Abstract
Background:
Models of health care coverage with varying degrees of patient cost-sharing have been shown to influence health care behaviors for chronic conditions including medication adherence. The effect of insurance cost-sharing subsidies on the probability of postoperative opioid refill, however, is unclear.
Methods:
This retrospective cohort study examined 100% Medicare claims data among patients (N = 21,781) ages 65 and older undergoing orthopedic procedures in Michigan between January 2013 and September 2016. Patients were classified based on the presence of low-income subsidy and on prior opioid exposure using Medicare Part D prescription files of drug events. We investigated the association of these factors with the probability of both initial and second postoperative opioid fill within 90 days from the date of discharge.
Results:
In this cohort, 84.6% of patients filled an initial opioid prescription, and 66.4% refilled an opioid prescription. Patients with a full low-income subsidy had greater odds of refill within the postoperative 90 days compared with those patients without a low-income subsidy (odds ratio 1.38, 95% confidence interval 1.18–1.60). Among opioid naïve patients with a full low-income subsidy, the adjusted refill rate was 61.3% (95% confidence interval 58.0–64.7%) compared with 57.6% (95% confidence interval 51.4–63.7%) among those with partial low-income subsidy and 54.2% (95% confidence interval 52.8–55.6%) among patients without low-income subsidy.
Conclusion:
Among Medicare patients undergoing orthopedic procedures, a full medication subsidy is associated with an increased probability of opioid refill when compared with no subsidy. Going forward, it is critical to lessen financial barriers to ensure all patients have equitable access to postoperative analgesia, including both opioid and nonopioid analgesics by decreasing the patient burden of cost-sharing.
Introduction
Opioid-related morbidity and mortality in the United States is well-described.1,2 With respect to the perioperative setting, multiple factors have contributed to uncoordinated and often excessive opioid prescribing.3 Guidelines based on opioid consumption after an operation have only been introduced recently, and routine disposal of unused opioids after surgical care remains uncommon.4–7 To date, the majority of research and policy has been directed at decreasing the amount of opioids prescribed at the point of care and at creating clinical practice guidelines to optimize safe storage and disposal.
Recently, the federal Council of Economic Advisers described the impact of decreased out-of-pocket cost of opioids in the first wave of the current opioid epidemic.8 Expansion of government insurance coverage in the 2000s combined with the increase in the number of generic opioids available to decrease the cost to patients for opioid medications. In many aspects of health care, financial factors influence behavior.9–11 Cost-sharing describes the phenomenon by which patients are responsible for paying a proportion of the cost of their medical care and prescription fills through copayments or deductibles.12 This effect is now being leveraged in the policies for the calendar year 2020 of the Centers for Medicare and Medicaid Services by decreasing cost-sharing—and therefore out-of-pocket cost to patients—to improve access to opioid-reversal agents.13 Previous work has shown increased insurance cost-sharing decreases the probability of medication fill, a proxy for medication adherence.14,15 Price elasticity, the strength of this effect, has been demonstrated in the Medicare population with antihypertensives such as angiotensin-converting enzyme inhibitors, with greater out-of-pocket costs being associated with less medication adherence.16,17 This effect has also been demonstrated to a lesser extent in opioid analgesics in the primary care setting.18 In the context of surgical care, however, in which opioids may be prescribed for both acute and acute-on-chronic pain on an as-needed basis, much less has been reported regarding the influence of insurance cost-sharing on the probability of the refill of opioid medication.
In this study, we examined the probability of postoperative refills among opioid-naïve and opioid-exposed Medicare patients in the state of Michigan undergoing orthopedic procedures. We specifically examined the effect of Part D subsidies for individuals meeting the criteria for low-income subsidy (LIS) as it relates to pharmaceutical claims. We hypothesized that patients with a cost-sharing subsidy, and therefore less cost to the patient, would be more likely to fill and subsequently refill their prescriptions for opioids.
Methods
Data source and study cohort
Our study cohort was drawn from fee-for-service Medicare Parts A, B, and D claims data among patients in the state of Michigan undergoing orthopedic operations: hip, knee, shoulder, and spine arthroplasty or fusion. All Medicare claims were examined from January 2013 through September 2016. We included adult patients ages 65 years and older who received Medicare benefits with a Part D prescription drug plan. We identified procedures based on Current Procedural Codes-4 (Supplement 1). Inclusion was restricted to those patients with continuous enrollment in Medicare Parts A, B, and D for 12 months before the admission date (presurgical period) and 90 days after discharge (postsurgical period). Patients were excluded if they (1) did not have continuous insurance enrollment as mentioned earlier, (2) had an additional operation within the 90 day postdischarge period as identified by Current Procedural Codes-4 codes, (3) stayed more than 30 days during the hospital admission for the surgery, (4) died within the 90 day, postsurgical period, or (5) did not undergo the procedure with an orthopedic surgeon or neurosurgeon.
Outcomes
The primary outcome measured in this study was the odds that a patient would refill a opioid prescription within 90 days of discharge after filling the initial postoperative prescription. The secondary outcome was the odds of an initial fill, defined as filling a postoperative opioid prescription between 14 days before admission and 3 days after discharge.19–23 Opioid prescription fills and refills were identified in Medicare Part D Prescription Drug Event data using National Drug Codes and converted to oral morphine equivalents using Centers for Disease Control conversion factors.24
Insurance cost-sharing
The primary exposure variable was the presence and level of LIS provided to the patient, defined as no subsidy, partial subsidy, or full subsidy, captured from the month during which the patient had their operation. Since its creation in 2006, Medicare Part D provides drug coverage for enrollees. These enrollees are required to pay premiums and copayments for this coverage; however, the LIS partially or completely decreases the costs to individuals who qualify.25
Patient Covariates
We obtained demographic information from Medicare denominator files for the year of the operative procedure, including age, sex, and race. Patients were grouped into 65 to 69, 70 to 74, 75 to 79, 80 to 84, and 85 years and older. We identified comorbid conditions using International Classification of Disease diagnosis codes versions 9 and 10 from claims within 1 year before the operation. Comorbid conditions were categorized by severity using the Charlson comorbidity index score.26 In addition, smoking status, mental health conditions (Supplement 2), and preoperative pain-related conditions (Supplement 3) were included as derived from diagnosis codes from claims data within 1 year before admission.23,27,28
Preoperative opioid exposure was characterized by the presence of preoperative opioid fills. Patients who had not filled a prescription for an opioid-containing medication between 12 months and 15 days before admission for the operation were classified as opioid naïve. Patients who had filled greater than 3 opioid prescriptions in the 3 consecutive months before operation (but not all were filled in the month immediately before the operation) or who had a 120 day supply or more of opioid medication within the period between 12 months and 15 days before admission were classified as chronic users. Patients who filled prescriptions within the period between 12 months and 15 days before admission but did not meet the criteria of chronic user were classified as intermittent opioid users.19 The initial postoperative opioid prescription size, in 10 tablets of 5 mg oxycodone, was also included as a covariate.
Statistical analysis
Descriptive statistics were generated for the study cohort. A separate, multilevel logistic regression was specified to examine the effect of insurance cost-sharing on the odds of a postoperative opioid refill and initial fill, adjusting for patient-level risk factors (age, sex, race, tobacco use, comorbidities, mental health disorders, and pain disorder) and preoperative opioid exposure. The interaction effects between cost-sharing and preoperative opioid exposure and between cost-sharing and type of operation were tested. A parsimonious model was selected after patient factors with a P value > .300 were excluded from the final model. The multilevel logistic regression model accounted for the correlation among patients of the same surgeon. Based on the regression model, we then estimated the rates of refill and initial fill by LIS and preoperative opioid exposure. Statistical analyses were performed using SAS version 9.4 (SAS Institute Inc, Cary, NC) and Stata version 14.1 (Stata-Corp, College Station, TX). Two-sided P < .05 was considered to be statistically significant. This study was deemed exempt by the Michigan Medicine Institutional Review Board.
Results
Sample Characteristics
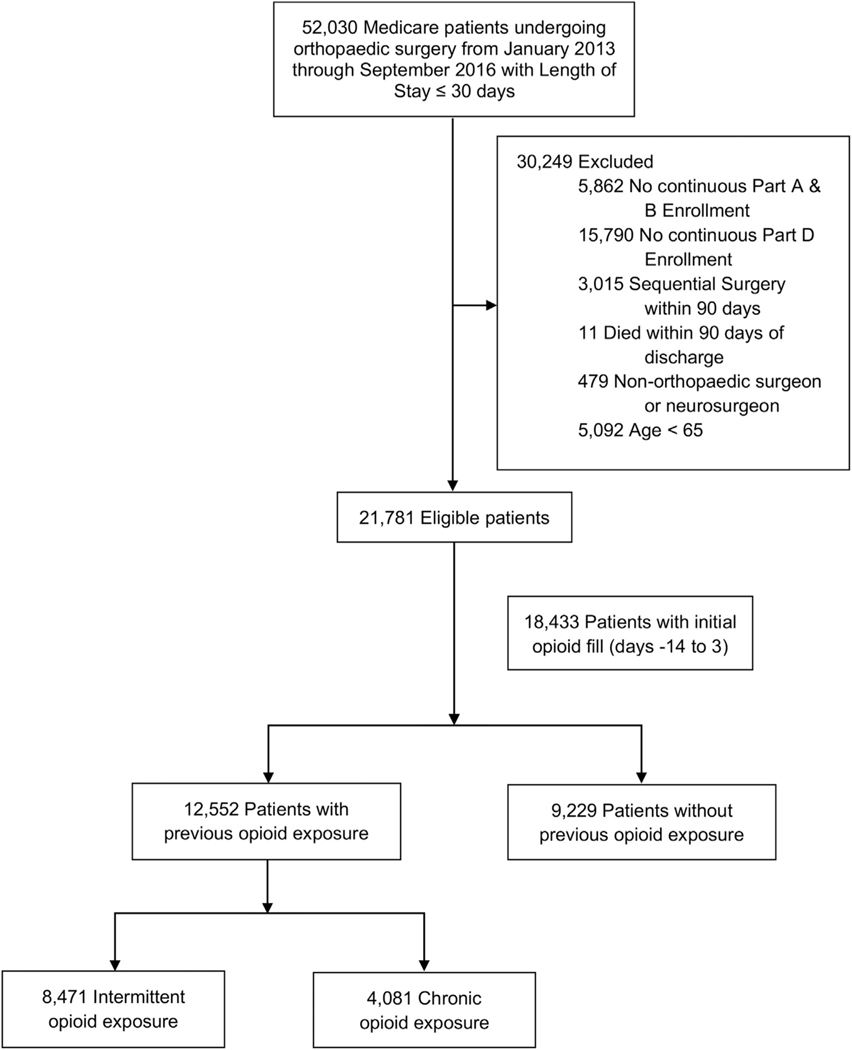
We identified 21,781 patients who met the study criteria (Fig 1). Overall, 5,058 (23.2%) underwent hip arthroplasty, 10,684 (49.1%) underwent knee arthroplasty, 2,074 (9.5%) underwent shoulder arthroplasty, and 3,965 (18.2%) underwent spine arthroplasty or fusion. (Table I) With respect to preoperative opioid exposure, 9,229 (42.4%) patients were opioid-naïve, 8,471 (38.9%) were intermittent opioid users, and 4,081 (18.7%) were chronic opioid users. Most patients (90.1%) did not receive a LIS. A small proportion of patients (4.2%) had a high comorbidity index (≥5). Over 8% of patients had depression, and 6.5% had anxiety disorder. Most patients (82.9%) had arthritis pain, the most common pain-related condition.
Fig 1.
Flow diagram of cohort.
Table I.
Study population characteristics: Number and % of total number of patients by LIS status
| All (N = 21,781) | No LIS (n = 19,619) | LIS (full subsidy, n = 1,763) | LIS (partial subsidy, n = 399) | P value | ||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||
| No. | % | No. | % | No. | % | |||
| Preoperative opioid use | < .001 | |||||||
| Naïve | 9,229 | 8,731 | 44.5% | 377 | 21.4% | 121 | 30.3% | |
| Intermittent | 8,471 | 7,745 | 39.5% | 591 | 33.5% | 135 | 33.8% | |
| Chronic | 4,081 | 3,143 | 16.0% | 795 | 45.1% | 143 | 35.8% | |
| Surgery | < .001 | |||||||
| Hip arthroplasty | 5,058 | 4,676 | 23.8% | 303 | 17.2% | 79 | 19.8% | |
| Knee arthroplasty | 10,684 | 9,663 | 49.3% | 840 | 47.6% | 181 | 45.4% | |
| Spine arthroplasty or fusion | 3,965 | 3,470 | 17.7% | 409 | 23.2% | 86 | 21.6% | |
| Shoulder arthroplasty | 2,074 | 1,810 | 9.2% | 211 | 12.0% | 53 | 13.3% | |
| Age | < .001 | |||||||
| 65–69 | 6,912 | 6,015 | 30.7% | 758 | 43.0% | 139 | 34.8% | |
| 70–74 | 7,196 | 6,550 | 33.4% | 535 | 30.3% | 111 | 27.8% | |
| 75–79 | 4,717 | 4,337 | 22.1% | 302 | 17.1% | 78 | 19.5% | |
| 80–84 | 2,236 | 2,056 | 10.5% | 132 | 7.5% | 48 | 12.0% | |
| 85+ | 720 | 661 | 3.4% | 36 | 2.0% | 23 | 5.8% | |
| Sex | < .001 | |||||||
| Male | 8,480 | 8,001 | 40.8% | 388 | 22.0% | 91 | 22.8% | |
| Female | 13,301 | 11,618 | 59.2% | 1,375 | 78.0% | 308 | 77.2% | |
| Race | < .001 | |||||||
| White | 19,979 | 18,347 | 93.5% | 1,301 | 73.8% | 331 | 83.0% | |
| Black | 1,024 | 709 | 3.6% | 269 | 15.3% | 46 | 11.5% | |
| Others | 574 | 381 | 1.9% | 173 | 9.8% | 20 | 5.0% | |
| Unknown | 204 | * | * | * | * | * | * | |
| Charlson comorbidity index | < .001 | |||||||
| 0 | 10,241 | 9,495 | 48.4% | 597 | 33.9% | 149 | 37.3% | |
| 1–2 | 8,295 | 7,383 | 37.6% | 754 | 42.8% | 158 | 39.6% | |
| 3–4 | 2,322 | 2,000 | 10.2% | 262 | 14.9% | 60 | 15.0% | |
| 5+ | 923 | 741 | 3.8% | 150 | 8.5% | 32 | 8.0% | |
| Tobacco use | 3,403 | 2,860 | 14.6% | 469 | 26.6% | 74 | 18.5% | < .001 |
| History of mental health disorder | ||||||||
| Adjustment disorder | 89 | * | * | * | * | * | * | |
| Anxiety | 1,419 | 1,150 | 5.9% | 219 | 12.4% | 50 | 12.5% | < .001 |
| Disruptive disorder | 33 | * | * | * | * | * | * | |
| Depression | 1,771 | 1,404 | 7.2% | 298 | 16.9% | 69 | 17.3% | < .001 |
| Personality disorder | 19 | * | * | * | * | * | * | |
| Suicide, self-harm | 21 | * | * | * | * | * | * | |
| Psychosis | 76 | * | * | * | * | * | * | |
| Other mental disorder | 132 | * | * | * | * | * | * | |
| Alcohol, substance disorder | 182 | * | * | * | * | * | * | |
| History of pain disorder | ||||||||
| Arthritis pain | 18,051 | 16,210 | 82.6% | 1,506 | 85.4% | 335 | 84.0% | .001 |
| Back pain | 6,637 | 5,850 | 29.8% | 654 | 37.1% | 133 | 33.3% | < .001 |
| Neck pain | 2,345 | 1,980 | 10.1% | 304 | 17.2% | 61 | 15.3% | < .001 |
| Other pain | 3,297 | 2,803 | 14.3% | 416 | 23.6% | 78 | 19.6% | < .001 |
The CMS cell size suppression policy sets minimum thresholds, 11 cases, for the display of CMS data. At least 1 of the 3 categories (ie, no LIS, LIS–full subsidy, LIS–partial subsidy) had cell size less than 11 cases.
Odds of initial opioid fill between 14 days before admission and 3 days after discharge
In this cohort, 18,433 (84.6%) patients filled an initial prescription for opioid medications (Table II). In multivariate analysis, patients with a full LIS were more likely to fill their opioid medication compared with patients without a LIS (adjusted odds ratio [aOR]: 1.29, 95% confidence interval [CI]: 1.10–1.51) after adjusting for patient characteristics, provider, and preoperative opioid exposure (Table III). This is reflected in the adjusted initial fill rate of 86.9% in opioid-naïve patients with a full LIS compared to 83.8% in opioid-naïve patients without any LIS (Fig 2).
Table II.
Number and % of total number of patients who filled initial postoperative prescription by LIS status
| All (N = 18,433) | No LIS (n = 16,544) | LIS (full subsidy, n = 1,551) | LIS (partial subsidy, n = 338) | P value | ||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||
| No. | % | No. | % | No. | % | |||
| Preoperative opioid use | < .001 | |||||||
| Naïve | 7,726 | 7,305 | 44.2% | 325 | 21.0% | 96 | 28.4% | |
| Intermittent | 7,159 | 6,524 | 39.4% | 515 | 33.2% | 120 | 35.5% | |
| Chronic | 3,548 | 2,715 | 16.4% | 711 | 45.8% | 122 | 36.1% | |
| Surgery | < .001 | |||||||
| Hip arthroplasty | 4,169 | 3,837 | 23.2% | 266 | 17.2% | 66 | 19.5% | |
| Knee arthroplasty | 9,240 | 8,333 | 50.4% | 753 | 48.5% | 154 | 45.6% | |
| Spine arthroplasty or fusion | 3,263 | 2,852 | 17.2% | 344 | 22.2% | 67 | 19.8% | |
| Shoulder arthroplasty | 1,761 | 1,522 | 9.2% | 188 | 12.1% | 51 | 15.1% | |
| Age | < .001 | |||||||
| 65–69 | 6,026 | 5,218 | 31.5% | 690 | 44.5% | 118 | 34.9% | |
| 70–74 | 6,118 | 5,570 | 33.7% | 453 | 29.2% | 95 | 28.1% | |
| 75–79 | 3,946 | 3,610 | 21.8% | 267 | 17.2% | 69 | 20.4% | |
| 80–84 | 1,774 | 1,627 | 9.8% | 112 | 7.2% | 35 | 10.4% | |
| 85+ | 569 | 519 | 3.1% | 29 | 1.9% | 21 | 6.2% | |
| Sex | < .001 | |||||||
| Male | 7,089 | 6,673 | 40.3% | 340 | 21.9% | 76 | 22.5% | |
| Female | 11,344 | 9,871 | 59.7% | 1,211 | 78.1% | 262 | 77.5% | |
| Race | < .001 | |||||||
| White | 16,915 | 15,484 | 93.6% | 1149 | 74.1% | 282 | 83.4% | |
| Black | 854 | 589 | 3.6% | 227 | 14.6% | 38 | 11.2% | |
| Others | 487 | 313 | 1.9% | 158 | 10.2% | 16 | 4.7% | |
| Unknown | 177 | * | * | * | * | * | * | |
| Charlson comorbidity index | < .001 | |||||||
| 0 | 8,697 | 8,053 | 48.7% | 8,053 | 33.8% | 120 | 35.5% | |
| 1–2 | 7,046 | 6,245 | 37.7% | 6,245 | 42.7% | 139 | 41.1% | |
| 3–4 | 1,928 | 1,648 | 10.0% | 1,648 | 14.9% | 49 | 14.5% | |
| 5+ | 762 | 598 | 3.6% | 598 | 8.6% | 30 | 8.9% | |
| Tobacco use | 2,856 | 2,371 | 14.3% | 422 | 27.2% | 63 | 18.6% | < .001 |
| Mental health disorder | ||||||||
| Adjustment disorder | 79 | * | * | * | * | * | * | |
| Anxiety | 1,217 | 987 | 6.0% | 190 | 12.3% | 40 | 11.8% | < .001 |
| Disruptive disorder | 24 | * | * | * | * | * | * | |
| Depression | 1,511 | 1,187 | 7.2% | 265 | 17.1% | 59 | 17.5% | < .001 |
| Personality disorder | 16 | * | * | * | * | * | * | |
| Suicide, self-harm | 18 | * | * | * | * | * | * | |
| Psychosis | 60 | * | * | * | * | * | * | |
| Other mental disorder | 109 | * | * | * | * | * | * | |
| Alcohol, substance disorder | 154 | * | * | * | * | * | * | |
| Pain disorder | ||||||||
| Arthritis pain | 15,267 | 13,651 | 82.5% | 1,330 | 85.8% | 286 | 84.6% | .004 |
| Back pain | 5,556 | 4,877 | 29.5% | 571 | 36.8% | 108 | 32.0% | < .001 |
| Neck pain | 1,921 | 1,614 | 9.8% | 257 | 16.6% | 50 | 14.8% | < .001 |
| Other pain | 2,763 | 2,327 | 14.1% | 372 | 24.0% | 64 | 18.9% | < .001 |
The CMS cell size suppression policy sets minimum thresholds, 11 cases, for the display of CMS data. At least 1 of the 3 categories (ie, no LIS, LIS–full subsidy, LIS–partial subsidy) had cell size less than 11 cases.
Table III.
Adjusted ORs for refill within 90 days after discharge and for initial fill
| Outcome: Refill | Outcome: Initial fill | |||
|---|---|---|---|---|
|
|
|
|||
| OR (95% CI) | P value | OR (95% CI) | P value | |
| Cost-sharing (ref: No LIS, no premium or copay subsidy) | ||||
| LIS with full subsidy | 1.39 (1.19–1.69) | < .001 | 1.29 (1.10–1.51) | .002 |
| LIS with partial subsidy | 1.18 (0.88–1.55) | .282 | 0.98 (0.74–1.30) | .896 |
| Preoperative opioid use (ref: Naïve) | ||||
| Intermittent | 1.93 (1.79–2.08) | < .001 | 1.10 (1.01–1.20) | .023 |
| Chronic | 11.93 (10.30–13.83) | < .001 | 1.34 (1.19–1.51) | < .001 |
| Surgery Type (ref: Hip replacement) | ||||
| Knee replacement | 3.39 (3.12–3.72) | < .001 | 1.30 (1.18–1.43) | < .001 |
| Spine surgery | 1.33 (1.15–1.54) | < .001 | 0.90 (0.77–1.05) | .169 |
| Shoulder surgery | 1.10 (0.95–1.27) | .205 | 1.14 (0.97–1.35) | .110 |
| Initial opioid prescription size* | 1.01 (1.00–1.01) | .009 | ||
| Age (Ref: 65–69) | ||||
| 70–74 | 0.88 (0.81–0.96) | .003 | 0.85 (0.77–0.94) | .001 |
| 75–79 | 0.74 (0.68–0.82) | < .001 | 0.76 (0.68–0.84) | < .001 |
| 80–84 | 0.66 (0.58–0.75) | < .001 | 0.57 (0.50–0.65) | < .001 |
| 85+ | 0.52 (0.43–0.64) | < .001 | 0.54 (0.44–0.66) | < .001 |
| Race (Ref: White) | ||||
| Black | 1.31 (1.08–1.58) | .006 | 0.82 (0.68–0.98) | .033 |
| Others | 0.88 (0.70–1.10) | .251 | 0.90 (0.71–1.14) | .384 |
| Unknown | 0.94 (0.66–1.32) | .707 | 1.09 (0.71–1.65) | .702 |
| Sex (ref: Male) | ||||
| Female | 1.26 (1.17–1.35) | < .001 | 1.08 (1.00–1.17) | .055 |
| Charlson comorbidity index (ref: CCI = 0) | ||||
| 1–2 | 1.04 (0.97–1.13) | .261 | 1.01 (0.93–1.10) | .749 |
| 3–4 | 1.01 (0.90–1.15) | .823 | 0.91 (0.80–1.03) | .141 |
| 5+ | 0.87 (0.72–1.05) | .147 | 0.86 (0.71–1.04) | .121 |
| Tobacco use (ref: Not use) | 1.10 (0.99–1.22) | .080 | 0.94 (0.84–1.04) | .235 |
| Mental health disorder | ||||
| Anxiety | 1.22 (1.04–1.44) | .018 | ||
| Depression | 1.15 (0.99–1.33) | .072 | ||
| Adjustment disorder | 1.50 (0.76–2.94) | .238 | ||
| Disruptive disorder | 0.42 (0.19–0.93) | .032 | ||
| Personality disorder | 0.40 (0.10–1.56) | .187 | ||
| Psychosis | 0.68 (0.37–1.27) | .225 | 0.70 (0.39–1.24) | .217 |
| Other mental health disorder | 1.39 (0.84–2.30) | .194 | ||
| Pain disorder | ||||
| Arthritis pain | 0.92 (0.84–1.01) | .083 | 0.92 (0.83–1.02) | .123 |
| Back pain | 1.07 (0.97–1.17) | .177 | ||
| Neck pain | 0.82 (0.72–0.93) | .002 | 0.85 (0.74–0.96) | .012 |
| Other pain | 1.11 (1.00–1.23) | .057 | 0.94 (0.84–1.05) | .276 |
CCI, Charlson comorbidity index.
In 10 tablets of 5 mg oxycodone; including prescriptions filled between 14 d.
Fig 2.
Initial postoperative opioid fills adjusted for patient attributes and surgical procedure and stratified by preoperative opioid exposure.
Preoperative opioid exposure increased the odds of filling the initial postoperative opioid prescription, which is reflected in the adjusted initial fill rate of 83.8% in opioid-naïve patients without a LIS compared with 86.3% in chronic opioid users without a LIS, and 86.9% in opioid-naïve patients with LIS compared to 89.0% in chronic opioid users with LIS (Fig 2). The odds of filling the initial prescription for chronic opioid users were 1.34 times (95% CI: 1.19–1.51) and for intermittent users were 1.10 times (95% CI: 1.01–1.20) that of opioid-naïve patients. Patients who underwent knee replacement were more likely to fill an initial prescription than hip replacement patients (aOR: 1.30, 95% CI: 1.18–1.43). Compared with patients between 65 and 69 years old, older patients were less likely to fill an initial prescription with odds ratios ranging from 0.85 (95% CI: 0.77–0.94) in patients between 70 to 74 years old to 0.54 (95% CI: 0.44–0.66) in patients 85+ years old (Table III). The adjusted initial fill rates were 87.1% for patients aged 65 to 69, compared to 85.3%, 83.8%, 79.7%, and 79% for ages 70 to 74, 75 to 79, 80 to 84, and 85+, respectively. Black patients had a lesser odds than white patients (aOR: 0.82, 95% CI: 0.68–0.98) of filling an initial prescription. While most patients had at least 1 preoperative pain-related condition, only neck pain was negatively associated with the odds of filling an initial prescription (aOR: 0.85, 95% CI: 0.74–0.96).
Odds and probability of opioid refill within 90 days after discharge
Of patients who had an initial opioid fill (n = 18,433, 84.6% of cohort), 12,236 (66.4%) patients refilled opioid prescriptions medications within 90 days of discharge. Unadjusted rates of refill were 51.4% (n = 2,143), 74.7% (6,902), 66.7% (2,176), and 57.7% (979) for hip, knee, spine, and shoulder arthroplasty, respectively. In multivariate analysis, patients who received a full LIS were more likely to refill a postoperative opioid prescription compared with patients without a LIS, aOR = 1.39 (95% CI: 1.19–1.62; P < .001) (Table III). There was no difference in odds of refill between partial LIS and no LIS (aOR: 1.17, 95% CI: 0.88–1.55). This finding is reflected in the comparison of adjusted refill rate between patients with a full LIS and patients without any LIS, stratified by operative procedure (Fig 3, a and b) and pooled (Fig 4). For example, among opioid-naïve patients, 61.3% of patients with a full LIS refilled a prescription compared to 54.2% of the counterpart patients without a LIS. There was no interaction between the type of operation and LIS.
Fig 3.
(A) Initial postoperative opioid fills adjusted for patient attributes and stratified by surgical procedure and preoperative opioid exposure. (B) Postoperative refills for opioid prescriptions adjusted for patient attributes and stratified by surgical procedure and preoperative opioid exposure.
Fig 4.
Postoperative refills for opioid prescriptions adjusted for patient attributes and surgical procedure and stratified by preoperative opioid exposure.
Compared with opioid-naïve patients, intermittent and chronic opioid users had greater odds of refilling a prescription (aOR: 1.93, 95% CI: 1.79–2.08, aOR: 11.93, 95% CI: 10.30–13.83, respectively) (Table III). Factors positively associated with refill included initial prescription size (aOR: 1.01, 95% CI: 1.00–1.01, P =.009) and younger age (Table III). Black patients were also more likely to refill within 90 days after discharge compared to white patients (aOR: 1.31, 95% CI: 1.08–1.58). The adjusted odds of refill for female patients were 1.26 times of male (95% CI: 1.17–1.35). Additionally, patients with anxiety were more likely to refill prescriptions (aOR: 1.22, 95% CI: 1.04–1.44).
Discussion
In this cohort of Medicare patients undergoing elective orthopedic procedures in the state of Michigan, we observed that for patients with a full LIS, postoperative opioids were price elastic – the lower cost of medication for these patients was associated with a greater likelihood of an opioid fill and refill when compared with patients without a LIS. These results are consistent with prior findings that demonstrate that decreased levels of cost-sharing are associated with an increase in medication fills. This observation may reflect the price elasticity for these medications, with greater out-of-pocket cost to the patient leading to decreased probability of refilling, possibly as a result of true financial barriers. It may also be that LIS is a strong indicator of socioeconomic disadvantage, which could be reflected in the results of this study. Taken together, our findings suggest that differential postoperative use of opioids may be associated with different access to, and ability to pay for, appropriate postoperative analgesia.
This analysis suggests that cost affects perioperative opioids similarly to medications in the setting of chronic disease, including opioids.14–18 The impact of cost on individual patient choice is likely multifactorial, with influences including patient disposable income and the impact on quality of life of condition under treatment.11,29–35 Prior investigations into the effects of cost-sharing on patient behavior have assessed the use of contraceptive devices and medications, including for medications of similar cost to patients as opioids, as well as adherence of patients to chronic disease medications and cancer treatments.14,15,18,36,37 These studies have illuminated the impact that cost has on patient decision-making and have broadly shown that shifting greater cost to the patient leads to decreased utilization of medication, albeit with varying strength of effect depending on the specific drug. In our study, when examining the impact of cost-sharing through the lens of postoperative pain management, we observed that the use of pain medication is similar to that of chronic disease medications.
Our findings of differences observed in refills after these orthopedic operations may reflect differences in access to appropriate postoperative analgesia. An example of this may be patient out-of-pocket cost in the context of recently implemented prescribing limits for Medicare Part D, whereby patients may have to pay additional copayments for clinically indicated refills.38 Moreover, given the well-documented history of disparities in access to appropriate pain management in the United States, these additional financial barriers may compound existing disparities in pain management.39–42 This observation holds true for opioid and nonopioid pain-management, as well as treatment for chronic pain and opioid-use disorder, the latter 2 of which are now being deployed as part of the 2020 policies of the Centers for Medicare and Medicaid Services.13 In the context of the potential impact that our findings demonstrate, there may be an opportunity to incentivize high-value care as it relates to postoperative analgesia. Prior research demonstrates notable gaps and variation in coverage for strategies of pain management.43 In this context, it is important to create care pathways that standardize prescribing and alleviate financial barriers to receiving effective analgesia. For example, the Michigan Pain-care Optimization Pathway leverages a multifaceted approach to pain management to encourage appropriate opioid prescribing alongside the use of opioid alternatives. This care pathway is coupled to patient and caregiver education regarding the use of opioids, as well as recommendations for safe storage and disposal. These pathways are acceptable to both patients and providers and a critical strategy to decrease unwarranted variation in opioid-prescribing and pain management by advocating for evidence-based guidelines for ensuring patients the analgesic standard of care. The adherence to these guidelines can be motivated by incentives from payers, including possibly by employing cost-sharing.44–46
There are several limitations inherent in this study. First, our analysis focuses on prescription fills, which may not reflect actual opioid use. We were also unable to account for secular trends in pain management during the study period or which specific prescriptions and fills or refills of opioids were clinically appropriate. The adoption of new practice patterns, including changes in postoperative duration of postoperative stay and advancements in the science of analgesia, may have varied by institution. Our findings were limited to Medicare enrollees using Part D who had continual insurance coverage for 12 months before and 90 days after the date of discharge in the state of Michigan. This population may not be representative of the Medicare-aged population as a whole, and our findings may not be generalizable to younger patient populations. We did not explore the effect of filling these medications on the probability of filling other chronic disease medications (for hypertension or hyperlipidemia, for example). It is possible that in the postoperative period, there are other effects on the probability of filling other medications that were not captured in this analysis. Restricting the preoperative fill window to 14 days may also miss some prescriptions given in anticipation of the procedure, but is necessary for defining prior opioid exposure and captured 97% of fills in this cohort. Additionally, the use of procedure codes for identification of surgical patients may be subject to misclassification leading to misidentification of the study population. The use of prior opioid fills to identify patients as opioid-naïve is similarly susceptible to this. There may also be bias inherent in conditioning the primary outcome variable on the presence of an initial fill or in unmeasured confounders that we could not account for in our model. Finally, this was a retrospective analysis and as such was unable to establish a causal relationship between greater levels of cost-sharing and decreased opioid fills. We did, however, observe an association between these variables which merits further investigation, especially as we begin to see implementation of policy which draws on this economic effect.
The results of this investigation demonstrate the potential for cost-sharing to impact patient opioid use postoperatively, as well as an opportunity to eliminate barriers to postoperative analgesia by decreasing cost-sharing. It is possible that this strategy could result in increased use from both clinically indicated and nonindicated prescription fills, or that patients without subsidies were able to decrease opioid use after the operation. Future work developing care pathways that integrate evidence-based guidelines will be the best option to achieve appropriate opioid prescribing alongside effective pain control.47 As such, cost-sharing has the potential to be a tool for influencing medication fills and consumption. More attention should be paid to standardizing and decreasing the financial barriers to appropriate pain medication in the postoperative period, including both appropriate opioid medications as well as alternatives, such as acetaminophen, nonsteroidal anti-inflammatory medications, gabapentinoids, and topical analgesics, to ensure patients a safe and comfortable recovery from their operation.
Supplementary Material
Acknowledgments
The authors would like to thank MICHR TL1 Grant Number TL1TR002242 for funding this work. We have no conflicts of interest to declare.
Funding/Support
Michael Kirsch was supported in this work through an educational and living stipend from Michigan Institute for Clinical and Health Research, United States (TL1 Grant Number TL1TR002242).
Footnotes
Conflict of interest/Disclosure
The authors have no related conflicts of interest to declare.
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at https://doi.org/10.1016/j.surg.2020.04.013.
References
- 1.Wilkerson RG, Kim HK, Windsor TA, Mareiniss DP. The opioid epidemic in the United States. Emerg Med Clin North Am. 2016;34:e1–e23. [DOI] [PubMed] [Google Scholar]
- 2.Dowell D, Arias E, Kochanek K, et al. Contribution of opioid-involved poisoning to the change in life expectancy in the United States, 2000–2015. JAMA. 2017;318:1065–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Waljee JF, Li L, Brummett CM, Englesbe MJ. Iatrogenic opioid dependence in the United States. Ann Surg. 2017;265:728–730. [DOI] [PubMed] [Google Scholar]
- 4.Howard R, Fry B, Gunaseelan V, et al. Association of opioid prescribing with opioid consumption after surgery in Michigan. JAMA Surg. 2019;154:e184234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hill MV, McMahon ML, Stucke RS, Barth RJ. Wide variation and excessive dosage of opioid prescriptions for common general surgical procedures. Ann Surg. 2017;265:709–714. [DOI] [PubMed] [Google Scholar]
- 6.Hill MV, Stucke RS, Billmeier SE, Kelly JL, Barth RJ. Guideline for discharge opioid prescriptions after inpatient general surgical procedures. J Am Coll Surg. 2018;226:996–1003. [DOI] [PubMed] [Google Scholar]
- 7.Hill MV, Stucke RS, McMahon ML, Beeman JL, Barth RJ. An educational intervention decreases opioid prescribing after general surgical operations. Ann Surg. 2018;267:468–472. [DOI] [PubMed] [Google Scholar]
- 8.Council of Economic Advisers (CEA). The role of opioid prices in the evolving opioid crisis; 2019. https://www.whitehouse.gov/wp-content/uploads/2019/04/The-Role-of-Opioid-Prices-in-the-Evolving-Opioid-Crisis.pdf.Accessed May 13, 2019.
- 9.Chernew ME, Rosen AB, Fendrick AM. Value-based insurance design. Health Aff (Millwood). 2007;26:w195–w203. [DOI] [PubMed] [Google Scholar]
- 10.Rice T, Matsuoka KY. The impact of cost-sharing on appropriate utilization and health status: A review of the literature on seniors. Med Care Res Rev. 2004;61: 415–452. [DOI] [PubMed] [Google Scholar]
- 11.Stuart B, Zacker C. Who bears the burden Of Medicaid drug copayment policies? Health Aff (Millwood). 1999;18:201–212. [DOI] [PubMed] [Google Scholar]
- 12.Ellis RP, McGuire TG. Supply-side and demand-side cost sharing in health care. J Econ Perspect. 1993;7:135–151. [DOI] [PubMed] [Google Scholar]
- 13.Centers for Medicare & Medicaid Services. Announcement of calendar year (CY) 2020 Medicare Advantage capitation rates and Medicare Advantage and Part D Payment policies and final call letter; 2019. https://www.cms.gov/Medicare/Health-Plans/MedicareAdvtgSpecRateStats/Downloads/Announcement2020.pdf.Accessed September 4, 2019.
- 14.Shen C, Zhao B, Liu L, Shih YT. Adherence to tyrosine kinase inhibitors among Medicare Part D beneficiaries with chronic myeloid leukemia. Cancer. 2018;124:364–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li P, Wong YN, Jahnke J, Pettit AR, Doshi JA. Association of high cost sharing and targeted therapy initiation among elderly Medicare patients with metastatic renal cell carcinoma. Cancer Med. 2018;7:75–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goldman DP, Joyce GF, Lawless G, Crown WH, Willey V. Benefit design and specialty drug use. Health Aff (Millwood). 2006;25:1319–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chernew M, Gibson TB, Yu-Isenberg K, Sokol MC, Rosen AB, Fendrick AM. Effects of increased patient cost sharing on socioeconomic disparities in health care. J Gen Intern Med. 2008;23:1131–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gatwood J, Gibson TB, Chernew ME, Farr AM, Vogtmann E, Fendrick AM. Price elasticity and medication use: cost sharing across multiple clinical conditions. J Manag Care Pharm. 2014;20:1102–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vu JV, Cron DC, Lee JS, et al. Classifying preoperative opioid use for surgical care. Ann Surg. 2018;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brescia AA, Waljee JF, Hu HM, et al. Impact of prescribing on new persistent opioid use after cardiothoracic surgery. Ann Thorac Surg. 2019;108:1107–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Santosa KB, Hu HM, Brummett CM, et al. New persistent opioid use among older patients following surgery: A Medicare claims analysis. Surgery. 2020;167:732–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Finney FT, Gossett TD, Hu HM, et al. New persistent opioid use following common forefoot procedures for the treatment of hallux valgus. J Bone Jt Surg Am. 2019;101:722–729. [DOI] [PubMed] [Google Scholar]
- 23.Brummett CM, Waljee JF, Goesling J, et al. New persistent opioid use after minor and major surgical procedures in US adults. JAMA Surg. 2017;152: e170504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Centers for Disease Control and Prevention (CDC). Calculating total daily dose of opioids for safer dosage; 2019. https://www.cdc.gov/drugoverdose/pdf/calculating_total_daily_dose-a.pdf.Accessed July 29, 2019.
- 25.Centers for Medicare and Medicaid Services (CMS). Guidance to States on the Low-Income Subsidy. https://www.cms.gov/Medicare/Eligibility-and-Enrollment/LowIncSubMedicarePresCov/Downloads/StateLISGuidance021009. pdf;2013, Accessed July 12, 2019.
- 26.Charlson ME, Pompei P, Ales K, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis. 1987;40.5:373–383. [DOI] [PubMed] [Google Scholar]
- 27.Lagisetty P, Bohnert A, Goesling J, et al. Care coordination for patients on chronic opioid therapy following surgery: A cohort study. Ann Surg; 2019. Available from: 10.1097/SLA.0000000000003235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hilliard PE, Waljee J, Moser S, et al. Prevalence of preoperative opioid use and characteristics associated with opioid use among patients presenting for surgery. JAMA Surg. 2018;153:929–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Osterbery L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353, 498–497. [DOI] [PubMed] [Google Scholar]
- 30.Tamblyn R, Laprise R, Hanley JA, et al. Adverse events associated with prescription drug cost-sharing among poor and elderly persons. JAMA. 2001;285(4):421–429. [DOI] [PubMed] [Google Scholar]
- 31.Murphy DA, Sarr M, Durako SJ, et al. , and the Adolescent Medicine HIV/AIDS Research Network. Barriers to HAART adherence among human immunodeficiency viruse-infected adolescents. Arch Pediatr Adolesc Med. 2003;157: 249–255. [DOI] [PubMed] [Google Scholar]
- 32.Lesen E, Andersson Sundell K, Carlsten A, Mardby A-C, Jonsson AK. Is the level of patient co-payment for medicines associated with refill adherence in Sweden? Eur J Public Health. 2014;24:85–90. [DOI] [PubMed] [Google Scholar]
- 33.Briesacher BA, Gurwitz JH, Soumerai SB. Patients at-risk for cost-related medication nonadherence: A review of the literature. J Gen Intern Med. 2007;22:864–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.George M, Freedman TG, Norfleet AL, Feldman HI, Apter AJ. Qualitative research-enhanced understanding of patients’ beliefs: Results of focus groups with low-income, urban, African American adults with asthma. J Allergy Clin Immunol. 2003;111:967–973. [DOI] [PubMed] [Google Scholar]
- 35.Ellis JJ, Erickson SR, Stevenson JG, Bernstein SJ, Stiles RA, Fendrick AM. Sub-optimal statin adherence and discontinuation in primary and secondary prevention populations: Should we target patients with the most to gain? J Gen Intern Med. 2004;19:638–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pace LE, Dusetzina SB, Fendrick AM, Keating NL, Dalton VK. The impact of out-of-pocket costs on the use of intrauterine contraception among women with employer-sponsored insurance. Med Care. 2013;51:959–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dalton VK, Carlos RC, Kolenic GE, et al. The impact of cost sharing on women’s use of annual examinations and effective contraception. Am J Obstet Gynecol. 2018;219:93.e1e93.e13. [DOI] [PubMed] [Google Scholar]
- 38.Centers for Medicare and Medicaid Services (CMS ). A Prescriber’s guide to the new Medicare Part D opioid overutilization policies for 2019; 2018. https://www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNMattersArticles/Downloads/se18016.pdf.Accessed July 29, 2019.
- 39.Institute of Medicine (US) Committee on Understanding and Eliminating Racial and Ethnic Disparities in Health Care. In: Smedley BD, Stith AY, Nelson AR, eds. Unequal Treatment: Confronting Racial and Ethnic Disparities in Health Care. Washington (DC): The National Academies Press; 2003. [PubMed] [Google Scholar]
- 40.Saha S, Freeman M, Toure J, Tippens KM, Weeks C, Ibrahim S. Racial and ethnic disparities in the VA health care system: A systematic review. J Gen Intern Med. 2008;23:654–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schoenfeld D, Mohn L, Agalliu I, Stern JM. Disparities in care among patients presenting to the emergency department for urinary stone disease. Urolithiasis; 2019. Available from: 10.1007/s00240-019-01136-y. [DOI] [PubMed] [Google Scholar]
- 42.Wheeler SM, Bryant AS. Racial and ethnic disparities in health and health care. Obstet Gynecol Clin North Am. 2017;44:1–11. [DOI] [PubMed] [Google Scholar]
- 43.Heyward J, Jones CM, Compton WM, et al. Coverage of nonpharmacologic treatments for low back pain among US public and private insurers. JAMA Netw Open. 2018;1:e183044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Howard R, Hallway A, Santos-Parker J, et al. Optimizing postoperative opioid prescribing through quality-based reimbursement. JAMA Netw Open. 2019;2: e1911619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hallway A, Vu J, Lee J, et al. Patient satisfaction and pain control using an opioid-sparing postoperative pathway. J Am Coll Surg. 2019;229:316–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Michigan Medicine. Managing your pain after surgery without opioids; 2019. http://www.med.umich.edu/1libr/Surgery/MPOPeducation-ManagingPainWithoutOpioids.pdf.Accessed October 31, 2019.
- 47.Vu JV, Howard RA, Gunaseelan V, Brummett CM, Waljee JF, Englesbe MJ. Statewide implementation of postoperative opioid prescribing guidelines. N Engl J Med. 2019;381:680–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.