Abstract
Q fever is a zoonotic disease caused by Coxiella burnetii. Most patients have non-specific symptoms at onset. In addition, routine diagnostic tests for C. burnetii are not sensitive, and the bacterium cannot grow in general culture medium. The diagnosis of Q fever therefore poses a challenge. This case study describes a man with a clear history of tick bite who had recurrent fever, pneumonia, and liver damage. Routine tests and bacterial cultures failed to clarify the pathogeny, but laboratory and imaging data suggested infection. After routine tests were exhausted, we detected the presence of C. burnetii in a whole blood sample using next-generation sequencing (NGS). To our knowledge, this is the first report of Q fever associated with Coxiella burnetii detected directly from blood samples in Lishui, China. NGS has revolutionized the diagnosis of infectious diseases, especially those caused by rare or newly discovered pathogens, and patient responses have finally proved its substantial benefits. NGS has important clinical significance for the early diagnosis of chronic Q fever. This proof-of-concept study is worthy of promotion in clinical practice.
Keywords: Coxiella burnetii, next-generation sequencing, Q fever, zoonosis, tick, infection, computed tomography
Introduction
In 1937, a fever of unknown cause was discovered in Queensland, Australia and was subsequently named Q fever. According to a seroepidemiological investigation, Q fever is distributed in all global regions excluding New Zealand,1 and it often occurs in pastoral, semi-agricultural, and semi-pastoral areas. Q fever is a natural tick-borne infectious disease caused by Coxiella burnetii. Q fever has a widespread prevalence, and it is listed as 1 of 13 global priority zoonoses. The most common route of infection is the inhalation of airborne particles or contaminated aerosols during processes such as parturition and slaughter.2 C. burnetii has large and small cell variants, both of which are infectious. The small cell variants are extremely stable in a variety of harsh environments.3 Organisms can be transmitted between animals without direct contact.4 Primary C. burnetii infection is asymptomatic or has only non-specific symptoms, such as recurrent fever, headache, night sweats, muscle soreness, and weight loss.5 Q fever can be classified as acute or chronic. Acute Q fever may be accompanied by atypical pneumonia or hepatitis. The incubation period for primary infection is 2 to 3 weeks before the onset of symptoms.6 The most common manifestations of chronic Q fever are endocarditis, granulomatous hepatitis, and osteomyelitis.7 Acute infection can progress to chronic Q fever, especially in the presence of immunodeficiency, pregnancy, or underlying heart disease. The disease is also widely recognized in patients with conditions associated with immunodeficiency, such as rheumatoid arthritis, systemic sclerosis, and non-Hodgkin lymphoma.8 Diagnosis is often delayed or missed because of its non-specific symptoms. Acute symptoms are quickly relieved after appropriate antibiotic treatment, whereas chronic disease requires long-term antibiotic treatment and disease monitoring. Chronic Q fever has a poor prognosis, and its mortality rate after 10 years is 10% to 20% when patients are treated.9
C. burnetii exhibits pantropism, being capable of invading the heart, lungs, liver, and other viscera. This report describes a patient with an acute onset and symptoms such as high fever, systemic fatigue, pneumonia, and liver damage. C. burnetii was ultimately detected in this patient via next-generation sequencing (NGS), and doxycycline effectively treated the condition. After symptomatic treatment, the patient’s febrile episodes were relieved.
Case presentation
Medical history and previous diagnosis
A 55-year-old farmer was admitted to a local county hospital (October 11–15, 2019) because of “repeated fever for 7 days” and a tick bite on his left leg more than 10 days before onset. Seven days before admission, the patient developed fever without an obvious cause accompanied by chills and obvious fatigue. Without special treatment, his body temperature normalized, but fever repeatedly recurred within 48 hours. After investigation, he was determined to be in good health, and he had no history of smoking or allergy. There were no abnormalities in his personal, marital, or familial history.
The findings of the examination performed after admission were as follows: temperature, 40°C (normal range, 36.3–37.2°C); respiratory rate, 20 breaths/minute (normal range, 16–18 breaths/minute); and blood pressure, 116/65 mmHg (normal range, 90–140/60–90 mmHg). No ecchymosis or bleeding points were found in the skin. The patient had slightly coarse sounds in both lungs, and no obvious dry and wet rales were detected. His heart rhythm was regular, but he exhibited pain in the liver on percussion and palpable lymph node enlargement in the groin.
The results of blood testing were as follows: total white blood cell count, 3.1 × 109/L (normal range, 5.0 ×109–10.0 × 109/L); neutrophil count, 2.48 × 109/L (normal range, 2.0 ×109–7.0 × 109/L); hemoglobin, 138 g/L (normal range, 120–160 g/L); platelet count, 112 × 109/L (normal range, 100 × 109–200 × 109/L); and C-reactive protein, 66.7 mg/L (normal range, <10 mg/L). The patient was negative for hepatitis B surface antigen, and urinalysis was normal. Chest computed tomography (CT) revealed mild inflammation in both lungs and emphysema.
The patient had previously been diagnosed with sepsis and pneumonia. Piperacillin 4.5 g was given every 8 hours for 2 days, but no significant improvement was achieved. The treatment was subsequently changed to Tienam injection 1.0 every 8 hours for 2 days, but there was no observable improvement. The patient relapsed, and he was then admitted to Lishui People’s Hospital (Lishui, China) for further treatment on October 16, 2019.
Physical examination
On admission to the Department of Respiratory and Critical Care Medicine, the patient’s laboratory data were as follows: temperature, 38°C; pulse, 67 beats/minute (normal range, 60–100 beats/minute); blood pressure, 113/69 mmHg; and respiratory rate, 21 breaths/minute. The patient was delirious, and he responded suspiciously to bending of his neck.
Laboratory examinations
A routine blood test was performed immediately after admission, and the hematology parameters were as follows: white blood cell count, 3.5 × 109/L; red blood cell count, 4.38 × 1012/L (normal range, 4.40 × 1012–5.60 × 1012/L); hemoglobin, 132 g/L ; platelet count, 61 × 109/L; and erythrocyte sedimentation rate, 17 mm/hour (normal range, 0–25 mm/hour). The results for coagulation indices were as follows: prothrombin time, 13.1 s (normal range, 10.8–13.0 s); and d-dimer, 4546 µg/L (normal range, <500 µg/L). The results of serum biochemistry for liver function were as follows: total protein, 53.4 g/L (normal range, 60–80 g/L); alanine aminotransferase, 285 U/L (normal range, 13–40 U/L); aspartate aminotransferase, 235 U/L (normal range, 12–30 U/L); total bile acids, 30.9 µmol/L (normal range, 0–10 µmol/L); and C-reactive protein, 130.8 mg/L. No blood plasmodia were detected under a microscope, and the patient was serologically negative for human immunodeficiency virus, hepatitis C, anti-Epstein–Barr virus IgG, syphilis, and pneumonia antibody. Both the Widal and Weil–Felix tests were negative. Urinalysis revealed a weakly positive signal for urinary microalbumin (>0.15 g/L; normal range, <0.02 g/L). Regarding routine cerebrospinal fluid tests, transparency was clear, and Pandy’s test revealed weak positivity for chloride (119 mmol/L; normal range, 120–130 mmol/L), total protein (0.64 g/L; normal range, 0.15–0.45 g/L), and IgG (95 mg/dL; normal range, 2.00–4.00 mg/dL). Cerebrospinal fluid and blood culture were sterile for 5 days.
Imaging examination
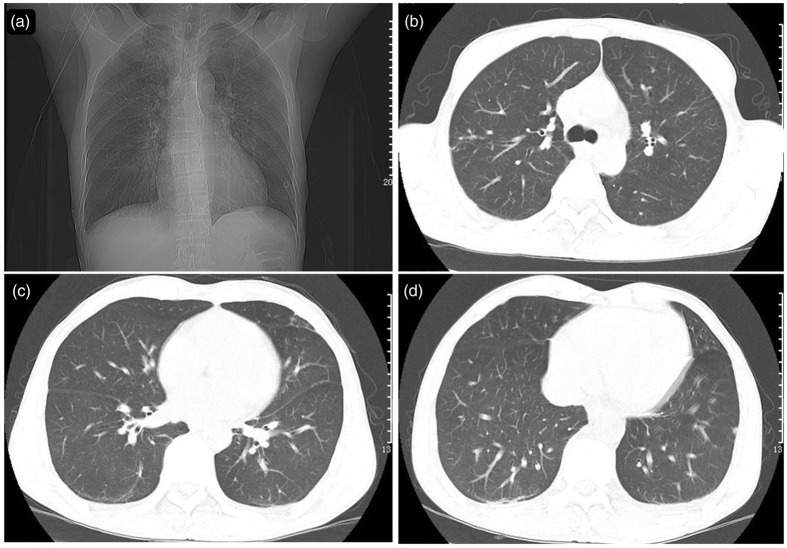
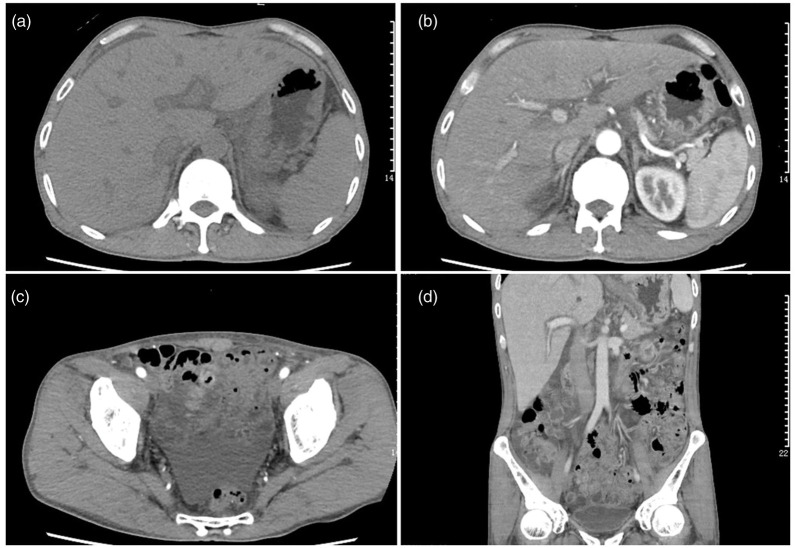
Repeat cardiac color Doppler ultrasound revealed mild mitral valve and tricuspid valve reflux and cardiac arrhythmia. Ultrasonic images of the prostate revealed calcification in the left renal cortex and prostate. Hepatobiliary and splenic ultrasound uncovered hepatomegaly with echogenicity, gallbladder wall thickening, and abdominal cavity effusion. Chest CT (Figure 1) performed in our hospital revealed inflammation in both lungs, multiple small nodules in the right and left lower lobes, patchy fibrosis in the right middle lung lobe and upper lobes of both lungs, and emphysema. Total abdominal plain CT and enhanced CT revealed the following findings: hepatomegaly, dilatation of intrahepatic lymphatic vessels, gallbladder wall edema, ascites, enlarged lymph nodes in the abdominal cavity and retroperitoneum, multiple low-density lesions in the spleen and possible splenic infarction, small cysts in caudate hepatic lobes and a small cyst in the left kidney, calcification foci in the prostate, and bilateral pleural effusion (Figure 2).
Figure 1.
Chest computed tomography. Large patches of high-density shadows were present in the upper lobes of the lungs, and the boundary was not clear. Solid nodules were observed in the middle lobe segment of the right lung and lower lobes of both lungs.
Figure 2.
Whole-abdomen plain computed tomography plus contrast-enhanced computed tomography. The liver caudate lobe was round without an enhanced low-density shadow with a diameter of approximately 7 mm. A patchy low-density shadow was observed on the edge of the spleen. A blotchy dense shadow was present in the prostate. Enlarged lymph nodes were observed in the abdominal cavity and retroperitoneum, the largest of which was approximately 13 × 23 mm2 in size. There was a flaky liquid density shadow in the abdominal pelvic cavity.
Next-generation sequencing
Although tests for pathogens were negative, the possibility of infection could not be eliminated. Therefore, further tests were needed to elucidate the causes of emphysema, hepatic cysts, and pleural effusion. Whole-blood samples were sent for metagenomic sequencing using the Illumina HiSeq platform (Illumina, San Diego, CA, USA). The nucleic acids of clinical samples were extracted to construct a sequencing library.
Results and treatment
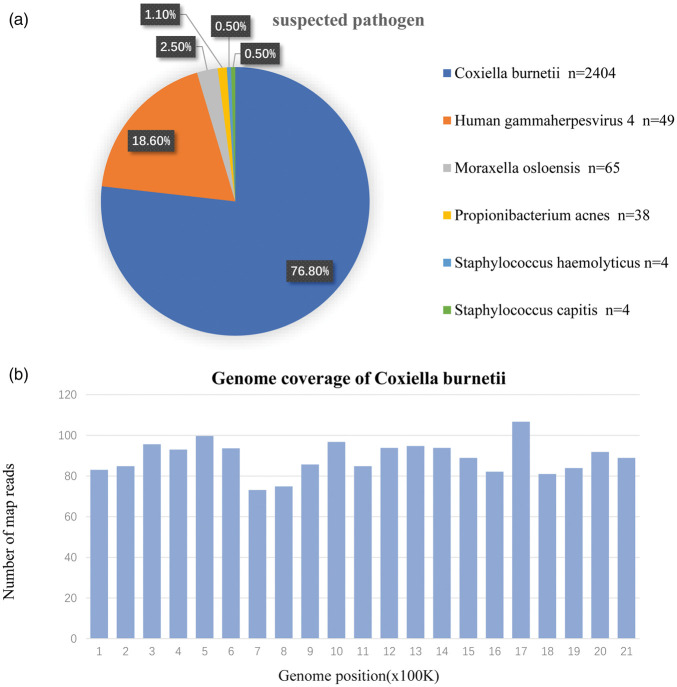
NGS identified Q fever associated with Coxiella infection on October 22, 2019 (Figure 3). Sequences were compared and analyzed using a special database of microorganisms. The similarity plot of sequencing result compared the C. burnetii to available sequences for C. burnetii at the nucleotide level. In total, 2404 reads mapped to C. burnetii in the reference database, and 49 reads corresponded to human herpesvirus type 4 (Epstein–Barr virus) and common skin microflora. C. burnetii reads were abundant in all microbial species. The known sequences covered most of the genome of C. burnetii.
Figure 3.
Next-generation sequencing results. A Coxiella burnetii strain had the highest similarity with the available strains. (a) The abundance of bacteria, fungi, viruses, and parasites was assessed. The relative abundance reflected the relative proportion of genomes in the corresponding classification of the pathogen. Approximately 76.8% of the genomes mapped to C. burnetii in the reference database. (b) The genome coverage denoted the proportion of obtained sequences occupying the entire genome.
Doxycycline hydrochloride enteric-coated capsules were administered orally (0.1 g twice daily), and a compound glycyrrhizin and reduced glutathione injection was used to protect the liver. Three days later, the patient had no fever or chills. The result of the repeat test was negative, and the patient was discharged from the hospital according to the standard procedure. After discharge, both doxycycline anti-infection and compound glycyrrhizin/doxycycline treatment were sustained. To date, the patient has reported no side effects of therapy.
Discussion
C. burnetii, a deadly bacterium that causes Q fever, is considered an emerging global concern because of its contagious nature. The most effective route of infection is intradermal inoculation by ticks. C. burnetii infection in humans is usually asymptomatic, or it presents as a mild disease with spontaneous recovery (fatigue, night sweats, and chills). Atypical pneumonia is also a primary clinical manifestation. Severe complications and even death may occur in patients with acute illness, especially in those with meningitis or myocarditis. Effective treatment and vaccines are available. However, if not treated appropriately, Q fever can become a chronic infection affecting multiple organs, including the bones, heart, and lungs.11
Concerning local treatment, diagnoses of sepsis and pneumonia were rendered according to the presence of mild percussion pain in the liver and infections in both lungs. Piperacillin–tazobactam and Tienam injection were administered , but no significant improvement was observed. After transfer to Lishui People’s Hospital, physical examination revealed positivity for meningeal irritation, laboratory analysis revealed liver and kidney dysfunction and inflammation, and imaging uncovered infection and small nodules in both lungs. Hepatomegaly, thickening of the gallbladder wall, effusion of the abdominal cavity, and lymphadenectasis indicated an inflammatory response associated with infection. Further efforts focused on the detection of etiologic microorganisms. Routine tests such as blood plasmodium, Epstein–Barr virus, syphilis, and HIV were negative. Cerebrospinal fluid and blood culture were sterile on day 5. Finally, whole blood was sent for sequencing, and NGS revealed Q fever associated with Coxiella infection. After treatment with doxycycline hydrochloride,12 the patient’s body temperature normalized, and his condition had generally improved after 3 days.
Current laboratory diagnostic methods mainly include antigen assays, bacterial isolation, and nucleic acid amplification testing. The sensitivities of culture and polymerase chain reaction (PCR) for Coxiella infection using serum or blood samples vary (40%–53% and 23%, respectively).7 Samples with low contamination rates and high bacterial counts can be submitted to direct microbial isolation via cell culture (Vero, HEL, HeLa, DH82, XTC2, and L929 cells) or yolk sac inoculation. Severely contaminated samples are usually inoculated into Guinea pigs and mice intraperitoneally. Specific culture techniques are also widely used (e.g., shell bottle method, Vero cell extract-based medium culture, ACCM-2, ACCM-D).8 However, C. burnetii is highly contagious in aerosol environments, and it can only be cultured and handled in biosafety level III laboratories.11 Serological methods include the Weil–Felix test, complement binding assays, immunofluorescence assays, immunoperoxidase assays, the latex agglutination test, and enzyme-linked immunosorbent assay (ELISA).6 Immunofluorescence and immunoperoxidase assays are laborious and inefficient to execute, and they may not be easily accessible in resource-poor environments. ELISA is the most commonly used serosurveillance assay, but it cannot differentiate between acute and chronic Q fever. Serology is the main and standard laboratory method for diagnosing Q fever. However, the use of serological methods is limited by the technique for obtaining antigens, and the inconsistency of analysis standards will also affect accuracy, thus hindering its widespread use.13 Molecular detection may be the preferred strategy. PCR can quickly screen a large number of samples with high sensitivity and specificity using target genes such as is1111, is30A com1, groEL, htpAB, 16s rRNA, and icd. Two PCR-based genotyping methods have gained widespread use, namely multi-locus variable number of tandem repeats analysis and multispacer sequence typing, which permit the typing of C. burnetii without bacterial isolation. Meanwhile, single-nucleotide polymorphisms genotyping has been explored recently.14 However, molecular assays using blood are only effective when the patient is bacteremic, and the success rates may be affected by low bacterial counts and the prior judgment of the investigator. The clinical manifestations of Q fever are not typical, and infection is not common. Differences in the strain specificity can lead to different symptoms. Therefore, the definition and diagnostic criteria of Q fever remain controversial.
Patients infected with C. burnetii have been reported globally and analyzed using different methods. Ivić15 reported a case of pericarditis that was serologically proved to involve chronic C. burnetii infection. Serological methods are suitable for countries in which the pathogen is endemic. Merhej16 found that abscess samples from the femoral region were positive using PCR. Keijmel17 retrospectively reported a regrettable case of death after infection with C. burnetii in 2016. A male patient had experienced inflammation since 2012, and was hospitalized in 2014 with a negative diagnosis of Q fever using PCR, the complement fixation assay, and ELISA. In 2015, he was diagnosed with chronic Q fever infection, an aneurysm, and an intravascular prosthesis. The patient died despite optimized treatment. Therefore, the early diagnosis and confirmation of Q fever are extremely valuable. If there are signs of disease, such as elevated inflammatory indicators, and routine culture is negative,18 then appropriate antibiotic therapy using an appropriate detection method can greatly reduce the mortality rate. Because there is no standard test in this case, sensitive NGS is recommended for the identification of unknown pathogenic microorganisms.
As an emerging high-throughput sequencing technology, NGS can be used to sequence all pathogens in a single sample without using any probes or primers, thus enabling the unbiased identification of all potential pathogens.19 More specific and effective treatment options depend on the development of precise and rapid methods for identifying Coxiella species. The important point is this platform is unbiased, and a variety of DNA sequences of bacteria,20 viruses,21 fungi,22 and parasites23 have been detected in clinical samples. In addition, NGS can also help guide the treatment of rare tumors24 and genetic diseases.25
As the applications of metagenomic sequencing increase, it is important for clinicians to fully understand the advantages and limitations of this approach. Culture, Gram staining, and biochemical and molecular detection are the traditional methods. For some microbes, especially fungi, these methods are usually slow. In addition, they sometimes do not necessarily provide relevant therapeutic guidance,26 and they are even considered “a guess” for the identification of infectious diseases. However, NGS also has several other disadvantages. NGS struggles to detect viruses and other non-bacterial organisms because the integrity of viral nucleic acids is compromised during the extraction process.
Conclusion
NGS is the most promising and sensitive diagnostic tool for Q fever, especially when the clinical manifestations are atypical, and all pathogens can be identified directly from samples in a single run in a hypothesis-free and culture-independent manner. For the early detection and diagnosis of clinically suspected infection, the diagnosis can be confirmed via NGS of blood samples, and appropriate antibacterial treatment and symptomatic support treatment can be provided as early as possible to reduce the mortality rate.
Footnotes
Declaration of conflicting interest: The authors declare that there is no conflict of interest.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by grants from the National Natural Science Foundation of China (No. 31701078), Research Fund of Chengdu Medical College (No. CYZ17-02), Research Fund of Non-coding RNA and Drug Discovery Key Laboratory of Sichuan Province (No. FB19-03), and College Students’ Innovative Entrepreneurial Training Plan Program (No. s201913705118).
Patient consent and ethics statement
The patient consented to treatment and provided written consent for publication of this study. The reporting of this study conforms to CARE guidelines.10 Ethical approval was not required due to the nature of this study (case report).
ORCID iDs
References
- 1.Hilbink F, Penrose M, Kovacova E, et al. Q fever is absent from New Zealand. Int J Epidemiol 1993; 22: 945–949. [DOI] [PubMed] [Google Scholar]
- 2.Wegdam-Blans MCA, Kampschreur LM, Delsing CE, et al. Chronic Q fever: review of the literature and a proposal of new diagnostic criteria. J Infect 2012; 64: 247–259. DOI: 10.1016/j.jinf.2011.12.014. [DOI] [PubMed] [Google Scholar]
- 3.Kersh GJ, Priestley R, Massung RF.Stability of Coxiella burnetii in stored human blood. Transfusion 2013; 53: 1493–1496. DOI: 10.1111/j.1537-2995.2012.03912.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hammami R, Bahloul A, Charfeddine S, et al. Q fever presenting as myocarditis. IDCases 2021; 23: e01056. DOI: 10.1016/j.idcr.2021.e01056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kampschreur LM, Delsing CE, Groenwold RHH, et al. Chronic Q fever in the Netherlands 5 years after the start of the Q fever epidemic: results from the Dutch chronic Q fever database. J Clin Microbiol 2014; 52: 1637–1643. DOI: 10.1128/JCM.03221-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eldin C, Mélenotte C, Mediannikov O, et al. From Q Fever to Coxiella burnetii Infection: a Paradigm Change. Clin Microbiol Rev 2017; 30: 115–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roothans D, Van Ierssel S, Moorkens G.A case of recurrent fever in an older man caused by Coxiella burnetii. Acta Clin Belg 2017; 72: 264–267. DOI: 10.1080/17843286.2016.1218177. [DOI] [PubMed] [Google Scholar]
- 8.Sahu R, Rawool D, Vinod V, et al. Current approaches for the detection of Coxiella burnetii infection in humans and animals. J Microbiol Methods 2020; 179: 106087. DOI: 10.1016/j.mimet.2020.106087. [DOI] [PubMed] [Google Scholar]
- 9.Million M, Thuny F, Richet H, et al. Long-term outcome of Q fever endocarditis: a 26-year personal survey. Lancet Infect Dis 2010; 10: 527–535. DOI: 10.1016/s1473-3099(10)70135-3. [DOI] [PubMed] [Google Scholar]
- 10.Gagnier JJ, Kienle G, Altman DG, et al. The CARE Guidelines: Consensus-based Clinical Case Reporting Guideline Development. Glob Adv Health Med 2013; 2: 38–43. DOI: 10.7453/gahmj.2013.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Neupane K, Kaswan D.Coxiella Burnetii. StatPearls. Treasure Island (FL): StatPearls Publishing Copyright © 2020, StatPearls Publishing LLC., 2020. [Google Scholar]
- 12.Lever MS, Bewley KR, Dowsett B, et al. In vitro susceptibility of Coxiella burnetii to azithromycin, doxycycline, ciprofloxacin and a range of newer fluoroquinolones. Int J Antimicrob Agents 2004; 24: 194–196. [DOI] [PubMed] [Google Scholar]
- 13.Robinson M, Satjanadumrong J, Hughes T, et al. Diagnosis of spotted fever group Rickettsia infections: the Asian perspective. Epidemiol Infect 2019; 147: e286. DOI: 10.1017/s0950268819001390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Das D, Malik S, Sahu R, et al. Loop-mediated isothermal amplification assay for detection of Coxiella burnetii targeting the com1 gene. J Microbiol Methods 2018; 155: 55–58. DOI: 10.1016/j.mimet.2018.11.011. [DOI] [PubMed] [Google Scholar]
- 15.Ivić I, Božić I, Ledina D.Coxiella burnetii chronic pericarditis: a case report. Neth J Med 2016; 74: 362–364. [PubMed] [Google Scholar]
- 16.Merhej V, Tattevin P, Revest M, et al. Q fever osteomyelitis: a case report and literature review. Comp Immunol Microbiol Infect Dis 2012; 35: 169–172. DOI: 10.1016/j.cimid.2011.12.008. [DOI] [PubMed] [Google Scholar]
- 17.Keijmel S, Raijmakers R, Schoffelen T, et al. A fatal case of disseminated chronic Q fever: a case report and brief review of the literature. Infection 2016; 44: 677–682. DOI: 10.1007/s15010-016-0884-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leahey PA, Tahan SR, Kasper EM, et al. Chronic Q-Fever (Coxiella burnetii) Causing Abdominal Aortic Aneurysm and Lumbar Osteomyelitis: A Case Report. Open Forum Infect Dis 2016; 3: ofv185. DOI: 10.1093/ofid/ofv185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guan H, Shen A, Lv X, et al. Detection of virus in CSF from the cases with meningoencephalitis by next-generation sequencing. J Neurovirol 2016; 22: 240–245. DOI: 10.1007/s13365-015-0390-7. [DOI] [PubMed] [Google Scholar]
- 20.Wu H, Wei J, Yu D.Application of NGS in Diagnosis of Tuberculous Pleurisy with Multiple Negative Tests: A Case Report. Infect Drug Resist 2020; 13: 3543–3550. DOI: 10.2147/IDR.S269779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang D, Tao X, Fei M, et al. Human encephalitis caused by pseudorabies virus infection: a case report. J Neurovirol 2020; 26: 442–448. DOI: 10.1007/s13365-019-00822-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang K, Yu C, Li Y, et al. Next-generation sequencing technology for detecting pulmonary fungal infection in bronchoalveolar lavage fluid of a patient with dermatomyositis: a case report and literature review. BMC Infect Dis 2020; 20: 608. DOI: 10.1186/s12879-020-05341-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roy M, Siddique N, Bathina B, et al. Rare Presentation of Pneumonitis in the Absence of Neurological Symptoms in an AIDS Patient and Use of Next-Generation Sequencing for Diagnosis. Eur J Case Rep Intern Med 2020; 7: 001862. DOI: 10.12890/2020_001862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tan DSP, Tan DSW, Tan IBH, et al. Recommendations to improve the clinical adoption of NGS-based cancer diagnostics in Singapore. Asia Pac J Clin Oncol 2020; 16: 222–231. DOI: 10.1111/ajco.13339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Latif H, Liu SV.Novel mutation with durable response to brigatinib-a case report. Transl Lung Cancer Res 2020; 9: 2145–2148. DOI: 10.21037/tlcr-20-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Long Y, Zhang Y, Gong Y, et al. Diagnosis of Sepsis with Cell-free DNA by Next-Generation Sequencing Technology in ICU Patients. Arch Med Res 2016; 47: 365–371. DOI: 10.1016/j.arcmed.2016.08.004. [DOI] [PubMed] [Google Scholar]