Abstract
Introduction
We aimed to compare the treatment patterns and oncological outcomes, including postoperative morbidity and chemotherapy use, between octogenarians and patients <80 years of age who underwent radical cystectomy for bladder cancer.
Methods
We conducted a retrospective analysis of 119 patients who underwent radical cystectomy for bladder cancer at our center between January 2013 and April 2019. Comorbidities, clinical and pathological data, 30-day postoperative morbidity, and perioperative chemotherapy use were compared between octogenarians (n=31) and younger patients (n=88). Cancer-specific and overall survival rates were estimated with the Kaplan-Meier method and compared between the groups.
Results
No significant differences were found between the age groups in the clinical and pathological findings, including Charlson comorbidity index, modified frailty index, albumin level, renal function, and TNM stage. The median followup for survivors was 19 months (interquartile range [IQR] 11–30). Major complications (Clavien-Dindo grade ≥3) and 30-day postoperative mortality rates did not differ between the age groups (p=0.3 and p=0.18, respectively). Despite no difference in baseline glomerular filtration rates, perioperative chemotherapy utilization rate was lower among octogenarians compared to younger patients (13% vs. 34%, p=0.03). Estimated two-year cancer-specific survival rates for octo-generians and younger patients were 40% and 75%, respectively. Similarly, estimated two-year overall survival rates were 30% and 69%, respectively. Both cancer-specific and overall survival rates were significantly lower in octogenarians (p=0.007 and p=0.001, respectively).
Conclusions
Radical cystectomy in octogenarians results in comparable short-term outcomes as in younger patients. However, in the elderly population, perioperative chemotherapy utilization rates are lower and survival is inferior.
Introduction
Bladder cancer (BC) is the fourth most common cancer in men and the fourth leading cause of cancer death among octogenarian males. Incidence rates increase with age, and the average age at diagnosis is 73.1,2 The observed rise in overall life expectancy, along with an increase in the age of BC patients, have resulted in an increase in long-term survivors and a corresponding growth in the cumulative expenditure reaching over $170 000 per patient during the course of illness.3
Radical cystectomy (RC) and urinary diversion is the primary treatment for patients presenting with muscle-invasive bladder cancer (MIBC). Moreover, the use of neoadjuvant chemotherapy is often indicated prior to RC, resulting in a significant overall survival (OS) benefit.4 However the procedure has been associated with high postoperative morbidity, including major complications in 15% of patients and a 30-day mortality rate of 1.5%.5 In this context, there are inconsistent data regarding the safety of RC among the elderly. While some studies reported older patients have comparable perioperative morbidity to younger patients,6–10 others suggest increased postoperative complications and a higher mortality rate among the former group.11–15 While acknowledging the oncological benefits of RC in all age groups,16 many urologists refrain from performing the procedure in elderly patients, particularly in octogenarians.17–19 This likely reflects concern from treatment-related morbidity and mortality. Less is known about perioperative chemotherapy (POC) administration patterns and its ensuing consequences among octogenarians undergoing RC.
In the current study, we aimed to characterize the postoperative morbidity and POC utilization patterns in octogenarians undergoing RC and compare them to younger patients.
Methods
Study population
After obtaining institutional review board approval, we reviewed our prospectively assembled institutional database to extract the medical records of 127 patients who underwent RC and urinary diversion between January 2013 and April 2019. We excluded seven patients who had cystectomy for non-malignant disease and one patient with metastatic disease at presentation, leaving 119 patients for analyses. All patients included in the study cohort were diagnosed with muscle-invasive or high-risk non-muscle-invasive BC refractory to conservative therapy. Metastatic disease was excluded by means of computed tomography and/or nuclear imaging prior to treatment.
Data collection
Preoperative clinical characteristics were collected and compared between octogenarians (n=31) and younger patients (n=88), focusing primarily on Charlson comorbidity index (CCI), modified frailty index (mFI), albumin levels (g/L) and estimated glomerular filtration rate (eGFR) calculated using the MDRD formula.20
All patients underwent RC and urinary diversion with curative intent. The type of urinary diversion used was based on the patient’s age, baseline clinical characteristics, and personal preference. Patients presenting with locally advanced disease, risk factors for urethral recurrence, or moderate-to-severe renal impairment were offered an ileal conduit urinary diversion. Patients were operated via an open approach and underwent extended pelvic lymph node (LN) dissection as well.
Patients with adequate renal function and purported locally advanced tumors on preoperative imaging were invariably offered cisplatin-based neoadjuvant chemotherapy. The other patients with muscle-invasive disease were considered candidates for neoadjuvant chemotherapy based on their general health status, comorbidities, renal function, and personal preference. eGFR was calculated for all patients in the first month after RC and adjuvant chemotherapy was offered to eligible patients who did not receive neoadjuvant therapy based on their final pathological findings, namely LN involvement and locally advanced features (pT3–4).
All RC specimens were reviewed by a dedicated genitourinary pathologist for cancer histology, TNM stage, surgical margin status, number and status of LN removed. Followup consisted of axial-imaging and blood workup every 6–12 months.
Outcomes and statistical analysis
The study outcomes included: 1) 30-day postoperative morbidity; 2) 30-day postoperative mortality; 3) OS; 4) cancer-specific-survival (CSS); and 5) rate of POC utilization. Postoperative morbidity was graded by the Clavien-Dindo (CD) classification system, with minor complications graded as 1–2, major complications graded as 3–4, and death graded as 5. The cause of death was revised and death due to BC or its treatment was categorized as cancer-specific mortality. POC included either neoadjuvant or adjuvant treatment. Chemotherapy toxicity was reported according to the common terminology criteria for adverse events (CTCAE), version 5.0.
Statistical comparisons were performed using the Fisher-Exact test for categorical variables, and the Mann-Whitney U test and Student’s t-test for continuous variables. OS and CSS were estimated with Kaplan-Meier method and log rank test was used to compare between groups. Survival was calculated from time of RC until death; patients alive at last followup were censored. CSS was calculated from time of RC until death from BC, and patients who died of other causes or who were alive at last followup were censored. Cox proportional hazard models were generated to calculate hazard ratios (HR), along with 95% confidence intervals (CI). All analyses were two-sided, and statistical significance was defined as p<0.05. SPSS v. 23 (IBM, U.S.) and R v. 3.5.1 (R Foundation for Statistical Computing, Vienna, Austria) were used to conduct the calculations.
Results
Clinical and pathological characteristics
The study cohort included 93 men and 26 women with a median age of 71 years (interquartile range [IQR] 66–79). The clinical and pathological characteristics of the cohort stratified by age groups are summarized in Table 1. No significant differences were observed among any of the clinical and pathological parameters between the age groups. Preoperative health and comorbidity indexes likewise did not differ significantly, reflecting a relatively homogenous population. Both age groups had a median CCI of 3 (IQR 2–4) with similar rates of CCI ≥4 (29% octogenarians vs. 27% younger group, p=0.82). Octogenarians had higher mFI values, however, this did not reach statistical significance. The median albumin value, a marker of nutritional and frailty status, was 39 g/L (IQR 35–42), representing a well-nourished population with no differences between age groups.
Table 1.
Clinical and pathological characteristics of the study cohort categorized by age group
| Overall (n=119) | Age ≥80 (n=31) | Age <80 (n=88) | p | |
|---|---|---|---|---|
| Age at surgery, median (IQR) | 71 (66–79) | 82.5 (80–85) | 68.5 (65–72) | <0.01 |
| Gender, M:F | 93:26 | 23:8 | 70:18 | 0.61 |
| CCI, median (IQR) | 3 (2–4) | 3 (2–4) | ||
| CCI, n (%) | 0.82 | |||
| 0–3 | 86 (72%) | 22 (71%) | 64 (73%) | |
| 4+ | 33 (28%) | 9 (29%) | 24 (27%) | |
| Albumin, g/L, median (IQR) | 39 (35–42) | 38.5 (33–41) | 39 (36–42) | 0.12 |
| eGFR at presentation*, median (IQR) | 70 (56–88) | 64 (56–86) | 73 (58–88) | 0.43 |
| mFI, n (%) | 0.08 | |||
| 0 | 31 (26%) | 3 (10%) | 28 (32%) | |
| 1 | 32 (27%) | 11 (35%) | 21 (24%) | |
| 2 | 33 (28%) | 8 (26%) | 25 (28%) | |
| 3+ | 23 (19%) | 9 (29%) | 14 (16%) | |
| Clinical T stage, n (%) | 0.68 | |||
| T1 | 20 (17%) | 4 (13%) | 16 (18%) | |
| T2 | 63 (53%) | 18 (58%) | 45 (51%) | |
| T3 | 24 (20%) | 4 (13%) | 20 (23%) | |
| T4 | 7 (6%) | 2 (6%) | 5 (6%) | |
| Tx | 5 (4%) | 3 (10%) | 2 (2%) | |
| Clinical N stage, n (%) | 0.22 | |||
| N0 | 102 (86%) | 23 (74%) | 79 (90%) | |
| N+ | 12 (10%) | 5 (16%) | 7 (8%) | |
| Nx | 5 (4%) | 3 (10%) | 2 (2%) | |
| Neobladder, n (%) | 10 (8%) | 0 | 10 (11%) | 0.06 |
| Number LN removed, median (IQR) | 18 (12–25) | 16 (11–20) | 18 (12–26) | 0.30 |
| Positive surgical margins, n (%) | 9 (7%) | 4 (13%) | 5 (6%) | 0.20 |
| Pathological T stage, n (%) | 0.69 | |||
| T0 | 26 (22%) | 9 (29%) | 17 (19%) | |
| T1 | 9 (7%) | 1 (3%) | 8 (9%) | |
| T2 | 23 (19%) | 5 (16%) | 18 (20%) | |
| T3 | 41 (34%) | 10 (32%) | 31 (35%) | |
| T4 | 20 (17%) | 6 (19%) | 14 (16%) | |
| Pathological N stage, n (%) | 0.57 | |||
| N0 | 82 (69%) | 19 (61%) | 63 (72%) | |
| N1 | 12 (10%) | 3 (10%) | 9 (10%) | |
| N2 | 20 (17%) | 7 (23%) | 13 (15%) | |
| Nx | 5 (4%) | 2 (6%) | 3 (3%) | |
| eGFR after RC*, median (IQR) | 75 (54–91) | 68 (53–86) | 76 (54–100) | 0.14 |
Units of mL/min/1.73 m2.
CCI: Charlson comorbidity index; eGFR: estimated glomerular filtration rate; IQR: interquartile range; LN: Lymph nodes; mFI: modified frailty index; RC: radical cystectomy.
RC was performed for high-risk non-muscle-invasive disease in 20 patients (17%). Thirty-one patients (26%) had locally advanced disease (cT3/pT4) and 12 patients (10%) were suspected of having nodal involvement at diagnosis. There was no difference in cTNM staging between age groups (Table 1). Pathological findings demonstrated T0 in 26 patients (22%), pT3/T4 disease in 61 patients (51%), and nodal involvement in 32 patients (27%). The median number of LN removed was 18 (IQR 12–25) and positive surgical margins (PSM) were found in nine patients (7%), including three with soft tissue PSM and six with urothelial PSM (two ureter, two urethra, two both). Adverse pathological features did not differ between age groups, specifically, rate of pT3/4 disease was 51% in both age groups, nodal involvement in octogenarians and younger patients was 33% and 25%, respectively (p=0.57), and PSM was apparent in 13% and 6% of patients, respectively (p=0.2). Orthotropic reconstruction with neobladder was performed in 10/88 patients of the non-octogenarian group, as all octogenarians underwent ileal conduit (p=0.06).
Postoperative morbidity and mortality
The overall 30-day postoperative complication rate was 71% (85/119 patients), with no difference in types of complications between age groups (Table 2). Urinary tract infections (20/119 patients, 17%), wound complications (13/119 patients, 12%), and prolonged ileus (9/119 patients, 8%) were the most common. Blood transfusions were administrated in the postoperative period to six patients (5%) due to symptomatic anemia. For the whole cohort, the 30-day postoperative major complications rate (CD grade 3–4) was 20% (24/119 patients) and mortality rate was 5% (6/119 patients), with no apparent differences between the age groups (Table 3). Major complications were observed in 13% of octogenarians and 23% of younger patients (p=0.3), and postoperative mortality rates were 10% and 3%, respectively (p=0.18) (Table 3).
Table 2.
30-day postoperative complication rates in the study cohort categorized by age group
| Type of complication | Overall (n=119) | Age ≥80 (n=31) | Age <80 (n=88) | p |
|---|---|---|---|---|
| Urinary tract infection | 20 (17%) | 8 (26%) | 12 (14%) | 0.16 |
| Wound complications* | 13 (12%) | 3 (10%) | 10 (11%) | 1 |
| Ileus | 9 (8%) | 2 (6%) | 7 (8%) | 1 |
| GI leak | 6 (5%) | 2 (6%) | 4 (5%) | 0.65 |
| Entero-vesical fistula | 1 (1%) | 0 | 1 (1%) | 1 |
| Blood transfusions | 6 (5%) | 2 (6%) | 4 (5%) | 0.65 |
| Cardiac | 6 (5%) | 2 (6%) | 4 (5%) | 0.65 |
| Urinary leakage | 5 (4%) | 0 | 5 (6%) | 0.32 |
| Significant electrolytes imbalance | 5 (4%) | 2 (6%) | 3 (3%) | 0.6 |
| Unexplained hemodynamic instability | 4 (3%) | 2 (6%) | 2 (2%) | 0.27 |
| Abscess** | 3 (3%) | 2 (6%) | 1 (1%) | 0.16 |
| Pulmonary | 3 (3%) | 1 (3%) | 1 (1%) | 0.45 |
| Lymphocele | 2 (2%) | 1 (3%) | 1 (1%) | 0.45 |
| Neurological | 2 (2%) | 0 | 2 (2%) | 1 |
| High output GI stoma | 1 (1%) | 0 | 1 (1%) | 1 |
| CDI | 1 (1%) | 0 | 1 (1%) | 1 |
| Thromboembolic event | 1 (1%) | 0 | 1 (1%) | 1 |
Surgical site infections, dehiscence, and perioperative ventral hernia.
Renal and/or abdominal.
CDI: Clostridium difficile infection; GI: gastrointestinal.
Table 3.
Postoperative morbidity and perioperative chemotherapy administration data categorized by age group
| Overall (n=119) | Age ≥80 (n=31) | Age <80 (n=88) | p | |
|---|---|---|---|---|
| 30-day perioperative morbidity, n (%) | 0.29 | |||
| CD 0 | 34 (28%) | 8 (26%) | 26 (29%) | |
| CD 1–2 (minor complications) | 55 (46%) | 16 (52%) | 39 (44%) | |
| CD 3–4 (major complications) | 24 (20%) | 4 (13%) | 20 (23%) | 0.30 |
| CD 5 (mortality) | 6 (5%) | 3 (10%) | 3 (3%) | 0.18 |
| Perioperative chemotherapy*, n (%) | 33 (28%) | 4 (13%) | 29 (33%) | 0.03 |
| Neoadjuvant | 19 (16%) | 3 (10%) | 16 (18%) | 0.39 |
| Adjuvant | 14 (12%) | 1 (3%) | 13 (15%) | 0.11 |
| Toxicity grade 3–4**, n (%) | 2 (50%) | 9 (31%) | 0.58 | |
| Pre-chemotherapy eGFR†, median (IQR) | 72 (62–102) | 98 (59–143) | 72 (64–101) | 0.63 |
None of the patients received both neoadjuvant and adjuvant treatments.
Percentages represent the relative proportion out of patients who received chemotherapy.
Units of mL/min/1.73 m2.
CD: Clavien-Dindo; eGFR: estimated glomerular filtration rate.
Perioperative chemotherapy
POC was administrated to 33 patients (28%), including 19 patients (16%) who received neoadjuvant therapy and 14 (12%) who received adjuvant therapy. Only 13% of octogenarians received POC compared to 33% in the younger group (p<0.05) (Table 3). POC utilization rates were significantly lower in octogenarians, mostly due to lower rate of adjuvant treatment. Severe chemotherapy-related toxicity rates (grade 3–4) were not different between the groups (p=0.58), which was reported in 50% of the octogenarians and 31% of younger patients.
Survival data
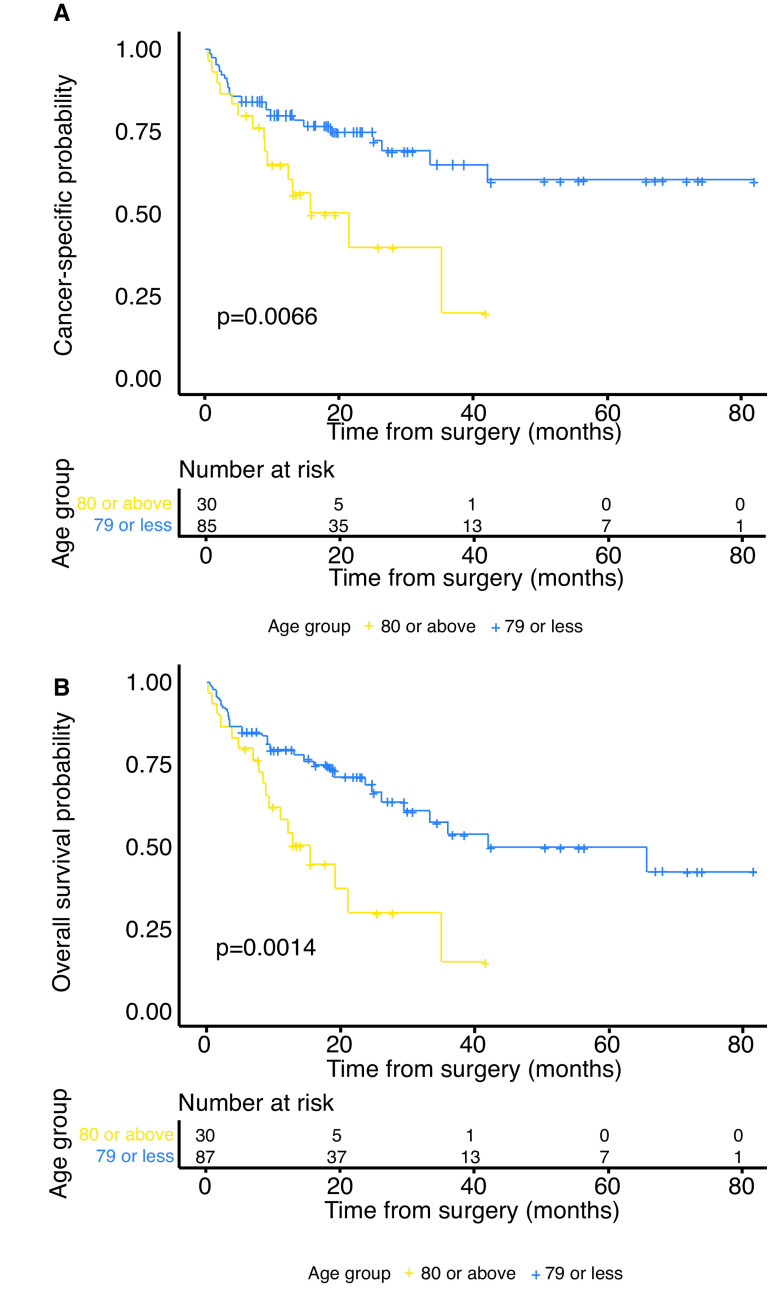
Two patients who were lost to followup were not included in survival analyses and two additional patients in whom we were unable to ascertain the cause of death were not included in the CSS analysis. The median followup for survivors was 19 months (IQR 11–30). By the end of followup, 48 patients had died, 38 of whom died from BC. Among the octogenarians, 18/30 (60%) patients died, 15 due to their disease. Among the younger patients, 30/87 (36%) died, 23 due to BC. Estimated one-year and two-year CSS rates were 65% (95% CI 50, 85) and 40% (95% CI 22, 73) for octogenarians and 80% (95% CI 72, 90) and 75% (95% CI 66, 86) for younger patients, respectively. Similarly, estimated one- and two-year OS rates were 58% (95% CI 42, 80) and 30% (95% CI 15, 61) for octogenarians and 80% (95% CI 71, 89) and 69% (95% CI 59, 81) for younger patients, respectively. Both CSS and OS were significantly lower in the older patients (p=0.007 and p=0.001, respectively) (Fig. 1).
Fig. 1.
Kaplan-Meier curves for (A) cancer-specific survival; and (B) overall survival categorized by age groups.
Discussion
In the current study we evaluated the role of RC and POC for treatment of BC in an elderly population over 80 years of age. Our findings suggest that octogenarians might not experience a higher rate of major complications after RC compared to younger patients, likely due to stringent selection criteria, which permit a cohort of octogenarians that resembles younger patients in terms of preoperative characteristics. On the other hand, we found that octogenarians are less likely to receive POC and have significantly lower CSS and OS rates, both of which should be considered when treating this population.
Previous studies highlighted the differences in the CCI of patients undergoing RC at different age groups.8,9,15 Donat et al demonstrated there was no difference in the 90-day postoperative major complications rate between octogenarians and younger patients, whereas the postoperative mortality among octogenarians was significantly higher (6.8% vs. 2.2%, p=0.01), possibly due to differences in baseline comorbidities.8 In addition, Berger et al and Hollenbeck et al concluded that not only age but rather CCI is a risk factor for postoperative morbidity and mortality in the elderly.12,15 These findings underscore the imperative for patient selection, particularly among octogenarians, to minimize postoperative complications and improve survival. One measure to facilitate this objective is the mFI, an 11-item score that grades the health status of older individuals and measures vulnerability to poor outcomes.21,22 The mFI was associated robustly with poor postoperative outcomes after RC, and mFI scores of ≥3 were associated with a greater likelihood of experiencing a major complication.23 In the current study, higher rates of mFI ≥3 were observed among octogenarians (29% vs. 16%), yet the difference was not statistically significant, likely reflecting our meticulous patient selection strategy contributing to the lack of difference in early postoperative outcomes.
Our observed 30-day postoperative major complication rate of 20% and 30-day mortality rate of 5% are consistent with prior publications in the field.24,25 The increase in postoperative morbidity over the last decade, since the data reported by Shabsigh et al in 2009, suggests variations in reporting methods and patient selection. In the current study, urinary tract infections (21%) and wound complications (12%) were the most commonly encountered postoperative complications. This is consistent with common complication rates reported in previous studies.5,24,25 Our blood transfusion rate (5%) was, however, lower than observed in previous studies (10–30%),8,24 which likely reflects our rigorous blood transfusion policy allowing the administration of packed cells only to patients with symptomatic anemia or hemoglobin levels below 7 g/dL.
The use of POC in patients with MIBC undergoing RC has been increasingly adopted. Neoadjuvant chemotherapy prolongs survival4 and does not affect postoperative morbidity after RC.26 Adjuvant treatment for selected patients with locally advanced disease or nodal involvement may prevent disease progression.27 Both strategies are endorsed by the European Association of Urology and the American Urological Association guidelines, with no age restriction. Nonetheless, many patients may fail to receive POC due to impaired renal function, unwarranted postoperative morbidity, and low compliance.28 In our study, octogenarians received less POC (13% vs. 33% in younger patients, p=0.03), which, excluding patient age, could not be entirely accounted for by patient or disease characteristics. Perioperative morbidity is also not likely the cause, as rates of major complications and mortality in the first 30 days after RC were not different. Finally, pre-treatment renal function, which is a paramount determinant of nephrotoxic chemotherapy eligibility, was also not significantly different between the age groups. We assume the lower rates of POC administration to octogenarians are related to physicians’ and patients’ shared decision influenced by age, estimated life expectancy, and concern from chemotherapy-related toxicity. The latter, however, has not been supported by any data. Notably, we did not observe a difference in chemotherapy toxicity between the age groups (p=0.58). As such, this should be less of a concern when recommending POC to patients at older age.
We report significantly lower CSS and OS rates among octogenarians. While a decrease in OS rates is expected given the age difference between the two groups, Donat et al and others reported CSS was not significantly different between the age groups.7,8 The significant decrease in CSS observed in our study could be, in part, due to a lower rate of POC in the older patients. It suggests that in order to optimize outcomes, management of MIBC in octogenarians should follow invariably the tenets of standard therapy, including RC with extended LN dissection and POC when indicated.
Limitations
The limitations of our study are derived mostly from its retrospective nature. First, the small cohort size and short followup preclude strong recommendations. Second, clinical considerations taken into account when selecting octogenarians for RC and, on the other hand, the outcomes of octogenarians who were not operated, were unavailable for analysis. Third, while elderly patients are known to be predisposed to prolonged delay in diagnosis and treatment of BC, resulting in poor oncological outcomes,29 we were unable to collect accurate data about the time interval between diagnosis and RC. Yet, lack of difference in clinical and pathological disease characteristics between the age groups might be used to refute a substantial delay. Fourth, quality-of-life parameters, which might be part of the considerations for RC and POC, were not included in our study. Lastly, while acknowledging the low rate of neoadjuvant and adjuvant chemotherapy in our cohort, these rates are comparable to those reported in retrospective analyses of the Surveillance, Epidemiology, and End Results database.30 With this in mind, our findings provide additional support to a more aggressive treatment approach, including RC and POC in well-selected octogenarians with BC.
Conclusions
Our findings suggest that with meticulous patient selection, octogenarian patients with BC can be safely offered RC, expecting similar early postoperative results compared to younger patients. Furthermore, when patients are carefully selected, chemotherapy-related side effects are not different between octogenarians and patients less than 80 years of age. Therefore, surgical treatment and POC should not be categorically precluded in the octogenarian population, but rather tailored according to patient and disease characteristics, estimated life expectancy, and shared decision-making between patients and physicians.
Acknowledgment
The authors would like to thank Mr. Ziv Bern for his assistance with the statistical analyses of the study.
Footnotes
Competing interests: The authors do not report any competing personal or financial interests related to this work.
This paper has been peer-reviewed.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7–30. doi: 10.3322/caac.21590. [DOI] [PubMed] [Google Scholar]
- 2.Antoni S, Ferlay J, Soerjomataram I, et al. Bladder cancer incidence and mortality: A global overview and recent trends. Eur Urol. 2017;71:96–108. doi: 10.1016/j.eururo.2016.06.010. [DOI] [PubMed] [Google Scholar]
- 3.Sloan FA, Yashkin AP, Akushevich I, et al. The cost to Medicare of bladder cancer care. Eur Urol Oncol. 2019;8 doi: 10.1016/j.euo.2019.01.015. S2588-9311(19)30016-1. [DOI] [PubMed] [Google Scholar]
- 4.Grossman HB, Natale RB, Tangen CM, et al. Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N Engl J Med. 2003;349:859–66. doi: 10.1056/NEJMoa022148. [DOI] [PubMed] [Google Scholar]
- 5.Shabsigh A, Korets R, Vora KC, et al. Defining early morbidity of radical cystectomy for patients with bladder cancer using a standardized reporting methodology. Eur Urol. 2009;55:164–74. doi: 10.1016/j.eururo.2008.07.031. [DOI] [PubMed] [Google Scholar]
- 6.De Groote R, Gandaglia G, Geurts N, et al. Robot-assisted radical cystectomy for bladder cancer in octogenarians. J Endourol. 2016;30:792–8. doi: 10.1089/end.2016.0050. [DOI] [PubMed] [Google Scholar]
- 7.Ito K, Kanno T, Sawada A, et al. Laparoscopic radical cystectomy in octogenarians: Analysis of a Japanese multicenter cohort. Int J Clin Oncol. 2019;24:1081–8. doi: 10.1007/s10147-019-01446-6. [DOI] [PubMed] [Google Scholar]
- 8.Donat SM, Siegrist T, Cronin A, et al. Radical cystectomy in octogenarians-does morbidity outweigh the potential survival benefits? J Urol. 2010;183:2171–7. doi: 10.1016/j.juro.2010.02.015. [DOI] [PubMed] [Google Scholar]
- 9.Yamanaka K, Miyake H, Hara I, et al. Significance of radical cystectomy for bladder cancer in patients over 80 years old. Int Urol Nephrol. 2007;39:209–14. doi: 10.1007/s11255-006-9122-5. [DOI] [PubMed] [Google Scholar]
- 10.Clark PE, Stein JP, Groshen SG, et al. Radical cystectomy in the elderly: Comparison of clinical outcomes between younger and older patients. Cancer. 2005;104:36–43. doi: 10.1002/cncr.21126. [DOI] [PubMed] [Google Scholar]
- 11.Leveridge MJ, Siemens DR, Mackillop WJ, et al. Radical cystectomy and adjuvant chemotherapy for bladder cancer in the elderly: A population-based study. Urology. 2015;85:791–8. doi: 10.1016/j.urology.2014.12.027. [DOI] [PubMed] [Google Scholar]
- 12.Hollenbeck BK, Miller DC, Taub D, et al. Identifying risk factors for potentially avoidable complications following radical cystectomy. J Urol. 2005;174:1231–7. doi: 10.1097/01.ju.0000173923.35338.99. [DOI] [PubMed] [Google Scholar]
- 13.Mendiola FP, Zorn KC, Gofrit ON, et al. Cystectomy in the ninth decade: Operative results and long-term survival outcomes. Can J Urol. 2007;14:3628–34. [PubMed] [Google Scholar]
- 14.Zakaria AS, Santos F, Tanguay S, et al. Radical cystectomy in patients over 80 years old in Quebec: A population-based study of outcomes. J Surg Oncol. 2015;111:917–22. doi: 10.1002/jso.23887. [DOI] [PubMed] [Google Scholar]
- 15.Berger I, Martini T, Wehrberger C, et al. Perioperative complications and 90-day mortality of radical cystectomy in the elderly (75+): A retrospective, multicenter study. Urol Int. 2014;93:296–302. doi: 10.1159/000357127. [DOI] [PubMed] [Google Scholar]
- 16.Chamie K, Hu B, DeVere White RW, et al. Cystectomy in the elderly: does the survival benefit in younger patients translate to the octogenarians? BJU Int. 2008;102:284–90. doi: 10.1111/j.1464-410X.2008.07636.x. [DOI] [PubMed] [Google Scholar]
- 17.Prout GR, Wesley MN, Yancik R, et al. Age and comorbidity impact surgical therapy in older bladder carcinoma patients: A population-based study. Cancer. 2005;104:1638–47. doi: 10.1002/cncr.21354. [DOI] [PubMed] [Google Scholar]
- 18.Koppie TM, Serio AM, Vickers AJ, et al. Age-adjusted Charlson comorbidity score is associated with treatment decisions and clinical outcomes for patients undergoing radical cystectomy for bladder cancer. Cancer. 2008;112:2384–92. doi: 10.1002/cncr.23462. [DOI] [PubMed] [Google Scholar]
- 19.Konety BR, Joslyn SA. Factors influencing aggressive therapy for bladder cancer: An analysis of data from the SEER program. J Urol. 2003;170:1765–71. doi: 10.1097/01.ju.0000091620.86778.2e. [DOI] [PubMed] [Google Scholar]
- 20.Levey AS, Coresh J, Greene T, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145:247. doi: 10.7326/0003-4819-145-4-200608150-00004. [DOI] [PubMed] [Google Scholar]
- 21.Farhat JS, Velanovich V, Falvo AJ, et al. Are the frail destined to fail? Frailty index as predictor of surgical morbidity and mortality in the elderly. J Trauma Acute Care Surg. 2012;72:1526–30. doi: 10.1097/TA.0b013e3182542fab. [DOI] [PubMed] [Google Scholar]
- 22.Revenig LM, Canter DJ, Taylor MD, et al. Too frail for surgery? Initial results of a large multidisciplinary prospective study examining preoperative variables predictive of poor surgical outcomes. J Am Coll Surg. 2013;217:665–70.e1. doi: 10.1016/j.jamcollsurg.2013.06.012. [DOI] [PubMed] [Google Scholar]
- 23.Chappidi MR, Kates M, Patel HD, et al. Frailty as a marker of adverse outcomes in patients with bladder cancer undergoing radical cystectomy. Urol Oncol. 2016;34:256.e1–6. doi: 10.1016/j.urolonc.2015.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Parekh DJ, Reis IM, Castle EP, et al. Robot-assisted radical cystectomy vs. open radical cystectomy in patients with bladder cancer (RAZOR): An open-label, randomized, phase 3, non-inferiority trial. Lancet. 2018;391:2525–36. doi: 10.1016/S0140-6736(18)30996-6. [DOI] [PubMed] [Google Scholar]
- 25.Bochner BH, Dalbagni G, Sjoberg DD, et al. Comparing open radical cystectomy and robot-assisted laparoscopic radical cystectomy: A randomized clinical trial. Eur Urol. 2015;67:1042–50. doi: 10.1016/j.eururo.2014.11.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Salminen AP, Koskinen I, Perez IM, et al. Neoadjuvant chemotherapy does not increase the morbidity of radical cystectomy: A10-year retrospective nationwide study. Eur Urol Oncol. 2018;1:525–30. doi: 10.1016/j.euo.2018.06.014. [DOI] [PubMed] [Google Scholar]
- 27.Leow JJ, Martin-Doyle W, Rajagopal PS, et al. Adjuvant chemotherapy for invasive bladder cancer: A 2013 updated systematic review and meta-analysis of randomized trials. Eur Urol. 2014;66:42–54. doi: 10.1016/j.eururo.2013.08.033. [DOI] [PubMed] [Google Scholar]
- 28.Donat SM, Shabsigh A, Savage C, et al. Potential impact of postoperative early complications on the timing of adjuvant chemotherapy in patients undergoing radical cystectomy: A high-volume tertiary cancer center experience. Eur Urol. 2009;55:177–85. doi: 10.1016/j.eururo.2008.07.018. [DOI] [PubMed] [Google Scholar]
- 29.Russell B, Liedberg F, Khan MS, et al. A systematic review and meta-analysis of delay in radical cystectomy and the effect on survival in bladder cancer patients. Eur Urol Oncol. 2019;3:239–49. doi: 10.1016/j.euo.2019.09.008. [DOI] [PubMed] [Google Scholar]
- 30.Macleod LC, Yabes JG, Yu M, et al. Trends and appropriateness of perioperative chemotherapy for muscle-invasive bladder cancer. Urol Oncol Semin Orig Investig. 2019;37:462–9. doi: 10.1016/j.urolonc.2019.04.006. [DOI] [PubMed] [Google Scholar]