Abstract
Background: Pancreatic cancer carries a devastating prognosis and is the fourth leading cause for cancer-related death in the United States and most European countries. Although one-third of patients receive a palliative third line therapy, the benefit of systemic therapy beyond second-line remains unclear. A plethora of clinical trials investigating novel drugs have failed over the past years. Due to the lack of established treatment regimens beyond second line, we offered nonpegylated liposomal doxorubicin, well known in other tumor entities, to pretreated pancreatic cancer patients requesting systemic therapy. Material and Methods: In this retrospective analysis, 28 patients with pancreatic carcinoma treated with nonpegylated liposomal doxorubicin (Myocet®) between 2012 and 2018 at our department were included. Results: For the majority of patients (n = 18, 64%), nonpeglyted liposomal doxorubicin was offered as a third-line therapy. Five patients received it as second line, four patients as fourth line, and one patient as fifth line of therapy. Half of the patients received at least a therapy cycle. The objective response rate to treatment was 7.1%. One patient had a period of radiologically confirmed stable disease with stable tumor markers. Another patient experienced partial remission. Conclusion: According to our findings the benefit of nonpegylated liposomal doxorubicin in pancreatic cancer beyond second line is limited.
Keywords: pancreatic cancer, palliative therapy, liposomal doxorubicin, chemotherapy, chemoresistance
Introduction
Despite a low incidence among solid tumors, pancreatic cancer (PC) represents the fourth leading cause of cancer-related death in the United States and most European countries.1,2 In fact, it is the least common solid tumor among the 10 most common types of cancer listed by the American Cancer Society in 2020.2 With a 5-year survival rate of only 9%, it carries a devastating prognosis and mortality rates remain unacceptably high.2 At diagnosis, most people are above the age of 70 years and PC is more common in men than in women.3,4
Curative surgery remains the standard therapy for localized PC whereas only 15% to 20% of patients present with technically resectable disease.5,6 Adjuvant chemotherapy is the standard therapeutic approach and/or—although efficacy is not yet clear—chemoradiotherapy (in case of R1 resection).7 Despite considerable progress in systemic therapy in advanced PC, the treatment intent for the latter patients remains palliative. However, the vast majority of phase III clinical trials investigating drugs for PC failed and only the results of three studies (investigating gemcitabine, nab-paclitaxel plus gemcitabine, and FOLFIRINOX) in the period from 1997 to 2015 should be considered practice changing.8–11 Despite the limited overall survival (OS) data in advanced PC, around 60% and 35% of patients receive a palliative second- and third-line therapy, respectively.12,13 The latter circumstance highlights the medical need for systemic therapeutic options beyond second line. Moreover, the clinical and OS benefit of a systemic third-line therapy remains unclear.
Preclinical studies suggest doxorubicin as a potential cytotoxic agent for PC and found that (pegylated) liposomal doxorubicin may yield better anti-tumor activity than free doxorubicin.14 Data on the efficacy of liposomal doxorubicin in advanced PC patients are sparse with only small patient cohorts.15,16 Based on the latter reports, we offered nonpegylated liposomal doxorubicin (Myocet®) to advanced PC patients beyond second-line at our tertiary cancer center.
Material and Methods
In this retrospective analysis, patients with advanced/metastatic PC undergoing palliative systemic therapy with liposomal nonpegylated doxorubicin (Myocet®) at our institution were included. All patients were over 18 years old and suffered from pathologically confirmed pancreatic adenocarcinoma. Treatment decisions were based on the European Society for Medical Oncology (ESMO) guidelines at the time of treatment and interdisciplinary tumor board decision.17 Patient data were obtained from medical records at the Department of Internal Medicine III, as well as from records from the Institute of Pathology. The study was conducted in accordance with the relevant guidelines and regulations. We retrospectively evaluated patient characteristics, Eastern Cooperative Oncology Group (ECOG) performance score, date of diagnosis, start of nonpegylated liposomal doxorubicin treatment, dose modifications during treatment, toxicity, response rates, and OS. Radiologic response assessment was based on computed tomography (CT) scans and if available on PET CT scans. Tumor response was classified as partial response, stable disease or progressive disease according to the World Health Organization criteria.18 OS under liposomal doxorubicin was defined as the period between the date of treatment-start with liposomal doxorubicin and the day of death from any cause. Dose modifications were made at the discretion of the treating physician. As per our institutional standard treatment was continued until disease progression or unacceptable toxicities.
Nonpegylated liposomal doxorubicin (Myocet®) cycle intervals varied between 2 to 4 weeks. Based on the ECOG performance status, 5 patients required a reduced initial dose.
At the time of data analysis all patients had succumbed to their PC disease. The analysis was approved by the Salzburg Ethics Commission (EP/73/789) and informed consent was waived by the decision of the same institution.
Statistics
Descriptive statistics was chosen to analyze the data. Data are presented with median and if appropriate with range.
The objective response rate (ORR) was calculated as the sum of the rates of and partial remission.
OS estimates were calculated using the Kaplan–Meier method.
Results
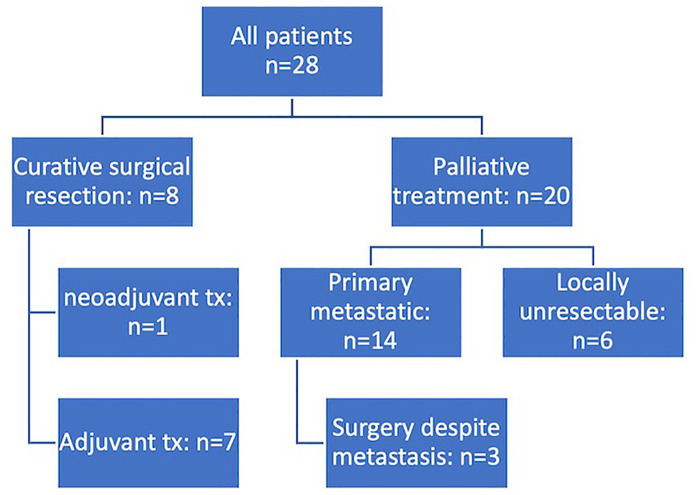
In total, 475 PC patients were treated between 2006 and 2020 at our institution. Between 2008 and 2018, 28 patients with PC received liposomal doxorubicin at our center. Distribution between male and female was balanced (13 vs 15 patients). Figure 1 depicts the prior surgical and systemic therapy approaches of the 28 patients undergoing liposomal doxorubicin therapy.
Figure 1.
Consort diagram showing the heterogeneous patient cohort at initial diagnosis (tx = treatment).
At the start of treatment with liposomal doxorubicin (Table 1), the median age was 67.5 years, ranging from 47 to 90 years. Liposomal doxorubicin was applied as palliative second line therapy in 5 patients (18%), third line in 18 patients (64%), fourth line in 4 patients (14%) and fifth line in 1 patient (4%). Prior palliative treatment included FOLFIRINOX (n = 18), gemcitabine plus nab-paclitaxel (n = 23), gemcitabine monotherapy (± antibody therapy within a clinical trial) (n = 4), nal-irinotecan plus leucovorin and 5-fluorouracil (5-FU) (n = 3) (with a total of 9 lines of diverse regimens).
Table 1.
Patient baseline characteristics prior to initiation of liposomal doxorubicin. (no. = number of patients, FOLFIRINOX = folinic acid, 5-fluorouracil, irinotecan, and oxaliplatin; 5-FU = 5-fluorouracil).
| Patient characteristics | Absolute number of patients | Percentage of patients (%) |
|---|---|---|
| Total number of patients | 28 | 100% |
| Age (years) | ||
| Median | 67.5 years | |
| Range | 46 to 89 years | |
| Sex (no.) | ||
| Male | 13 | 46% |
| Female | 15 | 54% |
| Prior palliative lines (no.) | ||
| FOLFIRINOX | 18 | 64% |
| Gemcitabine /nab-Paclitaxel | 23 | 82% |
| Gemcitabine mono (± experimental antibody) | 4 | 14% |
| Nal-Irinotecan/5-FU | 3 | 11% |
| Liposomal doxorubicin as palliative chemotherapy (no.) | ||
| 2nd line | 5 | 18% |
| 3rd line | 18 | 64% |
| 4th line | 4 | 14% |
| 5th line | 1 | 4% |
| Metastasis at start of liposomal doxorubicin (no.) | ||
| Hepatal | 18 | 64% |
| Pulmonary | 8 | 29% |
| Peritoneal | 5 | 18% |
| Bone | 1 | 4% |
| Adrenal | 1 | 4% |
| metastases in ≥ 1 organ | 6 | 21% |
Among the 28 patients 55 cycles of nonpegylated liposomal doxorubicin were administered (median: 1.5 cycles/patient). Fourteen (50%) patients received only 1 cycle, 7 patients received two cycles (25%), 5 patients received 3 cycles (18%), and 2 patients received 4 or more cycles. For patients receiving more than 1 cycle, a tri-weekly treatment interval was most frequently chosen (n = 11, 79%), followed by bi-weekly (n = 2, 14%) and monthly (n = 1, 7%) intervals, respectively.
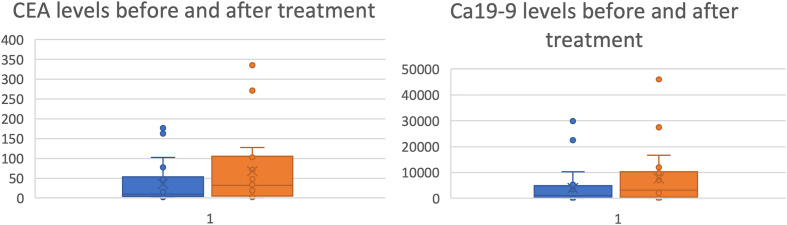
At the start of palliative treatment with liposomal doxorubicin liver metastases were most frequently detected (n = 18; 64%), followed by pulmonary (n = 8; 29%) and peritoneal (n = 5; 18%) metastases. The median level of carcinoembryonic antigen (CEA) was 11.4 ng/mL (range: 1.8-43 843) and the median level of CA 19 to 9 was 1031 U/mL (range: 7-38 787). After the last application of nonpegylated liposomal doxorubicin, the median CEA and CA19 to 9 levels rose to 33.6 ng/mL and 5418.5 U/mL, respectively (Figure 2).
Figure 2.
Box plot diagram demonstrating the tumor marker dynamics before the start of nonpegylated liposomal doxorubicin and after the last application. The median (50th percentile) is marked by the cross (X) and upper (75th percentile) and lower quartiles (25th percentile) are marked by the borders of the box. The whiskers show the minimal and maximal values (apart from outliers outside of the 1.5 of the interquartile range). CEA in ng/mL, CA19 to 9 in U/mL.
CEA: carcinoembryonic antigen
Radiologic response assessment was feasible in 14 patients (50%). Progressive disease was documented in 12 patients (43%). One patient achieved a stable disease (SD) and a partial remission (PR), respectively, yielding an objective response rate of 7.1% (=1/14). Thirteen patients succumbed prior to the radiologic re-assessment and 1 patient was lost to follow-up.
The OS from initial advanced PC diagnosis among the 28 patients receiving liposomal doxorubicin was 20.9 months since initial diagnosis. The OS from start of liposomal doxorubicin was 2.1 months (for OS, please refer to Table 2).
Table 2.
Clinical outcome among 28 patients undergoing non-pegylated liposomal doxorubicin therapy (no. = number of patients, OS = overall survival).
| Response to therapy | Number of patients (no.) or OS | Percentage (%) |
|---|---|---|
| Radiologic response (no.) | ||
| Progressive disease | 12 | 86 |
| Stable disease | 1 | 7 |
| Partial remission | 1 | 7 |
| No image available (no restaging) | 14 | 50 |
| OS (months) | ||
| OS under liposomal doxorubicin (months) | 2.1 | |
Table 3.
Medical complications (episodes) after start of liposomal doxorubicin. (no. p. = number of patients, LFP = liver function parameters).
| Medical complications | Number of events | Percentage (%) of patients |
|---|---|---|
| Median number of admissions | 1 | |
| Cardiotoxicity | 0 | 0 |
| Grade III or IV neutropenia | 3 | 11 |
| Infection requiring admission | 4 | 14 |
| Nausea | 1 | 4 |
| Increase of LFP | 1 | 4 |
| Alopecia | 1 | 4 |
No case of cardiotoxicity was reported. Three patients developed neutropenia grade III or IV. Four patients required hospital admission due to infectious complications. In addition to that, one patient suffered from alopecia, one experienced an increase in liver parameters and one patient developed nausea after therapy. The median number of hospitalizations was 1. In 12 cases, the admission was required due to a reduction of general condition or pain associated with the underlying disease. No reductions were necessitated after the initiation of nonpegylated liposomal doxorubicin therapy.
Discussion
In this retrospective analysis, 28 patients with pretreated advanced PC were treated with liposomal doxorubicin after failure of established palliative standard regimens. Around 35% of patients diagnosed with PC qualified for third-line palliative systemic therapy. However, a standard third-line palliative therapy has not been established so far in PC.12,13
Chemoresistance by a plethora of mechanisms remains a major challenge for treatment of PC.19 For example the extensive fibrosis surrounding tumor cells caused by cancer-associated fibroblasts inhibits drug delivery to tumor cells.20,21 Therefore, novel therapies have been extensively investigated.22 In a minority of patients, molecular alterations offer targeted therapy options: Programmed cell death protein 1 inhibitors in case of mismatch-repair deficiency (dMMR) or microsatellite instability (MSI-high), larotrectenib and entrectinib in case of tropomyosin receptor kinase (TRK)-fusion positivity, and the poly(adenosine diphosphate–ribose) polymerase inhibitor olaparib in case of germline BRCA1/2 mutations.23–26 Some examples of recently investigated approaches to target the stroma include hedgehog inhibitors, recombinant human hyaluronidase, CD40 agonists, vitamin D analogues, and pegylated recombinant human IL-10.27–31 Aiming at targeting the tumor's metabolism, selective inhibition of pyruvate and ketoglutarate dehydrogenase as well as the use of L-asparaginase have been investigated.32 Other more recent approaches used in clinical trials include masitinib plus gemcitabine, chemotherapy in combination with nivolumab, and the CD40 agonistic antibody sotigalimab.33–35
To our knowledge this retrospective analyses is the largest published cohort of patients with metastatic PC treated with liposomal doxorubicin. Furthermore, it is the first analysis report of nonpegylated liposomal doxorubicin efficacy among heavily pretreated patients. Two previous reports evaluated the anthracycline efficacy in 21 and 22 (with 16 evaluable for response) chemotherapy-naïve patients, respectively.15,16 In a phase II study by Halford et al published in 2001, 22 patients with unresectable PC were treated with pegylated liposomal doxorubicin.15 Syrigos et al administered a combination of liposomal doxorubicin and docetaxel to a cohort of 21 chemotherapy-naïve patients with unresectable PC.16 Data from the first study were inconclusive regarding the clinical benefit but 6 patients (38%) achieved stable disease after 2 cycles.15 In the second study, despite clinical improvement after treatment, it did not translate into a statistically significant progression free survival.16
We observed a partial remission in one patient and an SD in a second patient. Unfortunately, a disease progression was evident based on clinical, laboratory and radiologic findings among the remaining patients. The median OS of our study cohort was 2.1 months since the start of liposomal doxorubicin. If patients lost to follow up are included into the analysis until last time seen alive, these are (at least) 22.2 and 1.7 months, respectively. However, a slowdown of disease progression by the application of nonpegylated liposomal doxorubicin cannot be ruled out. We did not observe a clear objective benefit from the administration of liposomal doxorubicin. Although our analysis represents the largest patient cohort reported so far, the small sample size as well as the patient heterogeneity may be limitations of this analysis.
The efficacy of doxorubicin combined with other therapies such as L-DOS47 is currently being investigated in ongoing clinical trials (NCT04203641). L-DOS47 is an antibody–urease conjugate and liberates ammonia, which is thought to increase the microenvironment pH, thereby counteracting one of the tumor's resistance mechanisms. In addition to that, ammonia is cytotoxic and thereby thought to act synergistically with doxorubicin on tumor cells.
Furthermore, treatment with nonpegylated liposomal doxorubicin was generally tolerated in our cohort as evidenced by only a few adverse events during therapy.
Conclusion
Nonpegylated liposomal doxorubicin monotherapy did not achieve encouraging clinical outcome in pretreated pancreatic adenocarcinoma after failure of established treatment regimens. Our findings do not support its use beyond second line in daily clinical practice.
Acknowledgments
None
Abbreviations
- BRCA1/2
breast cancer 1/2, early-onset
- Ca19 to 9
carbohydrate antigen 19 to 9
- CEA
carcinoembryonic antigen
- CT
computed tomography
- dMMR
mismatch-repair deficiency
- ECOG
Eastern Cooperative Oncology Group
- ESMO
European Society for Medical Oncology
- FOLFIRINOX
5-fluorouracil (5-FU)
- leucovorin (LV)
irinotecan and oxaliplatin
- MSI
microsatellite instability
- nal-IRI
nanoliposomal pegylated irinotecan
- ORR
objective response rate
- OS
overall survival
- PARP
poly(adenosine diphosphate–ribose) polymerase
- PC
pancreatic cancer
- PD
progressive disease
- PD-1
Programmed cell death protein 1
- PET
positron emission tomography
- PR
partial remission
- SD
stable disease
- TRK
tropomyosin receptor kinase
- WHO
World Health Organization
Footnotes
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethics Statement: The analysis was approved by the Salzburg Ethics Commission (EP/73/789) and informed consent was waived by the decision of the same institution.
ORCID iD: Dominik Kiem https://orcid.org/0000-0002-8204-0334
References
- 1.Maisonneuve P. Epidemiology and burden of pancreatic cancer. Presse Med. 2019;48(3 Pt 2):e113-e123. doi: 10.1016/j.lpm.2019.02.030 [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70(1):7-30. doi: 10.3322/caac.21590 [DOI] [PubMed] [Google Scholar]
- 3.Rawla P, Sunkara T, Gaduputi V. Epidemiology of pancreatic cancer: global trends, etiology and risk factors. World J Oncol. 2019;10(1):10-27. doi: 10.14740/wjon1166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209-249. doi: 10.3322/caac.21660 [DOI] [PubMed] [Google Scholar]
- 5.Sabater L, Muñoz E, Roselló S, et al. Borderline resectable pancreatic cancer. Challenges and controversies. Cancer Treat Rev. 2018;68:124-135. doi: 10.1016/j.ctrv.2018.06.006 [DOI] [PubMed] [Google Scholar]
- 6.McGuigan A, Kelly P, Turkington RC, Jones C, Coleman HG, McCain RS. Pancreatic cancer: a review of clinical diagnosis, epidemiology, treatment and outcomes. World J Gastroenterol. 2018;24(43):4846-4861. doi: 10.3748/wjg.v24.i43.4846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coveler AL, Herman JM, Simeone DM, Chiorean EG. Localized pancreatic cancer: multidisciplinary management. Am Soc Clin Oncol Educ Book. 2016;35:e217-e226. doi: 10.1200/EDBK_160827 [DOI] [PubMed] [Google Scholar]
- 8.Burris HA, Moore MJ, Andersen J, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997;15(6):2403-2413. doi: 10.1200/JCO.1997.15.6.2403 [DOI] [PubMed] [Google Scholar]
- 9.Von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369(18):1691-1703. doi: 10.1056/NEJMoa1304369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX Versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364(19):1817-1825. doi: 10.1056/NEJMoa1011923 [DOI] [PubMed] [Google Scholar]
- 11.Thota R, Maitra A, Berlin JD. Preclinical rationale for the phase III trials in metastatic pancreatic cancer: is wishful thinking clouding successful drug development for pancreatic cancer? Pancreas. 2017;46(2):143-150. doi: 10.1097/MPA.0000000000000753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Taieb J, Prager GW, Melisi D, et al. First-line and second-line treatment of patients with metastatic pancreatic adenocarcinoma in routine clinical practice across Europe: a retrospective, observational chart review study. ESMO Open. 2020;5(1). doi: 10.1136/esmoopen-2019-000587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schlick K, Magnes T, Ratzinger L, et al. Novel models for prediction of benefit and toxicity with FOLFIRINOX treatment of pancreatic cancer using clinically available parameters. PLoS One. 2018;13(11):e0206688. doi: 10.1371/journal.pone.0206688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gabizon A, Martin F. Polyethylene glycol-coated (pegylated) liposomal doxorubicin. Rationale for use in solid tumours. Drugs. 1997;54(Suppl 4):15-21. doi: 10.2165/00003495-199700544-00005 [DOI] [PubMed] [Google Scholar]
- 15.Halford S, Yip D, Karapetis CS, et al. A phase II study evaluating the tolerability and efficacy of CAELYX (liposomal doxorubicin, Doxil) in the treatment of unresectable pancreatic carcinoma. Ann Oncol. 2001;12(10):1399-1402. doi: 10.1023/a:1012522120294 [DOI] [PubMed] [Google Scholar]
- 16.Syrigos KN, Michalaki B, Alevyzaki F, et al. A phase-II study of liposomal doxorubicin and docetaxel in patients with advanced pancreatic cancer. Anticancer Res. 2002;22(6b):3583-3588. [PubMed] [Google Scholar]
- 17.Ducreux M, Cuhna AS, Caramella C, et al. Cancer of the pancreas: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2015;26(Suppl 5):v56-v68. doi: 10.1093/annonc/mdv295 [DOI] [PubMed] [Google Scholar]
- 18.Miller AB, Hoogstraten B, Staquet M, Winkler A. Reporting results of cancer treatment. Cancer. 1981;47(1):207-214. [DOI] [PubMed] [Google Scholar]
- 19.Zeng S, Pöttler M, Lan B, Grützmann R, Pilarsky C, Yang H. Chemoresistance in pancreatic cancer. Int J Mol Sci. 2019;20(18). doi: 10.3390/ijms20184504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.von Ahrens D, Bhagat TD, Nagrath D, Maitra A, Verma A. The role of stromal cancer-associated fibroblasts in pancreatic cancer. J Hematol Oncol. 2017;10(1):76. doi: 10.1186/s13045-017-0448-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ansari D, Carvajo M, Bauden M, Andersson R. Pancreatic cancer stroma: controversies and current insights. Scand J Gastroenterol. 2017;52(6-7):641-646. doi: 10.1080/00365521.2017.1293726 [DOI] [PubMed] [Google Scholar]
- 22.Chiorean EG, Coveler AL. Pancreatic cancer: optimizing treatment options, new, and emerging targeted therapies. Drug Des Devel Ther. 2015;9:3529-3545. doi: 10.2147/DDDT.S60328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Le DT, Uram JN, Wang H, et al. PD-1 Blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015;372(26):2509-2520. doi: 10.1056/NEJMoa1500596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Drilon A, Fu S, Patel MR, et al. A phase I/Ib trial of the VEGFR-sparing multikinase RET inhibitor RXDX-105. Cancer Discov. 2019;9(3):384-395. doi: 10.1158/2159-8290.CD-18-0839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Doebele RC, Drilon A, Paz-Ares L, et al. Entrectinib in patients with advanced or metastatic NTRK fusion-positive solid tumours: integrated analysis of three phase 1-2 trials. Lancet Oncol. 2020;21(2):271-282. doi: 10.1016/S1470-2045(19)30691-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Golan T, Hammel P, Reni M, et al. Maintenance Olaparib for germline. N Engl J Med. 2019;381(4):317-327. doi: 10.1056/NEJMoa1903387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Olive KP, Jacobetz MA, Davidson CJ, et al. Inhibition of hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science. 2009;324(5933):1457-1461. doi: 10.1126/science.1171362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Provenzano PP, Cuevas C, Chang AE, Goel VK, Von Hoff DD, Hingorani SR. Enzymatic targeting of the stroma ablates physical barriers to treatment of pancreatic ductal adenocarcinoma. Cancer Cell. 2012;21(3):418-429. doi: 10.1016/j.ccr.2012.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Beatty GL, Chiorean EG, Fishman MP, et al. CD40 Agonists alter tumor stroma and show efficacy against pancreatic carcinoma in mice and humans. Science. 2011;331(6024):1612-1616. doi: 10.1126/science.1198443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sherman MH, Yu RT, Engle DD, et al. Vitamin D receptor-mediated stromal reprogramming suppresses pancreatitis and enhances pancreatic cancer therapy. Cell. 2014;159(1):80-93. doi: 10.1016/j.cell.2014.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hecht JR, Lonardi S, Bendell J, et al. Randomized phase III study of FOLFOX alone or with pegilodecakin as second-line therapy in patients with metastatic pancreatic cancer that progressed after gemcitabine (SEQUOIA). J Clin Oncol. Feb 2021;39(10):1108-1118. doi: 10.1200/JCO.20.02232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alistar A, Morris BB, Desnoyer R, et al. Safety and tolerability of the first-in-class agent CPI-613 in combination with modified FOLFIRINOX in patients with metastatic pancreatic cancer: a single-centre, open-label, dose-escalation, phase 1 trial. Lancet Oncol. 2017;18(6):770-778. doi: 10.1016/S1470-2045(17)30314-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hammel P, Fabienne P, Mineur L, et al. Erythrocyte-encapsulated asparaginase (eryaspase) combined with chemotherapy in second-line treatment of advanced pancreatic cancer: an open-label, randomized phase IIb trial. Eur J Cancer. 2020;124:91-101. doi: 10.1016/j.ejca.2019.10.020 [DOI] [PubMed] [Google Scholar]
- 34.O’Hara MH, O’Reilly EM, Varadhachary G, et al. CD40 Agonistic monoclonal antibody APX005M (sotigalimab) and chemotherapy, with or without nivolumab, for the treatment of metastatic pancreatic adenocarcinoma: an open-label, multicentre, phase 1b study. Lancet Oncol. 2021;22(1):118-131. doi: 10.1016/S1470-2045(20)30532-5 [DOI] [PubMed] [Google Scholar]
- 35.Ezenfis J, Hermine O, Group AS. Masitinib plus gemcitabine as first-line treatment of pancreatic cancer with pain: results from phase 3 study AB12005. J Clin Oncol. 2021;39(15)(suppl 15; abstr 4018):4018-4018. doi: 10.1200/JCO.2021.39.15_suppl.4018 [DOI] [Google Scholar]