Abstract
The Grb2 and Shc adapter proteins play critical roles in coupling activated growth factor receptors to several cellular signaling pathways. To assess the role of these molecules in mammary epithelial development and tumorigenesis, we have generated transgenic mice which individually express the Grb2 and Shc proteins in the mammary epithelium. Although mammary epithelial cell-specific expression of Grb2 or Shc accelerated ductal morphogenesis, mammary tumors were rarely observed in these strains. To explore the potential role of these adapter proteins in mammary tumorigenesis, mice coexpressing either Shc or Grb2 and a mutant form of polyomavirus middle T (PyV mT) antigen in the mammary epithelium were generated. Coexpression of either Shc or Grb2 with the mutant PyV mT antigen resulted in a dramatic acceleration of mammary tumorigenesis compared to parental mutant PyV mT strain. The increased rate of tumor formation observed in these mice was correlated with activation of the epidermal growth factor receptor family and mitogen-activated protein kinase pathway. These observations suggest that elevated levels of the Grb2 or Shc adapter protein can accelerate mammary tumor progression by sensitizing the mammary epithelial cell to growth factor receptor signaling.
The murine mammary gland represents a unique system to study the responsiveness of cells to diverse signals stimulating cell death, survival, proliferation, and differentiation. The control of mammary epithelial proliferation and differentiation is ultimately regulated by hormonal and peptide factors that exert their biological action through a variety of receptor molecules. Elevated expression of growth factors or their cognate receptors can result in deregulated mammary epithelial cell proliferation, which can ultimately progress to the malignant phenotype. For example, elevated expression of the ErbB-2/Neu receptor tyrosine kinase has been implicated in the genesis of a large proportion of human breast cancers (39, 40). Consistent with these observations, mammary epithelial expression of ErbB-2 in transgenic mice results in the efficient induction of mammary tumors (4, 14, 16, 25, 35). Whereas it is clear that oncogenes such as erbB-2 induce malignancy, the precise molecular mechanism by which this occurs is unclear.
One potential mechanism by which receptor tyrosine kinases (RTKs) can induce proliferation is through interaction with a number of Src homology 2 (SH2)- or protein tyrosine binding domain (PTB)-containing adapter proteins (27). Although adapter proteins such as Shc (Src homology and collagen) and Grb2 (growth factor receptor-bound protein 2) lack intrinsic enzymatic activity, they play an important role in connecting growth factors to specific signaling pathways (23, 28, 29, 32). Grb2 is a 25-kDa protein which contains a central SH2 domain flanked by two SH3 domains. Activation of RTKs can result in the direct recruitment of Grb2 via its SH2 domain to specific tyrosine-phosphorylated residues within the receptor. Subsequent recruitment of the guanine nucleotide exchange factor Sos to the plasma membrane via interaction with the SH3 domain of Grb2 results in nucleotide exchange on Ras and activation of the Ras/mitogen-activated protein kinase (MAPK) pathway (23, 32).
Another mechanism by which Grb2 can be indirectly recruited to RTKs is through its specific association with the Shc adapter protein. The human Shc gene is localized on chromosome 1q21 and encodes three distinct Shc isoforms. The p52 and p46 forms of Shc, which result from the use of distinct start translation sites, possess a conserved N-terminal PTB domain, a central collagen homology (CH-1) domain, and a C-terminal SH2 domain (3). The p66 form of Shc is generated by alternative splicing and encodes an additional N-terminal CH-2 domain. The association between Shc and Grb2 is mediated through the interaction of the Grb2 SH2 domain with two tyrosine phosphorylation sites present with the central CH-1 domain of Shc (tyrosines 239 and 240 and tyrosine 317) (13, 17, 32, 38, 45). Shc in turn can bind activated RTKs through a PTB domain that recognizes specific NPXY motifs within the receptor (1, 2, 5, 44). In addition to the PTB domain, Shc also possesses an SH2 domain, which is capable of interacting with a number of phosphotyrosine-containing proteins (12, 28).
There is considerable evidence implicating the Shc and Grb2 adapter proteins as critical functional components of oncogene-mediated signal transduction pathways. Indeed, elevated levels of Grb2 can be detected in a large percentage of human breast cancers and their derived cell lines (8, 46). Interestingly, in a subset of these breast tumors, the chromosomal regions encoding the Grb2 gene are amplified (8, 46). Moreover, the fact that Shc is constitutively phosphorylated in a high percentage of human breast tumors and breast cancer cell lines (30, 42) suggests that it is functionally involved in coupling RTKs to the Ras signaling pathway. Direct evidence for the involvement of the Grb2 adapter protein in mammary tumorigenesis has been derived from the recent observation that polyomavirus middle T (PyV mT) oncogene-expressing transgenic mice heterozygous for a Grb2 null allele demonstrate a significant delay in the onset of mammary tumors (6). Consistent with these observations, transgenic mice expressing a mutant PyV mT oncogene decoupled from the Shc and Grb2 adapter proteins in mammary epithelium exhibit a significant delay in the onset of mammary tumors compared to mice expressing the wild-type PyV mT oncogene (48). Taken together, these observations suggest that activation of the Shc and Grb2 adapter proteins plays a critical role in the induction of mammary tumors.
To further explore the role of the Shc and Grb2 adapter proteins in mammary tumor progression, transgenic mice expressing the Grb2 or Shc cDNA under the transcriptional control of the mouse mammary tumor virus (MMTV) promoter were generated. Female transgenic mice expressing these adapter proteins in the mammary epithelium were capable of nursing their litters. Whole-mount analyses of virgin mammary glands revealed that both the MMTV/Grb2 and MMTV/Shc strains exhibited evidence of enhanced tertiary branching. Specifically, the MMTV/Shc strains exhibited an increased number of terminal end buds, whereas the MMTV/Grb2 strains displayed enhanced side branching. Despite these mammary epithelial abnormalities, the MMTV/Shc mice rarely developed mammary tumors, while the MMTV/Grb2 strains failed to develop tumors during the observation period. To assess the importance of Grb2 and Shc in mammary tumorigenesis, these strains were interbred with transgenic mice expressing a mutant PyV mT antigen incapable of directly coupling to the Shc pathway. The results of these studies demonstrated that coexpression of either Grb2 or Shc with this mutant PyV mT oncogene resulted in accelerated tumor development, which was correlated with activation of the MAPK pathway.
MATERIALS AND METHODS
DNA constructions.
All transgene constructs were previously derived by inserting the appropriate cDNAs into the MMTV long terminal repeat (LTR) expression vector, p206 (25). The MMTV LTR component of p206 was derived from plasmid pA9 (20), and the simian virus 40 (SV40) transcriptional processing signals 3′ to the cDNA were derived from plasmid CDM8 (33). MTY250F was constructed by standard M13 mutagenesis of PyV mT and cloned into the HindIII and EcoRI sites of p206 to generate MMTV/MTY250F (48). MMTV/p52 Shc (herein referred to as MMTV/Shc) was generated by cloning the mouse p52 Shc cDNA into the HindIII and EcoRI sites of p206 and was provided by Venus Ka-Man Lai and Tony Pawson (Mount Sinai Hospital, Toronto, Ontario, Canada). Finally, MMTV/Grb2 was provided by R. Daly (Garvan Institute of Medical Research, Sidney, New South Wales, Australia) and was constructed by inserting into p206 the EcoRI fragment of the human Grb2 cDNA. To aid in the identification of tissue specificity of transgene expression, plasmid pASV was used to generate an antisense riboprobe (25). A phosphoglycerate kinase 1 internal control was obtained from M. Rudnicki (McMaster University) and contains the AccI/PstI fragment of phosphoglycerate kinase 1 in the appropriate sites of pSP64 (Promega).
Generation and identification of transgenic mice.
DNA was prepared for microinjection by digestion of pMMTV/Shc and MMTV/Grb2 with 4 U of SalI and SpeI per μg for 1.5 h. The DNA was subsequently electrophoresed through a 1% agarose gel, and the resultant fragment was purified as previously described (36). The night prior to injection, superovulated FVB/N mice were mated with FVB/N males (Taconic Farms, Germantown, N.Y.). Fertilized, one-cell embryos were isolated, and the pronuclei of the zygotes were injected with 0.5 to 1 pl of DNA (5 μg/ml). This was followed by oviduct transfer of viable embryos to pseudopregnant Swiss-Webster mice (Taconic Farms). The MMTV/MTY250F 5a strain was established as described previously (48).
To identify potential transgenic founders, DNA was extracted from 1.5-cm tail clippings of the progeny as previously described by Muller et al. (25). Briefly, tail clippings were digested overnight in PK (proteinase K) buffer (10 mM Tris [pH 8.0], 100 mM NaCl, 10 mM EDTA [pH 8.0], 0.5% sodium dodecyl sulfate [SDS], 0.2 mg of PK [Canadian Life Technologies, Burlington, Ontario, Canada] per ml). DNA was isolated by several buffer-saturated phenol-chloroform extractions and precipitated in 2 volumes of absolute ethanol–0.1 volume of 3 N sodium acetate. The nucleic acid pellet was resuspended in 50 μl of Tris-EDTA buffer (10 mM Tris [pH 8.0], 1 mM EDTA, RNase A [20 μg/ml]) to an approximate concentration of 1 mg/ml. A volume of 15 μl of the solution was digested with 30 U of BamHI at 37°C for 1.5 h. Digested samples were subsequently electrophoresed through 1.0% agarose gels. Gels were then denatured for 45 min to 1 h with 600 mM NaCl–200 mM NaOH followed by an equal duration of neutralization in 600 mM NaCl–1 M Tris (pH 7.5) under constant shaking at room temperature. Gels were then transferred to GeneScreen (Dupont) according to the method of Southern (41) and cross-linked to the filters by using a UV Stratalinker device (Stratagene, La Jolla, Calif.). Filters were prehybridized at 60°C for several hours in 0.1 mg of sheared salmon sperm DNA per ml, 5× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate), 0.5% SDS, and 5× Denhardt’s reagent. A [α-32P]dCTP-labelled SPA (SV40 polyadenylation) DNA probe was prepared by random priming (10) using the gel-purified 750-bp BamHI/EcoRI fragment from pSPA. Filters were hybridized with the SPA probe overnight. The next day, filters were washed with 150 mM sodium phosphate buffer–1% SDS once for 20 min at room temperature and once for 20 min at 60°C. After blotting dry, tail DNA restriction fragments hybridizing with the SPA probe were detected by autoradiography using Kodak X-Omat AR film (Kodak, Rochester, N.Y.).
RNA analyses.
To determine the tissue specificity of transgene expression and to select several lines that demonstrated a high degree of expression in the mammary gland, RNase protection analysis (24) was performed on mouse tissues. Briefly, RNA was isolated from various tissues by the method of Chirgwin et al. (7). To generate the antisense SPA riboprobe, pASV was linearized by HindIII digestion, and the gel-purified fragment (Geneclean; Biocan) was in vitro transcribed. The gel was dried, and tissues which expressed SV40-hybridizing transcripts were detected by autoradiography using Kodak X-Omat AR film. RNA was purified by phenol-chloroform extraction followed by precipitation in 2 volumes of absolute ethanol–0.1 volume of 3 N sodium acetate (pH 5.2). Finally, RNA was resuspended in 100:1 diethyl pyrocarbonate-water, and yield was determined by UV absorption at 260 nm (Uvikon).
Northern blot analysis of RNA was performed as follows. Thirty micrograms of total RNA in a volume of 6.75 μl was incubated with 3 μl of 10× MOPS (morpholinepropanesulfonic acid) buffer (200 mM MOPS, 50 mM sodium acetate, 10 mM EDTA), 5.25 μl of formaldehyde (37%), and 15 μl of deionized formamide for 15 min at 55°C. Samples were chilled on ice, and 3.3 μl of loading dye (50% glycerol, 1 mM EDTA, 1 mg of xylene cyanol FF per ml, 1 mg of bromophenol blue per ml) was added. Samples were electrophoresed on formaldehyde gels (1% agarose, 1× MOPS, 0.7% formaldehyde, 0.5 μg of ethidium bromide per ml) at 100 V in 1× MOPS buffer for approximately 2 h. Following electrophoresis, gels were washed twice for 5 min in distilled water, followed by two incubations in 20× SSC (3.0 M NaCl, 0.34 M sodium citrate). Gels were transferred overnight to Hybond-N membranes (Dupont) according to the method of Southern (41). Random-primed DNA probes were prepared according to the method of Feinberg and Vogelstein (10). [α-32P]CTP-labeled probes were added to 3 ml of hybridization buffer and incubated with the Hybond-N membranes overnight. Membranes were then blotted dry, and DNA-RNA hybrids were detected by autoradiography using Kodak X-Omat AR film.
Antibodies.
Antibodies used include a mouse monoclonal antibody to Shc, PG-797 (Santa Cruz product no. sc-967), and a rabbit polyclonal Shc antibody, S14630 (Transduction Laboratories). Antibodies used to detect Grb2 include a mouse monoclonal antibody, G16720 (Transduction Laboratories), and a rabbit polyclonal antibody, C-23 (Santa Cruz product no. sc-255). Tyrosine-phosphorylated proteins were specifically detected by using either a mouse monoclonal antibody, PY20 (Transduction Laboratories), or a rabbit polyclonal antibody, P11230 (Transduction Laboratories). Rabbit polyclonal antibodies specific to phosphorylated p44/42 MAPK (Thr202/Tyr204) and the unphosphorylated forms were obtained from New England Biolabs (product no. 9101S and 9102, respectively). ErbB-2 was detected by using a rabbit polyclonal antibody (Upstate Biotechnology), while the rabbit polyclonal antibody C-17 (Santa Cruz product no. sc-285) was used to identify ErbB-3. The epithelial cell-specific marker, cytokeratin-8 (34), was detected by using a 1:10 dilution of the rat hybridoma tissue culture supernatant containing TROMA-1 (generous gift from M. Rudnicki). 125I-conjugated goat anti-rat and goat anti-rabbit secondary antibodies were received from Dupont.
Protein extract preparation.
Mouse mammary gland tissues were flash frozen in liquid nitrogen and ground to a fine powder with a chilled mortar and pestle. Cells were lysed in 2 ml of either CHAPS {3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonate} lysis buffer (0.7% CHAPS, 50 mM Tris [pH 8.0], 50 mM NaCl) or 2 ml of radioimmunoprecipitation assay (RIPA) lysis buffer (1% Triton X-100, 0.1% SDS, 1% sodium deoxycholate, 10 mM sodium phosphate buffer [pH 7.2], 150 mM NaCl, 2 mM EDTA, 50 mM NaF) (where indicated) for 20 min on ice with agitation. Tyrosine phosphatase inhibitor (1 mM Na3VO4) and protease inhibitors (aprotinin [10 μg/ml] and leupeptin [10 μg/ml]) were freshly added to both CHAPS and RIPA lysis buffers before use, while serine/threonine phosphatase inhibitors (5 mM sodium pyrophosphate and 40 mM glycerophosphate) were freshly added to RIPA buffer only. Lysates were cleared twice by centrifugation at 13,000 rpm for 10 min at 4°C. When RIPA was used as the lysis buffer, lysates were first sheared with a 21-gauge needle before being cleared by centrifugation. Supernatants were then decanted, and protein concentration was determined by using a Bio-Rad Bradford assay kit.
Immunoblot analysis.
A total of 60 μg of total protein lysate was used for immunoblot analysis. To each lysate was added an equal volume of 2× SDS-polyacrylamide gel electrophoresis (PAGE) sample load buffer (62.5 mM Tris [pH 6.8], 2% SDS, 10% glycerol, 5% β-mercaptoethanol, 0.02% bromophenol blue), and samples were incubated at 95°C for 10 min. Proteins were electrophoresed first through a 1 mM SDS–polyacrylamide stacking gel (4.93% acrylamide, 0.017% bisacrylamide, 0.125 M Tris, 0.1% SDS, 0.1% ammonium persulfate, 0.1% N,N,N′,N′-tetramethylethylene diamine [TEMED] [pH 6.8]) followed by a 1-mm SDS-polyacrylamide resolving gel (8.7% acrylamide, 0.3% bisacrylamide, 0.375 M Tris, 0.1% SDS, 0.1% ammonium persulfate, 0.1% TEMED [pH 8.8]) at a constant voltage of 60 V. Proteins were electrophoretically transferred to polyvinylidene difluoride membranes (Immobilon-P; Millipore) for 2 h at 0.6 mA, using a Bio-Rad wet transfer apparatus and the appropriate buffer (20% methanol, 0.025 M Tris, 0.2 M glycine). Membranes were incubated overnight at 4°C or for 1 h at room temperature in 3% powdered skim milk in Tris-buffered saline (TBS; 20 mM Tris [pH 7.5], 150 mM NaCl, 5 mM KCl). Subsequently, membranes were incubated overnight at 4°C or for 2 h at room temperature in 3% milk–TBS containing primary antibody at 1:1,000 dilution (except P11230, which was used at 1:500). Membranes were then washed twice for 5 min in TBS–0.01% Tween 20 and once for 5 min in TBS. The appropriate 125I-conjugated secondary antibody (100 μCi) was then added to 50 ml of 3% milk–TBS and incubated with the membranes for 1 h at room temperature. The proteins of interest were then detected by autoradiography using Kodak X-Omat AR film or detected and quantitated by PhosphorImager (Molecular Dynamics, Sunnyvale, Calif.) analysis.
Immunoprecipitations.
Immunoprecipitations were performed by first preincubating the specific monoclonal antibody (2 μg of antibody per mg of total protein) with 20 μl of protein G-Sepharose Fast Flow (Pharmacia) in 800 μl of phosphate-buffered saline (PBS; 140 mM NaCl, 2.7 mM KCl, 4.3 mM Na2HPO4, 1.4 mM KH2PO4) for 2 h at 4°C on an end-over-end rotator. Bound antibodies were washed once with 1 ml of PBS and once with 1 ml of lysis buffer. Two milligrams of total protein lysate was added, and the volume was brought to 600 μl with lysis buffer. Beads were then washed three times in lysis buffer and resuspended in 60 μl of 2× SDS-PAGE sample load buffer. After incubating at 95°C for 10 min, samples were split 1/3 for Shc analysis and 2/3 for phosphotyrosine analysis.
Histological and whole-mount evaluation.
Necropsies were performed as described by Muller et al. (25), with both gross and microscopic examination being conducted. Upper left mammary fat pad tissues (3L) were removed from CO2-euthanized female mice and fixed in 4% paraformaldehyde (in PBS) overnight at 4°C. Tissues were transferred to and stored (4°C) in 70% ethanol the next day. Specimens were then blocked in paraffin, sectioned at 5 μm, and stained with hematoxylin and eosin by Pathology Research Services, McMaster University Medical Centre. Lungs of tumor-bearing mice were prepared and examined in an identical fashion.
Mammary glands were also analyzed by whole-mount preparation using the upper right mammary fat pad tissue (3R) (47). Briefly, glands were spread out on glass slides and allowed to air-dry overnight at room temperature. The next day, mammary glands were defatted overnight in acetone. The following day, glands were pressed between two glass slides and again transferred to fresh acetone to enhance the defatting process. This was followed by overnight staining in Harris’s modified hematoxylin (Fisher Scientific, Ottawa, Ontario, Canada). Glands were then destained in successive changes of acid-alcohol destain solution (1% concentrated HCl, 75% ethanol) until the epithelial component was seen in sharp contrast to the background of the fat pad. The stain was fixed for 1 min in 0.02% ammonium hydroxide; the specimens were placed in 75% ethanol for 1 h and transferred to 100% ethanol for several hours. The slides were then placed in xylenes, and the glands were cleared overnight. Finally, the glands were mounted in Permount (Fisher Scientific), and a coverslip was placed over the slide.
The development of mammary tumors was monitored in MMTV/MTY250F, MMTV/Shc/MTY250F, and MMTV/Grb2/MTY250F virgin female mice by regular palpation of all mammary fat pads. Necropsies were performed 2 months after the first detection of tumors by palpation. Histological and whole-mount analyses were conducted on mammary tumors, and lungs were histologically examined for the presence of metastases.
RESULTS
Generation and characterization of the MMTV/Grb2 and MMTV/Shc strains.
To derive transgenic mice expressing elevated levels of Grb2 and Shc, cDNAs encoding the 52-kDa form of Shc and 25-kDa form of Grb2 were placed under the transcriptional control of the MMTV promoter/enhancer and microinjected into one-cell mouse zygotes (Fig. 1). A total of 9 MMTV/Shc and 10 MMTV/Grb2 transgenic founder animals were generated (Table 1). To assess the tissue-specific pattern of expression of the transgene, 20 μg of total RNA was isolated from a variety of tissues and subjected to RNase protection analyses with an antisense riboprobe complementary to the SV40 component of the transgene. The results of these analyses revealed that two MMTV/Shc and five MMTV/Grb2 strains expressed the transgene in the mammary epithelium. In addition to these tissue sites, transgene specific expression was detected in salivary glands and the male reproductive tract (Table 1). Given the elevated levels of transgene expression observed in the Shc-3 and Grb2-6 strains, these strains were subjected to further molecular analyses.
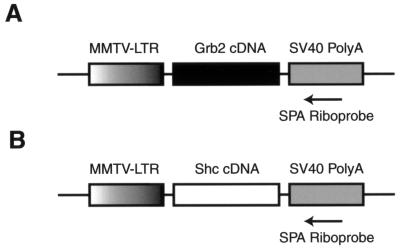
FIG. 1.
Schematic representations of the MMTV/Grb2 (A) and MMTV/Shc (B) transgenes. The shaded region represents the MMTV LTR. The solid region displays the cDNA encoding the human Grb2 protein. The gray region represents the SV40 splicing and polyadenylation signals. Also shown is the SPA riboprobe used to assess the tissue-specific pattern of transgene expression.
TABLE 1.
Transgene expression and tumor phenotype in MMTV/Shc and MMTV/Grb2 mice
| Line | Expressiona
|
Tumor phenotype (% penetrance) | Avg time (days) of tumor onset (days) ± SDb | ||||
|---|---|---|---|---|---|---|---|
| V.M.gl. | Sal.gl | Ovary | Testis | SV | |||
| Shc-1 | − | − | − | − | − | ||
| Shc-2 | − | − | − | − | − | ||
| Shc-3 | ++ | +/− | − | − | − | Focal papillary m.gl. adenocarcinoma (7%) | 403 ± 151 (n = 28) |
| Shc-4 | − | − | − | − | − | ||
| Shc-5 | − | − | − | − | − | ||
| Shc-6 | − | − | − | − | − | ||
| Shc-7 | − | − | − | − | − | ||
| Shc-8 | − | − | − | − | − | ||
| Shc-9 | + | − | − | − | − | ||
| Grb2-1 | +++ | +++ | − | + | +++ | ||
| Grb2-2 | − | − | ND | ND | |||
| Grb2-3 | ++ | ++ | − | ND | ND | ||
| Grb2-4 | + | + | ND | ND | ++ | ||
| Grb2-5 | − | − | − | ND | ND | ||
| Grb2-6 | +++ | +++ | − | − | +++ | ||
| Grb2-8 | − | − | − | ND | ND | ||
| Grb2-9 | + | + | − | ND | ND | ||
| Grb2-10 | + | + | − | ND | ND | ||
RNase protection analysis was performed on 20 μg of total RNA isolated from a variety of organs in MMTV/Shc and MMTV/Grb2 transgenic strains as described in Materials and Methods. Relative levels of transgene expression are indicated by − (not detected), +/− (very low), + (low), ++ (intermediate), and +++ (high). V.M.gl., virgin mammary gland; Sal.gl., salivary gland; SV, seminal vesicle; m.gl., mammary gland; ND, not determined.
Multiparous transgene carriers were monitored regularly for the appearance of tumors by physical palpation of mammary glands.
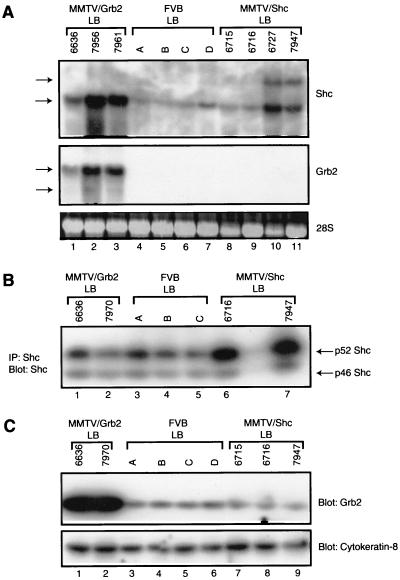
To gauge the extent of transgene expression, RNA and protein analyses were conducted on mammary gland tissues derived from female FVB/N, MMTV/Grb2-6, and MMTV/Shc-3 mice. To avoid differences in epithelial content, age-matched samples were harvested from lactating females and subjected to Northern blot analyses with either Grb2- or Shc-specific probes. The results of these analyses revealed a moderate but variable upregulation of Shc-specific transcripts in the mammary tissues of the MMTV/Shc mice (Fig. 2A, lanes 8 to 11). In addition to transcript comigrating with the endogenous Shc transcript, we have consistently observed a slower-migrating transcript in these tissues. Although the origin of the transcript is unclear, it likely derives from initiation of transcription from the MMTV-directed transcription start site. Interestingly, upregulation of endogenous Shc transcripts was also noted in the mammary epithelium of the MMTV/Grb2 strains (Fig. 2A, Shc, lanes 1 to 3). The observed differences in transcript levels in these samples were not due to differences in RNA loading since these samples displayed similar levels of 28S rRNA. Comparable Northern blot analyses revealed that elevated levels of Grb2-specific transcript could be detected in the mammary tissues derived from the MMTV/Grb2 animals (Fig. 2A, Grb2, lanes 1 to 3). In contrast, very low levels of endogenous Grb2 transcript could be detected in the mammary tissues of either FVB or MMTV/Shc transgenic mice (Fig. 2A, Grb2, lanes 4 to 11).
FIG. 2.
Expression of the MMTV/Shc-3 and MMTV/Grb2-6 strains. (A) Northern blot analyses of Shc and Grb2 mRNA levels. Total RNA (30 μg) was isolated from lactating mammary glands (LB) of MMTV/Grb2 (lanes 1 to 3), FVB (lanes 4 to 7), and MMTV/Shc (lanes 8 to 11) female mice. The gels were probed with Shc and Grb2 cDNAs, as indicated. Also shown is the 28S ribosomal marker. (B) Immunoblot (Blot)-immunoprecipitation (IP) analyses with Shc-specific antibodies. Immunoprecipitations were performed with 700 μg of total protein obtained from lactating glands from either MMTV/Grb2 (lanes 1 and 2), and FVB (lanes 3 to 5), or MMTV/Shc (lanes 6 and 7) females and immunoblotted with Shc-specific antisera. (C) Immunoblot analyses of Grb2 protein from lactating glands of MMTV/Grb2 (lanes 1 and 2), FVB (lanes 3 to 6), and MMTV/Shc (lanes 7 to 9) mice. The lower panel was probed with antibodies specific to cytokeratin-8.
To confirm that the observed upregulation of Grb2 and Shc transcripts corresponded to comparable levels of Grb2 and Shc protein, the protein lysates from the same samples were subjected to immunoblot analyses with Grb2- and Shc-specific antibodies. Immunoprecipitation of protein lysates with Shc-specific antisera followed by immunoblot analyses with Shc antibodies revealed that MMTV/Shc lysates expressed moderately elevated levels of the p52 isoform of Shc compared to either the MMTV/Grb2 or FVB protein lysates (Fig. 2B; compare lanes 1 to 5 to lanes 6 and 7). However, the levels of the 46-kDa form of Shc were comparable in all samples tested. Given that the MMTV transgene encodes the 52-kDa isoform of Shc, these observations are consistent with observed upregulation of Shc transgene transcripts observed in this strain.
Immunoblot analyses of the levels of Grb2 protein revealed that MMTV/Grb2 samples possessed dramatically elevated levels of Grb2 protein in the mammary epithelium compared to either the FVB or MMTV/Shc transgenic tissues (Fig. 2C; compare lanes 1 and 2 to lanes 3 to 9). The observed differences in Grb2 or Shc could not be attributed to differences in epithelial content since the protein lysates expressed comparable levels of the epithelial marker cytokeratin-8. Despite the elevated levels of Shc transcript observed in the MMTV/Grb2 strains, a concomitant increase in Shc protein was not observed. These observations indicate that the MMTV/Grb2 strains expressed dramatically elevated levels of Grb2 whereas the MMTV/Shc animals only moderately overexpressed Shc.
Aberrant mammary ductal morphogenesis in MMTV/Shc and MMTV/Grb2 transgenic mice.
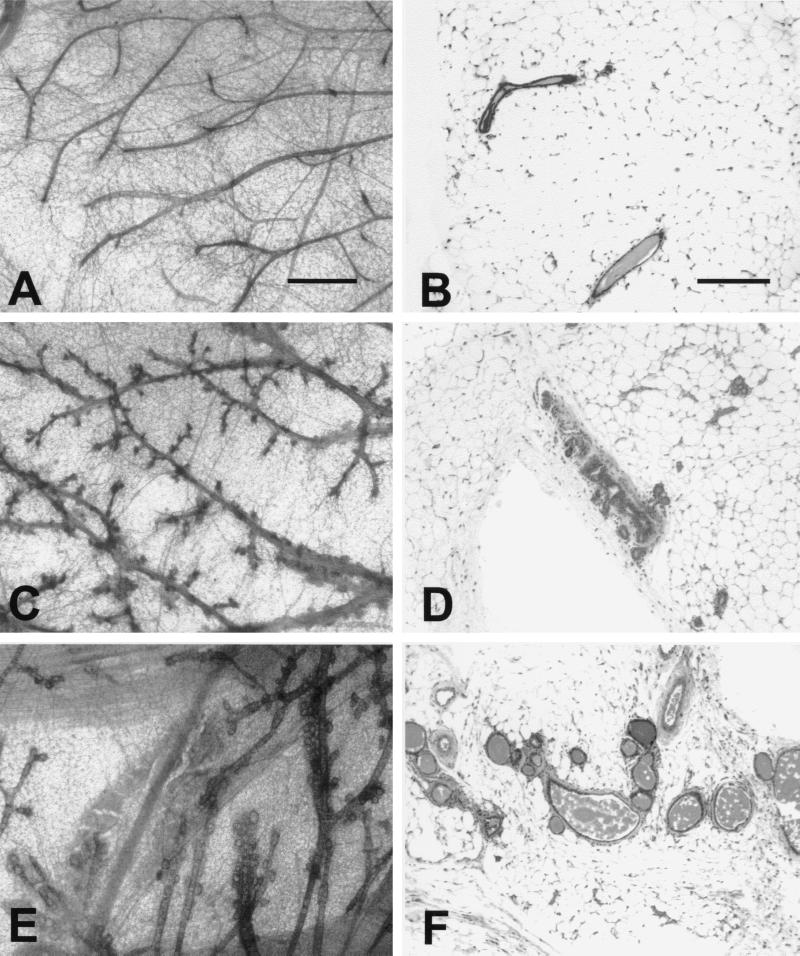
To ascertain whether elevated expression of either Shc or Grb2 resulted in abnormal mammary gland development, whole-mount analyses were conducted on virgin mammary glands. Virgin female mammary glands from MMTV/Shc and MMTV/Grb2 transgenic mice, along with nontransgenic FVB controls, were examined after necropsy by whole-mount and histological analyses at the end of puberty (Fig. 3). Mammary epithelial cell-specific expression of Shc in the mammary gland resulted in aberrant pubertal mammary ductal morphogenesis. In contrast to the wild-type FVB mammary glands, which displayed normal development, the mammary whole-mount preparations from the MMTV/Shc mice exhibited extensive side branching (compare Fig. 3A and B with Fig. 3C and D). This enhanced branching phenotype was also noted in whole-mount preparations derived from the MMTV/Grb2 strains (Fig. 3E and F). However, there are also subtle but distinct differences between the MMTV/Grb2 and MMTV/Shc mammary phenotypes. In particular, the mammary epithelium derived from the MMTV/Grb2 strains exhibited more extensive lobuloalveolar development than was observed in the MMTV/Shc strains. The differences between these strains were also evident at 14 weeks of age, when normal mammary gland ductal extension and branching has mostly ceased in the female mouse (19). Taken together, these data indicate that mammary epithelial cell-specific expression of either Grb2 or Shc can perturb the normal development of the murine mammary gland.
FIG. 3.
Histological analyses of virgin mammary glands derived from FVB, MMTV/Shc, and MMTV/Grb2 female mice. Photoimages of whole mounts (A, C, and E) and histological sections (B, D, and F) show mammary development in virgin FVB wild-type (A and B), Shc-3 (C and D), and Grb2-6 (E and F) females at 8.5 weeks after birth. Note the side branching and alveolar development in Shc-3 mammary gland (C and D) compared to the straight ductal structure of the wild-type gland (A and B). Note also that the Grb2-6 mammary gland has a more complex structure with more extensive lobuloalveolar development (E and F). The scale bars represent 0.5 mm (A, C, and E) and 200 μm (B, D, and F).
Although mammary epithelial expression of either Grb2 or Shc had pronounced effects on normal gland development, long-term monitoring of these strains revealed that induction of mammary tumors is extremely rare in either the MMTV/Grb2 or MMTV/Shc strain. Two of 28 multiparous MMTV/Shc females developed focal mammary tumors at 296 and 510 days, whereas none of the 15 Grb2 females had developed mammary tumors (Table 1). These data suggest that elevated expression of Grb2 or Shc is not sufficient to induce mammary tumors in these transgenic mice.
Elevated expression of Shc and Grb2 adapter proteins can accelerate tumor progression in transgenic mice expressing a mutant PyV mT oncogene.
The striking branching phenotype exhibited in the virgin female mammary glands suggested that elevated expression of Grb2 and Shc may influence neoplastic transformation. To further explore the role of Grb2 and Shc in tumor progression, we mated the MMTV/Grb2-6 and MMTV/Shc-3 lines with transgenic mice expressing a mutant PyV mT oncogene decoupled from the Shc signaling pathway (MTY250F-5a strain) (48). Unlike the wild-type PyV mT strains, which rapidly develop metastastic tumors, female carriers from the MTY250F-5a line develop focal mammary tumors with delayed kinetics (48). In addition, given that this model is also defective in Shc/Grb2 signaling, it presents a unique model system to examine the interaction of Shc and Grb2 adapter proteins with endogenously activated growth factor receptors. Indeed, we have previously demonstrated that tumor progression in the MTY250F strains is associated with increased expression of the ErbB-2 and ErbB-3 RTKs (48).
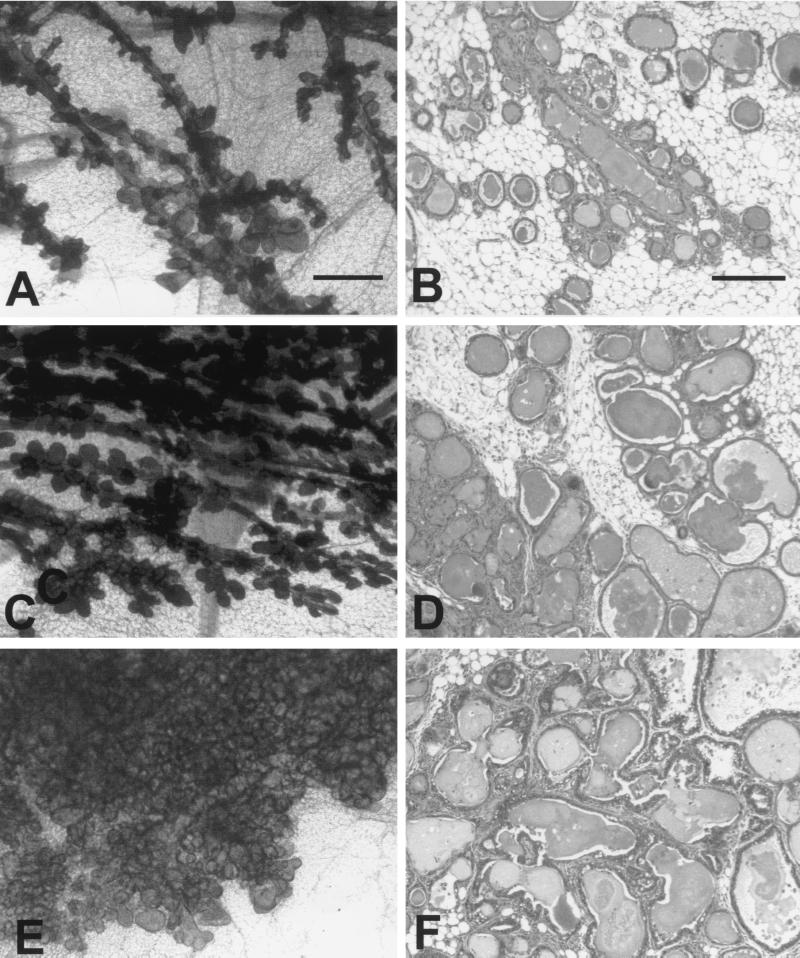
To assess whether either Grb2 or Shc could influence mammary tumor progression in the MTY250F strain, we initially performed whole-mount and histological analyses of 14-week-old monogenic MMTV/MTY250F (Fig. 4A and D) and bigenic MMTV/Shc/MTY250F (Fig. 4B and E) and MMTV/Grb2/MTY250F (Fig. 4C and 4F) virgin female mice. Although all three genotypes possess atypical epithelial and cystic alveolar hyperplasia, there was a dramatic difference in the degree of epithelial hyperplasia exhibited by the different genotypic combinations. In contrast to monogenic MMTV/MTY250F glands, MMTV/Shc/MTY250F hyperplasias exhibited a profound increase in lobuloalveolar development (Fig. 4C). In addition, a significant amount of inflammation and fibrosis was observed around the ducts (Fig. 4D). Detailed histological analysis of the MMTV/Shc/MTY250F epithelial hyperplasias also revealed larger nucleoli and more open chromatin. Although the MMTV/Grb2/MTY250F epithelial hyperplasias were cytologically similar to those in the Shc bigenic mice, the extent of lobuloalveolar development observed was dramatic. Indeed, in contrast to the other two genotypes, the alveoli from the MMTV/Grb2/MTY250F virtually filled the entire fat pad (Fig. 4E).
FIG. 4.
Histological analyses of virgin mammary glands derived from MMTV/MTY250F, MTY250F/Shc, and MTY250F/Grb2 female mice. Photoimages of whole mounts (A, C, and E) and histological sections (B, D, and F) show mammary development in virgin MMTV/MTY250F (A and B), MTY250F/Shc (C and D), and MTY250F/Grb2 (E and F) females 14 weeks after birth. Note the extensive side branching and lobuloalveolar development in the dual transgene carriers compared to the MMTV/MTY250F strain (A and B). The degree of lobuloalveolar development is greater in the MTY250F/Shc mammary gland (C and D) than in the monogenic MTY250F gland (A and B). However, the degree of lobuloalveolar development is the greatest in the MTY250F/Grb2 gland, virtually filling the fat pad with alveoli. The histological analysis reveals that all three transgenic glands have various degrees of fibrosis as well as lobuloalveolar development. At 14 weeks, cytological changes are present. The scale bars represent 0.5 mm (A and C) and 200 μm (B, D, and E).
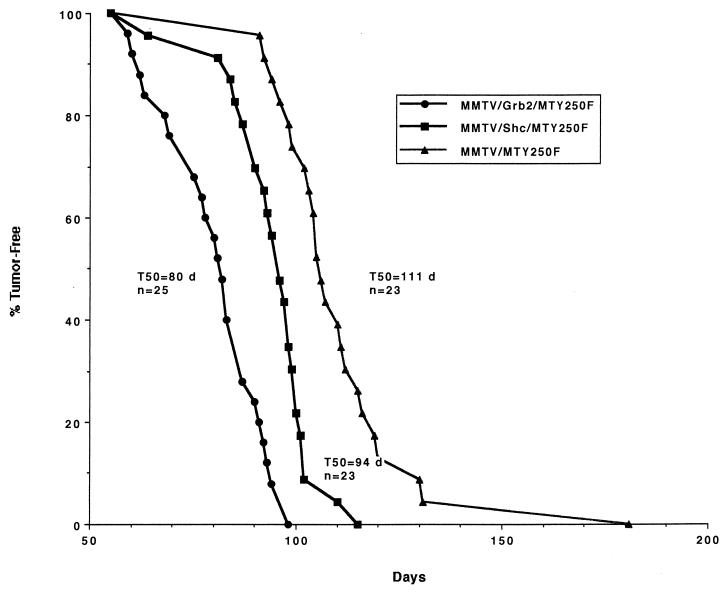
To assess whether the onset of tumors in these bigenic strains was affected by mammary epithelial expression of these adapter proteins, bigenic and monogenic virgin females were monitored for the onset of mammary tumors by physical palpation. Although coexpression of Shc or Grb2 with this mutant PyV mT oncogene did not recapitulate the phenotype exhibited by transgenic mice expressing wild-type PyV mT (15), ectopic expression of the adapter protein Shc or Grb2 significantly accelerated tumor onset in MTY250F transgenic females (Fig. 5). In contrast to the MMTV/MTY250F strain, which developed mammary tumors with an average latency of 111 days, the MMTV/Shc/MTY250F and MMTV/Grb2/MTY250F mice developed mammary tumors with average latencies of 94 and 80 days, respectively (Fig. 5).
FIG. 5.
Kinetics of tumor onset in the MMTV/MTY250F, MTY250F/Shc, and MTY250F/Grb2 strains. The number of animals analyzed for each strain (n) and the median age (days [d]) at which tumors were first palpable (T50) are indicated.
Biochemical characterization of mammary tumors expressing Shc and Grb2 adapter proteins.
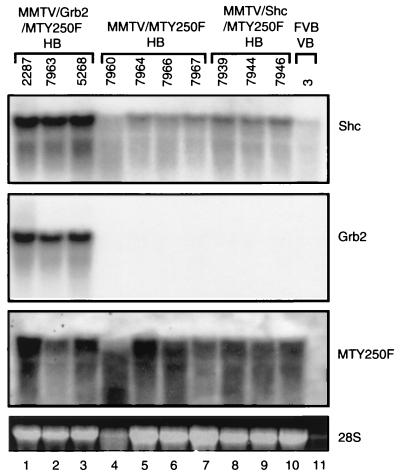
To confirm that the accelerated tumor onset observed in these crosses was due to coexpression of the mutant PyV mT oncogene and the respective adapter protein, both RNA and protein analyses were conducted on the hyperplastic and tumor tissues from these mice. Analyses of Shc transcript levels in epithelial hyperplasias derived from the various genotypes revealed that the MMTV/Shc/MTY250F hyperplasias expressed slightly elevated levels of Shc transcript compared to the parental MMTV/MTY250F samples (Fig. 6, Shc; compare lanes 8 to 10 and lanes 4 to 7). Interestingly, the MMTV/Grb2/MTY250F samples possessed a further threefold elevation of the endogenous Shc transcripts (Fig. 6, Shc, lanes 1 to 3). Consistent with previous Northern blot analyses (Fig. 2), analyses of the levels of Grb2 transcript revealed that the MMTV/Grb2/MTY250F samples expressed dramatically elevated levels of Grb2 transcript compared to either MMTV/Shc/MTY250F or MMTV/MTY250F tissues (Fig. 6, Grb2; compare lanes 1 to 3 to lanes 4 to 10). The observed differences in Grb2 and Shc transcript did not reflect differences in RNA loading since equal levels of 28S rRNA were observed in all samples (Fig. 6, 28S, lanes 1 to 10). Despite the differences in the levels in Grb2 and Shc transcripts in the various samples, all of the mammary hyperplasias expressed comparable levels of mutant PyV mT transcripts. Therefore, these observations suggest that the phenotypic differences observed between the various hyperplastic tissues (Fig. 3) do not reflect differences in the levels of mutant PyV mT transcripts but rather are a result of differences in the levels of expression of the Grb2 and Shc adapter molecules.
FIG. 6.
Northern blot analyses of Shc, Grb2, and MTY250F mRNA levels in hyperplastic mammary glands. Total cellular RNA was isolated from 14-week virgin Grb2/MTY250F (lanes 1 to 3), MTY250F (lanes 4 to 7), and Shc/MTY250F (lanes 8 to 10) hyperplastic mammary tissue (HB). Also included were virgin mammary glands (VB) obtained from an FVB female (lane 11). The gels were probed with Shc, Grb2, and MTY250F radiolabeled probes, as indicated at the right. Also shown for each sample is the 28S ribosomal species.
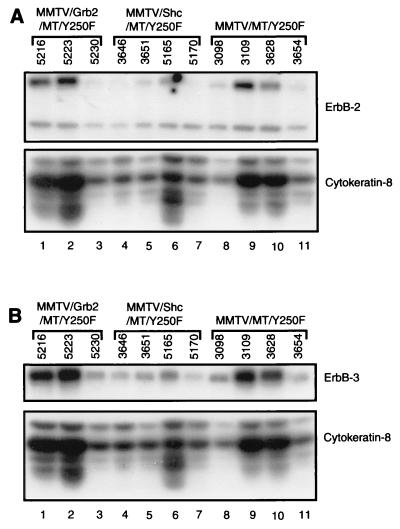
Another possible explanation for the accelerated tumor phenotype observed in the bigenic mice is through the indirect activation of growth factor receptor signaling. Indeed, we have previously demonstrated that tumor progression in the MMTV/MTY250F strain is associated with the induction of elevated levels of ErbB-2 and ErbB-3 growth factor receptors (48). To test this possibility, protein extracts from MMTV/Grb2/MTY250F, MMTV/Shc/MTY250F, and MMTV/MTY250F tumors were subjected to quantitative 125I immunoblot analyses with ErbB-2- and ErbB-3-specific antisera (Fig. 7). To control for variations in epithelial content, the same samples were also probed with a cytokeratin-8-specific antibody. After controlling for variations in epithelial content, quantitative comparison of the levels of ErbB-2 and ErbB-3 revealed that tumor samples from the various genotypic combinations possessed similar elevated levels of ErbB-2 and ErbB-3 proteins per epithelial cell. As for the MMTV/MTY250F strains, we observe a comparable increase in ErbB-2 and ErbB-3 levels during tumor progression in these bigenic mice (30a). Thus, the observed differences in tumor latency are not due to alteration in the levels of these activated growth factor receptors but rather reflect the elevated levels of Grb2 and Shc.
FIG. 7.
Expression of the EGFR family is not altered in the different intercrosses. ErbB-2 (A) and ErbB-3 (B) levels in mammary tumors derived from the MMTV/Grb2/MTY250F (lanes 1 to 3), MMTV/Shc/MTY250F (lanes 4 to 7), and MMTV/MTY250F (lanes 8 to 11) strains were measured by immunoblot analyses. Cytokeratin-8 levels in the samples (bottom) were determined to normalize for epithelial content.
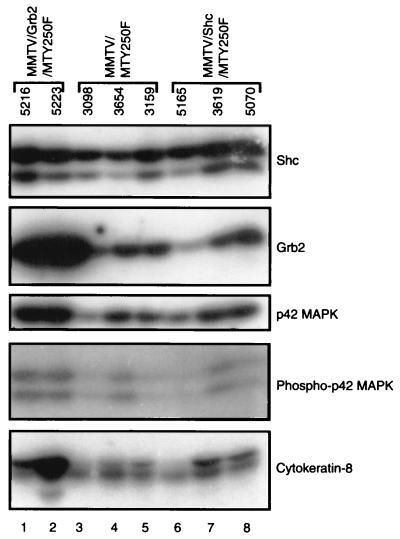
Given the ability of both Grb2 and Shc adapter molecules to couple ErbB-2 and ErbB-3 growth factor receptors to the Ras signaling pathway, we were also interested in assessing whether there was evidence of enhanced Ras signaling in tumor material coexpressing these adapter proteins. One measure of Ras activation is stimulation of the MAPK signaling pathway. To explore this possibility further, we performed quantitative 125I immunoblot analyses on these tumor samples with antisera specific to Shc, Grb2, p42 MAPK, phosphospecific p42 MAPK, and cytokeratin-8 antibodies. Consistent with Northern blot analyses (Fig. 6), dramatically elevated levels of Grb2 protein were noted in tumor samples derived from the MMTV/Grb2/MTY250F samples compared to either MMTV/Shc/MTY250F or parental MMTV/MTY250F samples (Fig. 8, Grb2; compare lanes 1 and 2 to lanes 3 to 8). Given the lower levels of expression of the Shc transgene, inspection of the levels of Shc protein failed to reveal significant differences in the levels of Shc protein expression. Quantitative analyses of the ratio of phosphospecific MAPK to the levels of MAPK protein revealed that MMTV/Grb2/MTY250F tumors had a twofold increase in the specific activity of MAPK compared to the parental MMTV/MTY250F tumor samples. In contrast, the MMTV/Shc/MTY250F samples possessed only slightly elevated levels of MAPK activity compared to the parental tumors. Taken together, these observations suggest that elevated levels of Grb2 in these tumors result in stimulation of the Ras signaling pathway.
FIG. 8.
Grb2 and Shc protein expression in hyperplastic and neoplastic mammary tissues. Total protein lysates prepared from virgin MMTV/Grb2/MTY250F (lanes 1 and 2), MMTV/MTY250F (lanes 3 to 5), and MMTV/Shc/MTY250F (lanes 6 to 8) hyperplasias were subjected to immunoblot analyses with Shc, Grb2, p42 MAPK, phospho specific p42 MAPK, and cytokeratin-8 antisera, as indicated at the right.
DISCUSSION
The Grb2 and Shc adapter proteins play critical roles in regulating the response of mammalian cells to a variety of proliferative and differentiation stimuli. To assess the importance of these adapter proteins in mammary gland development and tumorigenesis, we have generated separate transgenic strains expressing Shc or Grb2 under the transcriptional control of the MMTV promoter. Although female MMTV/Shc and MMTV/Grb2 mice appear to nurse their offspring normally, whole-mount analyses revealed that virgin glands exhibit enhanced ductal branching and lobuloalveolar development that also correlated with expression of either Shc or Grb2 transcript and encoded proteins. We have further demonstrated that mammary epithelial cell-specific expression of the Grb2 and Shc adapter proteins can accelerate mammary tumor development in transgenic mice expressing a mutant PyV mT oncogene decoupled from the Shc adapter protein. Tumor progression in mice coexpressing Grb2 and the mutant PyV mT oncogene was further correlated with a modest activation of the MAPK pathway. These observations argue that the levels of Grb2 and Shc adapter proteins can modulate the response of the mammary epithelial cell to growth factor and oncogenic stimuli.
The observation that elevated mammary epithelial cell-specific expression of Grb2 and Shc in transgenic mice can result in altered mammary epithelial morphogenesis has important implications in understanding the biological function of these adapter proteins in mammary gland development. Whole-mount analyses of virgin mammary glands isolated from MMTV/Grb2 and MMTV/Shc females have revealed that both strains display enhanced tertiary branching and lobuloalveolar development (Fig. 3). However, careful histological analyses of these strains revealed that the MMTV/Grb2 and MMTV/Shc strains display subtle differences in the nature of epithelial hyperplasias. Although both the MMTV/Shc and MMTV/Grb2 female mammary glands possess enhanced branching and lobuloalveolar development, the extent of lobuloalveolar development in the MMTV/Grb2 glands was more extensive (Fig. 3). Interestingly, the mammary phenotype exhibited by the transgenic strains closely resembles epithelial hyperplasias seen in the MMTV/heregulin strains (22). Given that members of the heregulin family of growth factors are ligands for the epidermal growth factor receptor (EGFR) family members, elevated expression of Shc and Grb2 may potentiate the action of the endogenous EGFR family during normal mammary gland development. In this regard, it has recently been demonstrated that stimulation of mammary organ culture systems with heregulins causes induction of alveolar structures resembling those seen within the MMTV/Grb2 and MMTV/Shc mice (26). These investigators further showed that the heregulin-induced alveolar phenotype was dependent on the activation of the MEK/MAPK (26). Given the importance of Shc and Grb2 in coupling the ErbB-2 and ErbB-3 receptors to the MAPK pathway, it is conceivable that the elevated levels of Shc sensitize the mammary epithelial cell to heregulin-mediated signals. Future crosses between the MMTV/adapter strains and MMTV/heregulin strains should allow this hypothesis to be tested.
Although mammary epithelial expression of either Shc or Grb2 was capable of altering normal mammary gland development, the occurrence of mammary tumors was extremely rare. Only 7% of MMTV/Shc female mice and none of the female MMTV/Grb2 strains developed mammary tumors (Table 1). In this regard, previous studies have demonstrated that elevated expression of Shc is capable of transforming fibroblasts (29), whereas comparable levels of Grb2 are incapable of mediating oncogenic transformation (43). Consistent with these observations, only the MMTV/Shc mice developed tumors despite the comparatively low levels of Shc expression observed in the mammary glands of these transgenic strains.
To further explore the role of these adapter proteins in mammary tumorigenesis, we have crossed the separate MMTV/Grb2 and MMTV/Shc strains with transgenic mice expressing a mutant form of PyV mT oncogene decoupled from the Shc adapter protein (MTY250F strain). Consistent with the effect of these adapter proteins on normal mammary gland development, mammary epithelial expression of either Grb2 or Shc adapter had a dramatic effect on tumor progression in the MTY250F strain. Indeed, whole-mount analyses of these bitransgenic strains revealed that elevated expression of Grb2 and Shc profoundly enhanced the abnormal lobuloalveolar hyperplasias induced by the MTY250F oncogene (Fig. 4). Moreover, comparison of the average times of onset of tumors revealed that elevated expression of these adapter proteins could significantly decrease the latency period required for tumor development (Fig. 5).
One potential explanation for accelerated tumor development in these strains is that an elevated level of either Grb2 or Shc sensitizes the mammary epithelial cell to endogenous growth factor receptor signaling pathways. For example, we have previously demonstrated that tumor progression in the mutant PyV mT strains involves upregulation of EGFR family members ErbB-2 and ErbB-3 (48). Consistent with these findings, mammary tumors derived from transgenic mice coexpressing the adapter proteins and mutant PyV mT strains also express elevated levels of these EGFR family members (Fig. 7). Although the levels of ErbB-2 and ErbB-3 protein were elevated in each of these tumors, quantitative PhosphorImager analyses of these blots revealed no significant differences between the various mutant samples. Therefore, the dramatic changes observed in the mammary glands of bigenic mice cannot simply be ascribed to elevated expression of these growth factor receptors but rather reflect increased expression of Grb2 and Shc.
Given the ability of ErbB-2 and ErbB-3 receptors to bind either Grb2 or Shc protein (9, 21, 30), the accelerated rates of tumor development may reflect an increased sensitivity of the mammary epithelial cell expressing these adapter proteins to growth factor stimulation. Consistent with this hypothesis, we have demonstrated that the tissues coexpressing Grb2 and mutant PyV mT oncogene possess elevated MAPK activity (Fig. 8). The marginal activation of MAPK activity observed in the samples coexpressing Shc and mutant PyV mT oncogene likely reflects the comparatively low levels of Shc observed in these samples. Whereas the levels of Shc are slightly elevated, we have observed a fourfold increase in the levels of tyrosine-phosphorylated Shc during tumor progression (30a). Another possible explanation for differences in MAPK activation between the Shc and Grb2 strains is that Grb2-coupled growth factor receptor may be more efficient in activating the MAPK pathway. Indeed, it has recently been reported that EGF stimulation of the MAPK pathway does not require Shc but is absolutely dependent on a functional Grb2 protein (18).
Further evidence supporting a role for these adapter proteins stems from the observation that a 50% reduction of Grb2 levels can have a profound effect on the induction of mammary tumors in transgenic mice expressing the wild-type PyV mT oncogene (6). Interestingly, whole-mount analyses of the mice carrying one of the knockout Grb2 alleles exhibit a defect in ductal morphogenesis compared to their wild-type siblings (6). These observations indicated that reduction of Grb2 dosage can also affect the ability of the mammary epithelial cell to respond to endogenous levels of growth factor stimulation.
Consistent with these observations related to transgenic mice, elevated expression of the Grb2 adapter protein is frequently observed in primary human breast cancers and their derived cell lines (8). In a more recent study, 50% of primary breast cancers exhibited a greater than twofold upregulation of Grb2 mRNA relative to normal breast epithelial cells (49). Interestingly, low-EGFR-expressing tumors expressed significantly higher Grb2 mRNA levels than high-EGFR-expressing tumors (49). Together these observations suggest that modulation of the dosage of Grb2 in breast cancer may play an important role in signal amplification from activated growth factor receptors. Further support for the importance of Shc and Grb2 adapter proteins in modulating the response to growth factor receptor stimulation stems from observations made in established cell lines. For example, elevated expression of Grb2 enhances both activation of Ras and MAPK in response to EGF (11, 43). In a similar fashion, elevated Grb2 expression results in an enhanced MAPK response to insulin (37). The increased activation of MAPK was further correlated with an increase in Grb2-Sos complex formation (37). Indeed, increased Grb2-Sos complex formation has also been noted in breast cancer cell lines expressing elevated Grb2 levels (8). Taken together, these observations suggest that the Grb2 and Shc adapter proteins could serve as important therapeutic targets in the treatment of human breast cancer.
ACKNOWLEDGMENTS
This work was supported by grants from The Cancer Research Society Inc. and the Canadian Breast Cancer Research Initiative awarded to W.J.M. This work was also partially supported by a Terry Fox Program project grant awarded to T.P. and a National Health and Medical Research Council of Australia grant awarded to R.D. V.K.-M.L. was supported by an NSERC fellowship, C.G.T. was supported by a CRS studentship, P.M.S. was supported by an MRC studentship. E.R.A. was supported by a CRS studentship, W.J.M. was supported by an MRC Scientist award, and T.P. was supported by a Distinguished Scientist Award. This work was also supported by a CBCRP award to R.D.C. (JB-0014).
We thank Judy Walls for technical contributions.
REFERENCES
- 1.Batzer A G, Blaikie P, Nelson K, Schlessinger J, Margolis B. The phosphotyrosine interaction domain of Shc binds an LXNPXY motif on the epidermal growth factor receptor. Mol Cell Biol. 1995;15:4403–4409. doi: 10.1128/mcb.15.8.4403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blaikie P A, Fournier E, Dilworth S M, Birnbaum D, Borg J P, Margolis B. The role of the Shc phosphotyrosine interaction/phosphotyrosine binding domain and tyrosine phosphorylation sites in polyoma middle T antigen-mediated cell transformation. J Biol Chem. 1997;272:20671–20677. doi: 10.1074/jbc.272.33.20671. [DOI] [PubMed] [Google Scholar]
- 3.Bonfini L, Migliaccio E, Pelicci G, Lanfrancone L, Pelicci P G. Not all Shc’s roads lead to Ras. Trends Biochem Sci. 1996;21:257–261. [PubMed] [Google Scholar]
- 4.Bouchard L, Lamarre L, Tremblay P J, Jolicoeur P. Stochastic appearance of mammary tumors in transgenic mice carrying the MMTV/c-neu oncogene. Cell. 1989;57:931–936. doi: 10.1016/0092-8674(89)90331-0. [DOI] [PubMed] [Google Scholar]
- 5.Campbell K S, Ogris E, Burke B, Su W, Auger K R, Druker B J, Schaffhausen B S, Roberts T M, Pallas D C. Polyoma middle tumor antigen interacts with SHC protein via the NPTY (Asn-Pro-Thr-Tyr) motif in middle tumor antigen. Proc Natl Acad Sci USA. 1994;91:6344–6348. doi: 10.1073/pnas.91.14.6344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheng A M, Saxton T M, Sakai R, Kulkarni S, Mbamalu G, Vogel W, Tortorice C G, Cardiff R D, Cross J C, Muller W J, Pawson T. Mammalian Grb2 regulates multiple steps in embryonic development and malignant transformation. Cell. 1998;95:793–803. doi: 10.1016/s0092-8674(00)81702-x. [DOI] [PubMed] [Google Scholar]
- 7.Chirgwin J M, Przybyla A E, MacDonald R J, Rutter W J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979;18:5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- 8.Daly R J, Binder M D, Sutherland R L. Overexpression of the Grb2 gene in human breast cancer cell lines. Oncogene. 1994;9:2723–2727. [PubMed] [Google Scholar]
- 9.Dankort D L, Wang Z, Blackmore V, Moran M F, Muller W J. Distinct tyrosine autophosphorylation sites negatively and positively modulate neu-mediated transformation. Mol Cell Biol. 1997;17:5410–5425. doi: 10.1128/mcb.17.9.5410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feinberg A P, Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983;132:6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- 11.Gale N W, Kaplan S, Lowenstein E J, Schlessinger J, Bar-Sagi D. Grb2 mediates the EGF-dependent activation of guanine nucleotide exchange on Ras. Nature. 1993;363:88–92. doi: 10.1038/363088a0. [DOI] [PubMed] [Google Scholar]
- 12.Gotoh N, Muroya K, Hattori S, Nakamura S, Chida K, Shibuya M. The SH2 domain of Shc suppresses EGF-induced mitogenesis in a dominant negative manner. Oncogene. 1995;11:2525–2533. [PubMed] [Google Scholar]
- 13.Gotoh N, Toyoda M, Shibuya M. Tyrosine phosphorylation sites at amino acids 239 and 240 of Shc are involved in epidermal growth factor-induced mitogenic signaling that is distinct from Ras/mitogen-activated protein kinase activation. Mol Cell Biol. 1997;17:1824–1831. doi: 10.1128/mcb.17.4.1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guy C T, Cardiff R D, Muller W J. Activated neu induces rapid tumor progression. J Biol Chem. 1996;271:7673–7678. doi: 10.1074/jbc.271.13.7673. [DOI] [PubMed] [Google Scholar]
- 15.Guy C T, Cardiff R D, Muller W J. Induction of mammary tumors by expression of polyomavirus middle T oncogene: a transgenic mouse model for metastatic disease. Mol Cell Biol. 1992;12:954–961. doi: 10.1128/mcb.12.3.954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guy C T, Webster M A, Schaller M, Parsons T J, Cardiff R D, Muller W J. Expression of the neu protooncogene in the mammary epithelium of transgenic mice induces metastatic disease. Proc Natl Acad Sci USA. 1992;89:10578–10582. doi: 10.1073/pnas.89.22.10578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harmer S L, DeFranco A L. Shc contains two Grb2 binding sites needed for efficient formation of complexes with SOS in B lymphocytes. Mol Cell Biol. 1997;17:4087–4095. doi: 10.1128/mcb.17.7.4087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hashimoto A, Kurosaki M, Gotoh N, Shibuya M, Kurosaki T. Shc regulates epidermal growth factor-induced activation of the JNK signaling pathway. J Biol Chem. 1999;274:20139–20143. doi: 10.1074/jbc.274.29.20139. [DOI] [PubMed] [Google Scholar]
- 19.Hennighausen L, Robinson G W. Think globally, act locally: the making of a mouse mammary gland. Genes Dev. 1998;12:449–455. doi: 10.1101/gad.12.4.449. [DOI] [PubMed] [Google Scholar]
- 20.Huang A L, Ostrowski M C, Berard D, Hager G L. Glucocorticoid regulation of the Ha-MuSV p21 gene conferred by sequences from mouse mammary tumor virus. Cell. 1981;27:245–255. doi: 10.1016/0092-8674(81)90408-6. [DOI] [PubMed] [Google Scholar]
- 21.Janes P W, Daly R J, deFazio A, Sutherland R L. Activation of the Ras signalling pathway in human breast cancer cells overexpressing erbB-2. Oncogene. 1994;9:3601–3608. [PubMed] [Google Scholar]
- 22.Krane I M, Leder P. NDF/heregulin induces persistence of terminal end buds and adenocarcinomas in the mammary glands of transgenic mice. Oncogene. 1996;12:1781–1788. [PubMed] [Google Scholar]
- 23.Lowenstein E J, Daly R J, Batzer A G, Li W, Margolis B, Lammers R, Ullrich A, Skolnik E Y, Bar-Sagi D, Schlessinger J. The SH2 and SH3 domain-containing protein GRB2 links receptor tyrosine kinases to ras signaling. Cell. 1992;70:431–442. doi: 10.1016/0092-8674(92)90167-b. [DOI] [PubMed] [Google Scholar]
- 24.Melton D A, Krieg P A, Rebagliati M R, Maniatis T, Zinn K, Green M R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984;12:7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Muller W J, Sinn E, Pattengale P K, Wallace R, Leder P. Single-step induction of mammary adenocarcinoma in transgenic mice bearing the activated c-neu oncogene. Cell. 1988;54:105–115. doi: 10.1016/0092-8674(88)90184-5. [DOI] [PubMed] [Google Scholar]
- 26.Niemann C, Brinkmann V, Spitzer E, Hartmann G, Sachs M, Naundorf H, Birchmeier W. Reconstitution of mammary gland development in vitro: requirement of c-met and c-erbB2 signaling for branching and alveolar morphogenesis. J Cell Biol. 1998;143:533–545. doi: 10.1083/jcb.143.2.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pawson T. SH2 and SH3 domains in signal transduction. Adv Cancer Res. 1994;64:87–110. doi: 10.1016/s0065-230x(08)60835-0. [DOI] [PubMed] [Google Scholar]
- 28.Pelicci G, Dente L, De Giuseppe A, Verducci-Galletti B, Giuli S, Mele S, Vetriani C, Giorgio M, Pandolfi P P, Cesareni G, Pelicci P G. A family of Shc related proteins with conserved PTB, CH1 and SH2 regions. Oncogene. 1996;13:633–641. [PubMed] [Google Scholar]
- 29.Pelicci G, Lanfrancone L, Grignani F, McGlade J, Cavallo F, Forni G, Nicoletti I, Pawson T, Pelicci P G. A novel transforming protein (SHC) with an SH2 domain is implicated in mitogenic signal transduction. Cell. 1992;70:93–104. doi: 10.1016/0092-8674(92)90536-l. [DOI] [PubMed] [Google Scholar]
- 30.Pelicci G, Lanfrancone L, Salcini A E, Romano A, Mele S, Grazia Borrello M, Segatto O, Di Fiore P P, Pelicci P G. Constitutive phosphorylation of Shc proteins in human tumors. Oncogene. 1995;11:899–907. [PubMed] [Google Scholar]
- 30a.Rauh, M., and W. J. Muller. Unpublished observations.
- 31.Ricci A, Lanfrancone L, Chiari R, Belardo G, Pertica C, Natali P G, Pelicci P G, Segatto O. Analysis of protein-protein interactions involved in the activation of the Shc/Grb-2 pathway by the ErbB-2 kinase. Oncogene. 1995;11:1519–1529. [PubMed] [Google Scholar]
- 32.Rozakis-Adcock M, McGlade J, Mbamalu G, Pelicci G, Daly R, Li W, Batzer A, Thomas S, Brugge J, Pelicci P G, et al. Association of the Shc and Grb2/Sem5 SH2-containing proteins is implicated in activation of the Ras pathway by tyrosine kinases. Nature. 1992;360:689–692. doi: 10.1038/360689a0. [DOI] [PubMed] [Google Scholar]
- 33.Seed B, Aruffo A. Molecular cloning of the CD2 antigen, the T-cell erythrocyte receptor, by a rapid immunoselection procedure. Proc Natl Acad Sci USA. 1987;84:3365–3369. doi: 10.1073/pnas.84.10.3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Semat A, Vasseur M, Maillet L, Brulet P, Darmon Y M. Sequence analysis of murine cytokeratin endo A (no. 8) cDNA. Evidence for mRNA species initiated upstream of the normal 5′ end in PCC4 cells. Differentiation. 1988;37:40–46. doi: 10.1111/j.1432-0436.1988.tb00794.x. [DOI] [PubMed] [Google Scholar]
- 35.Siegel P M, Ryan E D, Cardiff R D, Muller W J. Elevated expression of activated forms of Neu/ErbB-2 and ErbB-3 are involved in the induction of mammary tumors in transgenic mice: implications for human breast cancer. EMBO J. 1999;18:2149–2164. doi: 10.1093/emboj/18.8.2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sinn E, Muller W, Pattengale P, Tepler I, Wallace R, Leder P. Coexpression of MMTV/v-Ha-ras and MMTV/c-myc genes in transgenic mice: synergistic action of oncogenes in vivo. Cell. 1987;49:465–475. doi: 10.1016/0092-8674(87)90449-1. [DOI] [PubMed] [Google Scholar]
- 37.Skolnik E Y, Batzer A, Li N, Lee C H, Lowenstein E, Mohammadi M, Margolis B, Schlessinger J. The function of GRB2 in linking the insulin receptor to Ras signaling pathways. Science. 1993;260:1953–1955. doi: 10.1126/science.8316835. [DOI] [PubMed] [Google Scholar]
- 38.Skolnik E Y, Lee C H, Batzer A, Vicentini L M, Zhou M, Daly R, Myers M J, Jr, Backer J M, Ullrich A, White M F, et al. The SH2/SH3 domain-containing protein GRB2 interacts with tyrosine-phosphorylated IRS1 and Shc: implications for insulin control of ras signalling. EMBO J. 1993;12:1929–1936. doi: 10.1002/j.1460-2075.1993.tb05842.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Slamon D J, Clark G M, Wong S G, Levin W J, Ullrich A, McGuire W L. Human breast cancer: correlation of relapse and survival with the amplification of the HER2/neu oncogene. Science. 1987;235:177–182. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 40.Slamon D J, Godolphin W, Jones L A, Holt J A, Wong S G, Keith D E, Levin W J, Stuart S G, Udove J, Ullrich A, Press M F. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science. 1989;244:707–712. doi: 10.1126/science.2470152. [DOI] [PubMed] [Google Scholar]
- 41.Southern E M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975;98:503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- 42.Stevenson L E, Frackelton A R., Jr Constitutively tyrosine phosphorylated p52 Shc in breast cancer cells: correlation with ErbB2 and p66 Shc expression. Breast Cancer Res Treat. 1998;49:119–128. doi: 10.1023/a:1006007227747. [DOI] [PubMed] [Google Scholar]
- 43.Suen K L, Bustelo X R, Pawson T, Barbacid M. Molecular cloning of the mouse grb2 gene: differential interaction of the Grb2 adaptor protein with epidermal growth factor and nerve growth factor receptors. Mol Cell Biol. 1993;13:5500–5512. doi: 10.1128/mcb.13.9.5500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.van der Geer P, Pawson T. The PTB domain: a new protein module implicated in signal transduction. Trends Biochem Sci. 1995;20:277–280. doi: 10.1016/s0968-0004(00)89043-x. [DOI] [PubMed] [Google Scholar]
- 45.van der Geer P, Wiley S, Gish G D, Pawson T. The Shc adaptor protein is highly phosphorylated at conserved, twin tyrosine residues (Y239/240) that mediate protein-protein interactions. Curr Biol. 1996;6:1435–1444. doi: 10.1016/s0960-9822(96)00748-8. [DOI] [PubMed] [Google Scholar]
- 46.Verbeek B S, Adriaansen-Slot S S, Rijksen G, Vroom T M. Grb2 overexpression in nuclei and cytoplasm of human breast cells: a histochemical and biochemical study of normal and neoplastic mammary tissue specimens. J Pathol. 1997;183:195–203. doi: 10.1002/(SICI)1096-9896(199710)183:2<195::AID-PATH901>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 47.Vonderhaar B K, Greco A E. Lobulo-alveolar development of mouse mammary glands is regulated by thyroid hormones. Endocrinology. 1979;104:409–418. doi: 10.1210/endo-104-2-409. [DOI] [PubMed] [Google Scholar]
- 48.Webster M A, Hutchinson J N, Rauh M J, Muthuswamy S K, Anton M, Tortorice C G, Cardiff R D, Graham F L, Hassell J A, Muller W J. Requirement for both Shc and phosphatidylinositol 3′ kinase signaling pathways in polyomavirus middle T-mediated mammary tumorigenesis. Mol Cell Biol. 1998;18:2344–2359. doi: 10.1128/mcb.18.4.2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yip, S. S., A. J. Crew, J. M. W. Gee, R. Hui, R. W. Blamey, J. F. R. Robertson, R. I. Nicholson, R. L. Sutherland, and R. J. Daly. 1999. Unpublished observations.