ABSTRACT
The negative surface charge of brain microvessel endothelial cells is derived from the special composition of their membrane lipids and the thick endothelial surface glycocalyx. They are important elements of the unique defense systems of the blood–brain barrier. The tissue-specific properties, components, function and charge of the brain endothelial glycocalyx have only been studied in detail in the past 15 years. This review highlights the importance of the negative surface charge in the permeability of macromolecules and nanoparticles as well as in drug interactions. We discuss surface charge and glycoxalyx changes in pathologies related to the brain microvasculature and protective measures against glycocalyx shedding and damage. We present biophysical techniques, including a microfluidic chip device, to measure surface charge of living brain endothelial cells and imaging methods for visualization of surface charge and glycocalyx.
KEYWORDS: Blood-brain barrier, drug delivery, glycocalyx, nanoparticles, surface charge
1. Introduction
The blood-brain barrier (BBB) possesses several unique defense systems.1,2 Tight intercellular junctions between brain endothelial cells close the paracellular gap for cells and molecules, thus providing a physical barrier.3–5 Active efflux pumps and metabolic enzymes contribute to chemical protection against potentially neurotoxic molecules.6 The negative surface charge of endothelial cells forming cerebral capillaries represents an additional line of defense. In the early 1980s pioneering observations were made on the presence of anionic sites at the luminal surface of cerebral endothelium by labeling with positively charged colloidal iron by Zoltán Nagy.7–9 This negative surface charge of brain endothelial cells is derived from the special composition of their membrane lipids and the endothelial surface glycocalyx (Figure 1). The method to isolate viable and metabolically active microvessel fractions from brain, a breakthrough technique still used today to study the BBB in vitro, was developed by Ferenc Joó and his team in 1973.10 Studies on isolated brain microvessels revealed the biosynthesis of lipids at the BBB and that the phospholipids of brain endothelial cells contain relatively high amounts of n-3 and n-6 fatty acids.11 The fatty acid composition of the cerebral endothelial cells co-cultured with astrocytes more closely resembles that of brain microvessels: an increase in arachidonic acid (20:4 n-6) and docosahexaenoic acid (22:6 n-3) was observed in the endothelial cell phospholipid classes of phosphatidylcholine, phosphatidylethanolamine and phosphatidylserine.12 Two recent studies discovered that MFSD2A is constitutively expressed at the BBB and not only transfers lysophosphatidylcholine-docosahexaenoic acid but also plays a crucial role in BBB maturation and suppression of transendothelial vesicular transport.13 Other studies also confirmed that the brain endothelial cell plasma membrane is rich in different phospholipid species.14,15 This lipid composition is unique and due to its high content of phosphatidylinositol and phosphatidylserine more negatively charged, as compared to other endothelial cells.16
Figure 1.
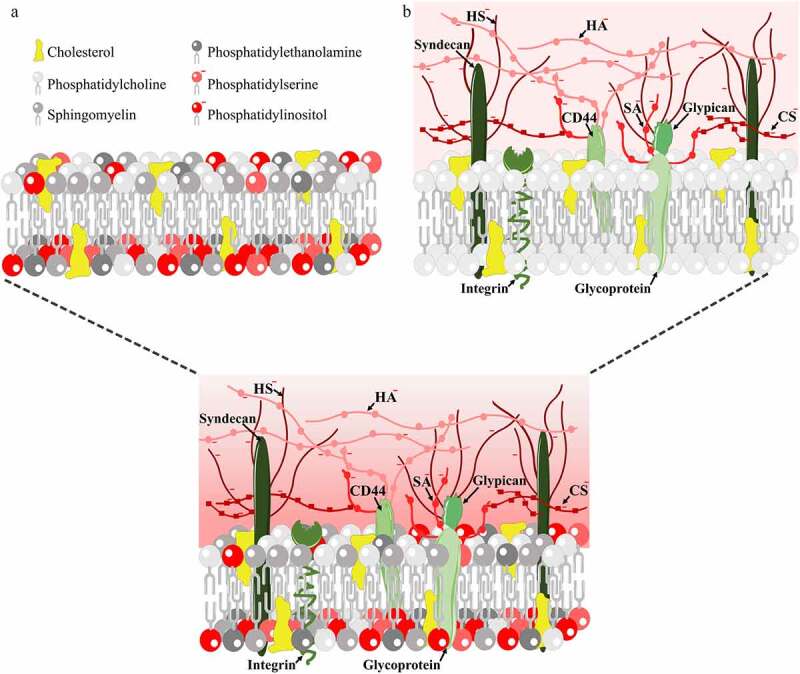
A. Structure of the lipid membrane of cells: headgroups of lipid species with negative charge are shown in red shades. B. Structure of the endothelial cell surface glycocalyx: core proteins are shown in green shades, negatively charged glucosaminolgycans and sialic acid are shown in red shades. Abbreviations: CD44: cluster of differentiation 44 glycoprotein, also known as hyaluronic acid receptor; CS: chondroitin sulfate; HA: hyaluronic acid; HS: heparan sulfate; SA: sialic acid
The brain endothelial surface glycocalyx is a sugar-protein matrix layer covering the luminal surface that is mainly composed of proteoglycans, glycoproteins, and glycosaminoglycans (GAGs), as detailed in a recent review.17 The endothelial glycocalyx acts as a mechanosensor to detect blood flow induced shear stress and modulates signaling pathways, gene expression, endothelial function and morphology.18,19 The basic structure of the glycocalyx consists of core proteins and long GAG side chains attached to them. The three negatively charged GAGs present in the glycocalyx of endothelial cells are hyaluronic acid, chondroitin sulfate and heparan sulfate (Figure 1).18 Heparan sulfate is the most abundant endothelial GAG, and is attached to core proteins such as transmembrane proteins syndecans and the membrane-bound protein glypican.18,19 The glycocalyx thickness of endothelial cells (0.2–5 µm) varies depending on the organ and different parts of the vascular bed20 as well as on the regions within cultured brain endothelial cells.21 Mostly sialo-glycoconjugates and heparan sulfate proteoglycans provide the negative charge barrier at the BBB as revealed by enzyme digestions combined with ultrastructural studies in mouse brain cortex.22
Most of our knowledge on the glycocalyx composition and structure of endothelial cells is based on the vascular endothelium of peripheral vessels.18 There are studies indicating that the surface charge and the glycocalyx at the level of the central nervous system (CNS) is different from other parts of the vascular bed. The surface charge of brain endothelial cells is more negative than that of other vascular endothelial cells.16 A recent in vivo study highlighted the denser structure of glycocalyx of brain microvessels as compared to those in the lung or the liver.23 However, the specific features of the glycocalyx and lipid composition at the level of BBB are not known in detail, especially compared to the vascular endothelium of other organs. In the present review, we aim to give an overview on brain endothelial surface charge and its relation to BBB functions and how we can examine it.
2. BBB surface charge and permeability of biomolecules and drugs
The structural complexity and the negative surface charge of the microvascular endothelial surface at the BBB not only provide an extra physical barrier, but also regulate the penetration of large biomolecules as well as therapeutic drugs to the CNS. In a recent study in mice using fluorescently labeled hydrophilic marker molecules with different size and two-photon microscopy three separate barriers were determined that influence the passive transport of small and large hydrophilic molecules through the BBB: the glycocalyx, the endothelium, and the extravascular compartment.24 The brain endothelial glycocalyx was well penetrated by the small hydrophilic molecules, but not by large dextrans (10 and 40 kDa). In addition to the glycocalyx, there are additional mechanisms at the BBB regulating the permeability to water and small solutes.25 Using a mathematical model, it was revealed that the basal membrane and astrocyte foot processes can prevent the permeability increase and maintain the stability of the BBB permeability even after glycocalyx damage, unlike in peripheral endothelial cells.25
It is well established that the physicochemical properties of molecules, among them lipophilicity and charge influence the passive permeability of molecules across the BBB.26–28 Negatively charged biomolecules, like sulfated dextrans cross brain endothelial cell layers in significantly lower amounts than neutral ones.29 Large, negatively charged polyanions, like the full length GAG heparin (~15 kDa) and the GAG mimetic pentosan polysulfate (3–6 kDa) do not cross the BBB in significant amount, while low molecular weight heparin oligomers (≤3 kDa) do.30 Cationization of albumin31 or anti-tetanus immunoglobulin fragments32 results in their better penetration across BBB models. In agreement with these experimental observations, an electrodiffusion model was described for the transport of charged molecules across the BBB.33 This mathematical model predicted that the increased negative charge of the BBB would greatly decrease the BBB permeability to negatively charged solutes but would increase that to positively charged ones.33
Charge can affect the permeability of a molecule across the BBB via different mechanisms, including affinity for transporters, protein binding, and adsorptive-mediated transcytosis.28 During adsorptive-mediated transcytosis at the BBB the first step is the binding of the biomolecules with net positive charge to the negatively charged endothelial surface followed by internalization.34 In this very first step, the ionic interactions drive membrane invagination and mediate adsorptive transcytosis. Both clathrin coated vesicles and caveolae participate in this energy-dependent process, which can be divided into three major steps, endocytosis, transcytosis and exocytosis.34 It has been recently discovered, that there are differences between vesicular trafficking in brain endothelial cells and in epithelial cells,35 although how adsorptive-mediated transcytosis at the BBB may differ from that of other endothelium is not known.
Cell-penetrating peptides, a large and heterogenous family with multiple positive charges, also use the adsorptive-mediated transcytosis pathway to enter cells and cross barriers.34 Several members of this highly cationic peptide family, like Tat or SynB were examined as vectors for brain delivery of different types of therapeutic molecules. SynB peptides increased the brain penetration of analgesic compounds dalargin and morphin-6-glucuronide, the cytostatic doxorubicin and the antibiotic benzylpenicillin in animal experiments.34 However, the chemical stability of Tat-conjugates and their ability to bind to brain endothelial cell membrane is cargo-dependent indicating that the efficacy of Tat conjugation to a peptide-based therapeutic for BBB transfer need to be evaluated case by case.36 We have recently demonstrated, that a highly positively charged cell penetrating peptide KLAL (also called MAP or PN159) not only shows cell entry, but opens intercellular tight junctions in culture models of the BBB and the intestinal epithelial barrier.37,38 This finding indicates that highly positively charged biomolecules may not only induce adsorptive-mediated transcytosis, but might interfere with other BBB functions.
Modification of the brain endothelial surface charge by cationic molecules
In the first study, which indicated that modification of the negative surface charge in the brain vasculature changes BBB permeability, rat brains were perfused with the polycation protamine sulfate.7 Protamine is an endogenous, arginine-rich nuclear protein, which is clinically used to neutralize the anticoagulant effects of heparin. Protamine treatment led to a decrease in the anionic sites on luminal surface of cerebral endothelium, elucidated by positively charged colloidal iron, and to the opening of the BBB to horseradish peroxidase enzyme (4.4 kDa).7 The polyanion heparin, a natural glycosaminoglycan, reversed the binding of colloidal iron to the brain endothelium, but did not change the increased permeability.7 A follow-up study revealed the opening of the brain endothelial tight junctions, suggesting that the paracellular pathway was also involved in the peroxidase extravasation.8 In a comparative study, intracarotid infusion of the polycation poly-L-arginine (12 kDa) increased the brain penetration of albumin the most, followed by poly-L-lysine (10 kDa) and protamine (4.5 kDa) in rats.39 In physiological conditions the BBB does not let plasma proteins to enter the brain parenchyma.2,28 A similar effect was seen in cultured mouse brain microvessel endothelial cells. Neutralization of the brain endothelial luminal surface charge by cationic ferritin, but not neutral ferritin, increased the permeability of the polar Evans blue dye (0.9 kDa).40 The transendothelial electrical resistance and permeability for dextran (20 kDa) was not changed.40
Another method to modify the overall negative charge of brain endothelial cell surface is to decrease of the anionicity of plasma membranes by the application of cationic lipophilic molecules. A cationic lipid probe, 1-[4-(trimethylamino)phenyl]-6-phenyl-1,3,5-hexatriene (TMA-DPH), which intercalates into the plasma membrane (Figure 2) made more positive the surface charge (zeta pontial) of brain endothelial cells, measured by laser-Doppler velocimetry.16,41 Kyotorphin, an endogenous dipeptide (L-Tyr-L-Arg), that does not cross the BBB was conjugated to ibuprofen, a lipophilic nonsteroidal antiinflammatory drug. Two derivatives, an amidated and a nonamidated were prepared. The amidated kyotorphin-ibuprofen, that is more positively charged, increased the zeta pontial of brain endothelial cells and had an analgesic effect in mice indicating brain penetration.41 Recently our group has demonstrated that lidocaine, a lipophilic and positively charged therapeutic molecule also made the surface charge of brain endothelial cells more positive.42 Lidocaine treatment slightly decreased the electrical resistance, but did not change the permeability of primary cell-based rat BBB co-culture model for the hydrophilic markers dextran (neutral, 10 kDa) and Lucifer yellow (negatively charged, 0.5 kDa).
Figure 2.
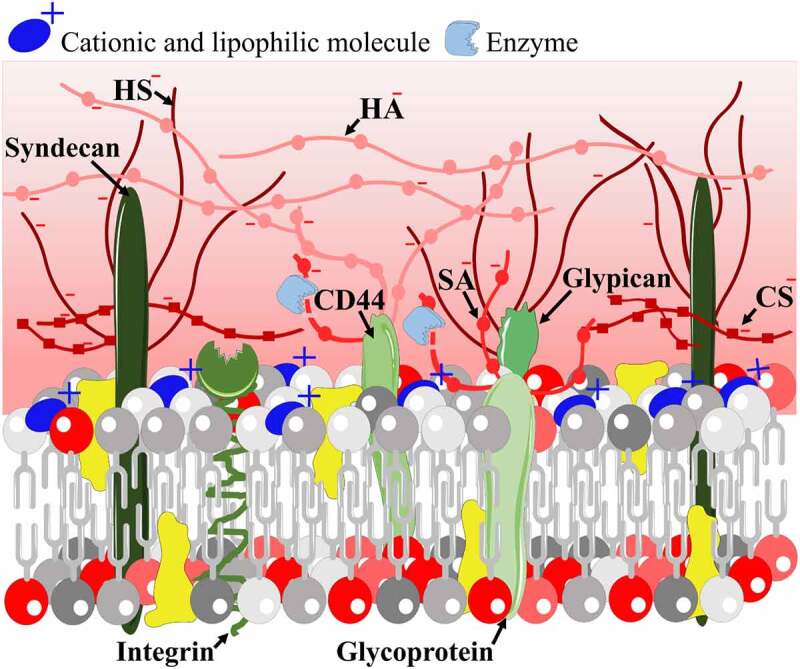
Modification of cellular surface charge by the insertion of a cationic and lipophilic molecule (blue) to the plasma membrane or by enzymatic cleavage of negatively charged residues from the surface glycocalyx (red shades)
Modification of the brain endothelial surface charge by enzymes cleaving the glycocalyx
The surface charge density of brain endothelial cells can be made more positive by using specific enzymes to digest off negatively charged residues from the glycocalyx (Figure 2). This observation was already made in the 1980s by electron microscopy, when neuraminidase, heparinase III and other enzymes were used to remove sialic acid and heparan sulfate from the luminal side of brain endothelial cells.22,43,44 Treatment with neuraminidase lowered the endothelial glycocalyx thickness while increasing the permeability to albumin in rat mesenteric microvessels.45 Recently, it was shown, that treatment with neuraminidase elevates the surface potential and decreases the sialic acid and N-acetyl neuraminic acid coverage on a human brain endothelial cell line.46 Neuraminidase treatment did not change the electrical resistance and permeability of the BBB culture model for hydrophilic markers dextran (4 and 10 kDa) and albumin (67.5 kDa),46 suggesting that BBB models can behave differently from other vascular endothelial models. In contrast, intravenously injected hyaluronidase increased BBB permeability for Evans blue in rats and aggravated brain edema following cardiac arrest,47 indicating a different role for hyaluronic acid within the glycocalyx in pathological conditions.
Modification of the surface charge by anionic molecules
There are no direct measurements available on how negatively charged polyanions like GAGs and GAG mimetics change the surface charge of endothelial cells. We hypothesize, that this might be the case from indirect experimental evidences. The polyanion GAG heparin reversed the disappearance of anionic sites from the luminal surface of brain capillaries when given after protamine treatment.7 A medicine containing natural GAGs, sulodexide, increased the thickness of glycocalyx in sublingual and retinal microvessels in patients.48 These data suggest an increased charge selectivity, which was proven experimentally for other polyanions. Orosomucoid or α-1-acid glycoprotein is a negatively charged plasma protein overexpressed in pathologies, like inflammation and hypertension. Orosomucoid, which is essential for capillary charge selectivity, is produced by human microvascular endothelial cells and exerts a protective effect by interacting with other components of the endothelial glycocalyx.49 When orosumucoid was present in the permeability assay buffer the permeability of the positively charged ribonuclease (14 kDa) was increased and the transfer of the negatively charged lactalbumin (14 kDa) was decreased in a mouse brain endothelial cell line model and in murine pial microvessels.50,51 Another study using a primary rat BBB model found a decrease in the permeability of the negatively charged small paracellular marker fluorescein (0.4 kDa) after orosomucoid treatment.52 In addition to charge effects, this barrier tightening was mediated by increased expression of tight junction proteins occludin and ZO-1.52
A GAG mimetic, pentosan polysulfate is a semi-synthetic, heparinoid-like drug.53 Pentosan is listed among the compounds protecting the glycocalyx, as it showed a beneficial effect in a diabetes experimental model by increasing GAG in tissues.54 As pentosan binds to the glycocalyx of circulating blood cells of patients, first it was used as an anticoagulant.53 Currently, pentosan is used for the therapy of osteoarthritis.55 In cultured rat brain endothelial cells the uptake of low-density lipoprotein, which is known to have a positive charge,56 was increased by pentosan treatment.57 We should add, that both receptor-mediated and adsorptive-mediated transport processes might have contributed to the increased cell entry in addition to charge. Pentosan also showed protective effects in primary cell-based BBB culture models: it decreased prion peptide toxicity58 and increased barrier integrity damaged by bacterial lipopolysaccharide treatment59 or by β-amyloid peptides.60
BBB surface charge and permeability of drugs
Medicinal chemistry utilizes multiple methods to promote brain delivery of different drugs by altering their physico-chemical properties, such as making the molecule more lipid soluble or more cationic. A good example for this electrostatic principle is a study on kyotorphin peptide, which is not transported across the BBB. When this dipeptide was conjugated to the lipophilic ibuprofen and was amidated, the complex gained a positive charge, and increased the surface charge of brain endothelial cells as compared to the negatively charged form of the conjugate.41 The amidated kyotorphin-ibuprofen also increased analgesic effect in mice suggesting a better penetration to the brain.41
This electrostatic principle certainly applies if the molecule passively crosses the BBB, however, the biological transport systems of brain endothelial cells may overrule it. Statins are highly negatively charged amphiphilic drugs, still several statins, especially the more lipophilic ones cross the BBB.61,62 One of the reasons of their good brain penetration is that these statins are substrates of organic anion transporting polypeptides. In a culture model of the BBB we observed the transfer of rosuvastatin, pravastatin and atorvastatin, in agreement with the expression of these carriers in the model.62 In the same study, we also demonstrated, that cationic drugs donepezil and tacrine, used in the treatment of Alzheimer’s disease, have higher permeability across the BBB model than the negatively charged statins.62 This difference did not only depend on the physicochemical properties, because these drugs also use solute carriers, namely the organic cation transporters. During aging and in neurodegenerative diseases brain endothelial surface glycocalyx changes were described, especially the thinning of this protective layer as well as the thickening of the basal membrane.17 The morphological alterations can potentially lead to a reduced surface charge which in turn may affect the permeability of cationic Alzheimer’s disease drugs, including donepezil, tacrine and memantine. Indeed, the brain penetration of two cationic molecules, memantine and propranolol were decreased in a transgenic mouse model of Alzheimer’s disease.63 These observations may be related to several factors: thicker basal membrane, changes in transporter expression and surface charge.
To better understand how cationic and lipophilic molecules may interact at the surface of brain endothelial cells, we have tested lidocaine in our recent study.42 Lidocaine, as an example of lipophilic drugs, interacts with the phospholipid membranes through an asymmetric insertion (Figure 2), and as we demonstrated changed brain endothelial surface charge to more positive and decreased the permeability of the positively charged hydrophobic molecule rhodamine 123.42 The effect was not related to inhibition of the P-glycoprotein. Our finding was corroborated with results demonstrating that lidocaine interferes with the BBB passage of cationic drugs pentazocine and naloxone in rats.64,65 These observations clearly indicate an electrostatic interaction of cationic drugs at the level of the BBB.
3. Nanoparticles, surface charge, and blood–brain barrier permeability
In the last several decades, the application of nanoparticles (NPs) for medical purposes has received significant attention from both researchers and funding agencies.66–68 NPs are innovative new tools to increase targeted drug delivery by improving the therapeutic efficacy of drug molecules, decrease the effective dose, and/or reduce the risk of systemic side effects.67 In the last 12 years there have been over 400 active, ongoing or recruiting clinical trials in which nanomedicines are investigated. Of these trials 65% is related to cancer applications.66 Currently more than 50 nanomedicine formulations have been approved for clinical use in various indications including cancer treatment, iron-replacement therapies, anesthetics, fungal treatments, macular degeneration and rare genetic diseases.66 There are still no approved nanomedicines for neurological diseases despite the many ongoing clinical trials. NPs are considered as potential therapeutic delivery platforms to overcome the BBB to reach CNS targets.69 The therapeutic effects of NPs are dependent on both physicochemical properties and specific targeting. Besides shape, size and functionalization, surface charge is an important factor that determines the cellular uptake and permeability of NPs across barriers.68,69 We focused on studies which measured the surface charge (zeta potential) of NPs and examined their interaction with the BBB either in culture models or in animal studies.
Non-targeted nanoparticles
The literature of NPs tested for BBB penetration in vitro or in vivo is large, but only few comparative studies are available studying specifically the charge of NPs. There is a controversy, how charge influences the penetration of neutral or charged NPs across the BBB. It is not very surprising, since we found large variations of zeta potentials for NPs considered as cationic, neutral or anionic (Table 1). Generally, positively charged nanoparticles are known to be more easily internalized than neutral or negatively charged NPs.70 The most widely used cationic NPs are mainly based on cationic polymers, such as the polysaccharide chitosan, or are coated with cationic lipids or cationic bovine serum albumin (Tables 1 and Tables 2).
Table 1.
Non-targeted nanoparticles with measured zeta potential tested for BBB permeability
| Nanoparticle type | Charge (mV) | Cargo | Size (nm) | Ref. |
|---|---|---|---|---|
| Emulsifying wax | cationic: +45; neutral: −14; anionic: −59 | - | 74–124 | 73 |
| Liposome | cationic: +70; neutral: +3; anionic: −10 | lipid tracers DiO, DiD, DiL | 80–200 | 76 |
| Liposome | cationic: +30; neutral: +2; anionic: −38 | simvastatin | 150–170 | 77 |
| Cyclodextrin | cationic: +4 & +14; anionic: −12 | - | 65–88 | 51 |
| Maltodextrin | cationic: +25; neutral: 0 | - | 60 | 72 |
| Nanotubes | cationic: +9; neutral: −8; anionic: −23 | - | 12–800 | 78 |
| Polystyrene | neutral: −1 to −8; anionic: −23 to −74 | - | 60–2000 | 80 |
| Silica & Qdots | cationic: −7; neutral: −4; anionic: −32 | - | 30–1500 | 79 |
Abbreviations: DiO: dioctadecyloxacarbocyanine perchlorate, DiD: dioctadecyl-tetramethylindodicarbocyanine chlorobenzene sulfonate salt, Dil: dioctadecyl-tetramethylindocarbocyanine perchlorate, Qdots: quantum dots.
Table 2.
Targeted nanoparticles with measured zeta potential tested for BBB permeability
| Nanoparticle type | Targeting ligand | Charge (mV) | Cargo | Size (nm) | Ref. |
|---|---|---|---|---|---|
| Carrier-mediated transport pathway | |||||
| Liposome | glutathione | −8 | doxorubicin | 95 | 85 |
| Niosome | alanine & glutathione | −7 | BSA | 103 | 86 |
| Niosome | alanine & glutathione | −5 | BSA | 115 | 87 |
| Niosome | glucosamine | −17 | antibody | 200 | 88 |
| Polystyrene | biotin & glutathione | −23 | - | 120 | 89 |
| Receptor-mediated transport pathway | |||||
| Gold | anti-TfRantibody | −4 | - | 73 | 90 |
| HSA | trasferrin | −28 | loperamide | 183 | 91 |
| HSA | insulin | −38 | loperamide | 153 | 92 |
| PLGA | ApoE | −12 | doxorubicin | 120 | 93 |
| SLN | transferrin | +4 | quinine | 126 | 94 |
| SLN | angiopep-2 | −16 | docetaxel | 111 | 95 |
| Adsorptive-mediated transport pathway | |||||
| Gelatin-siloxane | SynB | +32 | plasmid DNA | 194 | 96 |
| PEG-PLA | CBSA | −12 | coumarin-6 | 80–100 | 97,98 |
| BSA | −17 | ||||
| PLA | CBSA | −19 | sulpiride | 329 | 99 |
| PLA | Tat | +2 | ritonavir | 340 | 100 |
| SLN | RVG29 | −20 | quercetin | 201 | 101 |
Abbreviations: BSA: bovine serum albumin, anti-TfR antibody: anti-transferrin receptor antibody, HSA: human serum albumin, PLGA: poly(lactide-co-glycolide), ApoE: apolipoprotein E, SLN: solid lipid nanoparticles, PEG: poly(ethyleneglycol), PLA: poly(ethyleneglycol)–poly(lactide), CBSA: cationic bovine serum albumin, Tat: trans-activating transcriptor peptide from human immunodeficiency virus 1, RVG29: peptide fragment from rabies virus glycoprotein.
Significantly increased albumin transport was observed across a bovine brain endothelial co-culture model when albumin was loaded in cationic lipid-coated maltodextrin NPs.71 In another study also using cationic and neutral maltodextrin NPs and the same BBB model transcytosis did not differ significantly between the NP groups.72 The same conclusion can be drawn from experiments made with slightly cationic and anionic quaternary ammonium β-cyclodextrin using a mouse brain endothelial cell line model.51 Importantly, studies showed that NPs with positive surface charge (high zeta potential: 45 mV) and at high concentration might cause BBB toxicity in animal models.70,73 In addition, positively charged NPs have a rapid plasma clearance rate and high liver uptake resulting in lower residence time in brain microvessels and reduced brain delivery.74,75
More and more comparative studies demonstrate that the non-targeted carriers with neutral or sightly negative charge are more efficient for drug delivery across the BBB (Table 1).3,76–78,80 In a stroke model positively charged liposomes were not detected in brain or plasma 90 minutes after their administration, but simvastatin-loaded liposomes with neutral or negative zeta potential were able to reach the brain and accumulate specifically in the infarcted area.77 Moreover, neutral liposomes showed higher bioavailability in plasma after 4-hour treatment.77 Anionic multiwalled carbon nanotubes had the highest transport levels across hCMEC/D3, a simplified culture model of the human BBB, compared to neutral or cationic nanotubes.78 Negatively charged amino-Qdots (-32 mV) had significantly better penetration across a rat primary BBB co-culture model compared to Qdots with neutral or positive charge.79 Pegylated polystyrene NPs with neutral/slightly negative charge remained more stable in conditions relevant to the brain microenvironment than highly negative carboxyl-coated polystyrene NPs, and had higher diffusion capabilities in a brain tissue slice model.80 These results demonstrated that the colloidal stabilization of neutral or slightly negative charged NPs resulted in increased diffusive capability of the particles in the brain microenvironment.80
During the design of NP drug delivery systems, it is very important to take into account the interaction of serum or blood with NPs. The adsorption of serum proteins onto the NP surface, called protein corona, can cause a shift in zeta potential to slightly negative range regardless of the original surface charge (positive/neutral/negative) and modify the behavior of nanomaterials.74 This opsonization can be minimized with pegylation.74
Targeted nanoparticles
Although charged nanocarriers can show elevated cell penetration mainly in the periphery, there is no optimal CNS targeting without functionalization of NPs with BBB specific transporter ligands.69,81 Targeting ligands bind to their specific transporters on the surface of brain microvascular endothelial cells and enhance the entry of NPs into the brain tissue as molecular Trojan horses.82–85 The main endogenous influx transport systems of the BBB, such as the carrier mediated (CMT), the receptor mediated (RMT), and the adsorptive mediated (AMT) transport systems (Table 2) can be potentially exploited to shuttle nanocarriers into the brain.2
Glutathione (GSH) is one of the most successful BBB targeting ligand for NPs. The GSH-pegylated liposomal doxorubicin reached phase I/IIa clinical trials.102–103 Several studies proved the active cellular uptake of these slightly negatively charged (−8 mV) GSH-labeled liposomes demonstrating its BBB targeting efficacy.102–103 The transporter(s) of GSH at the brain microvasculature is unknown, it is assumed that it belongs to the CMT system.104 In a human in vitro BBB model, the more negatively charged (−24 mV) biotinylated GSH-labeled polystyrene NPs showed elevated BBB crossing compared to the non-targeted ones.89 Among the CMT targeted NPs, glucose-coated nanoparticles targeting the GLUT-1 transporter have been highly investigated as potential carriers of drugs across the BBB.105–109 The glucosamine coupled nonionic surfactant vesicles with negative zeta potential (−17 mV) were capable to target the GLUT-1 carrier and successfully cross the BBB.88 The small neutral amino acid, alanine is also a promising BBB targeting CMT ligand. Alanine and GSH dual-targeted niosomes with slightly negative charge (−7 mV) elevated NP permeability across the BBB both in a culture model and in mice compared to single ligand labeling.86 In a follow-up study, these dual-ligand decorated niosomes not only showed increased penetration across a BBB co-culture model, but enhanced the delivery of a large protein cargo into cultured cells of the neurovascular unit, namely brain endothelial cells, pericytes, astrocytes and neurons.87
Regarding the RMT systems of the BBB, transferrin receptor (TfR) is one of the most studied receptors with a potential for transporting drugs or drug-loaded NPs into the brain.110 TfRs are highly expressed on endothelial cells of the brain, but not on peripheral endothelial cells, which makes them an interesting target for drug delivery. Drug cargo of transferrin coupled NPs successfully crossed the BBB in animal studies: either they were highly negatively charged (−28 mV) human serum albumin nanocarriers91 or slightly positively charged solid lipid NPs (+4 mV).94 There was a dose-dependent impact of anti-TfR antibody labeling on the charge of gold nanocarriers: higher receptor density on the surface of NPs resulted in a more negative zeta potential (from −4 mV to –7 mV) as well as improved brain transport.90 Other RMT systems were also examined for CNS delivery of NPs: the highly negative ApoE, angiopep-2 or insulin functionalized nanocarriers targeting the low-density lipoprotein receptor-related protein-193,95 or the insulin receptor,92 respectively, proved to be successful drug delivery systems in experiments.
NPs functionalized with cationic proteins or cell-penetrating peptides enter the brain via AMT, based on the interaction of cationic carriers with the anionic surface of brain endothelial cells.34 Cationic bovine serum albumin (CBSA) conjugated with polymer NP loaded with coumarin-6, as compared to bovine serum albumin-labeled NPs, showed a higher brain penetration in mice98 and elevated cellular uptake and permeability in a rat BBB culture model.97 Importantly, the functionalization with CBSA does not change the negative charge of the NPs to positive, just shift the originally negative charge into less negative (Table 2).97,98 In the literature there is a huge amount of data confirming the effectivity of cell-penetrating peptides in drug delivery, although they are not BBB specific.36 Positively charged NPs targeted with different cell-penetrating peptides, like SynB or Tat showed improved BBB crossing.96,100 However, negatively charged NPs targeted with RVG peptide can also penetrate across a human BBB model effectively.101
In a recent study, our group demonstrated that the surface charge of brain endothelial cells has a direct role in the permeability of targeted vesicular NPs.86 In these experiments two approaches were used to make the endothelial surface charge more positive (Figure 2). Treatment of brain endothelial cells with neuraminidase, removing sialic acid residues from the endothelial surface glycocalyx, increased the cellular uptake of GSH and alanine dual-targeted NPs (−7 mV) compared with both the non-targeted and non-treated groups. Treatment with the cationic lipid TMA-DPH, which intercalates to the plasma membrane, also elevated the uptake of the NPs.86 These results indicate that surface charge at the BBB is important in the uptake mechanism of charged NPs and that internalization of NPs can be modulated by modification of the brain endothelial surface charge.
The overall message of these studies is that targeting specific BBB transport systems is more important for the successful brain delivery of NPs than zeta potential. In several cases, the targeting ligand on the surface of the NPs decreases the original charge of the particles and results better uptake in cells and crossing the BBB. It also seems that the density of targeting ligands on the surface of nanocarriers is also a more decisive factor as compared to charge. However, reliable comparative studies investigating the role of the charge in the case of targeted NPs are missing. Harmonization in the conditions of zeta potential measurements would also be needed to get more conclusive results in this underrepresented area of NP studies.
4. BBB glycocalyx changes in disease conditions: shedding and protection
The protective role of the surface sugar coat and the consequences of the damaged vascular endothelial surface glycocalyx were already explored in many diseases, such as atherosclerosis, stroke, hypertension, diabetes, cancer, inflammation, bacterial and viral infections, see extensive reviews.111–116 A number of studies examined diseases and peripheral vascular endothelium, but fewer concentrated on exploring the function, morphology and protection of brain microvascular endothelial glycocalyx in pathologies affecting the CNS. A review detailing brain microvessel extracellular matrix components, including glycocalyx molecules, and their function and synthesis at the level of the BBB in aging and neurodegeneration has just recently been published,17 therefore these conditions are not detailed in the present article.
Hypertension and stroke are the most common pathologies affecting the CNS. Early studies investigating the morphology and surface charge of brain endothelial cells in hypertensive rats showed that elevated blood pressure caused a loss of terminal sialic acid groups, which were quickly recovered after the elimination of the stressor.117 Brain endothelial glycocalyx was found to be damaged and BBB permeability increased for horseradish peroxidase in the hypothalamus and hippocampus of chronic hypertension- and stroke-prone rat models as compared to wild type Wistar rats.118,119 In stroke, one of the key modulators of BBB integrity and glycocalyx thickness was found to be the growth factor angiopoietin-2 (Ang-2).120 In physiological circumstances it facilitates the angiogenesis through a competitive binding to the Tie2 receptor on brain microvascular endothelial cells. In adult brains, this molecule is present mostly in tumor vessels. Ang-2 increased BBB permeability and reduced pericyte and glycocalyx coverage in gain-of-function mice, and its overexpression worsened stroke severity.120 Ang-1, a brain pericyte-derived mediator of BBB maturation and junctional tightness is antagonizing the effects of Ang-2.121 Ang-1 is also constitutively expressed in pericytes around mesenteric microvessels, and prevented vascular leakage stabilizing basal microvessel permeability possibly through the thickening of the glycocalyx and enriching its GAG components.122 It is possible, that Ang-1 may increase glycocalyx density at the BBB, too, but this has not been investigated yet. In a photothrombosis mouse model of stroke a lower level of heparan sulfate and chondroitin sulfate but an elevated activity of hyaluronidase and heparanase were found in the miscrovessels of ischemic brain tissue indicating glycocalyx damage of the brain endothelium.123 Glycocalyx changes were also observed in other CNS diseases. During epilepsy the decreased BBB integrity resulted in elevated seizure activity, partly because of possible changes at the glycocalyx.124 This hypothesis was later proven in a mouse study, where during status epilepticus glycocalyx was degraded, BBB permeability and brain edema were increased, and these effects were mitigated by the addition of heparin, a polyanion heparan sulfate GAG.125
Although there are many studies which detail the effects of stress, diabetes, cancer and infections on the endothelial glycocalyx in the periphery, only a few studies focused on the glycocalyx at the BBB. In a rat model perinatal stress elevated BBB permeability for WGA-horseradish peroxidase complex.126 Reversible thickening of the brain endothelial glycocalyx along with an increase of vesicular transport was also observed.126 Microvascular damage in several organs is well-known in diabetes,54 however the glycocalyx changes at the level of the microvascular endothelial cells in the brain are understudied. In a type-2 diabetes mouse model no increase in BBB permeability was observed, but the disruption of glycocalyx was demonstrated by cationic ferritin.127 Studies related to cancer cells and BBB have shown that in vitro soluble factors from a C6 glioma cell line could modulate the lectin-binding capacity, therefore the surface glycocalyx components of bovine and human brain endothelial cell lines.128 The secreted factors or the presence of non-small cell lung cancer cell lines caused an elevated E-selectin expression along with glycocalyx degradation in cultured human cerebral microvascular endothelial cells facilitating tumor cell adhesion.129 Regarding bacterial infections, the adherence of Haemophilus somnus to cultured bovine brain endothelial cells was mediated through heparin-binding and was reduced by enzyme digestion of the glycocalyx.130 Endothelial glycocalyx also plays an important role in virus binding and invasion of different organs. In severe SARS-CoV-2 infection generalized inflammation and vasculitis occurs, which damages mostly the lung.131 Based on a sepsis study in mice in which lung capillaries were more susceptible to inflammatory damage than those in the brain,23 it was hypothesized, that the thinner glycocalyx of lung microvessels can also be related to the prominent lung damage COVID-19.131
Glycocalyx components as biomarkers
The field studying the disruption of glycocalyx in different diseases and using the shedded sugar-protein elements as biomarkers is rising.114,115,132,133 There are only a handful studies focusing on glycocalyx elements as biomarkers in CNS diseases. Elevated level of hyaluronic acid in the cerebrospinal fluid was found in patients with vascular dementia, which was higher in individuals with preexisting white matter malformations or infarction.134 In a rat experimental autoimmune encephalomyelitis model GAGs chondroitin sulfate, hyaluronic acid and heparan sulfate could be detected in the blood, while the levels of syndecans were not different from the control.135 During sepsis proinflammatory cytokine levels, such as interleukin-1 and −8, and tumor necrosis factor-α were elevated, while levels of biomarkers reflecting BBB opening (glial fibrillary acidic protein, neuron-specific enolase or S100β protein) were also increased.136
Shedded heparan sulfate fragments could contribute to septic cognitive impairment by possibly crossing the BBB and inhibiting long-term potentiation causing memory deficits.137 This observation was also corroborated by another study from the same group describing that circulating heparan sulfates selectively penetrate to hippocampus tissue causing functional disturbances.138 During experimental hemorrhagia presence of syndecan-1 and heparan sulfate in the circulation could serve as reliable biomarkers of endothelial glycocalyx degradation.139 From these studies we can conclude that besides inflammatory, and other disease-specific markers, the detection of circulating glycocalyx components in body fluids can serve as markers of endothelial damage in CNS diseases, too.
Protection
The role of glycocalyx shedding in diseases is gaining more and more attention, still no specific therapy exists for the protection and regeneration of endothelial glycocalyx.54,140 Components of glycocalyx or plasma, containing albumin and other blood factors stabilizing endothelial glycocalyx, are used in experiments and in clinical studies for this purpose. Endothelial glycocalyx restoration was accelerated by the addition of sulodexide, a mixture of heparan and dermatan sulfates, in sepsis in vitro and in vivo48,141 and in diabetic patients.48 In a rat model of hemorrhagic shock microvascular endothelial glycocalyx was reconstructed and glycocalyx shedding was decreased using combinations of drugs and adjuvants, such as adenosine-lidocaine-magnesium or β-hydroxybutyrate-melatonin and poloxamer 188.142 Fresh frozen plasma was also successfully used to protect glycocalyx in hemorrhagic shock in experimental models as well as in patients.143,144 Albumin was also tested in experimental hemorrhagia, and stabilized vascular permeability, but was less effective to restore endothelial glycocalyx thickness as fresh frozen plasma.141 Pooled and pathogen-reduced plasma attenuated brain edema and at the same time reduced glycocalyx shedding in traumatic brain injury patients.145
Another approach is the use of colloids as high viscosity blood volume expanders. The administration of starch colloids after hemorrhage is debated due to their potential nephrotoxic and coagulation impairing effects.146 The direct effect of hydroxyethyl starch was tested in a mouse in vitro BBB model and exerted beneficial, barrier integrity elevating effect on brain capillary endothelial cells.147
In addition to fresh frozen plasma, albumin and starch colloids, glucocorticoids, which decrease inflammation, are clinically used to restore the function and composition of the endothelial surface glycocalyx.113 Glucocorticoids, such as hydrocortisone are well known to decrease BBB permeability26 and were also used to attenuate brain endothelial glycocalyx damage. Brain edema and BBB injury were decreased by hydrocortisone treatment after cardiac arrest and hypoxia in rats.47 Corticosteroids and antithrombin therapy prevented glycocalyx loss, reduced inflammation and prevented the elevation of BBB permeability in mice with cerebral malaria, while the inhibition of matrix metalloproteinases did not have an effect on the pathomechanism.148 We hypothesize that depending on the diseases and pathomechanisms different therapeutic approaches might be beneficial. More studies would be needed on CNS pathologies involving BBB impairment to find treatments to protect brain endothelial glycocalyx.
5. Measurement and visualization of BBB surface charge
In the previous parts of the review, we summarized the main sources and the physiological role of the negative surface charges in the BBB function. Here we would like to give a general biophysical background on their mechanism of action and their measurement, with special focus on biological barrier systems.
The actual surface charge pattern can be considered as a quasi-2D structure on the membrane-electrolyte interface Q(x,y, t), giving rise to an electric field, characterized by a field-strength vector space (E(r, t) and a potential space V(r, t), interacting with mobile charge carriers (molecules or ions). The (quasi-) static components of the electric field (E(r), V(r)), that do not depend on explicit time on short time scales (∂E/∂t ≈ 0, ∂V/∂t ≈ 0), stem from fixed charges at the membrane interface (forming a quasi-static Q(x,y) charge distribution), and give a framework of boundary conditions for dynamic processes determined by mobile charge carriers, such as ions or small molecules. Hence, among other types of physical interactions (such as the hydrophobic and van der Waals effects), the surface charge of the membrane controls the transport properties of the barrier to a large extent, therefore, the measurement and visualization of Q(x,y) is of primary importance in understanding the physiology of BBB. Mapping of Q(x,y) can be theoretically accomplished via measuring E(r) or V(r), which is, however, an extremely challenging task in a complex biological environment.
In the direction perpendicular to the hypothetical membrane surface (z), mobile counterions of the electrolyte forming the diffuse double layer screen the electric field of the “fix” (membrane-bound) charges, and in a low-potential limit, decrease V(z) exponentially by a space constant called Debye length (λD): V(z) = V0·exp(-z/λD), where V0 is the surface potential.149 Inside the membrane, the surface charge of the membrane lipids, together with water molecules occluded at the membrane surface, gives rise to the elusive dipole potential, which could not be measured precisely, so far, with the available experimental techniques.
From the electrolyte side, atomic force microscopy (AFM) and Kelvin probe force microscopy (KPFM) also known as surface potential microscopy, a noncontact variant of AFM, have been successfully used to map the surface charge of some biological organelles and macromolecules, attached to a rigid, solid surface.150,151 Based on the distance-dependence of the force curves along the z axis, characterizing the interaction of the charged tip and the surface charges, the longer-range electric forces could be discriminated from the shorter-range other interactions (the hydrophobic and van der Waals forces, as well as the Pauli-repulsion occurring due to overlapping electron clouds). In the case of living endothelial cells constituting BBB, however, the elastic deformation of the soft cell and the glycocalyx layer might well exceed the Debye-length, which, collapses to the subnanometer scale under physiological salt conditions,150 that makes such a discrimination difficult to accomplish. This must be among the primary reasons why surface force microscopic techniques have not been applied to map the electric charge of biological barriers, so far.
Recently, by a microelectrode technique using a micropipette-based measuring electrode, similar to a patch-clamp electrode, but immersed in the diffuse double layer, and a reference electrode in the bulk, the electric conductivity has been measured, probing the local charge density near the surface, proportional to the surface charge.152 The technique called scanning ion conductance microscopy (SICM) has, so far, been applied only to corn plant root-hair and human adipocyte cells. Although the method is technically challenging, and the lateral resolution of the method is limited, amongst others, by the size of the micro/nanopipette to a few 100 nanometers, it is a promising attempt to map the surface charge of living cells or cell layers, with high accuracy.
Laser-Doppler velocimetry
As far as biological barriers are concerned, however, it is not usually necessary to provide a detailed spatial information about the charge distribution at the interface, but an average value characteristic of surface charge density proves to be sufficient to understand the rule of thumb determining the relationship between surface charge properties and biological function. For this purpose, experiments utilizing electrokinetic phenomena can be applied. The measured electric parameter is generally the so-called zeta-potential (Vζ), that represents a sort of “damped” surface potential, due to the screening effect of the counterions in the diffuse double layer. By definition, the zeta-potential is the electric potential difference between the surface of the “shear layer” (that part of the interfacial fluid that remains attached to the surface under flow conditions, of a thickness δ) and the bulk, and it is proportional to the average surface charge density (σ), according to the linearized Grahame equation for low surface potentials: Vζ = (σ·λD/ε·ε0)·exp(-δ/λD), where ε0 and ε are absolute and relative electric permittivity, respectively.149
The usual way to measure zeta-potential of individual cells is a method borrowed from colloidics: laser-Doppler velocimetry (LDv).153 It measures the electrophoretic mobility of particles suspended in the electrolyte via detecting the Doppler-shift in the light scattered by the moving cells illuminated by two coherent beams of a narrow-band laser. The zeta-potential is calculated from Henry’s equation: Vζ = 3η·UE/(2 ε·ε0· f(κa)), where z is the zeta-potential, UE is the electrophoretic mobility, η is the viscosity, κ = 1/ λD, (i.e., the reciprocal of the Debye-length), “a” is the particle radius, and f(κa) is Henry’s function, which is ≈ 1.5 if κa >> 1, the so-called Smoluchowski limit, to a good approximation valid for cells in physiological salt.
Castanho et al. have measured the zeta-potential of various mammalian cells this way (Figure 3), and showed that the negative surface charge density of brain endothelial cells is much higher than that of other types of cells in the body, including peripheral vascular endothelial cells.16 Using the same technique, Santa Maria et al. have recently pointed out that treating brain endothelial cells with the cationic and lipophilic drug lidocain, considerably increased their zeta-potential (Figure 2), and in a separate experiment they showed that lidocaine also affected the permeability properties of BBB models for another cationic molecule.42 In addition to these observations we recently described using LDv measurements that the co-culture of human brain endothelial cells with pericytes along with the introduction of shear stress using fluid flow led to a more negative zeta potential and increased expression of glycocalyx-related genes.154
Figure 3.
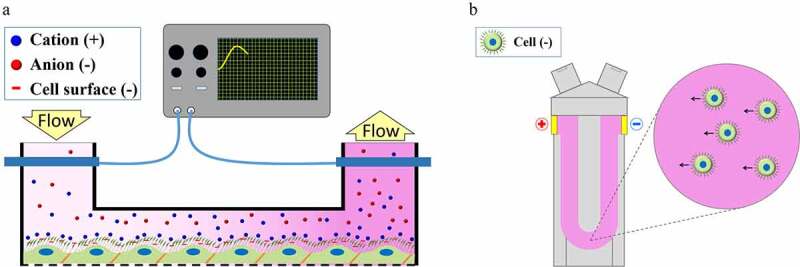
Laser-Doppler velocimetry and streaming potential to measure the zeta potential of brain endothelial cells (modified from46)
Although the LDv technique (Figure 3) works adequately on individual cells, it could not be adapted to the in situ measurement of zeta potential on confluent cell layers. In addition, as far as BBB is concerned, a common shortcoming of the LDv, AFM, KPFM, and SICM methods is that they are not applicable either in the inside of blood vessels for in vivo, or in microfluidic channels for in vitro measurements. Some other electrokinetic methods, on the other hand, that are utilizing fluid flow along solid surfaces, are suitable for such purposes.
Streaming potential and electro-osmosis techniques
Streaming potential (SP) is an electric potential difference developed in the longitudinal direction of a blood vessel or a microchannel, when the fluid (an electrolyte solution) filling it up moves by laminar flow. Under such conditions, the moving part of the counterion cloud of the diffuse double layer (carrying a net charge) is dragged by the flow, giving rise to an electric current and potential difference between a pair of electrodes separated alongside the channel. Under stationary conditions, the SP (Ustr) is proportional to the zeta potential, according to the Helmholtz–Smoluchowski equation: Ustr = (ε·ε0·Vζ /η·Ke)·ΔP, where Ke is the conductivity of the bulk solution, and ΔP is the pressure gradient driving the flow.
The electro-osmosis technique, which is the inverse electrokinetic phenomenon of streaming potential, was applied to determine the surface charge of different cultured cells.155 In this method, electric potential difference is applied along a microfluidic channel which induces a flow of the electrolyte solution. However, this type of biophysical measurement has not become widely used for biological systems.
The SP of different organs, including large blood vessels, was investigated by microelectrodes in animals or in ex vivo tissues. SPs generated by the bloodstream in rabbit aorta and vena cava were measured parallel to the vessel surface, and it was revealed that the endothelial surface was highly negatively charged at physiological pH.156 Technical advances in biomedical research, especially the introduction of microfluidic lab-on-a-chip devices, led to the extension of the streaming potential technique to investigate a culture model of the BBB. For these experiments we upgraded our lab-on-a-chip device developed for the study of culture models of different biological barriers157 with two Ag/AgCl electrodes and successfully measured the SP of confluent monolayers of brain endothelial cells (Figure 3).46
We have verified this novel method by comparing the streaming potential data measured in the chip device and zeta potential results by the LDv method. To change the negative surface charge of the BBB model we applied neuraminidase enzyme that cleaves sialic acid residues from the brain endothelial cell surface glycocalyx and lidocaine that makes the lipid membrane charge more positive (Figure 2). The changes in the surface charge could be measured by both the novel LOC device on cell monolayers and by LDv in cell suspensions.46 This device can be especially useful for studies on BBB culture and co-culture models to reveal how surface charge changes alter BBB functions in physiological or pathological conditions.
Imaging
Besides the biophysical measurements the primary methods of the investigation of glycocalyx composition, thickness, integrity or charge are techniques based on microscopical observation.20 The use of fluorescently labeled lectins, which bind specific sugars, is one of the most frequently performed method when labeling the glycocalyx in vivo or in vitro. To stain the glycocalyx of brain endothelial cells in rodents Griffonia (Bandeiraea) simplicifolia isolectin B4, specific for α-galactose, can be used,158 while in human brain microvessels Ulex europaeus lectin UEA-l binding to fucose gives good staining.159 Among lectins wheat germ agglutinin recognizing N-acetyl-d-glucosamine and sialic acid residues is often used to stain glycocalyx of cultured brain endothelial cells and it can be applied in both animal and human cells or samples.46,128,160–163 In a comparative study, brain microvessels and cultured brain endothelial cells were labeled with 14 lectins.164 This study found a similar labeling pattern of brain endothelial cells in vivo and in vitro. Recently, the composition and thickness of the glycocalyx in cultured brain endothelial cells was visualized and evaluated using immunocytochemistry followed by superresolution (STORM) microscopy.21 Recently, the ultrastructure of the glycocalyx in brain capillaries of mice was visualized with lanthanum-containing alkaline fixatives both in control conditions and in sepsis.23 The morphology and thickness of different cerebral vessels in live animals can be assessed using intravital microscopy165 or two-photon microscopy with or without lectin labeling.24,166
The above described techniques reveal the structure and composition of glycocalyx, but these methods do not provide information on the surface charge. To reveal the anionic sites at the level of the BBB, cationic probes were introduced and combined with transmission electron microscopy. The anionic sites at the luminal surface of cerebral endothelium could be labeled with positively charged colloidal iron in rat brains,7–9 cationic ferritin127,148,167,168 or by cationic gold and CBSA-gold conjugate particles in mouse brains.22,34 Cationic colloidal gold and cationized ferritin were also successfully used to reveal the surface anionic sites in cultured brain endothelial cells.40,169 Potentially other cationic probes, like Alcian blue, a copper containing dye, or the polycationic dye ruthenium red170 could also be used to reveal anionic sites at the BBB.
As compared to animal experimental and cell culture methods available to observe physiological and pathological changes of the glycocalyx, there are only limited resources to monitor endothelial glycocalyx integrity and changes in patients. A promising novel technique, sidestream dark field imaging was successfully used to assess glycocalyx thickness in sublingual blood vessels of volunteers,165 or in brain cortical and hippocampal microcirculation of patients.171 This method, especially during resective temporal lobe epilepsy surgeries, could enhance the evaluation of the status of the glycocalyx which is an important factor in BBB permeability. It would enable the better understanding of cerebral capillary glycocalyx thickness and function in different CNS pathologies.124,172
6. Future directions and perspectives
As compared to other biomedical fields, research on the vascular endothelial surface glycocalyx has not been in focus for many years. In the last 15 years its importance in pathologies affecting peripheral organs has become clear. The knowledge on endothelial cell glycocalyx mainly comes from studies on the heart, lungs, kidneys and gut vessels. Despite the growing number of papers on BBB and glycocalyx, there is still a gap between our understanding of this system in general and in the CNS. Many studies indicate that the glycocalyx at the BBB is different from those of other vascular endothelial cells, but the specific features of it are still to be discovered. The highly negative charge of the surface glycocalyx at the BBB cannot be neglected for drugs targeting the brain. Morphological changes, detection of shedded glycocalyx components and composition alterations need to be further investigated to gain a clearer picture in healthy brain and in CNS pathologies. We believe that the presented biophysical methods as well as the new microfluidic chip device will help to reach this goal.
Acknowledgments
The present work was supported by GINOP-2.2.1-15-2016-00007, GINOP-2.3.2-15-2016-00001, GINOP-2.3.2-15-2016-00037, GINOP-2.3.2-15-2016-00060, OTKA NNE 129617 as part of the M-Era.NET2 nanoPD project. SV is supported by the Hungarian Academy of Sciences Premium postdoctoral grant [Premium-2019-469]. FRW is currently supported by the National Research, Development and Innovation Office [PD-128480], by the János Bolyai Research Fellowship of the Hungarian Academy of Sciences [BO/00174/18], and by the Hungarian Ministry for Innovation and Technology’s New National Excellence Program Bolyai+ fellowship [UNKP-20-5-SZTE-672]. ARSM was supported by the European Training Network H2020-MSCA-ITN-2015 [675619] and UNKP-20-4-SZTE-593 scholarship. Drawings of Figure 1, 2were prepared with the basic outlines from Servier Medical Art (http://smart.servier.com/), licensed under a Creative Common Attribution 3.0 Generic License.
Funding Statement
This work was supported by the Hungarian Scientific Research Fund [PD-128480]; Magyar Tudományos Akadémia [Premium-2019-469]; Magyar Tudományos Akadémia [BO/00174/18]; Nemzeti Kutatási Fejlesztési és Innovációs Hivatal [GINOP-2.3.2-15-2016-00060]; Nemzeti Kutatási Fejlesztési és Innovációs Hivatal [OTKA NNE 129617]; Nemzeti Kutatási Fejlesztési és Innovációs Hivatal [GINOP-2.2.1-15-2016-00007]; Nemzeti Kutatási Fejlesztési és Innovációs Hivatal [GINOP-2.3.2-15-2016-00037]; Nemzeti Kutatási Fejlesztési és Innovációs Hivatal [GINOP-2.3.2-15-2016-00001]; Innovációs és Technológiai Minisztérium (HU) [UNKP-20-4-SZTE-593]; H2020 Marie Skłodowska-Curie Actions [H2020-MSCA-ITN-2015]; Innovációs és Technológiai Minisztérium (HU) [UNKP-20-5-SZTE-672].
Declaration of interest statement
We declare no conflict of interest regarding this manuscript.
References
- 1.Joó F.Endothelial cells of the brain and other organ systems: some similarities and differences. Prog Neurobiol. 1996;48(3):1–23. doi: 10.1016/0301-0082(95)00046-1. [DOI] [PubMed] [Google Scholar]
- 2.Abbott NJ. Blood-brain barrier structure and function and the challenges for CNS drug delivery. J Inherit Metab Dis. 2013;36(3):437–449. doi: 10.1007/s10545-013-9608-0. [DOI] [PubMed] [Google Scholar]
- 3.Turksen K, Troy TC . Barriers built on claudins. J Cell Sci. 2004; 117(Pt 12): 2435–2447. doi: 10.1242/jcs.01235. [DOI] [PubMed] [Google Scholar]
- 4.Deli MA. Potential use of tight junction modulators to reversibly open membranous barriers and improve drug delivery. Biochim Biophys Acta. 2009;1788(4):892–910. doi: 10.1016/j.bbamem.2008.09.016. [DOI] [PubMed] [Google Scholar]
- 5.Kaya M, Ahishali B. Basic physiology of the blood-brain barrier in health and disease: a brief overview. Tissue Barriers. 2021;9(1):1840913. doi: 10.1080/21688370.2020.1840913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Campos-Bedolla P, Walter FR, Veszelka S, Deli MA. Role of the blood-brain barrier in the nutrition of the central nervous system. Arch Med Res. 2014;45(8):610–638. doi: 10.1016/j.arcmed.2014.11.018. [DOI] [PubMed] [Google Scholar]
- 7.Nagy Z, Peters H, Hüttner I. Endothelial surface charge: blood-brain barrier opening to horseradish peroxidase induced by the polycation protamin sulfate. Acta Neuropathol Suppl. 1981;7:7–9. doi: 10.1007/978-3-642-81553-9_2. [DOI] [PubMed] [Google Scholar]
- 8.Nagy Z, Peters H, Hüttner I. Charge-related alterations of the cerebral endothelium. Lab Invest. 1983;49:662–671. [PubMed] [Google Scholar]
- 9.Nagy Z, Szabó M, Hüttner I. Blood-brain barrier impairment by low pH buffer perfusion via the internal carotid artery in rat. Acta Neuropathol. 1985;68(2):160–163. doi: 10.1007/BF00688639. [DOI] [PubMed] [Google Scholar]
- 10.Joó F, Karnushina I. A procedure for the isolation of capillaries from rat brain. Cytobios. 1973;8:41–48. [PubMed] [Google Scholar]
- 11.Joó F. The blood-brain barrier in vitro: the second decade. Neurochem Int. 1993;23(6):499–521. doi: 10.1016/0197-0186(93)90098-p. [DOI] [PubMed] [Google Scholar]
- 12.Bénistant C, Dehouck MP, Fruchart JC, Cecchelli R, Lagarde M. Fatty acid composition of brain capillary endothelial cells: effect of the coculture with astrocytes. J Lipid Res. 1995;36(11):2311–2319. doi: 10.1016/S0022-2275(20)39712-1. [DOI] [PubMed] [Google Scholar]
- 13.Betsholtz C. Physiology: Double function at the blood-brain barrier. Nature. 2014;509(7501):432–433. doi: 10.1038/nature13339 [DOI] [PubMed] [Google Scholar]
- 14.Tewes BJ, Galla HJ. Lipid polarity in brain capillary endothelial cells. Endothelium. 2001;8(3):207–220. doi: 10.1080/10623320109051566. [DOI] [PubMed] [Google Scholar]
- 15.Campbell SD, Regina KJ, Kharasch ED, Campbell SD, Regina KJ, Kharasch ED. Significance of lipid composition in a blood-brain barrier-mimetic PAMPA assay. J Biomol Screen. 2014;19(3):437–444. doi: 10.1177/1087057113497981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ribeiro MMB, Domingues MM, Freire JM, Santos NC, Castanho MARB. Translocating the blood-brain barrier using electrostatics. Front Cell Neurosci. 2012;6:44. doi: 10.3389/fncel.2012.00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reed MJ, Damodarasamy M, Banks WA. The extracellular matrix of the blood-brain barrier: structural and functional roles in health, aging, and Alzheimer’s disease. Tissue Barriers. 2019;7(4):1651157. doi: 10.1080/21688370.2019.1651157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fu BM, Tarbell JM. Mechano-sensing and transduction by endothelial surface glycocalyx: composition, structure, and function. Wiley Interdiscip Rev Syst Biol Med. 2013;5(3):381–390. doi: 10.1002/wsbm.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zeng Y, Zhang XF, Fu BM, Tarbell JM. The role of endothelial surface glycocalyx in mechanosensing and transduction. Adv Exp Med Biol. 2018;1097:1–27. doi: 10.1007/978-3-319-96445-4_1. [DOI] [PubMed] [Google Scholar]
- 20.Reitsma S, Slaaf DW, Vink H, van Zandvoort MA, oude Egbrink MG. The endothelial glycocalyx: composition, functions, and visualization. Pflugers Arch. 2007;454(3):345–359. doi: 10.1007/s00424-007-0212-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fan J, Sun Y, Xia Y, Tarbell JM, Fu BM, Tarbell J, Vink H. Endothelial surface glycocalyx (ESG) components and ultra-structure revealed by stochastic optical reconstruction microscopy (STORM). Biorheology. 2019;56(2–3):77–88. doi: 10.3233/BIR-180204. [DOI] [PubMed] [Google Scholar]
- 22.Vorbrodt AW. Ultracytochemical characterization of anionic sites in the wall of brain capillaries. J Neurocytol. 1989;18(3):359–368. doi: 10.1007/BF01190839. [DOI] [PubMed] [Google Scholar]
- 23.Ando Y, Okada H, Takemura G, Suzuki K, Takada C, Tomita H, Zaikokuji R, Hotta Y, Miyazaki N, Yano H, et al. Brain-specific ultrastructure of capillary endothelial glycocalyx and its possible contribution for blood brain barrier. Sci Rep. 2018;8(1):17523. doi: 10.1038/s41598-018-35976-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kutuzov N, Flyvbjerg H, Lauritzen M. Contributions of the glycocalyx, endothelium, and extravascular compartment to the blood-brain barrier. Proc Natl Acad Sci U S A. 2018;115(40):E9429–E9438. doi: 10.1073/pnas.1802155115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li G, Yuan W, Fu BM. A model for the blood-brain barrier permeability to water and small solutes. J Biomech. 2010;43(11):2133–2140. doi: 10.1016/j.jbiomech.2010.03.047. [DOI] [PubMed] [Google Scholar]
- 26.Deli MA, Ábrahám CS, Kataoka Y, Niwa M. Permeability studies on in vitro blood-brain barrier models: physiology, pathology, and pharmacology. Cell Mol Neurobiol. 2005;25(1):59–127. doi: 10.1007/s10571-004-1377-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Deli MA. Drug transport and the blood-brain barrier: chapter 8. Tihanyi K, Vastag M. editors. Solubility, delivery, and ADME problems of drugs and drug-candidates. ISBN: 978-1-60805-619-4. Bentham Science Publishers Ltd; 2011. 144–165. doi: 10.2174/978160805120511101010144 [DOI] [Google Scholar]
- 28.Banks WA. From blood-brain barrier to blood-brain interface: new opportunities for CNS drug delivery. Nat Rev Drug Discov. 2016;15(4):275–292. doi: 10.1038/nrd.2015.21. [DOI] [PubMed] [Google Scholar]
- 29.Sahagun G, Moore SA, Hart MN. Permeability of neutral vs. anionic dextrans in cultured brain microvascular endothelium. Am J Physiol. 1990;259(1Pt2):H162-H166. doi: 10.1152/ajpheart.1990.259.1.H162 [DOI] [PubMed]
- 30.Leveugle B, Ding W, Laurence F, Dehouck M-P, Scanameo A, Cecchelli R, Fillit H. Heparin oligosaccharides that pass the blood-brain barrier inhibit β-amyloid precursor protein secretion and heparin binding to β-amyloid peptide. J Neurochem. 1998;70(2):736–744. doi: 10.1046/j.1471-4159.1998.70020736.x. [DOI] [PubMed] [Google Scholar]
- 31.Smith KR, Borchardt RT. Permeability and mechanism of albumin, cationized albumin, and glycosylated albumin transcellular transport across monolayers of cultured bovine brain capillary endothelial cells. Pharm Res. 1989;6(6):466–473. doi: 10.1023/a:1015960205409. [DOI] [PubMed] [Google Scholar]
- 32.Girod J, Fénart L, Régina A, Dehouck M-P, Hong G, Schermann J-M, Cecchelli R, Roux F. Transport of cationized anti-tetanus Fab’2 fragments across an in vitro blood-brain barrier model: involvement of the transcytosis pathway. J Neurochem. 1999;73(5):2002–2008. doi: 10.1046/j.1471-4159.1999.02002.x. [DOI] [PubMed] [Google Scholar]
- 33.Li G, Fu BM. An electrodiffusion model for the blood-brain barrier permeability to charged molecules. J Biomech Eng. 2011;133(2):021002. doi: 10.1115/1.4003309. [DOI] [PubMed] [Google Scholar]
- 34.Hervé F, Ghinea N, Scherrmann J-M. CNS delivery via adsorptive transcytosis. AAPS J . 2008;10(3):455–472. doi: 10.1208/s12248-008-9055-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Toth AE, Holst MR, Nielsen MS. Vesicular transport machinery in brain endothelial cells: what we know and what we do not. Curr Pharm Des. 2020;26(13):1405–1416. doi: 10.2174/1381612826666200212113421. [DOI] [PubMed] [Google Scholar]
- 36.Kristensen M, Kucharz K, Felipe Alves Fernandes E, Strømgaard K, Schallburg NM, Cederberg HHC, Bach A, Ulrikkaholm Tofte-Hansen M, Irene Aldana Garcia B, Lauritzen M, et al. Conjugation of therapeutic PSD-95 inhibitors to the cell-penetrating peptide Tat affects blood-brain barrier adherence, uptake, and permeation. Pharmaceutics. 2020;12(7):661. doi: 10.3390/pharmaceutics12070661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bocsik A, Walter FR, Gyebrovszki A, Fülöp L, Blasig I, Dabrowski S, Ötvös F, Tóth A, Rákhely G, Veszelka S, et al. Reversible opening of intercellular junctions of intestinal epithelial and brain endothelial cells with tight junction modulator peptides. J Pharm Sci. 2016;105(2):754–765. doi: 10.1016/j.xphs.2015.11.018. [DOI] [PubMed] [Google Scholar]
- 38.Bocsik A, Gróf I, Kiss L, Ötvös F, Zsíros O, Daruka L, Fülöp L, Vastag M, Á K, Imre N, et al. Dual action of the PN159/KLAL/MAP peptide: increase of drug penetration across Caco-2 intestinal barrier model by modulation of tight junctions and plasma membrane permeability. Pharmaceutics. 2019;11(2):73. doi: 10.3390/pharmaceutics11020073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Westergren I, Johansson BB. Altering the blood-brain barrier in the rat by intracarotid infusion of polycations: a comparison between protamine, poly-L-lysine and poly-L-arginine. Acta Physiol Scand. 1993;149(1):99–104. doi: 10.1111/j.1748-1716.1993.tb09597.x. [DOI] [PubMed] [Google Scholar]
- 40.Hart MN, VanDyk LF, Moore SA, Shasby DM, Cancilla PA. Differential opening of the brain endothelial barrier following neutralization of the endothelial luminal anionic charge in vitro. J Neuropathol Exp Neurol. 1987;46(2):141–153. doi: 10.1097/00005072-198703000-00003. [DOI] [PubMed] [Google Scholar]
- 41.Ribeiro MMB, Pinto ART, Domingues MM, Serrano I, Heras M, Bardaji ER, Tavares I, Castanho MA. Chemical conjugation of the neuropeptide kyotorphin and ibuprofen enhances brain targeting and analgesia. Mol Pharm. 2011;8(5):1929–1940. doi: 10.1021/mp2003016. [DOI] [PubMed] [Google Scholar]
- 42.Santa-Maria AR, Walter FR, Valkai S, Brás AR, Mészáros M, Kincses A, Klepe A, Gaspar D, Castanho MARB, Zimányi L, et al. Lidocaine turns the surface charge of biological membranes more positive and changes the permeability of blood-brain barrier culture models. Biochim Biophys Acta Biomembr. 2019;1861(9):1579–1591. doi: 10.1016/j.bbamem.2019.07.008. [DOI] [PubMed] [Google Scholar]
- 43.Born GV, Palinski W. Unusually high concentrations of sialic acids on the surface of vascular endothelia. Br J Exp Pathol. 1985;66:543–549. [PMC free article] [PubMed] [Google Scholar]
- 44.dos Santos WL, Rahman J, Klein N, Male DK. Distribution and analysis of surface charge on brain endothelium in vitro and in situ. Acta Neuropathol. 1995;90(3):305–311. doi: 10.1007/BF00296515. [DOI] [PubMed] [Google Scholar]
- 45.Betteridge KB, Arkill KP, Neal CR, Harper SJ, Foster RR, Satchell SC, Bates DO, Salmon AHJ. Sialic acids regulate microvessel permeability, revealed by novel in vivo studies of endothelial glycocalyx structure and function. J Physiol. 2017;595(15):5015–5035. doi: 10.1113/JP274167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kincses A, Santa-Maria AR, Walter FR, Dér L, Horányi N, Lipka DV, Valkai S, Deli MA, Dér A. A chip device to determine surface charge properties of confluent cell monolayers by measuring streaming potential. Lab Chip. 2020;20(20):3792–3805. doi: 10.1039/D0LC00558D. [DOI] [PubMed] [Google Scholar]
- 47.Zhu J, Li X, Yin J, Hu Y, Gu Y, Pan S. Glycocalyx degradation leads to blood-brain barrier dysfunction and brain edema after asphyxia cardiac arrest in rats. J Cereb Blood Flow Metab. 2018;38(11):1979–1992. doi: 10.1177/0271678X17726062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Broekhuizen LN, Lemkes BA, Mooij HL, Meuwese MC, Verberne H, Holleman F, Schlingemann RO, Nieuwdorp M, Stroes ES, Vink H. Effect of sulodexide on endothelial glycocalyx and vascular permeability in patients with type 2 diabetes mellitus. Diabetologia. 2010;53(12):2646–2655. doi: 10.1007/s00125-010-1910-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sörensson J, Matejka GL, Ohlson M, Haraldsson B. Human endothelial cells produce orosomucoid, an important component of the capillary barrier. Am J Physiol. 1999;276(2):H530–H534. doi: 10.1152/ajpheart.1999.276.2.H530. [DOI] [PubMed] [Google Scholar]
- 50.Yuan W, Li G, Zeng M, Fu BM. Modulation of the blood-brain barrier permeability by plasma glycoprotein orosomucoid. Microvasc Res. 2010;80(1):148–157. doi: 10.1016/j.mvr.2010.03.011. [DOI] [PubMed] [Google Scholar]
- 51.Yuan W, Li G, Gil ES, Lowe TL, Fu BM. Effect of surface charge of immortalized mouse cerebral endothelial cell monolayer on transport of charged solutes. Ann Biomed Eng. 2010;38(4):1463–1472. doi: 10.1007/s10439-010-9920-x. [DOI] [PubMed] [Google Scholar]
- 52.Zhang S, Mark KS. α1-Acid glycoprotein induced effects in rat brain microvessel endothelial cells. Microvasc Res. 2012;84(2):161–168. doi: 10.1016/j.mvr.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Maffrand JP, Herbert JM, Bernat A, Defreyn G, Delebassee D, Savi P, Pinot JJ, Sampol J. Experimental and clinical pharmacology of pentosan polysulfate. Semin Thromb Hemost. 1991;17:186–198. [PubMed] [Google Scholar]
- 54.Tarbell JM, Cancel LM. The glycocalyx and its significance in human medicine. J Intern Med. 2016;280(1):97–113. doi: 10.1111/joim.12465. [DOI] [PubMed] [Google Scholar]
- 55.Kumagai K, Shirabe S, Miyata N, Murata M, Yamauchi A, Kataoka Y, Niwa M. Sodium pentosan polysulfate resulted in cartilage improvement in knee osteoarthritis - An open clinical trial. BMC Clin Pharmacol. 2010;10:7. doi: 10.1186/1472-6904-10-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dräger LJ, Julius U, Kraenzle K, Schaper J, Toepfer M, Zygan K, Otto V, Steinhagen-Thiessen E. DALI-the first human whole-blood low-density lipoprotein and lipoprotein (a) apheresis system in clinical use: procedure and clinical results. Eur J Clin Invest. 1998;28(12):994–1002. doi: 10.1046/j.1365-2362.1998.00395.x. [DOI] [PubMed] [Google Scholar]
- 57.Deli MA, Ábrahám CS, Takahata H, Katamine S, Niwa M. Pentosan polysulfate regulates scavenger receptor-mediated, but not fluid-phase, endocytosis in immortalized cerebral endothelial cells. Cell Mol Neurobiol. 2000;20(6):731–745. doi: 10.1023/a:1007007026500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Deli MA, Sakaguchi S, Nakaoke R, Ábrahám CS, Takahata H, Kopáček J, Shigematsu K, Katamine S, Niwa M. PrP fragment 106-126 is toxic to cerebral endothelial cells expressing PrPC. Neuroreport. 2000;11(17):3931–3936. doi: 10.1097/00001756-200011270-00064. [DOI] [PubMed] [Google Scholar]
- 59.Veszelka S, Pásztói M, Farkas AE, Krizbai I, Ngo TK, Niwa M, Ábrahám CS, Deli MA. Pentosan polysulfate protects brain endothelial cells against bacterial lipopolysaccharide-induced damages. Neurochem Int. 2007;50(1):219–228. doi: 10.1016/j.neuint.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 60.Deli MA, Veszelka S, Csiszár B, Tóth A, Á K, Csete M, Sipos A, Szalai A, Fülöp L, Penke B, et al. Protection of the blood-brain barrier by pentosan against amyloid-β-induced toxicity. J Alzheimers Dis. 2010;22(3):777–794. doi: 10.3233/JAD-2010-100759. [DOI] [PubMed] [Google Scholar]
- 61.Fong CW. Statins in therapy: understanding their hydrophilicity, lipophilicity, binding to 3-hydroxy-3-methylglutaryl-CoA reductase, ability to cross the blood brain barrier and metabolic stability based on electrostatic molecular orbital studies. Eur J Med Chem. 2014;85:661–674. doi: 10.1016/j.ejmech.2014.08.037. [DOI] [PubMed] [Google Scholar]
- 62.Veszelka S, Tóth A, Walter FR, Tóth AE, Gróf I, Mészáros M, Bocsik A, É H, Vastag M, Rákhely G, et al. Comparison of a rat primary cell-based blood-brain barrier model with epithelial and brain endothelial cell lines: gene expression and drug transport. Front Mol Neurosci. 2018;11:166. doi: 10.3389/fnmol.2018.00166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mehta DC, Short JL, Hilmer SN, Nicolazzo JA. Drug access to the central nervous system in Alzheimer’s disease: preclinical and clinical insights. Pharm Res. 2015;32(3):819–839. doi: 10.1007/s11095-014-1522-0. [DOI] [PubMed] [Google Scholar]
- 64.Suzuki T, Moriki Y, Goto H, Tomono K, Hanano M, Watanabe J. Investigation on the influx transport mechanism of pentazocine at the blood-brain barrier in rats using the carotid injection technique. Biol Pharm Bull. 2002;25(10):1351–1355. doi: 10.1248/bpb.25.1351. [DOI] [PubMed] [Google Scholar]
- 65.Suzuki T, Ohmuro A, Miyata M, Furuishi T, Hidaka S, Kugawa F, Fukami T, Tomono K. Involvement of an influx transporter in the blood-brain barrier transport of naloxone. Biopharm Drug Dispos. 2010;31(4):243–252. doi: 10.1002/bdd.707. [DOI] [PubMed] [Google Scholar]
- 66.Germain M, Caputo F, Metcalfe S, Tosi G, Spring K, Ako Å, Pottier A, Schiffelers R, Ceccaldi A, Schmid R. Delivering the power of nanomedicine to patients today. J Control Release. 2020;326:164–171. doi: 10.1016/j.jconrel.2020.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hua S, Mbc DM, Metselaar JM, Storm G. Current trends and challenges in the clinical translation of nanoparticulate nanomedicines: pathways for translational development and commercialization. Front Pharmacol. 2018;9:790. doi: 10.3389/fphar.2018.00790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sercombe L, Veerati T, Moheimani F, Wu SY, Sood AK, Hua S. Advances and challenges of liposome assisted drug delivery. Front Pharmacol. 2015;6:286. doi: 10.3389/fphar.2015.00286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kevadiya BD, Ottemann BM, Thomas MB, Mukadam I, Nigam S, McMillan J, Gorantla S, Bronich TK, Edagwa B, Gendelman HE. Neurotheranostics as personalized medicines. Adv Drug Deliv Rev. 2019;148:252–289. doi: 10.1016/j.addr.2018.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wechsler ME, Ramirez JEV, Peppas NA. 110th Anniversary: nanoparticle Mediated Drug Delivery for the Treatment of Alzheimer’s Disease: crossing the Blood–Brain Barrier. Ind Eng Chem Res. 2019;58(33):15079–15087. doi: 10.1021/acs.iecr.9b02196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fenart L, Casanova A, Dehouck B, Duhem C, Slupek S, Cecchelli R, Betbeder D. Evaluation of effect of charge and lipid coating on ability of 60-nm nanoparticles to cross an in vitro model of the blood-brain barrier. J Pharmacol Exp Ther. 1999;291:1017–1022. [PubMed] [Google Scholar]
- 72.Jallouli Y, Paillard A, Chang J, Sevin E, Betbeder D. Influence of surface charge and inner composition of porous nanoparticles to cross blood-brain barrier in vitro. Int J Pharm. 2007;344(1–2):103–109. doi: 10.1016/j.ijpharm.2007.06.023. [DOI] [PubMed] [Google Scholar]
- 73.Lockman PR, Koziara JM, Mumper RJ, Allen DD. Nanoparticle surface charges alter blood-brain barrier integrity and permeability. J Drug Target. 2004;12(9–10):635–641. doi: 10.1080/10611860400015936. [DOI] [PubMed] [Google Scholar]
- 74.Kristensen K, Engel TB, Stensballe A, Simonsen JB, Andresen TL. The hard protein corona of stealth liposomes is sparse. J Control Release. 2019;307:1–15. doi: 10.1016/j.jconrel.2019.05.042. [DOI] [PubMed] [Google Scholar]
- 75.Lombardo SM, Schneider M, Türeli AE, Türeli NG. Key for crossing the BBB with nanoparticles: the rational design. Beilstein J Nanotechnol. 2020;11:866–883. doi: 10.3762/bjnano.11.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.MacKay JA, Deen DF, Szoka FC. Distribution in brain of liposomes after convection enhanced delivery; modulation by particle charge, particle diameter, and presence of steric coating. Brain Res. 2005;1035(2):139–153. doi: 10.1016/j.brainres.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 77.Campos-Martorell M, Cano-Sarabia M, Simats A, Hernández-Guillamon M, Rosell A, Maspoch D, Montaner J. Charge effect of a liposomal delivery system encapsulating simvastatin to treat experimental ischemic stroke in rats. Int J Nanomedicine. 2016;11:3035–3048. doi: 10.2147/IJN.S107292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gonzalez-Carter D, Goode AE, Kiryushko D, Masuda S, Hu S, Lopes-Rodrigues R, Dexter DT, Shaffer MSP, Porter AE. Quantification of blood-brain barrier transport and neuronal toxicity of unlabelled multiwalled carbon nanotubes as a function of surface charge. Nanoscale. 2019;11(45):22054–22069. doi: 10.1039/c9nr02866h. [DOI] [PubMed] [Google Scholar]
- 79.Hanada S, Fujioka K, Inoue Y, Kanaya F, Manome Y, Yamamoto K. Cell-based in vitro blood-brain barrier model can rapidly evaluate nanoparticles’ brain permeability in association with particle size and surface modification. Int J Mol Sci. 2014;15(2):1812–1825. doi: 10.3390/ijms15021812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Curtis C, Toghani D, Wong B, Nance E. Colloidal stability as a determinant of nanoparticle behavior in the brain. Colloids Surf B Biointerfaces. 2018;170:673–682. doi: 10.1016/j.colsurfb.2018.06.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Saraiva C, Praça C, Ferreira R, Santos T, Ferreira L, Bernardino L. Nanoparticle-mediated brain drug delivery: overcoming blood-brain barrier to treat neurodegenerative diseases. J Control Release. 2016;235:34–47. doi: 10.1016/j.jconrel.2016.05.044. [DOI] [PubMed] [Google Scholar]
- 82.Wohlfart S, Gelperina S, Kreuter J. Transport of drugs across the blood-brain barrier by nanoparticles. J Control Release. 2012;161(2):264–273. doi: 10.1016/j.jconrel.2011.08.017. [DOI] [PubMed] [Google Scholar]
- 83.Masserini M. Nanoparticles for brain drug delivery. ISRN Biochem. 2013;2013:238428. doi: 10.1155/2013/238428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kreuter J. Drug delivery to the central nervous system by polymeric nanoparticles: what do we know? Adv Drug Deliv Rev. 2014;71:2–14. doi: 10.1016/j.addr.2013.08.008. [DOI] [PubMed] [Google Scholar]
- 85.Gaillard PJ, Appeldoom CCM, Dorland R, Van Kregten J, Manca F, Vugts DJ, Windhorst B, Gams VD, De Vries HE, Maussang D, et al. Pharmacokinetics, brain delivery, and efficacy in brain tumor-bearing mice of glutathione pegylated liposomal doxorubicin (2B3-101). PLoS One. 2014;9(1):e82331. doi: 10.1371/journal.pone.0082331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mészáros M, Porkoláb G, Kiss L, Pilbat A-M, Kóta Z, Kupihár Z, Kéri A, Galbács G, Siklós L, Tóth A, et al. Niosomes decorated with dual ligands targeting brain endothelial transporters increase cargo penetration across the blood-brain barrier. Eur J Pharm Sci. 2018;123:228–240. doi: 10.1016/j.ejps.2018.07.042. [DOI] [PubMed] [Google Scholar]
- 87.Porkoláb G, Mészáros M, Tóth A, Szecskó A, Harazin A, Szegletes Z, Ferenc G, Blastyák A, Mátés L, Rákhely G, et al. Combination of alanine and glutathione as targeting ligands of nanoparticles enhances cargo delivery into the cells of the neurovascular unit. Pharmaceutics. 2020;12(7):635. doi: 10.3390/pharmaceutics12070635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Woods S, O’Brien LM, Butcher W, Preston JE, Georgian AR, Williamson ED, Salguero FJ, Modino F, Abbott NJ, Roberts CW, et al. Glucosamine-NISV delivers antibody across the blood-brain barrier: optimization for treatment of encephalitic viruses. J Control Release. 2020;324:644–656. doi: 10.1016/j.jconrel.2020.05.048. [DOI] [PubMed] [Google Scholar]
- 89.Veszelka S, Mészáros M, Kiss L, Kóta Z, Páli T, Hoyk Z, Bozsó Z, Fülöp L, Tóth A, Rákhely G, et al. Biotin and glutathione targeting of solid nanoparticles to cross human brain endothelial cells. Curr Pharm Des. 2017;23(28):4198–4205. doi: 10.2174/1381612823666170727144450. [DOI] [PubMed] [Google Scholar]
- 90.Johnsen KB, Bak M, Melander F, Thomsen MS, Burkhart A, Kempen PJ, Andresen TL, Moos T. Modulating the antibody density changes the uptake and transport at the blood-brain barrier of both transferrin receptor-targeted gold nanoparticles and liposomal cargo. J Control Release. 2019;295:237–249. doi: 10.1016/j.jconrel.2019.01.005. [DOI] [PubMed] [Google Scholar]
- 91.Ulbrich K, Hekmatara T, Herbert E, Kreuter J. Transferrin- and transferrin-receptor-antibody-modified nanoparticles enable drug delivery across the blood-brain barrier (BBB). Eur J Pharm Biopharm. 2009;71(2):251–256. doi: 10.1016/j.ejpb.2008.08.021. [DOI] [PubMed] [Google Scholar]
- 92.Ulbrich K, Knobloch T, Kreuter J. Targeting the insulin receptor: nanoparticles for drug delivery across the blood-brain barrier (BBB). J Drug Target. 2011;19(2):125–132. doi: 10.3109/10611861003734001. [DOI] [PubMed] [Google Scholar]
- 93.Malinovskaya Y, Melnikov P, Baklaushev V, Gabashvili A, Osipova N, Mantrov S, Ermolenko Y, Maksimenko O, Gorshkova M, Balabanyan V, et al. Delivery of doxorubicin-loaded PLGA nanoparticles into U87 human glioblastoma cells. Int J Pharm. 2017;524(1–2):77–90. doi: 10.1016/j.ijpharm.2017.03.049. [DOI] [PubMed] [Google Scholar]
- 94.Gupta Y, Jain A, Jain SK. Transferrin-conjugated solid lipid nanoparticles for enhanced delivery of quinine dihydrochloride to the brain. J Pharm Pharmacol. 2007;59(7):935–940. doi: 10.1211/jpp.59.7.0004. [DOI] [PubMed] [Google Scholar]
- 95.Kadari A, Pooja D, Gora RH, Gudem S, Kolapalli VRM, Kulhari H, Sistla R. Design of multifunctional peptide collaborated and docetaxel loaded lipid nanoparticles for antiglioma therapy. Eur J Pharm Biopharm. 2018;132:168–179. doi: 10.1016/j.ejpb.2018.09.012. [DOI] [PubMed] [Google Scholar]
- 96.Tian X, Wei F, Wang T, Wang P, Lin X, Wang J, Wang D, Ren L. In vitro and in vivo studies on gelatin-siloxane nanoparticles conjugated with SynB peptide to increase drug delivery to the brain. Int J Nanomedicine. 2012;7:1031–1041. doi: 10.2147/IJN.S26541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lu W, Tan Y, Hu K, Jiang X. Cationic albumin conjugated pegylated nanoparticle with its transcytosis ability and little toxicity against blood-brain barrier. Int J Pharm. 2005;295(1–2):247–260. doi: 10.1016/j.ijpharm.2005.01.043. [DOI] [PubMed] [Google Scholar]
- 98.Lu W, Zhang Y, Tan YZ, Hu KL, Jiang XG, Fu SK. Cationic albumin-conjugated pegylated nanoparticles as novel drug carrier for brain delivery. J Control Release. 2005;107(3):428–448. doi: 10.1016/j.jconrel.2005.03.027. [DOI] [PubMed] [Google Scholar]
- 99.Parikh T, Bommana MM, Squillante E. Efficacy of surface charge in targeting pegylated nanoparticles of sulpiride to the brain. Eur J Pharm Biopharm. 2010;74(3):442–450. doi: 10.1016/j.ejpb.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 100.Rao KS, Reddy MK, Horning JL, Labhasetwar V. TAT-conjugated nanoparticles for the CNS delivery of anti-HIV drugs. Biomaterials. 2008;29(33):4429–4438. doi: 10.1016/j.biomaterials.2008.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Pinheiro RGR, Granja A, Loureiro JA, Pereira MC, Pinheiro M, Neves AR, Reis S. RVG29-functionalized lipid nanoparticles for quercetin brain delivery and Alzheimer’s disease. Pharm Res. 2020;37(7):139. doi: 10.1007/s11095-020-02865-1. [DOI] [PubMed] [Google Scholar]
- 102.Gaillard PJ. BBB crossing assessment and BBB crossing technologies in CNS Drug Discovery. Drug Discov Today Technol. 2016;20:1–3. doi: 10.1016/j.ddtec.2016.11.002. [DOI] [PubMed] [Google Scholar]
- 103.Rip J, Chen L, Hartman R, Van Den Heuvel A, Reijerkerk A, Van Kregten J, Van Der Boom B, Appeldoorn C, De Boer M, Maussang D, et al. Glutathione PEGylated liposomes: pharmacokinetics and delivery of cargo across the blood-brain barrier in rats. J Drug Target. 2014;22(5):460–467. doi: 10.3109/1061186X.2014.888070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zlokovic BV, Mackic JB, McComb JG, Weiss MH, Kaplowitz N, Kannan R. Evidence for transcapillary transport of reduced glutathione in vascular perfused guinea-pig brain. Biochem Biophys Res Commun. 1994;201(1):402–408. doi: 10.1006/bbrc.1994.1715. [DOI] [PubMed] [Google Scholar]
- 105.Dufes C, Schätzlein AG, Tetley L, Gray AI, Watson DG, Olivier J-C, Couet W, Uchegbu IF. Niosomes and polymeric chitosan based vesicles bearing transferrin and glucose ligands for drug targeting. Pharm Res. 2000;17(10):1250–1258. doi: 10.1023/a:1026422915326. [DOI] [PubMed] [Google Scholar]
- 106.Dufes C, Gaillard F, Uchegbu IF, Schätzlein AG, Olivier J-C, Muller J-M. Glucose-targeted niosomes deliver vasoactive intestinal peptide (VIP) to the brain. Int J Pharm. 2004;285(1–2):77–85. doi: 10.1016/j.ijpharm.2004.07.020. [DOI] [PubMed] [Google Scholar]
- 107.Gromnicova R, Davies HA, Sreekanthreddy P, Romero IA, Lund T, Roitt IM, Phillips JB, Male DK. Glucose-coated gold nanoparticles transfer across human brain endothelium and enter astrocytes in vitro. PLoS One. 2013;8(12):e81043. doi: 10.1371/journal.pone.0081043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gromnicova R, Kaya M, Romero IA, Williams P, Satchell S, Sharrack B, Male D. Transport of gold nanoparticles by vascular endothelium from different human tissues. PLoS One. 2016;11(8):e0161610. doi: 10.1371/journal.pone.0161610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Fatima N, Gromnicova R, Loughlin J, Sharrack B, Male D. Gold nanocarriers for transport of oligonucleotides across brain endothelial cells. PLoS One. 2020;15(9):e0236611. doi: 10.1371/journal.pone.0236611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Johnsen KB, Moos T. Revisiting nanoparticle technology for blood-brain barrier transport: unfolding at the endothelial gate improves the fate of transferrin receptor-targeted liposomes. J Control Release. 2016;222:32–46. doi: 10.1016/j.jconrel.2015.11.032. [DOI] [PubMed] [Google Scholar]
- 111.van den Berg BM, Nieuwdorp M, Stroes ES, Vink H. Glycocalyx and endothelial (dys) function: from mice to men. Pharmacol Rep. 2006;58:75–80. [PubMed] [Google Scholar]
- 112.Weinbaum S, Cancel LM, Fu BM, Tarbell JM. The glycocalyx and its role in vascular physiology and vascular related diseases. Cardiovasc Eng Technol. 202. 1;12(1):37–71. doi: 10.1007/s13239-020-00485-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Yilmaz O, Afsar B, Ortiz A, Kanbay M. The role of endothelial glycocalyx in health and disease. Clin Kidney J. 2019;12(5):611–619. doi: 10.1093/ckj/sfz042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Dogné S, Flamion B. Endothelial glycocalyx impairment in disease: focus on hyaluronan shedding. Am J Pathol. 2020;190(4):768–780. doi: 10.1016/j.ajpath.2019.11.016. [DOI] [PubMed] [Google Scholar]
- 115.Dogné S, Flamion B, Caron N. Endothelial glycocalyx as a shield against diabetic vascular complications: involvement of hyaluronan and hyaluronidases. Arterioscler Thromb Vasc Biol. 2018;38(7):1427–1439. doi: 10.1161/ATVBAHA.118.310839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Logsdon AF, Rhea EM, Reed M, Banks WA, Erickson MA. The neurovascular extracellular matrix in health and disease. Exp Biol Med (Maywood). 2020;10:1535370220977195. doi: 10.1177/1535370220977195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Nag S. Cerebral endothelial plasma membrane alterations in acute hypertension. Acta Neuropathol. 1986;70(1):38–43. doi: 10.1007/BF00689512. [DOI] [PubMed] [Google Scholar]
- 118.Ueno M, Sakamoto H, Tomimoto H, Akiguchi I, Onodera M, Huang CL, Kanenishi K. Blood-brain barrier is impaired in the hippocampus of young adult spontaneously hypertensive rats. Acta Neuropathol. 2004;107(6):532–538. doi: 10.1007/s00401-004-0845-z. [DOI] [PubMed] [Google Scholar]
- 119.Ueno M, Sakamoto H, Liao YJ, Onodera M, Huang CL, Miyanaka H, Nakagawa T. Blood-brain barrier disruption in the hypothalamus of young adult spontaneously hypertensive rats. Histochem Cell Biol. 2004;122(2):131–137. doi: 10.1007/s00418-004-0684-y. [DOI] [PubMed] [Google Scholar]
- 120.Gurnik S, Devraj K, Macas J, Yamaji M, Starke J, Scholz A, Sommer K, Di Tacchio M, Vutukuri R, Beck H, et al. Angiopoietin-2-induced blood-brain barrier compromise and increased stroke size are rescued by VE-PTP-dependent restoration of Tie2 signaling. Acta Neuropathol. 2016;131(5):753–773. doi: 10.1007/s00401-016-1551-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Sweeney MD, Ayyadurai S, Zlokovic BV. Pericytes of the neurovascular unit: key functions and signaling pathways. Nat Neurosci. 2016;19(6):771–783. doi: 10.1038/nn.4288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Salmon AH, Neal CR, Sage LM, Glass CA, Harper SJ, Bates DO. Angiopoietin-1 alters microvascular permeability coefficients in vivo via modification of endothelial glycocalyx. Cardiovasc Res. 2009;83(1):24–33. doi: 10.1093/cvr/cvp093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ko K, Suzuki T, Ishikawa R, Hattori N, Ito R, Umehara K, Furihata T, Dohmae N, Linhardt RJ, Igarashi K, et al. Ischemic stroke disrupts the endothelial glycocalyx through activation of proHPSE via acrolein exposure. J Biol Chem. 2020;295(52):18614–18624. doi: 10.1074/jbc.RA120.015105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Haeren RHL, Rijkers K, Schijns OEMG, Dings J, Hoogland G, Mamj VZ, Vink H, Van Overbeeke JJ. In vivo assessment of the human cerebral microcirculation and its glycocalyx: a technical report. J Neurosci Methods. 2018;303:114–125. doi: 10.1016/j.jneumeth.2018.03.009. [DOI] [PubMed] [Google Scholar]
- 125.Li X, Zhu J, Liu K, Hu Y, Huang K, Pan S. Heparin ameliorates cerebral edema and improves outcomes following status epilepticus by protecting endothelial glycocalyx in mice. Exp Neurol. 2020;330:113320. doi: 10.1016/j.expneurol.2020.113320. [DOI] [PubMed] [Google Scholar]
- 126.Gómez-González B, Larios HM, Escobar A. Increased transvascular transport of WGA-peroxidase after chronic perinatal stress in the hippocampal microvasculature of the rat. Int J Dev Neurosci. 2011;29(8):839–846. doi: 10.1016/j.ijdevneu.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 127.Yj L, Ueno M, Nakagawa T, Huang C, Kanenishi K, Onodera M, Sakamoto H. Oxidative damage in cerebral vessels of diabetic db/db mice. Diabetes Metab Res Rev. 2005;21(6):554–559. doi: 10.1002/dmrr.579. [DOI] [PubMed] [Google Scholar]
- 128.Neuhaus W, Germann B, Plattner VE, Gabor F, Wirth M, Noe CR. Alteration of the glycocalyx of two blood-brain barrier mimicking cell lines is inducible by glioma conditioned media. Brain Res. 2009;1279:82–89. doi: 10.1016/j.brainres.2009.05.010. [DOI] [PubMed] [Google Scholar]
- 129.Rai S, Nejadhamzeeigilani Z, Gutowski NJ, Whatmore JL. Loss of the endothelial glycocalyx is associated with increased E-selectin mediated adhesion of lung tumour cells to the brain microvascular endothelium. J Exp Clin Cancer Res. 2015;34:105. doi: 10.1186/s13046-015-0223-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Behling-Kelly E, Vonderheid H, Kim KS, Corbeil LB, Czuprynski CJ. Roles of cellular activation and sulfated glycans in Haemophilus somnus adherence to bovine brain microvascular endothelial cells. Infect Immun. 2006;74(9):5311–5318. doi: 10.1128/IAI.00614-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Okada H, Yoshida S, Hara A, Ogura S, Tomita H, Vascular endothelial injury exacerbates coronavirus disease 2019: the role of endothelial glycocalyx protection. Microcirculation. 2020;e12654. in press. doi: 10.1111/micc.12654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Pillinger NL, Kam P. Endothelial glycocalyx: basic science and clinical implications. Anaesth Intensive Care. 2017;45(3):295–307. doi: 10.1177/0310057X1704500305. [DOI] [PubMed] [Google Scholar]
- 133.Goligorsky MS, Sun D. Glycocalyx in endotoxemia and sepsis. Am J Pathol. 2020;190(4):791–798. doi: 10.1016/j.ajpath.2019.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Nägga K, Hansson O, Van Westen D, Minthon L, Wennström M. Increased levels of hyaluronic acid in cerebrospinal fluid in patients with vascular dementia. J Alzheimers Dis. 2014;42(4):1435–1441. doi: 10.3233/JAD-141200. [DOI] [PubMed] [Google Scholar]
- 135.DellaValle B, Manresa-Arraut A, Hasseldam H, Stensballe A, Rungby J, Larsen A, Hempel C. Detection of glycan shedding in the blood: new class of multiple sclerosis biomarkers? Front Immunol. 2018;9:1254. doi: 10.3389/fimmu.2018.01254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Piva S, McCreadie VA, Latronico N. Neuroinflammation in sepsis: sepsis associated delirium. Cardiovasc Hematol Disord Drug Targets. 2015;15(1):10–18. doi: 10.2174/1871529x15666150108112452. [DOI] [PubMed] [Google Scholar]
- 137.Hippensteel JA, Anderson BJ, Orfila JE, McMurtry SA, Dietz RM, Su G, Ford JA, Oshima K, Yang Y, Zhang F, et al. Circulating heparan sulfate fragments mediate septic cognitive dysfunction. J Clin Invest. 2019;129(4):1779–1784. doi: 10.1172/JCI124485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Zhang X, Han X, Xia K, Xu Y, Yang Y, Oshima K, Haeger SM, Perez MJ, McMurtry SA, Hippensteel JA, et al. Circulating heparin oligosaccharides rapidly target the hippocampus in sepsis, potentially impacting cognitive functions. Proc Natl Acad Sci U S A. 2019;116(19):9208–9213. doi: 10.1073/pnas.1902227116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Torres Filho IP, Torres LN, Salgado C, Dubick MA. Plasma syndecan-1 and heparan sulfate correlate with microvascular glycocalyx degradation in hemorrhaged rats after different resuscitation fluids. Am J Physiol Heart Circ Physiol. 2016;310(11):H1468–H1478. doi: 10.1152/ajpheart.00006.2016. [DOI] [PubMed] [Google Scholar]
- 140.Dobson GP. Trauma of major surgery: a global problem that is not going away. Int J Surg. 2020;81:47–54. doi: 10.1016/j.ijsu.2020.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Song JW, Zullo JA, Liveris D, Dragovich M, Zhang XF, Goligorsky MS. Therapeutic restoration of endothelial glycocalyx in sepsis. J Pharmacol Exp Ther. 2017;361(1):115–121. doi: 10.1124/jpet.116.239509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Torres Filho IP, Torres LN, Salgado C, Dubick MA. Novel adjunct drugs reverse endothelial glycocalyx damage after hemorrhagic shock in rats. Shock. 2017;48(5):583–589. doi: 10.1097/SHK.0000000000000895. [DOI] [PubMed] [Google Scholar]
- 143.Torres LN, Chung KK, Salgado CL, Dubick MA, Torres Filho IP. Low-volume resuscitation with normal saline is associated with microvascular endothelial dysfunction after hemorrhage in rats, compared to colloids and balanced crystalloids. Crit Care. 2017;21(1):160. doi: 10.1186/s13054-017-1745-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Haywood-Watson RJ, Holcomb JB, Gonzalez EA, Peng Z, Pati S, Park PW, Wang W, Zaske AM, Menge T, Kozar RA. Modulation of syndecan-1 shedding after hemorrhagic shock and resuscitation. PLoS One. 2011;6(8):e23530. doi: 10.1371/journal.pone.0023530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Genét GF, Bentzer P, Ostrowski SR, Johansson PI. Resuscitation with pooled and pathogen-reduced plasma attenuates the increase in brain water content following traumatic brain injury and hemorrhagic shock in rats. J Neurotrauma. 2017;34(5):1054–1062. doi: 10.1089/neu.2016.4574. [DOI] [PubMed] [Google Scholar]
- 146.Tuma M, Canestrini S, Alwahab Z, Marshall J. Trauma and endothelial glycocalyx: the microcirculation helmet? Shock (Augusta, Ga.). 2016;46(4):352–357. doi: 10.1097/SHK.0000000000000635. [DOI] [PubMed] [Google Scholar]
- 147.Gerhartl A, Hahn K, Neuhoff A, Friedl HP, Förster CY, Wunder C, Schick M, Burek M, Neuhaus W. Hydroxyethylstarch (130/0.4) tightens the blood-brain barrier in vitro. Brain Res. 2020;1727:146560. doi: 10.1016/j.brainres.2019.146560. [DOI] [PubMed] [Google Scholar]
- 148.Hempel C, Sporring J, Kurtzhals JAL. Experimental cerebral malaria is associated with profound loss of both glycan and protein components of the endothelial glycocalyx. Faseb J. 2019;33(2):2058–2071. doi: 10.1096/fj.201800657R. [DOI] [PubMed] [Google Scholar]
- 149.McLaughlin S. Electrostatic potentials at membrane-solution interfaces. Curr Top Membr Transp. 1977;9:71–144. doi: 10.1016/S0070-2161(08)60677-2. [DOI] [Google Scholar]
- 150.Butt HJ. Measuring local surface charge densities in electrolyte solutions with a scanning force microscope. Biophys J. 1992;63(2):578–582. doi: 10.1016/S0006-3495(92)81601-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Sotres J, Baró AM. AFM imaging and analysis of electrostatic double layer forces on single DNA molecules. Biophys J. 2010;98(9):1995–2004. doi: 10.1016/j.bpj.2009.12.4330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Perry D, Paulose Nadappuram B, Momotenko D, Voyias PD, Page A, Tripathi G, Frenguelli BG, Unwin PR. Surface charge visualization at viable living cells. J Am Chem Soc. 2016;138(9):3152–3160. doi: 10.1021/jacs.5b13153. [DOI] [PubMed] [Google Scholar]
- 153.Malher E, Martin D, Duvivier C, Volochine B, Stoltz JF. New device for determination of cell electrophoretic mobility using doppler velocimetry. Biorheology. 1982;19(5):647–654. doi: 10.3233/bir-1982-19506. [DOI] [PubMed] [Google Scholar]
- 154.Santa-Maria AR, Walter FR, Figueiredo R, Kincses A, Vigh JP, Heymans M, Culot M, Winter P, Gosselet F, Dér A, et al. Flow induces barrier and glycocalyx-related genes and negative surface charge in a lab-on-a-chip human blood-brain barrier model. J Cereb Blood Flow Metab. 2021;271678X21992638. in press doi: 10.1177/0271678X21992638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Fike RM, Van Oss CJ. Zeta-potentials of intact cell monolayers determined by electro-osmosis. In Vitro. 1976;12(6):428–436. doi: 10.1007/BF02806022. [DOI] [PubMed] [Google Scholar]
- 156.Sawyer PN, Himmelfarb E, Lustrin I, Ziskind H. Measurement of streaming potentials of mammalian blood vessels, aorta and vena cava, in vivo. Biophys J. 1966;6(5):641–651. doi: 10.1016/S0006-3495(66)86683-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Walter FR, Valkai S, Kincses A, Petneházi A, Czeller T, Veszelka S, Ormos P, Deli MA, Dér A. A versatile lab-on-a-chip tool for modeling biological barriers. Sens Actuators B Chem. 2016;222:1209–1219. doi: 10.1016/j.snb.2015.07.110. [DOI] [Google Scholar]
- 158.Szabó CA, Deli MA, Dung NTK, Joó F. Production of pure primary rat cerebral endothelial cell culture: a comparison of different methods. Neurobiology (Budapest). 1997;5:1–16. [PubMed] [Google Scholar]
- 159.Forsberg KME, Zhang Y, Reiners J, Ander M, Niedermayer A, Fang L, Neugebauer H, Kassubek J, Katona I, Weis J, et al. Endothelial damage, vascular bagging and remodeling of the microvascular bed in human microangiopathy with deep white matter lesions. Acta Neuropathol Commun. 2018;6(1):128. doi: 10.1186/s40478-018-0632-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Nag S. Ultrastructural localization of monosaccharide residues on cerebral endothelium. Lab Invest. 1985;52(5):553–558. doi: 10.1007/BF00688684. [DOI] [PubMed] [Google Scholar]
- 161.Nishida S, Akai F, Hiruma S, Maeda M, Tanji K, Hashimoto S. Experimental study of WGA binding on the endothelial cell surface in cerebral ischemia. Histol Histopathol. 1986;1(1):69–74 [PubMed] [Google Scholar]
- 162.Mazzetti S, Librizzi L, Frigerio S, De Curtis M, Vitellaro-Zuccarello L. Molecular anatomy of the cerebral microvessels in the isolated guinea-pig brain. Brain Res. 2004;999(1):81–90. doi: 10.1016/j.brainres.2003.11.032. [DOI] [PubMed] [Google Scholar]
- 163.Fischer D, Kissel T. Histochemical characterization of primary capillary endothelial cells from porcine brains using monoclonal antibodies and fluorescein isothiocyanate-labelled lectins: implications for drug delivery. Eur J Pharm Biopharm. 2001;52(1):1–11. doi: 10.1016/s0939-6411(01)00159-x. [DOI] [PubMed] [Google Scholar]
- 164.Fatehi MI, Gerhart DZ, Myers TG, Drewes LR. Characterization of the blood-brain barrier: glycoconjugate receptors of 14 lectins in canine brain, cultured endothelial cells, and blotted membrane proteins. Brain Res. 1987;415(1):30–39. doi: 10.1016/0006-8993(87)90266-6. [DOI] [PubMed] [Google Scholar]
- 165.Nieuwdorp M, Meuwese MC, Mooij HL, Ince C, Broekhuizen LN, Kastelein JJ, Stroes ES, Vink H. Measuring endothelial glycocalyx dimensions in humans: a potential novel tool to monitor vascular vulnerability. J Appl Physiol. 2008;104(3):845–852. doi: 10.1152/japplphysiol.00440.2007. [DOI] [PubMed] [Google Scholar]
- 166.Yoon J-H, Lee E-S, Jeong Y. In vivo imaging of the cerebral endothelial glycocalyx in mice. J Vasc Res. 2017;54(2):59–67. doi: 10.1159/000457799. [DOI] [PubMed] [Google Scholar]
- 167.Nag S. Cerebral endothelial surface charge in hypertension. Acta Neuropathol. 1984;63(4):276–281. doi: 10.1007/BF00687333. [DOI] [PubMed] [Google Scholar]
- 168.Ueno M, Tomita S, Nakagawa T, Ueki M, Iwanaga Y, Ono J, Onodera M, Huang CL, Kanenishi K, Shimada A, et al. Effects of aging and HIF-1alpha deficiency on permeability of hippocampal vessels. Microsc Res Tech. 2006;69(1):29–35. doi: 10.1002/jemt.20266. [DOI] [PubMed] [Google Scholar]
- 169.Vorbrodt AW, Trowbridge RS. Aluminum-induced alteration of surface anionic sites in cultured brain microvascular endothelial cells. Acta Neuropathol. 1993;86(4):371–377. doi: 10.1007/BF00369450. [DOI] [PubMed] [Google Scholar]
- 170.Hempel C, Hyttel P, Kurtzhals JA. Endothelial glycocalyx on brain endothelial cells is lost in experimental cerebral malaria. J Cereb Blood Flow Metab. 2014;34(7):1107–1110. doi: 10.1038/jcbfm.2014.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Haeren RHL, Sem VDV, Mamj VZ, Vink H, Van Overbeeke JJ, Hoogland G, Rijkers K. Assessment and imaging of the cerebrovascular glycocalyx. Curr Neurovasc Res. 2016;13(3):249–260. doi: 10.2174/1567202613666160504104434. [DOI] [PubMed] [Google Scholar]
- 172.Haeren RHL, Vink H, Staals J, Mamj VZ, Dings J, Van Overbeeke JJ, Hoogland G, Rijkers K, Schijns OEMG. Protocol for intraoperative assessment of the human cerebrovascular glycocalyx. BMJ Open. 2017;7(1):e013954. doi: 10.1136/bmjopen-2016-013954. [DOI] [PMC free article] [PubMed] [Google Scholar]


