Abstract
Objective
We performed a meta-analysis to create a quantitative estimate of the association between non-alcoholic fatty liver disease (NAFLD) and the risk of cardiac arrhythmia (including atrial fibrillation (AF), prolonged QT interval, premature atrial/ventricular contraction [PAC/PVC] and heart block).
Methods
A literature review was conducted using PubMed, Embase, Web of Science and the Cochrane Library database to identify observational studies of the link between NAFLD and cardiac arrhythmia. Effect sizes were expressed as odds ratios (ORs) or hazard ratios (HRs) with 95% confidence intervals (CIs). The method of analysis of AF was also analysed separately, according to the effect estimate (OR or HR).
Results
Nineteen studies of 7,012,960 individuals were included. NAFLD was independently associated with higher risks of AF (OR 1.71, 95% CI: 1.14–2.57; HR 1.12, 95% CI: 1.11–1.13), prolonged QT interval (OR 2.86, 95% CI: 1.64–4.99), PAC/PVC (OR 2.53, 95% CI: 1.70–3.78) and heart block (OR 2.65, 95% CI: 1.88–3.72). The heterogeneity of the data with respect to AF and prolonged QT was moderate on sensitivity analysis.
Conclusions
We found a significantly higher risk of cardiac arrhythmia in patients with NAFLD, but the observational design of the studies does not permit conclusions regarding causality.
Keywords: Non-alcoholic fatty liver disease, cardiac arrhythmia, atrial fibrillation, meta-analysis, prolonged QT interval, premature ventricular contraction, heart block
Introduction
Non-alcoholic fatty liver disease (NAFLD) encompasses a range of histological changes, from benign steatosis to non-alcoholic steatohepatitis (NASH). Accumulating evidence suggests that NAFLD is not merely a hepatic manifestation of metabolic syndrome: a strong bidirectional relationship between NAFLD and type 2 diabetes/metabolic syndrome has been shown.1 As living standards have improved, NAFLD has become the most prevalent chronic hepatic disease around the world,2 and it currently affects nearly 52% of Americans3 and 20.1% of Chinese people, according to a recent meta-analysis.4 NAFLD is also associated with chronic kidney disease, which may be associated with the future risk of cardiac arrhythmia.5 Furthermore, NAFLD is projected to be the most common indication for liver transplantation in the US during the next decade.6The pathogenesis of NAFLD has yet to be completely elucidated, but some risk factors of NAFLD have been identified, including obesity, diabetes and hyperlipidaemia, which are all associated with defects in metabolism.7 However, currently, only diet and lipid-lowering drugs are available for the prevention or inhibition of the development of NAFLD.
Accumulating evidence indicates that NAFLD is also associated with cardiac arrhythmia.8 NAFLD is a multi-system disorder, affecting a diverse range of extra-hepatic organs and organ systems, including the heart and the blood vessels. Its principal pathological features include not only alterations in hepatic structure and function, but also in the heart and the blood vessels, which increase the morbidity and mortality connected with cardiovascular disease (CVD), and previous studies have demonstrated that CVD is the leading cause of mortality in patients with NAFLD.9 The methods that are commonly used for the diagnosis of arrhythmia include standard electrocardiography (ECG), dynamic ECG and exercise testing. Standard ECG has become the most widely used method for the assessment of cardiac disease in community-based healthcare, because it is a simple, inexpensive, and objective way of identifying myocardial infarction and heart rhythm and conduction disturbances.
The mechanisms underpinning the link between NAFLD and arrhythmia are complex and not fully understood. However, there is compelling evidence that NAFLD is associated with several arrhythmias that can be assessed using ECG, such as atrial fibrillation (AF), heart block, QT interval prolongation, premature ventricular contraction (PVC) and premature atrial contraction (PAC).8 The most common tachyarrhythmia is AF, the incidence of which gradually increases with age. Heart block represents a block or delay in the conduction of action potentials emitted by pacemaker cells. QT interval is defined as the total duration of ventricular myocardial depolarization and repolarization, and prolongation of the QT interval is a significant predictor of cardiac death. PVC is the most common type of ventricular arrhythmia that is identified clinically, and refers to abnormalities in the heartbeat that are caused by premature impulses generated by an ectopic pacemaker, and might be associated with a sinus rhythm or an ectopic rhythm. PAC is the early generation of ectopic atrial beats, ahead of sinus rhythm. The early identification of these electrical abnormalities is important to prevent the development of further complications.
Recent meta-analyses have shown an association between NAFLD and AF, although the findings have been inconsistent.10,11 Moreover, the relationships between NAFLD and other types of cardiac arrhythmia have been poorly studied. Therefore, the purpose of the present study was to assess the relationship between NAFLD and the risk of cardiac arrhythmia by means of an integrative meta-analysis of the published observational studies.
Methods
Registration of the review protocol
The protocol for this systematic review and meta-analysis was registered in advance with PROSPERO (International Prospective Register of Systematic Reviews, no. CRD42021245860). Because of the nature of the study, the requirements for ethics approval and informed consent were waived by the Medical Ethics Committee of Zigong First People’s Hospital.
Search strategy
Two investigators (HG and XLL) searched for relevant studies in PubMed, Embase, and in the Web of Science and the Cochrane Library databases, from their inception to 28 March 2021. A comprehensive electronic search strategy was performed using a combination of medical subject headings (MeSH) and free text related to NAFLD and cardiac arrhythmias. The full electronic search strategies for all the databases are described in Appendix A. References cited in the studies identified were also examined. The search was restricted to studies that were conducted in humans, but there were no language restrictions. We performed a systematic review according to the Systematic Reviews and Meta-Analyses (PRISMA) guidelines,12 and because the studies were observational, we followed the Meta-analysis Of Observational Studies in Epidemiology (MOOSE) guidelines.13
Study selection
All the studies were assessed in blinded fashion by two investigators (HG and XLL). Disagreements were resolved by discussion. The inclusion criteria for a study were: (a) original observational study (cross-sectional, case-control or cohort study), (b) ‘NAFLD’ as the exposure factor, (c) incidence of cardiac arrhythmia (including AF, QT interval prolongation, heart block and PAC/PVC) as an outcome measure, (d) provision of an odds ratio (OR) or hazard ratio (HR) with a 95% confidence interval (95% CI), or ability to calculate one of these using univariate logistic regression analysis of events in the NAFLD and control groups, (e) identification of the methods of diagnosis of NAFLD and cardiac arrhythmia and (g) age ≥18 years. The exclusion criteria were: (a) case reports, abstracts, comments, reviews, letters or editorials, according to the title and abstract, (b) lack of exclusion of participants that consumed significant amounts of alcohol or had evidence of other chronic liver diseases, (c) missing key data and (d) duplication (if multiple studies from the same institution reported the same outcome, we chose the one with the largest sample size for inclusion).
Data extraction and quality assessment
For all the included studies, two researchers (HG and XLL) noted the first author, publication year, study country, study design, study size, age of the participants in the NAFLD and control groups, type of arrhythmia, methods used for the diagnosis of NAFLD and the cardiac arrhythmia, adjusted ORs or HRs with 95% CIs and the covariates that were adjusted for in multivariable regression analyses. If the any of the required parameters were unclear, the authors were contacted to clarify the information.
Two independent investigators (HG and XLL) assessed the risk of bias in the original studies. Any disagreements were resolved by discussion, including with the third author, until a consensus was reached. The Newcastle–Ottawa Quality Assessment Scale (NOS) was used to evaluate the quality of cohort studies and case–control studies.14 The quality of each included study was awarded between 1 and 9 stars, with 1 to 3 stars corresponding to low quality, 4 to 6 stars corresponding to unclear/moderate quality, and 7 to 9 stars corresponding to high quality.15,16 In addition, we assessed the quality of the included cross-sectional studies using the Agency for Healthcare Research and Quality (AHRQ) standards.17 Responses of ‘Yes’, ‘Unclear’ or ‘No’ were recorded for eleven criteria. Then, the study quality was graded as: low quality, score 0 to 3; moderate quality, score 4 to 7; and high quality, score 8 to 11.
Statistical analysis
The ORs/HRs and corresponding 95% CIs were used to evaluate the strength of the associations between NAFLD and the risk of cardiac arrhythmia. The statistical heterogeneity of the studies was assessed using Cochran’s Q test and I2 statistic, with I2 values of 25%, 50% and 75% representing low, moderate and high heterogeneity, respectively.18 I2 ≥ 50% and P < 0.1 was taken to imply significant heterogeneity, and the random-effects model was used. In addition, sensitivity analyses of the factors that might have led to heterogeneity were performed to identify the possible sources. Conversely, a fixed-effects model was used. Funnel plots and Egger’s test were used to identify potential publication bias. Statistical analyses were performed using Stata 16.0 (Stata Corp., College Station, TX, USA). Statistical significance was accepted with a two-sided P < 0.05.
Results
Search results
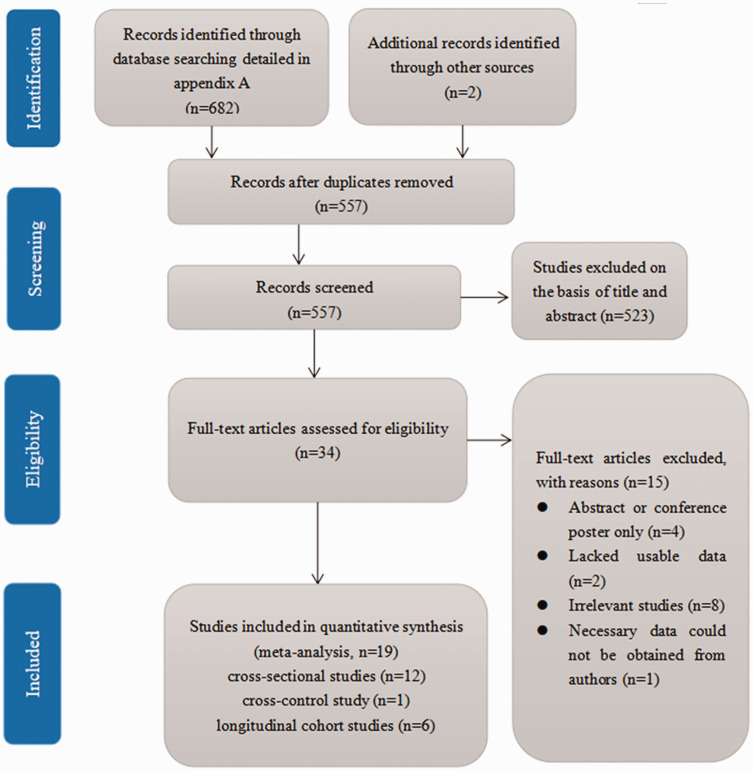
Our initial database search identified 684 studies. After the exclusion of 127 duplicate studies, the remaining 557 were subjected to title and abstract review. Five hundred and twenty-three articles were excluded at this stage because they were case reports, case series, reviews, animal studies or in vitro analyses, which left 34 studies for full-text article review. Fifteen of these were excluded because they were abstracts or conference posters (n = 4), lacked usable data (n = 2), were not relevant (n = 8) or because the required data could not be obtained from the authors (n = 1), as specified in the PRISMA flow diagram in Figure 1. Therefore, 19 eligible observational studies remained and were included in the meta-analysis.19–36
Figure 1.
PRISMA flow diagram for the identification of appropriate studies for inclusion in the meta-analysis.
Study characteristics
The principal characteristics and the quality assessment of the included studies are shown in Table 1. All the studies included were observational studies: there were 12 cross-sectional studies, one case–control study and six longitudinal cohort studies. Six of the 19 studies were conducted in Asia (Korea n = 4, China n = 2), nine in Europe (Germany n = 2, Finland n = 1, Italy n = 6) and four in the USA. A total of 7,012,960 adult participants were included in the meta-analysis, of whom 1,083,255 had NAFLD (15.45%) and 5,929,705 did not. NAFLD was diagnosed on the basis of the International Classification of Diseases (ICD) code or biopsy (n = 2 studies), ultrasonography or computed tomography (CT) (n = 11 studies), multi-detector CT (n = 1 study) or fatty liver index (FLI) (n = 4 studies), in the absence of significant alcohol consumption or other known causes of chronic liver disease. Diagnoses of cardiac arrhythmias were generally made on the basis of ECG, Holter monitoring, ICD code and/or medical history. The NOS scores of the seven observational studies (one case–control study and six longitudinal cohort studies) ranged from 5 to 9, with six high-quality studies and only one of medium quality. All of the cross-sectional studies (n = 12) were assessed using AHRQ, which showed that nine were of high quality and three were of moderate quality.
Table 1.
Details of the principal included observational (case–control, cross-sectional and longitudinal) studies of the relationship between NAFLD and the risk of cardiac arrhythmia, including the quality assessment.
| Study/Year of Publication | Country | Study design | NAFLD/no NAFLD | Age (years) | Type of cardiac arrhythmia | Method of cardiac assessment | Method of diagnosis of NAFLD | Quality assessment score | Parameters included in the multivariate model | OR/HR (95% CI) |
|---|---|---|---|---|---|---|---|---|---|---|
| Käräjämäki AJ, et al. 2015[19] | Finland | Longitudinal cohort study | 249/709 | NAFLD: 52.0 ± 6.0 no NAFLD: 51.0 ± 6.0 | AF | ICD-10 code | Ultrasonography | 8 | Age, sex, study group, diabetes, BMI, waist circumference, alcohol consumption, smoking, serum ALT, systolic blood pressure, QUICKI, left ventricular mass index, left atrial diameter, CAD, atrial natriuretic peptide, high-sensitivity C-reactive protein | HR: 1.88 (1.03–3.45) |
| Labenz C, et al. 2020[20] | Germany | Longitudinal cohort study | 22,048/22,048 | NAFLD: 55.6 ± 13.4 no NAFLD: 55.6 ± 13.4 | AF | ICD-10 code | ICD-10 code | 8 | MI, CHD, stoke | HR: 1.15 (1.04–1.26) |
| Lee SR, et al. 2021[21] | Korea | Longitudinal cohort study | 924,497/5,309,434 | NAFLD: 47.3 ± 12.7no NAFLD: 45.4 ± 14.4 | AF | ICD-10 code | FLI | 9 | Age, sex, hypertension, diabetes, dyslipidaemia, CKD, smoking, alcohol consumption, exercise, low income, systolic blood pressure, total cholesterol, fasting glucose | HR: 1.12 (1.11–1.13) |
| Long MT, et al. 2017[22] | USA | Longitudinal cohort study | 406/1654 | NAFLD: 59.2 ± 9.3no NAFLD: 58.9 ± 9.7 | AF | ECG, Holter monitoring | MDCT | 9 | Sex, age, systolic blood pressure, diastolic blood pressure, current smoking, use of antihypertensive medication, diabetes mellitus, history of heart failure, history of myocardial infarction | HR: 0.96 (0.64–1.45) |
| You SC, et al. 2016[23] | Korea | Longitudinal cohort study | 107,619/125,360 | Mean age: 49 | AF | ECG | FLI | 5 | Heart failure, serum creatinine, obesity, high systolic BP, impaired fasting glucose, dyslipidaemia. | HR: 1.13 (1.03–1.23) |
| Roh JH, et al. 2020[24] | Korea | Longitudinal cohort study | 1415/332,865 | NAFLD: 43.4 ± 11.5no NAFLD: 37.0 ± 11.6 | AF | ICD-10 code | FLI | 8 | Age, sex, clinical characteristics, diabetes, hypertension, heart failure, myocardial infarction | HR: 1.25 (1.13–1.39) |
| Targher G, et al. 2013[25] | Italy | Cross-sectional study | 281/119 | NAFLD: 63.0 ± 9.0no NAFLD: 64.0 ± 9.0 | AF | ECG, medical history | Ultrasonography | 9 | Age, sex, BMI, systolic BP, hypertension treatment, electrocardiographic PR interval, history of heart failure | OR: 4.96 (1.40–17.0) |
| Markus MR, et al. 2016[26] | Germany | Cross-sectional study | 937/2153 | Mean age: 52 | AF | ECG | Ultrasonography | 9 | Age, sex, body mass, height, alcohol consumption in the previous 30 days, smoking, systolic blood pressure, glycated haemoglobin, total cholesterol/HDL-C ratio, eGFR, chronic bronchitis, hyperthyroidism, current use of antihypertensive, hypoglycaemic or lipid-lowering medications, previous history of myocardial infarction or valvular heart diseases, left atrial diameter, LV mass, ejection fraction | OR: 1.20 (0.43–3.37) |
| Targher G, et al. 2013[27] | Italy | Cross-sectional study | 514/188 | NAFLD: 65.0 ± 13.0no NAFLD: 68.0 ± 14.0 | AF | ECG, medical history | Ultrasonography | 8 | Age, sex, systolic BP, HbA1c, estimated GFR, total cholesterol, electrocardiographic LVH, COPD, prior history of HF, VHD or hyperthyroidism, serum GGT activity, current use of antihypertensive drugs, insulin, digoxin, nitroderivates or anticoagulants | OR: 5.17 (2.05–13.0) |
| Whitsett M, et al. 2019[28] | USA | Cross-sectional study | 9108/ 111,812 | Biopsy- confirmed NASHmean age: 57 | AF | ICD-9 code | ICD-9 code, biopsy | 8 | Not applicable | Unadjusted OR: 2.13 (1.93–2.34) |
| Zhang Y, et al. 2018[29] | China | Cross-sectional study | 522/1166 | NAFLD: 71 (68–75)no NAFLD: 72 (68–77) | AF | ECG | Ultrasonography | 8 | Age, sex, systolic blood pressure, fasting plasma glucose, γ-glutamyl transpeptidase activity, high-density lipoprotein, triglycerides, total cholesterol, albumin | OR: 2.76 (1.32–5.77) |
| Long MT, et al. 2017[22] | USA | Cross-sectional study | 406/1654 | NAFLD: 59.2 ± 9.3no NAFL D: 58.9 ± 9.7 | AF | ECG, Holter monitoring | MDCT | 9 | Sex, age, systolic blood pressure, diastolic blood pressure, current smoking, use of antihypertensive medication, diabetes mellitus, history of heart failure, history of myocardial infarction | OR: 1.12 (0.58–2.18) |
| Pastori D, et al. 2020[30] | Italy | Cross-sectional study | 732/1003 | NAFLD: 73.7 ± 9.1no NAFLD: 75.6 ± 9.0 | AF | Medical history | FLI | 7 | Not applicable | Unadjusted OR: 1.33 (1.10–1.62) |
| Chung TH, et al. 2020[31] | Korea | Cross-sectional study | 179/585 | NAFLD: 50.1 ± 7.6no NAFL D: 45.7 ± 8.2 | Prolonged QT interval | ECG | Ultrasonography | 8 | Age, BMI, smoking status, regular exercise, mean arterial pressure, fasting plasma glucose, triglycerides, HDL-choleste rol, AST, ALT, calcium and potassium concentrations, menopausal status | OR: 2.05 (1.13–3.71) |
| Hung CS, et al. 2015[32] | Taiwan | Cross-sectional study | 12,891/ 18,225 | Overall: 50.1 ± 12.1 | Prolonged QT interval | ECG | Ultrasonography | 7 | Not applicable | Unadjusted OR: 1.30 (1.24–1.36) |
| Targher G, et al. 2014[33] | Italy | Cross-sectional study | 281/119 | Overall: 63.0 ± 10.0 | Prolonged QT interval | ECG | Ultrasonography | 7 | Age, sex, duration of diabetes, hypertension, presence of PAD, lower-limb sensory neuropathy, BMI, daily alcohol consumption, smoking, HbA1c, electrocardiographic LVH, CHD, CKD | OR: 2.26 (1.39–3.67) |
| Mantovani A, et al. 2016[34] | Italy | Cross-sectional study | 238/92 | NAFLD: 70.6 ± 7.9no NAFLD: 69.5 ± 8.4 | PVC | 24-h Holter monitoring | Ultrasonography | 8 | Age, sex, BMI, hypertension, smoking history, CKD, COPD, IHD, VHD, use of antiarrhythmic drugs, serum GGT activity, LV ejection fraction | OR: 3.01 (1.26–7.17) |
| Mantovani A, et al. 2017[35] | Italy | Cross-sectional study | 524/227 | Overall mean: 66 | Heart block | ECG | Ultrasonography | 9 | Age, sex, BMI, duration of diabetes, haemoglobin A1c, eGFR-EPI, macroalbuminuria, hypertension status, prior ischemic heart disease and mild-to-moderate valvular heart disease, PAD, diabetic retinopathy, lower-extremity sensory neuropathy, current use of statins or anti-platelet agents | OR: 3.04 (1.81–5.10) |
| Mangi MA, et al. 2017[36] | USA | Case–control study | 408/292 | NAFLD: 59.0 ± 13.6no NAFLD: 56.3 ± 17.5 | Heart block, AF, prolonged QT interval, PAC/PVC | ECG | Ultrasonography or CT | 8 | Age, sex, ethnicity, obesity, CAD, hypertension, diabetes mellitus, CHF, smoking, COPD, asthma, thyroid disorders, antipsychotic medicine, hyperlipidaemia | OR: 2.38 (1.51–3.73), Unadjusted OR: 0.97 (0.60–1.57), Unadjusted OR: 5.09 (2.92–8.86), Unadjusted OR: 2.42 (1.54–3.80), respect-ively |
Age (years) is expressed as mean ± SD, median (interquartile range) or percentage.
AF: atrial fibrillation; AST: aspartate aminotransferase; ALT: alanine aminotransferase; BMI: body mass index; BP: blood pressure; CT: computed tomography; CHF: congestive heart failure; CAD: coronary artery disease; CKD: chronic kidney disease; CHD: coronary heart disease; COPD: chronic obstructive pulmonary disease; eGFR: estimated glomerular filtration rate; eGFR-EPI: glomerular filtration rate estimated using the CKD-EPI equation; ECG: electrocardiography; FLI: fatty liver index; GGT: γ-glutamyltransferase; HbA1c: glycated haemoglobin; HF: heart failure; HDL-C: high-density lipoprotein-cholesterol; IHD: ischemic heart disease; LV: left ventricular; LVH: left ventricular hypertrophy; MI: myocardial infarction; MDCT: multi-detector computed tomography; NAFLD: non-alcoholic fatty liver disease; PAD: peripheral artery disease; PAC: premature atrial contraction; PVC: premature ventricular contraction; VHD: valvular heart disease.
Relationship between NAFLD and the risk of AF
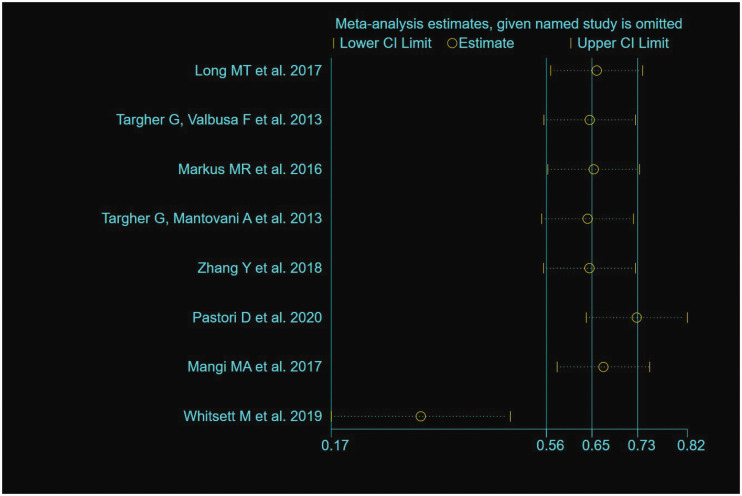
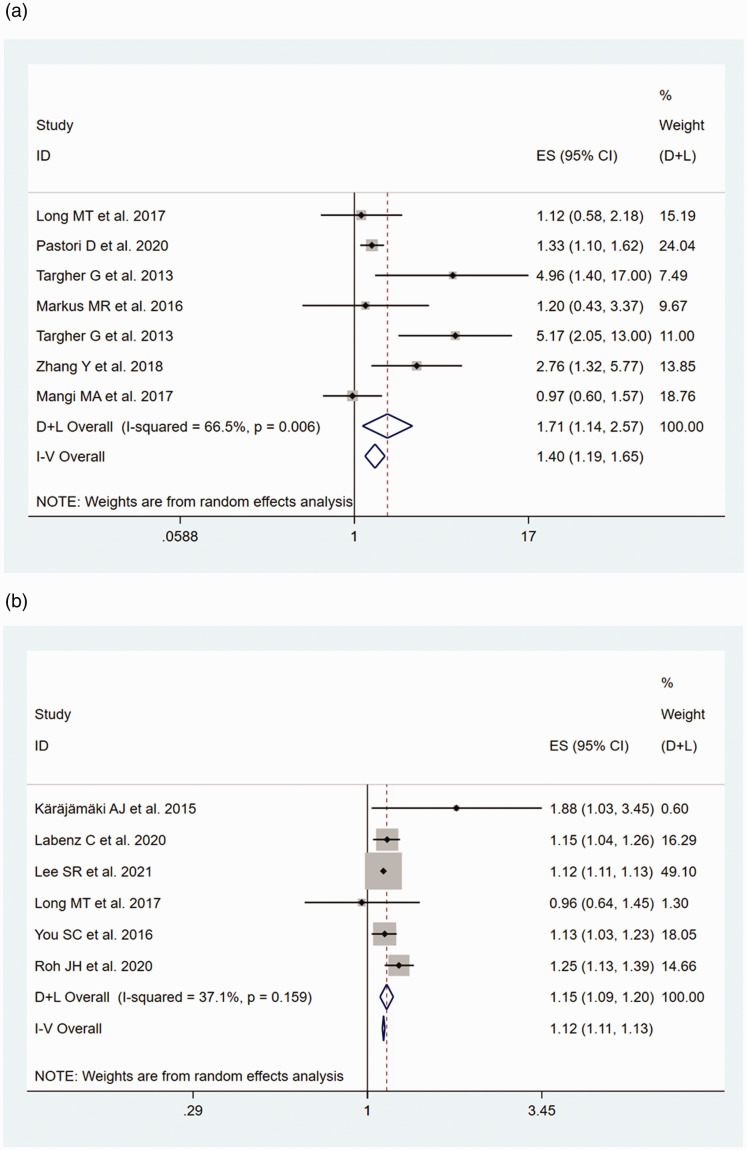
Of the 14 studies19–30,36 that assessed AF, an OR (95% CI) or HR (95% CI) was obtained from 8 (7 cross-sectional studies and 1 case–control study) and 6 (longitudinal cohort) studies, respectively. The heterogeneities of the OR and HR data were P < 0.001/I2 = 81.0% and P = 0.159/I2 = 37.1%, respectively. Because the OR estimate showed high heterogeneity (I2 = 81.0%), we also performed a sensitivity analysis. The study by Whitsett et al.28 was the main source of heterogeneity, according to the sensitivity analysis (Figure 2). Therefore, this study was excluded prior to the meta-analysis being performed. A pooled OR of 1.71 (95% CI, 1.14–2.57; random-effects model; I2 = 66.5%) was generated for the eight studies that produced estimates of the OR, and a pooled HR of 1.12 (95% CI, 1.11–1.13; fixed-effects model; I2 = 37.1%) was generated for the six cohort studies that produced estimates of the HR. The forest plots of this meta-analysis are shown as Figure 3.
Figure 2.
Sensitivity analysis of the odds ratio data for participants with atrial fibrillation. Data are point estimates and 95% confidence intervals for the omission of each named study from the analysis.
Figure 3.
Forest plots and pooled estimates of the relationship between NAFLD and the risk of AF, with corresponding 95% CIs and P-values, generated using fixed and random effects models. (a) OR data for participants with AF. (b) HR data for participants with AF.
AF, atrial fibrillation; CI, confidence interval; HR, hazard ratio; NAFLD, non-alcoholic fatty liver disease; OR, odds ratio.
Relationship between NAFLD and the risk of prolonged QT interval
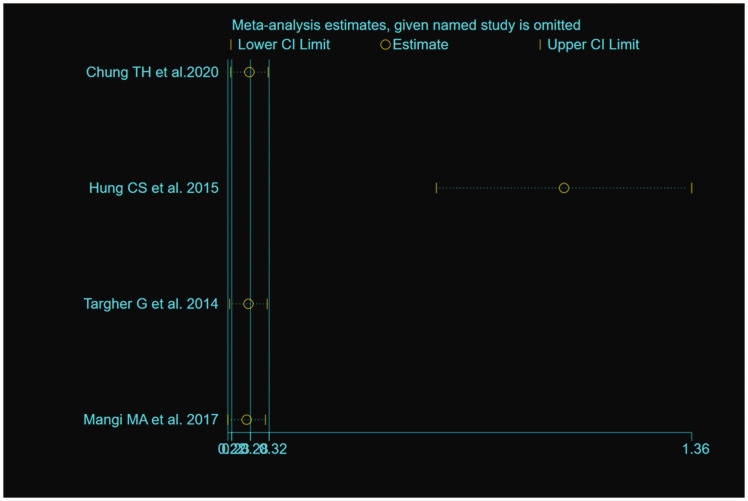
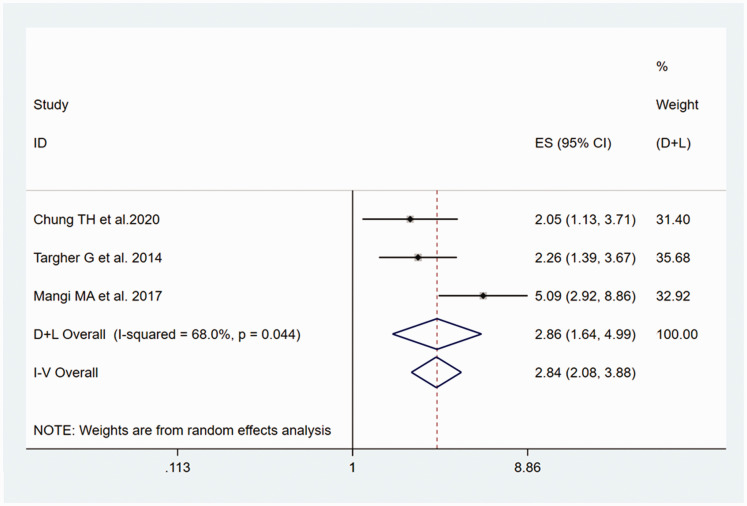
The outcome of the heterogeneity test was P<0.001/I2 = 90.0%, which indicated high heterogeneity of the four included studies.31–33,36 A sensitivity analysis showed that the study by Hung et al.32 was the principal cause of the heterogeneity (Figure 4). The result of the heterogeneity test was P = 0.044/I2 = 68.0% after excluding this study, which is consistent with moderate heterogeneity; therefore, the random-effects model was used. A significant association between NAFLD and the risk of prolonged QT interval was identified, with a pooled OR of 2.86 (95% CI, 1.64–4.99), as shown in Figure 5.
Figure 4.
Sensitivity analysis of the odds ratio data for participants with prolonged QT interval. Data are point estimates and 95% confidence intervals for the omission of each named study from the analysis.
Figure 5.
Forest plot of the estimated ORs, the corresponding 95% CIs and the P-values for the risk of prolonged QT interval in adult participants with NAFLD, compared with those without, generated using random effects models.
CI, confidence interval; NAFLD, non-alcoholic fatty liver disease; OR, odds ratio.
Relationship between NAFLD and the risk of PVC/PAC
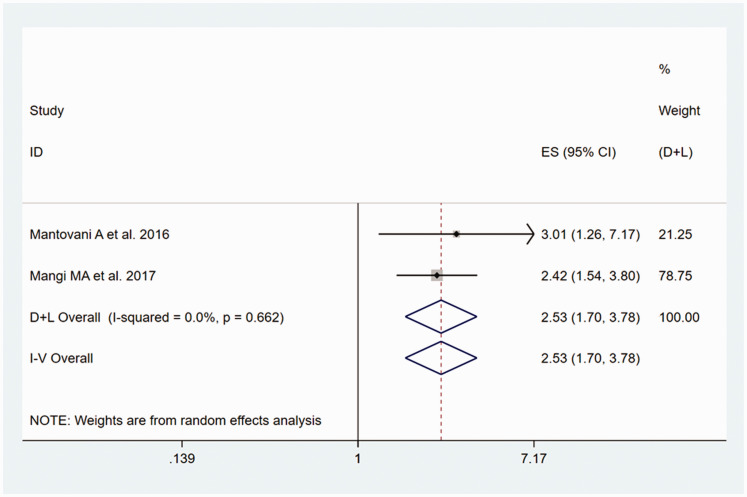
The outcome of the heterogeneity test was P = 0.662/I2 = 0.0%, which indicated no heterogeneity of the included two studies;34,36 therefore, the fixed-effects model was used for the analysis. The meta-analysis showed that there was a significantly higher risk of PVC/PAC in participants with NAFLD than in those without, with a pooled OR of 2.53 (95% CI, 1.70–3.78), as shown in Figure 6.
Figure 6.
Forest plot of the estimated ORs, corresponding 95% CIs and the P-values for the risk of PVC/PAC in adult participants with NAFLD, compared with those without, generated using fixed effects models.
CI, confidence interval; NAFLD, non-alcoholic fatty liver disease; OR, odds ratio; PVC/PAC, premature ventricular contraction/premature atrial contraction.
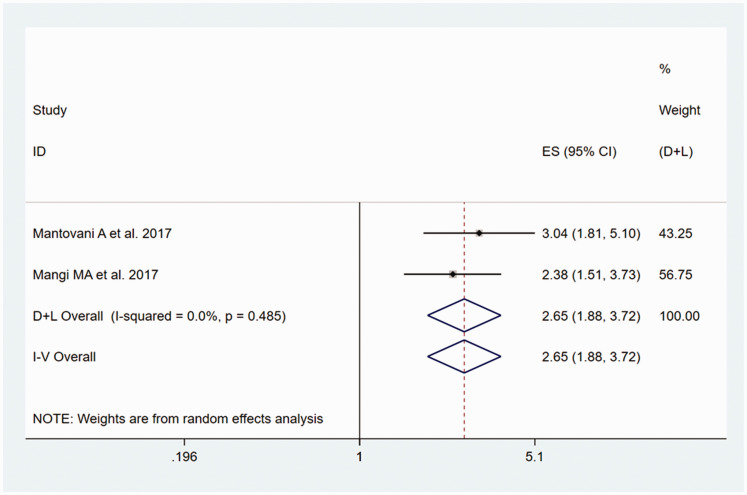
Relationship between NAFLD and the risk of heart block
The heterogeneity test showed that there was no heterogeneity of the two included studies,35,36 with P = 0.485/I2 = 0.0%. The fixed-effects meta-analysis generated a pooled OR for the participants with NAFLD versus those without of 2.65 (95% CI, 1.88–3.72), as shown in Figure 7.
Figure 7.
Forest plot of the estimated ORs, corresponding 95% CIs and P-values for the risk of heart block in adult participants with NAFLD, compared with those without, generated using fixed effects models.
NAFLD, non-alcoholic fatty liver disease; OR, odds ratio; CI, confidence interval.
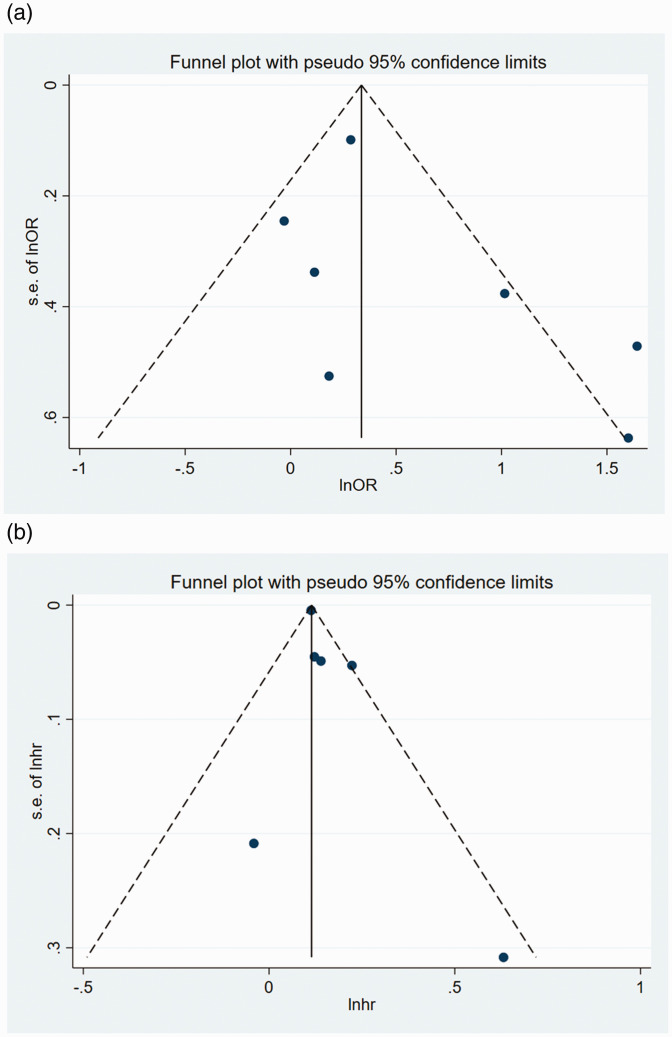
Evaluation of publication bias
Inverted funnel plots were next constructed to assess the OR and HR data for AF for publication bias. The shape of the funnel plots did not reveal obvious asymmetry (Figure 8(a) and (b)). In addition, Egger’s test generated p values of 0.219 and 0.223, respectively, for the OR and HR data with respect to AF. Publication bias was not assessed with respect to prolonged QT interval, PVC/PAC or heart block because of the small number of included studies.
Figure 8.
Funnel plots of studies reporting the risk of AF in adult participants with or without NAFLD. (a) OR data for participants with AF. (b) HR data for participants with AF. Pseudo-95% confidence intervals (dotted lines) are shown.
AF, atrial fibrillation; HR, hazard ratio; NAFLD, non-alcoholic liver disease; OR, odds ratio; s.e., standard error of the mean.
Discussion
In the present study, we performed a comprehensive search for observational studies in relevant databases to systematically characterize the relationship between NAFLD and the risk of cardiac arrhythmia. We found that NAFLD was significantly associated with an approximately two-fold higher risk of AF, independent of common established risk factors. However, two studies by Targher et al.25,27 showed a higher risk of AF in patients with diabetes and NAFLD than in those with diabetes but no NAFLD. This relationship was independent of age, sex, hypertension, glycated haemoglobin, circulating cholesterol concentration, left ventricular hypertrophy, prior history of heart failure, valvular heart disease, hyperthyroidism and other clinical risk factors for AF. Conversely, in the present study, we found that NAFLD was associated with only a 12% higher risk of AF, and there was no obvious heterogeneity of the included studies, which imbues this finding with a high level of credibility.
The present study has summarized the evidence to date regarding the relationship between NAFLD and AF, through a comprehensive review of the previously published original studies and meta-analyses. Similar to the present findings, Cai et al.11 conducted an analysis of six cohort studies and found that NAFLD was independently associated with a higher risk of incident AF (relative risk 1.19, 95% CI 1.04–1.31), independent of multiple cardiovascular risk factors. However, the authors did not include studies with case–control or cross-sectional designs, or those that did not adjust for other cardiovascular risk factors, which might have led to the exclusion of large-scale studies and weakened the evidence obtained. In another previous meta-analysis of nine observational studies (five cross-sectional and four longitudinal cohort studies), Mantovani et al.10 found that NAFLD was associated with a higher risk of AF (random-effects: OR=2.07, 95% CI 1.38–3.10; random-effects: HR=1.34, 95% CI 0.92–1.95). However, the results of this prior meta-analysis should be interpreted with caution, because these effect estimates were not separately obtained from the cross-sectional and longitudinal cohort studies and the type of effect estimate (OR or HR) obtained from the longitudinal cohort studies was not clearly discernible. A strength of the present study is that nine studies that were included in the previous meta-analysis were also included in the present meta-analysis. In addition, the number of participants in the 14 studies that were included in the present meta-analysis and showed an association with AF were approximately 19 times the number that were included in the meta-analysis conducted by Mantovani et al,10 and because the effect estimates (OR and HR) generated by the included studies (for example, cross-sectional or longitudinal cohort study) were clearly distinguished and analysed separately.
We also analysed the relationship between NAFLD and the risks of prolongation of the QT interval, PAC/PVC and heart block. The relationships of most of these cardiac arrhythmias were analysed in cross-sectional studies, with only one case–control study and no longitudinal cohort studies. Consequently, we were only able to show associations of NAFLD with higher risks of prolongation of the QT interval, PAC/PVC and heart block, and a causative link could not be established. Notably, participants with NAFLD exhibited much higher risks of cardiac arrhythmias (prolonged QT interval, PAC/PVC and heart block; by two-to-three-fold) than participants without, but there was a less than two-fold higher risk of AF. Therefore, future studies should focus not only on the risk of AF but also on the risk of other arrhythmias in patients with NAFLD. However, Mangi et al.36 showed evidence of a much higher risk of prolonged QT interval in patients with NAFLD (n = 1 study; unadjusted OR 5.09, 95% CI 2.92–8.86). This might be explained by potential confounding factors that had not been adjusted for because there were clear differences in the age, incidence of diabetics mellitus, BMI, blood pressure and incidence of cirrhosis between the participants with NAFLD and those without in this previous study. There was no heterogeneity identified in the previous two meta-analyses with respect to heart block and PAC/PVC, meaning that the pooled results were relatively reliable. Overall, we have confirmed that NAFLD is associated with a higher risk of cardiac arrhythmia, including AF. A previous meta-analysis showed that NAFLD is independently associated with the risk of fatal and non-fatal CVD, and that individuals with more ‘severe’ NAFLD were at higher risk.37 On this basis, and given that the prevalences of NAFLD and non-alcoholic steatohepatitis are increasing, a growing number of patients with liver disease will be affected by CVD and be candidates for cardiovascular therapies in the near future.38,39
The mechanisms linking NAFLD and cardiac arrhythmias remain unclear; however, they share multiple common risk factors that suggest relevant pathophysiological factors. NAFLD-related obesity, especially central obesity, leads to ectopic fat accumulation in the myocardium and pericardium, which results in functional and structural changes in the heart. Altered hepatic lipid metabolism might lead to greater production of atherogenic lipids, including very low-density lipoprotein, low-density lipoprotein and non-esterified fatty acids, during the onset and progression of NAFLD, which considerably increases the risk of cardiac arrhythmia.40 Furthermore, animal studies have shown that adipocytes in the posterior cardiac wall and epicardial tissue might affect the left atrial ion current and contribute to the generation of cardiac arrhythmias and other complications.41 Finally, Mantovani showed that pro-inflammatory factors (such as C-reactive protein, interleukin-6 and tumour necrosis factor-α), pro-fibrinogens (such as transforming growth factor-β), pro-oxidants and vasoactive and thrombotic factors (such as factor VIII, plasminogen activator inhibitor-1 and endothelin-1) are present at higher concentrations, which result in cardiac fibrosis and cardiac myocyte apoptosis during the onset and progression of NAFLD, through changes in the electrophysiological properties and structure of the myocardium, and the dysregulation of calcium homeostasis and connexins, which also alter electrical conduction through the myocardium42.
Several important potential limitations of the present meta-analysis should be mentioned. First, the included studies assessed hepatic steatosis using a variety of diagnostic methods, including ultrasonography, CT, FLI and ICD codes, which might have introduced heterogeneity into the analysis. Furthermore, liver biopsy is the current gold standard method for the diagnosis of NAFLD. Second, most of the studies used standard resting ECG, rather than 24-h Holter monitoring, to diagnose the arrhythmias; therefore, paroxysmal cardiac arrhythmias might have been missed in some of the participants. Third, adjustment for relevant potential confounding factors associated with cardiac arrhythmias was not performed in the generation of the partial effect estimates in the present study; and the included cohort studies, which are of superior quality with respect to study design, did not consider cardiac arrhythmias other than AF. Therefore, we might have failed to comprehensively evaluate the relationship of NAFLD with the risk of cardiac arrhythmia in patients. Fourth, the cross-sectional design of the analysis does not permit causality to be ascribed in the relationship between NAFLD and the risk of cardiac arrhythmia. Finally, few of the included studies assessed PAC/PVC and/or heart block, which placed the meta-analysis at the risk of some degree of publication bias.
The present study also had several strengths. Because of the sharp rises in the prevalences of NAFLD and cardiac arrhythmias worldwide, we believe that the results of this meta-analysis have important clinical implications. First, to the best of our knowledge, this was the first large-scale meta-analysis to evaluate the link between cardiac arrhythmias (including prolonged QT interval, PAC/PVC and heart block) and NAFLD. Second, For AF, we have extracted or calculated ORs (or HRs) and the related 95% CIs for each of the included studies, to increase the reliability of the results. Third, sensitivity analysis was also performed to identify factors that might have influenced the results. Finally, we conducted a comprehensive search of PubMed, Embase, the Web of Science database and the Cochrane Library to identify studies that investigated the relationship between NAFLD and the risk of cardiac arrhythmia. In addition, most of the studies included in the present meta-analysis were of high quality. All these factors strengthen our conclusions.
Conclusion
Our systematic review and meta-analysis have demonstrated a higher risk of cardiac arrhythmia, including AF, prolonged QT interval, PAC/PVC and heart block, in patients with NAFLD. Further prospective studies and basic research should be conducted to better understand the relationships between NAFLD and cardiac arrhythmias and the potential mechanisms involved.
Supplemental Material
Supplemental material, sj-pdf-1-imr-10.1177_03000605211047074 for Relationship between non-alcoholic fatty liver disease and cardiac arrhythmia: a systematic review and meta-analysis by Hang Gong, Xianli Liu and Fang Cheng in Journal of International Medical Research
Acknowledgements
We appreciate the contributions of all the physicians, co-workers and friends involved in this study, and thank the editors and reviewers for their help with this manuscript.
Footnotes
Author contributions: HG conceived and designed the study and collected data. HG participated in the design and coordination of the study and the analysis of the data. XLL drafted the manuscript. FC participated in the manuscript preparation and critical revision. All the authors read and approved the final version of the manuscript.
Declaration of conflicting interest: The authors declare that there is no conflict of interest.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
ORCID iD: Hang Gong https://orcid.org/0000-0001-5985-1254
References
- 1.Ballestri S, Zona S, Targher G, et al. Nonalcoholic fatty liver disease is associated with an almost twofold increased risk of incident type 2 diabetes and metabolic syndrome. Evidence from a systematic review and meta-analysis. J Gastroenterol Hepatol 2016; 31: 936–944. [DOI] [PubMed] [Google Scholar]
- 2.Nascimbeni F, Pais R, Bellentani S, et al. From NAFLD in clinical practice to answers from guidelines. J Hepatol 2013; 59: 859–871. [DOI] [PubMed] [Google Scholar]
- 3.Loomba R, Sanyal AJ.The global NAFLD epidemic. Nat Rev Gastroenterol Hepatol 2013; 10: 686–690. [DOI] [PubMed] [Google Scholar]
- 4.Li Z, Xue J, Chen P, et al. Prevalence of nonalcoholic fatty liver disease in mainland of China: a meta-analysis of published studies. J Gastroenterol Hepatol 2014; 29: 42–51. [DOI] [PubMed] [Google Scholar]
- 5.Cai X, Sun L, Liu X, et al. Non-alcoholic fatty liver disease is associated with increased risk of chronic kidney disease. Ther Adv Chronic Dis 2021; 12: 20406223211024361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goldberg D, Ditah IC, Saeian K, et al. Changes in the Prevalence of Hepatitis C Virus Infection, Nonalcoholic Steatohepatitis, and Alcoholic Liver Disease Among Patients with Cirrhosis or Liver Failure on the Waitlist for Liver Transplantation. Gastroenterology 2017; 152: 1090–1099.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fabbrini E, Sullivan S, Klein S.Obesity and Nonalcoholic Fatty Liver Disease: Biochemical, Metabolic, and Clinical Implications. Hepatology 2010; 51: 679–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mantovani A, Ballestri S, Lonardo A, et al. Cardiovascular Disease and Myocardial Abnormalities in Nonalcoholic Fatty Liver Disease. Dig Dis Sci 2016; 61: 1246–1267. [DOI] [PubMed] [Google Scholar]
- 9.Byrne CD, Targher G.NAFLD: a multisystem disease. J Hepatol 2015; 62: S47–S64. [DOI] [PubMed] [Google Scholar]
- 10.Mantovani A, Dauriz M, Sandri D, et al. Association between non-alcoholic fatty liver disease and risk of atrial fibrillation in adult individuals: An updated meta-analysis. Liver Int 2019; 39: 758–769. [DOI] [PubMed] [Google Scholar]
- 11.Cai X, Zheng S, Liu Y, et al. Nonalcoholic fatty liver disease is associated with increased risk of atrial fibrillation. Liver Int 2020; 40: 1594–1600. [DOI] [PubMed] [Google Scholar]
- 12.Page MJ, Moher D, Bossuyt PM, et al. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ 2021; 372: n160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000; 283: 2008–2012. [DOI] [PubMed] [Google Scholar]
- 14.Stang A.Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010; 25: 603–605. [DOI] [PubMed] [Google Scholar]
- 15.Li W, Huang A, Zhu H, et al. Gut microbiota-derived trimethylamine N-oxide is associated with poor prognosis in patients with heart failure. Med J Aust 2020; 213: 374–379. [DOI] [PubMed] [Google Scholar]
- 16.Cai X, Zhang Y, Li M, et al. Association between prediabetes and risk of all cause mortality and cardiovascular disease: updated meta-analysis. BMJ 2020; 370: m2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rostom A, Dubé C, Cranney A, et al. Celiac disease. Evid Rep Technol Assess (Summ) 2004; 104: 1–6. [PMC free article] [PubMed] [Google Scholar]
- 18.Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003; 327: 557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Käräjämäki AJ, Pätsi OP, Savolainen M, et al. Non-Alcoholic Fatty Liver Disease as a Predictor of Atrial Fibrillation in Middle-Aged Population (OPERA Study). PLoS One 2015; 10: e0142937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Labenz C, Huber Y, Michel M, et al. Impact of NAFLD on the Incidence of Cardiovascular Diseases in a Primary Care Population in Germany. Dig Dis Sci 2020; 65: 2112–2119. [DOI] [PubMed] [Google Scholar]
- 21.Lee SR, Han KD, Choi EK, et al. Nonalcoholic fatty liver disease and the risk of atrial fibrillation stratified by body mass index: a nationwide population-based study. Sci Rep 2021; 11: 3737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Long MT, Yin X, Larson MG, et al. Relations of Liver Fat with Prevalent and Incident Atrial Fibrillation in the Framingham Heart Study. J Am Heart Assoc 2017; 6: e005227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.You SC, Yang PS, Kim TH, et al. Non-alcoholic fatty liver disease is independently associated with new onset atrial fibrillation: a nationwide cohort study in Korea. J Am Coll Cardiol 2016; 67: 854. [Google Scholar]
- 24.Roh JH, Lee JH, Lee H, et al. Association between non-alcoholic fatty liver disease and risk of new-onset atrial fibrillation in healthy adults. Liver Int 2020; 40: 338–346. [DOI] [PubMed] [Google Scholar]
- 25.Targher G, Valbusa F, Bonapace S, et al. Non-alcoholic fatty liver disease is associated with an increased incidence of atrial fibrillation in patients with type 2 diabetes. PLoS One 2013; 8: e57183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Markus MR, Meffert PJ, Baumeister SE, et al. Association between hepatic steatosis and serum liver enzyme levels with atrial fibrillation in the general population: The Study of Health in Pomerania (SHIP). Atherosclerosis 2016; 245: 123–131. [DOI] [PubMed] [Google Scholar]
- 27.Targher G, Mantovani A, Pichiri I, et al. Non-alcoholic fatty liver disease is associated with an increased prevalence of atrial fibrillation in hospitalized patients with type 2 diabetes. Clin Sci (Lond) 2013; 125: 301–309. [DOI] [PubMed] [Google Scholar]
- 28.Whitsett M, Wilcox J, Yang A, et al. Atrial fibrillation is highly prevalent yet undertreated in patients with biopsy-proven nonalcoholic steatohepatitis. Liver Int 2019; 39: 933–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang Y, Li P, Miao M, et al. Nonalcoholic Fatty Liver Disease Is Associated with Increased Atrial Fibrillation Risk in an Elderly Chinese Population: A Cross-Sectional Study. Biomed Res Int 2018; 2018: 5628749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pastori D, Sciacqua A, Marcucci R, et al. Prevalence and Impact of Nonalcoholic Fatty Liver Disease in Atrial Fibrillation. Mayo Clin Proc 2020; 95: 513–520. [DOI] [PubMed] [Google Scholar]
- 31.Chung TH, Shim JY, Lee YJ.Nonalcoholic Fatty Liver Disease as a Risk Factor for Prolonged Corrected QT Interval in Apparently Healthy Korean Women. J Gastrointestin Liver Dis 2020; 29: 59–64. [DOI] [PubMed] [Google Scholar]
- 32.Hung CS, Tseng PH, Tu CH, et al. Nonalcoholic Fatty Liver Disease is Associated with QT Prolongation in the General Population. J Am Heart Assoc 2015; 4: e001820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Targher G, Valbusa F, Bonapace S, et al. Association of nonalcoholic fatty liver disease with QTc interval in patients with type 2 diabetes. Nutr Metab Cardiovasc Dis 2014; 24: 663–669. [DOI] [PubMed] [Google Scholar]
- 34.Mantovani A, Rigamonti A, Bonapace S, et al. Nonalcoholic Fatty Liver Disease Is Associated with Ventricular Arrhythmias in Patients with Type 2 Diabetes Referred for Clinically Indicated 24-Hour Holter Monitoring. Diabetes Care 2016; 39: 1416–1423. [DOI] [PubMed] [Google Scholar]
- 35.Mantovani A, Rigolon R, Pichiri I, et al. Nonalcoholic fatty liver disease is associated with an increased risk of heart block in hospitalized patients with type 2 diabetes mellitus. PLoS One 2017; 12: e0185459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mangi MA, Minhas AM, Rehman H, et al. Association of Non-alcoholic Fatty Liver Disease with Conduction Defects on Electrocardiogram. Cureus 2017; 9: e1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Targher G, Byrne CD, Lonardo A, et al. Non-alcoholic fatty liver disease and risk of incident cardiovascular disease: A meta-analysis. J Hepatol 2016; 65: 589–600. [DOI] [PubMed] [Google Scholar]
- 38.Cai J, Zhang XJ, Ji YX, et al. Nonalcoholic Fatty Liver Disease Pandemic Fuels the Upsurge in Cardiovascular Diseases. Circ Res 2020; 126: 679–704. [DOI] [PubMed] [Google Scholar]
- 39.Ballestri S, Capitelli M, Fontana MC, et al. Direct Oral Anticoagulants in Patients with Liver Disease in the Era of Non-Alcoholic Fatty Liver Disease Global Epidemic: A Narrative Review. Adv Ther 2020; 37: 1910–1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mantovani A.Nonalcoholic Fatty Liver Disease (NAFLD) and Risk of Cardiac Arrhythmias: A New Aspect of the Liver-heart Axis. J Clin Transl Hepatol 2017; 5: 134–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lin YK, Chen YC, Chen JH, et al. Adipocytes modulate the electrophysiology of atrial myocytes: implications in obesity-induced atrial fibrillation. Basic Res Cardiol 2012; 107: 293. [DOI] [PubMed] [Google Scholar]
- 42.Mantovani A.Nonalcoholic Fatty Liver Disease (NAFLD) and Risk of Cardiac Arrhythmias: A New Aspect of the Liver-heart Axis. J Clin Transl Hepatol 2017; 5: 134–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-imr-10.1177_03000605211047074 for Relationship between non-alcoholic fatty liver disease and cardiac arrhythmia: a systematic review and meta-analysis by Hang Gong, Xianli Liu and Fang Cheng in Journal of International Medical Research