–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RA, Lejuez CW, Strong DR, Kahler CW, Zvolensky MJ, Carpenter LL, … Price LH (2009). A prospective examination of distress tolerance and early smoking lapse in adult self-quitters. Nicotine and Tobacco Research,11(5), 493–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiesa A, Calati R, & Serretti A (2011). Does mindfulness training improve cognitive abilities? A systematic review of neuropsychological findings. Clinical Psychology Review, 31(3), 449–464. [DOI] [PubMed] [Google Scholar]
- Cox LS, Tiffany ST, & Christen AG (2001). Evaluation of the brief questionnaire of smoking urges (QSU-brief) in laboratory and clinical settings. Nicotine and Tobacco Research, 3(1), 7–16. [DOI] [PubMed] [Google Scholar]
- Daughters SB, Lejuez CW, Bornovalova MA, Kahler CW, Strong DR, & Brown RA (2005). Distress tolerance as a predictor of early treatment dropout in a residential substance abuse treatment facility. Journal of Abnormal Psychology, 114(4), 729–734. [DOI] [PubMed] [Google Scholar]
- Doan SN, Fuller-Rowell TE, & Evans GW (2012). Cumulative risk and adolescent’s internalizing and externalizing problems: The mediating roles of maternal responsiveness and self-regulation. Developmental Psychology, 48(6), 1529–1539. [DOI] [PubMed] [Google Scholar]
- Evans GW & Schamberg MA (2009). Childhood poverty, chronic stress, and adult working memory. Proceedings of the National Academy of Sciences USA, 106, 6545–6549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farris SG, DiBello AM, Allan NP, Hogan J, Schmidt NB, & Zvolensky MJ (2015). Evaluation of the Anxiety Sensitivity Index-3 among treatment-seeking smokers. Psychological Assessment, 27(3), 1123–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldner MT, Zvolensky MJ, Babson K, Leen-Feldner EW, & Schmidt NB (2008). An integrated approach to panic prevention targeting the empirically supported risk factors of smoking and anxiety sensitivity: Theoretical basis and evidence from a pilot project evaluating feasibility and short-term efficacy. Journal of Anxiety Disorder, 22(7), 1227–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyal M, Singh S, Sibinga EM, Gould NF, Rowland-Seymour A…Haythornthwaite JA (2014). Meditation programs for psychological stress and well-being: a systematic review and meta-analysis. JAMA Internal Medicine, 174, 357–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green KT, Beckham JC, Youssef N, & Elbogen EB (2014). Alcohol misuse and psychological resilience among US Iraq and Afghanistan era veterans. Addictive Behaviors, 39(2), 406–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamer RM, & Simpson PM (2009). Last observation carried forward versus mixed models in the analysis of longitudinal psychiatric clinical trials. American Journal of Psychiatry, 166, 639–641. [DOI] [PubMed] [Google Scholar]
- Hajek P, Belcher M, & Stapleton J (1987). Breath-holding endurance as a predictor of success in smoking cessation. Addictive Behaviors, 12(3), 285–288. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, & Fagerström KO (1991). The Fagerström Test for Nicotine Dependence: a revision of the Fagerström Tolerance Questionnaire. British Journal of Addiction, 86(9), 1119–1127. [DOI] [PubMed] [Google Scholar]
- Horenstein A, Potter CM, Heimberg RG (2018). How does anxiety sensitivity increase risk of chronic medical conditions? Clinical Psychology: Science and Practice, 25, e12248. [Google Scholar]
- Hox JJ (2010). Multilevel Analysis: Techniques and Application Routledge, NY. [Google Scholar]
- Jha AP, Stanley EA, Kiyonaga A, Wong L, & Gelfand L (2010). Examining the protective effects of mindfulness training on working memory capacity and affective experience. Emotion, 10, 54–64. [DOI] [PubMed] [Google Scholar]
- Kahler CW, Metrik J, Spillane NS, Day A, Leventhal AM, McKee SA, … Rohsenow DJ (2014). Acute effects of low and high dose alcohol on smoking lapse behavior in a laboratory analogue task. Psychopharmacology (Berl), 231, 4649–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendzor DE, Businelle MS, Costello TJ, Castro Y, Reitzel LR, Cofta-Woerpel LM,… Wetter DW (2010). Financial strain and smoking cessation among racially/ethnically diverse smokers. American Journal of Public Health, 100(4), 702–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendzor DE, Businelle MS, Mazas CA, Cofta-Woerpel LM, Reitzel LR, Vidrine JI, … Wetter DW (2009). Pathways between socioeconomic status and modifiable risk factors among African American smokers. Journal of Behavioral Medicine, 32(6), 545–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lantz PM, House JS, Mero RP, & Williams DR (2005). Stress, life events, and socioeconomic disparities in health: Results from the Americans’ changing lives study. Journal of Health and Social Behavior, 46(3), 274–288. [DOI] [PubMed] [Google Scholar]
- Leventhal AM, & Zvolensky MJ (2015). Anxiety, depression, and cigarette smoking: A transdiagnostic vulnerability framework to understanding emotion-smoking comorbidity. Psychological Bulletin, 141(1), 176–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludman EJ, Curry SJ, Grothaus LC, Graham E, Stout J, & Lozano P (2002). Depressive symptoms, stress, and weight concerns among African American and European American low-income female smokers. Psychology of Addictive Behaviors, 16(1), 68–71. [DOI] [PubMed] [Google Scholar]
- Mathur C, Erickson DJ, Stigler MH, Forster JL, & Finnegan JR Jr (2013). Individual and neighborhood socioeconomic status effects on adolescent smoking: A multilevel cohort-sequential latent growth analysis. American Journal of Public Health, 103, 543–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCracken LM & Keogh E (2009). Acceptance, mindfulness, and values based action may counteract fear and avoidance of emotions in chronic pain: An analysis of anxiety sensitivity. The Journal of Pain, 10(4), 408–415. [DOI] [PubMed] [Google Scholar]
- McHugh RK & Otto MW (2011). Domain-general and domain-specific strategies for the assessment of distress intolerance. Psychology of Addictive Behaviors, 25(4):745–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee SA (2009). Developing human laboratory models of smoking lapse behavior for medication screening. Addiction Biology, 14(1), 99–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee SA, Sinha R, Weinberger AH, Sofuoglu M, Harrison EL, Lavery M, & Wanzer J (2011). Stress decreases the ability to resist smoking and potentiates smoking intensity and reward. Journal of Psychopharmacology, 25(4), 490–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee SA, Weinberger AH, Shi J, Tetrault J, & Coppola S (2012). Developing and validating a human laboratory model to screen medications for smoking cessation. Nicotine and Tobacco Research, 14(11), 1362–1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLeod JD & Kessler RC (1990). Socioeconomic status differences in vulnerability to undesirable life events. Journal of Health and Social Behavior, 31, 162–72 [PubMed] [Google Scholar]
- Mercken L, Moore L, Crone MR, De Vries H, De Bourdeaudhuij I, Lien N, & Van Lenthe FJ (2012). The effectiveness of school-based smoking prevention interventions among low-and high-SES European teenagers. Health Education Research, 27(3), 459–469. [DOI] [PubMed] [Google Scholar]
- Murphy CM & MacKillop J (2014). Mindfulness as a strategy for coping with cue-elicited cravings for alcohol: an experimental examination. Alcoholism, Clinical and Experimental Research, 38(4), 1134–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mystkowski JL, Craske MG, & Echiverri AM (2002).Treatment context and return of fear in spider phobia. Behavior Therapy, 33(3), 399–416. [DOI] [PubMed] [Google Scholar]
- Mystkowski JL, Mineka S, Vernon LL, & Zinbarg RE (2003). Changes in caffeine states enhance return of fear in spider phobia. Journal of Consulting and Clinical Psychology, 71(2), 243–50. [DOI] [PubMed] [Google Scholar]
- Nielsen L, Riddle M, King JW, NIH Science of Behavior Change Implementation Team, Aklin WM, Chen W, … Weber W (2018). The NIH Science of Behavior Change Program: Transforming the science through a focus on mechanisms of change. Behaviour Research and Therapy, 101, 3–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble KG, Norman MF, & Farah MJ (2005). Neurocognitive correlates of socioeconomic status in kindergarten children Developmental Science, 8, 74–87. [DOI] [PubMed] [Google Scholar]
- Oikonomou MT, Arvanitis M, & Sokolove RL (2017). Mindfulness training for smoking cessation: A meta-analysis of randomized-controlled trials. Journal of Health Psychology, 22, 1841–1850. [DOI] [PubMed] [Google Scholar]
- Otto AR, Raio CM, Chiang A, Phelps EA, & Daw ND (2013). Working-memory capacity protects model-based learning from stress. Proceedings of the National Academy of Sciences of the USA, 110(52), 20941–20946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto MW, Eastman A, Lo S, Hearon BA, Bickel WK, Zvolensky M, … Doan SN (2016). Anxiety sensitivity and working memory capacity: Risk factors and targets for health behavior promotion. Clinical Psychology Review, 49, 67–78. [DOI] [PubMed] [Google Scholar]
- Otto MW, McHugh RK, Simon NM, Farach FJ, Worthington JJ, & Pollack MH (2010). Efficacy of CBT for benzodiazepine discontinuation in patients with panic disorder: Further evaluation. Behaviour Research and Therapy, 48, 720–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto MW, O’Cleirigh CM, & Pollack MH (2007). Attending to emotional cues for drug use: Bridging the gap between clinic and home behavior. NIDA Science and Practice Perspectives, 3(2), 48–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto MW, & Pollack MH (2009). Stopping anxiety medication (Therapist guide, 2nd ed.). New York: Oxford University Press. [Google Scholar]
- Otto MW, Pollack MH, Sachs GS, Reiter SR, Meltzer-Brody S, & Rosenbaum JF (1993). Discontinuation of benzodiazepine treatment: Efficacy of cognitive-behavior therapy for patients with panic disorder. American Journal of Psychiatry, 150, 1485–90. [DOI] [PubMed] [Google Scholar]
- Otto MW, Rosenfield D, Gorlin EI, Hoyt DL, Patten EJ, Bickel WK, Zvolensky MJ, & Doan SN (in press). Targeting cognitive and emotional regulatory skills for smoking prevention in low-SES youth: A randomized trial of mindfulness and working memory interventions. Addictive Behaviors [DOI] [PubMed]
- Patterson F, Jepson C, Loughead J, Perkins K, Strasser AA, Siegel S, … Lerman C (2010). Working memory deficits predict short-term smoking resumption following brief abstinence. Drug and Alcohol Dependence,106(1), 61–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patnode CD, O’Connor E, Whitlock EP, Perdue LA, Soh C, & Hollis J (2013). Primary care-relevant interventions for tobacco use prevention and cessation in children and adolescents: a systematic evidence review for the U.S. Preventive Services Task Force. Annals of Internal Medicine, 158(4), 253–260. [DOI] [PubMed] [Google Scholar]
- Raines EM, Rogers AH, Bakhshaie J, Viana AG, Lemaire C, Garza M, Mayorga NA, Ochoa-Perez M, & Zvolensky MJ (2018). Mindful attention moderating the effect of experiential avoidance in terms of mental health among Latinos in a Federally Qualified Health Center. Psychiatry Research, 270, 574–580. [DOI] [PubMed] [Google Scholar]
- Reitzel LR, Businelle MS, Kendzor DE, Li Y, Cao Y, Castro Y, … Wetter DW (2011). Subjective social status predicts long-term smoking abstinence. BMC Public Health, 11, 135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddle M, & Science of Behavior Change Working Group. (2015). News from the NIH: using an experimental medicine approach to facilitate translational research. Translational Behavioral Medicine, 5(4), 486–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roche DJ, Bujarski S, Moallem NR, Guzman I, Shapiro JR, & Ray LA (2014). Predictors of smoking lapse in a human laboratory paradigm. Psychopharmacology (Berl), 231(14), 2889–97. [DOI] [PubMed] [Google Scholar]
- Rogojanski J, Vettese LC, & Antony MM (2011). Role of sensitivity to anxiety symptoms in responsiveness to mindfulness versus suppression strategies for coping with smoking cravings. Journal of Clinical Psychology, 67(4), 439–45. [DOI] [PubMed] [Google Scholar]
- Sheffer C, Mackillop J, McGeary J, Landes R, Carter L, Yi R, … Bickel W (2012). Delay discounting, locus of control, and cognitive impulsiveness independently predict tobacco dependence treatment outcomes in a highly dependent, lower socioeconomic group of smokers. American Journal on Addictions, 21(3), 221–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffman S, Hickcox M, Paty JA, Gnys M, Richards T, & Kassel JD (1997). Individual differences in the context of smoking lapse episodes. Addictive Behaviors, 22, 797–811. [DOI] [PubMed] [Google Scholar]
- Siahpush M, Singh GH, Jones PR, & Timsina LR (2009). Racial/Ethnic and Socioeconomic Variations in Duration of Smoking: Results from 2003, 2006 and 2007 Tobacco Use Supplement of the Current Population Survey. Journal of Public Health, 32, 210–218. [DOI] [PubMed] [Google Scholar]
- Smits JAJ, Berry AC, Tart CD, & Powers MB (2008). The efficacy of cognitive-behavioral interventions for reducing anxiety sensitivity: A meta-analytic review. Behaviour Research Therapy, 46(9), 1047–54. [DOI] [PubMed] [Google Scholar]
- Smits JAJ, Otto MW, Powers MB, & Baird SO (2018). Anxiety sensitivity as a transdiagnostic treatment target. In Smits JAJ, Otto MW, Powers MB & Baird SO (Eds.) Anxiety sensitivity: A clinical guide to assessment and treatment San Diego, CA: Academic Press. [Google Scholar]
- Strong DR, Lejuez CW, Daughters SB, Marinello M, Kahler CW, & Brown RA (2003). The Computerized Mirror Tracing Task Version 1 Unpublished manuscript.
- Tang YY, Tang R, & Posner MI (2013). Brief meditation training induces smoking reduction. Proceedings of the National Academy of Sciences USA, 110(34), 13971–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor S, Zvolensky MJ, Cox BJ, Deacon B, Heimberg RG, Ledley DR, . . . Stewart SH (2007). Robust dimensions of anxiety sensitivity: development and initial validation of the Anxiety Sensitivity Index-3. Psychological Assessment, 19, 176–188. [DOI] [PubMed] [Google Scholar]
- Thomas BL, Larsen N, & Ayres JB (2003). Role of context similarity in ABA, ABC, and AAB renewal paradigms: Implications for theories of renewal and for treating human phobias. Learning and Motivation, 34, 410–436. [Google Scholar]
- Todd TP, Winterbauer NE, & Bouton ME (2012). Contextual control of appetite. Renewal of inhibited food-seeking behavior in sated rats after extinction. Appetite, 58(2), 484–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toelch U, & Ostwald D (2018). Digital open science-Teaching digital tools for reproducible and transparent research. PLoS biology, 16(7), e2006022. 10.1371/journal.pbio.2006022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toll BA, Katulak NA, & Mckee SA (2006). Investigating the factor structure of the Questionnaire on Smoking Urges-Brief (QSU-Brief). Addictive Behaviors, 31, 1231–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang TW, Asman K, Gentzke AS, Cullen KA, Holder-Hayes E, Reyes-Guzman C … King BA (2018). Tobacco Product Use Among Adults — United States, 2017. MMWR Morbidity Mortality Weekly Report, 67,1225–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsch SK, Smith SS, Wetter DW, Jorenby DE, Fiore MC, & Baker TB (1999). Development and validation of the Wisconsin Smoking Withdrawal Scale. Experimental and Clinical Psychopharmacology, 7(4), 354–61. [DOI] [PubMed] [Google Scholar]
- Witkiewitz K, Marlatt GA, & Walker D (2005). Mindfulness-Based Relapse Prevention for Alcohol and Substance Use Disorders. Journal of Cognitive Psychotherapy, 19, 211–28. [Google Scholar]
- Zvolensky MJ, Bakhshaie J, Garza M, Paulus DJ, Valdivieso J, Lam H, Bogiaizian D, Robels Z, Schmidt NB, & Vujanovic A (2015). Anxiety sensitivity and mindful attention in terms of anxiety and depressive symptoms and disorders among Latinos in primary care. Psychiatry Research, 1–2, 245–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zvolensky MJ, Paulus DJ, Langdon K, Robles Z, Garey L, Norton PJ, & Businelle MS (2017). Anxiety sensitivity explains associations between anxious arousal symptoms and smoking abstinence expectancies, perceived barriers to cessation, and problems experienced during past quit attempts among low-income smokers. Journal of Anxiety Disorders, 48, 70–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zvolensky MJ, Rosenfield D, Garey L, Kauffman BY, Langdon KJ, Powers MB, … Smits JAJ (2018). Does exercise aid smoking cessation through reductions in anxiety sensitivity and dysphoria? Health Psychology, 37(7), 647–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zvolensky MJ, Stewart SH, Vujanovic AA, Gavric D, & Steeves D (2009). Anxiety sensitivity and anxiety and depressive symptoms in the prediction of early smoking lapse and relapse during smoking cessation treatment. Nicotine and Tobacco Research, 11, 323–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zvolensky MJ, Yartz AR, Gregor K, Gonzalez A, & Bernstein A (2008). Interoceptive exposure-based cessation intervention for smokers high in anxiety sensitivity: A case series. Journal of Cognitive Psychotherapy, 22(4), 346–365. [Google Scholar]


