Abstract
Rationale: Pneumonia due to Pseudomonas aeruginosa (PA) is associated with high mortality and requires antipseudomonal treatment. Because PA can colonize the respiratory tract, the diagnosis of pathogenic PA involvement is challenging.
Objectives: To determine the prevalence of definitive and indeterminate PA infection in community-acquired pneumonia, to describe the clinical and microbiological profiles, and to estimate the burden of unnecessary antipseudomonal drug prescriptions.
Methods: We prospectively enrolled 2,701 patients with community-acquired pneumonia. Using stringent criteria for diagnosing PA pneumonia, we generated the following three groups: 1) definitive PA, 2) indeterminate PA, and 3) non-PA pneumonia.
Results: The prevalence of definitive PA pneumonia was 0.9% (n = 25), and that of indeterminate PA pneumonia was 4.9% (n = 131). Considerable clinical differences were observed among the groups. Patients with definitive PA pneumonia were more likely to have a history of tuberculosis and chronic obstructive pulmonary disease/bronchiectasis and had a higher 30-day mortality (28%) than patients with non-PA pneumonia. Patients with indeterminate PA pneumonia were more likely to have comorbidities than patients with non-PA pneumonia. More than half of the patients with indeterminate PA and 25% of the patients with non-PA pneumonia were treated with an antipseudomonal drug. No patients with definitive PA pneumonia had multidrug resistance.
Conclusions: In this population, the prevalence of community-acquired pneumonia due to PA was low. The clinical features and 30-day mortality rates of patients with indeterminate PA pneumonia were different from those of patients with definitive PA pneumonia. Most of the prescribed antipseudomonal drugs for patients with community-acquired pneumonia were potentially unnecessary.
Keywords: adult pneumonia, Pseudomonas aeruginosa, community-acquired pneumonia
Pseudomonas aeruginosa (PA), an aerobic and gram-negative rod-shaped bacterium, is ubiquitous and one of the most common pathogens isolated from patients with hospital-acquired pneumonia (1, 2). Although community-acquired pneumonia (CAP) due to PA is rare, PA is an independent risk factor associated with high 30-day mortality (3–5). Pneumonia due to PA (definitive PA pneumonia) is associated with prolonged hospitalization in the intensive care unit and extra costs (2, 6).
Antipseudomonal drugs are required for the initial treatment of PA pneumonia (2, 7). Because there is no consensus on the diagnostic criteria for PA pneumonia, the individual risk factors for PA vary across studies (8). The 2019 American Thoracic Society (ATS)/Infectious Diseases Society of America (IDSA) guideline recommends initiating antipseudomonal drugs after considering the local epidemiology and validated risk factors for culture-positive PA pneumonia, such as prior infection, recent hospitalization, and the use of parenteral antibiotics in the last 90 days (8). However, von Baum and colleagues (9) reported that approximately 1% of patients with CAP were culture positive for PA, of whom only 45% were regarded as having definitive PA pneumonia in Germany. Because of the small sample size, they did not conclusively determine which factors can differentiate definitive PA pneumonia from other cases of culture-positive PA pneumonia (indeterminate PA pneumonia). In Japan, PA was isolated from 3% to 6% of patients with CAP, but there are no data on the proportion of definitive PA pneumonia in this group (10, 11). Thus, the current practices regarding pneumonia treatment may lead to the overdiagnosis of PA pneumonia, resulting in overtreatment with antipseudomonal drugs (2). To avoid the unnecessary use of antipseudomonal drugs for nondefinitive PA pneumonia (i.e., indeterminate PA pneumonia and culture-negative PA pneumonia), clarification is needed regarding the clinical features of both groups.
Herein, we conducted this study to 1) determine the prevalence of definitive and indeterminate PA pneumonia among patients with CAP, 2) describe the clinical and microbiological profiles of definitive PA and indeterminate PA pneumonia, and 3) estimate the burden of unnecessary antipseudomonal drug prescriptions and describe the drug susceptibility of PA in Japan.
Methods
Study Design, Participants, and Data Collection
The current study was performed as part of the Adult Pneumonia Study Group-Japan (APSG-J), a multicenter prospective hospital-based surveillance for CAP in Japan. The APSG-J study enrolled 3,509 patients with pneumonia at four community-based acute hospitals in Japan from 28 September 2011 to 23 August 2014. For the current study, we included patients with pneumonia who had been enrolled in two community-based referral hospitals (Kameda Medical Center and Chikamori Hospital). A detailed description of the APSG-J methodology has been provided elsewhere (12). Briefly, all inpatients and outpatients were screened by hospital physicians, and eligible patients were identified using the following standardized case definition: patients aged ≥15 years with respiratory symptoms compatible with pneumonia (i.e., fever, chills or rigors, cough, sputum, pleuritic chest pain, dyspnea, and/or tachypnea) and new pulmonary infiltrates on chest X-ray or computed tomographic imaging. If a patient developed the disease more than 48 hours after hospitalization, the patient was classified as having hospital-acquired pneumonia and was not enrolled. Demographic and clinical information was collected through direct interviews with patients or their guardians and from reviews of medical charts using standardized data collection forms. Using the medical records, we defined oral steroid or immunosuppressant use as present regardless of dosage and defined chronic obstructive pulmonary disease (COPD) or bronchiectasis as present regardless of severity. For this study, further drug susceptibility in patients with pneumonia with PA was also collected at these study hospitals. Although antibiotic selection should be consistent with the guidelines, physicians can unrestrictedly prescribe antipseudomonal drugs in Japan.
Sample Processing and Definitions
All clinical specimens (i.e., respiratory specimens and blood cultures) were immediately transported to the microbiology laboratory at each hospital. Gram staining and culture were performed for all available respiratory samples, which were examined by semiquantitative or quantitative culture methods. PA isolates were considered definite causative pathogens when the following criteria were fulfilled: 1) PA was isolated from blood cultures and/or 2) a good-quality sputum sample (>25 polymorphonuclear cells and <10 epithelial cells per low-power field [total magnification, ×100]) containing gram-negative rods likely corresponding with PA with predominant growth in a culture of ≥1 × 106 cfu/ml (9) or a score of 3+ in a semiquantitative evaluation. Indeterminate PA was defined when PA was isolated from the sputum, but the criteria for definitive PA were not fulfilled. If PA was not isolated, the case was characterized as non-PA pneumonia. Drug susceptibility testing was performed according to the local protocol for minimum inhibitory concentration breakpoints and Clinical and Laboratory Standards Institute guidelines to determine antibiotic resistance. Antibiotic resistance to antipseudomonal drugs was evaluated (see Table E1 in the online supplement), and the resistance of PA was defined as follows: 1) if the isolate was resistant to at least one of the evaluated antibiotics, the PA was considered to be nonsusceptible and defined as drug resistant or 2) if the isolate was not susceptible to one or more antibiotic in three or more antipseudomonal categories, the PA was defined as multidrug resistant (MDR) (13). We defined overuse of antipseudomonal drugs if the physician did not de-escalate the antipseudomonal drug adequately referring to the sputum culture and Gram stain results.
Statistical Analysis
Patients were categorized into three groups according to PA culture positivity status (i.e., definitive PA, indeterminate PA, and non-PA). Multinomial logistic regression models were used to explore the factors associated with the three groups. For comparisons of definitive PA and non-PA pneumonia and of indeterminate PA and non-PA pneumonia, non-PA pneumonia was used as a baseline group. All samples were included in each model, and unadjusted and adjusted odds ratios and 95% confidence intervals were calculated. On the basis of clinical experience, we adjusted for age, sex, nursing home residency, COPD, past history of pneumonia, and CURB-65 scores in each model. CURB-65 scores (Confusion, Urea >7 mmol/l, Respiratory rate ≥30/min, low systolic (<90 mm Hg) or diastolic (≤60 mm Hg)Blood pressure, age ≥65 years) was calculated and one point given for each feature present. The treatment duration was compared using the Mann-Whitney U test. We accounted for missing data for covariates by using multiple imputations in all regression analyses (14). A P value of less than 0.05 was defined as statistically significant. All analyses were performed with Stata, version 14.0 (STATA Corp).
Ethics
This study was approved by the institutional review boards of the Institute of Tropical Medicine, Nagasaki University (number 11063070), Kameda Medical Center (number 11–025), and Chikamori Hospital (number 85). Written informed consent was obtained from the majority of the participants or their guardians. The requirement for obtaining written consent from all participants was waived by all institutional review boards because of the study’s observational nature, with no deviation from current medical practice. We used anonymized data for the analysis.
Results
Patient Characteristics
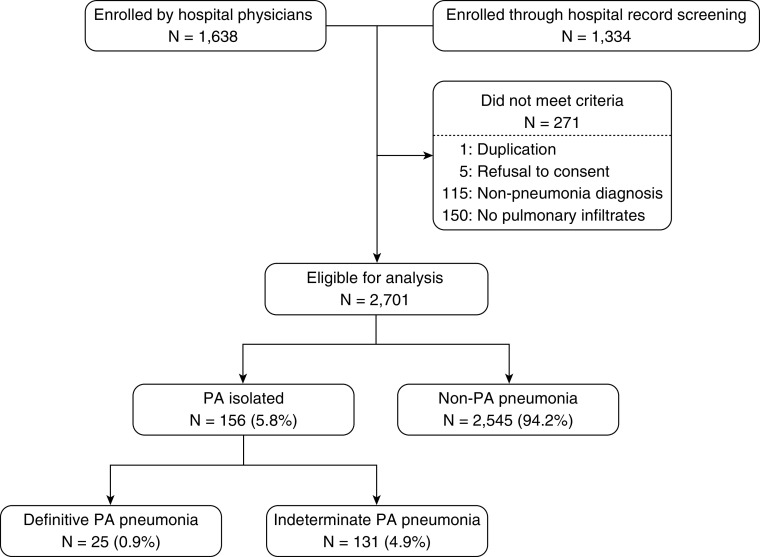
A total of 2,972 patients with CAP visited the two study hospitals. Of these, 271 patients were excluded for the following reasons: duplication (n = 1), refusal to participate in the study (n = 5), nonpneumonia diagnosis (n = 115), and the absence of pulmonary infiltrates (n = 150). After exclusion, 2,701 patients were eligible for our analysis (Figure 1).
Figure 1.
Study flow diagram. PA = Pseudomonas aeruginosa.
Among the 2,701 patients with pneumonia, PA was isolated from 156 patients; definitive and indeterminate PA pneumonia were diagnosed in 25 (0.9%) and 131 (4.9%) patients, respectively. Of the definitive PA strains, three were isolated from blood culture. The baseline clinical characteristics of the three groups, definitive PA, indeterminate PA, and non-PA pneumonia (n = 2,545), are summarized in Table 1. Approximately 40% of the participants were female. The median age was 76 years (interquartile range, 64–84 yr), and 72% of the patients were older than 65 years. A past history of tuberculosis was identified in 16% of the patients with definitive PA pneumonia but in only 3% and 4% of the patients with indeterminate PA and non-PA pneumonia, respectively. Chronic lung injury such as COPD/bronchiectasis was observed in 56% of patients with definitive PA pneumonia and in 31% of patients with indeterminate PA pneumonia, although it was observed in only 20% of patients with non-PA pneumonia.
Table 1.
Clinical characteristics of patients with definitive PA, indeterminate PA, and non-PA pneumonia
| Variable | Culture Positive for PA |
Culture Negative for PA Non-PA Pneumonia (n = 2,545) |
|
|---|---|---|---|
| Definitive PA (n = 25) | Indeterminate PA (n = 131) | ||
| Sociodemographic characteristics | |||
| Age >65 yr | 20 (80) | 100 (76) | 1,836 (72) |
| Sex, M | 15 (60) | 87 (66) | 1,522 (60) |
| Nursing home resident | 3 (12) | 32 (24) | 334 (13) |
| Comorbidities | |||
| Hypertension | 10 (40) | 38 (29) | 1,070 (42) |
| Diabetes | 6 (24) | 32 (24) | 539 (21) |
| Collagen vascular disease | 5 (20) | 19 (15) | 173 (7) |
| Neuromuscular disease | 2 (8) | 24 (18) | 165 (6) |
| Past history of tuberculosis | 4 (16) | 4 (3) | 112 (4) |
| COPD/bronchiectasis | 14 (56) | 40 (31) | 500 (20) |
| Body mass index, 0–18.49 | 7 (28) | 48 (37) | 563 (22) |
| Performance status ≥2 | 4 (16) | 57 (44) | 419 (16) |
| Home oxygen therapy | 6 (24) | 24 (18) | 114 (4) |
| Medications before hospitalization | |||
| Chronic oral steroids | 3 (12) | 15 (11) | 151 (6) |
| Inhaled corticosteroids | 1 (4) | 6 (5) | 199 (8) |
| Immunosuppressants | 0 (0) | 4 (3) | 37 (1) |
| Prior hospitalization* | 6 (24) | 44 (36) | 402 (16) |
| History of pneumonia | 9 (36) | 56 (43) | 443 (17) |
| Clinical presentation | |||
| Aspiration episode | 2 (8) | 32 (24) | 423 (17) |
| Temperature, ≤34.9°C or ≥40°C | 1 (4) | 9 (7) | 46 (2) |
| Albumin, <3.5 g/dl | 13 (52) | 82 (63) | 1,159 (46) |
| Chest radiograph findings | |||
| X-ray, bilateral infiltration | 5 (20) | 26 (20) | 385 (15) |
| Pleural effusion | 1 (4) | 6 (5) | 127 (5) |
| CURB-65, ≥3 | 5 (20) | 41 (31) | 573 (23) |
Definition of abbreviations: COPD = chronic obstructive pulmonary disease; PA = Pseudomonas aeruginosa.
Data are presented as n (%). CURB-65 scores (Confusion, Urea >7 mmol/l, Respiratory rate ≥30/min, low systolic (<90 mm Hg) or diastolic (≤60 mm Hg) Blood pressure, age ≥65 years) was calculated and one point given for each feature present.
Hospitalization ≥2 days in the last 90 days.
The factors associated with the PA categories were examined by adjusted multinomial regression (Table E2). The patients with definitive PA pneumonia were more likely to have a history of tuberculosis and COPD/bronchiectasis than the patients with non-PA pneumonia. The patients with indeterminate PA and non-PA pneumonia showed considerable clinical differences. Although the proportion of older patients was similar, the patients with indeterminate PA pneumonia were more likely than patients with non-PA pneumonia to be nursing home residents; to receive on oral steroids; to have neuromuscular disease, a low body mass index, a prior hospitalization in the last 90 days, a history of pneumonia, vital sign abnormality (i.e., body temperature ≤34.9 °C or ≥40°C), hypoalbuminemia; and to require help with activities of daily living. In contrast, patients with indeterminate PA pneumonia were less likely than patients with non-PA pneumonia to have hypertension.
Treatment, Prognosis, and Microbiological Findings
The treatment and prognosis of each group are shown in Table 2 (unadjusted model is shown in Table E3). Patients with indeterminate PA pneumonia were more likely to be hospitalized than patients with non-PA pneumonia. Higher proportions of patients with definitive PA (80%) and indeterminate PA (58%) pneumonia were initially treated with an antipseudomonal drug than those with non-PA pneumonia (25%) (see Table E4). Among the patients with indeterminate PA pneumonia, 76 (58%) were treated with antipseudomonal drugs, and 13 who were treated with nonantipseudomonal drugs were regarded by physicians as failing to respond to treatment. The median treatment duration was longer for patients with definitive PA pneumonia (median, 10 d; interquartile range, 6–18 d) and indeterminate PA pneumonia (median, 13 d; interquartile range, 7–14 d) than for those with non-PA pneumonia (median, 7 d; interquartile range, 6–11 d) (P = 0.046 and P < 0.001, respectively). The use of two antipseudomonal antibiotics for PA pneumonia was quite rare in both definitive PA (8%) and indeterminate PA (2%) pneumonia cases in our study. Patients with definitive PA pneumonia were more likely to need ventilator support and vasopressor administration than patients with non-PA pneumonia. In addition to PA, other pathogens were isolated in definitive PA (8%) and indeterminate PA (36%) pneumonia cases (see Table E5). Of the latter group, the most frequently isolated pathogen other than PA was Streptococcus pneumoniae (7%), followed by Haemophilus influenzae (7%), Moraxella catarrhalis (5%), and methicillin-susceptible Staphylococcus aureus (5%).
Table 2.
The treatments and prognoses of patients with definitive PA, indeterminate PA, and non-PA pneumonia
| Variable | Definitive PA Pneumonia aOR (95% CI) | Indeterminate PA Pneumonia aOR (95% CI) |
|---|---|---|
| Hospitalization | 1.09 (0.43–2.73) | 2.36 (1.40–3.97) |
| Treatment | ||
| Antipseudomonal drug | 12.22 (4.45–33.56) | 3.53 (2.37–5.25) |
| Two antipseudomonal drugs | 19.87 (3.64–108.53) | 2.26 (0.47–10.88) |
| Respirator | 5.40 (1.96–14.84) | 1.35 (0.67–2.72) |
| Vasopressor | 5.11 (1.52–15.34) | 0.83 (0.30–2.29) |
| Treatment duration >10 d | 2.65 (1.19–5.89) | 3.17 (2.19–4.60) |
| Treatment failure | 2.66 (0.89–7.96) | 3.34 (2.07–5.39) |
| Mortality | ||
| 14-d mortality | 13.04 (4.42–38.51) | 1.35 (0.59–3.11) |
| 30-d mortality | 9.89 (3.67–26.66) | 1.78 (0.94–3.36) |
Definition of abbreviations: aOR = adjusted odds ratio; CI = confidence interval; PA = Pseudomonas aeruginosa.
Multinomial logistic regression models were used to explore the clinical factors associated with the three groups (definitive PA, indeterminate PA, and non-PA pneumonia). For comparisons between definitive PA and non-PA pneumonia and between indeterminate PA and non-PA pneumonia, non-PA pneumonia was used as a baseline group. The model was adjusted for age, sex, nursing home residency, chronic obstructive pulmonary disease, past history of pneumonia, and CURB-65 scores (Confusion, Urea >7 mmol/l, Respiratory rate ≥30/min, low systolic (<90 mm Hg) or diastolic (≤60 mm Hg) Blood pressure, age ≥65 years). CURB-65 scores was calculated and one point given for each feature present.
In the acute phase, the case fatality rates of patients with definitive PA pneumonia at 14 days and 30 days (24% and 28%, respectively) were significantly higher than those of patients with non-PA pneumonia (4% and 5%, respectively), although the rates of patients with indeterminate PA pneumonia did not differ from those of patients with non-PA pneumonia.
Overall, antipseudomonal drugs were prescribed to 741 of the 2,701 (27.4%) patients with pneumonia. Among these 741 patients, 20 (2.7%) had definitive PA pneumonia, and 721 (97.3%) were treated with potentially unnecessary antipseudomonal drugs.
Drug Susceptibility
The drug susceptibility of the PA isolates is shown in Table 3. Four patients with definitive PA and eight patients with indeterminate PA had drug resistance. Of these, no patients with definitive PA pneumonia and four patients with indeterminate PA pneumonia had MDR PA; no isolates from patients with definitive PA pneumonia and only one isolate from a patient with indeterminate PA pneumonia showed resistance to carbapenems. Most isolates from patients with definitive PA pneumonia (80%) and indeterminate PA pneumonia (89%) were pansusceptible to all antipseudomonal antibiotics.
Table 3.
Drug susceptibility of PA
| Variable | Definitive PA (n = 25) | Indeterminate PA (n = 131) |
|---|---|---|
| Pansusceptible | 20 (80) | 116 (89) |
| Drug-resistant PA | 4 (16) | 8 (6) |
| MDR PA* | 0 (0) | 4 (3) |
| Carbapenem-resistant PA† | 0 (0) | 1 (1) |
Definition of abbreviations: MDR = multidrug-resistant; PA = Pseudomonas aeruginosa.
Data are presented as n (%).
MDR PA was also classified as drug-resistant PA.
Carbapenem-resistant PA was also classified as MDR PA and drug-resistant PA.
Discussion
We demonstrated that only 0.9% of CAP was due to PA, and that patients with definitive PA pneumonia had a higher 30-day mortality rate (28%) than those with non-PA pneumonia. The clinical features were considerably different among the patients in the different PA categories. Patients with definitive PA pneumonia were more likely to have a history of tuberculosis and COPD/bronchiectasis than patients with non-PA pneumonia. Furthermore, patients with indeterminate PA pneumonia were more likely to be frail, older adults with comorbidities than patients with non-PA pneumonia. More than half of the patients with indeterminate PA pneumonia and 25% of the patients with non-PA pneumonia were treated with an antipseudomonal drug.
The proportion of patients with PA infection has varied in previous studies. Our findings are in line with those of a multicenter study conducted in Germany by von Baum and colleagues (9) and those of a single-center study conducted in Spain by Cillóniz and colleagues (5), which reported that 0.4% (n = 22/5,130) and 1.4% (n = 77/5,384) of patients with CAP were classified as having definitive PA pneumonia, respectively. However, in a multicenter study in 54 countries, Restrepo and colleagues (15) reported that the prevalence of CAP due to PA was 4.2% (n = 133/3,193). The higher prevalence observed by Restrepo and colleagues (15) may be explained by different definitions of PA. They did not distinguish definitive PA pneumonia from indeterminate PA pneumonia among patients with CAP with positive PA isolates. If PA pneumonia is defined on the basis of only the isolation of PA from a sputum sample, PA colonization cases are very likely to be included (2). To identify definitive PA pneumonia, we used stringent criteria and classified PA pneumonia into two groups (definitive PA and indeterminate PA pneumonia) (9). We found that the majority of CAP cases with isolated PA were classified as indeterminate PA pneumonia, suggesting that the prevalence of true PA pneumonia may have been overestimated. In the 2019 ATS/IDSA guidelines, the risk factors for PA are presented as risk factors for culture-positive PA. Because the clinical features and mortality rates vary between definitive PA and indeterminate PA pneumonia, risk factors for PA pneumonia should be specified for only definitive PA pneumonia, and the proportions of definitive PA and indeterminate PA pneumonia should be locally validated in each area.
Patients with definitive PA pneumonia were more likely to have a history of tuberculosis and COPD/bronchiectasis than patients with non-PA pneumonia. This finding is compatible with the results of previous studies (5, 9). PA tends to colonize the injured lower respiratory tract, such as in patients with COPD/bronchiectasis and tuberculosis. PA was isolated from sputum in 4–15% of patients with COPD without pneumonia (16). In the current study, a strong association with chronic lung diseases was observed for definitive PA pneumonia. This result indicates that chronic lung diseases increase the risk of PA infection. Although a similar trend was also observed in patients with indeterminate PA pneumonia, patients with indeterminate PA pneumonia were more likely to be clinically frail than patients with non-PA pneumonia. This result suggests that, among patients with indeterminate PA pneumonia, the clearance of PA from the airway and an adequate host response to PA may be reduced in older adults (17).
In the current study, a quarter of the patients with non-PA pneumonia were initially treated with antipseudomonal drugs for a median of 6 days (interquartile range, 3–8 d), and treatment was de-escalated in only 21% of these patients. Although more than half of the patients with indeterminate PA pneumonia were treated with antipseudomonal drugs, treatment was de-escalated in 24%. Thus, antipseudomonal drugs might be overused in patients with indeterminate PA and non-PA pneumonia, although some patients with indeterminate PA potentially included pneumonia due to PA or other antibiotic-resistant bacteria. Clinicians may intend to target not only PA but also other atypical organisms or antibiotic-resistant bacteria with an antipseudomonal drug. Clinicians should note that the mortality rate was lower among patients with indeterminate PA pneumonia than among patients with definitive PA pneumonia in the acute phase. In an aging society, the proportion of patients with indeterminate PA pneumonia, including frail older adults, will increase. With respect to antimicrobial resistance, monitoring antimicrobial use is required not only for patients with non-PA pneumonia but also for patients with indeterminate PA pneumonia and should be employed to effectively reduce the usage of antipseudomonal drugs in the latter population.
Adequate de-escalation of antibiotics based on culture and Gram staining results is essential to reduce the drug resistance because the use of antipseudomonal β-lactam antibiotics leads to new drug resistance (18). Combining with a scoring system that has a high negative predictive value (19), Gram stain–guided empirical therapy is also useful for limiting the use of antipseudomonal drugs. Taniguchi and colleagues (20) reported that a Gram stain reduced the overuse of broad-spectrum antimicrobials and drug costs. A Gram stain is recommended only for adequate specimens, which are sometimes difficult to obtain (7). Thus, new tools in addition to Gram staining are needed to differentiate definitive PA from indeterminate PA pneumonia specifically and de-escalate adequately.
The proportion of MDR PA was lower in this study than in previous studies. In our study, the proportions of MDR PA were 0% (n = 0/25) and 3.1% (n = 4/131) in the definitive PA and indeterminate PA groups, respectively. However, the proportion of MDR PA was reported to be 32% (n = 22/68) by Cillóniz and colleagues (5) and 24.8% (n = 33/133) by Restrepo and colleagues (15). The difference may be dictated by area because more than half of the MDR PA cases in one multicenter study were reported in Europe (15). Because the proportion of MDR PA varies by country, local data are needed, as proposed in the ATS/IDSA 2019 guidelines. The treatment of PA pneumonia with two antipseudomonal drugs should be considered only in areas with a high proportion of MDR PA.
The present study has some limitations. First, we included patients in only two hospitals, and the average Japanese hospital may have thus not been represented. According to the Japan Nosocomial Infections Surveillance, MDR PA was rarely isolated (0.03%) in hospital patients in Japan in 2014; this finding of a low proportion of MDR PA is in line with our findings. Second, we intended to establish the true burden of definitive PA pneumonia, but no standardized criteria exist for distinguishing between definitive and indeterminate PA pneumonia. Thus, we used stringent criteria based on the Gram staining and culture results. Potential bias exists because patients with definitive PA pneumonia may be able to expectorate good sputum samples, whereas patients with indeterminate PA pneumonia may not be able to expectorate sputum. Likewise, a good sputum sample is more readily available from an intubated patient. Third, we evaluated clinical factors associated with PA infection using multinomial regression adjusted for potential confounders. However, because of the limited sample size, the 95% confidence intervals of our effect estimates require careful interpretation considering the multiple comparisons. Thus, a further study with a larger sample size is needed.
In conclusion, the prevalence of definitive PA pneumonia among patients with CAP was low, and the clinical features and outcomes were considerably different among the PA categories. The establishment of stringent criteria for diagnosing CAP is needed to avoid prescribing unnecessary antipseudomonal drugs.
Acknowledgments
Acknowledgment
The authors thank Rina Shiramizu, Kyoko Uchibori, and Yumi Araki (Department of Clinical Medicine, Institute of Tropical Medicine, Nagasaki University, Nagasaki, Japan) for their technical assistance. They also thank all the laboratory staff and collaborators at the participating hospitals, including Akihiro Toguchi, Tomo Yamada, and all staff at the infectious diseases and genetic testing centers (Department of Laboratory Medicine, Kameda Medical Center, Chiba, Japan) and Shiori Yoshinaga, Sayaka Yoshida, and Hitomi Morimoto (Department of Clinical Laboratory, Chikamori Hospital, Kochi, Japan).
Adult Pneumonia Study Group-Japan in Kameda Medical Center and Chikamori Hospital Collaborators: Masayuki Chikamori (Chikamori Hospital, Kochi, Japan), Naohisa Hamashige (Chikamori Hospital, Kochi, Japan), Naoto Hosokawa (Kameda Medical Center, Chiba, Japan), Norihiro Kaneko (Kameda Medical Center, Chiba, Japan), Naoko Katsurada (Kameda Medical Center, Chiba, Japan), Kei Nakashima (Kameda Medical Center, Chiba, Japan), Kaori Shibui (Kameda Medical Center, Chiba, Japan), Daisuke Suzuki (Kameda Medical Center, Chiba, Japan), Kenzo Tanaka (Kameda Medical Center, Chiba, Japan), Kentaro Tochitani (Kameda Medical Center, Chiba, Japan), and Yoshihito Otsuka (Kameda Medical Center, Chiba, Japan).
Footnotes
A complete list of the Adult Pneumonia Study Group-Japan in Kameda Medical Center and Chikamori Hospital collaborators may be found before the beginning of the References.
The Department of Clinical Medicine, Institute of Tropical Medicine, Nagasaki University received financial support for this study from Pfizer. The authors’ statements in the article are their own and not those of individuals holding official positions at the institution or those of the funding provider.
Author Contributions: Conceived and designed the experiments: E.S., M.S., M.I., and K.M. Performed the experiments: E.S., M.S., M.I., M.Y., M.A., and K.A. Analyzed the data: E.S., M.S., and K.M. Contributed reagents/materials/analysis tools: E.S. and M.S. Wrote the paper: E.S., M.S., K.A., and K.M.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1. Ding C, Yang Z, Wang J, Liu X, Cao Y, Pan Y, et al. Prevalence of Pseudomonas aeruginosa and antimicrobial-resistant Pseudomonas aeruginosa in patients with pneumonia in mainland China: a systematic review and meta-analysis. Int J Infect Dis. 2016;49:119–128. doi: 10.1016/j.ijid.2016.06.014. [DOI] [PubMed] [Google Scholar]
- 2. Fujitani S, Sun HY, Yu VL, Weingarten JA. Pneumonia due to Pseudomonas aeruginosa: part I: epidemiology, clinical diagnosis, and source. Chest. 2011;139:909–919. doi: 10.1378/chest.10-0166. [DOI] [PubMed] [Google Scholar]
- 3. Sibila O, Laserna E, Maselli DJ, Fernandez JF, Mortensen EM, Anzueto A, et al. Risk factors and antibiotic therapy in P. aeruginosa community-acquired pneumonia. Respirology. 2015;20:660–666. doi: 10.1111/resp.12506. [DOI] [PubMed] [Google Scholar]
- 4. Arancibia F, Bauer TT, Ewig S, Mensa J, Gonzalez J, Niederman MS, et al. Community-acquired pneumonia due to gram-negative bacteria and Pseudomonas aeruginosa: incidence, risk, and prognosis. Arch Intern Med. 2002;162:1849–1858. doi: 10.1001/archinte.162.16.1849. [DOI] [PubMed] [Google Scholar]
- 5. Cillóniz C, Gabarrús A, Ferrer M, Puig de la Bellacasa J, Rinaudo M, Mensa J, et al. Community-acquired pneumonia due to multidrug- and non-multidrug-resistant Pseudomonas aeruginosa. Chest. 2016;150:415–425. doi: 10.1016/j.chest.2016.03.042. [DOI] [PubMed] [Google Scholar]
- 6. Bou R, Lorente L, Aguilar A, Perpiñán J, Ramos P, Peris M, et al. Hospital economic impact of an outbreak of Pseudomonas aeruginosa infections. J Hosp Infect. 2009;71:138–142. doi: 10.1016/j.jhin.2008.07.018. [DOI] [PubMed] [Google Scholar]
- 7. Mandell LA, Wunderink RG, Anzueto A, Bartlett JG, Campbell GD, Dean NC, et al. Infectious Diseases Society of America; American Thoracic Society. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44:S27–S72. doi: 10.1086/511159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Metlay JP, Waterer GW, Long AC, Anzueto A, Brozek J, Crothers K, et al. Diagnosis and treatment of adults with community-acquired pneumonia: an official clinical practice guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med. 2019;200:e45–e67. doi: 10.1164/rccm.201908-1581ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. von Baum H, Welte T, Marre R, Suttorp N, Ewig S. CAPNETZ study group. Community-acquired pneumonia through Enterobacteriaceae and Pseudomonas aeruginosa: diagnosis, incidence and predictors. Eur Respir J. 2010;35:598–605. doi: 10.1183/09031936.00091809. [DOI] [PubMed] [Google Scholar]
- 10. Ishida T, Tachibana H, Ito A, Yoshioka H, Arita M, Hashimoto T. Clinical characteristics of nursing and healthcare-associated pneumonia: a Japanese variant of healthcare-associated pneumonia. Intern Med. 2012;51:2537–2544. doi: 10.2169/internalmedicine.51.7987. [DOI] [PubMed] [Google Scholar]
- 11. Shindo Y, Ito R, Kobayashi D, Ando M, Ichikawa M, Goto Y, et al. Central Japan Lung Study Group. Risk factors for 30-day mortality in patients with pneumonia who receive appropriate initial antibiotics: an observational cohort study. Lancet Infect Dis. 2015;15:1055–1065. doi: 10.1016/S1473-3099(15)00151-6. [DOI] [PubMed] [Google Scholar]
- 12. Morimoto K, Suzuki M, Ishifuji T, Yaegashi M, Asoh N, Hamashige N, et al. Adult Pneumonia Study Group-Japan (APSG-J) The burden and etiology of community-onset pneumonia in the aging Japanese population: a multicenter prospective study. PLoS One. 2015;10:e0122247. doi: 10.1371/journal.pone.0122247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Magiorakos AP, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18:268–281. doi: 10.1111/j.1469-0691.2011.03570.x. [DOI] [PubMed] [Google Scholar]
- 14. Sterne JA, White IR, Carlin JB, Spratt M, Royston P, Kenward MG, et al. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ. 2009;338:b2393. doi: 10.1136/bmj.b2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Restrepo MI, Babu BL, Reyes LF, Chalmers JD, Soni NJ, Sibila O, et al. GLIMP. Burden and risk factors for Pseudomonas aeruginosa community-acquired pneumonia: a multinational point prevalence study of hospitalised patients. Eur Respir J. 2018;52:1701190. doi: 10.1183/13993003.01190-2017. [DOI] [PubMed] [Google Scholar]
- 16. Murphy TF, Brauer AL, Eschberger K, Lobbins P, Grove L, Cai X, et al. Pseudomonas aeruginosa in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2008;177:853–860. doi: 10.1164/rccm.200709-1413OC. [DOI] [PubMed] [Google Scholar]
- 17. Gellatly SL, Hancock RE. Pseudomonas aeruginosa: new insights into pathogenesis and host defenses. Pathog Dis. 2013;67:159–173. doi: 10.1111/2049-632X.12033. [DOI] [PubMed] [Google Scholar]
- 18. Teshome BF, Vouri SM, Hampton N, Kollef MH, Micek ST. Duration of exposure to antipseudomonal β-lactam antibiotics in the critically ill and development of new resistance. Pharmacotherapy. 2019;39:261–270. doi: 10.1002/phar.2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ceccato A, Mendez R, Ewig S, de la Torre MC, Cilloniz C, Gabarrus A, et al. Validation of a prediction score for drug-resistant microorganisms in community-acquired pneumonia. Ann Am Thorac Soc. 2021;18:257–265. doi: 10.1513/AnnalsATS.202005-558OC. [DOI] [PubMed] [Google Scholar]
- 20. Taniguchi T, Tsuha S, Shiiki S, Narita M. Gram-stain-based antimicrobial selection reduces cost and overuse compared with Japanese guidelines. BMC Infect Dis. 2015;15:458. doi: 10.1186/s12879-015-1203-6. [DOI] [PMC free article] [PubMed] [Google Scholar]