Abstract
Rationale: Coronavirus disease (COVID-19) is an ongoing pandemic, in which obesity, hypertension, and diabetes have been linked to poor outcomes. Obstructive sleep apnea (OSA) is associated with these conditions and may influence the prognosis of adults with COVID-19.
Objectives: To determine the effect of OSA on clinical outcomes in patients with COVID-19.
Methods: The current prospective observational study was conducted in three hospitals in Istanbul, Turkey from March 10 to June 22, 2020. The participants were categorized as high-risk or low-risk OSA according to the Berlin questionnaire that was administered in the out-patient clinic, in hospital, or shortly after discharge from hospital blinded to the clinical outcomes. A modified high-risk (mHR)–OSA score based on the snoring patterns (intensity and/or frequency), breathing pauses, and morning/daytime sleepiness, without taking obesity and hypertension into account, were used in the regression models.
Results: The primary outcome was the clinical improvement defined as a decline of two categories from admission on a 7-category ordinal scale that ranges from 1 (discharged with normal activity) to 7 (death) on Days 7, 14, 21, and 28, respectively. Secondary outcomes included clinical worsening (an increase of 1 category), need for hospitalization, supplemental oxygen, and intensive care. In total, 320 eligible patients (median [interquartile range] age, 53.2 [41.3–63.0] yr; 45.9% female) were enrolled. In all, 121 (37.8%) were categorized as known (n = 3) or high-risk OSA (n = 118). According to the modified scoring, 70 (21.9%) had mHR-OSA. Among 242 patients requiring hospitalization, clinical improvement within 2 weeks occurred in 75.4% of the mHR-OSA group compared with 88.4% of the modified low-risk–OSA group (P = 0.014). In multivariate regression analyses, mHR-OSA (adjusted odds ratio [OR], 0.42; 95% confidence interval [CI], 0.19–0.92) and male sex (OR, 0.39; 95% CI, 0.17–0.86) predicted the delayed clinical improvement. In the entire study population (n = 320), including the nonhospitalized patients, mHR-OSA was associated with clinical worsening (adjusted hazard ratio, 1.55; 95% CI, 1.00–2.39) and with the need for supplemental oxygen (OR, 1.95; 95% CI, 1.06–3.59). Snoring patterns, especially louder snoring, significantly predicted delayed clinical improvement, worsening, need for hospitalization, supplemental oxygen, and intensive care.
Conclusions: Adults with mHR-OSA in our COVID-19 cohort had poorer clinical outcomes than those with modified low-risk OSA independent of age, sex, and comorbidities. Clinical trial registered with www.clinicaltrials.gov (NCT04363333).
Keywords: COVID-19, obstructive sleep apnea, clinical outcomes, hospitalization, intensive care
The ongoing outbreak of novel coronavirus disease (COVID-19) has critically worsened the lives of millions of people, causing a major public health crisis globally. According to the latest estimates by the World Health Organization, COVID-19 has affected more than 102 million people across 223 countries with more than 2.2 million deaths (as of February 2, 2021) (1). Turkey has reported the first case on March 10, 2020 (2), and the number of cases has increased rapidly. According to the latest estimates by the Ministry of Health, the Republic of Turkey, the number of confirmed cases has increased to more than 2.4 million with almost 26 thousand deaths (as of February 2, 2021) (2). The pandemic is still the largest health crisis globally, and many aspects of transmission, infection, and treatment remain unclear (3).
Based on the first reports from China and Italy, the most frequent comorbidities were hypertension, diabetes, and cardiovascular disease (4, 5) in addition to obesity based on the later reports (6, 7). Obstructive sleep apnea (OSA) is a condition characterized by intermittent partial or complete cessation of breathing during sleep, resulting in intermittent hypoxemia and arousal from sleep, and the condition is strongly associated with obesity, hypertension, and diabetes (8, 9). Recent research letters and short communications have suggested that individuals with OSA may be predisposed to COVID-19 infection and poor outcomes (10–12). A known OSA diagnosis was observed in retrospective analyses of the medical records of 12.3% and 9.5% of COVID-19 cases, respectively (6, 13). In the latter study, the COVID-19 cases with a known OSA diagnosis had a higher overall mortality rate than that among the control subjects (13). In the aforementioned study (7), addressing the role of diabetes mellitus on COVID-19 outcomes among 1,317 patients, concomitant OSA diagnosis was associated with an increased risk for mortality. To date, there is yet no information about a true prevalence of concomitant OSA among patients with COVID-19 given that objective sleep studies with polysomnography are not accurate in the face of an active contagious respiratory infection.
In the current work (the OSACOVID-19 [Obstructive Sleep Apnea and Covid-19] study), we aimed to address the occurrence of known/high-probability OSA, based on the Berlin questionnaire (BQ) (14), among patients with confirmed or suspected COVID-19 disease and evaluate the impact of high-risk OSA on short-term clinical outcomes.
Methods
Study Design, Setting, and Participants
The current multicenter, prospective, observational clinical trial was conducted in three hospitals (Koç University Hospital [KUH], Koç Healthcare American Hospital [KHAH], and Marmara University Hospital [MUH]) in Istanbul, Turkey. All participants were referred to the hospitals between March 10 and June 22, 2020 (KUH and KHAH) and between April 17 and June 21, 2020 (MUH) (Figure 1). Inclusion into the study started on April 16, 2020. Inclusion criteria were as follows: 1) being an adult with a diagnosis of COVID-19, 2) having the ability to read and speak, and 3) signed informed consent. Demographic data, comorbidities, clinical symptoms, laboratory and radiological findings, treatment, and outcomes were prospectively collected from the electronic health records per June 26, 2020. The participants answered to the BQ (see below), which was administered in the outpatient clinic, in hospital, or shortly after the hospital discharge between April 21 and July 7, 2020, blinded to the clinical outcomes. The clinical, laboratory, and radiological investigations are planned to be repeated, and polysomnographic, echocardiographic, and lung function evaluations will be performed within 12 months after the initial COVID-19 onset.
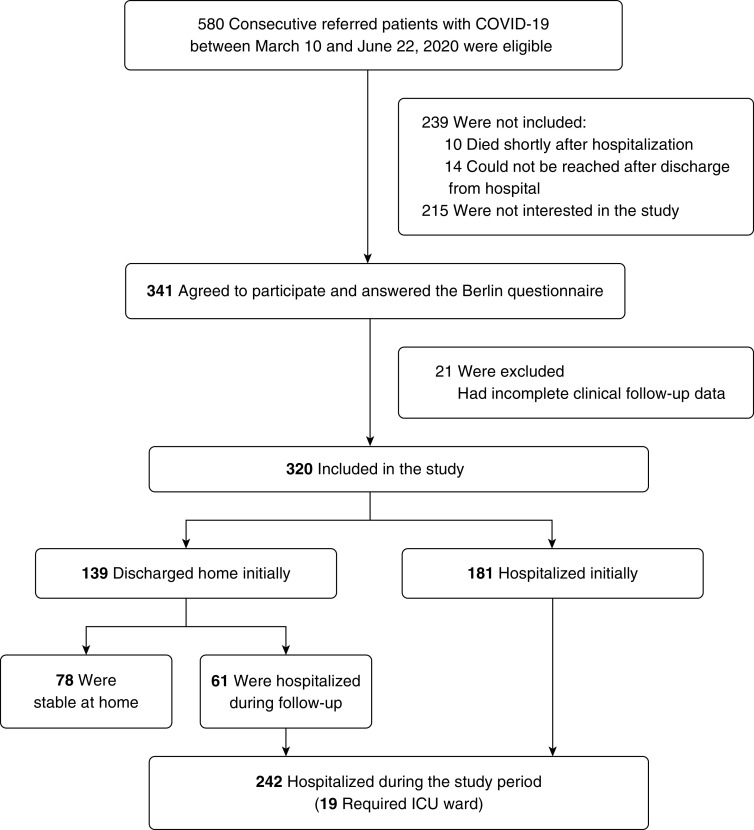
Figure 1.
Flow of patients through the study. COVID-19 = coronavirus disease; ICU = intensive care unit.
The Koç University Committee on Human Research approved the study protocol (approval nr 2020.140.IRB1.030; 04.15.2020), and written informed consent was provided from all participants. The trial was registered with ClinicalTrials.gov.
Data Collection and Definitions
All COVID-19 cases were confirmed by positive polymerase chain reaction (PCR) testing of nasopharyngeal specimens and/or clinical symptoms and radiologic findings suggestive of COVID-19 pneumonia (15). These were mainly the presence of ground-glass opacities, typically with a peripheral and subpleural distribution, and focal consolidation (15). The participants were defined as known OSA when there was a previous sleep study and/or the initiation of treatment documented by a physician. Others were classified as being at high risk or low risk for OSA based on the BQ, including 10 items in three categories: snoring severity, morning/daytime tiredness/sleepiness, and presence of hypertension and/or obesity (see Methods in the online supplement) (14). The questions were referred to the time preceding the patient’s COVID-19 onset. When available, the patient’s family or bed partner was asked to confirm the accuracy of responses to the questions about snoring, especially in cases with a “do not know” response. Patients were classified as high-risk OSA when they scored positive on two or more categories, whereas those who did not were categorized as low-risk OSA (14). The BQ has been widely used and validated in general populations (16) and clinical cohorts (17) as a screening tool for OSA (8). For the purpose of the current work for evaluation of the effect of high-risk OSA on the primary and secondary outcomes, we have developed a modified high-risk (mHR)–OSA score by ignoring obesity and hypertension and using three subcategories from the questionnaire: Subcategory 1, snoring patterns, such as snoring intensity (louder than talking or very loud [can be heard in adjacent rooms]) and snoring frequency (3–4 times a week or nearly every day), provided a positive score when the snoring intensity and/or frequency were positive. Subcategory 2, breathing pauses, provided a positive score when the response was “3–4 times a week” or “nearly every day”; and similarly, Subcategory 3, tiredness/sleepiness in the morning and/or daytime, provided a positive score when the response was “3–4 times a week” or “nearly every day.” Patients were classified as mHR-OSA when they scored positive on two or more subcategories, and the patients who did not score positive in any or only one subcategory were categorized as modified low-risk (mLR)–OSA. Demographics, comorbidities, and drugs at baseline and during the follow up were documented. Obesity was defined as a body mass index (BMI) of at least 30 kg/m2 (18).
Measurements
A 7-category ordinal scale was used for evaluation of short-term outcomes as previously described (19) as follows: Category 7, death; Category 6, intensive care unit (ICU) hospitalization and requiring invasive mechanical ventilation; Category 5, ICU hospitalization and not requiring invasive mechanical ventilation; Category 4, non-ICU hospitalization and requiring supplemental oxygen; Category 3, non-ICU hospitalization and not requiring supplemental oxygen; Category 2, not hospitalized but unable to resume normal activities; and Category 1, not hospitalized with the resumption of normal activities (19). The primary outcome was the clinical improvement defined as a decline of two categories from admission on the 7-category ordinal scale on Days 7, 14, 21, and 28, respectively. Secondary outcomes included worsening (an increase of one category), need for hospitalization, supplemental oxygen, and intensive care, respectively.
Bias
To avoid bias with the patient selection and the outcomes, the questionnaires were administrated by researchers (Y.C., S.A., and D.Y.) blinded to the clinical data and outcomes, which were completed by the other researchers blinded to the responses to the BQ.
Sample Size
To our best knowledge, there were no prospective reports published about the recovery rate of COVID-19 cases with concomitant OSA at the time of the planning of our study, and we hypothesized that the clinical improvement rate would be lower among the patients with OSA given the potential pathophysiological mechanisms involved in sleep-related breathing disorders (9). We assumed that the recovery rate of the patients with COVID-19 with known/high-risk OSA would be around 80% within 28 days of the hospitalization and around 90% among the cases with low-risk OSA. Based on this assumption, and an 80% power (1-β) with the type I error (α = 0.05), the sample size for the hospitalized participants was calculated as 196 for the primary outcome. For the secondary outcomes, after accounting for a 40% dropout rate in follow-up protocol, including the objective overnight sleep studies, we targeted at least 275 cases to be enrolled into the study. The sample size calculation was done by using MedCalc Statistical Software version 12.7.7 (Medcalc Software bvba; 2013).
Statistics
Statistical analysis was performed using IBM SPSS Statistics for Windows, version 26.0 (IBM Corp., 2020). The normality of continuous variables was investigated by Shapiro-Wilk’s test. Descriptive statistics were presented using mean and standard deviation for normally distributed variables and median (and interquartile range [IQR]) for the nonnormally distributed variables. Nonparametric statistical methods were used for values with skewed distribution. For comparison of two nonnormally distributed groups, the Mann-Whitney U test was used. For comparison of two normally distributed groups, Student’s t test was used. The χ2 test (Fisher’s exact test where available) was used for categorical variables and expressed as observation counts (and percentages). Evaluation of the change in percentages through time regarding the 7-category levels was performed with the McNemar test. Univariate factors associated with the clinical improvement on the 7-category scale at 7, 14, 21, and 28 days as well as the need for hospitalization, supplemental oxygen, and ICU ward were inspected by using logistic regression analysis. In addition, a time-dependent Cox model with a test for proportional hazards was used to estimate the impact of mHR-OSA on clinical worsening. In the multivariate analyses, the mHR-OSA as well as the snoring patterns were included in the models with age, sex, and BMI as covariates, with additional adjustments for hypertension, diabetes, and study site, depending on the results in the univariate analyses. Statistical significance was accepted when two-sided P < 0.05.
Results
Participants
As illustrated in Figure 1, 580 consecutive patients were referred to the study hospitals with a COVID-19 diagnosis confirmed by PCR testing of nasopharyngeal specimens and/or being exposed to patients with COVID-19, with clinical symptoms and radiologic findings suggestive for COVID-19 pneumonia, between April 16 and June 21, 2020. Ten patients died shortly after hospitalization, and 14 could not be reached after hospital discharge. Among 556 cases who were reached during or shortly after outpatient clinic visit or discharge from the hospital, 215 were not interested in the study. The patients who did not participate in the study were older than the included cases (median [IQR], 63.1 [48.5–75.2] yr versus 53.2 [41.3–63.0] yr) without any significant difference in sex distribution (45.4% vs. 45.9% were males). The reasons for unwillingness to participate were mainly being too tired, not willing to come to the hospital again for risk for reinfection, and/or personal concerns such as the risk for additional costs related to the follow-up procedures. Among 341 cases who agreed to participate, 21 were excluded because of incomplete clinical follow-up data. The majority of the participants (both hospitalized and not hospitalized) were recruited from KUH (n = 154) and KHAH (n = 100). The data from the MUH participants were collected during the hospitalization period (n = 66, of whom 47% were initially not hospitalized).
Descriptive Data
The median (IQR) age of the participants was 53.2 (41.3–63.0) years, and 173 (54.1%) were men. The median BMI was 27.4 (24.9–31.5) kg/m2, and 32 (10.0%) were current smokers (Table 1). The most frequent symptoms were fever, cough, fatigue, and dyspnea, and the most common comorbidities were hypertension (35.3%), obesity (33.8%), and diabetes (18.1%). Only three participants had a known OSA diagnosis (0.9%). In all, 302 patients (94.4%) presented radiologic findings supportive for COVID-19–pneumonia, and 194 (60.6%) were PCR positive. The hospitalized participants at referral were older than the patients who did not require hospitalization (median age, 55.5 [47.9–65.7] vs. 46.6 [37.5–58.1] yr; P < 0.001). Hypertension and diabetes were significantly more common among the hospitalized patients at referral (45.9% vs. 21.6% [P < 0.001]; 22.1 vs. 12.9 [P = 0.035], respectively). Other demographic characteristics and comorbidities did not differ significantly between the groups.
Table 1.
Characteristics of adults with COVID-19 and low-risk vs. known/high-risk OSA
| Low-Risk OSA (n = 199) |
Known/High-Risk OSA (n = 121) |
|
|---|---|---|
| Demographic characteristics | ||
| Age*, y | 51.6 (37.7–60.3) | 55.5 (47.7–66.1) |
| Age ⩾65 yr† | 32 (16.1) | 33 (27.3) |
| Male sex | 109 (54.8) | 64 (52.9) |
| BMI*, kg/m2 | 25.8 (23.4–28.7) | 31.4 (27.4–34.1) |
| Comorbidities | ||
| Hypertension* | 44 (22.1) | 69 (57.0) |
| Obesity* | 37 (18.6) | 71 (58.7) |
| Diabetes mellitus‡ | 25 (12.6) | 33 (27.3) |
| Coronary artery disease | 13 (6.5) | 15 (12.4) |
| COPD | 4 (2.0) | 4 (3.3) |
| Asthma† | 4 (2.0) | 9 (7.4) |
| Malignancy | 12 (6.0) | 5 (4.1) |
| Current smoking | 24 (12.1) | 8 (6.6) |
| Radiology and laboratory | ||
| Pneumonia | 188 (94.5) | 114 (94.2) |
| PCR positive | 114 (57.3) | 81 (66.9) |
| CRP†, mg/L | 21.3 (5.9–54.0) | 37.1 (9.6–65.4) |
| WBC count†, ×103/L | 5.2 (4.3–7.0) | 6.2 (4.7–8.3) |
| Neutrophile count†, ×103/L | 2.5 (0.4–3.6) | 2.8 (0.7–4.8) |
| Lymphocyte count, ×103/L | 0.8 (0.1–1.4) | 0.9 (0.2–1.4) |
| Platelet count, ×103/L | 198 (159–258) | 207 (158–258) |
| Hospitalization | ||
| At referral† | 104 (52.3) | 77 (63.6) |
| During the study period‡ | 139 (69.8) | 103 (85.1) |
| ICU during the study period‡ | 5 (2.5) | 14 (11.6) |
| Treatment | ||
| Chloroquine | 171 (85.9) | 110 (90.9) |
| Azithromycin | 94 (47.2) | 58 (47.9) |
| Favipiravir | 47 (23.6) | 41 (33.9) |
| Oseltamivir | 49 (24.6) | 25 (20.7) |
| Lopinavir | 3 (1.5) | 8 (6.6) |
| Tocilizumab | 24 (12.1) | 16 (13.2) |
| Systemic steroids | 2 (1.0) | 5 (4.1) |
| Anticoagulant† | 101 (50.8) | 76 (62.2) |
| Supplemental oxygen‡ | 51 (25.6) | 53 (43.8) |
| Berlin questionnaire symptoms | ||
| Louder snoring* | 5 (2.5) | 59 (48.8) |
| Frequent snoring (⩾3–4 times/wk)* | 10 (5.0) | 78 (64.5) |
| Subcategory 1*, louder and/or frequent snoring | 11 (5.5) | 99 (81.8) |
| Subcategory 2†, breathing pauses (⩾3–4 times/wk) | 7 (3.5) | 12 (9.9) |
| Subcategory 3*, morning and/or daytime sleepiness | 52 (26.1) | 72 (59.5) |
| Modified high-risk OSA*, at least 2 subcategories positive | 9 (4.5) | 61 (50.4) |
| Study sites‡ | ||
| KUH (n = 154) | 98 (49.2) | 56 (46.3) |
| KHAH (n = 100) | 70 (35.2) | 30 (24.8) |
| MUH (n = 66) | 31 (15.6) | 35 (28.9) |
Definition of abbreviations: BMI = body mass index; COPD = chronic obstructive pulmonary disease; COVID-19 = coronavirus disease; CRP = C-reactive protein; ICU = intensive care unit; KHAH = Koc Healthcare American Hospital; KUH = Koc University Hospital; MUH = Marmara University Hospital; OSA = obstructive sleep apnea; PCR = polymerase chain reaction; WBC = white blood cell.
Continuous variables are expressed as median (boundaries of interquartile ranges), and categorical variables are given in counts (percentages).
P < 0.001.
P < 0.05.
P < 0.01.
Outcome Data
In all, 121 (37.8%) were categorized as known OSA (n = 3) or high-risk OSA (n = 118) based on the BQ scores. The OSA cases were significantly older and had higher BMI than those with low-risk OSA, and obesity, hypertension, diabetes, and asthma were more common among the OSA group (Table 1). Other demographic characteristics and comorbidities did not differ significantly between the groups. At referral, 63.6% of the known/high-risk OSA cases versus 52.3% of low-risk OSA required hospitalization (P = 0.047), whereas the number of hospitalized cases during the whole study period were 85.1% versus 69.8%, respectively (P = 0.002). In the study population, 11.6% of the known/high-risk OSA cases versus 2.5% of the participants with low-risk OSA required ICU ward (P = 0.001) (Table 1).
The proportion of cases with a positive COVID-19 PCR test as well as radiological findings supportive for COVID-19–pneumonia did not differ significantly between the groups. C-reactive protein, white blood cell, and neutrophile counts were significantly higher in the known/high-risk OSA group compared with the low-risk OSA, whereas the lymphocyte and platelet counts were similar (Table 1).
During the study period, the most commonly used drug was chloroquine alone or in combination with azithromycin, both for nonhospitalized and hospitalized patients, based on the recommendations of the Ministry of Health at the beginning of the pandemic period (20). In all, 33.9% of the participants with known/high-risk OSA versus 23.6% of the cases with low-risk OSA received favipiravir (P = 0.046), whereas anticoagulants were used among 62.2% of the OSA cases versus 50.0% of the ones with low-risk OSA (P = 0.035). remdesivir was not available and not included in the therapy guidelines during the study period. Supplemental oxygen was required among 43.8% of the patients with known/high-risk OSA versus 25.6% of the participants with low-risk OSA (P = 0.001) (Table 1). All study sites followed the same therapy guidelines of the Ministry of Health (20) during the study period.
Based on the modified version of the BQ, the majority of the high-risk patients with OSA reported louder and/or frequent snoring (Subcategory 1) and morning/daytime sleepiness (Subcategory 3), whereas breathing pauses (Subcategory 2) were much less frequent (Table 1). Thus, based on the new scoring, not taking obesity and hypertension into account, 70 of the entire study population (21.9%) fulfilled the criteria for the mHR-OSA (Table 1).
Main Results
Primary outcome.
The distribution of 242 hospitalized patients falling into each category of the 7-category scale from admission to Day 28 is shown in Figure 2. The category changes were significant already on Day 7 (P = 0.001) and continued to be significantly different on Day 14 (P = 0.005). Clinical improvement (a decline of at least two categories) on Day 7 was observed among 47.5% of the mLR-OSA group compared with 34.4% of the cases with mHR-OSA (not significant). Corresponding rates on Day 14 were 88.4% among mLR-OSA compared with 75.4% of the cases with mHR-OSA (P = 0.014). On Day 21, 94.5% versus 91.8% of the cases were improved, respectively. Corresponding rates were 97.8% versus 96.7% on Day 28. In a univariate logistic regression model, male sex, hypertension, high-risk OSA, mHR-OSA, and the snoring patterns (loudness/frequency) were associated with delayed clinical improvement within 2 weeks, yet breathing pauses, morning/daytime sleepiness, age, BMI, obesity, diabetes, coronary artery disease, chronic obstructive pulmonary disease/asthma, and study sites were not (Table 2). In the adjusted logistic regression models, mHR-OSA (OR, 0.42; 95% CI, 0.19–0.92; P = 0.030) and male sex (OR, 0.39; 95% CI, 0.17–0.86; P = 0.020) remained significantly associated with the delayed clinical improvement within 2 weeks, adjusted for age, BMI, and hypertension (Figure 3A). Louder snoring (OR, 0.33; 95% CI, 0.15–0.75; P = 0.008) was also significant in the multivariate analysis, adjusted for age, BMI, sex, and hypertension (Figure 3B).
Figure 2.
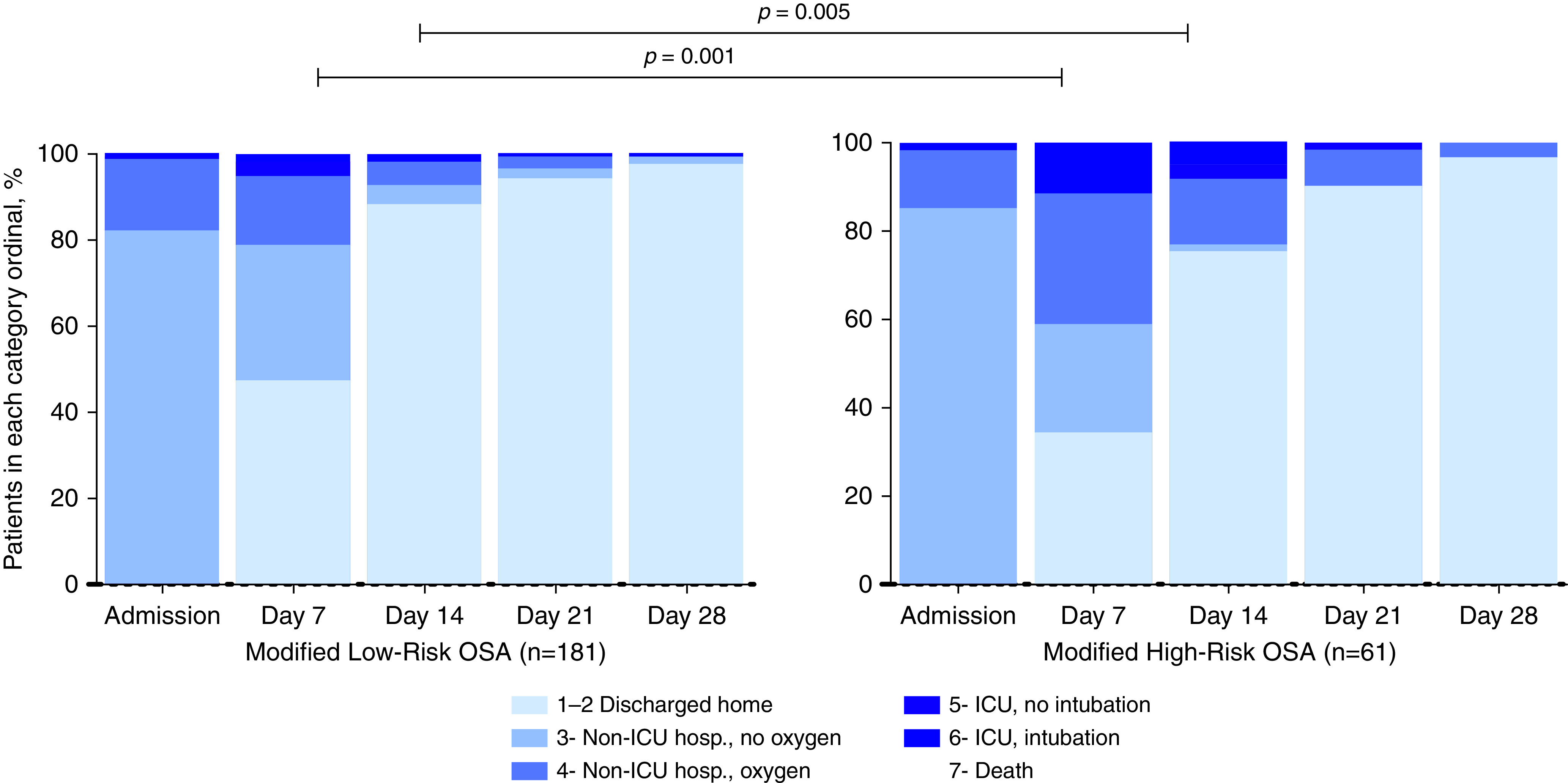
Distribution of proportion falling into each category of the 7-category scale from admission to Day 28 among 242 hospitalized patients with modified high-risk OSA and modified low-risk OSA. hosp. = hospitalization; ICU = intensive care unit; OSA = obstructive sleep apnea.
Table 2.
Unadjusted ORs (95% CIs) for variables associated with the clinical improvement within 2 weeks after hospitalization (n = 206 out of 242)
| Variables | OR | 95% CI | P Value |
|---|---|---|---|
| High-risk OSA | 0.41 | 0.20–0.85 | 0.017 |
| Modified high-risk OSA | 0.40 | 0.19–0.84 | 0.016 |
| Louder and/or frequent snoring | 0.40 | 0.20–0.82 | 0.013 |
| Louder snoring | 0.30 | 0.14–0.63 | 0.002 |
| Frequent snoring | 0.96 | 0.44–2.06 | 0.909 |
| Breathing pauses | 0.80 | 0.22–2.95 | 0.740 |
| Morning and/or daytime sleepiness | 0.60 | 0.29–1.22 | 0.155 |
| Age, y | 0.99 | 0.96–1.01 | 0.274 |
| Age ⩾65 yr | 0.81 | 0.37–1.80 | 0.607 |
| Male sex | 0.42 | 0.19–0.91 | 0.027 |
| BMI, kg/m2 | 1.00 | 0.94–1.07 | 0.937 |
| Obesity | 0.91 | 0.44–1.91 | 0.804 |
| Hypertension | 0.45 | 0.22–0.93 | 0.031 |
| Diabetes | 1.04 | 0.44–2.42 | 0.938 |
| Coronary artery disease | 1.32 | 0.37–4.65 | 0.670 |
| Asthma/COPD | 3.35 | 0.43–25.92 | 0.247 |
| Study sites: MUH vs. KHAH | 0.90 | 0.35–2.32 | 0.828 |
| Study sites: MUH vs. KUH | 0.67 | 0.29–1.54 | 0.344 |
Definition of abbreviations: BMI = body mass index; CI = confidence interval; COPD = chronic obstructive pulmonary disease; KHAH = Koc Healthcare American Hospital; KUH = Koc University Hospital; MUH = Marmara University Hospital; OR = odds ratio; OSA = obstructive sleep apnea.
Snoring parameters are from the Berlin Questionnaire. The bold typeface indicates significantly different (P < 0.05).
Figure 3.
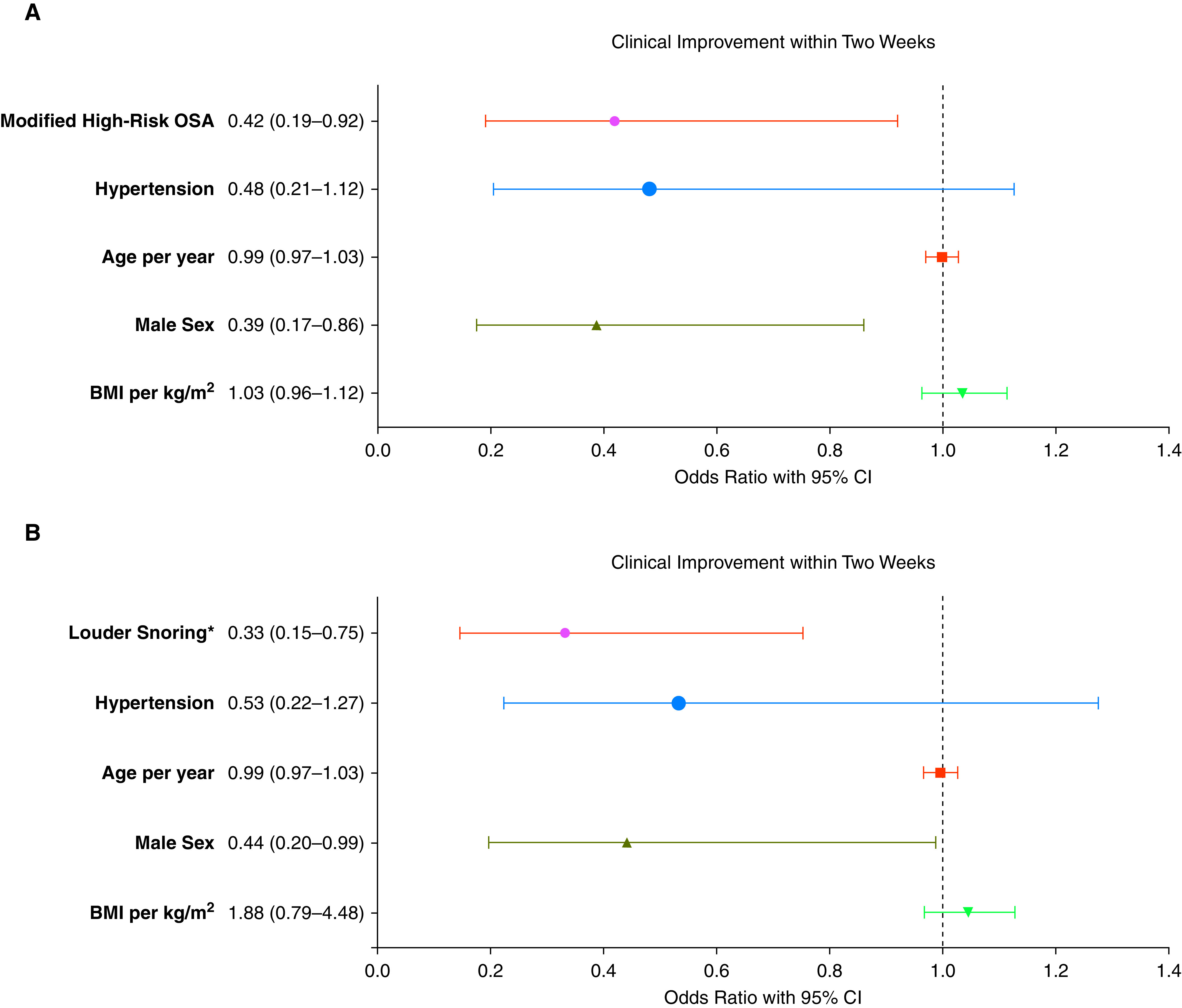
(A) Odds ratios with 95% CIs for the clinical improvement within 2 weeks for 242 hospitalized patients with coronavirus disease (COVID-19) in a multivariate logistic regression analysis (modified high-risk OSA adjusted for age, sex, BMI, and hypertension). (B) Odds ratios with 95% CIs for the clinical improvement within 2 weeks for 242 hospitalized patients with COVID-19 in a multivariate logistic regression analysis (louder snoring adjusted for age, sex, BMI, and hypertension). *Louder than talking/very loud (can be heard in adjacent rooms). BMI = body mass index; CI = confidence interval; OSA = obstructive sleep apnea.
Secondary outcomes.
As shown in Table 3, high-risk OSA (OR, 2.43), mHR-OSA (OR, 2.58), louder and/or frequent snoring (OR, 2.71), and louder snoring alone (OR, 2.63) were significantly associated with the need for hospitalization. Other significant variables were age (OR, 1.04), hypertension (OR, 3.94), and diabetes (OR, 7.35). The differences in ORs for the study sites were related to the patient recruitment from MUH, which was solely from the hospitalized patients. The need for supplemental oxygen was predicted by high-risk OSA, mHR-OSA, the snoring patterns, age, age ⩾65 yr, BMI, hypertension, and diabetes, respectively (Table 3). Because of the skewed distribution of hospitalized patients between the study sites, the patients requiring hospitalization at MUH had consequently higher ORs for the need for supplemental oxygen. The need for ICU ward was predicted by high-risk OSA (OR, 5.08), louder and/or frequent snoring (OR, 4.56), louder snoring alone (OR, 4.03), age (OR, 1.05), and hypertension (OR, 5.71). Other variables, including the study sites, were not associated with the need for an ICU ward (Table 3).
Table 3.
Unadjusted ORs (95% CIs) for variables associated with the secondary outcomes
| Variables | OR | 95% CI | P Value |
|---|---|---|---|
| Need for hospitalization in the entire study population (n = 242 out of 320) | |||
| High-risk OSA | 2.43 | 1.35–4.36 | 0.003 |
| Modified high-risk OSA | 2.58 | 1.22–5.49 | 0.013 |
| Louder and/or frequent snoring | 2.71 | 1.46–5.04 | 0.002 |
| Louder snoring | 2.63 | 1.20–5.81 | 0.016 |
| Frequent snoring | 1.64 | 0.89–3.04 | 0.114 |
| Breathing pauses | 2.87 | 0.65–12.7 | 0.165 |
| Morning and/or daytime sleepiness | 1.09 | 0.65–1.85 | 0.743 |
| Age, yr | 1.04 | 1.03–1.06 | <0.001 |
| Age ⩾65 yr | 3.87 | 1.60–9.36 | 0.003 |
| Male sex | 1.16 | 0.70–1.93 | 0.571 |
| BMI, kg/m2 | 1.03 | 0.98–1.08 | 0.291 |
| Obesity | 1.11 | 0.64–1.91 | 0.715 |
| Hypertension | 3.94 | 2.02–7.67 | <0.001 |
| Diabetes | 7.35 | 2.23–24.23 | 0.001 |
| Coronary artery disease | 0.35 | 0.10–1.18 | 0.091 |
| Asthma/COPD | 0.31 | 0.07–1.36 | 0.120 |
| Study sites: MUH vs. KHAH | 6.93 | 3.35–14.3 | <0.001 |
| Study sites: MUH vs. KUH | 50.1 | 6.78–370 | <0.001 |
| Need for supplemental oxygen in the entire study population (n = 104 out of 320) | |||
| High-risk OSA | 2.26 | 1.40–3.66 | 0.001 |
| Modified high-risk OSA | 2.62 | 1.52–4.52 | 0.001 |
| Louder and/or frequent snoring | 2.56 | 1.57–4.17 | <0.001 |
| Louder snoring | 2.56 | 1.46–4.48 | 0.001 |
| Frequent snoring | 1.79 | 1.07–2.97 | 0.026 |
| Breathing pauses | 1.55 | 0.61–3.99 | 0.360 |
| Morning and/or daytime sleepiness | 1.25 | 0.77–2.01 | 0.365 |
| Age, yr | 1.04 | 1.02–1.06 | <0.001 |
| Age ⩾65 yr | 1.78 | 1.02–3.12 | 0.043 |
| Male sex | 1.48 | 0.92–2.38 | 0.105 |
| BMI, kg/m2 | 1.09 | 1.04–1.14 | <0.001 |
| Obesity | 1.45 | 0.89–2.36 | 0.137 |
| Hypertension | 2.39 | 1.47–3.88 | <0.001 |
| Diabetes | 2.09 | 1.17–3.74 | 0.013 |
| Coronary artery disease | 1.39 | 0.62–3.07 | 0.424 |
| Asthma/COPD | 1.98 | 0.81–4.83 | 0.120 |
| Study sites: MUH vs. KHAH | 1.68 | 0.96–2.94 | 0.072 |
| Study sites: MUH vs. KUH | 4.08 | 2.21–7.54 | <0.001 |
| Need for ICU ward in the entire study population (n = 19 out of 320) | |||
| High-risk OSA | 5.08 | 1.78–14.5 | 0.002 |
| Modified high-risk OSA | 2.20 | 0.83–5.83 | 0.111 |
| Louder and/or frequent snoring | 4.56 | 1.68–12.4 | 0.003 |
| Louder snoring | 4.03 | 1.56–10.4 | 0.004 |
| Frequent snoring | 2.01 | 0.78–5.17 | 0.148 |
| Morning and/or daytime sleepiness | 1.45 | 0.57–3.69 | 0.429 |
| Age, yr | 1.05 | 1.02–1.08 | 0.004 |
| Age ⩾65 yr | 2.44 | 0.92–6.48 | 0.072 |
| Male sex | 2.50 | 0.88–7.12 | 0.086 |
| BMI, kg/m2 | 1.07 | 0.99–1.15 | 0.107 |
| Obesity | 2.31 | 0.91–5.85 | 0.080 |
| Hypertension | 5.71 | 2.00–16.3 | 0.001 |
| Diabetes | 2.21 | 0.80–6.08 | 0.125 |
| Coronary artery disease | 1.77 | 0.23–13.8 | 0.584 |
| Asthma/COPD | 1.28 | 0.16–10.1 | 0.814 |
| Study sites: MUH vs. KHAH | 1.83 | 0.64–5.20 | 0.260 |
| Study sites: MUH vs. KUH | 1.36 | 0.38–4.80 | 0.638 |
Definition of abbreviations: BMI = body mass index; CI = confidence interval; COPD = chronic obstructive pulmonary disease; ICU = intensive care unit; KHAH = Koc Healthcare American Hospital; KUH = Koc University Hospital; MUH = Marmara University Hospital; OR = odds ratio; OSA = obstructive sleep apnea.
Snoring parameters are from the Berlin Questionnaire. The bold typeface indicates significantly different (P < 0.05).
As shown in Table 4, mHR-OSA predicted the need for supplemental oxygen (OR, 1.95; 95% CI, 1.06–3.59) in the multivariate logistic regression analysis. Moreover, the snoring patterns (louder and/or frequent snoring) were significant predictors of need for hospitalization (adjusted OR, 2.14), supplemental oxygen (adjusted OR, 2.71), and ICU ward (adjusted OR, 3.05).
Table 4.
Adjusted ORs (95% CIs) for modified high-risk OSA and self-reported snoring parameters and secondary outcomes
| Variables | OR | 95% CI | P Value |
|---|---|---|---|
| Modified high-risk OSA | |||
| Need for hospitalization* | 2.06 | 0.45–5.03 | 0.112 |
| Supplemental oxygen* | 1.95 | 1.06–3.59 | 0.032 |
| ICU ward† | 1.53 | 0.54–4.31 | 0.419 |
| Louder and/or frequent snoring | |||
| Need for hospitalization* | 2.14 | 1.01–4.54 | 0.048 |
| Supplemental oxygen* | 2.71 | 1.46–5.04 | 0.002 |
| ICU ward† | 3.05 | 1.06–8.78 | 0.039 |
| Louder snoring | |||
| Need for hospitalization* | 1.74 | 0.68–4.47 | 0.248 |
| Supplemental oxygen* | 1.50 | 0.79–2.85 | 0.219 |
| ICU ward† | 2.54 | 0.91–7.10 | 0.074 |
| Frequent snoring | |||
| Need for hospitalization* | 1.29 | 0.60–2.78 | 0.511 |
| Supplemental oxygen* | 1.23 | 0.69–2.19 | 0.486 |
| ICU ward† | 1.42 | 0.53–3.84 | 0.487 |
Definition of abbreviations: CI = confidence interval; ICU = intensive care unit; OR = odds ratio; OSA = obstructive sleep apnea.
Snoring parameters are from the Berlin Questionnaire. The bold typeface indicates significantly different (P < 0.05)
Adjusted for age, sex, BMI, hypertension, diabetes and study center.
Adjusted for age, sex, BMI and hypertension.
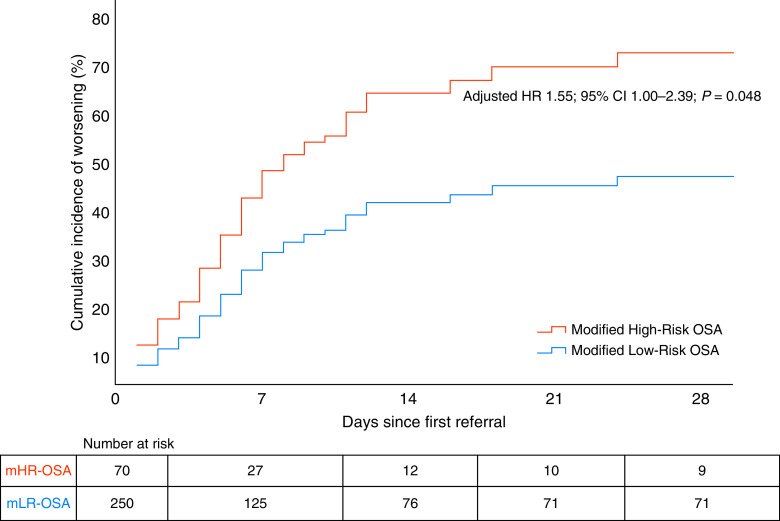
As illustrated in Figure 4, the cumulated incidence of clinical worsening over time was higher among the mHR-OSA cases in the entire study population (adjusted hazard ratio, 1.55; 95% CI, 1.00–2.39; P = 0.048). Similarly, there was an increased risk for clinical worsening among the cases with louder snoring versus silent snoring/no snoring (adjusted hazard ratio, 1.62; 95% CI, 1.04–2.52; P = 0.035).
Figure 4.
Cumulative incidence of clinical worsening in the entire study population of 320 cases with coronavirus disease (COVID-19) in a Cox proportional hazard model, adjusted for covariates. CI = confidence interval; HR = hazard ratio; mHR = modified high-risk; mLR = modified low-risk; OSA = obstructive sleep apnea.
Sensitivity analyses including only those with positive PCR test did not change the main findings of the study for the primary outcome as well as for the need for supplemental oxygen, whereas other differences in secondary outcomes did not reach significance in multivariate analyses, mainly because of the reduced sample size (see Tables E1–E3).
Discussion
The current study showed that 38% of adults with COVID-19 had a high-risk OSA based on the BQ, and 22% were categorized as having mHR-OSA when obesity and hypertension were not considered. The patients with mHR-OSA had an increased risk for delayed clinical improvement, clinical worsening, and need for supplemental oxygen compared with those with mLR-OSA, independent of age and the recognized risk factors of COVID-19 onset and prognosis. Moreover, snoring patterns (louder snoring and/or frequent snoring) were associated with delayed clinical improvement and clinical worsening as well as with the need for hospitalization, supplemental oxygen, and ICU ward.
To our best knowledge, the current multicenter study is the first prospective investigation addressing the impact of high-risk OSA on clinical outcomes. In one retrospective report, a known OSA diagnosis was observed among 12.3% of 463 cases (6) and among 9.5% of 4,668 patients in another retrospective study (13). In addition, medical records of 9,405 COVID-19 cases in the Chicago metropolitan area identified OSA among 15.3% of 3,185 patients compared with 3.4% of the nonhospitalized cases (12). After adjustment for diabetes, hypertension, and BMI, OSA was associated with an increased risk for hospitalization (OR, 1.65; 95% CI, 1.36–2.02) and respiratory failure (OR, 1.98; 95% CI, 1.65–2.37) (12). In a report by Cade and colleagues (13), patients with COVID-19 with a known OSA diagnosis had a higher overall mortality rate (11.7%) than the rate among the control subjects (6.9%) with an OR of 1.79 (95% CI, 1.31–2.45). In another study (7), addressing the influence of diabetes on the COVID-19 prognosis, concomitant OSA was associated with an increased risk (OR, 2.80; 95% CI, 1.46–5.38) for mortality among 1,317 cases.
To date, there is yet no information about a true prevalence of OSA among patients with COVID-19 infection given that objective sleep studies with polysomnography are not feasible at the time of COVID-19 onset. A known OSA diagnosis was prevalent only in three cases in our cohort, which highlights the fact that OSA is markedly underrecognized in the general population and clinical cohorts within the study hospitals’ recruitment area in Istanbul. One explanation might be the limited access to sleep laboratories in Turkey with long waiting lists owing to the payor reliance on polysomnography criteria for treatment coverage. As also emphasized in a recent editorial by Patel and Donovan (21) as well as in a systemic review by Miller and Cappuccio (22), the COVID-19 pandemic is now globally urging the need for new approaches beyond the polysomnography requirement for the management of OSA cases.
The main limitation of the study is the questionnaire-based evaluation of the high-risk OSA diagnosis, as objective tests are not accurate because of the aforementioned reasons during the pandemic. A recent review (8) has demonstrated that the average sensitivity value of the BQ is 77% (95% CI, 73–81%), whereas the specificity is 44% (95% CI, 38–51%). Hence, our results should be interpreted cautiously given that the BQ has not been validated in a cohort with an acute respiratory infection. Previously, a BQ-based Turkish population study estimated the OSA prevalence around 14% among 5,021 adults (16). Our results estimate the OSA occurrence to be much higher (38% of 320) among the clinical cases with COVID-19. The other limitation of the BQ is the items of Category 3 (obesity and hypertension), which complicate the statistical adjustments in multivariate analyses addressing the prognosis of patients with COVID-19. Therefore, a modified scoring based on the snoring patterns (louder and/or frequent snoring), breathing pauses, and morning and/or daytime tiredness/sleepiness was used in the current study. The prevalence of mHR-OSA was estimated to be around 22% when obesity and hypertension were not considered, and the increased risk for mHR-OSA remained significant in the multivariate models. The significant predictive effects of the snoring patterns, especially louder snoring, are indeed interesting. As also discussed in a recent paper from the SAVE (Sleep Apnea cardioVascular Endpoints) cohort (23), the self-reported snoring patterns predict stroke incidence in high-risk patients with OSA. The authors also highlighted the controversy over whether snoring is a symptom or a surrogate marker of OSA. Furthermore, it was debated whether the adverse cardiovascular effects are driven by obstructive events and hypoxemia or from the trauma of vibrations owing to snoring per se (23). In line with these approaches, our findings provide further insights into the relationship between OSA and COVID-19 infection. The individuals with louder snoring might be more prone to be infected in COVID-19 and develop pneumonia with a poorer prognosis because of the trauma around the upper airway muscles caused by vibrations. It may also be argued that the COVID-19 infection per se may increase the collapsibility of the upper airway muscles, which may trigger or worsen OSA. Thus, the association between OSA and COVID-19 onset as well as prognosis might be bidirectional.
In our study sample, 60.6% were PCR positive, whereas 94.4% had radiological findings suggestive for COVID-19–pneumonia. As stated in a recent focused review (24), the sensitivity of the PCR testing ranges from 38% to 88%, with a specificity of almost 100% for detection of COVID-19 (25–28). It has been suggested that a single negative result must be interpreted with caution, and a repeat test may be needed to increase the diagnostic accuracy (29). Even two negative PCR results were reported by the same researchers among 21% of the patients that were diagnosed with active COVID-19 infection (29). Computed tomography has been widely used as an important complement to PCR for diagnosing pneumonia in the pandemic context (15, 30). Hence, the PCR positivity rate in our study sample is comparable with the studies reported in the recent review (24) and in line with the suggested diagnostic guidelines for COVID-19 infection (15). As shown in the online supplement, sensitivity analyses including only those with positive PCR test did not change the main findings of the study for the primary outcome as well as for the need for supplemental oxygen, whereas other differences in secondary outcomes did not reach significance in multivariate analyses, mainly because of the reduced sample size.
We should also acknowledge that our results are attributable to mild to moderate COVID-19 cases and not generalizable to patients with severe COVID-19 given that the study included both nonhospitalized and hospitalized cases and the ones volunteering to answer the BQ. The cases who did not want to participate were mainly older and sicker than the ones who volunteered. This may partly explain the higher prevalence estimates of OSA in our cohort, and a self-selection bias can therefore not be excluded. Nevertheless, the significant impact of the mHR-OSA on short-term outcomes is not volunteer driven, and the comorbidity and outcome data were collected by researchers blinded to the answers of the BQ.
Our results are in line with the previous reports demonstrating age, male sex, hypertension, and diabetes as risk factors for prevalent COVID-19 and worse outcomes. The mean age of our study population was relatively lower than the published studies from other countries, which may reflect the general lockdown for adults older than 65 years and other preventive travel restrictions during the first months of the pandemic (20). The same reasons might also explain the relatively low number of patients with concomitant chronic cardiac and pulmonary diseases at baseline in the current population.
Several potential mechanisms linking OSA to increased risk for predisposition to COVID-19 infection and poor outcomes have been discussed recently (10, 11, 22). OSA, particularly with concomitant obesity, could potentially worsen hypoxemia and the cytokine storm that occurs in patients with COVID-19 (10). Other supposed mechanisms include myocardial injury involving the angiotensin-converting enzyme-2 signaling pathways, systemic inflammation, and hypercoagulability (11). OSA could be a trigger of COVID-19 infection, and once the disease occurred, it could contribute to worsening of prognosis, especially among the cases with hypertension and diabetes. Moreover, many patients with COVID-19 suffer pulmonary fibrosis, which itself is associated with future development of OSA (22).
Conclusions
Our results indicate that patients with COVID-19 with mHR-OSA are at increased risk for delayed clinical improvement, clinical worsening, and need for supplemental oxygen compared with those with mLR-OSA. As previously mentioned (21, 22), the COVID-19 pandemic is now globally urging the need for new approaches beyond the polysomnography requirement for the management of OSA cases. The further follow up of the current sample with clinical, laboratory, and radiological investigations in addition to objective sleep recordings would provide further insights into the clinical usefulness of the modified BQ as a screening tool during the COVID-19 onset and into the association between OSA and long-term COVID-19 outcomes.
Acknowledgments
Acknowledgment
The authors thank the use of the services and facilities of the Koç University Research Center for Translational Medicine, funded by the Presidency of Turkey, Presidency of Strategy and Budget.
Footnotes
A complete list of the OSACOVID-19 Study Collaborators may be found before the beginning of the References.
Data collected for the study, including deidentified individual participant data, will be made available to others within 6 months after the publication of this article, as will additional related documents (study protocol, statistical analysis plan, and informed consent form), for academic purposes (e.g., meta-analyses), on request to the corresponding author (yuksel.peker@lungall.gu.se) and with a signed data access agreement.
Author Contributions: Y.P. designed the study. Y.C., S.A., and D.Y. assessed the Berlin questionnaire and data entry. S.R.I., B.B., F.K., F.I.U., L.T., B.Ç., A.B.Ö., E.A., S.İ., C.A., M.K., H.B., B.D.Ç., and B.Ç. performed the patient recruitment and clinical follow-up. All collaborators contributed to the clinical management of the patients with COVID-19. Y.P., Y.C., and A.B. performed the statistical analysis. Y.P. prepared the manuscript and drafted the article. All authors interpreted the data. Y.P. takes full responsibility for the work as a whole, including the study design, access to data, and the decision to submit and publish the manuscript. All authors approved this manuscript in its final form.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Author disclosures are available with the text of this article at www.atsjournals.org.
Contributor Information
Collaborators: the OSACOVID-19 Study Collaborators, Önder Ergönül, Şiran Keske, Suda Tekin, Pelin İrkören, Mehmet Karaca, Bilgin Sait, Nahit Çakar, Evren Şentürk, Gülay Kır, Semra Ugur, Ayla Esin, Fatma Yurdakul, Boğaç Özserezli, İpek Erus, Zeynep Atçeken, Saide Aytekin, Gökhan Erdoğan, Nur Konyalilar, and Özgecan Kayalar
References
- 1.World Health Organization. Coronavirus disease (COVID-19 pandemic). WHO; 2020 [accessed 2021 Feb 2]. Available from: https://www.who.int/emergencies/diseases/novel-coronavirus-2019
- 2.Ministry of Health, Republic of Turkey (MoH-TR) https://covid19.saglik.gov.tr
- 3. Wiersinga WJ, Rhodes A, Cheng AC, Peacock SJ, Prescott HC. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. JAMA. 2020;324:782–793. doi: 10.1001/jama.2020.12839. [DOI] [PubMed] [Google Scholar]
- 4. Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Grasselli G, Zangrillo A, Zanella A, Antonelli M, Cabrini L, Castelli A, et al. COVID-19 Lombardy ICU Network. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy region, Italy. JAMA. 2020;323:1574–1581. doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Suleyman G, Fadel RA, Malette KM, Hammond C, Abdulla H, Entz A, et al. Clinical characteristics and morbidity associated with coronavirus disease 2019 in a series of patients in metropolitan Detroit. JAMA Netw Open. 2020;3:e2012270. doi: 10.1001/jamanetworkopen.2020.12270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cariou B, Hadjadj S, Wargny M, Pichelin M, Al-Salameh A, Allix I, et al. CORONADO investigators. Phenotypic characteristics and prognosis of inpatients with COVID-19 and diabetes: the CORONADO study. Diabetologia. 2020;63:1500–1515. doi: 10.1007/s00125-020-05180-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gottlieb DJ, Punjabi NM. Diagnosis and management of obstructive sleep apnea: a review. JAMA. 2020;323:1389–1400. doi: 10.1001/jama.2020.3514. [DOI] [PubMed] [Google Scholar]
- 9. Javaheri S, Barbe F, Campos-Rodriguez F, Dempsey JA, Khayat R, Javaheri S, et al. Sleep apnea: types, mechanisms, and clinical cardiovascular consequences. J Am Coll Cardiol. 2017;69:841–858. doi: 10.1016/j.jacc.2016.11.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. McSharry D, Malhotra A. Potential influences of obstructive sleep apnea and obesity on COVID-19 severity. J Clin Sleep Med. 2020;16:1645. doi: 10.5664/jcsm.8538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tufik S, Gozal D, Ishikura IA, Pires GN, Andersen ML. Does obstructive sleep apnea lead to increased risk of COVID-19 infection and severity? J Clin Sleep Med. 2020;16:1425–1426. doi: 10.5664/jcsm.8596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Maas MB, Kim M, Malkani RG, Abbott SM, Zee PC. Obstructive sleep apnea and risk of COVID-19 infection, hospitalization and respiratory failure. Sleep Breath. doi: 10.1007/s11325-020-02271-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cade BE, Dashti HS, Hassan SM, Redline S, Karlson EW. Sleep apnea and COVID-19 mortality and hospitalization. Am J Respir Crit Care Med. 2020;202:1462–1464. doi: 10.1164/rccm.202006-2252LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Netzer NC, Stoohs RA, Netzer CM, Clark K, Strohl KP. Using the Berlin Questionnaire to identify patients at risk for the sleep apnea syndrome. Ann Intern Med. 1999;131:485–491. doi: 10.7326/0003-4819-131-7-199910050-00002. [DOI] [PubMed] [Google Scholar]
- 15. Hani C, Trieu NH, Saab I, Dangeard S, Bennani S, Chassagnon G, et al. COVID-19 pneumonia: a review of typical CT findings and differential diagnosis. Diagn Interv Imaging. 2020;101:263–268. doi: 10.1016/j.diii.2020.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Demir A, Ardic S, Firat H, Karadeniz D, Aksu M, Ucar Z, et al. Prevalence of sleep disorders in the Turkish adult population epidemiology of sleep study. Sleep Biol Rhythms. 2015;13:298–308. [Google Scholar]
- 17. van Oosten EM, Hamilton A, Petsikas D, Payne D, Redfearn DP, Zhang S, et al. Effect of preoperative obstructive sleep apnea on the frequency of atrial fibrillation after coronary artery bypass grafting. Am J Cardiol. 2014;113:919–923. doi: 10.1016/j.amjcard.2013.11.047. [DOI] [PubMed] [Google Scholar]
- 18. Obesity: preventing and managing the global epidemic: report of a WHO consultation. World Health Organ Tech Rep Ser. 2000;894:i–xii, 1–253. [PubMed] [Google Scholar]
- 19. Wang Y, Fan G, Horby P, Hayden F, Li Q, Wu Q, et al. CAP-China Network. Comparative outcomes of adults hospitalized with seasonal influenza A or B virus infection: application of the 7-category ordinal scale. Open Forum Infect Dis. 2019;6:ofz053. doi: 10.1093/ofid/ofz053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Demirbilek Y, Pehlivantürk G, Özgüler ZÖ, Alp Meşe E. COVID-19 outbreak control, example of ministry of health of Turkey. Turk J Med Sci. 2020;50:489–494. doi: 10.3906/sag-2004-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Patel SR, Donovan LM. The COVID-19 pandemic presents an opportunity to reassess the value of polysomnography. Am J Respir Crit Care Med. 2020;202:309–310. doi: 10.1164/rccm.202005-1546ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Miller MA, Cappuccio FP. A systematic review of COVID-19 and obstructive sleep apnoea. Sleep Med Rev. 2021;55:101382. doi: 10.1016/j.smrv.2020.101382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Li J, McEvoy RD, Zheng D, Loffler KA, Wang X, Redline S, et al. Self-reported snoring patterns predict stroke events in high-risk patients with OSA: post hoc analyses of the SAVE study. Chest. 2020;158:2146–2154. doi: 10.1016/j.chest.2020.05.615. [DOI] [PubMed] [Google Scholar]
- 24. Roshkovan L, Chatterjee N, Galperin-Aizenberg M, Gupta N, Shah R, Barbosa EM, Jr, et al. The role of imaging in the management of suspected or known COVID-19 pneumonia: a multidisciplinary perspective. Ann Am Thorac Soc. 2020;17:1358–1365. doi: 10.1513/AnnalsATS.202006-600FR. [DOI] [PubMed] [Google Scholar]
- 25. Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. China Medical Treatment Expert Group for Covid-19. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382: 1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Liu R, Han H, Liu F, Lv Z, Wu K, Liu Y, et al. Positive rate of RT-PCR detection of SARS-CoV-2 infection in 4880 cases from one hospital in Wuhan, China, from Jan to Feb 2020. Clin Chim Acta. 2020;505: 172–175. doi: 10.1016/j.cca.2020.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bernheim A, Mei X, Huang M, Yang Y, Fayad ZA, Zhang N, et al. Chest CT findings in coronavirus disease-19 (COVID-19): relationship to duration of infection. Radiology. 2020;295:200463. doi: 10.1148/radiol.2020200463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Watson J, Whiting PF, Brush JE. Interpreting a covid-19 test result. BMJ. 2020;369:m1808. doi: 10.1136/bmj.m1808. [DOI] [PubMed] [Google Scholar]
- 29. Xiao AT, Tong YX, Zhang S. False negative of RT-PCR and prolonged nucleic acid conversion in COVID-19: rather than recurrence. J Med Virol. 2020;92:1755–1756. doi: 10.1002/jmv.25855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fang Y, Zhang H, Xie J, Lin M, Ying L, Pang P, et al. Sensitivity of chest CT for COVID-19: comparison to RT-PCR. Radiology. 2020;296:E115–E117. doi: 10.1148/radiol.2020200432. [DOI] [PMC free article] [PubMed] [Google Scholar]