Abstract
Objectives
Chronic venous disease (CVeD) is a multifactorial and debilitating condition that has a high prevalence in Western countries and an important associated socioeconomic burden. Varicose veins (VVs) are the most common manifestations of CVeD. Pathologically, many morphological and functional changes have been described in VVs, which most notably affect venous wall integrity. Previous studies have found several molecular alterations that negatively affect normal cell signaling pathways. Insulin receptor substrate (IRS)-4 is a central adaptor protein that is closely related to insulin/insulin-like growth factor-1 signaling upstream, phosphatidylinositol 3-kinase/Akt or mitogen-activated protein kinases downstream, and other proteins. These molecular pathways have been implicated in CVeD pathogenesis. Thus, the aim of our study was to identify the role of IRS-4 in VV tissue.
Methods
We conducted a histopathological study to analyze IRS-4 protein expression in CVeD patients compared with healthy controls.
Results
Our results demonstrate a significant increase in IRS-4 expression in VV tissue.
Conclusions
IRS-4 may be implicated in CVeD development and progression. Therefore, IRS-4 could be a potential diagnostic or therapeutic target for patients with this condition.
Keywords: Chronic venous disease, insulin receptor substrate-4, varicose vein, insulin-like growth factor/phosphatidylinositol 3-kinase signaling, insulin, mitogen-activated protein kinase, therapeutic target, diagnostic
Introduction
Chronic venous disease (CVeD) is a debilitating multifactorial venous condition that primarily affects the lower limbs. CVeD is also involved in many metabolic disorders and hemodynamic changes, including during pregnancy, and it can negatively affect a patient’s quality of life.1,2 Clinical manifestations of CVeD include varicose veins (VVs), telangiectasias, and reticular veins, and severe clinical manifestations, such as edema, lipodermatosclerosis, and venous ulceration. In the early stages of CVeD, symptoms usually go unnoticed by patients. Despite its underdiagnosis, CVeD is the most common vascular disease in humans with an estimated prevalence of 60% to 70%.3 Consequently, this disease places a significant economic burden on healthcare systems.4
Among the most important risk factors for CVeD are advanced age, obesity, female sex, pregnancy, family history, and taller stature.5–7 Both genetic and environmental factors contribute to changes in the biophysical properties of veins.8
In VV pathology, prolonged venous hypertension and valvular incompetence is thought to induce a series of signaling processes, including immune cell activation and proinflammatory cytokine release,9,10 which result in venous wall remodeling by stress regulators.11 Another school of thought hypothesizes that changes in the venous wall may precede these events. Several alternative hypotheses about the etiology of CVeD have been proposed, including exposure to hypoxia and profound extracellular matrix remodeling.12,13 All of these etiologies could promote important disruptions in cell signaling that could lead to venous arterialization as well as smooth muscle cell hypertrophy and hyperplasia in VVs.14 In summary, several mechanisms appear to be involved in the cellular processes that result in CVeD pathology. Effectively, insulin receptor substrate (IRS)-4 may be involved in the above changes because it is an important modulator of several cellular pathways and metabolic events.15–17
IRS-4, similar to other insulin receptor substrates (IRS-1 to IRS-6), acts as an adaptor protein that mediates cell signaling from the insulin receptor (IR) and insulin-like growth factor (IGF)-1 receptor (IGF-1R). Both insulin and IGF-1 regulate IRS-4 expression through activation of IR/IGF-1R. IRS-4 is expressed in various tissue types, but its expression in healthy tissue is maintained at low levels.18 Consequently, altered IRS-4 expression may be associated with cellular disturbances, such as impaired growth and glucose dysmetabolism.19 Thus, histopathological studies of IRS-4 have demonstrated its promise because IRS-4 overexpression could be used as a prognostic tool20 or as a therapeutic target.21 The aim of this study was to evaluate the role of IRS-4 in venous wall changes in patients with CVeD using immunohistochemistry (IHQ) techniques.
Patients and methods
Study design
Patients with a clinical diagnosis of CVeD were enrolled into this cross-sectional study and divided into the CVeD group and the healthy venous (HV) group.
The inclusion criteria were as follows: women and men diagnosed with CVeD; body mass index (BMI) less than or equal to 25 kg/m2; with and without venous reflux in the great saphenous vein; signed informed consent; and commitment to undergo follow-up during the pre- and postoperative periods and to provide tissue samples. The exclusion criteria were as follows: patients with venous malformations or arterial insufficiency; patients without access to their clinical history; pathologies that could affect the cardiovascular system (infectious diseases, diabetes, hypertension, and dyslipidemia); patients with toxic habits; and those who were uncertain of participating in the continued monitoring. The clinical diagnosis of CVeD and the evaluation of venous reflux were based on a noninvasive color Doppler ultrasound (7.5–10 MHz) of the superficial and deep vein systems. The Classification System for Chronic Venous Disorders (CEAP), which is based on clinical, etiologic, anatomic, and pathophysiologic data, was applied previously to the venous extraction.22 All patients had a CEAP score ≥1 (C1, n = 9; C2, n = 19; C3, n = 7). Saphenous vein segments for the HV group were verified during organ extraction for bypass surgery without CVeD, and the same section was used as in the CVeD group.
This study was conducted in accordance with basic ethical principles (autonomy, harmlessness, beneficence, and distributive justice); its development followed the standards of Good Clinical Practice and the principles defined in the Declaration of Helsinki (2013) and the Oviedo Convention (1997). The collected data and information complied with the current legislation on data protection (Organic Law 3/5 December 2018 on the Protection of Personal Data and the Guarantee of Digital Rights and Regulation (EU) 2016/679). This project (FIS-PI18/00912) was approved (March 2017) by the Clinical Research Ethics Committee of the Gómez-Ulla-UAH Defence Hospital (37/17). Written informed consent was provided by all patients who were enrolled in this study.
Sample processing
Following saphenectomy, the entire great saphenous vein was removed. Fragments of the extracted vein were placed into two sterile tubes, one containing a solution of RNAlater (Ambion, Austin, TX, USA) and the other containing minimum essential medium (MEM) with 1% antibiotic/antimycotic solution (Thermo Fisher Scientific, Waltham, MA, USA). The samples were processed under a Telstar AV 30/70 Müller class II 220V 50 MHz laminar flow hood (Grupo Telstar SA, Terrasa, Spain) in a sterile environment. The samples incubated in the RNAlater solution were stored at −80°C until they were used in gene expression analyses. The samples incubated in the MEM solution were used immediately for histological analyses.
Samples were washed and hydrated several times with antibiotic-free MEM to eliminate erythrocytes and isolate venous tissue. The samples were cut into fragments and fixed in F13 (60% ethanol, 20% methanol, 7% polyethylene glycol, and 13% distilled water). After the necessary fixation time, the samples were dehydrated in a series of graded ethanol baths and embedded in paraffin. Once the wax had infiltrated the tissue, the paraffin block was formed. Next, the tissue-embedded paraffin blocks were cut into fine slices that were 5 μm thick using an HM 350 S rotary microtome (Thermo Fisher Scientific). The slices were then mounted on slides that were treated with a 10% polylysine solution to ensure tissue adherence to the slide.23
Immunohistochemistry
The antigen–antibody reaction was detected using the avidin–biotin complex method with peroxidase or alkaline phosphatase as a chromogen, in accordance with the protocol described by Ortega et al.24 Non-specific binding sites were blocked by incubation with 3% bovine serum albumin (BSA) blocking solution in phosphate-buffered saline (PBS) for 30 minutes at room temperature. Samples were then incubated overnight with the primary antibody diluted in 3% BSA and PBS at 4°C (Table 1b). Next, samples were incubated with biotin-bound secondary antibody diluted in PBS for 90 minutes at room temperature (Table 1c).
Table 1.
a. Primer sequences used in quantitative reverse transcription polymerase chain reaction and temperature. Primary (b) and secondary (c) antibody used in immunohistochemical studies that were performed using the dilution and protocol specifications shown below.
| a | |||||||
| Gene | Forward sequence (5ʹ→3ʹ) | Reverse sequence (5ʹ→3ʹ) | Tm | ||||
| GAPDH | GGAAGGTGAAGGTCGGAGTCA | GTCATTGATGGCAATATCCACT | 60°C | ||||
| IRS-4 | CCCACACATGACCAGAGAGA | CTGACTGTCTGGGTTCAGCA | 61°C | ||||
| b | |||||||
| Antigen | Species | Dilution | Supplier | Protocol specifications | |||
| IRS-4 | Rabbit | 1.50 | Abcam (ab5262) | Triton 0.1% in PBS, 10 minutes, before incubation with blocking solution | |||
| c | |||||||
| Antigen | Species Dilution | Dilution | Supplier | Protocol specifications | |||
| IgG (Rabbit) | Mouse | 1:1000 | Sigma (RG-96/B5283) | — | |||
Tm, temperature; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; IRS-4, insulin receptor substrate; IgG, immunoglobulin G; PBS, phosphate-buffered saline; —, no specifications.
Samples were then incubated with the avidin–peroxidase conjugate ExtrAvidin®-Peroxidase (Sigma-Aldrich, St. Louis, MO, USA) diluted 1:200 in PBS for 60 minutes at room temperature, followed by incubation with the chromogenic substrate diaminobenzidine (DAB) (Kit DAB, SK-4100; Vector Laboratories, Burlingame, CA, USA). The chromogenic substrate was prepared immediately before exposure (5 mL of distilled water, two drops of buffer, four drops of DAB, and two drops of hydrogen peroxide). This technique resulted in a brown-colored stain. To obtain contrast with the nucleus staining, samples were incubated with Carazzi hematoxylin for 5 to 15 minutes. Samples were then mounted in an aqueous medium using plasdone. In the IHQ assay of each sample, sections from the same tissue were used as a negative control where the primary solution was replaced with blocking solution.
Microscopy
The prepared samples were visualized using a Zeiss Axiophot light microscope (Carl Zeiss, Oberkochen, Germany) equipped with an AxioCam HRc digital camera (Carl Zeiss). Given the biological importance of the proteins of interest, histological results were evaluated using the expression intensity for the IHQ staining on a scale of 1 to 3. Thus, patient histological samples were classified as negative (0) or positive (1–3) on the basis of the IRS-Score method.21 For each established group of subjects, seven microscopic fields were randomly selected and examined in each of the five sections. Sample observation and quantification was performed independently by two investigators.
Gene expression analyses
Expression of the gene of interest was studied using quantitative reverse transcription polymerase chain reaction (RT-qPCR). The amount of IRS-4 cDNA (Thermo Fisher Scientific) was quantified in each sample. RNA extraction was performed using the guanidine–phenol–chloroform isothiocyanate method, as described by Ortega et al.25
The primers that were used were designed using the Primer-BLAST tool and the Auto-Dimer application.26 The StepOnePlus™ system with the relative standard curve method was used to perform qPCR. Five microliters of each sample, which had been previously diluted with nuclease-free water, were mixed with 10 µL of intercalating agent iQ™ SYBR® Green Supermix (Bio-Rad Laboratories, Hercules, CA, USA), 1 µL of forward primer, 1 µL of reverse primer, and 3 µL of DNase and RNase-free water. The 20-µL solutions were analyzed in a MicroAmp® 96-well plate (Applied Biosystems-Life Technologies, Foster City, CA, USA). The final results were normalized and compared with the housekeeping gene, glyceraldehyde 3-phosphate dehydrogenase (GAPDH; Table 1a). The data obtained for each gene were interpolated using the standard curve. The samples were tested in triplicate, the standard curve was tested in duplicate, and the remaining two wells were filled with negative controls.
Statistical analysis and interpretation
For the statistical analysis, the Mann–Whitney U test was performed using GraphPad Prism® 5.1 software (Graphpad Software, San Diego, CA, USA). The data are provided as the median with the interquartile range (IQR). Error bars in the figures represent the IQR. Additionally, p < 0.05 was considered to be significant, and values were identified as significant at *p < 0.05, **p < 0.005, and ***p < 0.001.
Results
There were 35 patients with a clinical diagnosis of CVeD (median age, 47.0 years; IQR, 27.0–68.0 years) and 27 HV control patients (median age, 45.0 years; IQR, 23.0–66.0 years) who were enrolled into this cross-sectional study.
IRS-4 mRNA expression
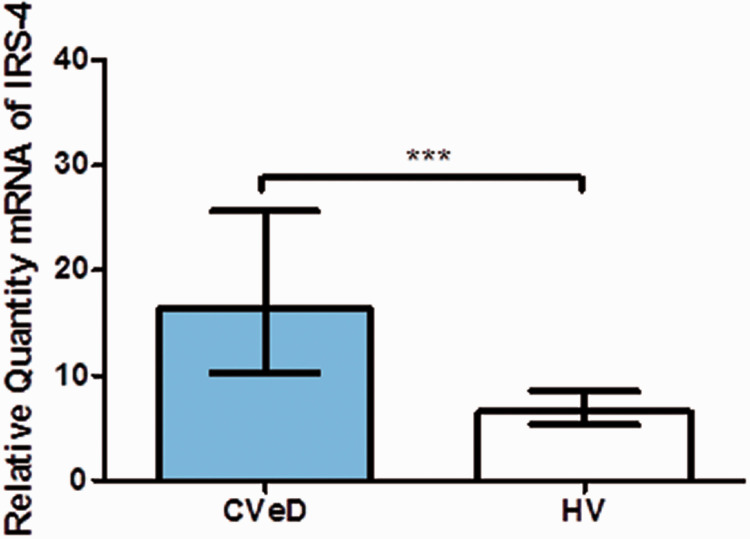
IRS-4 gene expression analysis showed a significant difference between the two study groups. The median IRS-4 mRNA expression in the CVeD group was higher (16.360 [4.695–37.157]) than that of the HV group (6.565 [1.270–21.010]; p < 0.001; Figure 1).
Figure 1.
IRS-4 mRNA expression in CVeD and HV in arbitrary units. ***p < 0.0001.
IRS-4, insulin receptor substrate; CVeD, chronic venous disease; HV, healthy venous controls.
IRS-4 protein levels
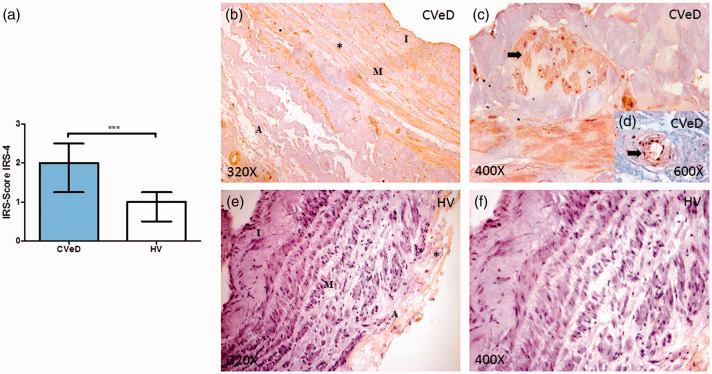
Protein expression was studied using IHQ techniques and the IRS-Score method, which also revealed a significant difference between the experimental groups. CVeD patients had significantly higher IRS-4 protein levels (2.000 [0.750–3.000]) compared with controls (1.000 [0.250–2.500]; p < 0.0001; Figure 2a). Microscopic analysis revealed that in CVeD patients, IRS-4 was localized in the three venous wall layers (Figure 2b, asterisk). However, IRS-4 expression in the tunica adventitia of vascular structures, such as arterioles, was pronounced in the CVeD group (Figure 2d, arrow) while low IRS-4 expression was shown in the tunica adventitia of the venous wall in the HV group (Figure 2e). In most of the HV samples, IRS-4 expression was not observed in the tunica media (Figure 2f). Furthermore, IRS-4 protein was also expressed in the venous wall smooth muscle (Figure 2c, arrow).
Figure 2.
(a) IRS-4 protein expression level score in the venous wall of patients with CVeD and HV. (b) Histological image showing IRS-4 protein expression in the three venous wall layers of CVeD patients. (c) Histological image showing IRS-4 protein expression in smooth muscle cells from CVeD patients. (e) Histological image showing IRS-4 protein expression in an arteriole from the tunica adventitia of CVeD patients. (e–f) Histological images showing limited IRS-4 protein expression in the tunica adventitia in healthy tissue from HV controls. ***p < 0.0001.
IRS-4, insulin receptor substrate; CVeD, chronic venous disease; HV, healthy venous controls; I, tunica intima; M, tunic media; A, tunica adventitia.
Discussion
CVeD is a highly prevalent and debilitating condition that encompasses a wide variety of molecular alterations in the venous wall.27,28 Our work is the first to demonstrate increased IRS-4 expression in VVs and implicate IRS-4 in CVeD pathophysiology.
IRS-4 is a central adaptor protein that is activated by insulin/IGF-1 binding to cellular receptors. Consistent with our results, previous studies have described a noteworthy increase of IGF-1 and IGF-1R in venous walls in CVeD patients.29 Previously, we demonstrated that abnormal expression of pregnancy-associated plasma protein A (PAPP-A) and stanniocalcin 2 (STC-2) results in increased IGF-1 bioavailability in tissues. This evidence supports the importance of IGF-1/IGF-1R in the pathogenesis of CVeD.30 IRS-4 may also negatively regulate the expression of other IRS-family proteins via competitive inhibition.31,32 Thus, IRS-4 hyperactivation could be a downstream effect of IGF-1/IGF-1R binding that occurs in the venous wall of CVeD patients.
IRS-4 is crucial for the activation of multiple downstream effectors, and it could be described as following a unique and specific signaling pathway.33 Phosphatidylinositol 3-kinase (PI3K) is the best characterized molecule that is related to IRS-4 and its functionality. Previous research has described the important role of IRS-4 in PI3K activation, even in the absence of upstream effectors.34 Abnormal IRS-4/PI3K activation has been associated with increased proliferation and cellular alterations in pathological conditions,35 and some authors have proposed IRS-4 as a potential molecular target.36 In previous research, we elucidated important alterations in PI3K/Akt and mitogen-activated protein kinase (MAPK) pathways in the venous wall of CVeD patients. The aberrant signaling of these pathways was strongly associated with premature tissue aging, which was accompanied by increased hypoxia and inflammation.37 IRS-4 was also associated with MAPK pathway upregulation.38 In human skeletal muscle cells, the IRS4 mechanism of action is very different compared with both IRS-1 and IRS-2, which are rapidly phosphorylated on tyrosine in response to insulin.39 In human myoblasts, no tyrosine phosphorylation of IRS-4 was observed in response to either insulin or IGF-I, but a sustained increase in IRS-4 tyrosine phosphorylation was observed in myocytes that were subjected to osmotic stress. This suggests that there is an important role for IRS-4 in stress situations in muscle cells. Furthermore, high MAPK pathway activation has been associated with the advanced stages of CVeD,27,40 and thus, it is probable that IRS-4 is involved in the pathogenesis of this condition. This may explain how tunica adventitia structures such as arterioles show high IRS-4 expression levels. Several studies have shown how proteins that are related to the MAPK pathway are expressed in vascular structures in the adventitial tunica, which could be explained by the damage that is caused by factors such as mechanical pressure.30,37,40,41
Conclusions
Our results reveal a physiological role for IRS-4 in CVeD pathogenesis. The data suggest that IRS-4 acts as an important adaptor protein in the venous wall, forming a complex pathway involving IGF-1/PAPP-A/STC-2 as upstream effectors and the PI3K and MAPK pathways as downstream effectors. Further studies are required to clarify additional regulatory components that are modulated by IRS-4. However, our study provides the initial data to support the possible use of IRS-4 targeting to restrict its various downstream effectors and thereby limit CVeD progression.
Footnotes
Informed consent statement: Written informed consent was obtained from all subjects involved in the study.
Institutional review board statement: This study was conducted in accordance with the guidelines of the Declaration of Helsinki and approved by the Clinical Research Ethics Committee of the Central University Hospital of Defense-UAH (37/17).
Data availability statement: The data used to support the findings of the present study are available from the corresponding author upon request.
Declaration of conflicting interest: The authors declare that there is no conflict of interest.
Funding: The authors disclosed receipt of the following financial support for the research, authorship and/or publication of this article: This study (FIS-PI18/00912) was supported by the Instituto de Salud Carlos III (grant no. Estatal de I + D + I 2013–2016) and co-financed by the European Development Regional Fund “A way to achieve Europe” and B2017/BMD-3804 MITIC-CM (Comunidad de Madrid). Halekulani S.L. Oscar Fraile-Martinez had a predoctoral fellowship from the University of Alcalá throughout this work (522505).
ORCID iDs: Miguel A Ortega https://orcid.org/0000-0003-2588-1708
Silve Barrena https://orcid.org/0000-0003-2715-1979
References
- 1.Jacobs BN, Andraska EA, Obi AT, et al. Pathophysiology of varicose veins. J Vasc Sur Venous Lymphat Disord 2017; 5: 460–467. doi:10.1016/j.jvsv.2016.12.014. [DOI] [PubMed] [Google Scholar]
- 2.Ortega MA, Asúnsolo Á, Álvarez-Rocha MJ, et al. Remodelling of collagen fibres in the placentas of women with venous insufficiency during pregnancy. Histol Histopathol 2018; 33: 567–576. doi:10.14670/HH-11-948. [DOI] [PubMed] [Google Scholar]
- 3.Raffetto JD, Mannello F.Pathophysiology of chronic venous disease. Int Angiol a J Int Union Angiol 2014; 33: 212–221. [PubMed] [Google Scholar]
- 4.Nicolaides AN, Labropoulos N.Burden and suffering in chronic venous disease. Adv Ther 2019; 36(Suppl 1): 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fukaya E, Flores AM, Lindholm D, et al. Clinical and genetic determinants of varicose veins: prospective, community-based study of ≈500 000 individuals. Circulation 2018; 138: 2869–2880. doi:10.1161/CIRCULATIONAHA.118.035584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Criqui MH, Denenberg JO, Bergan J, et al. Risk factors for chronic venous disease: the San Diego Population Study. J Vasc Surg 2007; 46: 331–337. doi:10.1016/j.jvs.2007.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.García-Honduvilla N, Asúnsolo Á, Ortega MA, et al. Increase and redistribution of sex hormone receptors in premenopausal women are associated with varicose vein remodelling. Oxid Med Cell Longev 2018; 2018: 3974026. doi:10.1155/2018/3974026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saberianpour S, Modaghegh MHS, Rahimi H, et al. Role of mechanosignaling on pathology of varicose vein. Biophys Rev 2021; 13: 139–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Castro-Ferreira R, Cardoso R, Leite-Moreira A, et al. The role of endothelial dysfunction and inflammation in chronic venous disease. Ann Vasc Surg 2018; 46: 380–393. [DOI] [PubMed] [Google Scholar]
- 10.Segiet OA, Brzozowa-Zasada M, Piecuch A, et al. Biomolecular mechanisms in varicose veins development. Ann Vasc Surg 2015; 29: 377–384. [DOI] [PubMed] [Google Scholar]
- 11.Pfisterer L, König G, Hecker M, et al. Pathogenese der varizenbildung – Lehren aus der biomechanik. Vasa - J Vasc Dis 2014; 43: 88–99. [DOI] [PubMed] [Google Scholar]
- 12.Somers P, Knaapen M.The histopathology of varicose vein disease. Angiology 2006; 57: 546–555. [DOI] [PubMed] [Google Scholar]
- 13.Horecka A, Hordyjewska A, Biernacka J, et al. Intense remodeling of extracellular matrix within the varicose vein: the role of gelatinases and vascular endothelial growth factor. Ir J Med Sci 2021; 190: 255–259. doi:10.1007/s11845-020-02289-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Surendran S, Ramegowda KS, Suresh A, et al. Arterialization and anomalous vein wall remodeling in varicose veins is associated with upregulated FoxC2-Dll4 pathway. Lab Investig 2016; 96: 399–408. doi:10.1038/labinvest.2015.167. [DOI] [PubMed] [Google Scholar]
- 15.Dörpholz G, Murgai A, Jatzlau J, et al. IRS4, a novel modulator of BMP/Smad and Akt signalling during early muscle differentiation. Sci Rep 2017; 7: 1–17. doi:10.1038/s41598-017-08676-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shaw LM.The insulin receptor substrate (IRS) proteins: at the intersection of metabolism and cancer. Cell Cycle 2011; 10: 1750–1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Qu BH, Karas M, Koval A, et al. Insulin receptor substrate-4 enhances insulin-like growth factor-I-induced cell proliferation. J Biol Chem 1999; 274: 31179–31184, doi:10.1074/jbc.274.44.31179. [DOI] [PubMed] [Google Scholar]
- 18.Sesti G, Federici M, Hribal ML, et al. Defects of the insulin receptor substrate (IRS) system in human metabolic disorders. FASEB J 2001; 15: 2099–2111. doi:10.1096/fj.01-0009rev. [DOI] [PubMed] [Google Scholar]
- 19.Fantin VR, Wang Q, Lienhard GE, et al. Mice lacking insulin receptor substrate 4 exhibit mild defects in growth, reproduction, and glucose homeostasis. Am J Physiol - Endocrinol Metab 2000; 278: E127–E133. doi:10.1152/ajpendo.2000.278.1.e127. [DOI] [PubMed] [Google Scholar]
- 20.Sanmartín-Salinas P, Toledo-Lobo MV, Noguerales-Fraguas F, et al. Overexpression of insulin receptor substrate-4 is correlated with clinical staging in colorectal cancer patients. J Mol Histol 2018; 49: 39–49. doi:10.1007/s10735-017-9745-0. [DOI] [PubMed] [Google Scholar]
- 21.Sanmartín-Salinas P, Lobo MDVT, Noguerales-Fraguas F, et al. Insulin receptor substrate-4 is overexpressed in colorectal cancer and promotes retinoblastoma–cyclin-dependent kinase activation. J Gastroenterol 2018; 53: 932–944. [DOI] [PubMed] [Google Scholar]
- 22.Lurie F, Passman M, Meisner M, et al. The 2020 update of the CEAP classification system and reporting standards. J Vasc Surg Venous Lymphat Disord 2020; 8: 342–352. doi: 10.1016/j.jvsv.2019.12.075 [DOI] [PubMed] [Google Scholar]
- 23.Ortega MA, Romero B, Asúnsolo Á, et al. Pregnancy-associated venous insufficiency course with placental and systemic oxidative stress. J Cell Mol Med 2020; 24: 4157–4170. doi: 10.1111/jcmm.15077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.García-Honduvilla N, Ortega MA, Asúnsolo Á, et al. Placentas from women with pregnancy-associated venous insufficiency show villi damage with evidence of hypoxic cellular stress. Hum Pathol 2018; 77: 45–53. doi: 10.1016/j.humpath.2018.03.022. [DOI] [PubMed] [Google Scholar]
- 25.Ortega MA, Saez MA, Fraile-Martínez O, et al. Increased angiogenesis and lymphangiogenesis in the placental villi of women with chronic venous disease during pregnancy. Int J Mol Sci 2020; 21: 2487. doi: 10.3390/ijms21072487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vallone PM, Butler JM.AutoDimer: a screening tool for primer-dimer and hairpin structures. Biotechniques 2004; 37: 226–231. doi: 10.2144/04372ST03. [DOI] [PubMed] [Google Scholar]
- 27.Ortega MA, Asúnsolo Á, Pekarek L, et al. Histopathological study of JNK in venous wall of patients with chronic venous insufficiency related to osteogenesis process. Int J Med Sci 2021; 18: 1921–1934. doi:10.7150/ijms.54052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ortega MA, Fraile-Martínez O, Pekarek L, et al. Defective expression of the peroxisome regulators PPARα receptors and lysogenesis with increased cellular senescence in the venous wall of chronic venous disorder. Histol Histopathol 2021; 18322. doi:10.14670/HH-18-322. [DOI] [PubMed] [Google Scholar]
- 29.Bruczko-Goralewska M, Romanowicz L, Baczyk J, et al. Peptide growth factors and their receptors in the vein wall. J Investig Med 2019; 67: 1149–1154. doi:10.1136/jim-2019-001075. [DOI] [PubMed] [Google Scholar]
- 30.Ortega MA, Fraile-Martínez O, Asúnsolo Á, et al. Chronic venous disease patients showed altered expression of IGF-1/PAPP-A/STC-2 axis in the vein wall. Biomed Res Int 2020; 2020: 6782659. doi:10.1155/2020/6782659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tsuruzoe K, Emkey R, Kriauciunas KM, et al. Insulin receptor substrate 3 (IRS-3) and IRS-4 impair IRS-1- and IRS-2-mediated signaling. Mol Cell Biol 2001; 21: 26–38. doi:10.1128/mcb.21.1.26-38.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ortega MA, Fraile-Martínez O, Saez MA, et al. Abnormal proinflammatory and stressor environmental with increased the regulatory cellular IGF-1/PAPP-A/STC and Wnt-1/β-Catenin canonical pathway in placenta of women with chronic venous disease during pregnancy. Int J Med Sci 2021; 18: 2814–2827. doi: 10.7150/ijms.58992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sanmartín-Salinas P, Guijarro LG.Overexpression of IRS-4 correlates with procaspase 3 levels in tumoural tissue of patients with colorectal cancer. J. Oncol 2018; 2018: 3812581. doi:10.1155/2018/3812581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hoxhaj G, Dissanayake K, MacKintosh C.Effect of IRS4 levels on PI 3-kinase signalling. PLoS One 2013; 8: e73327. doi:10.1371/journal.pone.0073327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xia Z, Zhang N, Ding D.Proliferation and migration of hepatoblastoma cells are mediated by IRS-4 via PI3K/Akt pathways. Int J Clin Exp Med 2014; 15: 3763–3769. [PMC free article] [PubMed] [Google Scholar]
- 36.Ikink GJ, Hilkens J.Insulin receptor substrate 4 (IRS4) is a constitutive active oncogenic driver collaborating with HER2 and causing therapeutic resistance. Mol Cell Oncol 2017; 4: e1279722. doi:10.1080/23723556.2017.1279722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ortega MA, Asúnsolo Á, Leal J, et al. Implication of the PI3K/Akt/mTOR pathway in the process of incompetent valves in patients with chronic venous insufficiency and the relationship with aging. Oxid Med Cell Longev 2018; 2018: 1495170. doi:10.1155/2018/1495170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cuevas EP, Escribano O, Chiloeches A, et al. Role of insulin receptor substrate-4 in IGF-I-stimulated HEPG2 proliferation. J Hepatol 2007; 46: 1089–1098. doi:10.1016/j.jhep.2007.01.031. [DOI] [PubMed] [Google Scholar]
- 39.Schreyer S, Ledwig D, Rakatzi I, et al. Insulin receptor substrate-4 is expressed in muscle tissue without acting as a substrate for the insulin receptor. Endocrinology 2003; 144: 1211–1218. doi: 10.1210/en.2002-220723. [DOI] [PubMed] [Google Scholar]
- 40.Ortega MA, Asúnsolo Á, Romero B, et al. Unravelling the role of mapks (erk1/2) in venous reflux in patients with chronic venous disorder. Cells Tissues Organs 2018; 206: 272–281. doi:10.1159/000500449. [DOI] [PubMed] [Google Scholar]
- 41.Ortega MA, Fraile-Martínez O, García-Montero C, et al. Tissue remodelling and increased DNA damage in patients with incompetent valves in chronic venous insufficiency. J Cell Mol Med 2021. doi: 10.1111/jcmm.16711. [DOI] [PMC free article] [PubMed] [Google Scholar]