Abstract
Recent studies have shown that the p300/CREB binding protein (CBP)-associated factor (PCAF) is involved in transcriptional activation. PCAF activity has been shown strongly associated with histone acetyltransferase (HAT) activity. In this report, we present evidence for a HAT-independent transcription function that is activated in the presence of the human T-cell leukemia virus type 1 (HTLV-1) Tax protein. In vitro and in vivo GST-Tax pull-down and coimmunoprecipitation experiments demonstrate that there is a direct interaction between Tax and PCAF, independent of p300/CBP. PCAF can be recruited to the HTLV-1 Tax responsive element in the presence of Tax, and PCAF cooperates with Tax in vivo to activate transcription from the HTLV-1 LTR over 10-fold. Point mutations at Tax amino acid 318 (TaxS318A) or 319 to 320 (Tax M47), which have decreased or no activity on the HTLV-1 promoter, are defective for PCAF binding. Strikingly, the ability of PCAF to stimulate Tax transactivation is not solely dependent on the PCAF HAT domain. Two independent PCAF HAT mutants, which knock out acetyltransferase enzyme activity, activate Tax transactivation to approximately the same level as wild-type PCAF. In contrast, p300 stimulation of Tax transactivation is HAT dependent. These studies provide experimental evidence that PCAF contains a coactivator transcription function independent of the HAT activity on the viral long terminal repeat.
Eukaryotic transcription control is achieved through a hierarchy of regulatory components. One of central focus in eukaryotic transcription at present is the function of coactivators such as CREB binding protein (CBP), p300, and PCAF. CBP and p300 are present in a variety of multicellular organisms from Caenorhabditis elegans to humans but are not present in yeast. Their function is essential since a homozygous deletion of the gene is lethal (75). Moreover, the proteins appear to be limiting in the cell since the loss of one allele can cause facial and limb abnormalities and mental retardation associated with the Rubinstein-Taybi syndrome (56). p300/CBP interacts with a wide variety of activators (see review in reference 65), including CREB (15, 41), c-Myb (18), c-Jun (5), MyoD (81), p53 (3, 30, 44, 62), adenovirus oncoprotein E1A (21), Tax (41), Tat (7, 47), and nuclear hormone receptors (13, 36) in a ligand-dependent manner. PCAF, a p300/CBP-associated factor, also interacts with a growing number of activators, such as muscle differentiation factor MyoD (59), retinoic acid receptor-retinoid hormone X receptor heterodimer (9), E1A (60), nuclear factor Y (NF-Y) (17), and Tat (7). Transcription activators can recruit these coactivators to upstream promoter elements, resulting in the enhancement of transcription (9, 41, 59).
Biochemical studies show that transcriptionally active chromatin is usually hyperacetylated (see reviews in references 72 and 73). The acetylation at lysine residues within the N termini of nucleosomal histones neutralizes the basic charge of the lysine and loosens the integrity of nucleosomes. p300, CBP, and PCAF all possess intrinsic histone acetyltransferase activity (HAT) (4, 53, 74). Further, it has been shown that p300/CBP and PCAF can activate selective promoters via intrinsic HAT activity (9, 39, 59, 60), suggesting that the histone acetylation may be important for the activation. Several observations, however, suggest that the activation mechanism may be complicated and distinct for each of the proteins. First, there are differences between their HAT activities in terms of the acetylation sites on histones (61), as well as the specificity for promoter activation. Second, p300/CBP and PCAF have been found to acetylate nonhistone proteins. p300/CBP acetylates general transcription factors TFIIE and TFIIF (33), tumor suppressor p53 (29, 44), transcription factor GATA-1 (10), and enhanceosome component HMG I(Y) (50). PCAF can also acetylate p53, but at a different site (45), and chromosomal protein HMG-17 (32). Third, p300 and CBP contain multiple activation domains and contact basal transcription factors TATA binding protein (TBP) and TFIIB (19, 42, 71). This indicates that p300/CBP may activate transcription through both recruitment and modification of histones and general transcription factors. Last, it has been reported that holo-PCAF is in a complex with more than 20 polypeptides including TBP-associated factors, human ADA2, and yeast ADA3, which appears to be different from the holo-polymerase II complex (52). Overall, the detailed mechanism of how p300/CBP and PCAF activate transcription from specific promoters remains to be elucidated.
Human T-cell lymphotrophic virus type 1 (HTLV-1) is a human retrovirus that causes adult T-cell leukemia (58, 80) and the degenerative neuromuscular disease tropical spastic paraparesis or HTLV-1-associated myelopathy (24, 54). The HTLV-1 proviral DNA encodes a 40-kDa protein, Tax, which is critical in HTLV-1 transformation (27, 28). The Tax protein not only regulates HTLV-1 gene expression (34, 55, 68) but also influences cellular gene expression (see reviews in references 12, 78, and 79). Tax has been shown previously to interact with p300/CBP, and CBP can activate Tax-mediated HTLV-1 transcription in vitro (38, 41).
In this study, we have investigated the function of PCAF in HTLV-1 transcription regulation. We found that PCAF can be recruited to the HTLV-1 Tax responsive element (TRE) site through direct interaction with Tax and enhance Tax-mediated HTLV-1 transcription. PCAF domain analysis suggests that the C terminus of PCAF is responsible for Tax binding and that this binding is independent of p300/CBP. Point mutations at Tax amino acid 318 (TaxS318A) or 319 to 320 (Tax M47), which decrease binding to PCAF, also decrease the functional interaction of Tax and PCAF. PCAF can increase Tax-dependent HTLV-1 transcription comparable to the activity of p300/CBP. Uniquely, while the HAT domain of p300 is required for HTLV-1 transcription stimulation, the PCAF HAT domain is not necessary for the activation. Our results reveal the important role of PCAF in HTLV-1 transcription and suggest a possible mechanism of PCAF activation distinct from the HAT activity.
MATERIALS AND METHODS
Protein purification.
Vectors for glutathione S-transferase (GST)–Tax truncations (including GST–Tax 151-353, GST–Tax 245-353, and GST–Tax 1-244) were generated by subcloning corresponding PCR products into pGEX-3X (Pharmacia). pGST-TaxS318A was obtained by subcloning the BamHI fragment of IEXS318A (63) into pGEX-3X. The GST-Tax protein and GST-Tax mutants were expressed from Escherichia coli and purified as described previously (16). The CREB protein was expressed from the pET-15b vector (kindly provided by R. Goodman’s laboratory) under the T7 promoter. Transformed E. coli BL21(DE3)(pLysS) was induced by 1 mM isopropyl-β-d-thiogalactopyranoside for 3 h. The protein was then purified through Mono Q and heparin columns and dialyzed in buffer E (25 mM Tris-HCl [pH 7.5], 50 mM NaCl, 0.1% Triton X-100, 5% glycerol, 1 mM dithiothreitol [DTT]). The Tax protein with a six-histidine tag at the C terminus (TaxH6) was purified from E. coli as previously described (82), with slight modification. HB101 cells containing plasmid pTaxH6 were grown, harvested, treated with lysozyme, and sonicated in buffer A (100 mM Tris-HCl [pH 8.0], 100 mM NaCl, 2.0 mM β-mercaptoethanol, 2 mM EDTA, 1 mM phenylmethylsulfonyl fluoride [PMSF]). Two 25% (wt/vol) ammonium sulfate precipitations were performed, and precipitates were dissolved in and dialyzed against buffer B (20 mM Tris-HCl [pH 7.8], 500 mM NaCl, 5 mM imidazole). Samples were loaded onto a Ni2+-charged HiTrap chelating column (Pharmacia) and eluted with a 60 to 400 mM imidazole gradient. The fractions of TaxH6 protein were pooled and dialyzed against buffer C (50 mM Tris-HCl [pH 8.0], 100 mM NaCl, 2 mM β-mercaptoethanol, 50% glycerol). The CBP(1-682) and M47 proteins and purification protocol have been described previously (38). PCAF protein and PCAF truncations PCAF(1-390), PCAF(Δ65-464), PCAF(1-529), PCAF(Δ579-608), PCAF(Δ609-623), and PCAF(352-832) were expressed as Flag-tagged fusion proteins in baculovirus-infected cells and purified through an anti-Flag M2 column (74).
Biotinylated DNA pull-down assay.
A 5′-biotinylated 76-base oligonucleotide (5′-AATTCCGTTGACGACAACCCCTCAGGCGTTGACGACAACCCAGATCTGAGGTCCACTTCGCTATATATTCCCCGAG 3′) was annealed to its antisense strand to form a double-stranded DNA. The sequence is the same as used in pTRE-1Id, containing two copies of promoter-proximal 21-bp repeats from the wild-type HTLV-1 long terminal repeat (LTR) inserted upstream of the chicken ovalbumin TATA box (20). Indicated amount of proteins were incubated with DNA in 100 μl of buffer [50 mM Tris (pH 7.6), 50 mM NaCl, 0.5 mM EDTA, 1 mM DTT, 5 mM MgCl2, 0.1% Triton, 5% glycerol, 2.5 mg of bovine serum albumin (BSA) per ml, 10 μg of poly(dI-dC) per ml] for 1 h at 30°C (41); 10 μl of streptavidin-coupled Dynabeads (Dynal) was added, and the mixture was incubated for 1 h at room temperature. Beads were washed four times with binding buffer without BSA and poly(dI-dC). Proteins were eluted by sodium dodecyl sulfate (SDS) loading buffer and loaded onto an SDS–4 to 20% gel. Immunoblotting was performed with PCAF polyclonal (74), anti-Tax (hybridoma 168B17-46-92; AIDS Research and Reference Reagent Program), CREB polyclonal (raised against full-length recombinant CREB), and anti-CBP polyclonal (38) antibodies.
GST-Tax fusion protein pull-down assay.
Four picomoles GST-Tax, GST-Tax truncations, or GST was incubated with 3 pmol of Flag-tagged PCAF or PCAF truncations in 50 μl of GST binding buffer (50 mM Tris-HCl, 150 mM NaCl, 0.5% NP-40, 1 mM PMSF, 1 mM DTT, 5% glycerol) at 30°C for 1 h; 50 μl of glutathione-Sepharose (50% slurry; Pharmacia) was added, and the mixture was incubated for 1 h at 4°C. Complexes were washed with GST washing buffer (50 mM Tris-HCl, 150 mM NaCl, 1.0% NP-40, 1 mM PMSF, 1 mM DTT, 5% glycerol) four times and eluted in loading buffer by boiling for 4 min. Components were subjected to SDS-polyacrylamide gel electrophoresis (PAGE) on a 4 to 20% gel and analyzed by anti-Flag M2 antibody immunoblotting.
In vitro and in vivo co-IP.
For the in vitro co-IP, 4 pmol of TaxH6 or M47 was incubated with 2 pmol of either Flag-PCAF or CBP(1-682) protein in buffer A (50 mM Tris-HCl [pH 7.6], 50 mM NaCl, 0.5 mM EDTA, 1 mM DTT, 5 mM MgCl2, 0.1% Triton, 5% glycerol, 2.5 mg of BSA per ml) for 1 h at 4°C; 1 μl of anti-Flag M2 monoclonal antibody or 4 μl of anti-CBP polyclonal antibody was added, and the mixture was incubated for 2 h. Complexes were bound to 25 μl of protein A/G-agarose beads (Calbiochem) by rocking for 2 h. The agarose beads were washed for three times with buffer B (50 mM Tris-HCl [pH 7.6], 150 mM NaCl, 1% NP-40, 1 mM EDTA, 5% glycerol) and boiled in loading buffer. SDS-PAGE and Western blotting were performed to analyze the proteins. HTLV-1-transformed T-cell C81 nuclear extracts were made in lysis buffer (20 mM HEPES [pH 7.3], 400 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1 mM DTT, 1 mM PMSF); 1.1 mg of nuclear extract (precleared with control rabbit immunoglobulin G [IgG]) was incubated with 50 μl antibody-bound protein A/G-coupled beads (beads were prebound with each of 5 μg of anti-Tax polyclonal antibody, anti-PCAF polyclonal antibody, or rabbit control IgG) in IP buffer (25 mM HEPES [pH 7.3], 2 mM EDTA, 130 mM NaCl, 0.1% NP-40, 1 mM DTT, 1 mM AEBSF, 10 μg of leupeptin per ml, 2 μg of aprotinin per ml, 10 μg of pepstatin A per ml) at 4°C for 3 h. Beads were washed with IP buffer four times and eluted in SDS-PAGE loading buffer.
Transfection and CAT assay.
Reporter plasmid pU3RCAT containing HTLV-1 LTR and Tax expression vector pCTax were described previously (16). PCAF and p300 expression vectors for transfection assays were as follows. pcx-PCAF (74) and pCI-Flag-PCAF (59) express the full-length PCAF. Vectors pCI-PCAF(Δ65-464), pCI-PCAF(1-529), pCI-PCAF(Δ579-608), and pCI-PCAF(Δ609-624) (59, 74) express Flag-tagged PCAF truncations. Plasmids pCI-p300, pCI-p300(Δ1472-1522), and pCI-p300(Δ1603-1653) express p300 and truncations (59). IEX (Tax) and IEXTaxS318A were described previously (63). All plasmids were purified by using a CsCl gradient or Qiagen kit. Indicated amounts of plasmid were electroporated into 7.5 × 106 Jurkat cells (or 5 × 106 NIH 3T3 cells), and cells were harvested after 24 h. All plasmid amounts in transfections were normalized with carrier DNA. Chloramphenicol acetyltransferase (CAT) assays were performed as described previously (37).
Free histone acetylation assay.
The assay is similar to that described previously (53). Purified PCAF or PCAF deletion mutants (100 ng) were used to acetylate 1 μg of free histone mixture (H1, H2, H3, and H4) (Sigma).
RESULTS
Tax physically interacts with PCAF in vitro independently of p300/CBP.
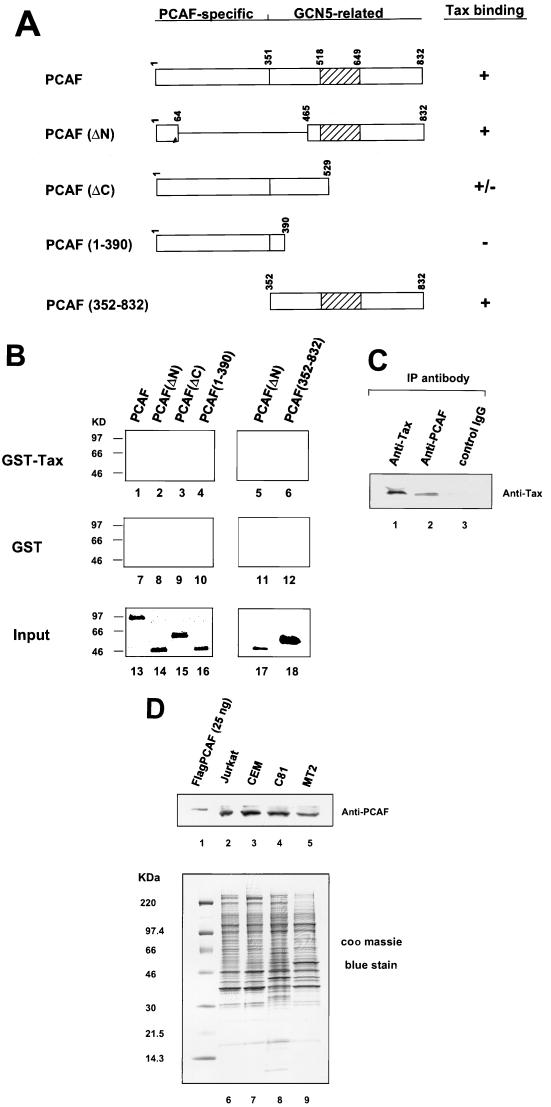
To determine whether Tax and PCAF interact and to localize the domain of PCAF required for Tax interaction, we analyzed the binding of wild-type and PCAF deletion mutants in an in vitro binding assay (Fig. 1A). PCAF protein was found to interact with GST-Tax but not the control GST protein (Fig. 1B, lanes 1 and 7). Similarly, the PCAF mutant lacking amino acids 65 to 464 [PCAF(ΔN)] binds to GST-Tax, but not the GST control, as efficiently as wild-type PCAF (lanes 1 and 2). A PCAF truncation containing amino acids 1 to 529 [PCAF(ΔC)] binds specifically but less efficiently to Tax (lane 3). PCAF(352-832) binds to Tax as well as PCAF(ΔN) (compare lanes 5 and 6). In contrast, a PCAF mutant containing N-terminal amino acids 1 to 390 failed to interact with Tax (lane 4). These results suggest that amino acids 465 to 529 of PCAF are important for the interaction with Tax. The fact that the C-terminal PCAF deletion [PCAF(ΔC)] showed weaker binding to Tax suggests that the carboxyl terminus of PCAF may contribute to the stability of the binding.
FIG. 1.
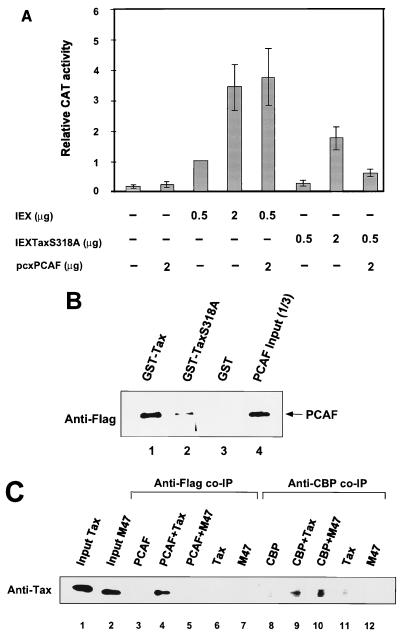
In vitro and in vivo binding assays showing that Tax associates with the C-terminal part of PCAF. (A) Schematic of PCAF. Hatched regions represent the HAT domain. (B) GST-Tax pull-down experiment. Flag-tagged PCAF and PCAF truncations were detected by anti-Flag M2 antibody Western blotting. Lanes 1 to 6, GST-Tax pull-down; lanes 7 to 12, GST control; lanes 13 to 18, input representing one-fourth of the amount added to the binding reaction. (C) In vivo co-IP of Tax with PCAF. Extracts from HTLV-1-transformed C81 cells were incubated with anti-Tax polyclonal, anti-PCAF polyclonal, or control IgG antibody to precipitate Tax and associated proteins. Immunoprecipitates were subjected to SDS-PAGE, a polyvinylidene transferred to membrane, and analyzed by Western blotting with an anti-Tax antibody. (D) PCAF expression levels in T cells and HTLV-1-transformed cells. Extracts from Jurkat, CEM, C81, and MT2 cells were subjected to SDS-PAGE and analyzed by Western blotting with an anti-PCAF antibody (lanes 1 to 5). Lane 1 is 25 ng of purified Flag-PCAF protein. The bottom panel represent a Coomassie blue-stained gel to demonstrate that equivalent amounts of protein were added to the gel.
We then determined whether PCAF could be coimmunoprecipitated with purified Tax without the GST tag. Following incubation of PCAF and TaxH6, a PCAF polyclonal antibody was used to coimmunoprecipitate proteins associated with PCAF. An anti-Tax monoclonal antibody was used to probe for the presence of Tax. Consistent with the results presented above, Tax can be specifically coimmunoprecipitated with PCAF protein (data not shown). These experiments provide further evidence that Tax and PCAF interact in vitro. Since the assays are done with purified proteins, these results further demonstrate that the interaction between Tax and PCAF is independent of p300 and CBP.
To demonstrate that Tax interacts with PCAF in vivo, the HTLV-1-transformed T-cell line C81, which constitutively expresses Tax, was used. Nuclear extracts were prepared and immunoprecipitated with either anti-Tax, anti-PCAF, or control IgG. The immunoprecipitates were washed four times with buffer containing 0.1% NP-40 and eluted in SDS-PAGE loading buffer (for details, see Materials and Methods). The results of the Western blot demonstrate that Tax is specifically coimmunoprecipitated with endogenous PCAF (Fig. 1C, lane 2).
Given the fact that HTLV-1 Tax interacts with endogenous PCAF, it was of interest to determine whether the cellular PCAF level was increased in HTLV-1-transformed cells. Sixty-microgram aliquots of nuclear extracts from normal T cells (Jurkat and CEM) and HTLV-1-transformed cells (C81 and MT2) were subjected to SDS-PAGE and analyzed by Western blotting with an anti-PCAF antibody (Fig. 1D, lanes 2 to 5). Lane 1 is a control purified Flag-PCAF sample (25 ng) which runs slightly higher than endogenous PCAF. Similar levels of PCAF expression were observed in the extracts from T cells and HTLV-1-transformed cells. Lanes 6 to 9 represent the Coomassie blue stain of 20 μg of each lysate to demonstrate that the same amount of protein was loaded for each sample.
PCAF is recruited to the HTLV-1 TRE site by Tax and stabilizes the DNA-Tax complex.
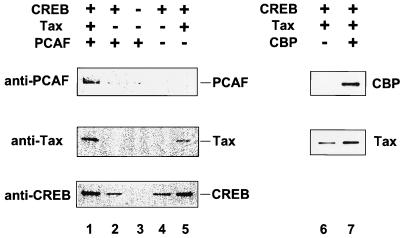
The HTLV-1 LTR contains three cis-acting regulatory elements, designated the 21-bp repeats, each of which contains a cyclic AMP response element (CRE) (11, 23, 49, 55). Members of the CREB transcription factor family bind to the CRE element. Tax protein binds indirectly to DNA through interaction with the sequence-specific CREB binding proteins (2, 6, 46, 70); 21-bp mutations which abolish CREB binding also abolish Tax binding (77). Tax activates HTLV-1 gene expression by facilitating the DNA binding of CREB, as well as the binding of coactivators such as CBP, through direct protein-protein interactions (22, 25, 41, 76, 83). The binding of p300/CBP stabilizes the CREB/Tax complex on DNA (43). To determine whether PCAF is involved in HTLV-1 promoter activation, we next examined whether PCAF can be recruited to the HTLV-1 LTR in the presence of Tax. For these studies, we used a 5′-biotin-labeled DNA probe containing two copies of the promoter-proximal HTLV-1 21-bp repeat. The biotinylated probe was incubated with recombinant, purified PCAF (Flag-PCAF) in the presence or absence of purified CREB and Tax proteins. Following incubation, the protein-DNA complexes were pulled down with avidin beads and subjected to SDS-PAGE and Western blot analysis with anti-PCAF or anti-CBP, anti-Tax, and anti-CREB antibodies (Fig. 2). In the absence of Tax and PCAF, a basal level of CREB binding was detected (Fig. 2, bottom, lane 4). Consistent with previous observations, Tax was found to associate with the DNA in the presence of CREB and increased the level of CREB binding (middle and bottom, lanes 4 and 5) (22, 25, 76, 83). The addition of PCAF alone or in combination with Tax did not increase CREB binding (lanes 1 and 2). We did observe, however, that the binding of Tax was increased approximately two- to threefold in the presence of CREB and PCAF (middle; compare lanes 1 and 5). Of importance, we found that while PCAF did not associate with purified CREB on the DNA, a significant level of PCAF binding was observed in the presence of CREB and Tax (top, lanes 1 and 2). PCAF does not bind to the DNA complex in the presence of only Tax or CREB plus Tax mutant M47 (data not shown). These results suggest that like CBP, PCAF can be recruited to the HTLV-1 TRE in the presence of Tax protein.
FIG. 2.
Biotinylated HTLV-1 TRE DNA pull-down experiment in which 0.2 μg of 5′-biotinylated DNA was incubated with different combinations of 20 pmol of CREB, TaxH6, Flag-PCAF, and CBP(1-682) in 100 μl of buffer as outlined in Materials and Methods. Western blotting with anti-PCAF polyclonal (top, lanes 1 to 5), anti-Tax (middle, lanes 1 to 7), anti-CREB polyclonal (bottom, lanes 1 to 5), and anti-CBP (top, lanes 6 and 7) antibodies was used to analyze the components in the pulled down DNA-protein complex.
To compare the abilities of PCAF and CBP to increase the binding of Tax to the CREB complex, we did a parallel DNA binding assay using purified CBP(1-682). This fragment of CBP contains the binding site for CREB and Tax and can stimulate transcription from the HTLV-1 LTR (38). CBP(1-682) was incubated with the biotinylated probe in the absence or presence of Tax, using the same conditions as for PCAF binding. In agreement with previous studies (41), CBP is recruited to DNA in the presence of Tax and CREB but not CREB alone (Fig. 2, lane 7; data not shown). The presence of CBP results in a slight enhancement of Tax binding (lanes 6 and 7). This result is also consistent with the finding of Lenzmeier et al. (43). The apparent discrepancy between this result and a previous report from this laboratory analyzing the Tax-CBP interaction is most likely due to the difference in protein binding assays (38). A consistent but small increase in Tax binding is observed with the biotinylated oligonucleotide binding assay but not the anisotropy assay (38). Our results demonstrate that PCAF and CBP increase Tax binding to the HTLV-1 TRE site to similar levels.
PCAF stimulates Tax-mediated HTLV-1 transcription.
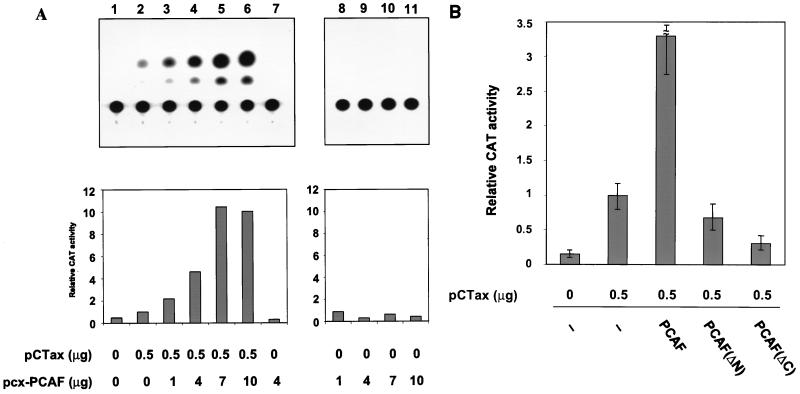
To determine the significance of the interaction between PCAF and Tax, we used transient transfection assays with Jurkat T lymphocytes and the HTLV-1 LTR-CAT plasmid pU3RCAT to see whether PCAF has any effect on Tax-mediated transcription. Subsaturating amounts of Tax were transfected into the cells, either alone or in the presence of increasing amounts of the PCAF expression plasmid. Tax alone can stimulate HTLV-1 LTR expression (Fig. 3A; compare lanes 1 and 2). Interestingly, cotransfection of increasing amounts of the PCAF expression plasmid resulted in a significant increase in CAT activity (Fig. 3A, lanes 3 to 6). Quantitation of the CAT activity shows up to a 10-fold stimulation in Tax transactivation. Transfection of PCAF alone does not increase transcription from the HTLV-1 LTR (Fig. 3A, lane 7 to 11). The levels of Tax protein expression were similar in the absence or presence of overexpressed PCAF, demonstrating that increased transcription was not due to stimulation of Tax expression (see below). We performed the same experiment with NIH 3T3 cells, which have a low level of p300/CBP (7), and observed a similar pattern of stimulation (data not shown).
FIG. 3.
PCAF stimulates Tax-mediated HTLV-1 transcription. (A) Human T-lymphocyte Jurkat cells were transiently transfected with 5 μg of HTLV-1–CAT reporter plasmid and pCTax and pcx-PCAF expression vectors as indicated. Cells were harvested after 24 h of incubation as described in Materials and Methods, and 1 μg of lysate was used for CAT assay. CAT activity was normalized to protein concentration. A bar graph representing the quantitative analysis of the CAT assay is shown below. The relative activities presented are calculated as fold activity, with the activity in the presence of Tax alone equal to 1. (B) Activity of PCAF mutants on activation of the HTLV-1 promoter. Four micrograms of each PCAF plasmid and 0.5 μg of pCTax were cotransfected into Jurkat cells with the HTLV-1 LTR reporter pU3RCAT. CAT assays were performed as described above. The results are the average of three independent assays.
Next, we used the PCAF truncation expression vectors in the transfection assays on the HTLV-1 promoter. As shown in Fig. 3C, neither PCAF(ΔN) nor PCAF(ΔC) activated HTLV-1 transcription, suggesting that both the N and C termini of PCAF are important for Tax activation. This result is also consistent with previous published data that deletions within the N or C terminus of PCAF abrogate the ability of PCAF to enhance the Sp1-driven reporter gene (40).
PCAF binds to the carboxyl terminus of Tax.
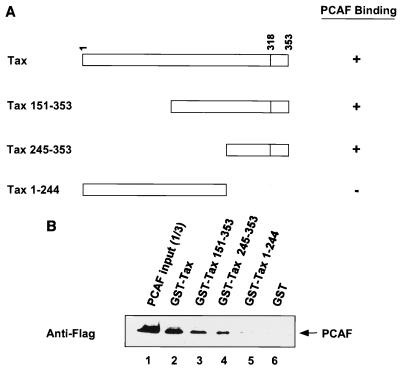
To map the PCAF binding domain on Tax, wild-type and Tax deletion mutants as GST fusion proteins were analyzed for the ability to bind to PCAF (Fig. 4A). Figure 4B represents a Western blot analysis using anti-Flag antibody to detect the pulled-down PCAF protein. The results of the binding assay demonstrate that GST-Tax (lane 2), GST–Tax 151-353 (lane 3), and GST–Tax 245-353 (lane 4) bind to PCAF with comparable efficiencies. In contrast, GST–Tax 1-244 failed to interact with the PCAF protein (lane 5).
FIG. 4.
Mapping the domain of Tax required for interaction with PCAF. (A) Schematic of Tax deletion mutants. (B) PCAF binds to the carboxy-terminal domain of Tax. PCAF and purified GST-Tax fusion proteins were incubated, pulled down with glutathione beads, and analyzed by Western blot analysis using an anti-Flag antibody.
As a control for the above experiments, we performed a CREB binding assay to show that GST–Tax 1-244 is functional. Although both GST-Tax and GST–Tax 1-244 bind CREB protein weakly, probably due to steric hindrance of the N-terminal CREB binding region by the GST tag (1), similar amounts of CREB protein were pulled down by GST-Tax and GST–Tax 1-244 (data not shown). CREB binding to both proteins was significantly higher than that observed with the GST control. Our results suggest, therefore, that PCAF interacts with the carboxyl terminus of Tax between amino acids 245 and 353.
PCAF fails to bind and functionally interact with Tax mutants S318A and M47.
To correlate the in vitro PCAF-Tax binding with the in vivo activation, we screened several Tax point mutations within the PCAF binding region (245 to 353), using transient transfection assays (63). The results with one of these mutants, TaxS318A, which has previously been shown to be partially defective in transactivation of the HTLV-1 LTR, are presented in Fig. 5A. Consistent with the original description of the mutant, we found that TaxS318A had approximately 50% of the activity of wild-type Tax protein (Fig. 5A; compare lanes 3 and 4 with lanes 6 and 7). Consistent with the results presented above, cotransfection of wild-type Tax (IEXTax) and PCAF stimulated HTLV-1 promoter activity approximately fourfold over that observed with Tax alone (lanes 3 and 5). In contrast, cotransfection of TaxS318A and PCAF did not result in a significant increase over the level observed with TaxS318A alone (lanes 6 and 8).
FIG. 5.
Tax mutants S318A and M47 fail to interact with PCAF in vitro and in vivo. (A) Jurkat transfection and CAT assay using IEXTax and IEX-TaxS318A. The pU3RCAT reporter was transfected with either wild-type IEXTax or mutant IEX-TaxS318A in the absence and presence of PCAF as indicated. Cells were then incubated for 24 h, and extracts were prepared as described in Materials and Methods. The relative activities presented are calculated as fold activity. (B) PCAF interacts weakly with Tax mutant S318A. PCAF was incubated with either GST-Tax or GST-TaxS318A, pulled down with glutathione beads, and assayed by Western blot analysis. (C) Tax mutant M47 (L319R-L320S) fails to interact with PCAF. Wild-type or M47 Tax was incubated with Flag-tagged PCAF or CBP(1-682) and subsequently immunoprecipitated with either an anti-Flag or anti-CBP antibody. Immunoprecipitates were run on an SDS-gel, and Western blot analysis was performed with an anti-Tax antibody. Lanes 1 and 2 represent one-fourth of the Tax or M47 protein added to the in vitro binding assay.
We next subcloned TaxS318A into a GST vector and used the purified fusion protein in a PCAF binding assay. As shown in Fig. 5B, GST-TaxS318A interacts weakly with PCAF compared to wild-type Tax (compare lanes 1 and 2). Densitometric quantitation of the bands indicates that TaxS318A binds approximately sixfold less efficiently to PCAF than wild-type Tax. These results show a parallel relationship between PCAF-Tax association and Tax or PCAF activation of the HTLV-1 promoter.
Tax mutant M47 (L319R-L320S) is mutated at a position adjacent to TaxS318A. M47 is an important mutant in that it fails to activate HTLV-1 transcription and is defective for transformation (66). To test the ability of M47 protein to interact with PCAF, we used an in vitro co-IP assay. Wild-type and M47 Tax were incubated with PCAF and immunoprecipitated with anti-Flag monoclonal antibody. The immunoprecipitate was washed with IP buffer, and the presence of Tax protein was determined by Western blot analysis with an anti-Tax antibody. The results of this experiment demonstrate that the M47 protein is defective for interaction with PCAF. Although the same amounts of Tax and the M47 mutant were added to the assay, approximately 10-fold less M47 protein coimmunoprecipitated with PCAF (Fig. 5C, lanes 1, 2, 4, and 5).
For comparison, we have analyzed the ability of wild-type and M47 Tax to interact with CBP. The purified Tax proteins were incubated with CBP and then immunoprecipitated with an anti-CBP polyclonal antibody. The immunoprecipitate was washed with IP buffer, and the presence of Tax protein was determined by Western blot analysis with an anti-Tax antibody. Both wild-type and M47 Tax were found to interact with the CBP (Fig. 5C, lanes 9 and 10). These results clearly show that M47 Tax binds to CBP but not PCAF. We suggest, therefore, that the defect in M47 activation of the HTLV-1 promoter may be in part due to the weak interaction with PCAF.
We have also tested the functional interaction of M47 Tax and PCAF in transient transfection assays. PCAF did not stimulate transcription from the HTLV-1 LTR in the presence of M47 Tax, just as PCAF failed to stimulate transcription in the presence of Tax S318A (Fig. 5A; data not shown). Thus, two Tax mutants which fail to interact with PCAF in vitro also fail to interact with PCAF in vivo.
PCAF and p300 stimulate Tax transactivation of the HTLV-1 LTR to similar levels.
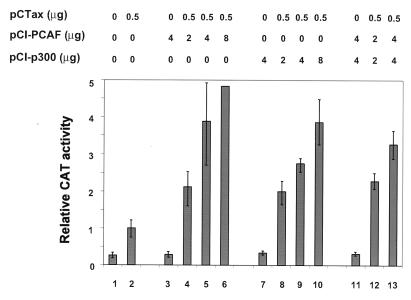
It has been reported that p300 and CBP are involved in the activation of Tax-mediated HTLV-1 transcription (38, 41). It was of interest, therefore, to compare the activation of PCAF and p300/CBP on HTLV-1 transcription. pCI-PCAF and pCI-p300 expression vectors, in which PCAF and p300 were cloned downstream of the same promoter, were used to overexpress Flag-tagged proteins in Jurkat T lymphocytes. We observed that PCAF activates Tax transactivation of the HTLV-1 LTR (Fig. 6, lanes 3 to 6) to a level similar to that seen with p300 (lanes 7 to 10). To see if PCAF and p300 can synergistically activate the HTLV-1 promoter, we cotransfected Tax, PCAF, and p300 (lanes 11 to 13). We did not observe a synergistic level of activation when both PCAF and p300 were cotransfected with Tax. Moreover, coexpression of both did not significantly increase transcription over that observed with either of the proteins alone.
FIG. 6.
Comparison of the activities of PCAF and p300 as coactivators on the HTLV-1 promoter. Tax, PCAF, and p300 expression vectors were cotransfected with 5 μg of pU3RCAT into Jurkat cells as indicated. The relative activities presented are calculated as fold activity, with the activity in the presence of Tax alone equal to 1. The values represent the average of three independent experiments.
HAT activity of PCAF is not necessary for the activation of HTLV-1 transcription.
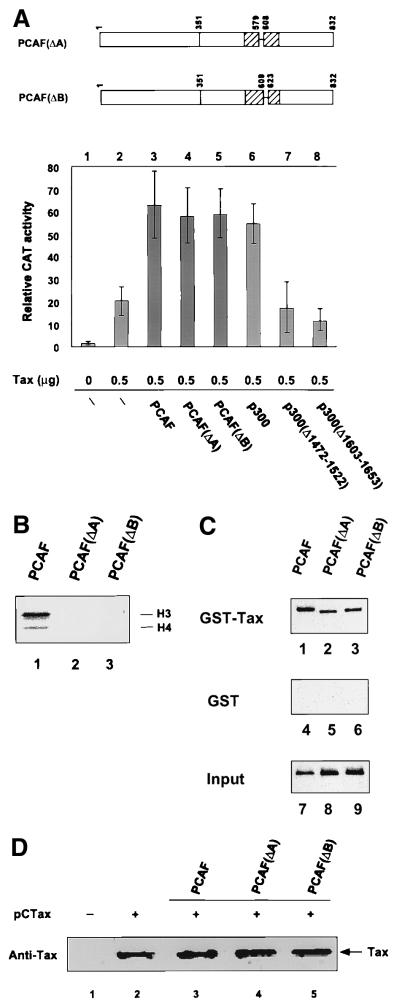
PCAF, p300, and CBP are HATs. HAT enzymatic activity is required for activation of at least some promoters, since mutation of the HAT domain inhibits transcription (7, 9, 39, 59, 69). It was of importance to determine if PCAF and p300 HAT activity was required for Tax transactivation. Two PCAF truncations of the HAT domain, PCAF(Δ579-608) and PCAF(Δ609-623), which have deletions at acetyl coenzyme A binding motifs A and B (51), were used in the transfection assays and are referred to below as PCAF(ΔA) and PCAF(ΔB). Interestingly, both pCI-PCAF(ΔA) and pCI-PCAF(ΔB) activate HTLV-1 transcription to a level comparable to that seen with wild-type PCAF (Fig. 7A; compare lanes 3, 4, and 5). To confirm and unambiguously demonstrate that HAT enzyme activity was indeed knocked out by the mutants, the activity of recombinant PCAF proteins carrying identical mutations were checked by an acetylation assay for free histones. The results presented in Fig. 7B demonstrate that in fact both PCAF(ΔA) and PCAF(ΔB) are free of any HAT activity (Fig. 7B, lane 2 and 3), consistent with previous work (59). The in vitro binding between Tax and the two PCAF HAT mutants was checked in the GST-Tax binding assay as shown in Fig. 7C. Both of the proteins bind to the Tax protein (lanes 2 and 3), similar to the level observed with wild-type PCAF (lane 1). The levels of Tax expression in the cell extracts in the presence of overexpressed PCAF, PCAF(ΔA), and PCAF(ΔB) were determined. No difference was observed, as shown by the anti-Tax Western blot (Fig. 7D). Together, these results suggest that PCAF can activate HTLV-1 transcription through a transcriptional activation domain which is independent of the HAT activity.
FIG. 7.
Effect of the PCAF HAT domain on HTLV-1 transcription. (A) Jurkat cells were transfected Tax and PCAF, PCAF HAT mutants, p300, or p300 HAT mutants as indicated, and CAT assays were performed as described in Materials and Methods. PCAF(ΔA) contains a deletion of amino acids 579 to 608; PCAF(ΔB) contains a deletion of amino acids 609 to 623. Both deletion mutants lack HAT activity. Two p300 mutants containing deletions with the p300 HAT domain, amino acids 1472 to 1522 and 1603 to 1653, were compared to wild-type p300. The PCAF and p300 proteins were expressed from the same pCI vector. (B) Histone acetylation assay. Both PCAF(ΔA) and PCAF(ΔB) were expressed as Flag-tagged fusion proteins in baculovirus-infected cells and purified through an anti-Flag affinity column. The positions of histones H3 and H4 in the gel are indicated. (C) Interaction of GST-Tax with PCAF and PCAF deletion mutants. The GST binding assays were performed as described in Materials and Methods. GST-bound proteins were subjected to SDS-PAGE, and Western blot analysis was performed with an anti-Flag M2 antibody. The top, middle, and bottom panels represent GST-Tax-bound, GST-bound, and input PCAF protein, respectively. The bottom panel represents one-fourth of the PCAF added to the GST-Tax or GST binding assays. (D) PCAF, PCAF(ΔA), and PCAF(ΔB) do not increase Tax expression level. Jurkat cells were cotransfected with 5 μg of pCTax with either control plasmid (lane 2), PCAF (lane 3), PCAF(ΔA) (lane 4), or PCAF(ΔB) (lane 5). Nuclear extracts were made, and 60-μg aliquots of the proteins were subjected to SDS-PAGE and analyzed by anti-Tax Western blotting.
In parallel experiments, we examined the functional characteristics of p300 proteins containing deletion mutants of the HAT domain. Two p300 truncations of the HAT domain at 1472 to 1522 and 1603 to 1653 were used (59). Similar to the PCAF HAT mutants, the p300 proteins have been shown to lack HAT activity (59). In contrast to the results observed with PCAF, mutations in the p300 HAT domain abolished the p300 activation of Tax transactivation of the HTLV-1 LTR (Fig. 7A, lanes 7 and 8).
We also performed experiments with PCAF and p300 function on a MyoD-driven promoter. In agreement with previous studies, we observed that both wild-type and p300 HAT mutants [p300(Δ1472-1522) and p300(Δ1603 to 1653)] activate transcription (59). In contrast, PCAF HAT mutants [PCAF(ΔA) and PCAF(ΔB)] fail to activate transcription. These results argue strongly that the requirement for PCAF or p300 HAT dependency is promoter specific.
DISCUSSION
In this study, we have demonstrated an important role for PCAF in Tax transactivation of the viral LTR. In vitro binding studies indicate that PCAF can be recruited to the HTLV-1 promoter, independently of p300/CBP, through direct interaction with Tax. The correlation between in vitro Tax-PCAF binding assays and in vivo cotransfection assays suggest that PCAF activate through direct interaction with Tax. Tax mutant TaxS318A, which decreases binding to PCAF, also decreases cooperating with PCAF to activate the HTLV-1 promoter in cotransfection assays. Another important Tax mutant, M47, that cannot activate HTLV-1 transcription also fails to bind PCAF. PCAF stimulates Tax transactivation as efficiently as p300, and cotransfection of both coactivators does not significantly increase transcription over the level seen with either one alone. Strikingly, while the HAT activity of p300 is required for the HTLV-1 transcription, the HAT domain of PCAF is dispensable for Tax-mediated activation. These results suggest overlapping but distinct roles of p300 and PCAF in HTLV-1 transcription. Most important, our data demonstrate that a non-HAT activity of PCAF is important for viral LTR transcription.
Previous studies have shown that p300/CBP and PCAF form a coactivator complex to facilitate gene transcription (14). It does not appear, however, that PCAF and p300/CBP cooperate for Tax transactivation. No synergistic effect was observed between p300 and PCAF on HTLV-1 transcription. The independent function of PCAF is consistent with the previous observation that PCAF retains activator function independently of p300/CBP. Reid et al. (60) have shown that PCAF stimulates transcription in yeast, which has no p300/CBP. Moreover, an N-terminal deletion of PCAF which removes the interaction site for CBP was able to activate transcription from the Rous sarcoma virus promoter in mammalian cells.
The finding that p300 and PCAF both activate HTLV-1 gene expression raises the question as to why multiple coactivators are involved in HTLV-1 transcription regulation. One possibility is that p300 and PCAF provide alternative mechanisms to ensure that the activation occurs. The activity may be dependent on the relative levels of p300/CBP and PCAF in the cell. Western blot analysis of cell extracts show that levels of both p300 and PCAF vary between cell types (data not shown). The activation mechanism may also depend on the chromatin configuration. It is possible that there are subtle changes from one cell type to another that would favor or require a particular coactivator function. It will be of interest to define which coactivator plays a major role in HTLV-1 LTR regulation in different cell lines or different stages of the cell cycle.
It appears that the requirement of the PCAF and p300 HAT domain for transcriptional activity is likely promoter dependent. PCAF has been shown to activate MyoD, MDR1, and retinoic acid-dependent activation of promoters through its HAT domain (9, 35, 59). Similarly, Reid et al. have shown that the p300/CBP-independent activation function of PCAF is also dependent on the HAT activity (60). In contrast, Kurzus et al. have reported that PCAF activation of a CRE-LacZ promoter construct is not dependent on the PCAF HAT domain (39). Similarly, PCAF activation of the HTLV-1 LTR is retained when the HAT domain is deleted. Moreover, Munish et al. observe no correlation between beta interferon expression and PCAF acetylation with HMG I(Y) as the substrate (50). A recent paper shows that a HAT-deficient PCAF mutant can increase cyclin D1-estrogen receptor activity (48). The promoter-specific configuration of transcription factors may determine the requirement for specific acetyltransferase activity. These studies also bring up the interesting possibility that PCAF, as a member of the GCN5 family, contains, in addition to the HAT domain, a transcription activation domain that expands the functions of PCAF as a coactivator.
It is interesting to consider the possible mechanism by which PCAF regulates transcription other than its acetylation activity. It has been reported that PCAF is involved in the elongation process by contacting the elongation-competent, hyperphosphorylated form of RNA polymerase II complex (14). PCAF has also been reported to be a component of non-holo-polymerase II-type complex, the PCAF histone acetylase complex, which resembles the yeast SAGA complex (26, 52). It is possible, therefore, that while PCAF HAT activity is not essential, PCAF activates HTLV-1 transcription by recruiting other components into the PCAF histone acetylase complex. While our results suggest that the PCAF HAT domain is not necessary for HTLV-1 transcription, this does not rule out the possibility that other proteins within the complex provide HAT activity in vivo.
p300/CBP proteins contain multiple activation domains which interact with the basal transcription factors, including TBP and TFIIB. It was originally suggested that the coactivators function by connecting sequence-specific activators with the basal transcription machinery and aiding the formation of the preinitiation complex. This concept, however, was simplistic in light of observations that CBP and p300 have HAT activity. Although the HAT activity of p300 is not required for transcription activation of promoters such as the MyoD and retinoic acid receptor promoters, it is required for Tax transactivation of the HTLV-1 LTR. These results emphasize that similar to findings for PCAF, the mechanism of p300 activation and the requirement for HAT activity are dependent on the promoter. An intriguing question is the target of HAT activity. It will be of interest to determine whether p300 HAT activity is required for acetylation of histones or general transcription factors such as TFIIE or TFIIF.
Tax mutant M47 (L319R-L320S) is an interesting and important Tax mutant which is defective in transactivating the HTLV-1 LTR via the CREB pathway (66). Interestingly, the fusion of different domains of Tax to the Gal4 DNA binding domain suggest that a transactivation domain exist within the carboxyl terminus of Tax (64). The activation domain does not likely represent the binding domain for CREB, since protein binding studies have indicated that the NH2 terminus of Tax is critical for interaction with CREB (1) and that the M47 mutation does not affect this interaction. Moreover, the interaction of Tax with p300/CBP does not seem to be affected by the mutation in M47 Tax (8, 31) (Fig. 5C). In fact, investigators have recently mapped the CBP interaction domain close to the amino terminus of Tax (31). The results presented in this study clearly demonstrate that amino acids including and surrounding the M47 site dramatically affect PCAF binding. Our results suggest that the defect in CREB transactivation exhibited by M47 may reflect a decrease in the ability to assimilate PCAF into the CREB-Tax-DNA complex. It is important to also note that M47 Tax has been reported to be defective in transformation of certain cell types including rat fibroblasts (67). The ability of Tax to bind PCAF may, therefore, be linked to the transformation potential of the Tax protein in certain cell types.
PCAF may also play an important role in Tax transactivation of cellular genes induced through the NF-Y pathway. NF-Y has been shown to play an important role in the cell cycle regulation of cyclin A and cdc2, in addition to its role as a key transcription factor for a variety of cellular genes including the major histocompatibility complex (MHC) class II gene. We have previously reported that Tax increases transcription from the MHC class II gene through direct interaction with the NF-Y subunit NF-YB (57). The recent observation that NF-Y is associated with the PCAF coactivator (17) suggests that Tax transactivation of the MHC class II promoter may be PCAF dependent. Studies are in progress to analyze the importance of PCAF in Tax-mediated MHC class II gene regulation.
ACKNOWLEDGMENTS
We thank Pier Lorenzo Puri for providing MyoD expression vectors and p21 promoter plasmid. We are grateful to Oliver John Semmes for providing IEXTax, IEXS318A, and other Tax mutation expression vectors. We thank Janet Duvall for editorial assistance in preparation of the manuscript.
REFERENCES
- 1.Adya N, Giam C Z. Distinct regions in human T-cell lymphotropic virus type I tax mediate interactions with activator protein CREB and basal transcription factors. J Virol. 1995;69:1834–1841. doi: 10.1128/jvi.69.3.1834-1841.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adya N, Zhao L J, Huang W, Boros I, Giam C Z. Expansion of CREB’s DNA recognition specificity by Tax results from interaction with Ala-Ala-Arg at positions 282-284 near the conserved DNA-binding domain of CREB. Proc Natl Acad Sci USA. 1994;91:5642–5646. doi: 10.1073/pnas.91.12.5642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Avantaggiati M L, Ogryzko V, Gardner K, Giordano A, Levine A S, Kelly K. Recruitment of p300/CBP in p53-dependent signal pathways. Cell. 1997;89:1175–1184. doi: 10.1016/s0092-8674(00)80304-9. [DOI] [PubMed] [Google Scholar]
- 4.Bannister A J, Kouzarides T. The CBP co-activator is a histone acetyltransferase. Nature. 1996;384:641–643. doi: 10.1038/384641a0. [DOI] [PubMed] [Google Scholar]
- 5.Bannister A J, Oehler T, Wilhelm D, Angel P, Kouzarides T. Stimulation of c-Jun activity by CBP: c-Jun residues Ser63/73 are required for CBP induced stimulation in vivo and CBP binding in vitro. Oncogene. 1995;11:2509–2514. [PubMed] [Google Scholar]
- 6.Beimling P, Moelling K. Direct interaction of CREB protein with 21 bp Tax-response elements of HTLV-ILTR. Oncogene. 1992;7:257–262. [PubMed] [Google Scholar]
- 7.Benkirane M, Chun R F, Xiao H, Ogryzko V V, Howard B H, Nakatani Y, Jeang K T. Activation of integrated provirus requires histone acetyltransferase. p300 and P/CAF are coactivators for HIV-1 Tat. J Biol Chem. 1998;273:24898–24905. doi: 10.1074/jbc.273.38.24898. [DOI] [PubMed] [Google Scholar]
- 8.Bex F, Yin M J, Burny A, Gaynor R B. Differential transcriptional activation by human T-cell leukemia virus type 1 Tax mutants is mediated by distinct interactions with CREB binding protein and p300. Mol Cell Biol. 1998;18:2392–2405. doi: 10.1128/mcb.18.4.2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blanco J C, Minucci S, Lu J, Yang X J, Walker K K, Chen H, Evans R M, Nakatani Y, Ozato K. The histone acetylase PCAF is a nuclear receptor coactivator. Genes Dev. 1998;12:1638–1651. doi: 10.1101/gad.12.11.1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boyes J, Byfield P, Nakatani Y, Ogryzko V. Regulation of activity of the transcription factor GATA-1 by acetylation. Nature. 1998;396:594–598. doi: 10.1038/25166. [DOI] [PubMed] [Google Scholar]
- 11.Brady J, Jeang K T, Duvall J, Khoury G. Identification of p40x-responsive regulatory sequences within the human T-cell leukemia virus type I long terminal repeat. J Virol. 1987;61:2175–2181. doi: 10.1128/jvi.61.7.2175-2181.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brady J N. Biology of HTLV-1:host cell interactions. In: Hollsberg P, Hafler D A, editors. Human T-cell lymphotropic virus type I. Chichester, England: John Wiley & Sons Ltd.; 1996. pp. 79–112. [Google Scholar]
- 13.Chakravarti D, LaMorte V J, Nelson M C, Nakajima T, Schulman I G, Juguilon H, Montminy M, Evans R M. Role of CBP/P300 in nuclear receptor signalling. Nature. 1996;383:99–103. doi: 10.1038/383099a0. [DOI] [PubMed] [Google Scholar]
- 14.Cho H, Orphanides G, Sun X, Yang X J, Ogryzko V, Lees E, Nakatani Y, Reinberg D. A human RNA polymerase II complex containing factors that modify chromatin structure. Mol Cell Biol. 1998;18:5355–5363. doi: 10.1128/mcb.18.9.5355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chrivia J C, Kwok R P S, Lamb N, Haglwara M, Montminy M R, Goodman R H. Phosphorylated CREB binds specifically to the nuclear protein CBP. Nature. 1993;365:855–859. doi: 10.1038/365855a0. [DOI] [PubMed] [Google Scholar]
- 16.Clemens K E, Piras G, Radonovich M F, Choi K S, Duvall J F, DeJong J, Roeder R, Brady J N. Interaction of the human T-cell lymphotropic virus type 1 tax transactivator with transcription factor IIA. Mol Cell Biol. 1996;16:4656–4664. doi: 10.1128/mcb.16.9.4656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Currie R A. NF-Y is associated with the histone acetyltransferases GCN5 and P/CAF. J Biol Chem. 1998;273:1430–1434. doi: 10.1074/jbc.273.3.1430. [DOI] [PubMed] [Google Scholar]
- 18.Dai P, Akimaru H, Tanaka Y, Hou D X, Yasukawa T, Kanei-Ishii C, Takahashi T, Ishii S. CBP as a transcriptional coactivator of c-Myb. Genes Dev. 1996;10:528–540. doi: 10.1101/gad.10.5.528. [DOI] [PubMed] [Google Scholar]
- 19.Dallas P B, Yaciuk P, Moran E. Characterization of monoclonal antibodies raised against p300: both p300 and CBP are present in intracellular TBP complexes. J Virol. 1997;71:1726–1731. doi: 10.1128/jvi.71.2.1726-1731.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Duvall J F, Kashanchi F, Cvekl A, Radonovich M F, Piras G, Brady J N. Transactivation of the human T-cell lymphotropic virus type 1 Tax1-responsive 21-base-pair repeats requires holo-TFIID and TFIIA. J Virol. 1995;69:5077–5086. doi: 10.1128/jvi.69.8.5077-5086.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eckner R, Ewen M E, Newsome D, Gerdes M, DeCaprio J A, Lawrence J B, Livingston D M. Molecular cloning and functional analysis of the adenovirus E1A-associated 300-kD protein (p300) reveals a protein with properties of a transcriptional adaptor. Genes Dev. 1994;8:869–884. doi: 10.1101/gad.8.8.869. [DOI] [PubMed] [Google Scholar]
- 22.Franklin A A, Kubik M F, Uittenbogaard M N, Brauweiler A, Utaisincharoen P, Matthews M A, Dynan W S, Hoeffler J P, Nyborg J K. Transactivation by the human T-cell leukemia virus Tax protein is mediated through enhanced binding of activating transcription factor-2 (ATF-2) ATF-2 response and cAMP element-binding protein (CREB) J Biol Chem. 1993;268:21225–21231. [PubMed] [Google Scholar]
- 23.Fujisawa J, Toita M, Yoshida M. A unique enhancer element for the trans activator (p40tax) of human T-cell leukemia virus type I that is distinct from cyclic AMP- and 12-O-tetradecanoylphorbol-13-acetate-responsive elements. J Virol. 1989;63:3234–3239. doi: 10.1128/jvi.63.8.3234-3239.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gessain A, Barin F, Vernant J C, Gout O, Maurs L, Calender A, de The G. Antibodies to human T-lymphotropic virus type-I in patients with tropical spastic paraparesis. Lancet. 1985;ii:407–410. doi: 10.1016/s0140-6736(85)92734-5. [DOI] [PubMed] [Google Scholar]
- 25.Giebler H A, Loring J E, van Orden K, Colgin M A, Garrus J E, Escudero K W, Brauweiler A, Nyborg J K. Anchoring of CREB binding protein to the human T-cell leukemia virus type 1 promoter: a molecular mechanism of Tax transactivation. Mol Cell Biol. 1997;17:5156–5164. doi: 10.1128/mcb.17.9.5156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grant P A, Schieltz D, Pray-Grant M G, Steger D J, Reese J C, Yates III J R, Workman J L. A subset of TAF(II)s are integral components of the SAGA complex required for nucleosome acetylation and transcriptional stimulation. Cell. 1998;94:45–53. doi: 10.1016/s0092-8674(00)81220-9. [DOI] [PubMed] [Google Scholar]
- 27.Grassmann R, Berchtold S, Radant I, Alt M, Fleckenstein B, Sodroski J G, Haseltine W A, Ramstedt U. Role of human T-cell leukemia virus type 1 X region proteins in immortalization of primary human lymphocytes in culture. J Virol. 1992;66:4570–4575. doi: 10.1128/jvi.66.7.4570-4575.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grassmann R, Dengler C, Muller-Fleckenstein I, Fleckenstein B, McGuire K, Dokhelar M C, Sodroski J G, Haseltine W A. Transformation to continuous growth of primary human T lymphocytes by human T-cell leukemia virus type I X-region genes transduced by a Herpesvirus saimiri vector. Proc Natl Acad Sci USA. 1989;86:3351–3355. doi: 10.1073/pnas.86.9.3351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gu W, Roeder R G. Activation of p53 sequence-specific DNA binding by acetylation of the p53 C-terminal domain. Cell. 1997;90:595–606. doi: 10.1016/s0092-8674(00)80521-8. [DOI] [PubMed] [Google Scholar]
- 30.Gu W, Shi X L, Roeder R G. Synergistic activation of transcription by CBP and p53. Nature. 1997;387:819–823. doi: 10.1038/42972. [DOI] [PubMed] [Google Scholar]
- 31.Harrod R, Tang Y, Nicot C, Lu H S, Vassilev A, Nakatani Y, Giam C Z. An exposed KID-like domain in human T-cell lymphotropic virus type 1 Tax is responsible for the recruitment of coactivators CBP/p300. Mol Cell Biol. 1998;18:5052–5061. doi: 10.1128/mcb.18.9.5052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Herrera J E, Sakaguchi K, Bergel M, Trieschmann L, Nakatani Y, Bustin M. Specific acetylation of chromosomal protein HMG-17 by PCAF alters its interaction with nucleosomes. Mol Cell Biol. 1999;19:3466–3473. doi: 10.1128/mcb.19.5.3466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Imhof A, Yang X J, Ogryzko V V, Nakatani Y, Wolffe A P, Ge H. Acetylation of general transcription factors by histone acetyltransferases. Curr Biol. 1997;7:689–692. doi: 10.1016/s0960-9822(06)00296-x. [DOI] [PubMed] [Google Scholar]
- 34.Jeang K T, Brady J, Radonovich M, Duvall J, Khoury G. p40X transactivation of the HTLV-1 LTR promoter. In: Cullen B, Gage L P, Siddiqi M A Q, Skalka A M, Weissbach H, editors. Mechanisms of the control of gene expression. New York, N.Y: Alan R. Liss, Inc.; 1988. pp. 181–189. [Google Scholar]
- 35.Jin S, Scotto K W. Transcriptional regulation of the MDR1 gene by histone acetyltransferase and deacetylase is mediated by NF-Y. Mol Cell Biol. 1998;18:4377–4384. doi: 10.1128/mcb.18.7.4377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kamei Y, Xu L, Heinzel T, Torchia J, Kurokawa R, Gloss B, Lin S C, Heyman R A, Rose D W, Glass C K, Rosenfeld M G. A CBP integrator complex mediates transcriptional activation and AP-1 inhibition by nuclear receptors. Cell. 1996;85:403–414. doi: 10.1016/s0092-8674(00)81118-6. [DOI] [PubMed] [Google Scholar]
- 37.Kashanchi F, Duvall J F, Brady J N. Electroporation of viral transactivator proteins into lymphocyte suspension cells. Nucleic Acids Res. 1992;20:4673–4674. doi: 10.1093/nar/20.17.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kashanchi F, Duvall J F, Kwok R P S, Lundblad J, Goodman R, Brady J N. The coactivator CBP stimulates human T-cell lymphotropic virus type I Tax transactivation in vitro. J Biol Chem. 1998;51:34646–34652. doi: 10.1074/jbc.273.51.34646. [DOI] [PubMed] [Google Scholar]
- 39.Korzus E, Torchia J, Rose D W, Xu L, Kurokawa R, McInerney E M, Mullen T M, Glass C K, Rosenfeld M G. Transcription factor-specific requirements for coactivators and their acetyltransferase functions. Science. 1998;279:703–707. doi: 10.1126/science.279.5351.703. [DOI] [PubMed] [Google Scholar]
- 40.Krumm A, Madisen L, Yang X J, Goodman R, Nakatani Y, Groudine M. Long-distance transcriptional enhancement by the histone acetyltransferase PCAF. Proc Natl Acad Sci USA. 1998;95:13501–13506. doi: 10.1073/pnas.95.23.13501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kwok R P, Laurance M E, Lundblad J R, Goldman P S, Shih H, Connor L M, Marriott S J, Goodman R H. Control of cAMP-regulated enhancers by the viral transactivator Tax through CREB and the co-activator CBP. Nature. 1996;380:642–646. doi: 10.1038/380642a0. [DOI] [PubMed] [Google Scholar]
- 42.Kwok R P S, Lundblad J R, Chrivia J C, Richards J P, Bachinger H P, Brennan R G, Roberts S G E, Green M R, Goodman R H. Nuclear protein CBP is a coactivator for the transcription factor CREB. Nature. 1998;370:223–226. doi: 10.1038/370223a0. [DOI] [PubMed] [Google Scholar]
- 43.Lenzmeier B A, Giebler H A, Nyborg J K. Human T-cell leukemia virus type 1 Tax requires direct access to DNA for recruitment of CREB binding protein to the viral promoter. Mol Cell Biol. 1998;18:721–731. doi: 10.1128/mcb.18.2.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lill N L, Grossman S R, Ginsberg D D J, Livingston D M. Binding and modulation of p53 by p300/CBP coactivators. Nature. 1997;387:823–827. doi: 10.1038/42981. [DOI] [PubMed] [Google Scholar]
- 45.Liu L, Scolnick D M, Trievel R C, Zhang H B, Marmorstein R, Halazonetis T D, Berger S L. p53 sites acetylated in vitro by PCAF and p300 are acetylated in vivo in response to DNA damage. Mol Cell Biol. 1999;19:1202–1209. doi: 10.1128/mcb.19.2.1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Marriott S J, Boros I, Duvall J F, Brady J N. Indirect binding of human T-cell leukemia virus type I Tax1 to a responsive element in the viral long terminal repeat. Mol Cell Biol. 1989;9:4152–4160. doi: 10.1128/mcb.9.10.4152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Marzio G, Tyagi M, Gutierrez M I, Giacca M. HIV-1 tat transactivator recruits p300 and CREB-binding protein histone acetyltransferases to the viral promoter. Proc Natl Acad Sci USA. 1998;95:13519–13524. doi: 10.1073/pnas.95.23.13519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McMahon C, Suthiphongchai T, DiRenzo J, Ewen M E. P/CAF associates with cyclin D1 and potentiates its activation of the estrogen receptor. Proc Natl Acad Sci USA. 1999;96:5382–5387. doi: 10.1073/pnas.96.10.5382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Montminy M R, Bilezikjian L M. Binding of a nuclear protein to the cyclic-AMP response element of the somatostatin gene. Nature. 1987;328:175–178. doi: 10.1038/328175a0. [DOI] [PubMed] [Google Scholar]
- 50.Munshi N, Merika M, Yie J, Senger K, Chen G, Thanos D. Acetylation of HMG I(Y) by CBP turns off IFN beta expression by disrupting the enhanceosome. Mol Cell. 1998;2:457–467. doi: 10.1016/s1097-2765(00)80145-8. [DOI] [PubMed] [Google Scholar]
- 51.Neuwald A F, Landsman D. GCN5-related histone N-acetyltransferases belong to a diverse superfamily that includes the yeast SPT10 protein. Trends Biochem Sci. 1997;22:154–155. doi: 10.1016/s0968-0004(97)01034-7. [DOI] [PubMed] [Google Scholar]
- 52.Ogryzko V V, Kotani T, Zhang X, Schlitz R L, Howard T, Yang X J, Howard B H, Qin J, Nakatani Y. Histone-like TAFs within the PCAF histone acetylase complex. Cell. 1998;94:35–44. doi: 10.1016/s0092-8674(00)81219-2. [DOI] [PubMed] [Google Scholar]
- 53.Ogryzko V V, Schiltz R L, Russanova V, Howard B H, Nakatani Y. The transcriptional coactivators p300 and CBP are histone acetyltransferases. Cell. 1996;87:953–959. doi: 10.1016/s0092-8674(00)82001-2. [DOI] [PubMed] [Google Scholar]
- 54.Osame M, Usuku K, Izumo S, Ijichi N, Amitani H, Igata A, Matsumoto M, Tara M. HTLV-1 associated myelopathy, a new clinical entity. Lancet. 1986;i:1031–1032. doi: 10.1016/s0140-6736(86)91298-5. . (Letter.) [DOI] [PubMed] [Google Scholar]
- 55.Paskalis H, Felber B K, Pavlakis G N. Cis-acting sequences responsible for the transcriptional activation of human T-cell leukemia virus type I constitute a conditional enhancer. Proc Natl Acad Sci USA. 1986;83:6558–6562. doi: 10.1073/pnas.83.17.6558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Petrij F, Giles R H, Dauwerse H G, Saris J J, Hennekam R C, Masuno M, Tommerup N, van Ommen G J, Goodman R H, Peters D J. Rubinstein-Taybi syndrome caused by mutations in the transcriptional co-activator CBP. Nature. 1995;376:348–351. doi: 10.1038/376348a0. [DOI] [PubMed] [Google Scholar]
- 57.Pise-Masison C A, Dittmer J, Clemens K E, Brady J N. Physical and functional interaction between the human T-cell lymphotropic virus type 1 Tax1 protein and the CCAAT binding protein NF-Y. Mol Cell Biol. 1997;17:1236–1243. doi: 10.1128/mcb.17.3.1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Poiesz B J, Ruscetti F W, Gazdar A F, Bunn P A, Minna J D, Gallo R C. Detection and isolation of type C retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma. Proc Natl Acad Sci USA. 1980;77:7415–7419. doi: 10.1073/pnas.77.12.7415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Puri P L, Sartorelli V, Yang X J, Hamamori Y, Ogryzko V V, Howard B H, Kedes L, Wang J Y, Graessmann A, Nakatani Y, Levrero M. Differential roles of p300 and PCAF acetyltransferases in muscle differentiation. Mol Cell. 1997;1:35–45. doi: 10.1016/s1097-2765(00)80005-2. [DOI] [PubMed] [Google Scholar]
- 60.Reid J L, Bannister A J, Zegerman P, Martinez-Balbas M A, Kouzarides T. E1A directly binds and regulates the P/CAF acetyltransferase. EMBO J. 1998;17:4469–4477. doi: 10.1093/emboj/17.15.4469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schiltz R L, Mizzen C A, Vassilev A, Cook R G, Allis C D, Nakatani Y. Overlapping but distinct patterns of histone acetylation by the human coactivators p300 and PCAF within nucleosomal substrates. J Biol Chem. 1999;274:1189–1192. doi: 10.1074/jbc.274.3.1189. [DOI] [PubMed] [Google Scholar]
- 62.Scolnick D M, Chehab N H, Stavridi E S, Lien M C, Caruso L, Moran E, Berger S L, Halazonetis T D. CREB-binding protein and p300/CBP-associated factor are transcriptional coactivators of the p53 tumor suppressor protein. Cancer Res. 1997;57:3693–3696. [PubMed] [Google Scholar]
- 63.Semmes O J, Jeang K T. Mutational analysis of human T-cell leukemia virus type I Tax: regions necessary for function determined with 47 mutant proteins. J Virol. 1992;66:7183–7192. doi: 10.1128/jvi.66.12.7183-7192.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Semmes O J, Jeang K T. Definition of a minimal activation domain in human T-cell leukemia virus type I Tax. J Virol. 1995;69:1827–1833. doi: 10.1128/jvi.69.3.1827-1833.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shikama N, Lyon J, La Thangue N B. The p300/CBP family: integrating signals with transcription factors and chromatin. Trends Cell Biol. 1999;7:230–236. doi: 10.1016/S0962-8924(97)01048-9. [DOI] [PubMed] [Google Scholar]
- 66.Smith M R, Greene W C. Identification of HTLV-1 tax trans-activator mutants exhibiting novel transcriptional phenotypes. Genes Dev. 1990;4:1875–1885. doi: 10.1101/gad.4.11.1875. . (Errata, 5:150, 1999, and 9:2324, 1995.) [DOI] [PubMed] [Google Scholar]
- 67.Smith M R, Greene W C. Type I human T cell leukemia virus tax protein transforms rat fibroblasts through the cyclic adenosine monophosphate response element binding protein/activating transcription factor pathway. J Clin Investig. 1991;88:1038–1042. doi: 10.1172/JCI115364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sodroski J G, Rosen C A, Haseltine W A. Trans-acting transcriptional activation of the long terminal repeat of human T lymphotropic viruses in infected cells. Science. 1984;225:381–385. doi: 10.1126/science.6330891. [DOI] [PubMed] [Google Scholar]
- 69.Struhl K. Histone acetylation and transcriptional regulatory mechanisms. Genes Dev. 1998;12:599–606. doi: 10.1101/gad.12.5.599. [DOI] [PubMed] [Google Scholar]
- 70.Suzuki T, Fujisawa J, Toita M, Yoshida M. The trans-activator Tax of human T-cell leukemia virus type 1 (HTLV-1) interacts with cAMP-responsive element (CRE) binding and CRE modulator proteins that bind to the 21-base-pair enhancer of HTLV-1. Proc Natl Acad Sci USA. 1993;90:610–614. doi: 10.1073/pnas.90.2.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Swope D L, Mueller C L, Chrivia J C. CREB-binding protein activates transcription through multiple domains. J Biol Chem. 1996;271:28138–28145. doi: 10.1074/jbc.271.45.28138. [DOI] [PubMed] [Google Scholar]
- 72.Turner B M. Decoding the nucleosome. Cell. 1993;75:5–8. [PubMed] [Google Scholar]
- 73.Wolffe A P, Pruss D. Targeting chromatin disruption: transcription regulators that acetylate histones. Cell. 1996;84:817–819. doi: 10.1016/s0092-8674(00)81059-4. [DOI] [PubMed] [Google Scholar]
- 74.Yang X J, Ogryzko V V, Nishikawa J, Howard B H, Nakatani Y. A p300/CBP-associated factor that competes with the adenoviral oncoprotein E1A. Nature. 1996;382:319–324. doi: 10.1038/382319a0. [DOI] [PubMed] [Google Scholar]
- 75.Yao T P, Oh S P, Fuchs M, Zhou N D, Ch’ng L E, Newsome D, Bronson R T, Li E, Livingston D M, Eckner R. Gene dosage-dependent embryonic development and proliferation defects in mice lacking the transcriptional integrator p300. Cell. 1998;93:361–372. doi: 10.1016/s0092-8674(00)81165-4. [DOI] [PubMed] [Google Scholar]
- 76.Yin M J, Gaynor R B. Complex formation between CREB and Tax enhances the binding affinity of CREB for the human T-cell leukemia virus type 1 21-base-pair repeats. Mol Cell Biol. 1996;16:3156–3168. doi: 10.1128/mcb.16.6.3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yin M J, Gaynor R B. HTLV-1 21 bp repeat sequences facilitate stable association between Tax and CREB to increase CREB binding affinity. J Mol Biol. 1996;264:20–31. doi: 10.1006/jmbi.1996.0620. [DOI] [PubMed] [Google Scholar]
- 78.Yoshida M. HTLV-1 oncoprotein Tax deregulates transcription of cellular genes through multiple mechanisms. J Cancer Res Clin Oncol. 1995;121:521–528. doi: 10.1007/BF01197764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yoshida M, Seiki M. Recent advances in the molecular biology of HTLV-1: trans-activation of viral and cellular genes. Annu Rev Immunol. 1987;5:541–559. doi: 10.1146/annurev.iy.05.040187.002545. [DOI] [PubMed] [Google Scholar]
- 80.Yoshida M, Seiki M, Yamaguchi K, Takatsuki K. Monoclonal integration of human T-cell leukemia provirus in all primary tumors of adult T-cell leukemia suggests causative role of human T-cell leukemia virus in the disease. Proc Natl Acad Sci USA. 1984;81:2534–2537. doi: 10.1073/pnas.81.8.2534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yuan W, Condorelli G, Caruso M, Felsani A, Giordano A. Human p300 protein is a coactivator for the transcription factor MyoD. J Biol Chem. 1996;271:9009–9013. doi: 10.1074/jbc.271.15.9009. [DOI] [PubMed] [Google Scholar]
- 82.Zhao L J, Giam C Z. Interaction of the human T-cell lymphotrophic virus type I (HTLV-I) transcriptional activator Tax with cellular factors that bind specifically to the 21-base-pair repeats in the HTLV-1 enhancer. Proc Natl Acad Sci USA. 1991;88:11445–11449. doi: 10.1073/pnas.88.24.11445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhao L J, Giam C Z. Human T-cell lymphotropic virus type I (HTLV-I) transcriptional activator, Tax, enhances CREB binding to HTLV-I 21-base-pair repeats by protein-protein interaction. Proc Natl Acad Sci USA. 1992;89:7070–7074. doi: 10.1073/pnas.89.15.7070. [DOI] [PMC free article] [PubMed] [Google Scholar]