Abstract
Objective:
To determine methamphetamine positivity and copositivity with other drugs in urine drug test (UDT) results geographically through time.
Methods:
This cross-sectional study of UDT results from January 1, 2014, through December 31, 2019, included patient specimens submitted by health care professionals across the United States. The analysis used LC-MS/MS to detect cocaine, heroin, alcohol, marijuana and nonprescribed methamphetamine, fentanyl, methadone, buprenorphine, benzodiazepines, and other opioids. Logistic regression was used to evaluate association of demographic features and model yearly methamphetamine detection patterns across US census divisions. Odds ratios (OR) from logistic modeling were used to evaluate the impact of methamphetamine positivity on the spatio-temporal detection patterns of additional nonprescribed or illicit drugs.
Results:
The probability of being positive for methamphetamine increased nationally from 0.010 [0.010–0.011] in 2014 to 0.044 [0.042–0.046] in 2019, a 340% increase after correction for demographic covariates. The highest predicted positivity rate was in male patients, 25- to 34-years-old, from the West North Central division and from substance use disorder treatment centers. Nationally, copositivity ORs for fentanyl, heroin, and other opioids with methamphetamine were highest in 2019. Increases in ORs from 2014 through 2019 were statistically significant for heroin (P = 0.024) and fentanyl (P = 0.0085). Copositivity ORs for methamphetamine and other substances varied by census division.
Conclusions:
The probability of being positive for methamphetamine in UDT increased nationwide between 2014 and 2019. Not all census divisions are increasing at the same rate. Copositivity with additional substances is increasing in some census divisions, which further increases the risk of overdose and poor treatment outcomes.
Keywords: drug overdose deaths, drug trends, methamphetamine, substance use disorder, urine drug test
Methamphetamine, like many psychoactive drugs, has exhibited a rise and fall in popularity that has affected the United States in waves. Numerous factors influence rates of methamphetamine use, including availability of other psychoactive drugs and measures combatting its use. Historically, methamphetamine (and other amphetamines) was used by soldiers during World War II to diminish appetite and fatigue. Use was largely contained by the Comprehensive Drug Abuse Prevention and Control Act of 1970, although methamphetamine continued to be illicitly manufactured by motorcycle gangs in California and Oregon throughout the 1970s. In the 1980s, manufacturing and use began to expand with importation of methamphetamine into Hawaii from Southeast Asia. It was not until the 1990s, however, that methamphetamine use exploded, primarily due to increased production by small laboratories in rural areas and importation and distribution from “superlabs” in Mexico and Southern California.1
Methamphetamine use increased significantly in the early 1990s and peaked around late 2007. The DEA named it “the greatest drug threat nationwide by the highest percentages of law enforcement agencies” from 2004 to 2008.2 During this same time period, the DEA was assessing a newer, emerging threat: that of prescription opioid misuse.
From 2008 until 2012, methamphetamine use, demand, and availability remained relatively stable.3 One of the main contributors to the decrease of domestic production and availability of methamphetamine was the Combat Methamphetamine Act passed in 2005, limiting sales and access to ephedrine and pseudoephedrine, which are precursors to methamphetamine production.4 After 2012, the DEA began again to report an increase in drug seizures at the Southwest border.5 Most methamphetamine in US markets now originates in Mexico, where manufacturing has evolved from an ephedrine-based product into one created by less controlled precursor chemicals, such as phenyl-2-propanone (P2P).4
Methamphetamine is again quickly becoming one of the most concerning psychoactive drugs used in the US. According to the 2018 National Survey on Drug Use and Health, approximately 1.9 million Americans used methamphetamine in the past year; among adults age 26 years and older, this number grew significantly compared to 2016 and 2017 survey data, increasing by 45.5% between 2016 and 2018.6
More concerning is that drug overdose deaths involving psychostimulants, including methamphetamine, have increased nearly five-fold from 2012 through 2018.7 The combination of methamphetamine with other drugs increases the risk of overdose. In 2017, 50.4% of psychostimulant-related overdoses involved an opioid.8 Coinvolvement of synthetic opioids, namely fentanyl, has become particularly troubling. A 2019 study identified a 798% increase in urine drug test (UDT) positivity rates for nonprescribed fentanyl among results positive for methamphetamine between January 2013 and September 2018.9
Increasing methamphetamine use and related consequences may be due to a changing landscape of methamphetamine availability and chemical qualities. According to the National Forensic Laboratory Information System, methamphetamine was the top identified drug submitted to state and local laboratories by law enforcement in 2018, comprising 24% of total drug reports.10 Many forms of the drug are widely available throughout the West and Midwest, though traffickers are seeking to establish a new customer base in nontraditional markets, including the Northeast.4 Availability in certain parts of the country has increased dramatically, including a 1600% reported increase between 2015 and 2019 by 23 drug task forces in Ohio and Northern Kentucky.11 The type of methamphetamine identified is often of a higher purity and potency than years past while prices are lower. For example, in 2007, methamphetamine purity was 56.4% with a price per pure gram of $152; a decade later, purity jumped to 96.9% with a price per pure gram of $56.12,4
Knowing the methamphetamine landscape is shifting and has important public health implications, in this analysis, the authors identify national and regional trends of UDT results for methamphetamine in a population seeking health care, as well as copositivity with select drugs and drug classes. Identifying trends in methamphetamine use through near real-time urine drug testing data may help ascertain areas of the country most affected by the methamphetamine use crisis and better inform those involved in care and prevention efforts. In addition to providing timely analysis of use, the inclusion of copositivity helps shed light on the likelihood of various drug combinations with methamphetamine, which may have implications in the treatment of acute toxicity and substance use disorders (SUDs).
METHODS
Data
We conducted a cross-sectional study of UDT results from January 1, 2014, to December 31, 2019, from patient specimens submitted for testing by health care professionals (HCP) as part of routine care based on medical necessity. Specimens were collected from health care practices from all 50 states and the District of Columbia. A single specimen for each patient was selected based on the earliest collection date to remove repeated measurements. The study used a convenience sample of 2 million randomly selected adult patient specimens with test orders for definitive UDT for methamphetamine by liquid chromatography-tandem mass spectrometry (LC-MS/MS). The LC-MS/MS testing method is a laboratory-developed test with performance characteristics determined by Millennium Health, San Diego, California, which is certified by the Clinical Laboratory Improvement Amendments and accredited by the College of American Pathologists for high-complexity testing. Millennium Health performed all LC-MS/MS testing for the study. In addition to methamphetamine, the following drugs and/or drug classes were tested for in a subset of patient specimens (analytes and metabolites tested in parentheses): cocaine (benzoylecgonine), fentanyl (fentanyl, norfentanyl), heroin (6-MAM), alcohol (ethyl glucuronide, ethyl sulfate), marijuana (cTHC), methadone (methadone, EDDP), buprenorphine (buprenorphine, norbuprenorphine), other opioids (codeine, hydrocodone, norhydrocodone, hydromorphone, morphine, oxycodone, noroxycodone, oxymorphone), and benzodiazepines (alpha-hydroxyalprazolam, 7-amino-clonazepam, nordiazepam, oxazepam, temazepam, and lorazepam). If any parent analyte or metabolite within a drug class was detected, the drug or drug class was considered positive for that specimen. All analytes/metabolites in the analysis were ordered and had valid test results for the specimen. When ordering UDTs, HCPs report a patient's prescribed medications. All results consistent with a patient's prescribed medications were excluded; only results for nonprescribed drugs were included.
The study protocol was approved by the Aspire Independent Review Board and includes a waiver of consent for the use of deidentified data. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
Statistical Analysis
Logistic regression was performed to evaluate the association of demographic features with methamphetamine detection. Collection year, clinic location (US census division),13 sex, age (discretized into 18–24, 25–34, 35–44, 45–54, 55–64, and >65-year-olds), and health care specialty were modeled as explanatory variables. Health care specialty is a 6-level categorization designed to describe the clinical practice of the ordering physician. Specialty classification was initially chosen by Millennium Health and subsequently verified by the ordering HCP. Health care specialty was added as a potentially confounding covariate in the regression model to account for potential changes in the sample. An interaction term for collection year and US census division was included to help better understand the changing nature of methamphetamine use over time and across divisions. Type-III analysis of deviance was performed to evaluate significance of each model factor using likelihood ratio tests. Least square mean predictions (marginal probabilities) for all factors in the logistic model were estimated along with Sidak-corrected 95% confidence interval (CI) values. Tukey-corrected P values were calculated for each factor level comparison.
Regression models were also constructed for the nonmethamphetamine drugs and drug classes tested. In addition to collection year, US census division, sex, age, and health care specialty, the detection status of methamphetamine (positive or negative) was added as an explanatory variable to each of the nine independent models. Detection status of the given drug class was treated as the dependent variable. A 3-way interaction term for collection year, US census division, and methamphetamine detection were included in the model to better understand spatio-temporal drug copositivity patterns in the methamphetamine population. Adjusted odds ratios (aOR) and Sidak-corrected 95% CIs for the detection status of methamphetamine were estimated for each year and census division. The aOR represents the odds of being positive for a given drug class in the methamphetamine-positive population compared to the odds in the methamphetamine-negative population. This aOR was used to determine if methamphetamine detection status was correlated with detection of a given drug class across years and census divisions. A Mann-Kendall correlation test was used to look for statistically significant increasing or decreasing trends in the aOR over time.
R statistical software version 3.5.0 (R Project for Statistical Computing) was used for data analysis. Statistical significance was set at P less than 0.05, and all tests were 2-tailed.
RESULTS
Study Population Demographics
Two million methamphetamine UDT results from individual patients, received between January 1, 2014, and December 31, 2019, were analyzed (Table 1). Of the analyzed specimens, 106,405 (5.32%) were positive for nonprescribed methamphetamine. The median age (interquartile range) was 44 (19–69) years for the entire sample population and 35 (19–51) years for the methamphetamine-positive population. The sample population was 54.72% female compared to 49.11% for the methamphetamine-positive population. The largest number of specimens came from pain management practices (28.47%) and primary care physicians (24.05%). However, SUD treatment centers (46.97%) and behavioral health (19.98%) were the most frequent specialties with specimens positive for methamphetamine. Although the greatest number of specimens were from the South Atlantic (19.16%) and East North Central (16.95%) divisions, the largest number of methamphetamine positives were found in the Pacific (32.94%) and Mountain (13.27%) divisions. This is concordant with reports of higher methamphetamine availability in western states.4
TABLE 1.
Characteristics of UDT Specimens Tested Between January 1, 2014 and December 31, 2019
| Specimens Tested, No. (%) | ||
| Characteristics | Total Population | Methamphetamine-Positive Population |
| Unique Patient Specimens | 2,000,000 (100.00%) | 106,405 (100.00%) |
| Sex | ||
| Female | 1,094,479 (54.72%) | 52,258 (49.11%) |
| Male | 905,521 (45.28%) | 54,147 (50.89%) |
| Age | ||
| Age, median [IQR], y | 44 [19–69] | 35 [19–51] |
| 18–24 | 181,599 (9.08%) | 11,317 (10.64%) |
| 25–34 | 444,191 (22.21%) | 38,643 (36.32%) |
| 35–44 | 383,014 (19.15%) | 27,731 (26.06%) |
| 45–54 | 391,064 (19.55%) | 17,546 (16.49%) |
| 55–64 | 346,078 (17.30%) | 9190 (8.64%) |
| 65+ | 236,741 (11.84%) | 1515 (1.42%) |
| US Census Division | ||
| East North Central | 338,960 (16.95%) | 10,434 (9.81%) |
| East South Central | 224,077 (11.20%) | 13,488 (12.68%) |
| Mid Atlantic | 172,955 (8.65%) | 1570 (1.48%) |
| Mountain | 230,342 (11.52%) | 14,117 (13.27%) |
| New England | 43,050 (2.15%) | 463 (0.44%) |
| Pacific | 310,555 (15.53%) | 35,055 (32.94%) |
| South Atlantic | 383,123 (19.16%) | 9127 (8.58%) |
| West North Central | 92,792 (4.64%) | 13,015 (12.23%) |
| West South Central | 204,146 (10.21%) | 9136 (8.59%) |
| Health Care Practice Specialty | ||
| Behavioral Health | 260,518 (13.03%) | 21,262 (19.98%) |
| Multispecialty and Other | 114,164 (5.71%) | 5636 (5.30%) |
| OBGYN | 94,506 (4.73%) | 1314 (1.23%) |
| Pain Management | 569,398 (28.47%) | 9890 (9.29%) |
| Primary Care Physician | 481,034 (24.05%) | 18,321 (17.22%) |
| Substance Use Disorder Treatment | 480,380 (24.02%) | 49,982 (46.97%) |
| Ordered Specimen Tests, No. (% Raw Positivity Rate) | ||
| Definitive UDT Results | Total Population | Methamphetamine-Positive Population |
| Methamphetamine | 2,000,000 (5.32%) | 106,405 (100%) |
| Other Opioids | 1,266,914 (21.54%) | 76,338 (38.53%) |
| THC | 1,774,320 (20.10%) | 93,704 (43.55%) |
| Benzodiazepines | 1,568,632 (16.84%) | 81,984 (23.58%) |
| Alcohol | 1,351,166 (17.00%) | 80,544 (18.94%) |
| Buprenorphine | 1,301,621 (6.89%) | 68,214 (13.15%) |
| Cocaine | 1,938,686 (4.74%) | 96,208 (9.25%) |
| Fentanyl | 1,502,367 (2.64%) | 78,510 (9.03%) |
| Heroin | 1,748,861 (2.20%) | 92,272 (12.48%) |
| Methadone | 1,771,528 (2.04%) | 85,658 (4.16%) |
Other opioids include codeine, hydrocodone, hydromorphone, morphine, oxycodone, and oxymorphone.
IQR indicates interquartile range; UDT, urine drug testing.
In addition to methamphetamine, 9 drugs and drug classes were evaluated. Testing for additional drugs and drug classes was not ordered in all 2 million specimens (Table 1). Cocaine (n = 1,938,686), THC (n = 1,774,320), and methadone (n = 1,771,528) were ordered most frequently.
Other opioids and THC were detected most frequently in both the total sample population and the methamphetamine-positive population; however, there were clear differences in drug and drug class positivity rates for the methamphetamine-positive population relative to the total sample population. Heroin shows the largest percent difference, with a positivity rate of 12.48% in the methamphetamine-positive group compared with 2.20% positivity in the total sample population (Table 1).
Methamphetamine Usage
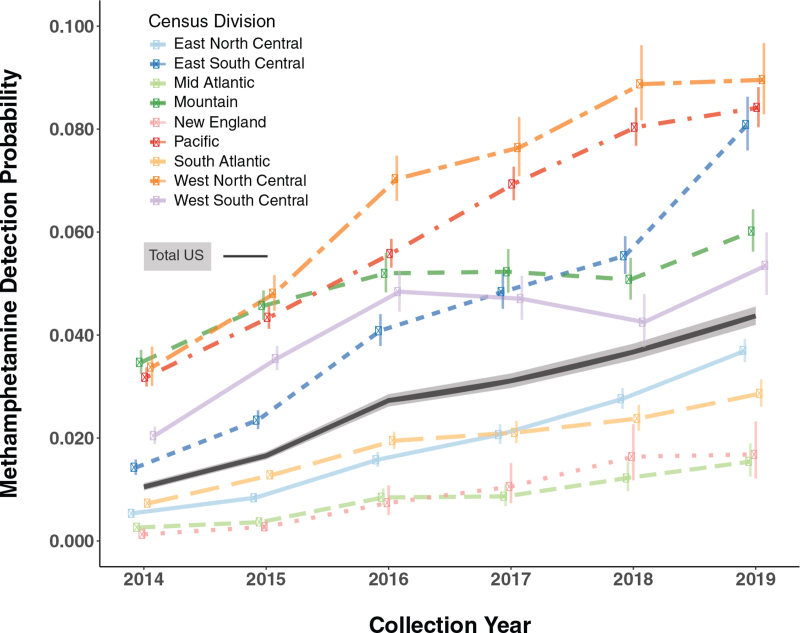
A logistic regression model of methamphetamine detection, including collection year, US census division, sex, age, and health care specialty as explanatory factors, was highly significant (X2 = 121,595, df = 64, LRT P = <2.2e-16, McFadden Pseudo-R2 = 0.81, Table 2). The marginal predicted probability of being positive for methamphetamine increased from 0.010 [0.010–0.011] in 2014 to 0.044 [0.042–0.046] in 2019, a 340% increase in total US methamphetamine positivity over 6 years. Notably, predicted detection ranged from 0.064 [0.062–0.066] for the West North Central division to 0.007 [0.006–0.008] for the New England division, indicating a wide range of geographical methamphetamine positivity during the study period. Age significantly impacted methamphetamine, with 25- to 34-year-old patients showing the highest predicted positivity. Males were found to have a slightly higher methamphetamine positivity rate than females; however, it was not significant. Patient specimens from SUD treatment centers had the highest predicted positivity (0.050 [0.049–0.051]) and OB/GYN specimens the lowest (0.008 [0.008–0.009]).
TABLE 2.
Logistic Regression Results for Methamphetamine Detection
| Least Square Mean Estimates (Main Effects) | ||||
| Factor Level | Analysis of Deviance (Type III Tests)∗ | Treatment Level | Probability (95% CI)† | Tukey Relationship‡ |
| Year Collected | (ChiSq = 4073.449, df = 5, P value = 0) | |||
| 2014 | 0.010 [0.010–0.011] | a | ||
| 2015 | 0.017 [0.016–0.017] | b | ||
| 2016 | 0.027 [0.026–0.029] | c | ||
| 2017 | 0.031 [0.030–0.033] | d | ||
| 2018 | 0.037 [0.035–0.038] | e | ||
| 2019 | 0.044 [0.042–0.046] | f | ||
| US Census Division | (ChiSq = 7727.541, df = 8, P value = 0) | |||
| New England | 0.007 [0.006–0.008] | a | ||
| Mid Atlantic | 0.007 [0.007–0.008] | a | ||
| East North Central | 0.016 [0.015–0.016] | b | ||
| South Atlantic | 0.017 [0.017–0.018] | c | ||
| East South Central | 0.038 [0.037–0.039] | d | ||
| West South Central | 0.040 [0.038–0.041] | d | ||
| Mountain | 0.049 [0.047–0.050] | e | ||
| Pacific | 0.058 [0.056–0.059] | f | ||
| West North Central | 0.064 [0.062–0.066] | g | ||
| Sex | (ChiSq = 0.688, df = 1, P value = 0.407) | |||
| Female | 0.025 [0.024–0.025] | a | ||
| Male | 0.025 [0.024–0.026] | a | ||
| Patient Age | (ChiSq = 16391.032, df = 5, P value = 0) | |||
| 18-24 | 0.035 [0.033–0.036] | d | ||
| 25-34 | 0.048 [0.047–0.049] | f | ||
| 35-44 | 0.043 [0.042–0.044] | e | ||
| 45-54 | 0.031 [0.031–0.032] | c | ||
| 55-64 | 0.020 [0.019–0.021] | b | ||
| 65+ | 0.005 [0.005–0.006] | a | ||
| Health Care Specialty | (ChiSq = 15468.194, df = 5, P value = 0) | |||
| OBGYN | 0.008 [0.008–0.009] | a | ||
| Pain Management | 0.015 [0.015–0.016] | b | ||
| Primary Care Physician | 0.029 [0.028–0.029] | c | ||
| Multispecialty and Other | 0.033 [0.031–0.034] | d | ||
| Behavioral Health | 0.039 [0.038–0.041] | e | ||
| Substance Use Disorder Treatment | 0.050 [0.049–0.051] | f | ||
| Year Collected:US Census Division | (ChiSq = 2445.313, df = 40, P value = 0) | |||||
| Least Square Mean Estimates (Interaction Effect) Probability (95% CI) | ||||||
| US Census Division | 2014 | 2015 | 2016 | 2017 | 2018 | 2019 |
| New England | 0.001 [0.001–0.003] | 0.003 [0.002–0.004] | 0.007 [0.005–0.011] | 0.011 [0.007–0.015] | 0.016 [0.012–0.023] | 0.017 [0.012–0.023] |
| Mid Atlantic | 0.003 [0.002–0.003] | 0.004 [0.003–0.004] | 0.008 [0.007–0.010] | 0.009 [0.007–0.011] | 0.012 [0.010–0.015] | 0.015 [0.013–0.019] |
| East North Central | 0.005 [0.005–0.006] | 0.008 [0.008–0.009] | 0.016 [0.014–0.017] | 0.021 [0.019–0.023] | 0.028 [0.026–0.030] | 0.037 [0.035–0.039] |
| South Atlantic | 0.007 [0.007–0.008] | 0.013 [0.012–0.014] | 0.019 [0.018–0.021] | 0.021 [0.019–0.023] | 0.024 [0.021–0.026] | 0.029 [0.026–0.031] |
| East South Central | 0.014 [0.013–0.016] | 0.024 [0.022–0.025] | 0.041 [0.038–0.044] | 0.048 [0.045–0.052] | 0.055 [0.052–0.059] | 0.081 [0.076–0.086] |
| West South Central | 0.020 [0.019–0.022] | 0.035 [0.033–0.038] | 0.048 [0.045–0.053] | 0.047 [0.043–0.052] | 0.043 [0.038–0.048] | 0.054 [0.048–0.060] |
| Mountain | 0.035 [0.032–0.037] | 0.046 [0.043–0.049] | 0.052 [0.048–0.056] | 0.052 [0.048–0.057] | 0.051 [0.047–0.055] | 0.060 [0.056–0.064] |
| Pacific | 0.032 [0.030–0.034] | 0.043 [0.041–0.046] | 0.056 [0.053–0.059] | 0.069 [0.066–0.073] | 0.080 [0.077–0.084] | 0.084 [0.080–0.088] |
| West North Central | 0.034 [0.030–0.038] | 0.048 [0.045–0.052] | 0.070 [0.066–0.075] | 0.076 [0.071–0.082] | 0.089 [0.082–0.096] | 0.090 [0.083–0.097] |
A logistic regression model of methamphetamine detection was performed containing collection year, US census division, sex, age, and health care specialty main effects and collection year by US census division interaction effects was fit. The total model fit was significant (ChiSq = 121,595, df = 64, LRT P = <2.2e-16, McFadden Pseudo-R2 = 0.81). Type III Likelihood ratio tests were performed for main and interaction effects.
Least square mean predictions (marginal probabilities) and Sidak-corrected confidence intervals were calculated as odds values and transformed to probability estimates.
Tukey corrected p values were estimated for each pairwise comparison of treatment level within a main or interaction effect. A compact letter display was used to evaluate pairwise comparisons for significance at the 5% level. Treatment levels with the same letter are not significantly different.
The interaction term for collection year and census division in the model was found to be significant, leading to an evaluation of the predicted methamphetamine detection probabilities across divisions and years (Fig. 1, Table 2). The significant interaction suggests that not all divisions are changing at the same rate. For example, positivity in the East South Central division is increasing rapidly and surpassed both the Mountain and West South Central divisions after 2018. The East South Central division had the third highest positivity in 2019 and could surpass the West North Central and Pacific divisions in 2020 if similar trends remain in effect. While the East North Central, South Atlantic, New England, and Mid Atlantic divisions remain lower than the national average, they all continue to increase at similar rates, suggesting that methamphetamine is growing in most divisions of the country (Table 2, Fig. 1).
FIGURE 1.
Predicted methamphetamine detection probabilities for US census divisions. Notes: Predicted probabilities (95%CI) for methamphetamine detection were calculated for each US census division and collection year using the logistic regression model summarized in Table 2. The black line and confidence band represents Total US prediction probabilities derived from the collection year main effect.
Codetection of Drug Classes With Methamphetamine Usage
Nine independent regression models were constructed for each of the nonmethamphetamine drugs or drug classes using the same demographic features as above. In addition, methamphetamine detection status (positive or negative) was added as an explanatory factor. For each regression, the detection status of the drug or drug class of interest was modeled as the dependent variable. A 3-way interaction, and all possible 2-way interactions, of collection year, census division, and methamphetamine detection status were included in each model. All models were found to be highly significant based on individual likelihood ratio tests (see Table, Supplemental Digital Content 1).
We evaluated the aOR from each of the nine copositivity models to determine if methamphetamine positivity was associated with positivity for any of the nine drugs and drug classes evaluated (Table 3). For each census division and year, an aOR was calculated. If the aOR is significantly greater than 1, the odds of being positive for the drug or drug class is greater than the odds in the methamphetamine-negative population. Many of the aOR CIs do not overlap one, indicating statistical significance and that the methamphetamine-positive population has higher illicit and non-prescribed drug positivity than the methamphetamine-negative population.
TABLE 3.
Copositivity Analysis – Methamphetamine Positive Versus Negative Population Odds Ratios
| Region/Year | Benzodiazepines | Buprenorphine | Cocaine | Alcohol | Fentanyl | Heroin | Methadone | Opioids | THC |
| US Total | |||||||||
| 2014 | 1.86 (1.49–2.33)‡ | 1.7 (1.34–2.16)‡ | 2.44 (1.97–3.03)‡ | 1.38 (1.09–1.74)‡ | 1.11 (0.67–1.84) | 4.38 (3.25–5.91)‡ | 2.23 (1.49–3.35)‡ | 2.59 (2.13–3.14)‡ | 2.65 (2.21–3.19)‡ |
| 2015 | 1.83 (1.61–2.08)‡ | 1.64 (1.39–1.94)‡ | 1.93 (1.66–2.25)‡ | 1.01 (0.84–1.22) | 1.69 (1.33–2.13)‡ | 4.2 (3.5–5.05)‡ | 1.65 (1.11–2.45)† | 2.8 (2.49–3.16)‡ | 2.47 (2.18–2.8)‡ |
| 2016 | 2.01 (1.77–2.28)‡ | 1.56 (1.31–1.85)‡ | 2.21 (1.91–2.56)‡ | 1.11 (0.93–1.33) | 2.15 (1.71–2.69)‡ | 5.38 (4.42–6.56)‡ | 1.78 (1.34–2.35)‡ | 2.95 (2.58–3.38)‡ | 2.44 (2.15–2.78)‡ |
| 2017 | 1.79 (1.55–2.07)‡ | 1.92 (1.62–2.26)‡ | 2.26 (1.94–2.64)‡ | 1.1 (0.94–1.29) | 2.95 (2.43–3.57)‡ | 5.41 (4.18–7.01)‡ | 2.19 (1.62–2.97)‡ | 2.59 (2.23–3)‡ | 2.74 (2.41–3.12)‡ |
| 2018 | 1.87 (1.64–2.14)‡ | 2.01 (1.68–2.39)‡ | 2.54 (2.17–2.97)‡ | 1.04 (0.88–1.23) | 4.05 (3.36–4.88)‡ | 7.5 (5.75–9.78)‡ | 1.6 (1.02–2.51)∗ | 3.41 (2.96–3.92)‡ | 2.82 (2.5–3.19)‡ |
| 2019 | 1.6 (1.38–1.85)‡ | 2.01 (1.71–2.37)‡ | 2.43 (2.1–2.81)‡ | 1.09 (0.95–1.26) | 4.35 (3.73–5.08)‡ | 8.09 (6.31–10.36)‡ | 1.8 (1.24–2.6)‡ | 3.41 (2.98–3.89)‡ | 2.62 (2.34–2.93)‡ |
| East North Central | |||||||||
| 2014 | 1.88 (1.19–2.98)‡ | 2.33 (1.26–4.34)‡ | 2.18 (1.23–3.85)‡ | 1.04 (0.54–2.01) | 1.9 (0.57–6.32) | 3.83 (2.08–7.06)‡ | 3.24 (1.55–6.77)‡ | 3.54 (2.21–5.65)‡ | 2.48 (1.65–3.71)‡ |
| 2015 | 2.06 (1.52–2.8)‡ | 1.92 (1.33–2.78)‡ | 2.48 (1.78–3.45)‡ | 0.83 (0.53–1.3) | 3.56 (2.3–5.49)‡ | 3.13 (2.19–4.47)‡ | 1.67 (0.8–3.5) | 3.64 (2.71–4.89)‡ | 2.25 (1.68–3)‡ |
| 2016 | 1.99 (1.48–2.66)‡ | 1.92 (1.39–2.66)‡ | 2.93 (2.18–3.94)‡ | 0.9 (0.61–1.31) | 3.9 (2.83–5.36)‡ | 3.48 (2.49–4.88)‡ | 0.9 (0.42–1.91) | 3.4 (2.6–4.44)‡ | 2.18 (1.67–2.84)‡ |
| 2017 | 1.81 (1.3–2.51)‡ | 2.31 (1.64–3.24)‡ | 2.81 (2.03–3.89)‡ | 0.92 (0.62–1.38) | 4.99 (3.56–7.01)‡ | 4.14 (2.69–6.38)‡ | 1.92 (0.9–4.08) | 3.05 (2.28–4.09)‡ | 2.36 (1.81–3.07)‡ |
| 2018 | 1.66 (1.27–2.17)‡ | 2.06 (1.59–2.68)‡ | 2.62 (2.06–3.35)‡ | 0.92 (0.68–1.25) | 5.38 (4.23–6.83)‡ | 4.76 (3.31–6.85)‡ | 0.97 (0.45–2.08) | 3.4 (2.72–4.25)‡ | 2.22 (1.81–2.72)‡ |
| 2019 | 1.72 (1.37–2.14)‡ | 1.75 (1.39–2.21)‡ | 2.15 (1.76–2.62)‡ | 0.82 (0.64–1.06) | 4.35 (3.64–5.21)‡ | 4.57 (3.34–6.24)‡ | 1.67 (1.22–2.3)‡ | 3.53 (2.94–4.24)‡ | 2.12 (1.8–2.5)‡ |
| East South Central | |||||||||
| 2014 | 2.86 (2.07–3.95)‡ | 2.69 (1.71–4.22)‡ | 2.19 (1.3–3.67)‡ | 1.34 (0.76–2.34) | 1.79 (0.53–6) | 3.14 (1.11–8.92)† | 2.79 (1.46–5.36)‡ | 2.15 (1.53–3.03)‡ | 2.74 (2.01–3.74)‡ |
| 2015 | 2.45 (1.93–3.11)‡ | 1.98 (1.43–2.73)‡ | 1.78 (1.22–2.6)‡ | 1.32 (0.93–1.87) | 1.91 (0.98–3.72) | 4.07 (2.44–6.8)‡ | 2.35 (1.41–3.91)‡ | 2.4 (1.88–3.07)‡ | 3.19 (2.55–3.99)‡ |
| 2016 | 2.31 (1.82–2.92)‡ | 1.65 (1.2–2.27)‡ | 1.89 (1.33–2.7)‡ | 1.24 (0.91–1.68) | 3.69 (2.42–5.64)‡ | 5.38 (3.39–8.52)‡ | 2.29 (1.15–4.57)† | 2.39 (1.89–3.01)‡ | 2.88 (2.32–3.58)‡ |
| 2017 | 2.16 (1.74–2.7)‡ | 2.04 (1.51–2.77)‡ | 1.99 (1.46–2.71)‡ | 1.25 (0.94–1.67) | 4.8 (3.52–6.56)‡ | 5.85 (3.76–9.1)‡ | 1.46 (0.7–3.05) | 2.8 (2.26–3.47)‡ | 3.46 (2.84–4.22)‡ |
| 2018 | 1.83 (1.47–2.29)‡ | 1.91 (1.45–2.53)‡ | 1.81 (1.35–2.43)‡ | 1.02 (0.79–1.33) | 4.71 (3.68–6.04)‡ | 5.51 (3.69–8.24)‡ | 1.25 (0.56–2.77) | 3.27 (2.67–4.01)‡ | 3.41 (2.84–4.1)‡ |
| 2019 | 1.62 (1.26–2.09)‡ | 1.84 (1.42–2.4)‡ | 1.68 (1.23–2.29)‡ | 0.99 (0.75–1.29) | 3.94 (3.13–4.96)‡ | 4.63 (3.05–7.01)‡ | 1.12 (0.6–2.09) | 2.85 (2.3–3.55)‡ | 2.79 (2.3–3.39)‡ |
| Mid Atlantic | |||||||||
| 2014 | 2.74 (1.57–4.76)‡ | 2.45 (1.27–4.76)‡ | 2.63 (1.43–4.81)‡ | 1.5 (0.62–3.63) | 0.92 (0.1–8.31) | 2.89 (1.38–6.05)‡ | 1.73 (0.7–4.27) | 2.49 (1.4–4.43)‡ | 2.49 (1.35–4.59)‡ |
| 2015 | 2.14 (1.21–3.78)‡ | 2.66 (1.26–5.6)‡ | 2.37 (1.26–4.47)‡ | 0.56 (0.17–1.83) | 2.98 (1–8.86)∗ | 4.15 (2.04–8.44)‡ | 1.57 (0.58–4.24) | 3.81 (2.21–6.57)‡ | 2.14 (1.13–4.06)† |
| 2016 | 2.6 (1.44–4.7)‡ | 2.09 (0.93–4.67) | 3.01 (1.54–5.86)‡ | 1.12 (0.5–2.51) | 6.16 (2.91–13.02)‡ | 6.36 (3–13.49)‡ | 1.58 (0.38–6.54) | 3.91 (2.06–7.44)‡ | 2.03 (1.08–3.78)† |
| 2017 | 2.19 (0.94–5.06) | 3.01 (1.2–7.57)† | 2.94 (1.24–6.98)‡ | 0.83 (0.28–2.49) | 4.92 (1.7–14.27)‡ | 7.57 (2.48–23.08)‡ | 2.24 (0.41–12.34) | 2.01 (0.86–4.66) | 2.21 (1.06–4.61)∗ |
| 2018 | 3 (1.32–6.83)‡ | 5.64 (2.29–13.92)‡ | 3.07 (1.23–7.64)‡ | 0.8 (0.28–2.33) | 6.38 (2.39–17.07)‡ | 7.71 (1.95–30.45)‡ | 2.03 (0.22–18.99) | 3.34 (1.45–7.71)‡ | 2.38 (1.15–4.94)† |
| 2019 | 1.92 (0.83–4.43) | 6.04 (2.69–13.6)‡ | 2.87 (1.27–6.49)‡ | 1.14 (0.49–2.69) | 6.17 (2.47–15.38)‡ | 4.78 (1.04–21.91)∗ | 2 (0.21–18.92) | 2.12 (0.91–4.95) | 1.8 (0.95–3.42) |
| Mountain | |||||||||
| 2014 | 1.43 (1.15–1.79)‡ | 1.29 (0.77–2.16) | 2.87 (1.97–4.19)‡ | 1.39 (1.07–1.79)‡ | 0.55 (0.15–1.97) | 7.8 (5.32–11.44)‡ | 2.23 (1.43–3.47)‡ | 2.04 (1.61–2.58)‡ | 2.35 (1.91–2.88)‡ |
| 2015 | 1.51 (1.22–1.86)‡ | 1.61 (1.04–2.49)† | 2.13 (1.44–3.17)‡ | 1.24 (0.98–1.58) | 0.61 (0.21–1.74) | 11.85 (8.64–16.26)‡ | 2.44 (1.58–3.76)‡ | 2.73 (2.21–3.38)‡ | 2.21 (1.83–2.68)‡ |
| 2016 | 1.79 (1.35–2.35)‡ | 1.74 (1.05–2.9)† | 2.63 (1.72–4.03)‡ | 1.29 (0.97–1.73) | 0.55 (0.15–2) | 10.67 (7.32–15.56)‡ | 3.25 (2.19–4.83)‡ | 3.12 (2.37–4.12)‡ | 2.42 (1.93–3.04)‡ |
| 2017 | 1.63 (1.2–2.22)‡ | 1.88 (1.12–3.18)‡ | 2.88 (1.84–4.5)‡ | 1.38 (1.01–1.88)∗ | 1.3 (0.53–3.16) | 12.7 (8.47–19.04)‡ | 5.72 (3.32–9.87)‡ | 3.66 (2.75–4.85)‡ | 2.23 (1.75–2.85)‡ |
| 2018 | 1.79 (1.31–2.44)‡ | 2.69 (1.63–4.43)‡ | 3.25 (2.01–5.26)‡ | 1.45 (1.08–1.94)‡ | 1.92 (0.93–3.96) | 19.84 (12.96–30.37)‡ | 3.35 (1.93–5.8)‡ | 3.75 (2.86–4.92)‡ | 2.15 (1.69–2.73)‡ |
| 2019 | 1.3 (0.99–1.71) | 2.15 (1.49–3.12)‡ | 3 (2–4.5)‡ | 1.17 (0.92–1.51) | 3.62 (2.5–5.25)‡ | 18.56 (12.28–28.07)‡ | 2.89 (1.76–4.73)‡ | 3.92 (3.15–4.88)‡ | 1.88 (1.54–2.3)‡ |
| New England | |||||||||
| 2014 | 0.83 (0.07–9.7) | 3.3 (0.34–32.53) | 4.64 (0.55–39.38) | 1.92 (0.18–20.27) | 1.45 (0.02–127.64) | 4.94 (0.31–79.97) | 1.49 (0.02–128.79) | 3.04 (0.39–23.86) | 1.89 (0.27–13.33) |
| 2015 | 1.27 (0.34–4.7) | 1.09 (0.25–4.68) | 2.47 (0.63–9.65) | 0.62 (0.1–3.64) | 6.64 (1.76–25.07)‡ | 3.33 (0.67–16.49) | 0.49 (0.01–39.24) | 2.59 (0.8–8.33) | 1.53 (0.45–5.2) |
| 2016 | 2.87 (0.85–9.69) | 1.38 (0.32–5.93) | 3.5 (0.98–12.47) | 0.77 (0.13–4.64) | 9.75 (3.25–29.28)‡ | 6.8 (1.18–39.09)† | 1.58 (0.12–21.37) | 3.21 (0.86–12.01) | 1.73 (0.48–6.2) |
| 2017 | 2.05 (0.57–7.42) | 2.18 (0.62–7.68) | 3.07 (0.91–10.43) | 0.84 (0.23–3.09) | 9.15 (3.15–26.57)‡ | 2.81 (0.27–28.84) | 1.36 (0.1–18.47) | 1.88 (0.49–7.26) | 3.14 (0.97–10.16) |
| 2018 | 2.54 (0.86–7.48) | 2.24 (0.6–8.43) | 4.56 (1.61–12.91)‡ | 0.58 (0.14–2.43) | 11.21 (4.23–29.68)‡ | 3.76 (0.43–32.6) | 0.43 (0.01–35.32) | 3.73 (1.12–12.38)† | 3.12 (1.1–8.87)∗ |
| 2019 | 1.8 (0.49–6.53) | 2.65 (0.7–10.01) | 3.23 (1.06–9.88)∗ | 0.83 (0.24–2.92) | 8.57 (3.19–23)‡ | 10.81 (1.51–77.61)‡ | 1.02 (0.04–24.2) | 6.16 (2.03–18.71)‡ | 2.64 (0.98–7.14) |
| Pacific | |||||||||
| 2014 | 1.5 (1.25–1.8)‡ | 1.23 (0.86–1.76) | 2.34 (1.65–3.32)‡ | 1.4 (1.15–1.71)‡ | 0.65 (0.26–1.66) | 11.64 (8.55–15.86)‡ | 2.21 (1.54–3.17)‡ | 3.46 (2.9–4.13)‡ | 2.64 (2.24–3.11)‡ |
| 2015 | 1.51 (1.29–1.78)‡ | 1.68 (1.28–2.22)‡ | 2.28 (1.69–3.07)‡ | 1.33 (1.12–1.57)‡ | 0.89 (0.42–1.9) | 11.75 (8.92–15.48)‡ | 1.9 (1.32–2.73)‡ | 3.81 (3.27–4.44)‡ | 2.49 (2.17–2.86)‡ |
| 2016 | 1.21 (1.01–1.46)∗ | 1.42 (1.06–1.9)‡ | 2.38 (1.77–3.2)‡ | 1.22 (1.03–1.44)‡ | 0.86 (0.44–1.69) | 13.08 (10.06–17)‡ | 1.95 (1.33–2.85)‡ | 3.99 (3.4–4.68)‡ | 2.19 (1.91–2.5)‡ |
| 2017 | 1 (0.83–1.22) | 1.75 (1.37–2.24)‡ | 1.81 (1.35–2.44)‡ | 1.17 (1–1.38)∗ | 1.59 (0.97–2.62) | 17.13 (12.87–22.8)‡ | 2.4 (1.65–3.49)‡ | 4.58 (3.93–5.34)‡ | 2.18 (1.93–2.48)‡ |
| 2018 | 0.99 (0.81–1.22) | 1.62 (1.27–2.08)‡ | 1.95 (1.42–2.67)‡ | 1.16 (0.99–1.37) | 2.66 (1.74–4.08)‡ | 20.74 (16.05–26.81)‡ | 2.48 (1.72–3.58)‡ | 6.85 (5.87–8)‡ | 2.18 (1.92–2.48)‡ |
| 2019 | 0.86 (0.68–1.08) | 1.69 (1.33–2.15)‡ | 1.95 (1.38–2.76)‡ | 1.14 (0.97–1.35) | 4.14 (2.76–6.22)‡ | 33.17 (24.38–45.13)‡ | 2.23 (1.42–3.49)‡ | 8.09 (6.83–9.57)‡ | 2.17 (1.9–2.48)‡ |
| South Atlantic | |||||||||
| 2014 | 2.11 (1.62–2.74)‡ | 1.43 (0.97–2.1) | 2.33 (1.7–3.21)‡ | 1.2 (0.82–1.76) | 1.2 (0.45–3.17) | 2.8 (1.56–5)‡ | 2.52 (1.57–4.07)‡ | 2.74 (2.09–3.59)‡ | 2.5 (1.94–3.22)‡ |
| 2015 | 1.92 (1.56–2.38)‡ | 1.28 (0.92–1.79) | 1.61 (1.22–2.12)‡ | 1.07 (0.81–1.41) | 1.38 (0.76–2.51) | 1.81 (1.19–2.75)‡ | 1.67 (1.06–2.63)† | 2.27 (1.84–2.8)‡ | 2.56 (2.09–3.12)‡ |
| 2016 | 1.86 (1.4–2.46)‡ | 1.36 (0.87–2.11) | 2.11 (1.53–2.91)‡ | 1.03 (0.72–1.47) | 1.92 (1.15–3.2)‡ | 2.58 (1.57–4.23)‡ | 1.55 (0.9–2.69) | 2.45 (1.85–3.25)‡ | 2.31 (1.8–2.96)‡ |
| 2017 | 1.61 (1.12–2.3)‡ | 1.86 (1.13–3.07)‡ | 1.75 (1.19–2.58)‡ | 0.92 (0.57–1.5) | 1.96 (1.18–3.23)‡ | 2.42 (1.29–4.55)‡ | 1.85 (0.94–3.65) | 2.13 (1.51–3.01)‡ | 2.33 (1.7–3.18)‡ |
| 2018 | 1.43 (0.98–2.1) | 1.22 (0.71–2.08) | 2.45 (1.68–3.59)‡ | 1.2 (0.76–1.88) | 2.55 (1.6–4.09)‡ | 4.72 (2.52–8.83)‡ | 1.78 (0.82–3.87) | 2.37 (1.66–3.37)‡ | 2.73 (2–3.73)‡ |
| 2019 | 1.62 (1.15–2.27)‡ | 1.33 (0.85–2.07) | 2.55 (1.81–3.6)‡ | 1.05 (0.73–1.5) | 3.36 (2.27–4.97)‡ | 5.84 (3.35–10.16)‡ | 1.76 (0.9–3.41) | 2.32 (1.71–3.14)‡ | 2.56 (1.94–3.37)‡ |
| West North Central | |||||||||
| 2014 | 1.77 (1.22–2.57)‡ | 0.68 (0.28–1.63) | 1.22 (0.55–2.69) | 1.27 (0.82–1.97) | 2.13 (0.53–8.58) | 2.43 (0.71–8.32) | 1.43 (0.66–3.11) | 1.96 (1.32–2.91)‡ | 3.23 (2.34–4.47)‡ |
| 2015 | 1.73 (1.38–2.18)‡ | 1.44 (0.83–2.49) | 0.98 (0.6–1.6) | 1.36 (1.06–1.75)‡ | 1.39 (0.54–3.55) | 2.99 (1.81–4.93)‡ | 1.19 (0.69–2.05) | 2.49 (1.96–3.16)‡ | 2.9 (2.38–3.54)‡ |
| 2016 | 1.67 (1.35–2.06)‡ | 1.34 (0.82–2.19) | 0.93 (0.61–1.41) | 1.22 (0.98–1.53) | 2.09 (1.3–3.37)‡ | 2.66 (1.74–4.07)‡ | 1.08 (0.64–1.82) | 2.63 (2.11–3.28)‡ | 3.11 (2.61–3.7)‡ |
| 2017 | 1.91 (1.43–2.55)‡ | 1.4 (0.82–2.41) | 1.45 (0.81–2.62) | 1.53 (1.14–2.04)‡ | 3.46 (1.95–6.16)‡ | 6.43 (2.89–14.31)‡ | 1.85 (0.81–4.23) | 2.44 (1.79–3.35)‡ | 3.16 (2.52–3.97)‡ |
| 2018 | 2.06 (1.43–2.96)‡ | 2.06 (1.15–3.66)‡ | 2.4 (1.17–4.9)‡ | 1.27 (0.86–1.86) | 10.38 (5.35–20.13)‡ | 18.37 (6.46–52.22)‡ | 1.82 (0.62–5.37) | 4.46 (3.01–6.61)‡ | 3.44 (2.62–4.52)‡ |
| 2019 | 1.71 (1.21–2.41)‡ | 1.36 (0.76–2.41) | 2.66 (1.56–4.54)‡ | 1.59 (1.15–2.19)‡ | 5.6 (3.44–9.11)‡ | 7.1 (3.42–14.75)‡ | 2.62 (1.1–6.25)† | 2.94 (2.04–4.24)‡ | 4.01 (3.12–5.15)‡ |
| West South Central | |||||||||
| 2014 | 2.74 (2.07–3.62)‡ | 1.54 (0.83–2.85) | 2.79 (1.89–4.12)‡ | 1.52 (1.05–2.2)† | 0.6 (0.1–3.65) | 5.59 (2.13–14.66)‡ | 3.32 (1.68–6.56)‡ | 2.38 (1.76–3.22)‡ | 4.11 (3.23–5.22)‡ |
| 2015 | 2.22 (1.8–2.75)‡ | 1.58 (0.95–2.63) | 1.91 (1.36–2.68)‡ | 1.22 (0.93–1.6) | 0.78 (0.23–2.63) | 3.09 (1.34–7.14)‡ | 3.3 (1.9–5.73)‡ | 2.1 (1.66–2.66)‡ | 3.57 (2.96–4.29)‡ |
| 2016 | 2.29 (1.71–3.07)‡ | 1.32 (0.73–2.41) | 1.73 (1.06–2.82)† | 1.37 (0.98–1.91) | 0.59 (0.12–2.88) | 4.89 (1.86–12.85)‡ | 3.23 (1.59–6.56)‡ | 2.07 (1.5–2.86)‡ | 3.68 (2.88–4.71)‡ |
| 2017 | 2.16 (1.58–2.97)‡ | 1.3 (0.68–2.48) | 2.32 (1.5–3.59)‡ | 1.3 (0.91–1.84) | 1.12 (0.38–3.26) | 2.28 (0.74–6.99) | 2.91 (1.29–6.58)‡ | 1.86 (1.32–2.62)‡ | 4.29 (3.28–5.62)‡ |
| 2018 | 2.34 (1.55–3.51)‡ | 0.97 (0.41–2.29) | 1.77 (0.92–3.41) | 1.24 (0.78–1.97) | 1.18 (0.34–4.14) | 2.75 (0.81–9.28) | 2.36 (0.76–7.34) | 1.64 (1.04–2.56) † | 4.61 (3.21–6.63)‡ |
| 2019 | 2.34 (1.55–3.55)‡ | 1.6 (0.81–3.15) | 2.2 (1.22–3.98)‡ | 1.3 (0.83–2.04) | 2.2 (0.9–5.37) | 5.3 (1.82–15.5)‡ | 1.73 (0.41–7.24) | 2.18 (1.42–3.35)‡ | 4.88 (3.44–6.91)‡ |
A logistic regression model for each of the nine independent drug or drug classes was performed containing collection year, US census division, sex, age, health care specialty, methamphetamine detection status (positive or negative) main effects and collection year by US census division by methamphetamine detection status interaction effects. The drug or drug class detection was modeled as the response variable. Odds ratios (OR) were calculated for each model. OR estimates and 95% Sidak-corrected confidence intervals represent the odds of being positive for the response drug in the methamphetamine-positive population divided by the odds in the methamphetamine-negative population. Higher OR values represent higher copositivity values. OR was calculated for the Total US population and each individual US census division for each year of the study. Other opioids include codeine, hydrocodone, hydromorphone, morphine, oxycodone, and oxymorphone.
P < 0.05.
P < 0.01.
P < 0.001.
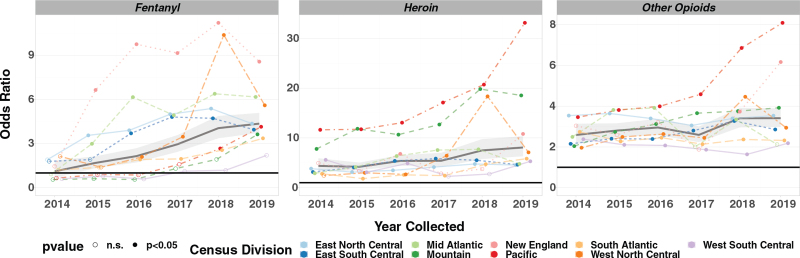
Nationally, aOR values are significantly greater than 1 for most years and across several drugs and drug classes (Table 3). Fentanyl, heroin, and other opioids had the highest overall aORs nationally in 2019, and these aORs increased from 2014 to 2019 (Fig. 2, Table 3). A Kendall correlation test showed that the increases over time in aOR for methamphetamine copositivity with heroin (P = 0.024) and fentanyl (P = 0.0085) were statistically significant (see Table, Supplemental Digital Content 2, showing Kendall correlation test results for each of copositive drugs). Although national aORs for buprenorphine and other opioids also increased between 2014 and 2019, these changes were not significant. Decreases in national aORs for benzodiazepines, cocaine, alcohol, methadone, and THC were not significant.
FIGURE 2.
Copositivity analysis – methamphetamine positive versus negative population OR. Notes: OR were calculated for logistic regression models of fentanyl, heroin, and other opioids. OR estimates represent the odds of being positive for the response drug in the methamphetamine-positive population divided by the odds in the methamphetamine-negative population. Higher OR values represent higher copositivity values. OR was calculated for the total US population and each individual US census division for each year of the study. Solid circles represent OR significantly higher than 1 based on a Tukey corrected P value (P < 0.05). Open circles represent OR not significantly higher than 1 (n.s.). n.s. indicates not significant; OR, odds ratios.
Geographically, the data reveal a number of interesting temporal trends. For example, several significant trends were noted for the Pacific division, which had the highest aORs for heroin and other opioids in 2019. For example, heroin was detected with a probability of 0.095 [0.086–0.106] in the methamphetamine-positive population versus 0.003 [0.003–0.004] in the methamphetamine-negative group (see Figure and Table, Supplemental Digital Content 3 and 4, which illustrate predicted probabilities for copositives drugs). This differential means that methamphetamine-positive individuals in the Pacific division were 33.17 [24.38–45.13] times more likely to be positive for heroin than methamphetamine-negative individuals in 2019 (Table 3, Fig. 2). The increase in aOR values for heroin in the Pacific division was statistically significant (P = 0.0085), as were increases in aOR for fentanyl (P = 0.0242) and other opioids (P = 0.0085) (see Table, Supplemental Digital Content 2). Though aORs for fentanyl were higher for New England, Mid Atlantic, West North Central, and East North Central in 2019 than in the Pacific, all 3 decreased after 2018 (Table 3, Fig. 2).
DISCUSSION
These data indicate a 340% nationwide increase between 2014 and 2019 in probability for methamphetamine-positivity in UDT after model adjustment. The highest predicted positivity rate was in male patients, 25- to 34-years-old, from the West North Central division and from SUD treatment centers. Although the West North Central division had the highest predicted methamphetamine positivity in 2019, positivity for the East South Central is rapidly rising and may surpass the West North Central and Pacific divisions in 2020.
These findings are concerning for several reasons.14 Methamphetamine use can have severe consequences for the user, resulting in short-term and long-term health effects, including overdose and death. Because of stimulation of cardiovascular and central nervous systems, use may lead to heart attack or stroke.15 Methamphetamine overdose may result in increased body temperature and convulsions, which when left untreated, may result in death.14 Unfortunately, treating a methamphetamine overdose is challenging because there is no specific antidote, as is the case for opioids with the opioid antagonist naloxone. With acute intoxication, in which the user may be experiencing agitation and psychotic symptoms, supportive measures are often used, including placing the patient in a calm, quiet space and use of a benzodiazepine or antipsychotic.15
Binge use of methamphetamine is common to sustain the drug's effects and increases risk of acute psychosis and the need for intervention by law enforcement or medical personnel. Tolerance can build following prolonged exposure, which may lead to withdrawal when drug exposure is reduced. Withdrawal-related effects may include insomnia, anxiety, depression, and intense cravings.15 Other long-term consequences include anhedonia, mood disturbances, and psychotic features, such as paranoia and hallucinations, that may remain for months or years.14
Another challenge associated with methamphetamine is that, unlike opioid use disorder, there are no FDA-approved medications indicated for the treatment of methamphetamine use disorder. Thus, treatment is limited to off-label use of medications, such as bupropion, methylphenidate, modafinil, or mirtazapine, or nonpharmacologic therapy, such as cognitive-behavioral therapy or contingency management. Although both forms of therapy are widely accepted, relapse rates remain high with over 50% of participants expected to relapse within 6 to 9 months following treatment.15 Given that the highest predicted positivity for methamphetamine in this study was in specimens from SUD treatment centers, it is particularly important to monitor for use of this substance and improve the treatment of methamphetamine use disorder. Strategies to prolong time to relapse include longer time in treatment and posttreatment involvement in self-health or other relapse-prevention programs.16 Researchers funded by the National Institute on Drug Abuse are investigating several options for treating methamphetamine use disorder, including medications targeting various systems, such as dopamine, as well as transcranial magnetic stimulation, neurofeedback, vaccines, and antibodies that would use the immune system to block methamphetamine from reaching the brain.14 In light of rising methamphetamine availability and use with other substances, this research will be key to addressing the public health consequences.
Although methamphetamine use is increasing, the rate of those receiving treatment for methamphetamine use disorder has not changed significantly.6 Barriers to accessing treatment services may include availability, affordability, knowledge of how to seek treatment, lack of perceived need for treatment, and stigma associated with methamphetamine use.17,18 Psychosocial factors, including stigma, are some of the primary barriers to treatment of methamphetamine use disorder.18
When methamphetamine is combined with other substances, additional challenges arise, including increased risk of overdose death, worse treatment engagement and outcomes, riskier health behaviors, and elevated psychological distress.8,19 Currently, clinical guidelines focus on solitary SUDs, which makes it more difficult to develop treatment strategies for those with multiple SUDs.19 This study demonstrated significant copositivity for several substances with the odds being highest for heroin, fentanyl, and other opioids in 2019. Adjusted OR for copositive drugs and drug classes vary considerably by geography, with those in the Pacific census division being at highest risk for copositive methamphetamine and heroin or other opioids in 2019. Methamphetamine combinations with opioids can be lethal, particularly when the user is opioid-naïve or unaware of the presence of other substances.19 While copositivity of fentanyl and methamphetamine seems to be declining in certain divisions, the increasing national OR and widespread availability of these substances necessitates caution and continued monitoring.
This study highlights the importance of carefully evaluating patients using methamphetamine for polysubstance use to tailor and adjust their treatment plan. The increasing combination of methamphetamine and opioids will require education and overdose prevention efforts that may not have previously reached the nonopioid user population or the HCPs who care for them. Examples include harm reduction strategies, warnings about the increased risk of overdose, and access to naloxone. Health care practitioners may need to prepare to treat more cases of mixed intoxication, withdrawal, and methamphetamine-induced psychosis. Lastly, there is a need to develop public health strategies to overcome barriers to accessing treatment for methamphetamine and polysubstance use that improve patient engagement and effectiveness of treatment outcomes.18
Limitations
Data are limited to a population of patients seeking health care and may not reflect the general population. Individuals included in the analysis may have had an incomplete or inaccurate medication list, which would result in the inclusion of some subjects expected to be positive for prescribed analytes. The study used a convenience sample and patient characteristics changed through time. We attempted to account for this variation by modeling sex, age, and health care specialty because of their strong impact on nonprescribed drug positivity. It is possible that these covariates were insufficient to remove all potential confounding and/or selection bias. The
“other opioids” class of drugs studied includes morphine, which is a potential metabolite of heroin. Lastly, the designated health care specialty may include patient populations that would also fit a different specialty, such as patients with cooccurring mental health and SUDs being treated at a behavioral health practice versus a SUD treatment center.
CONCLUSIONS
The probability of being positive for methamphetamine in UDT increased nationwide between 2014 and 2019. Copositivity among the methamphetamine-positive population, particularly with opioids, increases the risk of overdose and poor treatment outcomes. Clinicians and public health officials should be aware of the potential health implications associated with methamphetamine use and support strategies to improve health outcomes and the treatment of methamphetamine use disorder.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Footnotes
Supported by Millennium Health, LLC.
Dr. Twillman is a consultant of Millennium Health. Drs. Dawson, LaRue, Guevara, Huskey and Mr. Whitley are employees of Millennium Health, LLC, San Diego, California.
Supplemental digital content is available for this article.
REFERENCES
- 1.Gonzales R, Mooney L, Rawson RA. The methamphetamine problem in the United States. Annu Rev Public Health 2010; 31:385–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. US Drug Enforcement Administration, National Drug Intelligence Center. National Prescription Drug Threat Assessment. Available at: https://www.justice.gov/archive/ndic/pubs33/33775/33775p.pdf. Published April 2009. Accessed April 6, 2020. [Google Scholar]
- 3. US Drug Enforcement Administration. 2013 National Drug Threat Assessment Summary. Available at: https://www.dea.gov/documents/2013/10/01/2013-national-drug-threat-assessment. Accessed April 6, 2020. [Google Scholar]
- 4. US Drug Enforcement Administration. 2019 National Drug Threat Assessment. Available at: https://www.dea.gov/documents/2020/01/30/2019-national-drug-threat-assessment. Accessed March 3, 2020. [Google Scholar]
- 5. US Drug Enforcement Administration. 2014 National Drug Threat Assessment Summary. Available at: https://www.dea.gov/sites/default/files/2018-07/dir-ndta-unclass.pdf. Accessed April 6, 2020. [Google Scholar]
- 6. Substance Abuse and Mental Health Services Administration. Key substance use and mental health indicators in the United States: Results from the 2018 National Survey on Drug Use and Health. HHS Publication No. PEP19–5068, NSDUH Series H–54. Rockville, MD: Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services Administration; 2019. [Google Scholar]
- 7.Hedegaard H, Miniño AM, Warner M. Drug Overdose Deaths in the United States, 1999-2018. NCHS Data Brief, no 356. Hyattsville, MD: National Center for Health Statistics; 2020. [Google Scholar]
- 8.Kariisa M, Scholl L, Wilson N, Seth P, Hoots B. Drug overdose deaths involving cocaine and psychostimulants with abuse potential — United States, 2003–2017. MMWR Morb Mortal Wkly Rep 2019; 68:388–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.LaRue L, Twillman RK, Dawson E, et al. Rate of fentanyl positivity among urine drug test results positive for cocaine or methamphetamine. JAMA Netw Open 2019; 2(4):e192851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. US Drug Enforcement Administration, Diversion Control Division. National Forensic Laboratory Information System: NFLIS-Drug 2018 annual report. Available at: https://www.nflis.deadiversion.usdoj.gov/DesktopModules/ReportDownloads/Reports/NFLIS-Drug-AR2018.pdf. Accessed March 3, 2020. [Google Scholar]
- 11. DeMio T. Meth is back and flooding the streets of Ohio and Kentucky, and it's uglier than ever. Cincinnati Enquirer. February 13, 2020. Available at: https://www.cincinnati.com/story/news/2020/02/13/meth-opioids-new-epidemic-of-addiction/4551991002/. Accessed March 3, 2020. [Google Scholar]
- 12. US Drug Enforcement Administration. 2016 National Drug Threat Assessment summary. Available at: https://www.dea.gov/sites/default/files/2018-07/DIR-001-17_2016_NDTA_Summary.pdf. Accessed March 3, 2020. [Google Scholar]
- 13. Census Regions and Divisions of the United States. US Census Bureau. Available at: https://www2.census.gov/geo/pdfs/maps-data/maps/reference/us_regdiv.pdf. Accessed April 23, 2020. [Google Scholar]
- 14. Methamphetamine. National Institute on Drug Abuse Web site. Available at: https://www.drugabuse.gov/publications/methamphetamine/how-methamphetamine-misused. Published April 1, 2019. Accessed March 3, 2020. [Google Scholar]
- 15.Ling W, Mooney L, Haglund M. Treating methamphetamine use disorder. Curr Psychiatry 2014; 13(9):36–44. [Google Scholar]
- 16.Brecht ML, Herbeck D. Time to relapse following treatment for methamphetamine use: a long-term perspective on patterns and predictors. Drug Alcohol Depend 2014; 139:18–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Semple SJ, Grant I, Patterson TL. Utilization of drug treatment programs by methamphetamine users: the role of social stigma. Am J Addict 2005; 14(4):367–380. [DOI] [PubMed] [Google Scholar]
- 18.Cumming C, Troeung L, Young JT, Kelty E, Preen DB. Barriers to accessing methamphetamine treatment: a systematic review and meta-analysis. Drug Alcohol Depend 2016; 168:263–273. [DOI] [PubMed] [Google Scholar]
- 19.Wang L, Min JE, Krebs E, et al. Polydrug use and its association with drug treatment outcomes among primary heroin, methamphetamine, and cocaine users. Int J Drug Policy 2017; 49:32–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.