ABSTRACT
Glioblastoma (GBM) is one of the deadliest and aggressive forms of brain cancer. Environmental and intrinsic factors such as Western Diet and advanced age can function as powerful accelerants to the progression of GBM. Recently, we discovered that pre-clinical GBM models subject to an obesogenic and age-accelerating high fat diet (HFD) presented with hyperaggressive GBM phenotypes, including treatment-refractory cancer stem cell (CSC) enrichment. Mechanistically, HFD suppressed production of the gasotransmitter hydrogen sulfide (H2S) and its downstream sulfhydration signaling in the brain. Likewise, we observed dramatic loss of sulfhydration in brains of GBM patients. Importantly, we showed the tumor suppressive effects of H2S against GBM in cell culture and in vivo. Here, we discuss these recent findings and provide insight into how they can be leveraged to improve treatment modalities, prognosis, and quality of life for GBM patients.
KEYWORDS: Glioblastoma (GBM), hydrogen sulfide (H2S), cancer stem cells (CSCS), sry transcription factor 2 (SOX2), high fat diet (HFD), oleic acid (OA), cystathionine gamma-lyase (CGL), cystathionine beta-synthase (CBS), mercaptopyruvate sulfurtransferase (MPST), sulfhydration, transsulfuration
While cancer in its many forms can arise at any stage of life, the majority of malignant cancers present with increased incidence at advanced ages. This is consistent with the hypothesis that advanced age increases factors contributing to and/or decreases factors suppressing the onset and progression of cancer. The aging-related association particularly holds true for glioblastoma (GBM), the most common primary malignant brain tumor, with the median age of diagnosis being 65 years.1 Even with dedicating over half a century into biological studies and clinical approaches for GBM, it remains uniformly lethal despite aggressive approaches focused on surgical debulking of the tumor combined with radiation and chemotherapy with temozolomide. With median survival being 16–21 months and only 2–3% of patients surviving 5 years post-diagnosis,2 it is evident that novel treatment paradigms must be devised.
Despite aging being a major risk factor for GBM, little research investigating the underlying aging-related mechanisms into GBM initiation and progression have been pursued. Likewise, aging-accelerating metabolic alterations are emerging as key drivers of GBM, with epidemiological and correlative evidence supporting a link between metabolic syndrome and GBM.3 However, the effects of obesity- and diet-associated metabolic alterations on GBM and the microenvironment, and if they are directly shared with aging, are unexplored. Thus, investigating how these environmental and intrinsic factors impact GBM from a molecular perspective may provide the missing pieces for devising novel curative treatments and methodologies against GBM.
Recently, our laboratories addressed these unknowns by studying obesogenic high fat diet (HFD)-induced changes in GBM cancer stem cells (CSCs), tumor progression, tumor microenvironment, and metabolic heterogeneity that ultimately confer treatment resistance to GBM.4 We employed syngeneic mouse models and human patient–derived GBM models in a series of in vivo experiments in which mice were fed a control diet or a HFD. The HFD tumor-bearing animals succumbed to disease rapidly, which coincided with increased tumor cell proliferation and higher tumor initiation frequency. In the brain tumor microenvironment, the HFD mice had increased CSC frequency as read out by the protein expression of the pluripotent transcription factor SRY (Sex determining region Y)-Box 2, also known as SOX2. CSC enrichment was also increased in patient-derived GBM in vitro models treated with mono-unsaturated fatty acids.
Using mass spectrometry-based non-targeted lipidomic analysis, we tested the hypothesis that the HFD results in intracerebral lipid enrichment, which in turn increases proliferation and self-renewal within the tumor cell population. We identified saturated, mono-, or di-unsaturated lipid species enrichment specifically within tumors isolated from HFD-fed mice compared to controls. Additionally, excess saturated lipids acted directly on tumor cells to enhance GBM progression and increase CSC frequency.
Examination into the molecular mechanisms for how lipids could impact GBM focused on sulfur amino acid metabolism and hydrogen sulfide (H2S) production pathways through the enzymes Cystathionine beta-synthase (CBS), Cystathionine gamma-lyase (CGL), and Mercaptopyruvate sulfurtransferase (MPST). While at first these pathways appeared to be non-canonical with traditional lipid-related metabolic pathways, we believed it would be an intriguing avenue to explore due to HFD consumption inhibiting production of the gasotransmitter H2S in a tissue specific manner5 along with H2S playing both oncogenic and tumor suppressive roles dependent on cell and tissue type. By mining epidemiologic datasets, we identified decreased mRNA expression of CBS and MPST in tumor biopsies from GBM patients, and no changes in CGL compared to non-cancerous controls. However, suppressed CGL enzymatic activity has been reported across multiple grades of astrocytoma and GBM.6 These findings suggest a tumor-suppressive role for H2S in GBM, thus we pursued this theory by testing a variety of in vitro, in vivo, and GBM specimens.
In the in vitro cell culture assays, we assessed proliferation of GBM cells treated with the CGL chemical inhibitor propargylglycine (PAG). Treatment with PAG inhibited H2S production in GBM cells, induced hyperproliferation, and provided resistance against the standard-of-care chemotherapeutic temozolomide. Conversely, H2S supplementation via sodium hydrosulfide (NaHS) or the chemical donor GYY4137 suppressed GBM cell viability. We followed up these results by testing whether H2S perturbations result in functional metabolic consequences in GBM cells. Inhibition of H2S synthesis increased GBM cell bioenergetics and metabolic plasticity at both resting state and after exposure to the mono-unsaturated fat oleic acid. Thus, H2S serves as a tumor suppressor in these GBM culture models, and its loss elevates cellular metabolic function and provides treatment resistance.
In the in vivo tests, in addition to the HFD tumor-bearing animals succumbing to disease rapidly, having increased tumor cell proliferation and CSC enrichment, they also had losses in brain CBS expression, H2S synthesis, and H2S-induced protein sulfhydration (aka persulfidation) modifications and signaling. H2S rescue experiments via daily NaHS administration were applied to GBM tumor-bearing mice, which resulted in arrested GBM tumor development and marked reduction in SOX2 expression. These findings link HFD consumption to the loss of GBM suppressive H2S. We further investigated the degree obesity contributes to HFD-induced GBM acceleration utilizing the leptin deficient obese spontaneous mutation LepOB mutant mouse, which exhibits the physiological hallmarks of metabolic syndrome, including obesity, even while on a standard control diet due to hyperphagic food consumption. Despite being obese, the LepOB mice did not experience GBM acceleration, poor survival outcomes, or reductions in H2S production compared to wildtype mice. Thus, inhibition of tumor suppressive H2S synthesis required HFD consumption independent of the obesity phenotype.
Analysis of tumor biopsies from human GBM patients versus non-cancerous controls mirrored the results obtained in mice, with GBM tissue producing 50% less H2S. Leveraging a recently developed sulfhydration detection assay,7 we further analyzed these specimens to generate differential cancer versus non-cancer sulfhydrome profiles. Protein sulfhydration within the GBM specimens was decreased compared to controls, in which >400 discrete proteins lost this modification in GBM. These >400 proteins are involved in numerous molecular pathways, including carbon metabolism, pyruvate and amino acid metabolism, oxidative phosphorylation, and glycolysis. Thus, loss in H2S production and protein sulfhydration under GBM and HFD may represent a broad-spectrum molecular reprogramming that enables metabolic flexibility, particularly to the CSCs, needed for growth and treatment resistance (Figure 1).
Figure 1.
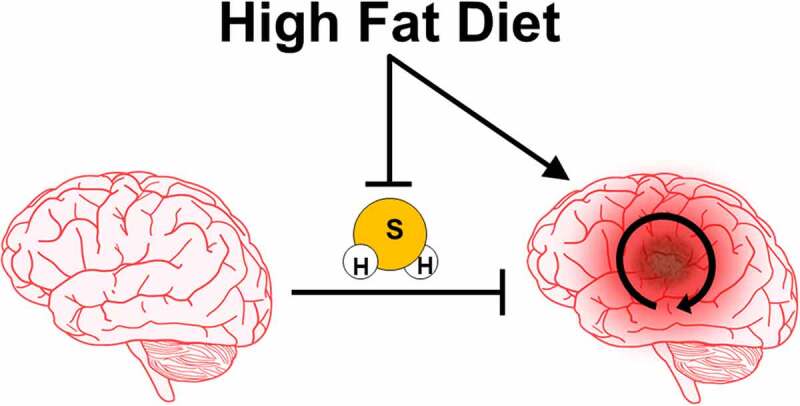
Graphical model for high fat diet (HFD) driving glioblastoma (GBM) initiation and progression. HFD drives glioblastoma initiation, progression, and cancer stem cell (CSC) renewal and enrichment via perturbing tumor lipid accumulation and inhibiting tumor suppressive hydrogen sulfide (H2S) production and downstream protein sulfhydration signaling. Loss of H2S production and signaling ultimately promotes aggressive GBM cell growth and survival and allows for metabolic flexibility and treatment resistance
Translating our findings for clinical use, we propose that replenishing and/or supplementing H2S in conjunction with standard of care may offer improved management of GBM. Identifying feasible, safe, and effective approaches to boost H2S are thus needed. While our approach, direct H2S supplementation via NaHS or GYY41374, would be the most straightforward, due to the volatility and relatively short half-life of these H2S donors, their long-term use in humans may have limitations. Thus, approaches enhancing endogenous enzymatic production, which is normally lost under aging, HFD, and GBM, may be the best route. A possible pharmacological intervention to stimulate endogenous production could be the use of thyroid hormone lowering drugs, such as the United States Food and Drug Administration (FDA) approved propylthiouracil (PTU). Notably, PTU was used in combination with chemotherapy in a phase I/II GBM clinical trial almost 20 years ago at the Cleveland Clinic Brain Tumor Center.8 Although patients treated with PTU experienced significantly longer survival, this line of investigation was set aside due to lack of a clear mechanism. However, more recent work showed the feasibility of oral PTU administration to enhance H2S production,9 thus providing a possible mechanism in the former clinical study. Dietary interventions that alter the rate of aging such as caloric restriction (CR), fasting, or sulfur amino acid restriction, all augment endogenous H2S production and/or sulfhydration in various tissues including the brain.7 While dietary restrictions may appear counterintuitive in the management of cancer, their use has been favorably reported in several preclinical and clinical trials in combination with current standards of care.10
In conclusion, we demonstrated that a HFD resulted in shifts to lipid and sulfhydration profiles in the brain and GBM microenvironment. These shifts resulted in a favorable oncogenic setting driving CSC enrichment, chemotherapy resistance, and GBM progression. However, reversing declines in H2S production via pharmacological, dietary, and lifestyle interventions may provide the needed arsenal in battling GBM when combined with standards of care.
Acknowledgments
We would like to thank members of the Lathia and Hine labs for their discussions, contributions, and insights leading to the completions of this work.
Funding Statement
Funding was provided by the Glioblastoma Research Organization (to CH), and pilot funding from the Case Comprehensive Cancer Center (to DJS, JDL, and CH).
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- 1.Ladomersky E, Scholtens DM, Kocherginsky M, Hibler EA, Bartom ET, Otto-Meyer S, Zhai L, Lauing KL, Choi J, Sosman JA, et al. The Coincidence Between Increasing Age, Immunosuppression, and the Incidence of Patients With Glioblastoma. Front Pharmacol. 2019;10:1. doi: 10.3389/fphar.2019.00200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ostrom QT, Gittleman H, Liao P, Vecchione-Koval T, Wolinsky Y, Kruchko C, Barnholtz-Sloan JS.. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2010-2014. Neuro-oncology. 2017;19:v1–3. doi: 10.1093/neuonc/nox158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rogers LR, Ostrom QT, Schroer J, Vengoechea J, Li L, Gerson S, Nock CJ, Machtay M, Selman W, Lo S, Sloan AE, et al.Association of metabolic syndrome with glioblastoma: a retrospective cohort study and review. Neurooncol Pract. 2020;7:541–548. doi: 10.1093/nop/npaa011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Silver DJ, Roversi GA, Bithi N, Wang SZ, Troike KM, Neumann CKA, Ahuja GK, Reizes O, Brown JM, Hine C, et al. Severe consequences of a high-lipid diet include hydrogen sulfide dysfunction and enhanced aggression in glioblastoma. J Clin Invest. 2021. doi: 10.1172/JCI138276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peh MT, Anwar AB, Ng DSW, Atan MSBM, Kumar SD, Moore PK. Effect of feeding a high fat diet on hydrogen sulfide (H2S) metabolism in the mouse. Nitric Oxide. 2014;41:138–145. doi: 10.1016/j.niox.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 6.Wrobel M, Czubak J, Bronowicka-Adamska P, Jurkowska H, Adamek D, Papla B. Is development of high-grade gliomas sulfur-dependent? Molecules. 2014;19(12):21350–21362. doi: 10.3390/molecules191221350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bithi N, Link C, Henderson YO, Kim S, Yang J, Li L, Wang R, Willard B, Hine C. Dietary restriction transforms the mammalian protein persulfidome in a tissue-specific and cystathionine gamma-lyase-dependent manner. Nat Commun. 2021;12:1745. doi: 10.1038/s41467-021-22001-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hercbergs AA, Goyal LK, Suh JH, Reddy CA, Cohen BH, Steven GH, Reddy SK, Peereboom DM, Elson PJ, Gupta MK, et al. Propylthiouracil-induced chemical hypothyroidism with high-dose tamoxifen prolongs survival in recurrent high grade glioma: a phase I/II study. Anticancer Res. 2003;23:617–626. [PubMed] [Google Scholar]
- 9.Hine C, Kim H-J, Zhu Y, Harputlugil E, Longchamp A, Matos MS, Ramadoss P, Bauerle K, Brace L, Asara JM, et al. Hypothalamic-pituitary axis regulates hydrogen sulfide production. Cell Metab. 2017;25(6):1320–1333 e1325. doi: 10.1016/j.cmet.2017.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brandhorst S, Longo VD. Fasting and caloric restriction in cancer prevention and treatment. Recent Results Cancer Res. 2016;207(241–266). doi: 10.1007/978-3-319-42118-6_12. [DOI] [PMC free article] [PubMed] [Google Scholar]


