Supplemental Digital Content is available in the text.
Keywords: coronavirus, coronavirus disease 2019, non-invasive venous waveform analysis, severe acute respiratory syndrome coronavirus 2
OBJECTIVES:
Due to the rapid rate of severe acute respiratory syndrome coronavirus 2 transmission and the heterogeneity of symptoms of coronavirus disease 2019, expeditious and effective triage is critical for early treatment and effective allocation of hospital resources.
DESIGN:
A post hoc analysis of respiratory data from non-invasive venous waveform analysis among patients enrolled in an observational study was performed.
SETTING:
Vanderbilt University Medical Center.
PATIENTS:
Peripheral venous waveforms were recorded from admission to discharge in enrolled coronavirus disease 2019–positive patients and healthy age-matched controls.
INTERVENTIONS:
Data were analyzed in LabChart 8 to transform venous waveforms to the frequency domain using fast Fourier transforms. The peak respiratory frequency was normalized to the peak cardiac frequency to generate a respiratory non-invasive venous waveform analysis respiratory index. Paired Fisher exact tests were used to compare each patient’s respiratory non-invasive venous waveform analysis respiratory index at admission and discharge. A nonparametric one-way analysis of variance was used for multiple comparisons between patients with coronavirus disease 2019 and healthy controls for respiratory non-invasive venous waveform analysis respiratory index.
MEASUREMENTS AND MAIN RESULTS:
Fifty coronavirus disease 2019–positive patients were enrolled between April 2020, and September 2020, and 45 were analyzed; 34 required supplemental oxygen and 11 did not. The respiratory non-invasive venous waveform analysis respiratory index was significantly higher for the 34 patients with coronavirus disease 2019 who received supplemental oxygen (median, 0.27; interquartile range, 0.11—1.28) compared with the 34 healthy controls (median, 0.06; interquartile range, 0.03–0.14) (p < 0.01). For patients with coronavirus disease 2019 who received supplemental oxygen, respiratory non-invasive venous waveform analysis respiratory index was significantly lower at hospital discharge (p = 0.02; 95% CI, 0.10–1.9) compared with hospital admission (median = 0.12; interquartile range, 0.05–0.56). For patients with coronavirus disease 2019, a respiratory non-invasive venous waveform analysis respiratory index of 0.64 demonstrated sensitivity of 92%, specificity of 47%, and positive predictive value of 93% for predicting requirement of supplemental oxygen during the hospitalization.
CONCLUSIONS:
Respiratory non-invasive venous waveform analysis respiratory index represents a novel physiologic respiratory measurement with a promising ability to triage early care and predict the need for oxygen support therapy in coronavirus disease 2019 patients.
The novel coronavirus (severe acute respiratory syndrome coronavirus 2 [SARS-CoV-2]) has produced a global pandemic that continues to cripple both economies and healthcare systems (1, 2). Although most patients with SARS-CoV-2 experience only mild symptoms, a subset will experience life-threatening respiratory failure (2). Evaluation and management of coronavirus disease 2019 (COVID-19) depends on the severity of the disease (2). Unfortunately, the ability to determine whose symptoms will remain mild and whose will progress to severe respiratory failure is difficult and often requires observation in a hospital setting (2). Rapid and effective triage is critical for early treatment and effective allocation of hospital resources (3).
Non-Invasive Venous waveform Analysis (NIVA) is a promising monitoring approach to assess volume in both adults and children (4–7). NIVA uses a piezoelectric sensor on the venous plexus on the volar aspect of the wrist to capture a venous waveform signal. The signal is then deconvoluted into two components with a fast Fourier transformation (FFT) into the frequency domain. The cardiac component (fundamental frequency of the pulse rate [f0]) and harmonics of the cardiac component are used to derive a value that estimates pulmonary artery occlusion pressure (PAOP), the best clinical measure of intravascular volume (5, 8). Signals were collected from COVID-19 patients to assess volume status and the need for invasive measurements of volume state. Post hoc analysis of these data was used to analyze and evaluate the fundamental frequency of the respiratory rate (fR0) of the venous waveform and the relationship of the respiratory component to the need for oxygen support therapy. Use of the respiratory component of the venous waveform might provide a rapid, noninvasive, inexpensive test to identify patients with COVID-19 at risk for requiring oxygen therapy.
For this study, a post hoc analysis of the NIVA waveforms was performed to evaluate the respiratory component of the venous waveforms. The hypothesis was that differences in the respiratory component of the venous waveform could be detected and would stratify COVID-19 patients based on need for oxygen support therapy.
METHODS
This observational study was approved by ADVARRA (www.advarra.com; Avient Corporation, Avon Lake, OH) Institutional Review Board (IRB) (IRB approval number: MOD00777155) secondary to institutional conflict with Vanderbilt University Medical Center (VUMC). The protocol enrolled 50 patients hospitalized with COVID-19 to investigate venous waveforms (NIVA scores) (5) during the hospital stay to determine volume overload and a need for invasive measurements to assess volume overload. These waveforms were interrogated post hoc to specifically assess the respiratory component of the venous waveform. Control data were obtained using previously recorded waveforms from a database of healthy volunteers without any diagnosis of pulmonary disease (i.e., asthma, chronic obstructive pulmonary disease, emphysema, pulmonary fibrosis, sarcoidosis, or restrictive lung disease).
NIVA scores and their associated raw venous waveform were obtained with a prototype NIVA device (VoluMetrix, LLC, Nashville, TN) (Fig. 1), at the same time as standard vital signs by the patient’s assigned bedside nurse. The cardiac component (f0) and harmonics of the cardiac component recorded with the NIVA device (Fig. 1) were used to obtain a NIVA score using a proprietary algorithm. This NIVA score represents a value that is a PAOP equivalent (5, 8). Respiratory rates (RRs) were obtained during physical examination by a provider. Pulse rate (PR) was obtained using either pulse oximetry and/or electrocardiogram readings (Philips, Amsterdam, the Netherlands).
Figure 1.
Picture rendering of the non-invasive venous waveform analysis (NIVA) device. The NIVA device is composed of a wristband used to capture the venous waveforms using a piezoelectric sensor encased in Versaflex as well as a main device where the signal is amplified and digitally processed on a microcontroller. The measured venous waveform contains the cardiac and respiratory harmonics in coronavirus disease 2019 and control subjects.
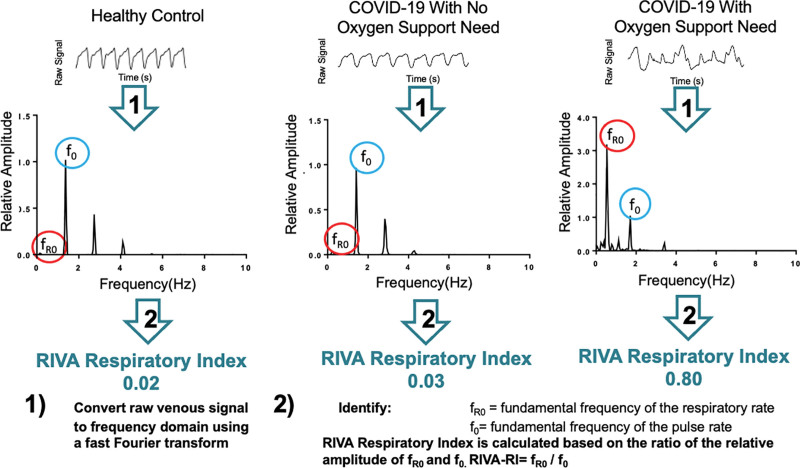
Respiratory data analysis of the venous waveforms was performed by study personnel (L.V., K.H.) blinded to the subject’s oxygen needs. Data were exported from the NIVA device (4–7) via a universal serial bus cable to a personal computer and analyzed using LabChart 8 software to transform the data to the frequency domain using FFTs. Respiratory and cardiac harmonics from the power spectrum (Figs. 2 and 3) were investigated for peaks associated with the respiratory (fR0) and cardiac cycles (f0) throughout the hospitalization. These “peaks” are defined by what created them, the respiratory and/or cardiac systems, and were confirmed by their frequencies relationship to the RR and PR, respectively. By definition, 1 hertz (Hz = 1/s) in the power spectrum is 60 beats and/or breaths per minute. Therefore, respiratory and cardiac harmonics were confirmed by correlation with a subjects’ RR and PR. The amplitude of the cardiac frequency, f0, of the waveform was relatively consistent throughout and was used to normalize the respiratory frequency, fR0, to provide the Respiratory non-Invasive Venous waveform Analysis - Respiratory Index (RIVA-RI): fR0/f0 (RIVA-RI) (Fig. 2).
Figure 2.
Representative respiratory non-invasive venous waveform analysis (RIVA) respiratory signals from subjects with low risk to high risk of oxygen support need (left to right). Raw signals are transformed from the time domain (top) to the frequency domain (bottom) (1). The relative amplitude of the respiratory rate (fundamental frequency of the respiratory rate [fR0]) compared with the relative amplitude of the pulse rate (fundamental frequency of the pulse rate [f0]) is used to calculate a RIVA respiratory index (RIVA-RI). COVID-19 = coronavirus disease 2019.
Figure 3.
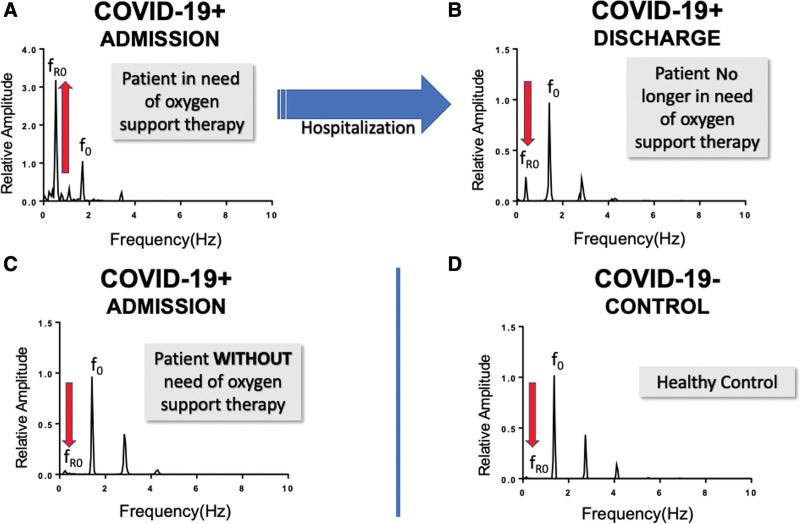
Representative examples of fundamental frequency of the respiratory rate (fR0) amplitude changes in the frequency domain recorded in coronavirus disease 2019–positive (COVID-19+) subjects. The fast Fourier transform (FFT) of venous waveform recorded using non-invasive venous waveform analysis in COVID-19+ subjects admitted requiring oxygen support therapy (A) and at time of discharge when oxygen support was no longer required (B). A representative FFT of a COVID-19+ subject who did not require any oxygen support therapy (C) and, for comparison, the FFT of a coronavirus disease 2019–negative (COVID-19–) healthy control subject (D). f0 = fundamental frequency of the pulse rate.
Each RIVA-RI value was then categorized into either admission with discharge/death patients who received any level of oxygen support therapy (nasal cannula, high-flow, OptiFlow [Fisher & Paykel, Auckland, New Zealand], noninvasive positive pressure, and/or mechanical ventilation) at any point prior to discharge or death and compared with those patients who did not need oxygen support therapy.
Paired Fisher exact test was used to compare the RIVA-RI at admission and discharge. To compare admission to all other groups, a nonparametric one-way analysis of variance (ANOVA) was used with multiple comparisons between groups, for RIVA-RI, RR, and pulse oximetry hemoglobin oxygen saturation (Spo2). The venous waveform (1K window) was deconvoluted using a FFT into frequencies corresponding to the heart rate (f0) and RR (fR0). Statistical analysis was conducted using GraphPad Prism 13 (La Jolla, CA).
RESULTS
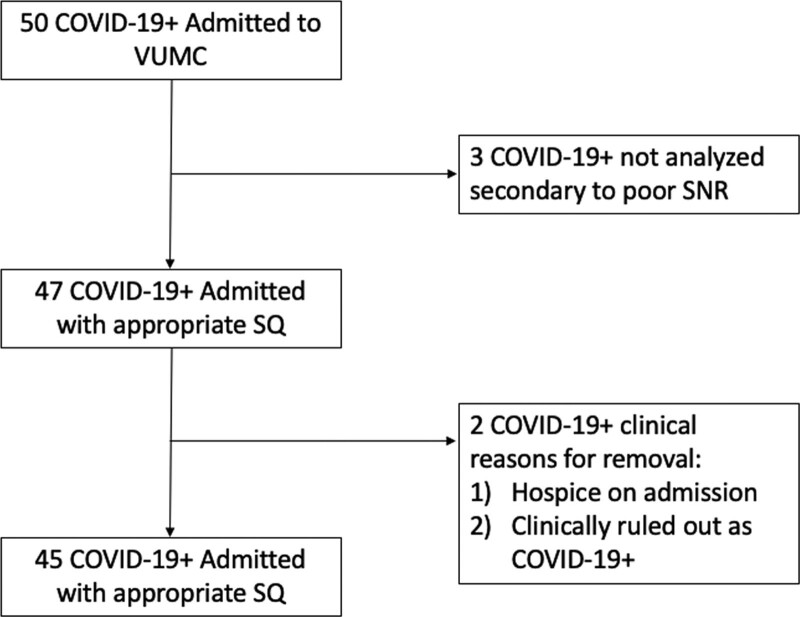
A total of 50 patients with COVID-19 admitted to VUMC between April 2020 and September 2020 were enrolled in the study. Three patients were excluded during signal analysis secondary to poor signal to noise ratio (SNR)—for these patients, there was elevated baseline noise in the Fourier window which led to a low/uninterpretable SNR (Fig. 4). Two additional patients were excluded based on clinical history—one patient was transferred to the VUMC palliative care unit with no escalation of care (including supplemental oxygen therapy) during hospitalization, and the other was diagnosed with COVID-19 infection 13 days prior to admission to VUMC for a non-COVID complaint (Fig. 4). Data from 262 patients evaluated in the VUMC Cardiac Catheterization Laboratory during right heart catheterization (RHC) between November 2019 and November 2020 were used as COVID-19–negative (COVID-19–) controls. These control patients were initially screened for indications for RHC: 1) diagnostic, 2) heart failure, and 3) postheart transplant. COVID-19– controls were obtained from the patients in groups 2 and 3 as these patients had no documented pulmonary chief complaint prompting RHC and no significant prior cardiopulmonary history (n = 171). Patients were further screened for any additional pulmonary comorbidities, including asthma, chronic obstructive pulmonary disease, pulmonary hypertension, and obstructive sleep apnea (OSA). This left a total of 45 COVID-19– control patients for inclusion in the study. Eleven of these patients were excluded during signal analysis due to poor signal quality with low SNR as described above for a total of 34 COVID-19– controls for analysis.
Figure 4.
Flow diagram of patient enrollment and analysis. COVID-19+= coronavirus disease 2019 positive, SNR = signal quality, SQ = signal quality, VUMC = Vanderbilt University Medical Center.
Demographics and clinical characteristics are outlined in Table 1 (Supplemental Digital Content, http://links.lww.com/CCX/A808). There was a significant difference (p < 0.05) between median body mass index of COVID-19–positive (COVID-19+) patients with oxygen support needs (median = 33.22; 25–75% interquartile range [IQR], 28.07–36.22) and COVID-19– control subjects (median = 26.92; 25–75% IQR, 24.96–29.91). There was also a significant difference (Table 1, Supplemental Digital Content, http://links.lww.com/CCX/A808) (p < 0.05) in the following comorbidities between groups: higher incidence of hyperlipidemia and chronic kidney disease (CKD) (CKD/end stage renal disease) in the COVID-19– control group; higher incidence of heart disease, prior malignancy, OSA, and asthma in the COVID-19+ patients who required oxygen support during hospitalization; and higher occurrence of liver disease in the COVID-19+ patients who did not require oxygen support during hospitalization.
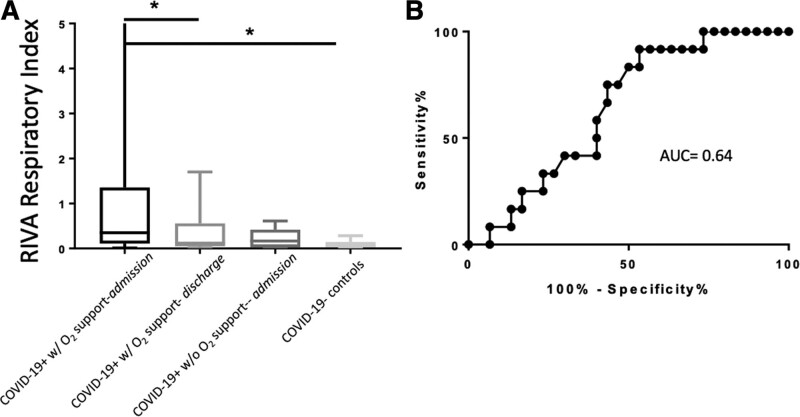
The RIVA-RI for COVID-19+ patients requiring oxygen support during hospitalization (median = 0.27; 25–75% IQR, 0.11–1.28; n = 34) was significantly higher (one-way ANOVA p < 0.01; 95% CI, 0.4008–2.037) (Fig. 5A) than the RIVA-RI for COVID-19– controls (median = 0.06; 25–75% IQR, 0.03–0.14; n = 34) (Fig. 5A). The RIVA-RI for COVID-19+ patients requiring oxygen support was also significantly higher (p = 0.02; 95% CI, 0.1023–1.939) (Fig. 5A) than the RIVA-RI for those same patients at time of discharge (median = 0.12; 25–75% IQR, 0.05–0.56; n = 24) (Fig. 5A). There was no significant difference (p = 0.09; 95% CI, –0.1242 to 2.265) (Fig. 5A) in the RIVA-RI for COVID-19+ patients requiring oxygen support during hospitalization and COVID-19+ patients who did not require oxygen support during hospitalization (median = 0.2; 25–75% IQR, 0.04–0.44; n = 11) (Fig. 5A).
Figure 5.
Respiratory non-invasive venous waveform analysis respiratory index (RIVA-RI) for coronavirus disease 2019–positive (COVID-19+) patients and coronavirus disease 2019–negative (COVID-19–) controls (A) and area under the curve (AUC) for predicting oxygen requirement (B). The amplitude of the respiratory signal relative to the amplitude of the cardiac signal was derived from the venous waveforms after fast Fourier transformation, and this ratio represents the RIVA-RI (A). The RIVA-RI for COVID-19+ patients admitted to the hospital and requiring oxygen support during hospitalization (median = 0.27, n = 34) was significantly higher (p < 0.01; 95% CI, 0.4008–2.037) than the RIVA-RI for COVID-19– controls (median = 0.06; n = 34). The RIVA-RI for COVID-19+ patients who required oxygen support was also significantly higher (p = 0.02; 95% CI, 0.1023–1.939) than the RIVA-RI for those same patients at time of discharge (median = 0.12; n = 24). The RIVA-RI was not significantly different (p = 0.09; 95% CI, –0.1242 to 2.265) for COVID-19+ patients who required oxygen support during hospitalization and those COVID-19+ patients who never required oxygen support during hospitalization (median = 0.2; n = 11). Statistical analysis was completed using analysis of variance with multiple comparisons between groups. Horizontal bars with star (*) demonstrate statistical significance. The ability of the RIVA-RI for predicting the need for oxygen support (B) during admission for COVID-19+ patients demonstrated an AUC of 0.64 (95% CI, 0.48–0.81). At a RIVA-RI value of greater than or equal to 0.6 had a 92% sensitivity (95% CI, 61.52–99.79%) and 47% specificity (95% CI, 28.34–65.67%) for predicting need for oxygen support during admission for COVID-19+ patients.
A receiver operating characteristic (ROC) curve with an area under the curve (AUC) of 0.64 (Fig. 5B) was used to determine a cutoff value for RIVA-RI as a predictor for requiring supplemental oxygen therapy during hospitalization in COVID-19+ patients. This value was selected to maximize the sensitivity of the RIVA-RI in order to most prioritize appropriately prediction of an oxygen requirement in this patient subset. A RIVA-RI of greater than or equal to 0.6 was selected based on a 92% sensitivity (95% CI, 61.52–99.79%) and 47% specificity (95% CI, 28.34–65.67%). The positive predictive value (PPV) of a RIVA-RI of greater than or equal to 0.6 for a COVID-19+ patient’s need for oxygen support was 93%.
NIVA Scores
There was no significant difference (Table 1, Supplemental Digital Content, http://links.lww.com/CCX/A808) in the median admission NIVA score between COVID-19+ admitted to the hospital requiring oxygen support (n = 27; median NIVA, 12; 25–75% IQR, 12–14), COVID-19+ patients who did not require oxygen support during hospitalization (n = 10; median NIVA, 10.5; 25–75% IQR, 9.25–12.5), and COVID-19– controls (n = 31; median NIVA, 12; 25–75% IQR, 9–17). The COVID-19+ patients who required oxygen support’s median NIVA score at admission (12; 25–75% IQR, 12–14) was not significantly different (p = 0.52) than their discharge median NIVA score (12; 25–75% IQR, 11–12). A ROC curve with an AUC of 0.54 demonstrated how the admission NIVA score predicted the need for oxygen support therapy (Fig. 1, Supplemental Digital Content, http://links.lww.com/CCX/A809).
RR
There was no significant difference (p = 0.13; 95% CI, –0.7309 to 8.28) in the RR between COVID-19+ patients who required oxygen support during hospitalization (median = 20; 25–75% IQR, 19–25; n = 34) (Fig. 2, Supplemental Digital Content, http://links.lww.com/CCX/A810) and those who did not require oxygen support during hospitalization (median = 17; 25–75% IQR, 16–18; n =10) (Fig. 2, Supplemental Digital Content, http://links.lww.com/CCX/A810) or between COVID-19+ patients at admission to the hospital (median = 20; 25–75% IQR, 19–25; n = 34) (Fig. 2, Supplemental Digital Content, http://links.lww.com/CCX/A810) and at discharge (median = 19; 25–75% IQR, 17–20; n = 27; p = 0.66; 95% CI, –1.944 to 4.974) (Fig. 2, Supplemental Digital Content, http://links.lww.com/CCX/A810). There was a significant difference between the RR at both admission and discharge of COVID-19+ patients who required oxygen support therapy compared with COVID-19– controls (p < 0.001; 95% CI, 2.536–8.727 and p = 0.0084; 95% CI, 0.8066–7.426, respectively) (Fig. 2, Supplemental Digital Content, http://links.lww.com/CCX/A810).
Oxygen Saturation
COVID-19+ patients who required oxygen support during hospitalization had a significantly lower Spo2 at admission (median = 93%; 25–75% IQR, 91–95%; n = 34; p < 0.01; 95% CI, 2.536–8.727) (Fig. 2, Supplemental Digital Content, http://links.lww.com/CCX/A810) and at discharge (median = 93%; 25–75% IQR, 92–95%; n = 27; p < 0.01; 95% CI, 0.8066–7.426) (Fig. 2, Supplemental Digital Content, http://links.lww.com/CCX/A810) than COVID-19+ patients who did not require oxygen support during hospitalization (median = 96%; 25–75% IQR, 94–98%; n = 10) (Fig. 2, Supplemental Digital Content, http://links.lww.com/CCX/A810).
DISCUSSION
Given the speed of respiratory deterioration that occurs in COVID-19+ patients (3), rapid, accurate, noninvasive monitoring for triage of oxygen support therapy represents an unmet clinical need. To date, there are only two noninvasive monitoring approaches for assessing respiratory distress, RR, and Spo2. Whether it be with COVID-19 patients or patients with other pulmonary conditions, there are no validated and accepted criteria that are predictive of the need of oxygen support or the type of administration (9). This leads to considerable differences among clinicians with regard to care for patients with acute respiratory disease, such as COVID-19 (10).
This study was designed with the primary objective of assessing the volume status of COVID-19+ patients upon arrival by measuring their NIVA scores. The NIVA scores, PAOP equivalent values (5), demonstrated that COVID-19+ patients did not present to the hospital in a volume overloaded state (Table 1, Supplemental Digital Content, http://links.lww.com/CCX/A808). The NIVA scores also did not predict the need for oxygen support therapy (Fig. 1, Supplemental Digital Content, http://links.lww.com/CCX/A809). Although there were five COVID-19+ patients who came to the hospital in volume overloaded states (NIVA > 18, consistent with a PAOP > 18 mm Hg), all of which did require oxygen (Fig. 3, Supplemental Digital Content, http://links.lww.com/CCX/A811), the admitted COVID-19+ subjects who required oxygen support therapy ranged from “dry” to “wet” in their volume states. Compared with controls, there was not a statistically significant difference (Table 1, Supplemental Digital Content, http://links.lww.com/CCX/A808). Also, for the COVID-19+ patients who required oxygen support therapy, the median NIVA score at discharge was the same (NIVA score = 12) as the median admission score. Obviously, volume control is an important treatment goal with any respiratory distressed state—this appears to be well achieved, based on median NIVA scores, in the studied patient population. When NIVA scores are visualized across the range RIVA-RI values obtained using a bubble plot, there is an interesting distribution of NIVA scores along the range of RIVA-RI values (Fig. 3, Supplemental Digital Content, http://links.lww.com/CCX/A811). There were five patients with NIVA scores above 18 at time of admission, none were actively diuresed during their hospital course. Two did receive their home hydrochlorothiazide in the 5 days of admission, but it was not continued throughout either patient’s hospital stays. These five patients’ need for oxygen support therapy very well could have been exaggerated secondary to their elevated volume state and just not appreciated by clinicians. Of note, no advanced imaging or monitoring was performed on any of the five patients with a NIVA score that was consistent with congestion.
In the secondary analysis of data collected during the observational cohort study focusing on the respiratory component of the venous waveforms (fR0) as compared to the fundamental frequency of the PR (f0) of four subject categories, there was a novel objective measure of respiratory distress, RIVA-RI. The RIVA-RI approach to monitoring respiratory distress appears to be a promising discovery that is data driven. In the healthy controls, 34 subjects with no known pulmonary disease demonstrated a range of 0 to less than 0.3. No healthy control demonstrated a RIVA-RI at or above 0.3. COVID-19+ subjects who never required oxygen support therapy had a range with a minimum value of 0.01 and a maximum value of 0.6. Although no healthy controls ever had a RIVA-RI above 0.3, both categories of COVID-19+ subjects had a RIVA-RI greater than 0.3; however, no COVID-19+ patient who did not require oxygen support therapy had a RIVA-RI above 0.6. When taking the two maximum values into account, there appears to be a “respiratory distress” intermediate range of RIVA-RI from 0.3 to 0.6. COVID-19+ subjects who required oxygen support therapy had a range of a 0.01 minimum and a maximum of 11. Interestingly, no COVID-19+ patient who did not require oxygen support went above the RIVA-RI of 0.6, whereas subjects who did require oxygen support therapy did. When a ROC curve analysis was performed ROC (AUC = 0.64) (Fig. 5B) for these data, it demonstrated that when a cutoff of 0.6 is used, there is a sensitivity for detecting the need for oxygen support therapy of 92% with a strong PPV of 93%.
Although some screening tests optimized for sensitivity tend to have higher false-positive rates, RIVA-RI’s PPV of 93% demonstrates this novel signal’s promise and potential ability to be sensitive without this limitation. Therefore, a RIVA-RI less than 0.3, along with signs and symptoms, would aid clinical decisions in “ruling out” a patient as one who will be requiring oxygen support therapy and if the RIVA-RI is above 0.6, a clinician could feel confident that the patient will likely require subsequent oxygen support.
The practical use of the RIVA-RI would be in triage and hospital settings. To date, the data points available to clinicians when determining the severity of a patient’s respiratory distress are extremely limited. Therefore, the RIVA-RI would be a welcomed addition to a barren field. It carries the potential to aid a clinician in determining the severity of respiratory distress and prediction of clinical trajectory. For example, a 66-year-old patient with COVID-19 comes in complaining of mild shortness of breath, with a moderately elevated RR (22 breaths/min), Spo2 of 94%, and examination that does not demonstrate any signs of respiratory distress. This would normally be a difficult triage decision. Do you use valuable resources and admit for observation or send home where they could deteriorate without healthcare personnel immediately available? Now, let us say that same patient had a RIVA-RI of 1.0. Based on the data, there is high likelihood they will deteriorate to the point of requiring oxygen support therapy with a sensitivity of 92% and PPV of 93%. Therefore, the admission of this patient is further justified. The opposite could be said if the RIVA RI was 0.2; they would be unlikely to require oxygen support therapy, so a home-based plan may be appropriate. This is a just an elementary example of the potential clinical utility of this innovative device to aid clinicians in their heroic care of COVID-19 patients.
One significant limitation to this study was the inability to capture the venous waveforms prior to the initiation of oxygen support therapy. Each subject was enrolled after admission to the COVID-19 unit at VUMC. Venous waveforms were obtained both prior to and as the patient was on oxygen support therapy. Therefore, there could have been improvement on the RIVA-RI as the subject was placed on oxygen therapy. When analyzed in subjects who were later placed on oxygen support, there was a noticeable and significant lowering of the RIVA-RI. Although this certainty cannot be assumed, this could be a reason for the lack of statistical significance between the COVID-19+ subjects who did require oxygen support therapy median RIVA-RI and COVID-19+ subjects who did not require oxygen support therapy median RIVA-RI. Future studies will need to be designed to obtain the RIVA-RI prior to the initiation of oxygen therapy and follow the RIVA-RI in a more continuous fashion. Future development will include device and signal optimization specific to the respiratory venous waveform to improve signal acquisition and reduce the number of poor signals that require exclusion from analysis.
Also, the admission data were not normally distributed which did not allow for parametric statistical considerations to be made, this prevented statistical significance from being achieved in the study of RIVA.
Finally, this was a secondary, post hoc analysis of an observational study investigating venous waveforms and volume status; thus, treatment decisions were not made using the device, and the study was not designed specifically to investigate the respiratory harmonics in an admitted COVID-19 patient. Future studies will need to be designed to appropriately investigate the predictive nature of RIVA toward predicting oxygen support therapy need, as well as allow for a more granular analysis of RIVA and respiratory disease states/therapies. Also, future work will need to exist to optimize the device for respiratory venous waveform capture and, simultaneously, perform automated analysis to provide a RIVA-RI that can be used clinically.
CONCLUSIONS
The peripheral venous waveform signal is able to be captured noninvasively in hospitalized COVID-19 patients. RIVA-RI is a promising, novel physiologic measurement with the potential ability to predict the need for oxygen support therapy in COVID-19 patients. With the increasing need for efficient and correct triage, RIVA monitoring could aid clinicians in caring for patients both at home and at the hospital and potentially prevent unnecessary hospitalizations.
ACKNOWLEDGMENTS
We would like to thank all the care partners, nurses, and physicians who worked tirelessly in the coronavirus disease 2019 unit at Vanderbilt University Medical Center. Not only did they provide excellent care to patients, but they also supported this research. It was through their hard work that this study and novel discovery were achieved.
Supplementary Material
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccejournal).
Supported, in part, by grants from the National Institutes of Health: R01HL148244-01 (to Dr. Alvis) and R44HL140669-01 (to Dr. Hocking).
Dr. Alvis is CSO at VoluMetrix and an inventor on intellectual property in the field of venous waveform analysis assigned to Vanderbilt and licensed to VoluMetrix and is married to the COO. Dr. Brophy is the Founder and CMO of VoluMetrix and an inventor on intellectual property in the field of venous waveform analysis assigned to Vanderbilt and licensed to VoluMetrix. Dr. Hocking is Founder, CEO, and President of VoluMetrix and an inventor on intellectual property in the field of venous waveform analysis assigned to Vanderbilt and licensed to VoluMetrix. The remaining authors have disclosed that they do not have any conflicts of interest.
Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
REFERENCES
- 1.Klompas M. Coronavirus disease 2019 (COVID-19): Protecting hospitals from the invisible. Ann Intern Med. 2020; 172:619–620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gandhi RT, Lynch JB, Del Rio C. Mild or moderate Covid-19. N Engl J Med. 2020; 383:1757–1766 [DOI] [PubMed] [Google Scholar]
- 3.Liang W, Yao J, Chen A, et al. Early triage of critically ill COVID-19 patients using deep learning. Nat Commun. 2020; 11:3543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alvis BD, McCallister R, Polcz M, et al. Non-invasive venous waveform analysis (NIVA) for monitoring blood loss in human blood donors and validation in a porcine hemorrhage model. J Clin Anesth. 2020; 61:109664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alvis BD, Polcz M, Huston JH, et al. Observational study of noninvasive venous waveform analysis to assess intracardiac filling pressures during right heart catheterization. J Card Fail. 2020; 26:136–141 [DOI] [PubMed] [Google Scholar]
- 6.Alvis BD, Polcz M, Miles M, et al. Non-invasive venous waveform analysis (NIVA) for volume assessment in patients undergoing hemodialysis: An observational study. BMC Nephrol. 2020; 21:194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sobey JH, Reddy SK, Hocking KM, et al. Non-invasive venous waveform analysis (NIVA) for volume assessment during complex cranial vault reconstruction: A proof-of-concept study in children. PLoS One. 2020; 15:e0235933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wise ES, Hocking KM, Polcz ME, et al. Hemodynamic parameters in the assessment of fluid status in a porcine hemorrhage and resuscitation model. Anesthesiology. 2021; 134:607–616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roca O, Caralt B, Messika J, et al. An index combining respiratory rate and oxygenation to predict outcome of nasal high-flow therapy. Am J Respir Crit Care Med. 2019; 199:1368–1376 [DOI] [PubMed] [Google Scholar]
- 10.Delbove A, Darreau C, Hamel JF, et al. Impact of endotracheal intubation on septic shock outcome: A post hoc analysis of the SEPSISPAM trial. J Crit Care. 2015; 30:1174–1178 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.