Abstract
Objective
We aimed to evaluate the prognostic value of circulating tumor cells (CTCs) and the impact of intraoperative tumor manipulation on CTCs in colorectal cancer (CRC) patients.
Methods
We performed a prospective study on 40 patients with CRC stages I to IV who received curative surgery using the no-touch technique. Flow cytometry was used to identify CTCs in peripheral blood samples (4 mL/sample) collected at two surgical moments: skin incision (T1) and after surgical resection (T2). A threshold of ≥4 CTCs/4 mL blood was established for considering patients CTC positive.
Results
In the univariate analysis, CTC evaluation at T2 was correlated with female sex, vascular invasion, tumor localization in the colon and metastatic lymph nodes. In the multivariate analysis, only female sex and colon cancer maintained statistical significance. At a medium follow-up of 15 months (1–25 months), the mortality rate was 10% (n = 4), with no significant differences between the overall survival of T1 or T2 CTC-positive and CTC-negative patients.
Conclusions
Flow cytometry is a feasible CTC identification technique in CRC, and although surgical manipulation has no influence on CTC numbers, CTCs may serve as a prognostic and predictive factor.
Keywords: Colorectal cancer, circulating tumor cell, flow cytometry, liquid biopsy, surgical resection, intraoperative tumor manipulation
Introduction
Circulating tumor cells (CTCs) are neoplastic cells that originate in primary tumors, distant metastases or local recurrences.1 A liquid biopsy in colorectal cancer (CRC) enables the analysis of circulating DNA, which helps inform and optimize patient management in terms of screening, diagnosis, treatment and follow-up.2 Epithelial-specific markers, such as epithelial cell adhesion molecule and cytokeratin (CK),3 have been used for CTC detection, and multiple identification techniques and protocols have been developed.4,5
In CRC, the persistence of CTCs in peripheral blood after radical surgery is associated with disease progression and poor responses to adjuvant therapies. Furthermore, 24-hour persistence following resection is regarded as an independent prognostic marker for local recurrence and reduced specific survival.6,7 In addition, the persistence of increased CTC numbers following chemotherapy is associated with worse disease progression compared with patients whose CTCs decreased to low levels.8 A laparoscopic approach was found to lead to significantly lower CTC counts compared with open surgery as a result of medial to lateral dissection and was associated with higher CTC counts in portal blood compared with peripheral blood.9 The detection of CTCs in the peripheral blood of a CRC patient is a statistically significant prognostic factor associated with a poor prognosis, unlike CTCs detected in mesenteric or portal blood detection.10 The persistence of CTCs for at least 24 hours after surgical resection is associated with both recurrence11 and poor survival outcomes.12 In patients with positive lymph nodes, CTCs counts of ≥50% have been reported, with a significantly increased liver metastatic rate and reduced disease-free survival (DFS) in CTC-positive patients and aggressive disease progression in non-metastatic CTC-positive patients.13,14 Regarding the prognostic value of CTCs identified with the CellSearch System, researchers have observed a significantly higher incidence of CTCs in patients with distant and liver metastasis and a significantly lower response rate during treatment in CTC-positive patients.15 Flow cytometry has also been used as a CTC detection method in CRC,16 but only a limited number of studies performed the CTC count analysis in relation to surgical resection.17–23 The aim of our study was to evaluate the prognostic value of CTC counts in the peripheral blood of CRC patients and determine the impact of intraoperative tumor manipulation on CTC numbers.
Materials and methods
Patients and data collection
We performed a prospective study that included patients with CRC operated on at the 1st Surgical Oncology Unit of the Regional Institute of Oncology, Iasi, Romania. According to the inclusion criteria, the research subjects were adult patients with confirmed CRC stages I to IV who had undergone curative surgery and signed an informed consent form regarding their participation in this study. The exclusion criteria were as follows: no informed consent, palliative surgical treatment (bypass/biopsy), impossible follow-up and associated immune disease. All patients were enrolled between May and December 2018.
The same surgical team operated on all patients included in this study using the no-touch approach and the principles of oncological resection with primary vascular ligation. Follow-up was conducted in accordance with international guidelines and consisted of a thorough clinical, biological, imaging and endoscopic evaluation. This study was approved by the Ethics Committee of the “Grigore T. Popa” University of Medicine and Pharmacy Iasi and the Regional Institute of Oncology Iasi, Romania. All patients signed an informed consent form at the time of enrollment in full awareness of the risks and agreed to their participation in the study.
Identification of CTCs using flow cytometry
For CTC identification, peripheral blood samples (4 mL/sample) were drawn using a cubital vein puncture at two surgical times: before (T1-upon skin incision, after general anesthesia with oral intubation) and after surgical resection of the tumor and specimen removal (T2-after intraoperative evaluation, tumor mobilization and vascular ligation).
The samples, equivalent in size to two complete blood count tests, were collected into ethylenediaminetetraacetic acid-containing tubes and processed within 24 hours following the Euro Flow Bulk Lysis protocol.24,25 The peripheral blood was first lysed for 15 minutes on a roller using Bulk Lysis solution (CYT-BL, Cytognos Flow Cytometry Solutions, Salamanca, Spain). Next, the samples were centrifuged (800 × g, 10 minutes, room temperature) and the cellular pellet was washed with erythrocyte lysis solution (FACS Lyse, 349202, BD Bioscience, San Jose, CA, USA, provided by Novaintermed, Romania) supplemented with 0.5% bovine serum albumin (BSA, A7906-10CT, Sigma-Aldrich, St Louis, MO, USA, provided by Redox, Romania). After washing the cell surface, cells were stained with CD3-peridinin chlorophyll protein cyanine 5.5 (CD3-PerCP-Cy5.5, 552852, BD Pharmingen, BD Bioscience, San Jose, CA, USA, provided by Novaintermed), CD8-phycoerythrin-cyanine 7 (CD8-PE-Cy7, 557746, BD Pharmingen) and CD45-allophycocyanin (CD45-APC, C7230, Dako, CA, USA, provided by Redox) in the dark for 15 minutes to identify lymphocytes. The samples were then washed and incubated with Fix&Perm solution A (GAS-002, Nordic MUbio, Susteren, The Netherlands, provided by Proton, Romania) for 15 minutes at room temperature while protected from light. Next, the samples were washed with BSA, then incubated for 15 minutes with Fix&Perm solution B and the labeled intracellular markers CK20-fluorescein isothiocyanate (FITC) (ABIN 11170, Antibodies-online GmbH, Aachen, Germany, provided by Proton) and CK7-phycoerythrin (PE) (SC-23876, Santa Cruz Biotechnology, Dallas, TX, USA, provided by Proton) in the dark. After staining, the samples were washed, centrifuged (540 × g, 5 minutes, room temperature) and finally resuspended in the acquisition buffer (BD FACS Flow solution). Up to 10 × 106 cells were acquired for each sample on a FACS NAVIOS flow cytometer (Beckman Coulter Life Sciences, Indianapolis, IN, USA), and the data were analyzed using Infinicyt software (Cytognos Flow Cytometry Solutions) by the same immunologist who processed all samples.
A threshold of CTC-positive patients was established at ≥4 CTCs/4 mL blood, whereas patients with between 1 and 3 CTCs/4 mL of blood were considered CTC negative.
Statistical analysis
Excel 2013 (Microsoft Corporation, Redmond, WA, USA) was used for descriptive statistics. Univariate analyses of categorical covariates were performed using the χ2 or Fisher’s exact test. P-values <0.05 were considered significant. Multivariate analyses were performed using the Statistical Package for IBM SPSS Statistics for Windows ver. 21.0 (IBM Corp., Armonk, NY, USA), and only covariates with p<0.05 were included.
The overall survival (OS) was measured as the time elapsed between the baseline blood collection and the patient’s death, and progression-free survival (PFS) was recorded as the time elapsed between the baseline blood collection and the time when disease recurrence was detected. Survival was determined using the Kaplan–Meier method, and the log-rank test was used for comparisons.
Results
Gating strategies for cell population identification in CRC
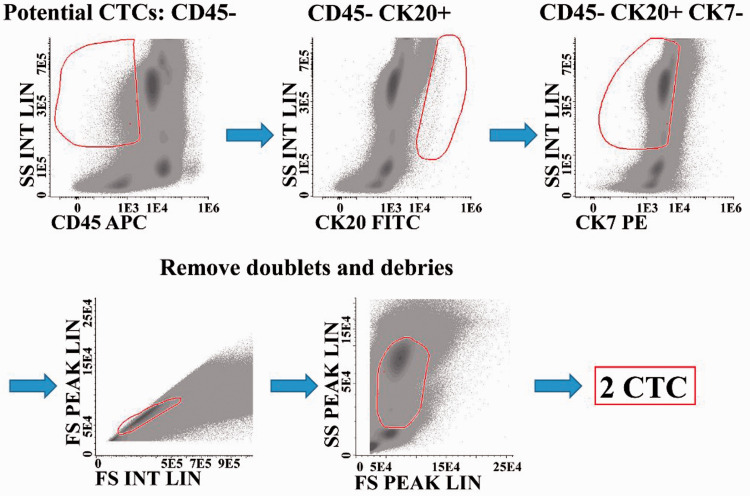
CTC identification started with the selection of CD45− cells on the CD45/side scatter (SSC) plot, followed by the selection of CK20+ CK7− cells (CK20/SSC and CK7/SSC plots). Next, debris and dead cells were removed using forward scatter (FSC)/SSC and forward scatter-height (FSC-H )/forward scatter-area (FSC-A) plots. Cell populations were identified using CD45 expression and the indicated markers. Debris and dead cells were excluded using FSC-H/FSC-A and CD45/SSC plots, and then the following strategy was used: granulocytes were identified as CD45dim/bright/SSCint/high cells, monocytes as CD45dim/bright/SSCint cells, eosinophils as CD45bright/SSChigh and CK20+ cells, lymphocytes as CD45bright/SSClow cells, T lymphocytes as CD45bright/SSClow and CD3+ cells and CD8+ T cells as CD45bright/SSClow, CD3+ and CD8+ cells. In addition, B and natural killer cells were obtained by subtracting the T cells from the number of total lymphocytes. A complete blood count was obtained for each sample using a PENTRA XLR machine (PENTRA XLR, provided by Horiba, Japan), and the white blood cell number was inputted into the Profile/Configure/Statistics in the Infinicyt program (Cytognos Flow Cytometry Solutions) to obtain the cells/mL for all cell populations identified (Figure 1).
Figure 1.
Gating strategy to identify rare CTCs. The CTCs were identified in peripheral blood samples collected before and after surgery from patients with confirmed CRC stages I to IV. The selection started in both samples with CD45− cells on CD45/SSC plots, followed by CK20+ CK7− cells (CK20/SSC and CK7/SSC plots). Next, debris and dead cells were removed using FSC/SSC and FSC-H/FSC-A plots. Finally, the potential CTC cells were identified.
CTCs, circulating tumor cells; CD45, lymphocyte common antigen; SS, side scatter; CK20, keratin 20; CK7, cytokeratin 7; APC, allophycocyanin; FITC, fluorescein isothiocyanate; PE, phycoerythrin; FS, forward scatter; H, height; A, area.
General cohort characteristics
We included 40 patients with CRC stages I to IV. Their average age was 63.94 years, ranging from 39 to 80 years old. The cohort consisted of 19 men (47.5%) and 21 women (52.5%). Tumor localization was predominantly in the colon (n = 20, 50%), followed by the rectum (n = 18, 45%) and the colorectal junction (n = 2, 5%). In 13 cases (32.5%), the patients had undergone neoadjuvant treatment, including chemotherapy in three cases, radiochemotherapy in nine and radiotherapy alone in one case (Table 1).
Table 1.
General characteristics of the 40 patients.
| Characteristics | N (% of 40 patients) |
|---|---|
| Age, years | 63.94 (range, 39–80) |
| <65 years old | 17 (42.5) |
| ≥65 years old | 23 (57.5) |
| Male | 19 (47.5) |
| Female | 21 (52.5) |
| Pathological type | |
| Adenocarcinoma | 35 (87.5) |
| Mucinous | 5 (12.5) |
| Localization | |
| Colon | 20 (50) |
| Right | 9 (22.5) |
| Splenic angle | 3 (7.5) |
| Sigmoid | 8 (20) |
| Rectum | 18 (45) |
| Upper | 1 (2.5) |
| Middle | 10 (25) |
| Lower | 7 (17.5) |
| Junction | 2 (5) |
| Neoadjuvant treatment | 13 (32.5) |
| Chemotherapy | 3 (7.5) |
| Radiochemotherapy | 9 (22.5) |
| Radiotherapy | 1 (2.5) |
| Surgery type | |
| Right hemicolectomy | 9 (22.5) |
| Upper left segmental colectomy | 3 (7.5) |
| Lower left segmental colectomy | 7 (17.5) |
| Anterior rectal resection with mesorectum transection | 3 (7.5) |
| Very low rectal resection with TME | 5 (12.5) |
| Hartmann’s procedure | 2 (5) |
| Hartmann’s procedure with TME | 6 (15) |
| Extralevator abdominoperineal resection | 5 (12.5) |
| Colostomy | 17 (42.5) |
| Ileostomy | 2 (5) |
TME, total mesorectum excision.
The pathological reports showed the presence of adenocarcinoma in 35 cases (87.5%) and mucinous adenocarcinoma in only five patients (12.5%) (Table 1). Patient staging showed a predominance of stage III (n = 20, 50%) and stage II (n = 11, 27.5%), and a complete pathologic response after neoadjuvant therapy being was reported in only one case. In eight patients, distant metastasis was also present, with liver (n = 5, 12.5%), pulmonary (n = 2, 5%) and peritoneal (n = 1, 2.5%) localization (Table 2).
Table 2.
Staging and pathological characteristics in included patients.
| Staging | N (% of 40 patients) |
|---|---|
| Depth of invasion | |
| T0 | 1 (2.5) |
| T2 | 2 (5) |
| T3 | 33 (82.5) |
| T4 | 4 (10) |
| Lymph node involvement | |
| N0 | 16 (40) |
| N1 | 16 (40) |
| N2 | 8 (20) |
| Distant metastasis | |
| M0 | 32 (80) |
| M1 | 8 (20) |
| M-Liver | 5 (12.5) |
| M-Pulmonary | 2 (5) |
| M-Peritoneal | 1 (2.5) |
| TNM Stage | |
| 0 | 1 (2.5) |
| II | 11 (27.5) |
| III | 20 (50) |
| IV | 8 (20) |
| VELIPI | |
| Lymphatic invasion | 25 (62.5) |
| Vascular invasion | 17 (42.5) |
| Perineural invasion | 15 (37.5) |
| Grading | |
| Well differentiated (G1) | 2 (5) |
| Moderately differentiated (G2) | 26 (65) |
| Poorly differentiated (G3) | 3 (7.5) |
| Undifferentiated (Gx) | 8 (20) |
Surgical characteristics: no-touch technique and intraoperative findings
The surgical technique applied was predominantly the no-touch procedure following the oncological principles and primary tumor ligation with no or minimum tumor mobilization. The same surgical team operated on all patients included in this study, without variation in the surgical method. The most common procedure was right hemicolectomy (n = 9, 22.5%), followed by lower left segmental colectomy (n = 7, 17.5%) and Hartmann’s procedure with total mesorectum excision (n = 6, 15%). A colostomy was performed on 17 patients (42.5%), and a protective ileostomy for colorectal anastomosis was considered necessary in two cases (Table 1).
The mean operating time was 110 minutes (SD 42.1), and the mean blood loss was 276.154 mL (20–1800 mL, SD = 274.04). The mean time between T1 and T2 sampling was 27.48 minutes (15–70 minutes, SD = 15.176).
There was no statistical association between the number of CTCs, duration of the surgeries, loss of blood, time elapsed between the collection of the two blood samples or CTC values at T1 and T2.
CTC evaluation in relation to clinical and pathological factors and the impact on clinical outcomes
The mean number of CTCs was 6.43 cells/4 mL blood (SD = 5.6) at T1 and 7.83 (SD = 5.9) at T2, without statistical differences between patients when grouped based on intraoperative tumor manipulation (p = 0.17).
At a cut-off value of ≥4 CTCs, a positive baseline CTC count was detected at T1 in 24 patients (60%) and T2 in 29 patients (72.5%).
At T2, the mean number of CTCs was 10.24 (SD = 5.1) in positive patients and 1.45 (SD = 1.12) in the negative group, with significant differences between the positive and negative patients (p<0.001). No statistical differences were observed at T1.
Clinical and biological correlations with CTCs at T2 showed a tendency towards a significant association between the CTC-positive patients and the harvesting of lymph nodes (p = 0.06) (Table 3).
Table 3.
Clinical and biological characteristics for colorectal cancer patients who underwent an operation.
| Factor | T2-CTC positive(≥4 CTCs), (n = 29) | T2-CTC negative(1–3 CTCs), (n = 11) | p-value1 |
|---|---|---|---|
| Age, years | 64.83 (9.77) | 63.18 (11.32) | 0.612 |
| Preoperative lymphocyte count | 1.71 (0.98) | 1.82 (0.62) | 0.39 |
| Preoperative platelets | 292.97 (91.28) | 359.45 (152.01) | 0.12 |
| Preoperative neutrophils | 4.48 (1.66) | 5.70 (2.09) | 0.23 |
| Lymph node harvest | 28.86 (19.34) | 39.45 (17.87) | 0.06 |
| Positive lymph node harvest | 1.31 (2.19) | 3.27 (3.77) | 0.09 |
| Preoperative CEA | 19.59 (74.56) | 92.61 (202.87) | 0.18 |
| Preoperative CA19.9 | 253.61 (1269) | 101.03 (206, 36) | 0.53 |
| Preoperative albumin | 4.37 (0.7) | 4.70 (0.29) | 0.16 |
1Mann–Whitney test.
CEA, carcinoembryonic antigen; CA19.9, carbohydrate antigen 19-9; CTCs, circulating tumor cells.
According to the univariate analysis, radiochemotherapy appeared to influence the levels of T1 CTC positive numbers (p = 0.06), whereas at T2, CTC numbers were correlated with female sex (p = 0.049), the presence of vascular invasion (p = 0.03), tumor localization in the colon segment (p = 0.04) and positive lymph nodes (more than one metastatic lymph node) (p = 0.04). In the multivariate analysis, female sex and tumor localization in the colon maintained statistical significance (p = 0.03 and p = 0.01, respectively) in T2 CTC-positive patients, whereas vascular invasion and positive lymph nodes were no longer statistically significant (Table 4).
Table 4.
Factors associated with intraoperative positive CTCs in colorectal cancer patients in T1 and T2 based on univariate and multivariate analyses.
| T1 |
T2 |
||||
|---|---|---|---|---|---|
| Univariate Analysisa |
Univariate Analysisa |
Multivariate Analysisb |
|||
| Independent variables | p-value | p-value | Odds ratio | 95%CI | p-value |
| Sex | |||||
| Female vs Male | 0.35 | 0.049 | 0.11 | 0.02–0.76 | 0.03 |
| Preoperative chemotherapy | 0.26 | 0.55 | |||
| Preoperative radiochemotherapy | 0.06 | 0.39 | |||
| Preoperative radiotherapy | 0.40 | 0.27 | |||
| T stage | |||||
| T1 vs T2 vs T3 vs T4 | 0.82 | 0.73 | |||
| Lymphovascular invasion | |||||
| Yes vs No | 1.01 | 0.72 | |||
| Stage | |||||
| Stage I vs II vs III vs IV | 0.84 | 0.36 | |||
| Vascular invasion | |||||
| Yes vs No | 1.01 | 0.03 | NS | ||
| Perineural invasion | |||||
| Yes vs No | 1 | 0.71 | |||
| Metastatic disease | |||||
| Yes vs No | 1.01 | 0.25 | |||
| Tumor grade | |||||
| Grade 1 vs Grade 2 vs Grade 3 | 0.28 | 0.44 | |||
| Tumor location | |||||
| Colon vs rectum | 0.20 | 0.04 | 9.18 | 1.22–69.02 | 0.01 |
| Positive lymph nodes | |||||
| 0–1 vs > 1 | 0.74 | 0.04 | NS | ||
aChi-square test or Fisher’s exact test when appropriate; b Multivariate logistic regression; Hosmer–Lemeshow p = 0.22 (>0.05).
CI, confidence interval; NS, not significant.
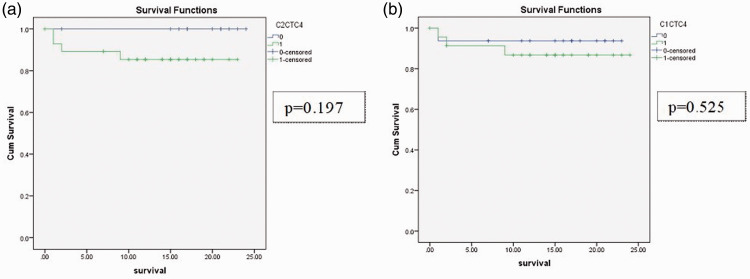
The OS analyzed using Kaplan–Meier curves showed no significant differences between the CTC-positive and CTC-negative patients at T2 and T1 at a median follow-up of 15 months (range 1–25 months) with a 10% mortality rate (n = 4) (Figure 2).
Figure 2.
Kaplan–Meier overall survival curves of patients with confirmed CRC stages I to IV who had undergone curative surgery. (a) CTC-positive versus negative patients at T2; (b) CTC-positive versus negative patients at T1.
Cum, cumulative; CTC, circulating tumor cells; C2CTC4, CTC positive versus negative patients at T2 with CTCs ≥4; C1CTC4, CTC positive versus negative patients at T1 with CTCs ≥4.
Discussion
In CRC, blood samples obtained via a liquid biopsy can be used to analyze CTCs, circulating tumor DNA and exosomes, thereby providing valuable additional information about the tumor.26 Making CTC detection part of routine clinical practice could aid in decision making in terms of systemic oncological therapies in metastatic CRC patients, and the direct molecular analysis of CTCs has been proposed as a means of identifying therapeutic resistance.27
After a systematic search for published research available on PubMed based on PRISMA criteria, we found a small number of studies correlating flow cytometry CTC identification in CRC with different surgical moments.17–23 In all of these studies, CTC detection was performed using between 7.5 and 10 mL of peripheral blood, resulting in the identification of 0 to 9 CTCs, and the patients with >3 CTCs were considered CTC-positive.17–23 Flow cytometry in CTC detection takes up to 2 hours; therefore, it is time consuming compared with the fast and affordable high-sensitivity techniques used for the detection of other rare cell types. A new protocol based on a CTC identification procedure taking less than one hour has recently been reported.28
In our study, we used 4 mL of blood per sample collected at two distinct surgical moments to evaluate the influence of intraoperative tumor manipulation on the number of CTCs when the no-touch technique was performed. The sample size of 4 mL was decided based on the technical validation performed in our lab for CTC identification, and because patients were more likely to participate in the study if the amount of blood collected per sample was as small as possible. We also performed statistical analysis for the total amount of CTCs identified in 8 mL of blood in each patient, but no statistical results were obtained.
The correlations between the type of surgery performed and the number of CTCs were based on the fact that in CRC, primary vascular ligation and the no-touch procedure are standards of practice to reduce tumor dissemination. Our final results did not reveal any statistical differences in CTC numbers at the time of skin incision or after vascular ligation. Because of the tumor stages in our patient group, no patient could be operated on using an entirely laparoscopic approach. Therefore, the impact of minimal tumor manipulation could not be evaluated.
Based on the prognostic value of CTCs in CRC, changes to patient management were proposed, taking into consideration CTC identification before and after one round of chemotherapy prior to surgery but without compelling results in terms of survival.29
The persistence of CTCs in peripheral blood three days after surgery can predict tumor recurrence and patient survival in stage II to III colon cancer, and if included in statistical prognostic models, CTCs can be useful to identify patients at high risk of recurrence and justify a more aggressive treatment with improved survival.30
During the resection of liver metastases in CRC, patients with CTC-positive preoperative samples had a relapse rate of 100% compared with a 65% relapse rate in negative patients. In addition, DFS and OS were poorer in the CTC-positive group.31 Moreover, patients with liver metastases with R0 (macroscopically)/R1 (microscopically) resection margins and circulating tumor DNA before surgery had a shorter OS compared with patients without CTCs identified preoperatively.32 In our cohort, metastatic disease was not associated with CTC-positive patients, possibly due to the reduced number of patients with stage IV disease included in the analysis.
In studies that focused on sex differences in CRC patients, female sex hormones were found to decrease the risk of colon cancer in premenopausal and postmenopausal women when hormonal replacement therapy was used, thereby providing a protective effect and better survival compared with men.33 In our cohort, the statistical correlation between the female sex and CTCs at T2 was significant in both univariate and multivariate analyses, and this advantageous hormonal status may be a possible explanation.
Regarding the impact of tumor localization, significant differences between the CTC numbers in right versus left colon cancers were reported in one study, with higher numbers identified in the former, the highest prognostic impact with reduced time to progression for left-sided tumors34 and no differences compared with rectal cancer. In rectal cancer, the analysis of CTCs can be used to evaluate the complete pathologic response, and the expression of specific proteins in CTCs can play an important role in predicting resistance to neoadjuvant radiochemotherapy in patients with locally advanced rectal cancer, allowing for the non-surgical management of patients with complete pathologic response.35
In our study, only the presence of tumors in the colon was statistically correlated with T2 CTC positivity in the univariate, and especially, in the multivariate analyses. No statistical differences were observed in terms of CTC numbers and tumor localization on colon topography, possibly due to a reduced number of patients with tumors in each location. However, the response to neoadjuvant therapy in rectal cancer could not be evaluated because only a small number of patients who received this type of treatment were included.
In addition to the above, it has been reported that CTC presence is significantly associated with lymphatic invasion, T stage, distant metastasis, TNM stage and tumor de-differentiation (all p<0.05),36 and specifically, the presence of CTCs before chemotherapy is associated with reduced PFS (34.8 vs 53.6 months) and OS (36.2 vs. 61.6 months).37 In our study, at a cut-off of >4 CTCs, only vascular invasion as noted in the pathological reports was statistically associated with T2 CTC-positive patients, which is consistent with the results reported in other studies.38 An explanation for this association may that angiogenesis is essential for tumor growth, and vascular invasion represents a progression pathway with CTC dissemination and a high risk for distant metastasis.
In terms of survival, there were no significant differences between the OS of T2 CTC-positive versus CTC-negative patients. However, this should be interpreted with caution because of the relatively short follow-up of patients and their unexpected long-term survival, with the median survival not being able to be accurately determined from Kaplan–Meier curves.
Regarding the involvement of the lymphatic system, the assessment of micrometastasis in sentinel lymph nodes in CRC combined with CTC identification in peripheral blood can help identify patients at high risk of relapse, for whom adjuvant chemotherapy may be beneficial.39 In our study, the presence of metastasis in more than one lymph node was statistically correlated with CTC positivity at T2 in the univariate analysis.
The assessment of peripheral blood elements, such as CTCs, circulating tumor DNA, microRNAs or long non-coding RNAs, using a liquid biopsy may improve both screening and diagnosis in CRC with significant value in predicting recurrence, metastasis and chemotherapy resistance and minimal residual disease evaluation.40
As part of a liquid biopsy, exosomes are considered important biomarkers because they play a role in tumor progression and metastasis and are carriers for the genetic material in primary tumor cells.26
Conclusions
In the future, the widespread application of a liquid biopsy can be achieved after standardization of the technique and validation in clinical trials with high reproducibility of the results. Personalized medicine based on genetic information obtained through a liquid biopsy could change clinical practice and the management of patients with multiple neoplasms, including CRC. The better stratification of patients in the same tumor stage may allow us to identify those at higher risk of relapse who require more aggressive therapy.
In our study, CTCs were correlated with the female sex, colon cancer, vascular invasion and positive lymph nodes. Surgical manipulation appeared to have no influence on CTC numbers, and no statistical differences were found between the time of skin incision and specimen removal during surgery. Flow cytometry was a feasible and robust technique for CTC identification in CRC, but it requires a competent professional. Overall, CTCs have the potential to serve as a prognostic and predictive factor, but research is still needed for procedural validation and inclusion in routine clinical practice. The main limitations of our study are the small size of the cohort and the relatively short follow-up period, which prevented us from providing estimates on the relationship between CTCs and patient survival.
Footnotes
Declaration of conflicting interest: The authors declare that there is no conflict of interest.
Funding: This scientific study was financed by the “Grigore T. Popa” University of Medicine and Pharmacy Iasi as part of a research contract (no. 29028/28.12.2016).
ORCID iD: Dragoș-Viorel Scripcariu https://orcid.org/0000-0002-9945-6328
References
- 1.Lampignano R, Schneck H, Neumann M, et al. Enrichment, isolation and molecular characterization of EpCAM-begative circulating tumor Cells. Adv Exp Med Biol 2017; 994: 181–203. [DOI] [PubMed] [Google Scholar]
- 2.Nadal C, Winder T, Gerger A, et al. Future perspectives of circulating tumor DNA in colorectal cancer. Tumour Biol 2017; 39: 1010428317705749. [DOI] [PubMed] [Google Scholar]
- 3.Mavroudis D.Circulating cancer cells. Ann Oncol 2010; 21: vii95–vii100. [DOI] [PubMed] [Google Scholar]
- 4.Duda DG, Cohen KS, Scadden DT, et al. A protocol for phenotypic detection and enumeration of circulating endothelial cells and circulating progenitor cells in human blood. Nat Protoc 2007; 2: 805–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mund JA, Estes ML, Yoder MC, et al. Flow cytometric identification and functional characterization of immature and mature circulating. Arterioscler Thromb Vasc Biol 2012; 32: 1045–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Torino F, Bonmassar E, Bonmassar L, et al. Circulating tumor cells in colorectal cancer patients. Cancer Treat Rev 2013; 39: 759–772. [DOI] [PubMed] [Google Scholar]
- 7.Sundling KE, Lowe AC.Circulating tumor cells: overview and opportunities in cytology. Adv Anat Pathol 2019; 26: 56–63. [DOI] [PubMed] [Google Scholar]
- 8.Huang X, Gao P, Song Y, et al. Relationship between circulating tumor cells and tumor response in colorectal cancer patients treated with chemotherapy: a meta-analysis. BMC Cancer 2014; 14: 976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wind J, Tuynman JB, Tibbe AGJ, et al. Circulating tumour cells during laparoscopic and open surgery for primary colonic cancer in portal and peripheral blood. Eur J Surg Oncol 2009; 35: 942–950. [DOI] [PubMed] [Google Scholar]
- 10.Rahbari NN, Aigner M, Thorlund K, et al. Meta-analysis shows that detection of circulating tumor cells indicates poor prognosis in patients with colorectal cancer. Gastroenterology 2010; 138: 1714–1726. [DOI] [PubMed] [Google Scholar]
- 11.Peach G, Kim C, Zacharakis E, et al. Prognostic significance of circulating tumour cells following surgical resection of colorectal cancers: a systematic review. Br J Cancer 2010; 102: 1327–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tan Y, Wu H.The significant prognostic value of circulating tumor cells in colorectal cancer: A systematic review and meta-analysis. Curr Probl Cancer 2018; 42: 95–106. [DOI] [PubMed] [Google Scholar]
- 13.Katsuno H, Zacharakis E, Aziz O, et al. Does the presence of circulating tumor cells in the venous drainage of curative colorectal cancer resections determine prognosis? A meta-analysis. Ann Surg Oncol 2008; 15: 3083–3091. [DOI] [PubMed] [Google Scholar]
- 14.Lu Y, Wang P, Peng J, et al. Meta-analysis reveals the prognostic value of circulating tumour cells detected in the peripheral blood in patients with non-metastatic colorectal cancer. Sci Rep 2017; 7: 905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang X, Gao P, Song Y, et al. Meta-analysis of the prognostic value of circulating tumor cells detected with the CellSearch System in colorectal cancer. BMC Cancer 2015; 15: 202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lu Y, Liang H, Yu T, et al. Isolation and characterization of living circulating tumor cells in patients by immunomagnetic negative enrichment coupled with flow cytometry. Cancer 2015; 121: 3036–3045. [DOI] [PubMed] [Google Scholar]
- 17.Tsavellas G, Huang A, Mccullough T, et al. Flow cytometry correlates with RT-PCR for detection of spiked but not circulating colorectal cancer cells. Clin Exp Metastasis 2002; 19: 495–502. [DOI] [PubMed] [Google Scholar]
- 18.Cohen SJ, Alpaugh RK, Gross S, et al. Isolation and characterization of circulating tumor cells in patients with metastatic colorectal cancer. Clin Colorectal Cancer 2006; 6: 125–132. [DOI] [PubMed] [Google Scholar]
- 19.Galizia G, Gemei M, Orditura M, et al. Postoperative detection of circulating tumor cells predicts tumor recurrence in colorectal cancer patients. J Gastrointest Surg 2013; 17: 1809–1818. [DOI] [PubMed] [Google Scholar]
- 20.Tseng J, Yang C, Liang S, et al. Interleukin-17A modulates circulating tumor cells in tumor draining vein of colorectal cancers and affects metastases. Clin Cancer Res 2014; 20: 2885–2897. [DOI] [PubMed] [Google Scholar]
- 21.Tralhão JG, Hoti E, Serôdio M, et al. Perioperative tumor cell dissemination in patients with primary or metastatic colorectal cancer. Eur J Surg Oncol 2010; 36: 125–129. [DOI] [PubMed] [Google Scholar]
- 22.Bahnassy AA, Salem SE, Mohanad M, et al. Prognostic significance of circulating tumor cells (CTCs) in Egyptian non-metastatic colorectal cancer patients: A comparative study for four different techniques of detection (Flow cytometry, CellSearch, Quantitative Real-time PCR and Cytomorphology). Exp Mol Pathol 2019; 106: 90–101. [DOI] [PubMed] [Google Scholar]
- 23.Fang C, Fan C, Wang C, et al. Prognostic value of CD133+ CD54+ CD44+ circulating tumor cells in colorectal cancer with liver metastasis. Cancer Med 2017; 6: 2850–2857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Flores-Montero J, Sanoja-Flores L, Paiva B, et al. Next generation flow for highly sensitive and standardized detection of minimal residual disease in multiple myeloma. Leukemia 2017; 31: 2094–2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Theunissen P, Mejstrikova E, Sedek L, et al. Standardized flow cytometry for highly sensitive MRD measurements in B-cell acute lymphoblastic leukemia. Blood 2017; 129: 347–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Scripcariu V, Scripcariu DV, Filip B, et al. “Liquid biopsy” - Is it a feasible option in colorectal cancer? Chirurgia (Bucur) 2019; 114: 162–166. [DOI] [PubMed] [Google Scholar]
- 27.Marcuello M, Vymetalkova V, Neves RPL, et al. Circulating biomarkers for early detection and clinical management of colorectal cancer. Mol Aspects Med 2019; 69: 107–122. [DOI] [PubMed] [Google Scholar]
- 28.Lopresti A, Malergue F, Bertucci F, et al. Sensitive and easy screening for circulating tumor cells by flow cytometry. JCI Insight 2019; 5: pii: 128180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rothé F, Maetens M, Rouas G, et al. CTCs as a prognostic and predictive biomarker for stage II/III Colon Cancer: A companion study to the PePiTA trial. BMC Cancer 2019; 19: 304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang D, Yang Y, Jin L, et al. Prognostic models based on postoperative circulating tumor cells can predict poor tumor recurrence-free survival in patients with stage II-III colorectal cancer. J Cancer 2019; 10: 4552–4563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arrazubi V, Mata E, Antelo ML, et al. Circulating tumor cells in patients undergoing resection of colorectal cancer liver metastases. Clinical utility for long-term outcome: A prospective trial. Ann Surg Oncol 2019; 26: 2805–2811. [DOI] [PubMed] [Google Scholar]
- 32.Bidard FC, Kiavue N, Ychou M, et al. Circulating tumor cells and circulating tumor DNA detection in potentially resectable metastatic colorectal cancer: a prospective ancillary study to the Unicancer Prodige-14 Trial. Cells 2019; 8: 516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Majek O, Gondos A, Jansen L, et al. Sex differences in colorectal cancer survival: population-based analysis of 164,996 colorectal cancer patients in Germany. PLoS One 2013; 8: e68077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nicolazzo C, Raimondi C, Gradilone A, et al. Circulating tumor cells in right-and left-sided colorectal cancer. Cancers (Basel) 2019; 11: 1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Troncarelli Flores BC, Souza e Silva V, Ali Abdallah E, et al. Molecular and kinetic analyses of circulating tumor cells as predictive markers of treatment response in locally advanced rectal cancer patients. Cells 2019; 8: 641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang L, Zhou S, Zhang W, et al. Circulating tumor cells as an independent prognostic factor in advanced colorectal cancer: a retrospective study in 121 patients. Int J Colorectal Dis 2019; 34: 589–597. [DOI] [PubMed] [Google Scholar]
- 37.Romiti A, Raffa S, Di Rocco R, et al. Circulating tumor cells count predicts survival in colorectal cancer patients. J Gastrointestin Liver Dis 2014; 23: 279–284. [DOI] [PubMed] [Google Scholar]
- 38.Baek DH, Kim GH, Song GA, et al. Clinical potential of circulating tumor cells in colorectal cancer: A prospective study. Clin Transl Gastroenterol 2019; 10: e00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Koyanagi K, Bilchik AJ, Saha S, et al. Prognostic relevance of occult nodal micrometastases and circulating tumor cells in colorectal cancer in a prospective multicenter trial. Clin Cancer Res 2008; 14: 7391–7396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vacante M, Ciuni R, Basile F, et al. The liquid biopsy in the management of colorectal cancer: an overview. Biomedicines 2020; 8: 308. [DOI] [PMC free article] [PubMed] [Google Scholar]