Abstract
A previously unrecognized Rickettsia species was isolated in 1976 from a pool of Ixodes pacificus ticks collected in 1967 from Tillamook County, Oregon, USA. The isolate produced low fever and mild scrotal oedema following intraperitoneal injection into male guinea pigs (Cavia porcellus). Subsequent serotyping characterized this isolate as distinct from recognized typhus and spotted fever group Rickettsia species; nonetheless, the isolate remained unevaluated by molecular techniques and was not identified to species level for the subsequent 30 years. Ixodes pacificus is the most frequently identified human-biting tick in the western United States, and as such, formal identification and characterization of this potentially pathogenic Rickettsia species is warranted. Whole-genome sequencing of the Tillamook isolate revealed a genome 1.43 Mbp in size with 32.4 mol% G+C content. Maximum-likelihood phylogeny of core proteins places it in the transitional group of Rickettsia basal to both Rickettsia felis and Rickettsia asembonensis. It is distinct from existing named species, with maximum average nucleotide identity of 95.1% to R. asembonensis and maximum digital DNA–DNA hybridization score similarity to R. felis at 80.1%. The closest similarity at the 16S rRNA gene (97.9%) and sca4 (97.5%/97.6% respectively) is to Candidatus ‘Rickettsia senegalensis’ and Rickettsia sp. cf9, both isolated from cat fleas (Ctenocephalides felis). We characterized growth at various temperatures and in multiple cell lines. The Tillamook isolate grows aerobically in Vero E6, RF/6A and DH82 cells, and growth is rapid at 28 °C and 32 °C. Using accepted genomic criteria, we propose the name Rickettsia tillamookensis sp. nov., with the type strain Tillamook 23. Strain Tillamook 23 is available from the Centers for Disease Control and Prevention Rickettsial Isolate Reference Collection (WDCM 1093), Atlanta, GA, USA (CRIRC accession number RTI001T) and the Collection de Souches de l’Unité des Rickettsies (WDCM 875), Marseille, France (CSUR accession number R5043). Using accepted genomic criteria, we propose the name Rickettsia tillamookensis sp. nov., with the type strain Tillamook 23 (=CRIRC RTI001=R5043).
Keywords: Rickettsia, Rickettsia tillamookensis, Ixodes pacificus
Introduction
In 1976, investigators at the Rocky Mountain Laboratory (RML) in Hamilton, MN, described the isolation and preliminary characterization of a previously unrecognized Rickettsia species obtained from western blacklegged (Ixodes pacificus) ticks collected from a rural area of Tillamook County in northwestern Oregon in 1967 [1]. When 80 male guinea pigs (Cavia porcellus) were inoculated intraperitoneally with a 10% yolk sac suspension of the Tillamook isolate, 40 of the animals developed 1–2 days of low-grade fever and mild scrotal swelling was observed in 21 of the guinea pigs, suggesting that the novel agent was mildly virulent [1]. In 1981, investigators applied a mouse serotyping technique to characterize 72 strains of Rickettsia species from diverse sources and demonstrated that the Tillamook isolate represented a unique serotype, distinct from all known typhus and spotted fever group Rickettsia species [2]. Additional isolates similar or identical to the Tillamook isolate were subsequently cultivated from individual specimens of I. pacificus ticks collected from Sonoma and Monterey counties in Northern California during 1979–1980, and designated as ‘Tillamook-like’ strains CA 277 and CA 288, respectively [3, 4]. Since then, no work has been performed to further characterize the Tillamook isolate. More recently, other Rickettsia species, designated phylotypes G021 and G022, have been detected in I. pacificus ticks collected from the western United States [5–7]. Phylotype G021, a near-ubiquitously distributed endosymbiont in I. pacificus, has recently been isolated and appears to be a species distinct from the Tillamook isolate [7, 8], whereas the identity of phylotype G022 has not yet been determined. Ixodes pacificus is widely distributed and is the most frequently identified human-biting tick in the western United States [9]; therefore the pathogenic potential of Tillamook isolate warrants its further characterization and formal identification among other members of the genus Rickettsia.
Isolation and whole genome sequencing
Records documenting the cultivation and passage history of the Tillamook isolate were kindly provided by Ted Hackstadt at RML. These records designated the original strain as Tillamook 23. The primary isolate was cultivated in embryonated chicken eggs from a pool of nine female I. pacificus ticks [1]. An aliquot of the isolate that had been maintained in chicken yolk sacs for 23 passages and in Vero cells for seven passages was provided to the Centers for Disease Control and Prevention (CDC) in 1991 and was retained in liquid nitrogen until 2017. The revived isolate was cultivated at 32 °C in a 5 % CO2-in-air atmosphere for an additional six passes in Vero E6 cells. Infected cells from a T150 cell culture flask were disrupted by vortexing for 30 s with autoclaved rock tumbler grit (Lortone) to lyse the host Vero cells. After allowing the grit to settle, the lysate was passed through a 2 µm filter to remove any remaining intact host cells. To each 1 ml lysate was added 10 µl DNase I (Sigma-Aldrich) at a concentration of 1.4 mg ml−1. Following a 30 min incubation at room temperature, the lysate was centrifuged at 11 000 g for 10 min at 4 °C, and the supernatant removed. The pellet was resuspended in 1 ml 300 mM sucrose. The process of centrifugation, removal of supernatant and resuspension in the sucrose solution was repeated two additional times. The final pellet was resuspended in 10 µl 300 mM sucrose, mixed gently with 600 µl Gentra PureGene (Qiagen) lysis buffer, and incubated at 80 °C for 10 min to lyse the rickettsial cells. The lysate was cooled to room temperature then mixed with 3 µl RNase A (Qiagen) and incubated 37 °C for 45 min. The lysate was cooled on ice and mixed with 200 µl Gentra PureGene (Qiagen) protein precipitation buffer. After a 5 min incubation on ice, the mixture was centrifuged at 15 000 g for 1 min at 4 °C. The supernatant was removed and mixed with an equal volume of buffer-saturated phenol–chloroform–isoamyl alcohol (Sigma-Aldrich). The mixture was transferred to a phase lock gel microfuge tube and centrifuged at 11 000 g for 1 min. The aqueous layer was transferred to a new phase lock tube, mixed with an equal volume of chloroform and centrifuged 15 000 g for 1 min. The aqueous layer was transferred to a new microfuge tube, mixed with 600 µl ice-cold isopropanol and incubated on ice for 30 min. The mixture was then centrifuged at 11 000 g for 10 min at 4 °C. The isopropanol was removed and the pellet was washed twice in 600 µl 70% ethanol. Following the second wash, the ethanol was removed, the purified pellet of bacterial DNA was air-dried overnight then resuspended in 50 µl double-distilled H2O.
DNA isolated from the Tillamook 23-infected Vero cells was purified and concentrated with AMPure XP paramagnetic beads (Beckman Coulter), and used to prepare libraries for Pacific Biosciences (PacBio) sequencing using a SMRTbell Express library kit (version 2.0) with a starting DNA quantity of 73 ng. Libraries were run at 10 pM on the PacBio Sequel instrument in continuous long read mode using the Sequel Binding Kit 3.0 for 10 h. The 199 806 resulting reads [subread N50=4501] generated by Sequel2 were first processed by ICS 8.0 [defaults] and exported to fastq format using bam2fastq [defaults] (SmrtLink 8.0). Flye 2.6 [10] [--meta -g 100 m --pacbio-raw] was used to assemble the fastq reads resulting in 19 contigs with N50 641 316. blast+ version 2.8 [11] [e=0.01] was used to classify the contigs as rickettsial (n=9), mycoplasma (n=6) and Chlorocebus aethiops (n=3). Minimap2 [12] [-axe map-pb] was used to map the fastq reads to rickettsial contigs only, and mapped reads were extracted to fastq using SAMtools version 1.9 [13] [ fastq -F 4]. Flye 2.6 [-g 2 m --asm-coverage 100 --pacbio-raw] was then used to assemble the extracted mapped reads and resulted in a single circularized contig of 1 438 965 bp with mean coverage of 1343×. Arrow [defaults] (Smrtlink 8.0) was used to polish the contig. The final assembly obtained after polishing was 1 438 973 bp and the G+C content was 32.4 mol%. IGV version 2.8 [14, 15] was used to visualize the raw reads mapped to the final assembly to check for mis-assemblies and collapsed repeats, of which none were found.
Tillamook 23 DNA was prepared for shotgun sequencing using a Nextera XT (Illumina) library preparation kit and sequenced for 2×250 bp paired-end reads on an Illumina MiSeq sequencer. Reference alignment to the PacBio-assembled genome indicated 43× mean coverage after removal of reads from contaminating primate (Vero E6 cell) DNA. PacBio reads were indexed using BWA-MEM [16] [-t 14]. SAMtools [13, 17] [ view - -Sb | samtools sort - -@14 -o] was used to align, view, map and sort the final file, and to index the final file for polishing. Pilon [18] [--genome --fix all --changes --frags --threads 14 --output] was used to polish the genome using PacBio reads and the final file from the SAMtools step. Annotation of the polished genome was done using Prokka [19].
Assembly completeness and annotation accuracy were assessed using Benchmarking Universal Single-Copy Orthologs (BUSCO) software [20], using lineage dataset rickettsiales odb10 (2019-04-20) in ‘genome’ mode. Of 364 BUSCO, 99.5% were recovered as complete (one as a duplicate), and one each fragmented and missing BUSCOs were detected. The fragmented CDS (H6P87_00685; glutamine synthase) was likely mis-annotated due to an overlap of reading frames with an upstream CDS and was corrected. The missing BUSCO, ATP-dependent chaperone clpB was confirmed as absent in Tillamook 23, part of a larger regional deletion including an oxidoreductase and five hypothetical proteins in R. felis URRWXCal2T (NC_007109). This chaperone is also annotated as a pseudogene in R. felis LSU-Lb (NZ_JSEL01000000). Rickettsial palindromic elements (RPEs) [21] were annotated on the Tillamook 23 genome using the ‘Transfer Annotations’ function in Geneious Prime [22] using R. felis URRWXCal2T (NC_007109) as reference. Individual RPEs were curated manually.
Phylogeny
The 16S rRNA, GltA, OmpA, OmpB, Sca4 and 17 kDA (htr) genes from Tillamook 23 were searched by using blast against the NCBI Genbank database to determine similar sequences among isolates without standing in nomenclature or for which whole genome data was not available (Table 1). The closest identity at the 16S rRNA gene (97.9 %) was shared for Candidatus ‘Rickettsia senegalensis’ [23] from cat fleas (Ctenocephalides felis), a member of the R. felis group, and Rickettsia sp. cf9, again from cat fleas. These isolates had the highest identity at sca4 (>97%) of any for which sequence was available. A further rickettsial isolate (Rickettsia sp. CF26B/US) from C. felis was 97.8% identical at the 16S rRNA gene locus. A rickettsial isolate from Mansonia mosquitoes (Rickettsia sp. A12.2646) was also similar at 97.6% identity and was highly similar at gltA and the 17 kDa gene (99.2 %, 99.8% identity, respectively). Among valid species within the transitional group, Tillamook 23 has the highest (96.3 %) 16S rRNA gene identity with R. felis URRWXCal2T, followed by R. hoogstraalii CroaticaT (96.1 %), R. australis Cutlack (96.0 %), R. asembonensis F30 (95.8 %) and R. akari HartfordT (95.2 %).
Table 1.
Rickettsia species available in GenBank with high identity of individual genes to Tillamook 23
|
Rickettsia species |
16s rRNA (%) |
gltA (%) |
ompA (%) |
ompB (%) |
sca4 (%) |
17 kDa (%) |
Country |
Vector |
Reference |
|---|---|---|---|---|---|---|---|---|---|
|
Uncultured Rickettsia sp. clone P4 |
– |
96.7 |
95.1 |
– |
– |
– |
Turkey |
Haemaphysalis parva |
[46] |
|
Rickettsia sp. RF2125 |
– |
97.2 |
– |
– |
– |
– |
Vietnam |
Ctenocephalides felis |
[47] |
|
Rickettsia sp. genotype RF2125 isolate T29C9 |
– |
96.9 |
– |
– |
– |
– |
Thailand |
Ctenocephalides felis |
[48] |
|
Rickettsia sp. genotype RF2125 isolate AL866-1 |
– |
96.7 |
94 |
– |
– |
– |
USA |
Ctenocephalides felis |
[49] |
|
Rickettsia endosymbiont of Haemaphysalis sulcata |
– |
96.7 |
– |
– |
– |
96.4 |
Croatia |
Hemaphysalis sulcata |
[32] |
|
Rickettsia sp. clone: RT2 |
– |
95.6 |
– |
– |
– |
97.8 |
Indonesia |
Amblyomma varanense |
[50] |
|
Rickettsia sp. 777 c |
– |
– |
– |
88.8 |
– |
97.8 |
Australia |
Amblyomma fimbriatum Koch |
[51] |
|
Candidatus ‘Rickettsia senegalensis’ isolate Cf_US_0036D |
97.9 |
– |
– |
88.7 |
97.6 |
– |
USA |
Ctenocephalides felis |
[31] |
|
Rickettsia sp. cf9 |
97.9 |
|
|
92 |
97.5 |
|
USA |
Ctenocephalides felis |
Genbank: DQ287314-5, DQ379483 |
|
Rickettsia endosymbiont of Ctenocephalides felis isolate CF26B/US |
97.8 |
97.2 |
92.8 |
93.5 |
95.4 |
|
USA |
Ctenocephalides felis |
[52] |
|
Rickettsia sp. A12.2646 |
97.6 |
99.2 |
|
89.6 |
93.5 |
99.8 |
Korea |
Mansonia uniformis |
[53] |
|
Rickettsia secondary symbiont Curculio camelliae |
96.1 |
– |
– |
– |
– |
– |
Japan/SE Asia |
Curculionini camilliae |
[54] |
|
Rickettsia secondary symbiont Curculio hilgendorfi |
96.1 |
– |
– |
– |
– |
– |
Japan/SE Asia |
Curculionini hilgendorfi |
[54] |
|
Candidatus ‘Rickettsia senegalensis’ strain PU01-02 |
96 |
96.7 |
– |
89.5 |
96.1 |
– |
Senegal |
Ctenocephalides felis |
[23] |
|
Rickettsia asiatica Maytaro1284 DNA |
95.9 |
95.3 |
89.2 |
86.9 |
86 |
95.6 |
Japan |
Ixodes ovatus |
[55] |
|
Rickettsia sp. DnS28 strain DnS28 |
95.9 |
94.7 |
90.3 |
– |
– |
– |
Russia |
Dermacentor nuttallii |
[56] |
|
Rickettsia sp. RpA4 strain RpA4 |
95.9 |
94.9 |
90.4 |
– |
– |
– |
Russia |
Rhipicephalus pumilio |
[56] |
|
Rickettsia sp. TwKM01 |
95.7 |
94.5 |
89.3 |
89 |
85.5 |
94.4 |
Taiwan |
Rhipicephalus haemaphysaloides |
[57] |
|
Rickettsia sp. DnS14 |
95.7 |
94.8 |
90.3 |
– |
– |
– |
Russia |
Dermacentor nuttallii |
[56] |
|
Candidatus ‘Rickettsia wissemanii’ isolate G1329 |
95.6 |
94.5 |
89.4 |
86.2 |
85.3 |
– |
French Guiana |
Ornithodoros hasei |
[58] |
|
Rickettsia sp. Chad |
95.5 |
94.2 |
87.8 |
– |
– |
– |
Chad |
Unknown |
[59] |
|
Candidatus ‘Rickettsia barbariae’ |
95.4 |
94.1 |
86 |
88.3 |
84.9 |
– |
Italy |
Rhipicephalus turanicus |
[60] |
|
Rickettsia sp. IRS4 |
95.4 |
94.8 |
90.3 |
– |
87.5 |
– |
Slovakia |
Ixodes ricinus |
[61] |
Validity of Tillamook 23 as a novel species was determined using guidelines established by Diop et al. [8], namely a digital DNA–DNA hybridization (dDDH) score <92.3% and/or orthology-derived average nucleotide identity (OrthoANI) score of <99.2% with the type strain of a valid Rickettsia species. The dDDH values were calculated using the GGDC web server (http://ggdc.dsmz.de/distcalc2.php). OrthoANI values were calculated using the program OrthoANI version 0.93.1 GUI [24]. Both analyses included the same set of 78 valid Rickettsia genomes detailed in Diop et al. [8]. The highest dDDH scores under formulae 1 and 3 were to R. felis Pedreira, at 80.1 and 77.7%, respectively, and to R. asembonensis NMRCiiTT under formula 2 (57.7%). Maximum OrthoANI at 95.1% was to R. asembonensis NMRCiiTT.
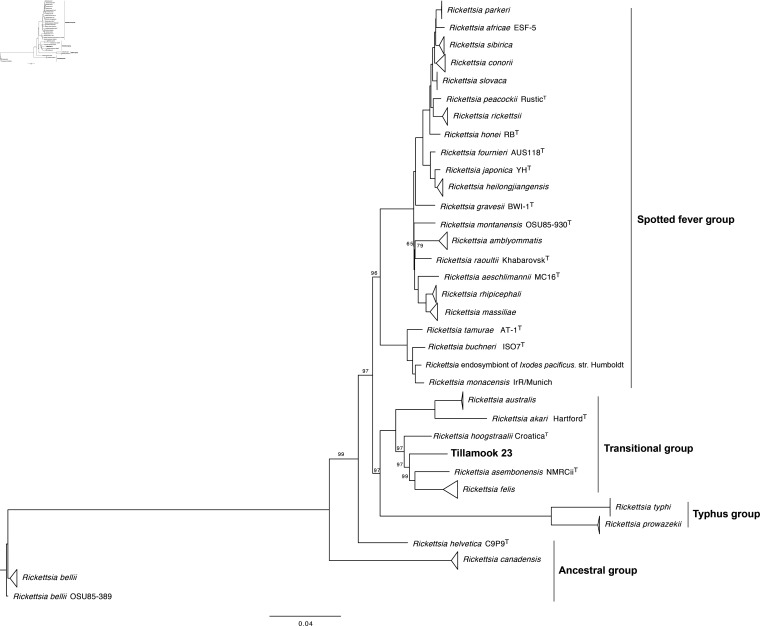
A core set of orthologous protein sequences was created using ProteinOrtho software [25] [-sim=0.8 -identity=15 -cov=25]. The core set consisted of 555 proteins per strain for the 78 Rickettsia described by Diop et al. [8] as well as Tillamook 23. Maximum-likelihood phylogenetic analysis was performed using Phyml software [26] under the JTT+gamma model and 500 bootstraps. Tree topology was reconstructed using FigTree software [27]. Tillamook 23 is placed with high bootstrap support as the lone member of a relatively deep node within the transitional group, intermediate between R. hoogstraalii and R. asembonensis (Fig. 1).
Fig. 1.
Phylogenetic tree reconstructed with 555 concatenated core proteins using ProteinOrtho software from 78 Rickettsia complete genomes from GenBank (see [8]) and Tillamook 23. Phylogenetic analysis was run using Phyml software for maximum-likelihood with options JTT and gamma models and 500 bootstraps (shown as percentages in nodes; all nodes had 100% bootstrap support except where otherwise noted). Tree topology was constructed using FigTree software. Clades with three or more strains of the same species are collapsed. The tree is rooted on Rickettsia bellii OSU85-389.
Two additional Rickettsia species identified in I. pacificus ticks, phylotypes G021, or ‘Rickettsia monacensis’ Humboldt, and G022 [5, 7], are genetically distinct from Tillamook 23 at the 16S rRNA gene (95.6/95.6% identity, respectively), gltA (95.3/96.2 %) and ompA (79.6/81.4 %). Tillamook 23 therefore represents a third unique Rickettsia species harboured by I. pacificus.
Genomic features
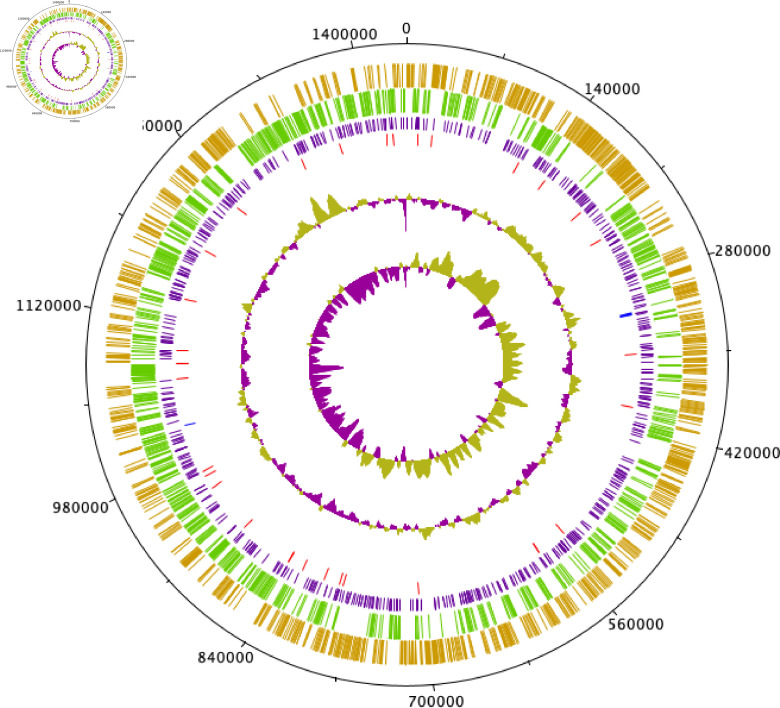
The genome of Tillamook 23 consists of a single 1 438 973 bp chromosome with a G+C content of 32.4 mol% (Fig. 2). No plasmids were detected during assembly. A total of 1346 coding sequences were predicted. One copy each of 16S, 23S and 5S rRNA genes were detected, as were 33 tRNAs and one tmRNA.
Fig. 2.
Circular representation (Circular-Plot [62]) of the Tillamook 23 genome. From outermost size marker ring (black), the first and second inner rings show CDS on forward and reverse strands, respectively, with gene functions sorted by colour. Third ring (purple) is Rickettsial palindromic elements (RPEs). Fourth and fifth rings indicate positions of tRNA (red) and rRNA (blue) genes, respectively. GC plot and skew are indicated on sixth and seventh inner rings, respectively.
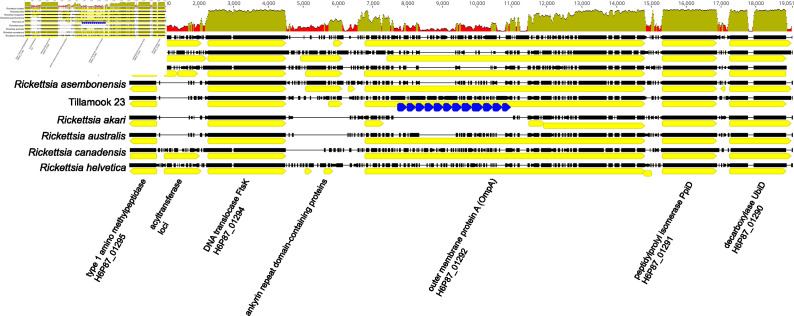
Genome analysis places Tillamook 23 within a lineage of Rickettsia species designated as transitional group rickettsiae [28]. This lineage is characterized notably by diverse representation of primary arthropod hosts that include mites (R. akari) [29], fleas (R. felis and R. asembonensis) [30, 31], argasid ticks (R. hoogstraalii) [32], ixodid ticks (R. australis and Tillamook 23) ([33], data herein) and wasps [34]. Transitional group rickettsiae share more common orthologous groups with spotted fever group Rickettsia species than with the typhus group or ancestral lineages [28]. Tillamook 23 possesses nominally full-length copies of the six surface cell antigen (sca) genes known to be functionally involved with host cell receptor interactions [ompA (sca0), sca1, sca2, sca3, sca4 and ompB (sca5)], as well as intact copies of multiple other rickettsial effector protein genes including pat1, pat2, tlyC, pld, vapC, ralF, rickA, rarp1 and rarp2 [35]. OmpA is conserved among the spotted fever group rickettsiae [36] and likely plays a role in adhesion [37]. The Tillamook 23 ompA ORF is considerably longer than other transitional group rickettsiae at 7308 bp (R. australis is next-longest with 5811 bp in strain Cutlack) and possesses a series of 12 full and one partial ~255 bp tandem repeats (Fig. 3). This repeat unit has ~97% identity with tandem repeats in ompA of R. australis and lower (75–76%) identity with tandem repeats in sca7 of R. felis URRWXCal2T and in an orthologous CDS to ompA in R. asiatica Maytaro1284 (NZ_AP019563). Tandem repeats are a common feature of adhesion proteins in the Rickettsiales [38] and other bacterial groups, and may play a role in adhesion specificity. Inconsistency in amplifying ompA with conventional primer sets has been reported for other Rickettsia species, particularly those now considered as members of the transitional group [32, 39–41] and ompA was reported absent in R. asiatica [42]. Indeed, the locus identified here and in most manual annotations as ompA is frequently annotated in available genomes as an autotransporter CDS by automated annotation software. Conventional primers [39, 40, 43] designed for amplifying ompA target this CDS (H6P87_01292 in Tillamook 23) and orthologs of this fragment consistently map between ftsK and ppiD in annotated genomes (see Fig. 3). Although ompA is present in Tillamook 23, in silico analysis (present work) suggests that sequence variability in binding sites will preclude its amplification by several commonly used primer sets targeting this locus [39, 40, 43].
Fig. 3.
Multiple alignment (mafft [63]) of ompA-containing region for ancestral rickettsiae (R. helvetica, R. canadensis), transitional group rickettsiae and R. rickettsii. Sequence identity graph (50 bp sliding window) is shown at top. Multiple CDS predicted in the ompA region for R. akari (NC_009881) are annotated as hypothetical proteins. 255 bp tandem repeat elements in Tillamook 23 are shown as blue arrows. Aligned gap positions are shown as thin horizontal lines.
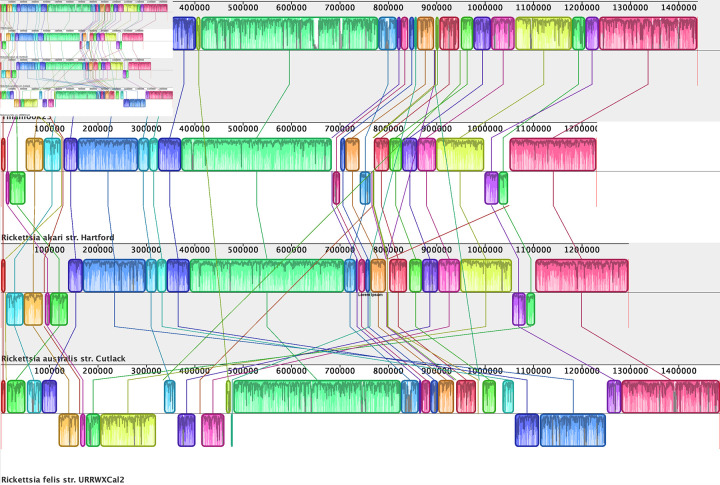
Synteny of Tillamook 23 with other completed genomes in the transitional group was examined with progressiveMauve [44]. R. asembonensis and R. hoogstraalii are in draft assembly stage and were therefore not included in this analysis. The genome of Tillamook 23 has extensive synteny with R. akari HartfordT and R. australis Cutlack, with only two small regional inversions between Tillamook 23 and R. australis. Synteny with the more closely similar R. felis was less, with several inversions and rearrangements noted (Fig. 4).
Fig. 4.
Genomic synteny (progressiveMauve [44]) of Tillamook 23 vs. available Rickettsia transitional group complete genomes Rickettsia akari HartfordT (NC_009881), Rickettsia australis Cutlack (NC_017058) and Rickettsia felis URRWXCal2T (NC_00710).
Intracellular growth
Growth of Tillamook 23 in Vero E6 cells was evaluated at various temperatures by molecular and microscopical methods. Three 25 cm2 tissue culture flasks containing a semi-confluent monolayer of Vero E6 cells were each inoculated with 1×108 genome equivalents (GEs) of bacterial cells removed from a common culture of Tillamook 23 grown at 32 °C. Inocula were quantified using a qPCR assay targeting the 23S rRNA gene of Rickettsia [45]. Flasks were incubated separately at 28, 32 and 37 °C in a 5% CO2-in-air atmosphere. From each flask, a 200 µl sample of the supernatant was removed daily for 9 days. Total DNA was extracted from each sample using a Qiagen DNEasy kit and rickettsial DNA was quantified as described previously. Rickettsial growth, as assessed by DNA quantification was similar at each temperature for first 2 days post infection (1.95–2.5×106 GE ml−1 supernatant). Beginning on day three, rickettsial DNA amounts increased at 28 and 32 °C, and followed this general trend until the end of the evaluation on day nine, at which point rickettsial DNA levels had increased at 28 °C to 1.91×107 GE ml−1 supernatant and at 32 °C to 2.38×107 GE ml−1 supernatant. By day nine, considerable cytopathic effects (host cell destruction) to the monolayers were evident, and abundant intracytoplasmic rickettsiae were observed microscopically in the Vero E6 cells incubated at 28 and 32°C when stained by acridine orange. There was no observable increase in rickettsial genome numbers when grown at 37 °C (2.05–4.31×106 GE ml−1 supernatant). Neither appreciable cytopathic effects nor stainable intracellular rickettsiae were identified in Vero E6 cells incubated at this temperature. To examine growth of Tillamook 23 in different host cells, a culture of Tillamook 23-infected Vero E6 cells was disrupted using sterile glass beads. The disrupted cellular material was passed sequentially through a series of 18G, 20G and 25G hypodermic needles to disrupt the eukaryotic cells and release the bacteria. The suspension was subsequently passed through a 0.45 µm filter to remove any remaining intact Vero cells and 1 ml of the host cell-free rickettsiae was inoculated separately into 75 cm2 cell culture flasks containing semi-confluent monolayers of Vero E6, D.Mel.2 (Drosophila melanogaster), C6/36 (Aedes albopictus), RF/6A (Macaca mulatta) or DH82 (Canis familiaris) cells. Vero E6, RF/6A and DH82 cultures were grown at 32 °C in a 5% CO2-in-air atmosphere, the C6/36 culture was grown at 28 °C in 5% CO2-in-air atmosphere and the D.Mel.2 culture was grown at room temperature in ambient air. The cultures were examined weekly for 21 days and assessed microscopically for cytopathic effects, and by using acridine orange stain to evaluate intracellular localization of rickettsiae. After 21 days, abundant rickettsiae and cytopathic effects were evident in cultures of Vero E6, RF/6A and DH82 cells, but neither cytopathic effects nor rickettsiae were detected in C6/36 or D.mel.2 cell cultures.
Description of Rickettsia tillamookensis sp. nov.
Rickettsia tillamookensis [till.a.mook.en′sis. N.L. fem. adj. tillamookensis from Tillamook County, Oregon, USA from where a pool of western blacklegged (Ixodes pacificus) ticks were collected in 1967 and were used to obtain the primary isolate in 1976 [1]].
Obligate intracellular bacterium characterized morphologically as small rods, diplococci and diplobacilli that stain well with Giménez stain (Fig. 5). Grows aerobically in Vero E6, RF/6A and DH82 cells. Growth in Vero E6 cells is rapid at 28 and 32 °C. The whole genome is 1.43 Mb in size and has a G+C content of 32.4 mol%. ANI and dDDH analyses indicate that R. tillamookensis is distinct from all other Rickettsia species by established genomic criteria [8]. R. tillamookensis belongs to the transitional group rickettsiae and is most closely related to R. asembonensis (OrthoANI and dDDH formula 2) and R. felis strain Pedreira (dDDH formulae 1 and 3). The type strain, R. tillamookensis Tillamook 23T, is mildly pathogenic when inoculated intraperitoneally into guinea pigs and can be lethal when inoculated intravenously in mice. The DDBJ/ENA/GenBank accession for the 16S rRNA gene and genome sequences of R. tillamookensis Tillamook 23T are MW523993 and CP060138, respectively. Rickettsia tillamookensis Tillamook 23T is available from the Centers for Disease Control and Prevention Rickettsial Isolate Reference Collection (WDCM 1093), Atlanta, GA, USA (CRIRC accession number RTI001T) and the Collection de Souches de l’Unité des Rickettsies (WDCM 875), Marseille, France (CSUR accession number R5043). The version of whole genome sequence described in this paper is version 1. The type strain, Tillamook 23T (=CRIRC RTI001=R5043), was isolated in 1976 from Ixodes pacificus ticks collected in Oregon, USA.
Fig. 5.
Rickettsia tillamookensis strain Tillamook 23, grown in Vero E6 cells at 32 °C, showing intracytoplasmic localization of small diplococcal and diplobacillary bacteria. Giménez stain, original magnification ×250.
Funding information
This work was funded in part by NIH grant 1R01AI136035 as part of the joint NIH-NSF-USDA Ecology and Evolution of Infectious Diseases program. The funding source did not have involvement in study design, data analysis, or decision to publish.
Acknowledgements
The authors thank Ulrike Munderloh, University of Minnesota, for providing the technique used to purify rickettsial DNA for whole genome sequencing, and to Joy Hecht, CDC, for purifying the rickettsial DNA. Additionally, the authors would like to thank Dr. Aharon Oren of The Hebrew University of Jerusalem for advice regarding bacterial nomenclature. The findings and conclusions are those of the authors and do not necessarily reflect the official position of the Centers for Disease Control and Prevention.
Author contributions
D.G., conceptualization, methodology, formal analysis, investigation, resources, data curation, writing-original, writing-review and editing, visualization, supervision, funding acquisition. S.K., methodology, formal analysis, investigation, resources, data curation, writing-original, writing-review and editing, visualization. S.G., methodology, formal analysis, investigation, data curation, writing-original, writing-review and editing. D.B., methodology, formal analysis, writing-review and editing. L.R., methodology, formal analysis, writing-review and editing. C.P., conceptualization, methodology, formal analysis, investigation, resources, writing-original, writing-review and editing, visualization.
Conflicts of interest
The authors declare that there are no conflicts of interest.
Footnotes
Abbreviations: ANI, average nucleotide identity; BUSCO, Benchmarking Universal Single-Copy Orthologs; dDDH, digital DNA–DNA hybridization; GE, genome equivalent; RPE, Rickettsial palindromic element.
References
- 1.Hughes LE, Clifford CM, Gresbrink R, Thomas LA, Keirans JE. Isolation of a spotted fever group Rickettsia from the Pacific Coast tick, Ixodes pacificus, in Oregon. Am J Trop Med Hyg. 1976;25:513–516. doi: 10.4269/ajtmh.1976.25.513. [DOI] [PubMed] [Google Scholar]
- 2.Philip RN, Casper EA, Burgdorfer W, Gerloff RK, Hughes LE, et al. Serologic typing of rickettsiae of the spotted fever group by microimmunofluorescence. J Immunol. 1978;121:1961–1968. [PubMed] [Google Scholar]
- 3.Philip RN, Lane RS, Casper EA. Serotypes of tick-borne spotted fever group rickettsiae from western California. Am J Trop Med Hyg. 1981;30:722–727. doi: 10.4269/ajtmh.1981.30.722. [DOI] [PubMed] [Google Scholar]
- 4.Lane R, Philip R, Casper E. In: Rickettsiae and Rickettsial Diseases. Burgdorfer W, Anacker R, editors. New York, NY: Academic Press; 1981. Ecology of tick-borne agents in California. II. Further observations on rickettsiae. [Google Scholar]
- 5.Phan JN, CR L, Bender WG, Smoak III RM, Zhong J. Molecular detection and identification of Rickettsia species in Ixodes pacificus in California. Vector-Borne and Zoonotic Diseases. 2011;11:957–961. doi: 10.1089/vbz.2010.0077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheng D, Vigil K, Schanes P, Brown RN, Zhong J. Prevalence and burden of two rickettsial phylotypes (G021 and G022) in Ixodes pacificus from California by real-time quantitative PCR. Ticks and Tick-borne Diseases. 2013;4:280–287. doi: 10.1016/j.ttbdis.2012.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alowaysi M, Chen J, Stark S, Teague K, LaCourse M, et al. Isolation and characterization of a Rickettsia from the ovary of a western black-legged tick, Ixodes pacificus . Ticks and Tick-borne Diseases. 2019;10:918–923. doi: 10.1016/j.ttbdis.2019.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Diop A, El Karkouri K, Raoult D, Fournier PE. Genome sequence-based criteria for demarcation and definition of species in the genus Rickettsia . Int J Syst Evol Microbiol. 2020;70:1738–1750. doi: 10.1099/ijsem.0.003963. [DOI] [PubMed] [Google Scholar]
- 9.Furman DP, Loomis EC. The Ticks of California (Acari: Ixodida) Univ of California Press; 1984. [Google Scholar]
- 10.Kolmogorov M, Yuan J, Lin Y, Pevzner PA. Assembly of long, error-prone reads using repeat graphs. Nat Biotechnol. 2019;37:540–546. doi: 10.1038/s41587-019-0072-8. [DOI] [PubMed] [Google Scholar]
- 11.Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 12.Li H. Minimap2: pairwise alignment for nucleotide sequences. Bioinformatics. 2018;34:3094–3100. doi: 10.1093/bioinformatics/bty191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li H. A statistical framework for SNP calling, mutation discovery, association mapping and population genetical parameter estimation from sequencing data. Bioinformatics. 2011;27:2987–2993. doi: 10.1093/bioinformatics/btr509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Robinson JT, Thorvaldsdóttir H, Winckler W, Guttman M, Lander ES, et al. Integrative genomics viewer. Nat Biotechnol. 2011;29:24–26. doi: 10.1038/nbt.1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thorvaldsdóttir H, Robinson JT, Mesirov JP. Integrative Genomics Viewer (IGV): high-performance genomics data visualization and exploration. Brief Bioinform. 2013;14:178–192. doi: 10.1093/bib/bbs017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li H, Durbin R. Fast and accurate short read alignment with Burrows–Wheeler transform. Bioinformatics. 2009;25:1754–1760. doi: 10.1093/bioinformatics/btp324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, et al. The sequence alignment/map format and SAMtools. Bioinformatics. 2009;25:2078–2079. doi: 10.1093/bioinformatics/btp352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Walker BJ, Abeel T, Shea T, Priest M, Abouelliel A, et al. Pilon: An integrated tool for comprehensive microbial variant detection and genome assembly improvement. PloS one. 2014;9:e112963. doi: 10.1371/journal.pone.0112963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seemann T. Prokka: rapid prokaryotic genome annotation. Bioinformatics. 2014;30:2068–2069. doi: 10.1093/bioinformatics/btu153. [DOI] [PubMed] [Google Scholar]
- 20.Seppey M, Manni M, Zdobnov EM. BUSCO: Assessing Genome Assembly and Annotation Completeness. Gene Prediction: Springer; 2019. pp. 227–245. [DOI] [PubMed] [Google Scholar]
- 21.Ogata H, Audic S, Barbe V, Artiguenave F, Fournier PE, et al. Selfish DNA in protein-coding genes of Rickettsia . Science. 2000;290:347–350. doi: 10.1126/science.290.5490.347. [DOI] [PubMed] [Google Scholar]
- 22.Kearse M, Moir R, Wilson A, Stones-Havas S, Cheung M, et al. Geneious Basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics. 2012;28:1647–1649. doi: 10.1093/bioinformatics/bts199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mediannikov O, Aubadie-Ladrix M, Raoult D. Candidatus “Rickettsia senegalensis” in cat fleas in Senegal. New Microbes New Infect. 2014;3:24–28. doi: 10.1016/j.nmni.2014.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee I, Kim YO, Park SC, Chun J. Orthoani: An improved algorithm and software for calculating average nucleotide identity. Int J Syst Evol Microbiol. 2016;66:1100–1103. doi: 10.1099/ijsem.0.000760. [DOI] [PubMed] [Google Scholar]
- 25.Lechner M, Findeiß S, Steiner L, Marz M, Stadler PF, et al. Proteinortho: detection of (co-) orthologs in large-scale analysis. BMC Bioinformatics. 2011;12:1–9. doi: 10.1186/1471-2105-12-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guindon S, Delsuc F, Dufayard JF, Gascuel O. Estimating Maximum Likelihood Phylogenies with PhyML. Springer: Bioinformatics for DNA sequence analysis; 2009. pp. 113–137. [DOI] [PubMed] [Google Scholar]
- 27.Rambaut A. Figtree, a graphical viewer of phylogenetic trees [Internet] 2014 http://treebioedacuk/software/figtree
- 28.Gillespie JJ, Williams K, Shukla M, Snyder EE, Nordberg EK, et al. Rickettsia phylogenomics: unwinding the intricacies of obligate intracellular life. PloS one. 2008;3:e2018. doi: 10.1371/journal.pone.0002018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huebner RJ, Jellison WL, Pomerantz C. Rickettsialpox, a newly recognized rickettsial disease; isolation of a Rickettsia apparently identical with the causative agent of rickettsialpox from Allodermanyssus sanguineus, a rodent mite. Public Health Rep. 1946;61:1677–1682. [PubMed] [Google Scholar]
- 30.Ogata H, Renesto P, Audic S, Robert C, Blanc G, et al. The genome sequence of Rickettsia felis identifies the first putative conjugative plasmid in an obligate intracellular parasite. PLoS Biol. 2005;3:e248. doi: 10.1371/journal.pbio.0030248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maina AN, Luce-Fedrow A, Omulo S, Hang J, Chan T-C, et al. Isolation and characterization of a novel Rickettsia species (Rickettsia asembonensis sp. nov.) obtained from cat fleas (Ctenocephalides felis . Int J Syst Evol Microbiol. 2016;66:4512–4517. doi: 10.1099/ijsem.0.001382. [DOI] [PubMed] [Google Scholar]
- 32.Duh D, Punda-Polic V, Avsic-Zupanc T, Bouyer D, Walker DH, et al. Rickettsia hoogstraalii sp. nov., isolated from hard-and soft-bodied ticks. Int J Syst Evol Microbiol. 2010;60:977–984. doi: 10.1099/ijs.0.011049-0. [DOI] [PubMed] [Google Scholar]
- 33.Campbell RW, Domrow R. Rickettsioses in Australia: isolation of Rickettsia tsutsugamushi and R. australis from naturally infected arthropods. Trans R Soc Trop Med Hyg. 1974;68:397–402. doi: 10.1016/0035-9203(74)90156-4. [DOI] [PubMed] [Google Scholar]
- 34.Weinert LA, Werren JH, Aebi A, Stone GN, Jiggins FM. Evolution and diversity of Rickettsia bacteria. BMC Biol. 2009;7:1–15. doi: 10.1186/1741-7007-7-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Narra HP, Sahni A, Walker DH, Sahni SK. Recent research milestones in the pathogenesis of human rickettsioses and opportunities ahead. Future Microbiol. 2020;15:753–765. doi: 10.2217/fmb-2019-0266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Noriea NF, Clark TR, Hackstadt T. Targeted knockout of the Rickettsia rickettsii OmpA surface antigen does not diminish virulence in a mammalian model system. mBio. 2015;6:e00323–15. doi: 10.1128/mBio.00323-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li H, Walker DH. rOmpA is a critical protein for the adhesion of Rickettsia rickettsii to host cells. Microb Pathog. 1998;24:289–298. doi: 10.1006/mpat.1997.0197. [DOI] [PubMed] [Google Scholar]
- 38.de la Fuente J, Garcia-Garcia JC, Barbet AF, Blouin EF, Kocan KM. Adhesion of outer membrane proteins containing tandem repeats of Anaplasma and Ehrlichia species (Rickettsiales: Anaplasmataceae) to tick cells. Vet Microbiol. 2004;98:313–322. doi: 10.1016/j.vetmic.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 39.Fournier PE, Roux V, Raoult D. Phylogenetic analysis of spotted fever group rickettsiae by study of the outer surface protein rOmpA. Int J Syst Evol Microbiol. 1998;48:839–849. doi: 10.1099/00207713-48-3-839. [DOI] [PubMed] [Google Scholar]
- 40.Regnery RL, Spruill CL, Plikaytis BD. Genotypic identification of rickettsiae and estimation of intraspecies sequence divergence for portions of two rickettsial genes. J Bacteriol. 1991;173:1576–1589. doi: 10.1128/jb.173.5.1576-1589.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stenos J, Walker DH. The rickettsial outer-membrane protein A and B genes of Rickettsia australis, the most divergent rickettsia of the spotted fever group. Int J Syst Evol Microbiol. 2000;50:1775–1779. doi: 10.1099/00207713-50-5-1775. [DOI] [PubMed] [Google Scholar]
- 42.Thu MJ, Qiu Y, Yamagishi J, Kusakisako K, Ogata S, et al. Complete genome sequence of Rickettsia asiatica strain Maytaro1284, a member of spotted fever group rickettsiae isolated from an Ixodes ovatus tick in Japan. Microbiol Resour Announc. 2019;8:e00886–19. doi: 10.1128/MRA.00886-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Roux V, Fournier PE, Raoult D. Differentiation of spotted fever group rickettsiae by sequencing and analysis of restriction fragment length polymorphism of PCR-amplified DNA of the gene encoding the protein rOmpA. J Clin Microbiol. 1996;34:2058–2065. doi: 10.1128/jcm.34.9.2058-2065.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Darling AE, Mau B, Perna NT. progressiveMauve: multiple genome alignment with gene gain, loss and rearrangement. PloS one. 2010;5:e11147. doi: 10.1371/journal.pone.0011147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kato CY, Chung IH, Robinson LK, Austin AL, Dasch GA, et al. Assessment of real-time PCR assay for detection of Rickettsia spp. and Rickettsia rickettsii in banked clinical samples. J Clin Microbiol. 2013;51:314–317. doi: 10.1128/JCM.01723-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brinkmann A, Hekimoğlu O, Dinçer E, Hagedorn P, Nitsche A, et al. A cross-sectional screening by next-generation sequencing reveals Rickettsia, Coxiella, Francisella, Borrelia, Babesia, Theileria and Hemolivia species in ticks from Anatolia. Parasites & Vectors. 2019;12:1–13. doi: 10.1186/s13071-018-3277-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Parola P, Sanogo O, Lerdthusnee K, Zeaiter Z, Chauvancy G, et al. Identification of Rickettsia spp. and Bartonella spp. in fleas from the Thai‐Myanmar border. Ann N Y Acad Sci. 2003;990:173–181. doi: 10.1111/j.1749-6632.2003.tb07359.x. [DOI] [PubMed] [Google Scholar]
- 48.Phoosangwalthong P, Hii SF, Kamyingkird K, Kengradomkij C, Pinyopanuwat N, et al. Cats as potential mammalian reservoirs for Rickettsia sp. genotype RF2125 in Bangkok, Thailand. Vet Parasitol Reg Stud Reports. 2018;13:188–192. doi: 10.1016/j.vprsr.2018.07.001. [DOI] [PubMed] [Google Scholar]
- 49.Šlapeta Š, Šlapeta J. Molecular identity of cat fleas (Ctenocephalides felis) from cats in Georgia, USA carrying Bartonella clarridgeiae, Bartonella henselae and Rickettsia sp. RF2125. Vet Parasitol Reg Stud Reports. 2016;3:36–40. doi: 10.1016/j.vprsr.2016.06.005. [DOI] [PubMed] [Google Scholar]
- 50.Supriyono. Takano A, Kuwata R, Shimoda H, Hadi UK, et al. Detection and isolation of tick-borne bacteria (Anaplasma spp., Rickettsia spp., and Borrelia spp.) in Amblyomma varanense ticks on lizard (Varanus salvator . Microbiol Immunol. 2019;63:328–333. doi: 10.1111/1348-0421.12721. [DOI] [PubMed] [Google Scholar]
- 51.Vilcins I-ME, Fournier P-E, Old JM, Deane E. Evidence for the presence of Francisella and spotted fever group rickettsia DNA in the tick Amblyomma fimbriatum (Acari: Ixodidae), Northern Territory, Australia. J Med Entomol. 2009;46:926–933. doi: 10.1603/033.046.0427. [DOI] [PubMed] [Google Scholar]
- 52.Krueger L, Bai Y, Bennett S, Fogarty C, Sun S, et al. Identification of zoonotic and vector-borne infectious agents associated with opossums (Didelphis Virginiana) in residential neighborhoods of Orange County, California. vertebrate_pest_conference. 2016;27 doi: 10.5070/V427110386. [DOI] [Google Scholar]
- 53.Maina AN, Klein TA, Kim H-C, Chong S-T, Yang Y, et al. Molecular characterization of novel mosquito-borne Rickettsia spp. from mosquitoes collected at the demilitarized zone of the Republic of Korea. PloS one. 2017;12:e0188327. doi: 10.1371/journal.pone.0188327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Toju H, Tanabe AS, Notsu Y, Sota T, Fukatsu T. Diversification of endosymbiosis: replacements, co-speciation and promiscuity of bacteriocyte symbionts in weevils. ISME J. 2013;7:1378–1390. doi: 10.1038/ismej.2013.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Thu MJ, Qiu Y, Matsuno K, Kajihara M, Mori-Kajihara A, et al. Diversity of spotted fever group rickettsiae and their association with host ticks in Japan. Sci Rep. 2019;9:1–10. doi: 10.1038/s41598-018-37836-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rydkina E, Roux V, Fetisova N, Rudakov N, Gafarova M, et al. New rickettsiae in ticks collected in territories of the former Soviet Union. Emerging Infect Dis. 1999;5:811. doi: 10.3201/eid0506.990612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tsui P-Y, Tsai K-H, Weng M-H, Hung Y-W, Liu Y-T, et al. Molecular detection and characterization of spotted fever group Rickettsiae in Taiwan. Am J Trop Med Hyg. 2007;77:883–890. [PubMed] [Google Scholar]
- 58.Tahir D, Socolovschi C, Marié J-L, Ganay G, Berenger J-M, et al. New Rickettsia species in soft ticks Ornithodoros hasei collected from bats in French Guiana. Ticks and Tick-borne Diseases. 2016;7:1089–1096. doi: 10.1016/j.ttbdis.2016.09.004. [DOI] [PubMed] [Google Scholar]
- 59.Fournier PE, Xeridat B, Raoult D. Isolation of a rickettsia related to Astrakhan fever rickettsia from a patient in Chad. Ann N Y Acad Sci. 2003;990:152–157. doi: 10.1111/j.1749-6632.2003.tb07356.x. [DOI] [PubMed] [Google Scholar]
- 60.Mura A, Masala G, Tola S, Satta G, Fois F, et al. First direct detection of rickettsial pathogens and a new rickettsia,‘Candidatus Rickettsia barbariae’, in ticks from Sardinia, Italy. Clin Microbiol Infect. 2008;14:1028–1033. doi: 10.1111/j.1469-0691.2008.02082.x. [DOI] [PubMed] [Google Scholar]
- 61.Sekeyova Z, Fournier P, Řeháček J, Raoult D. Characterization of a new spotted fever group rickettsia detected in Ixodes ricinus (Acari: Ixodidae) collected in Slovakia. J Med Entomol. 2000;37:707–713. doi: 10.1093/jmedent/37.5.707. [DOI] [PubMed] [Google Scholar]
- 62.Carver T, Thomson N, Bleasby A, Berriman M, Parkhill J. DNAPlotter: circular and linear interactive genome visualization. Bioinformatics. 2009;25:119–120. doi: 10.1093/bioinformatics/btn578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Katoh K, Standley DM. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol Biol Evol. 2013;30:772–780. doi: 10.1093/molbev/mst010. [DOI] [PMC free article] [PubMed] [Google Scholar]