Abstract
Rationale: Sarcopenia is associated with disability and death. The optimal definition and clinical relevance of sarcopenia in lung transplantation remain unknown.
Objectives: To assess the construct and predictive validity of sarcopenia definitions in lung transplant candidates.
Methods: In a multicenter prospective cohort of 424 lung transplant candidates, we evaluated limited (muscle mass only) and expanded (muscle mass and quality) sarcopenia definitions from the European Working Group on Sarcopenia in Older People 2 (EWGSOP2), the Foundation for the National Institutes of Health (FNIH), and a cohort-specific distribution-based lowest quartile definition. We assessed construct validity using associations with conceptually related factors. We evaluated the relationship between sarcopenia and frailty using generalized additive models. We also evaluated associations between sarcopenia definitions and key pretransplant outcomes, including disability (quantified by the Lung Transplant Valued Life Activities scale [range, 0–3; higher scores = worse disability; minimally important difference, 0.3]) and waitlist delisting/death, by multivariate linear and Cox regression, respectively.
Results: Sarcopenia prevalence ranged from 6% to13% by definition used. The limited EWGSOP2 definition demonstrated the highest construct validity, followed by the expanded EWGSOP2 definition and both limited and expanded FNIH and lowest quartile definitions. Sarcopenia exhibited a linear association with the risk of frailty. The EWGSOP2 and expanded lowest quartile definitions were associated with disability, ranging from 0.20 to 0.25 higher Lung Transplant Valued Life Activities scores. Sarcopenia was associated with increased risk of waitlist delisting or death by the limited and expanded lowest quartile definitions (hazard ratio [HR], 3.8; 95% confidence interval [CI], 1.4–9.9 and HR, 3.5; 95% CI, 1.1–11.0, respectively) and the EWGSOP2 limited definition (HR, 2.8; 95% CI, 0.9–8.6) but not with the three other candidate definitions.
Conclusions: The prevalence and validity of sarcopenia vary by definition; the EWGSOP2 limited definition exhibited the broadest validity in lung transplant candidates. The linear relationship between low muscle mass and frailty highlights sarcopenia’s contribution to frailty and also questions the clinical utility of a sarcopenia cut-point in advanced lung disease. The associations between sarcopenia and important pretransplant outcomes support further investigation into using body composition for candidate risk stratification.
Keywords: sarcopenia, frailty, lung transplantation
The number of patients aged 65 years or older with end-stage lung disease undergoing lung transplantation is rising dramatically (1, 2). Worldwide, the proportion of patients within this age group at the time of lung transplantation increased from 3.3% in 2005 to almost 17% in 2017 (1). Growth has been even more dramatic in the United States, where this population represented only 4% of new lung transplant recipients in 2002 compared with 32% in 2017 (2).
Concurrent to this shift toward transplant in older individuals, postoperative morbidity and resource use have also increased, with some studies also showing a rise in post-transplant mortality (3, 4). Older age is an independent risk factor for discharge to an inpatient rehabilitation facility after lung transplantation (3, 5), unplanned 30-day readmission after transplant surgery (2), and less health-related quality of life (HRQL) benefit derived from transplant compared with younger recipients (6). These findings are particularly relevant given the expected median survival of only 3 years for lung transplant recipients aged 65 years and older (7).
In light of these trends, lung transplant centers worldwide are struggling to identify which older patients will have improved functional status, HRQL, or prolonged survival after transplantation. Measures of physiologic age, rather than chronologic age, may help to identify patients at increased risk of morbidity and mortality in lung transplantation. Efforts to test concepts from geriatric medicine in this population have shown that frailty—a state of low physiologic reserve—is associated with increased risks of disability, readmission after lung transplant surgery, and mortality before and after lung transplantation (8–10). Although these findings have identified a novel risk factor for poor transplant outcomes, pathobiological causes underlying these associations are unknown. This knowledge deficit hinders our understanding of frailty in advanced lung disease and impedes the development of targeted interventions to improve outcomes in lung transplantation.
Sarcopenia, defined as low skeletal muscle mass combined with poor muscle quality, may represent one factor underlying these associations. Believed to be a key physical component of frailty (11), sarcopenia is associated with physical disability, falls, and death in older adults (12, 13). It is associated with morbidity and mortality before and after other solid organ transplants (14–16). Decreased skeletal muscle mass and poor muscle quality have been observed in lung transplant candidates (17, 18) and are associated with mortality in patients with chronic obstructive pulmonary disease (COPD) (19, 20). Weak grip strength is also associated with longer length of stay after lung transplant surgery (21). Thus, sarcopenia may serve as a driver of poor outcomes in lung transplantation.
Although sarcopenia is conceptually straightforward, operationalizing its measurement is not. Like frailty, numerous operational definitions of sarcopenia have been proposed. None, however, have been validated in advanced lung disease. Identifying a valid operational definition of sarcopenia in lung transplant candidates could provide consistency in reporting prevalence across future studies, enable testing of the association between sarcopenia and transplant outcomes, and allow for evaluation of interventions to improve sarcopenia. In this prospective cohort study of patients with advanced lung disease awaiting lung transplantation, we aimed to establish the prevalence of sarcopenia in lung transplant candidates, test the construct validity of existing definitions of sarcopenia, examine sarcopenia’s relationship with frailty, and evaluate associations between sarcopenia and clinical outcomes such as pretransplant disability and waitlist delisting or death.
Methods
Study Design, Setting, and Participants
This study analyzed participants in the Lung Transplant Body Composition Study, a multicenter prospective cohort study examining the impact of preoperative body composition on lung transplant outcomes. Adult lung transplant candidates aged 18 years or older are being recruited at the University of California, San Francisco; the University of Pennsylvania; and Columbia University Medical Center. The study period began in June 2017 and is ongoing. Institutional review boards at each center approved this study. Study participants provided written informed consent.
Sarcopenia Definitions and Measurement
Originally described as an age-related decline in skeletal muscle mass, recent operational definitions of sarcopenia have expanded to require the simultaneous presence of decreased skeletal muscle mass and poor muscle quality (either weak muscle strength or functional limitation) (22). These broadened definitions reflect increasing recognition of contributions from both mass and quality in determining maximal force generated by skeletal muscle. Several operational definitions have been proposed by consensus statements. Little evidence exists, however, regarding the validity of applying these in advanced lung disease or whether one definition is superior to the rest. As a result, we elected to test the most promising candidate definitions.
A priori, three pairs of sarcopenia definitions (six total) were evaluated. Each pair included a “limited” definition of sarcopenia that consisted of low muscle mass alone as well as an “expanded” definition that included both low muscle mass and poor muscle quality. The pairs included definitions from the European Working Group on Sarcopenia in Older People 2 (EWGSOP2), the Foundation for the National Institutes of Health (FNIH) Sarcopenia Project, and a distribution-based definition derived from our study cohort (22–24). The EWGSOP2 definition proposed cut-points derived from normative references of healthy adults age 18 to 90 years (23). The cut-point for appendicular skeletal muscle mass was set at two standard deviations (SDs) below the mean reference value (25), the cut-point for grip strength was set at 2.5 SDs below the mean reference value (26), and the gait speed cut-point of ≤0.8 m/s was derived from studies demonstrating an association between a gait speed of ≤0.8 m/s and increased mortality in adults age ≥65 (27). The FNIH Sarcopenia Project pooled nine existing data sources to identify diagnostic thresholds for sarcopenia (24). Because the EWGSOP2 and FNIH definitions were derived in community-dwelling older populations, the proposed cutoffs may not be generalizable to patients with advanced lung disease. Thus, we elected to test a third distribution-based definition derived from our study cohort, using the lowest quartile of each sarcopenia component. The six sarcopenia definitions evaluated are described in Table 1.
Table 1.
Description of sarcopenia definitions
| Muscle Mass (Men) | Muscle Mass (Women) | Grip Strength (Men) | Grip Strength (Women) | Gait Speed | |||
|---|---|---|---|---|---|---|---|
| EWGSOP2 | |||||||
| Limited* | ASM/ht2 < 7.0 kg/m2 | ASM/ht2 < 5.5 kg/m2 | — | — | — | — | — |
| Expanded† | and | <27 kg | <16 kg | or | ≤0.8 m/s | ||
| FNIH | |||||||
| Limited | ASMBMI < 0.8 | ASMBMI < 0.512 | — | — | — | — | — |
| Expanded | and | <26 kg | <16 kg | or | ≤0.8 m/s | ||
| Lowest Quartile | |||||||
| Limited | ASM/ht2 < 7.4 kg/m2 | ASM/ht2 < 5.8 kg/m2 | — | — | — | — | — |
| Expanded | and | <27 kg | <14 kg | or | ≤0.8 m/s |
Definition of abbreviations: ASM = appendicular skeletal muscle mass; BMI = body mass index; EWGSOP2 = European Working Group on Sarcopenia in Older People 2; FNIH = Foundation for the National Institutes of Health; Lowest Quartile = distribution-based lowest quartile of sarcopenic study participants.
Limited definitions require only appendicular skeletal muscle mass below specified cut-point for diagnosis of sarcopenia.
Expanded definitions require both appendicular skeletal muscle mass and either grip strength or gait speed below specified cut-points for diagnosis of sarcopenia.
Skeletal muscle mass was quantified by bioelectrical impedance analysis (InBody S10 [InBody]). InBody S10 quantifies the skeletal muscle mass of the bilateral upper and lower extremities, which are summed to generate a measure of appendicular skeletal muscle mass. InBody S10 measurements demonstrate test–retest reliability (intraclass correlation coefficient, 0.96) (28) and strong correlation with whole-body dual energy X-ray absorptiometry (r = 0.94) (29). For lowest quartile and EWGSOP2 definitions, muscle mass is expressed as appendicular skeletal muscle mass (ASM) divided by height squared (ASM/ht2). For the FNIH definition, muscle mass is expressed as ASM divided by body mass index (BMI) (ASMBMI). Measures of muscle quality included grip strength and gait speed. The average of three isometric grip strength attempts was used, quantified using a hydraulic handheld dynamometer (Jamar [Patterson Medical/Performance Health]) with the participant seated, shoulder adducted, and neutrally rotated and elbow flexed at 90°. The average of two gait speed attempts was used, which were measured while the participant walked 4.57 m on a flat surface. Frailty (by Short Physical Performance Battery [SPPB] and Fried Frailty Phenotype [FFP]), grip strength, gait speed, and measures of body composition were measured by research coordinators at each site. Research staff adhered to defined study protocols detailed in a manual of operating procedures. New coordinators underwent rigorous training on the performance of these measures under observation by an experienced coordinator until deemed proficient. Regular quality control checks were performed to minimize the potential for measurement differences attributable to study staff across sites.
Measures of Construct Validity
Because a gold-standard definition of sarcopenia in advanced lung disease is lacking, we undertook a multistep process to identify the most valid definition. We first assessed construct validity, the degree to which a definition effectively measures its intended entity. For construct validity, we evaluated the convergent and discriminant validity of the six sarcopenia definitions described above. Convergent validity assesses correlations with conceptually related variables. Discriminant validity examines correlations (or anticipated lack thereof) with conceptually unrelated variables. The following components were examined: 6-minute walk distance (6MWD), physical frailty (SPPB), and Short Form 12 (SF12) Physical and Mental Health Component Summary scores (SF12-PCS and SF12-MCS, respectively), measures that focus on HRQL (SF12 range, 0–100; higher scores indicate better HRQL; minimally important difference [MID], 5) (30, 31). Because sarcopenia is believed to represent a physical component of frailty, a frailty domain unrelated to physical fitness (the Trails B Test, an assessment of cognitive limitation) was tested as a variable for discriminant validity (32). We hypothesized that skeletal muscle mass would positively correlate with 6MWD, SPPB frailty score, and SF12-PCS and would not correlate with Trails B Test score or SF12-MCS.
Participants completed study visits upon study enrollment and subsequently at 3-month intervals. These visits included measures of body composition, grip strength, gait speed, 6MWD, frailty, cognition, and survey measures of disability and HRQL. Forty-seven patients were on either mechanical ventilation or extracorporeal membrane oxygenation before transplant. Frailty was measured in 40 of these patients during their most recent visit before transplant; frailty assessment was not able to be performed for the seven patients who were hospitalized at the time of their last scheduled clinic visit before transplant. Because the instrument used to measure body composition is portable and the measurements can be performed even when a patient is hospitalized, body composition assessment was performed on all 47 patients. For patients who underwent transplantation, the most proximal values before transplant surgery were used. For patients who did not undergo transplantation, the most proximal values at the time of dataset analysis were used.
Frailty
We evaluated the association between sarcopenia and two operational physical frailty measures (SPPB and FFP). SPPB was used in binary (SPPB ≤ 7 is frail) and continuous (range, 0–12; lower scores reflect increased frailty) forms (33). FFP was used in binary (FFP ≥ 3 is frail) and continuous (range, 0–5; higher scores reflect increased frailty) forms (34). The FFP physical activity domain was assessed using the Duke Activity Status Index rather than Minnesota Leisure Time Activity scale, a modification shown to improve FFP construct and predictive validity in advanced lung disease (35).
Outcome Variables
Physical disability was quantified using the Lung Transplant Valued Life Activities (LT-VLA) scale, a 15-item measure of disability validated for use in advanced lung disease and lung transplantation (range, 0–3; higher scores reflect greater disability; a change of 0.3 is considered clinically meaningful). The LT-VLA scale measures disability across a broad spectrum of daily activities, including obligatory (required for survival), committed (focused on social roles such as working or caring for others), and discretionary (activities of leisure) categories (36).
Frailty and sarcopenia measures were not provided to the clinical team and thus were not factored into clinical decision-making regarding removal from the lung transplant waitlist. Dates of waitlist death or delisting because of being deemed too ill for transplantation were abstracted from the medical record at the time the dataset was analyzed.
Analytical Approach
To evaluate construct validity, we used Pearson correlation coefficients to measure the magnitude and direction of association between ASM/ht2 or ASMBMI and our variables of interest. Next, we assessed “known-groups” construct validity by Student’s t test for SPPB, SF12-PCS, and SF12-MCS and Hodge-Lehman tests for 6WMD and Trails B Test. Known-groups testing allows for comparison of the relative construct validity of each definition. Using this method, the proportion of hypotheses met by each definition can be compared. Construct validity is considered to be strong if at least 75% of hypotheses are met (37). We hypothesized that sarcopenia would be associated with shorter 6MWD and worse SF12-PCS and SPPB frailty score and would not be associated with Trails B Test score or SF12-MCS. We tested agreement between the candidate sarcopenia definitions using Cohen’s κ.
Next, we evaluated the association between appendicular skeletal muscle mass index (ASM/ht2) and frailty by SPPB and FFP using generalized additive models adjusted for age, sex, and diagnosis. Although prior literature has demonstrated that sarcopenia is associated with frailty, most studies have tested this relationship using dichotomous definitions of sarcopenia, imposing a potentially artificial threshold on sarcopenia as “present” or “absent.” Whether a clear threshold or a more graded relationship exists between muscle mass and risk of frailty has not previously been established in advanced lung disease. Generalized additive models allowed us to model the relationship between muscle mass and frailty and to model whether that relationship was linear or nonlinear.
Sarcopenia is a well-established risk factor for physical disability in other populations. As a limited proxy for sarcopenia, low BMI has been associated with increased risk of waitlist death but is prone to misclassification. Identifying associations between sarcopenia and clinical outcomes would begin to elucidate sarcopenia’s clinical relevance in advanced lung disease and transplantation. Thus, to assess the relationship between sarcopenia and waitlist disability and death, we tested associations between our candidate sarcopenia definitions and these outcomes by linear regression and Cox proportional hazard modeling, respectively. All models were adjusted for age, sex, race, and diagnosis. There were no significant interactions between candidate sarcopenia definitions and covariates included in the models.
For our primary models of predictive validity, we aimed to estimate the total effect of regressing sarcopenia on disability or waitlist delisting/death. In these models, we adjusted for age, sex, race, and diagnosis but did not adjust for other markers of disease severity and transplant urgency that may lie on the causal pathway between sarcopenia and disability and waitlist delisting/death. For example, some of these markers, such as weight loss (low BMI), low albumin, exercise intolerance (6MWD), or the Lung Allocation Score itself likely reflect both sarcopenia (causal pathway) and advanced lung disease (Figure E2 in the online supplement).
We recognized that some may be interested in the relationship between sarcopenia and disability and waitlist delisting/death independent of a measure of disease severity. Thus, in secondary models, we also adjusted for the Lung Allocation Score, calculated at the time of body composition measurement (Table E4).
For death, we employed a composite outcome of waitlist delisting because of being too ill or death, as we have done previously (9). We compared cumulative probabilities of death/delisting between sarcopenic and nonsarcopenic groups using Kaplan-Meier methods. For Cox models, nonproportionality was tested using martingale residuals.
Not all subjects completed their pretransplant visits. Overall, the rate of missing data was small (21/424 [=5%] for the frailty assessment and 60/424 [=14%] for the survey on pretransplant disability and HRQL). We imputed missing values based on reasons for missingness. Missing data were considered missing at random (MAR) if a subject did not complete the frailty assessment or the survey for reasons other than health (for example, he/she missed the clinic appointment because of inclement weather, left the clinic before completing the frailty assessment or survey because of traffic, refused assessment for non–health-related reasons, or was unable to answer the survey because of limited English proficiency). A frailty assessment or survey was categorized as missing not at random if the subject was hospitalized or too ill to complete it. For data MAR, we performed 20-fold multiple imputation. Because the distribution of missing data was not balanced across centers, an indicator for centers was included in the multiple imputation model. The assumption of missing completely at random (MCAR) is stronger than MAR and is relatively rare. Because multiple imputation can be applied on data both MAR and MCAR, we did not test whether our data was MAR or MCAR specifically. For data missing not at random, we imputed with the median of the worst quartile of observed values, as we have done previously (38). Patients lacking both grip strength and gait speed (n = 26) were included only in the analyses involving the limited definitions of sarcopenia. Analyses were performed using R (version 3.6.1, R Foundation) and SAS (version 9.4, SAS Institute).
Results
Of 424 adult lung transplant candidates, 197 (46%) were female, with a median age of 57 years (interquartile range [IQR], 52–66); 326 (77%) were White, 35 (8%) were Asian, 29 (7%) were Black/African American, 34 (8%) identified as another race, and 66 (16%) were Hispanic. Most participants had interstitial lung disease (n = 283; 67%), followed by COPD (n = 85; 20%) (Table 2). The median follow-up time was 10.7 months (IQR, 5.2–15.3).
Table 2.
Demographics and baseline characteristics by sarcopenia status
| Variable | Limited Definitions |
|||||
|---|---|---|---|---|---|---|
| EWGSOP2 |
FNIH |
Lowest Quartile |
||||
| Nonsarcopenic | Sarcopenic | Nonsarcopenic | Sarcopenic | Nonsarcopenic | Sarcopenic | |
| Number of subjects | 366 | 58 | 372 | 52 | 320 | 104 |
| Age, yr | 57.9 (11.9) | 53.7 (14.2) | 57.1 (12.5) | 59.1 (10.2) | 58.2 (11.9) | 54.7 (12.9) |
| Sex, F | 170 (46.4) | 27 (46.6) | 181 (48.7) | 16 (30.8) | 150 (46.9) | 47 (45.2) |
| Race | ||||||
| White | 292 (79.8) | 34 (58.6) | 293 (78.8) | 33 (63.5) | 261 (81.6) | 65 (62.5) |
| Black | 27 (7.4) | 2 (3.4) | 29 (7.8) | 0 (0) | 24 (7.5) | 5 (4.8) |
| Asian | 21 (5.7) | 14 (24.1) | 26 (7.0) | 9 (17.3) | 18 (5.6) | 17 (16.3) |
| Other | 26 (7.1) | 8 (13.8) | 24 (6.5) | 10 (19.2) | 17 (5.3) | 17 (16.3) |
| Hispanic | 51 (13.9) | 15 (25.9) | 46 (12.4) | 20 (38.5) | 37 (11.6) | 29 (27.9) |
| Disease diagnosis* | ||||||
| COPD | 73 (19.9) | 12 (20.7) | 80 (21.5) | 5 (9.6) | 64 (20.0) | 21 (20.2) |
| PAH | 22 (6.0) | 2 (3.4) | 21 (5.6) | 3 (5.8) | 18 (5.6) | 6 (5.8) |
| CF | 24 (6.6) | 8 (13.8) | 32 (8.6) | 0 (0) | 19 (5.9) | 13 (12.5) |
| IPF | 247 (67.5) | 36 (62.1) | 239 (64.2) | 44 (84.6) | 219 (68.4) | 64 (61.5) |
| BMI, kg/m2 | 26.8 (4.2) | 21.4 (3.5) | 25.7 (4.5) | 28.1 (3.6) | 27.2 (4.1) | 22.4 (3.6) |
| SF12-PCS | 27.2 (9.3) | 26.0 (9.3) | 27.1 (9.5) | 26.4 (8.4) | 27.1 (9.3) | 27.0 (9.4) |
| 6MWD, m | 280 [175–366] | 203 [109–298] | 279 [175–366] | 200 [110–298] | 278 [179–366] | 246 [127–324] |
| LAS | 42.8 (13.9) | 48.5 (18.8) | 42.9 (14.5) | 48.3 (16.1) | 42.4 (13.1) | 47.1 (18.5) |
| SPPB score | 9.9 (2.7) | 8.3 (3.9) | 9.8 (2.9) | 9.3 (3.1) | 10.0 (2.6) | 8.9 (3.6) |
| Trails B Test score | 92 [69–119] | 104 [76–139] | 92 [69–119] | 109 [76–141] | 88 [68–115] | 106 [77–142] |
| SF12-MCS | 48.1 (10.7) | 48.8 (13.4) | 47.9 (11.0) | 50.2 (11.5) | 48.1 (10.7) | 48.2 (12.1) |
| Variable |
Expanded Definitions |
|||||
|
EWGSOP2 |
FNIH |
Lowest Quartile |
||||
| Nonsarcopenic | Sarcopenic | Nonsarcopenic | Sarcopenic | Nonsarcopenic | Sarcopenic | |
| Number of subjects | 367 | 36 | 378 | 25 | 353 | 50 |
| Age, yr | 57.5 (12.1) | 56.6 (12.2) | 57.2 (12.4) | 61.0 (6.8) | 57.4 (12.1) | 57.5 (12.6) |
| Sex, F | 169 (46.0) | 19 (52.8) | 177 (46.8) | 11 (44.0) | 166 (47.0) | 22 (44.0) |
| Race | ||||||
| White | 292 (79.6) | 19 (52.8) | 297 (78.6) | 14 (56.0) | 282 (79.9) | 29 (58.0) |
| Black | 24 (6.5) | 2 (5.6) | 26 (6.9) | 0 (0) | 25 (7.1) | 1 (2.0) |
| Asian | 25 (6.8) | 7 (19.4) | 27 (7.1) | 5 (20.8) | 23 (6.5) | 9 (18.0) |
| Other | 26 (7.1) | 8 (22.2) | 28 (7.4) | 6 (24.0) | 23 (6.5) | 11 (22.0) |
| Hispanic | 53 (14.4) | 11 (30.6) | 54 (14.4) | 8 (33.3) | 48 (13.6) | 16 (32.0) |
| Disease diagnosis* | ||||||
| COPD | 76 (20.7) | 7 (19.4) | 80 (21.2) | 3 (12.0) | 75 (21.2) | 8 (16.0) |
| PAH | 23 (6.3) | 1 (2.8) | 23 (6.1) | 1 (4.0) | 22 (6.2) | 2 (4.0) |
| CF | 27 (7.4) | 3 (8.3) | 30 (7.9) | 0 (0) | 27 (7.6) | 3 (6.0) |
| IPF | 241 (65.7) | 25 (69.4) | 245 (64.8) | 21 (84.0) | 229 (64.9) | 37 (74.0) |
| BMI, kg/m2 | 26.5 (4.4) | 21.6 (3.6) | 25.9 (4.5) | 27.5 (4.2) | 26.5 (4.4) | 22.6 (3.8) |
| SF12-PCS | 27.3 (9.4) | 25.8 (9.0) | 27.2 (9.5) | 26.8 (8.2) | 27.4 (9.5) | 26.0 (8.3) |
| 6MWD, m | 283 [183–367] | 185 [100–276] | 282 [182–366] | 175 [93–226] | 282 [183–366] | 198 [119–312] |
| LAS | 41.7 (12.3) | 48.2 (16.7) | 41.7 (12.4) | 50.9 (16.4) | 41.5 (11.9) | 48.2 (17.5) |
| SPPB score | 10.0 (2.7) | 7.8 (3.8) | 9.9 (2.8) | 8.4 (3.4) | 10.1 (2.7) | 8.1 (3.6) |
| Trails B Test score | 92 [69–119] | 105 [85–144] | 92 [69–118] | 117 [95–157] | 90 [69–117] | 110 [81–150] |
| SF12-MCS | 48.2 (10.8) | 47.6 (12.7) | 48.2 (11.0) | 47.8 (11.8) | 48.2 (11.1) | 48.4 (10.7) |
Definition of abbreviations: 6MWD = 6-minute walk distance; BMI = body mass index; CF = cystic fibrosis; COPD = chronic obstructive pulmonary disease; EWGSOP2 = European Working Group on Sarcopenia in Older People 2; FNIH = Foundation for the National Institutes of Health; IPF = idiopathic pulmonary fibrosis; IQR = interquartile range; LAS = Lung Allocation Score (measured at time of body composition assessment); Lowest Quartile = distribution-based lowest quartile of sarcopenic study participants; PAH = pulmonary arterial hypertension; SF12-MCS = Short Form 12 Mental Health Composite Score; SF12-PCS = Short Form 12 Physical Health Composite Score; SPPB = Short Physical Performance Battery.
Data are presented as n (%), mean ± SD, or median [IQR].
Disease diagnoses used for calculation of LAS.
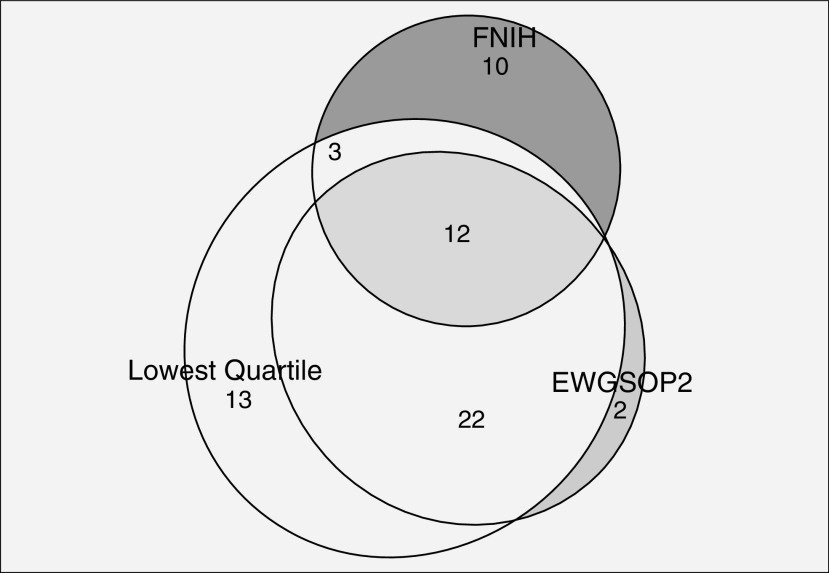
Depending on the definition used, the prevalence of sarcopenia varied. For example, 12% of participants had sarcopenia by the limited FNIH Sarcopenia Project definition, and 6% had sarcopenia by the expanded version. Thirteen percent of participants had sarcopenia by the limited EWGSOP2 definition, and 8% of participants had sarcopenia by the expanded version. By definition, the prevalence of sarcopenia by limited lowest quartile cut-point was 25% (Table 2). Notably, only 12 participants (3%) met criteria for sarcopenia by all three expanded definitions (Figure 1). Although the proportion of patients deemed to have sarcopenia by the limited FNIH and EWGSOP2 definitions was similar, agreement was minimal (Cohen’s κ = 0.32; P < 0.0001). Agreement between the expanded FNIH and EWGSOP2 definitions was also minimal (κ = 0.35; P < 0.0001). Those labeled sarcopenic by an EWGSOP2 definition appeared younger, with a lower BMI and a higher likelihood of being female compared with those labeled sarcopenic by an FNIH definition (Table 2). However, agreement between the limited versions of the EWGSOP2 and lowest quartile definitions was stronger (Cohen’s κ = 0.66; P < 0.0001), as was agreement between the expanded versions of these definitions (Cohen’s κ = 0.77; P < 0.0001) (Table E3).
Figure 1.
Circles display total number of patients deemed sarcopenic by each expanded definition: EWGSOP2 (35), FNIH (24), Lowest Quartile (52). EWGSOP2 = European Working Group on Sarcopenia in Older People 2; FNIH = Foundation for the National Institutes of Health; Lowest Quartile = distribution-based lowest quartile of sarcopenic study participants.
Construct Validity
The 6MWD and SPPB correlated with both ASM/ht2 and ASMBMI. SF12-PCS correlated with ASMBMI but not with ASM/ht2. No measure of muscle mass correlated with Trails B Test score or SF12-MCS (Table 3). Although the magnitudes of correlations were small, muscle mass correlated with a majority of variables believed to be conceptually related to sarcopenia and did not correlate with variables believed to be conceptually unrelated to sarcopenia.
Table 3.
Correlations between muscle mass and conceptually related functional and health-related quality of life variables
| Variable | ASM/ht2 | ASMBMI |
|---|---|---|
| 6MWD | 0.16* | 0.21* |
| Frailty (SPPB) | 0.15* | 0.14* |
| SF12-PCS | 0.11 | 0.13* |
| Trails B Test score | −0.05 | −0.09 |
| SF12-MCS | −0.02 | 0.001 |
Definition of abbreviations: 6MWD = 6-minute walk distance; ASM = appendicular skeletal muscle mass; BMI = body mass index; EWGSOP2 = European Working Group on Sarcopenia in Older People 2; FNIH = Foundation for the National Institutes of Health; SF12-MCS = Short Form 12 Mental Health Composite Score; SF12-PCS = Short Form 12 Physical Health Composite Score; SPPB = Short Physical Performance Battery.
ASM/ht2 was used in lowest quartile and EWGSOP2 definitions. ASMBMI was used in FNIH definitions.
Statistically significant at P < 0.05. Testing by Pearson correlation coefficient.
Known-group testing revealed differences in construct validity across definitions. Within the limited definitions, EWGSOP2 exhibited the greatest validity, with 80% of hypotheses maintained, followed equally by the expanded EWGSOP2 definition and both forms of the FNIH and lowest quartile definitions (60% maintained) (Table 4).
Table 4.
Tests of convergent and discriminant validity of sarcopenia definitions using conceptually related physiologic and functional variables
| Convergent Validity | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Variable | Limited Definitions |
|||||||||
| EWGSOP2 |
FNIH |
Lowest Quartile |
||||||||
| Nonsarcopenic | Sarcopenic | P Value | Nonsarcopenic | Sarcopenic | P Value | Nonsarcopenic | Sarcopenic | P Value | ||
| 6MWD, m | 280 [175–366] | 203 [109–298] | <0.01* | 279 [175–366] | 200 [110–298] | <0.01* | 278 [179–366] | 246 [127–324] | 0.01* | |
| SPPB | 9.9 ± 2.7 | 8.3 ± 3.9 | <0.01* | 9.8 ± 2.9 | 9.3 ± 3.1 | 0.27 | 10.0 ± 2.6 | 8.9 ± 3.6 | <0.01* | |
| SF12-PCS | 27.2 ± 9.3 | 26.0 ± 9.3 | 0.40 | 27.1 ± 9.5 | 26.4 ± 8.4 | 0.66 | 27.1 ± 9.3 | 27.0 ± 9.4 | 0.94 | |
|
Expanded Definitions |
||||||||||
|
EWGSOP2 |
FNIH |
Lowest quartile |
||||||||
| Variable | Nonsarcopenic | Sarcopenic | P Value | Nonsarcopenic | Sarcopenic | P Value | Nonsarcopenic | Sarcopenic | P Value | |
| 6MWD, m | 283 [183–367] | 185 [100–276] | <0.01* | 282 [182–366] | 175 [93–226] | <0.01* | 282 [183–366] | 198 [119–312] | <0.01* | |
| SPPB | 10.0 ± 2.7 | 7.8 ± 3.8 | <0.01* | 9.9 ± 2.8 | 8.4 ± 3.4 | <0.01* | 10.1 ± 2.7 | 8.1 ± 3.6 | <0.01* | |
| SF12-PCS | 27.3 ± 9.4 | 25.8 ± 9.0 | 0.42 | 27.2 ± 9.5 | 26.8 ± 8.2 | 0.83 | 27.4 ± 9.5 | 26.0 ± 8.3 | 0.41 | |
|
Discriminant Validity |
||||||||||
|
Limited Definitions |
||||||||||
|
EWGSOP2 |
FNIH |
Lowest Quartile |
||||||||
| Variable | Nonsarcopenic | Sarcopenic | P Value | Nonsarcopenic | Sarcopenic | P Value | Nonsarcopenic | Sarcopenic | P Value | |
| Trails B Test, s | 92 [69–119] | 104 [76–139] | 0.07 | 92 [69–119] | 109 [76–141] | 0.053 | 88 [68–115] | 106 [77–142] | <0.01* | |
| SF12-MCS | 48.1 ± 10.7 | 48.8 ± 13.4 | 0.67 | 47.9 ± 11.0 | 50.2 ± 11.5 | 0.24 | 48.1 ± 10.7 | 48.2 ± 12.1 | 0.96 | |
|
Expanded Definitions |
||||||||||
|
EWGSOP2 |
FNIH |
Lowest Quartile |
||||||||
| Variable | Nonsarcopenic | Sarcopenic | P Value | Nonsarcopenic | Sarcopenic | P Value | Nonsarcopenic | Sarcopenic | P Value | |
| Trails B Test, s | 92 [69–119] | 105 [85–144] | 0.03* | 92 [69–118] | 117 [95–157] | 0.01* | 90 [69–117] | 110 [81–150] | 0.01* | |
| SF12-MCS | 48.2 ± 10.8 | 47.6 ± 12.7 | 0.75 | 48.2 ± 11.0 | 47.8 ± 11.8 | 0.89 | 48.2 ± 11.1 | 48.4 ± 10.7 | 0.89 | |
Definition of abbreviations: 6MWD = 6-minute walk distance; EWGSOP2 = European Working Group on Sarcopenia in Older People 2; FNIH = Foundation for the National Institutes of Health; Lowest Quartile = distribution-based lowest quartile of sarcopenic study participants; SF12-MCS = Short Form 12 Mental Health Composite Score; SF12-PCS = Short Form 12 Physical Health Composite Score; SPPB = Short Physical Performance Battery.
Statistically significant at P < 0.05. “Known-groups” validity testing by Student’s t and Hodge-Lehman tests.
Sarcopenia as a Physical Component of Frailty
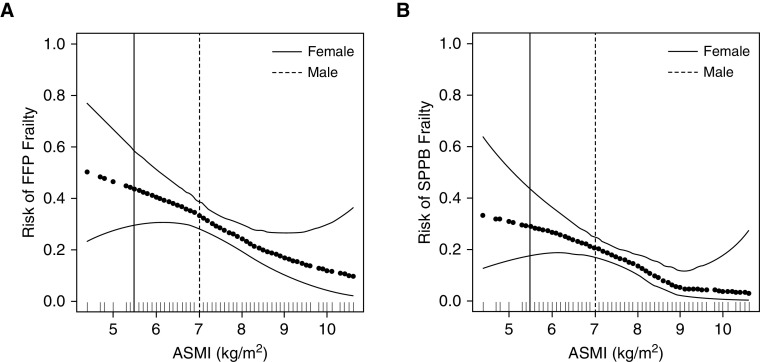
After controlling for age, sex, and diagnosis, the relationship between ASM and risk of frailty by both SPPB and FFP appeared to be linear (P < 0.01) (Figure 2). Notably, these models did not identify a threshold for muscle mass above which the risk of frailty plateaued.
Figure 2.
(A and B) Risk of frailty by Fried frailty phenotype (FFP) (A) and short physical performance battery (SPPB) (B) by appendicular skeletal muscle mass index (ASMI). Generalized additive models; horizontal dark dotted black line represents the effect estimates. Surrounding thin lines represent 95% confidence bands. Full-height solid and dashed vertical lines represent sex-specific ASMI cut-points for sarcopenia diagnosis. Each vertical line in the rug plot along the x-axis represents a single study subject. Models are adjusted for age, sex, and diagnosis.
Predictive Validity
By both EWGSOP2 definitions and the expanded lowest quartile definition, sarcopenia was independently associated with higher pretransplant disability. Sarcopenia by the limited lowest quartile definition showed an association with similar magnitude of effect and CI, although it was not statistically significant. Other definitions were not associated with LT-VLA disability (Table 5). In secondary analyses including Lung Allocation Score as a covariate, the associations between sarcopenia and disability were modestly attenuated (Table E4).
Table 5.
Associations between sarcopenia and pretransplant LT-VLA disability and waitlist delisting or death
| Pretransplant Disability | Waitlist Delisting or Death | |
|---|---|---|
| Definition | Regression Estimate (95% CI) | Hazard Ratio (95% CI) |
| EWGSOP2 | ||
| Limited | 0.20* (−0.0001 to 0.41) | 2.84 (0.94 to 8.55) |
| Expanded | 0.25† (−0.0004 to 0.51) | 1.25 (0.25 to 6.10) |
| FNIH | ||
| Limited | −0.03 (−0.24 to 0.18) | 0.98 (0.26 to 3.70) |
| Expanded | 0.16 (−0.12 to 0.45) | 1.00 (0.12 to 8.21) |
| Lowest Quartile | ||
| Limited | 0.07 (−0.09 to 0.23) | 3.75‡ (1.42 to 9.93) |
| Expanded | 0.23‡ (0.01 to 0.44) | 3.46‡ (1.09 to 10.97) |
Definition of abbreviations: CI = confidence interval; EWGSOP2 = European Working Group on Sarcopenia in Older People 2; FNIH = Foundation for the National Institutes of Health; Lowest Quartile = distribution-based lowest quartile of sarcopenic study participants; LT-VLA = Lung Transplant-Specific Valued Life Activities Disability Scale.
Associations between sarcopenia and pretransplant disability evaluated using linear regression. LT-VLA MID = 0.3. Associations between sarcopenia definitions and composite outcome of waitlist delisting or death evaluated using Cox proportional hazards models. One patient who was enrolled after transplant was excluded from Cox analyses. All analyses adjusted for age, sex, race, and diagnosis.
P = 0.0501.
P = 0.054.
Statistically significant at P < 0.05.
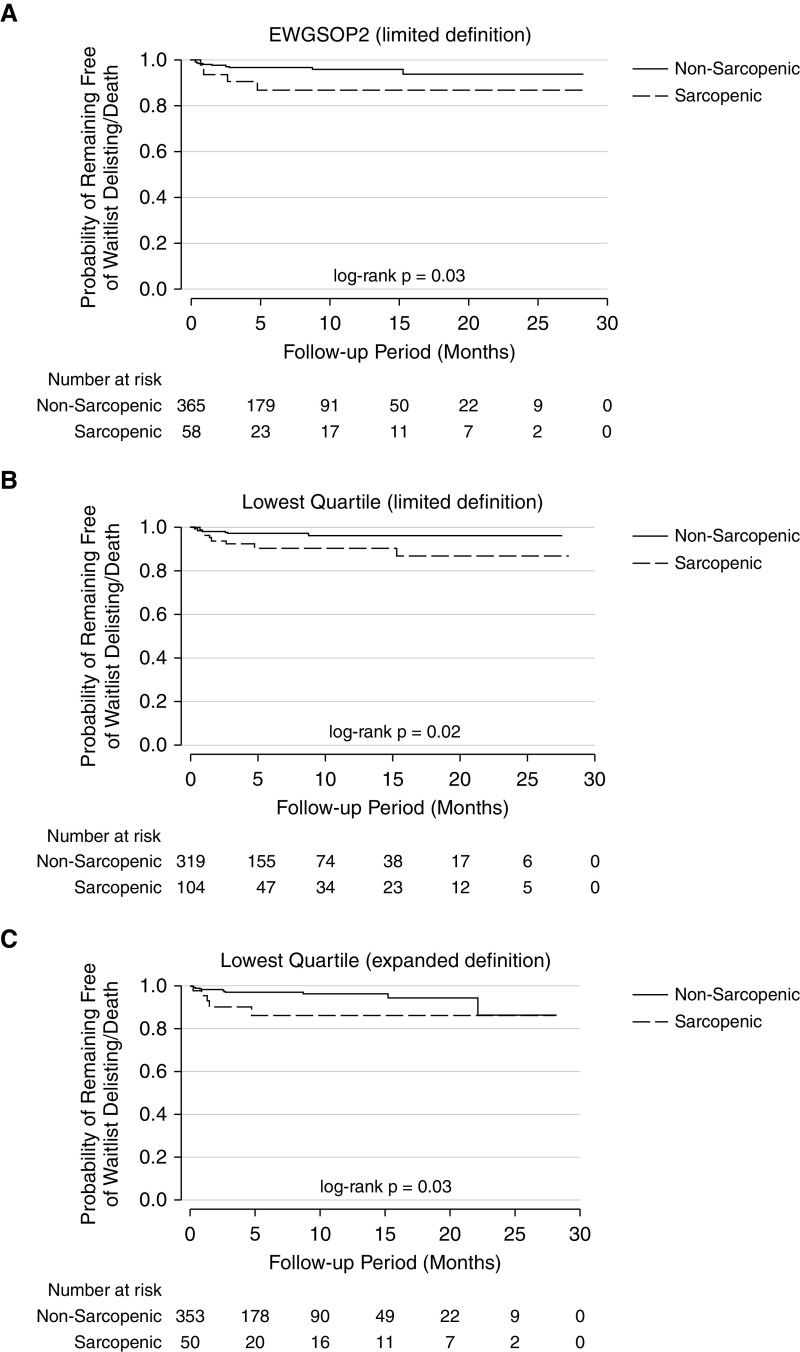
In unadjusted analyses, sarcopenia by the limited EWGSOP2 definition and both the limited and expanded lowest quartile definitions was associated with a higher cumulative probability of delisting/death (Figure 3). In adjusted models, sarcopenia by limited and expanded lowest quartile definitions was associated with a threefold increased risk of delisting or death (hazard ratio [HR], 3.75; 95% confidence interval [CI], 1.42–9.93 for the limited definition and HR, 3.46; 95% CI, 1.09–10.97 for the expanded definition). Similarly, the EWGSOP2 limited and lowest quartile expanded definitions were associated with trends toward similar magnitudes of increased risk. For example, the limited EWGSOP2 definition was associated with a 2.84-fold increased risk of delisting or death, although the CI crossed unity (95% CI, 0.94–8.55) (Table 5). In secondary analyses including Lung Allocation Score as a covariate, the associations between sarcopenia and waitlist delisting/death were moderately, though not entirely, attenuated and were no longer statistically significant (Table E4).
Figure 3.
Time to waitlist delisting or death of sarcopenic patients by (A) limited EWGSOP2, (B) limited Lowest Quartile, and (C) expanded Lowest Quartile definitions. EWGSOP2 = European Working Group on Sarcopenia in Older People 2; Lowest Quartile = distribution-based lowest quartile of sarcopenic study participants.
Discussion
In testing six definitions of sarcopenia within a multicenter cohort of adult lung transplant candidates, we found that sarcopenia prevalence varied by definition (6–13%). Not surprisingly, prevalence was lower when definitions of sarcopenia included measures of both muscle mass and quality compared with muscle mass alone. We found that existing sarcopenia definitions demonstrate a range of construct validity when applied to lung transplant candidates. The limited (muscle mass alone) EWGSOP2 definition was found to be most valid in this population. Both EWGSOP2 definitions and the expanded lowest quartile definition were associated with greater pretransplant disability. Sarcopenia by the limited and expanded lowest quartile definitions was associated with increased risk of waitlist delisting or death, and there was a trend toward increased risk by the limited EWGSOP2 definition. These results suggest that assessment of muscle mass alone may be sufficient when measuring sarcopenia in lung transplant candidates and when risk-stratifying patients by sarcopenia status for certain transplant outcomes.
The specific population of individuals labeled sarcopenic varied from one sarcopenia definition to the next. These differences highlight the limitations of adopting and applying definitions without validating them in the population of interest. The variation in these populations means that a patient labeled sarcopenic by one definition may not be sarcopenic by another; in turn, risk stratification for the pretransplant outcomes examined may vary by the definition applied. These findings underscore the importance of further testing the predictive validity of sarcopenia definitions in larger cohorts and with longer-term outcomes before implementing these candidate definitions in clinical decision-making. The apparent linear relationship between decreased muscle mass and increased risk of frailty supports sarcopenia’s role as a physical component of frailty; as an individual becomes more sarcopenic, his or her risk of frailty also increases. This relationship has not previously been demonstrated in advanced lung disease. Notably, the linear relationship between muscle mass and frailty without evidence of a threshold challenges the clinical utility of defining a set cut-point for muscle mass below which a patient is deemed “sarcopenic.” Rather, if muscle mass is weighed in clinical decision-making, our findings argue that muscle mass would be better considered on a continuum rather than dichotomizing sarcopenia as present or absent.
The magnitudes of correlations between muscle mass and conceptually related variables of interest were weak. Although speculative, it is possible that the relative contribution of sarcopenia to factors such as 6MWD, frailty, and SF12-PCS may be modest in the setting of end-stage lung disease. Furthermore, our cohort reflects a unique population of patients with end-stage lung disease who are deemed candidates for lung transplantation. It is likely that they represent a less overtly frail and debilitated group than the general population of adults with end-stage lung disease. These findings highlight limitations and potential risks of applying sarcopenia definitions derived in other populations to candidates for lung transplantation. They also underscore the importance of assessing the validity of potential definitions of sarcopenia before implementing them into clinical or research practice. We caution against extrapolating our findings to adults with end-stage lung disease not being considered for lung transplantation.
Our findings contribute to emerging literature focused on identifying more advanced approaches to evaluating body composition in candidates for solid organ transplantation. Currently, BMI is the most common clinically employed measure of body composition. Many consider obesity (BMI ≥ 30 or ≥35) and underweight status (BMI ≤18.5) to be relative contraindications to transplantation on the basis of associations with mortality after lung transplantation (39). In these instances, BMI is employed as a surrogate for either total body adipose tissue (obesity) or undernourishment, malnourishment, or sarcopenia (underweight). Importantly, BMI fails to directly quantify muscle and fat, resulting in misclassification of both obesity and sarcopenia (40–42). In addition, patients with elevated BMI may have both excess adipose tissue and low muscle mass (a state referred to as “sarcopenic obesity”) (42). Our findings raise the possibility that directly quantifying muscle mass and adipose tissue could improve the precision and accuracy of candidate risk stratification before transplantation.
This study has limitations. Our study cohort is composed of patients already listed or near listing for lung transplantation; whether our findings can be generalized to other patients with lung disease is unknown. Idiopathic pulmonary fibrosis is the predominant diagnosis within our study population; thus, our study cohort may not be representative of other transplant candidate populations. Unmeasured covariates may explain associations seen between sarcopenia and pretransplant disability or waitlist delisting/death. For example, it is possible that, in a subset of patients, corticosteroid use contributed to our findings. When controlling for Lung Allocation Score (LAS), the association between some definitions of sarcopenia and waitlist delisting/death may be mediated by some factors already included in the LAS. However, the magnitude of the associations, despite controlling for LAS, does suggest that directly measuring body composition may add clinically relevant information not collected as part of the routine clinical evaluation. Finally and importantly, this study did not investigate the impact of sarcopenia on outcomes after lung transplantation. Clarifying this impact and the relevance of sarcopenia across clinically important strata of lung transplant candidates is needed before sarcopenia can be used to inform patient management.
Our study has notable strengths. We examined a large multicenter prospective cohort. Sample size permitted us to control for several covariates when analyzing associations between sarcopenia and pretransplant outcomes. Use of a distribution-based definition of sarcopenia derived from our study population allowed for comparison of construct validity and absolute cut-points for sarcopenia diagnosis between this definition and existing definitions from geriatric literature.
In summary, we found that the prevalence, construct validity, and predictive validity of sarcopenia vary depending on the operational definition used. We also identified a linear relationship between muscle mass and risk of frailty. Future studies should evaluate associations between sarcopenia and post-transplant morbidity and mortality as well as whether interventions to improve muscle mass and function improve clinical outcomes.
Acknowledgments
Acknowledgment
The authors thank the patients at the University of California, San Francisco, the University of Pennsylvania, and the Columbia University Medical Center who participated in the Lung Transplant Body Composition Study.
Footnotes
Supported by National Heart, Lung, and Blood Institute grants R01-HL134851 (P.P.K. and J.P.S.), K24-HL115354 (J.D.C.), U01-HL145435 (J.D.C.), R01-HL087115 (J.D.C.), and R01-HL151552 (J.G.). and Veterans Affairs Merit Award CX002011 (J.G.).
Author Contributions: J.A.M., Y.G., and J.P.S. made substantial contributions to the creation and design of this work. J.A.M. wrote the first draft of the manuscript. Y.G. and J.P.S. revised the manuscript for important intellectual content. All authors made substantial contributions to the acquisition, analysis, or interpretation of data for the work. All authors approved the manuscript. All authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1. Lund LH, Khush KK, Cherikh WS, Goldfarb S, Kucheryavaya AY, Levvey BJ, et al. International Society for Heart and Lung Transplantation. The registry of the international society for heart and lung transplantation: thirty-fourth adult heart transplantation report-2017; focus theme: allograft ischemic time. J Heart Lung Transplant. 2017;36:1037–1046. doi: 10.1016/j.healun.2017.07.019. [DOI] [PubMed] [Google Scholar]
- 2. Courtwright A, Cantu E. Lung transplantation in elderly patients. J Thorac Dis. 2017;9:3346–3351. doi: 10.21037/jtd.2017.08.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Maxwell BG, Mooney JJ, Lee PH, Levitt JE, Chhatwani L, Nicolls MR, et al. Increased resource use in lung transplant admissions in the lung allocation score era. Am J Respir Crit Care Med. 2015;191:302–308. doi: 10.1164/rccm.201408-1562OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Maxwell BG, Levitt JE, Goldstein BA, Mooney JJ, Nicolls MR, Zamora M, et al. Impact of the lung allocation score on survival beyond 1 year. Am J Transplant. 2014;14:2288–2294. doi: 10.1111/ajt.12903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tang M, Mawji N, Chung S, Brijlal R, Lim Sze How JK, Wickerson L, et al. Factors affecting discharge destination following lung transplantation. Clin Transplant. 2015;29:581–587. doi: 10.1111/ctr.12556. [DOI] [PubMed] [Google Scholar]
- 6. Genao L, Whitson HE, Zaas D, Sanders LL, Schmader KE. Functional status after lung transplantation in older adults in the post-allocation score era. Am J Transplant. 2013;13:157–166. doi: 10.1111/j.1600-6143.2012.04299.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yusen RD, Christie JD, Edwards LB, Kucheryavaya AY, Benden C, Dipchand AI, et al. International Society for Heart and Lung Transplantation. The registry of the international society for heart and lung transplantation: thirtieth adult lung and heart-lung transplant report: 2013; focus theme: age. J Heart Lung Transplant. 2013;32:965–978. doi: 10.1016/j.healun.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 8. Courtwright AM, Zaleski D, Gardo L, Ahya VN, Christie JD, Crespo M, et al. Causes, preventability, and cost of unplanned rehospitalizations within 30 days of discharge after lung transplantation. Transplantation. 2018;102:838–844. doi: 10.1097/TP.0000000000002101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Singer JP, Diamond JM, Gries CJ, McDonnough J, Blanc PD, Shah R, et al. Frailty phenotypes, disability, and outcomes in adult candidates for lung transplantation. Am J Respir Crit Care Med. 2015;192:1325–1334. doi: 10.1164/rccm.201506-1150OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Singer JP, Diamond JM, Anderson MR, Katz PP, Covinsky K, Oyster M, et al. Frailty phenotypes and mortality after lung transplantation: a prospective cohort study. Am J Transplant. 2018;18:1995–2004. doi: 10.1111/ajt.14873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cesari M, Landi F, Vellas B, Bernabei R, Marzetti E. Sarcopenia and physical frailty: two sides of the same coin. Front Aging Neurosci. 2014;6:192. doi: 10.3389/fnagi.2014.00192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yeung SSY, Reijnierse EM, Pham VK, Trappenburg MC, Lim WK, Meskers CGM, et al. Sarcopenia and its association with falls and fractures in older adults: a systematic review and meta-analysis. J Cachexia Sarcopenia Muscle. 2019;10:485–500. doi: 10.1002/jcsm.12411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yang M, Hu X, Wang H, Zhang L, Hao Q, Dong B. Sarcopenia predicts readmission and mortality in elderly patients in acute care wards: a prospective study. J Cachexia Sarcopenia Muscle. 2017;8:251–258. doi: 10.1002/jcsm.12163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tandon P, Ney M, Irwin I, Ma MM, Gramlich L, Bain VG, et al. Severe muscle depletion in patients on the liver transplant wait list: its prevalence and independent prognostic value. Liver Transpl. 2012;18:1209–1216. doi: 10.1002/lt.23495. [DOI] [PubMed] [Google Scholar]
- 15. Streja E, Molnar MZ, Kovesdy CP, Bunnapradist S, Jing J, Nissenson AR, et al. Associations of pretransplant weight and muscle mass with mortality in renal transplant recipients. Clin J Am Soc Nephrol. 2011;6:1463–1473. doi: 10.2215/CJN.09131010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Garonzik-Wang JM, Govindan P, Grinnan JW, Liu M, Ali HM, Chakraborty A, et al. Frailty and delayed graft function in kidney transplant recipients. Arch Surg. 2012;147:190–193. doi: 10.1001/archsurg.2011.1229. [DOI] [PubMed] [Google Scholar]
- 17. Maury G, Langer D, Verleden G, Dupont L, Gosselink R, Decramer M, et al. Skeletal muscle force and functional exercise tolerance before and after lung transplantation: a cohort study. Am J Transplant. 2008;8:1275–1281. doi: 10.1111/j.1600-6143.2008.02209.x. [DOI] [PubMed] [Google Scholar]
- 18. Kyle UG, Nicod L, Romand JA, Slosman DO, Spiliopoulos A, Pichard C. Four-year follow-up of body compostion in lung transplant patients. Transplantation. 2003;75:821–828. doi: 10.1097/01.TP.0000054689.50879.36. [DOI] [PubMed] [Google Scholar]
- 19. Swallow EB, Reyes D, Hopkinson NS, Man WD, Porcher R, Cetti EJ, et al. Quadriceps strength predicts mortality in patients with moderate to severe chronic obstructive pulmonary disease. Thorax. 2007;62:115–120. doi: 10.1136/thx.2006.062026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Marquis K, Debigaré R, Lacasse Y, LeBlanc P, Jobin J, Carrier G, et al. Midthigh muscle cross-sectional area is a better predictor of mortality than body mass index in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2002;166:809–813. doi: 10.1164/rccm.2107031. [DOI] [PubMed] [Google Scholar]
- 21. Rozenberg D, Singer LG, Herridge M, Goldstein R, Wickerson L, Chowdhury NA, et al. Evaluation of skeletal muscle function in lung transplant candidates. Transplantation. 2017;101:2183–2191. doi: 10.1097/TP.0000000000001754. [DOI] [PubMed] [Google Scholar]
- 22. Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, et al. European Working Group on Sarcopenia in Older People. Sarcopenia: European consensus on definition and diagnosis: report of the European working group on sarcopenia in older People. Age Ageing. 2010;39:412–423. doi: 10.1093/ageing/afq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyère O, Cederholm T, et al. Writing Group for the European Working Group on Sarcopenia in Older People 2 (EWGSOP2), and the Extended Group for EWGSOP2. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48:16–31. doi: 10.1093/ageing/afy169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Studenski SA, Peters KW, Alley DE, Cawthon PM, McLean RR, Harris TB, et al. The FNIH sarcopenia project: rationale, study description, conference recommendations, and final estimates. J Gerontol A Biol Sci Med Sci. 2014;69:547–558. doi: 10.1093/gerona/glu010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gould H, Brennan SL, Kotowicz MA, Nicholson GC, Pasco JA. Total and appendicular lean mass reference ranges for Australian men and women: the Geelong osteoporosis study. Calcif Tissue Int. 2014;94:363–372. doi: 10.1007/s00223-013-9830-7. [DOI] [PubMed] [Google Scholar]
- 26. Dodds RM, Syddall HE, Cooper R, Benzeval M, Deary IJ, Dennison EM, et al. Grip strength across the life course: normative data from twelve British studies. PLoS One. 2014;9:e113637. doi: 10.1371/journal.pone.0113637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Studenski S, Perera S, Patel K, Rosano C, Faulkner K, Inzitari M, et al. Gait speed and survival in older adults. JAMA. 2011;305:50–58. doi: 10.1001/jama.2010.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ling CH, de Craen AJ, Slagboom PE, Gunn DA, Stokkel MP, Westendorp RG, et al. Accuracy of direct segmental multi-frequency bioimpedance analysis in the assessment of total body and segmental body composition in middle-aged adult population. Clin Nutr. 2011;30:610–615. doi: 10.1016/j.clnu.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 29. Faria SL, Faria OP, Cardeal MD, Ito MK. Validation study of multi-frequency bioelectrical impedance with dual-energy X-ray absorptiometry among obese patients. Obes Surg. 2014;24:1476–1480. doi: 10.1007/s11695-014-1190-5. [DOI] [PubMed] [Google Scholar]
- 30. Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473–483. [PubMed] [Google Scholar]
- 31. McHorney CA, Ware JE, Jr, Raczek AE. The MOS 36-Item Short-Form Health Survey (SF-36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. Med Care. 1993;31:247–263. doi: 10.1097/00005650-199303000-00006. [DOI] [PubMed] [Google Scholar]
- 32.Army Individual Test Battery. Manual of directions and scoring. War Department, Washington, DC: Adjunct General’s Office; 1944 [Google Scholar]
- 33. Guralnik JM, Simonsick EM, Ferrucci L, Glynn RJ, Berkman LF, Blazer DG. et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49:M85–M94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 34. Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, et al. Cardiovascular Health Study Collaborative Research Group. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–M156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 35. Baldwin MR, Singer JP, Huang D, Sell J, Gonzalez WC, Pollack LR, et al. Refining low physical activity measurement improves frailty assessment in advanced lung disease and survivors of critical illness. Ann Am Thorac Soc. 2017;14:1270–1279. doi: 10.1513/AnnalsATS.201612-1008OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Singer JP, Blanc PD, Dean YM, Hays S, Leard L, Kukreja J, et al. Development and validation of a lung transplant-specific disability questionnaire. Thorax. 2014;69:437–442. doi: 10.1136/thoraxjnl-2013-204557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Terwee CB, Bot SD, de Boer MR, van der Windt DA, Knol DL, Dekker J, et al. Quality criteria were proposed for measurement properties of health status questionnaires. J Clin Epidemiol. 2007;60:34–42. doi: 10.1016/j.jclinepi.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 38. Kolaitis NA, Soong A, Shrestha P, Zhuo H, Neuhaus J, Katz PP, et al. Improvement in patient-reported outcomes after lung transplantation is not impacted by the use of extracorporeal membrane oxygenation as a bridge to transplantation. J Thorac Cardiovasc Surg. 2018;156:440–448, e2. doi: 10.1016/j.jtcvs.2018.01.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lederer DJ, Wilt JS, D’Ovidio F, Bacchetta MD, Shah L, Ravichandran S, et al. Obesity and underweight are associated with an increased risk of death after lung transplantation. Am J Respir Crit Care Med. 2009;180:887–895. doi: 10.1164/rccm.200903-0425OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Shah NR, Braverman ER. Measuring adiposity in patients: the utility of body mass index (BMI), percent body fat, and leptin. PLoS One. 2012;7:e33308. doi: 10.1371/journal.pone.0033308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Gonzalez MC, Pastore CA, Orlandi SP, Heymsfield SB. Obesity paradox in cancer: new insights provided by body composition. Am J Clin Nutr. 2014;99:999–1005. doi: 10.3945/ajcn.113.071399. [DOI] [PubMed] [Google Scholar]
- 42. Choi KM. Sarcopenia and sarcopenic obesity. Korean J Intern Med. 2016;31:1054–1060. doi: 10.3904/kjim.2016.193. [DOI] [PMC free article] [PubMed] [Google Scholar]