Abstract
The potential to differentiate into different cell lines, added to the easy and cost-effective method of extraction, makes adipose-derived stem cells (ADSCs) an object of interest in lymphedema treatment. Our study’s goal was to conduct a comprehensive systematic review of the use of ADSCs in lymphatic tissue engineering and regeneration. On July 23, 2019, using PubMed/MEDLINE, Cochrane Clinical Answers, Cochrane Central Register of Controlled Trials, and Embase databases, we conducted a systematic review of published literature on the use of ADSCs in lymphatic tissue engineering and regeneration. There were no language or time frame limitations, and the following search strategy was applied: ((Adipose stem cell) OR Adipose-derived stem cell)) AND ((Lymphedema) OR Breast Cancer Lymphedema). Only original research manuscripts were included. Fourteen studies fulfilled the inclusion criteria. Eleven studies were experimental (in vitro or in vivo in animals), and only three were clinical. Publications on the topic demonstrated that ADSCs promote lymphangiogenesis, and its effect could be enhanced by modulation of vascular endothelial growth factor-C, interleukin-7, prospero homeobox protein 1, and transforming growth factor-β1. Pilot clinical studies included 11 patients with breast cancer-related lymphedema, and no significant side effects were present at 12-month follow-up. Literature on the use of ADSCs in lymphatic tissue engineering and regeneration demonstrated promising data. Clinical evidence is still in its infancy, but the scientific community agrees that ADSCs can be useful in regenerative lymphangiogenesis. Data collected in this review indicate that unprecedented advances in lymphedema treatment can be anticipated in the upcoming years.
Keywords: Adipose-derived mesenchymal stem cells, Regenerative medicine, Lymphatic vessels, Lymphedema
INTRODUCTION
Secondary lymphedema is a chronic debilitating disease characterized by the accumulation of interstitial fluid due to a disruption of the lymphatic circulation [1]. Radiation and lymphadenectomy related to cancer treatment are the most common causes [2]. A recent meta-analysis demonstrated that one in every five breast cancer patients develops some degree of lymphedema [3]. The risk of lymphedema is particularly higher among those treated with axillary lymph node dissection and regional lymph node irradiation [4]. The diagnosis of lymphedema is usually clinical, identifying discrepancies in volume, shape and skin between limbs [5]. Traditional treatments such as complete decongestive therapy are palliative in their nature and may even impact patient quality of life [6]. Surgical treatment such as lymphovenous anastomosis and lymph node transfer have been used for patients with moderate to severe lymphedema that does not respond to traditional treatment, but it is still considered experimental [7]. This mixed scenario of a highly prevalent but still incurable disease creates a meaningful opportunity for discoveries in regenerative medicine.
Adipose-derived stem cells (ADSCs) are promoting substantial advances in regenerative medicine, which is reflected by the growing number of publications and clinical trials exploring their use [8,9]. Recent studies have demonstrated ADSCs capacity to differentiate into lymphatic endothelial cells (LECs) and secrete lymphangiogenic growth factors [10]. It is generally agreed that they are easily extracted, cost-effective, and have few ethical considerations [9]. Nonetheless, the scientific evidence in favor of implementing ADSCs in the clinical routine is still pending [11]. In addition, the scientific community has concerns about its safety profile in oncologic patients [11]. Therefore, we conducted a systematic review of publications exploring the use of ADSCs in lymphatic tissue engineering and regeneration for lymphedema treatment. We hypothesized that ADSCs could regenerate lymphedematous tissue. Current studies appear to support the notion that the use of ADSCs in lymphatic regeneration could help lymphedema patients in the future.
METHODS
Search strategy
On July 23, 2019, two reviewers (DB and MTH) independently conducted electronic searches using PubMed/MEDLINE, Cochrane Clinical Answers, Cochrane Central Register of Controlled Trials, and Embase databases, without language or time frame limitations. Disagreements regarding article identification and final selection for inclusion were resolved by another reviewer (AJF). The following were used as either keywords or Medical Subject Headings in all combinations in the search strategy: (((Adipose stem cell) OR Adipose-derived stem cell)) AND ((Lymphedema) OR Breast Cancer Lymphedema))). The compiled reference lists were compared and reviewed for potential relevance. The bibliographies of included studies were also examined for articles not acquired through initial search queries. After all reviewers had completed the systematic literature review, additional verification by a medical librarian trained in systematic reviews was requested and received to ensure the most comprehensive review of all published studies meeting these criteria. This study complied with the guidelines outlined in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA).
Selection criteria
Eligibility criteria included studies reporting data on the use of ADSCs in lymphatic tissue engineering and regeneration. We included studies in vitro, in vivo in animals, and human work. We excluded papers that assessed ADSCs without a direct relationship to lymphatic regeneration (e.g., a paper identifying differences between ADSCs in lymphedema and healthy tissue). Abstracts, presentations, correspondence, editorials, and systematic reviews were excluded.
Data extraction and processing
Extracted data included the year of publication, country, type of study, origin of ADSCs, lymphedema model, additional agents and factors associated with ADSCs in the study, main findings, and conclusions. Data extraction from articles, tables, and figures was performed by two reviewers (DB and MTH), with the accuracy of data entry confirmed by an additional reviewer (AJF).
RESULTS
Studies characteristics
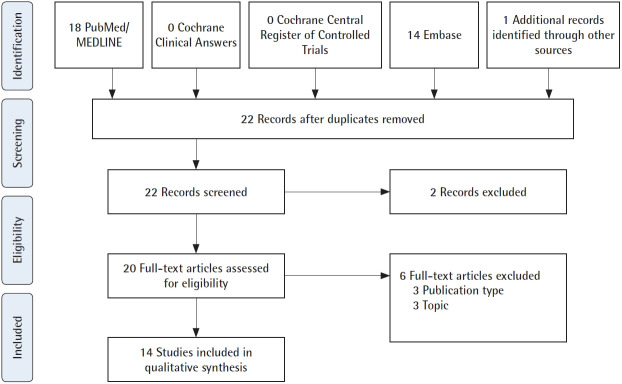
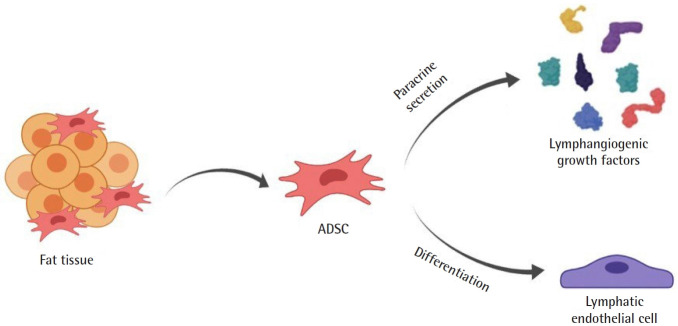
Of 22 papers found in the literature, 14 fulfilled the study inclusion criteria (Fig. 1). Authors carried out studies on the use of ADSCs in lymphatic tissue engineering and regeneration in three types of studies: in vitro, in vivo in animals, and human work. Only three studies were clinical, with 11 patients [12-14]. Two mechanisms have been proposed to explain the lymphangiogenic potential of ADSCs: paracrine secretion and direct differentiation (Fig. 2). Authors have also explored mechanisms to enhance the lymphangiogenic potential of ADSCs [15-19]. The strategy most often employed was the enhancement of media used to culture the ADSCs, to which vascular endothelial growth factor (VEGF)-C or interleukin (IL)-7 was added [16,17]. Moreover, it was also proposed cutaneous hydrogel for VEGFC delivery and viral-mediated transfection for induction of prospero homeobox protein 1 (Prox-1) overexpression or transforming growth factor (TGF)-β1 inhibition [15,16,18].
Fig. 1.
Flowchart of article identification and final selection. Article identification following Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.
Fig. 2.
Proposed Mechanisms for adipose-derived stem cells (ADSCs) lymphangiogenesis. ADSCs have two lymphangiogenesis mechanisms: paracrine secretion and direct differentiation into lymphatic endothelial cells.
In vitro studies
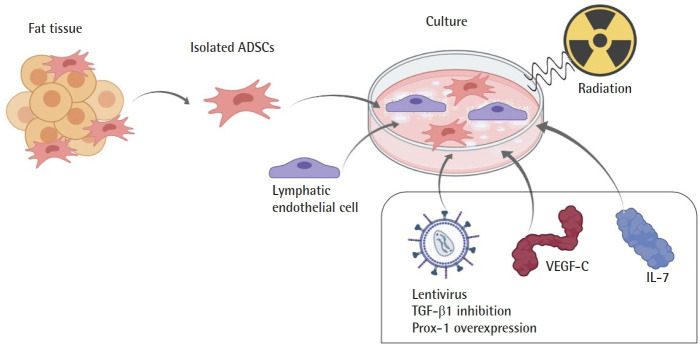
In vitro studies have been conducted to determine whether ADSC could be applicable in lymphatic tissue engineering (Table 1, Fig. 3). Authors co-cultured LEC and ADSCs to determine if ADSCs have direct effects on LECs in vitro. Strassburg et al. [17] co-cultured human ADSCs and LECs in a proportion of 50:50 cells, demonstrating that ADSCs can induce messenger RNA (mRNA) expression of lymphatic markers and promote lymphatic proliferation and migration. Takeda et al. [20] demonstrated that ADSCs promoted proliferation and migration of LECs, resulting in longer lymphatic tubes than controls. They compared the co-culture of LEC with ADSCs (1 × 104 cells/cm2) or VEGF-C, observing that the increase in lymphangiogenesis by ADSCs is greater than VEGF-C alone (P < 0.001). It was also demonstrated that lymphangiogenic factors from ADSCs were upregulated under serum-starved conditions [20]. Saijo et al. [21] conducted an in vitro study to test the effects of ionizing radiation on dermal LECs co-cultured with human ADSCs. They noticed that factors secreted by ADSCs, such as VEGF-A, VEGF-C, and hepatocyte growth factor, promoted lymphangiogenesis regardless of radiation. Moreover, radiation increased paracrine effects by increasing the expression of fibroblast growth factor in a dose-dependent manner [21].
Table 1.
Summary of in vitro studies
| Author (year) | ADSCs origin | Additional agents | Delivery | Route of delivery | Lymphangiogenesis or LE improvement | Paracrine action | Differentiation into LEC |
|---|---|---|---|---|---|---|---|
| Takeda et al. (2015) [20] | Animal | - | - | - | Yes | Yes | No |
| Strassburg et al. (2016) [17] | Human | VEGF-C | Culture media | - | Yes | Yes | No |
| Deng et al. (2017) [18] | Human | Prox-1 | Virus | - | Yes | Yes | Yes |
| Saijo et al. (2019) [21] | Human | Irradiation | - | Yes | Yes | No | |
| Sun et al. (2019) [19] | Human | IL-7 | Culture media | - | Yes | Yes | Yes |
| Yan et al. (2011) [16] | Animal | VEGF-C | Culture media | Implanted Matrigel plug | Yes | Yes | Yes |
| TGF-β1 inhibition | Virus |
ADSCs, adipose-derived stem cells; LE, lymphedema; LEC, lymphatic endothelial cell; VEGF, vascular endothelial growth factor; Prox-1, prospero homeobox protein 1; IL, interleukin; TGF, transforming growth factor.
Fig. 3.
In vitro studies on the use of adipose-derived stem cells (ADSCs) for lymphatic regeneration. Isolated ADSCs were cultured by authors who investigated different perspectives, such as the impact of co-culturing it with lymphatic endothelial cells, growth factors, and radiation. VEGF, vascular endothelial growth factor; IL, interleukin; TGF, transforming growth factor; Prox-1, prospero homeobox protein 1.
In 2011, Yan et al. [16] hypothesized that ADSC could promote lymphangiogenesis in response to changes in the balance between pro-and anti-lymphangiogenic cytokines. ADSC harvested from mice (1 × 106 cells) were cultured in a media that was enriched with VEGF-C for 48 hours or that received adenoviral transfection to induce TGF-β1 inhibition. They noticed that short term stimulation of ADSCs with VEGF-C increased in vitro expression of Prox-1, VEGF-A, and VEGF-C compared to the control group. Expressed values were notably higher when TGF-β1 inhibition was done in combination with VEGF-C stimulation [16].
Deng et al. [18] sought to determine if overexpression of Prox1, induced by transfection of lentiviral vectors, in human ADSCs could promote differentiation into LECs in vitro. They noticed an increase of LEC-specific markers, pointing out increased mRNA and protein levels of podoplanin, lymphatic vessel endothelial hyaluronan receptor 1 (LYVE-1), and VEGF-3. Moreover, tube-like structures were formed by these differentiated ADSCs in the matrix gel [18]. Sun et al. [19] aimed to investigate the effect of IL-7 on ADSC differentiation into LECs. They conducted an in vitro study using human ADSCs (5 × 105 cells) cultured by an induced medium with IL-7. They noticed an increase in RNA and protein levels of LEC markers (Prox-1, LYVE‐1, podoplanin, and VEGF‐3) in the ADSCs cultured with IL-7 and suggested that this cytokine enhances ADSC differentiation into LECs [19].
In vivo studies in animals
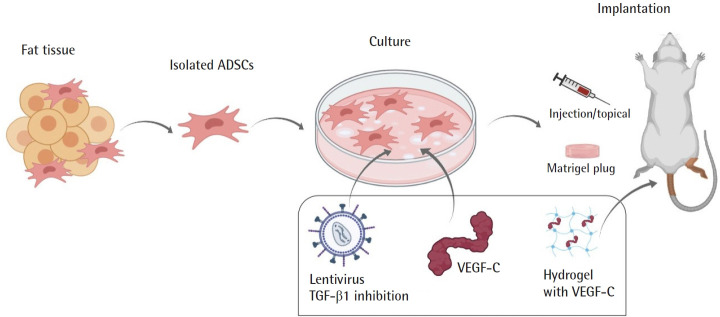
In vivo studies in animals were conducted on mice models of secondary lymphedema (Table 2, Fig. 4). Shimizu et al. [22] conducted a study to investigate whether the injection of ADSCs can promote lymphangiogenesis in vivo on a murine tail lymphedema model. They implanted 2 × 106 cells of freshly isolated mice ADSC on the first postoperative day of lymphedema induction. Compared to the control group, mice that received an injection of ADSCs presented greater lymphatic capillary density (P < 0.01), tissue expression of VEGF-C (P < 0.01) and plasma levels of VEGF-C (P < 0.05). They proposed that ADSC stimulates lymphangiogenesis through secretion of VEGF-C and recruitment of lymphatic endothelial progenitor cells (marrow-derived M2 macrophages). Yoshida et al. [23] conducted an in vivo study on a murine hindlimb lymphedema model, in which they injected different concentrations of ADSCs (no ADSCs, 1 × 106 cells, 1 × 105 cells, and 1 × 105 cells). They noted an indirect increase in the number of lymphatic vessels measured through cells expressing LYVE-1, VEGF-C, and VEGFR-3. Recovery from circumferential deterioration was significantly higher in the group receiving an injection of a greater amount of ADSCs (1 × 106 stem cells) (P < 0.05) [23].
Table 2.
Summary of in vivo studies in animals
| Author (year) | Study | ADSCs origin | Cultured | Additional agents | Delivery | Route of delivery | Lymphangiogenesis or LE improvement | Paracrine action | Differentiation into LEC |
|---|---|---|---|---|---|---|---|---|---|
| Yan et al. (2011) [16] | In vitro and in vivo animals (mice) | Animal | Yes | VEGF-C | Culture media | Implanted Matrigel plug | Yes | Yes | Yes |
| Hwang et al. (2011) [15] | In vivo in animals (mice) | Human | Yes | VEGF-C | Gelatin hydrogel | Injection | Yes | Yes | Yes |
| Shimizu et al. (2012) [22] | In vivo in animals (mice) | Animal | No | - | - | Injection | Yes | Yes | No |
| Ackermann et al. (2015) [25] | In vivo in animals (mice) | Animal | Yes | - | - | Topical | No | Yes | No |
| Yoshida et al. (2015) [23] | In vivo in animals (mice) | Animal | Yes | - | - | Injection | Yes | Yes | No |
| Hayashida et al. (2017) [24] | In vivo in animals (mice) | Animal | No | - | - | Injection | Yes | Yes | No |
ADSCs, adipose-derived stem cells; LE, lymphedema; LEC, lymphatic endothelial cell; VEGF, vascular endothelial growth factor.
Fig. 4.
In vivo animal studies on the use of adipose-derived stem cells (ADSCs) for lymphatic regeneration. Isolated ADSCs were administered to mice after being co-cultured with growth factors. VEGF, vascular endothelial growth factor; TGF, transforming growth factor.
Hayashida et al. [24] conducted an in vivo study on mice to investigate the effect of combining injection of ADSCs (1.0 × 104 cells) with vascularized lymph node transplant, a physiologic surgery to treat lymphedema. They noticed that the injection of ADSCs promoted an increase in lymphatic vessels (LYVE-1 immunoreactivity) and preservation of transplanted lymph nodes (P < 0.05). They were able to prove the function of local lymphatic circulation assessing the lymphatic metastasis’ velocity through an injection of B16 melanoma cells. The group treated with ADSCs developed lymph node metastases at a faster rate compared to the control group [24].
Ackermann et al. [25] conducted an in vivo study assessing the lymphangiogenic potential of mice ADSCs and human platelet-rich plasma (PRP) separately on a murine tail lymphedema model. PRP, ADSCs (1 × 105 cells), or saline were topically applied to the wounds. Animals that received PRP or ADSCs presented a faster wound healing. However, regarding lymphangiogenesis, only the administration of PRP demonstrated significant results (P < 0.01). ADSC-treated mice had nearly the same density of lymphatic vessels compared to the control group, and no improvement of lymphedema was seen on day 14 of their experiment. They mentioned that possible explanations for the absence of a lymphangiogenic effect of ADSCs were the short observation period of the study (14 days was not enough to leverage ADSC antifibrotic potential) and insufficient stimulation of ADSCs by VEGF-C [25].
Enhancement of ADSCs lymphangiogenesis in vivo was an object of consideration by authors. In the same study described above, Yan et al. [16] also conducted an in vivo study in mice using ADSCs that were cultured with VEGF-C or adenoviral transfection for TGF-β1 inhibition. Matrigel plug was used to transplant 5 × 105 cells in the animals. They found a significant increase in lymphangiogenic response, the number of proliferating cells, and the number of donor cells for ADSCs cultured with VEGF-C (P < 0.01). Those cells also presented podoplanin, a lymphangiogenic marker, while the cells without stimulation did not. This result was also potentiated by TGF-β1 inhibition [16]. Hwang et al. [15] aimed to determine if the therapeutic outcomes of human ADSC injection could increase with a topical gelatin hydrogel containing VEGF-C. Murine hindlimb lymphedema model was the choice for their experiment. ADSCs were implanted at a density of 1 × 104 cells/mL. Their experiment demonstrated that combined therapy (ADSCs plus VEGF-C hydrogel) had decreased dermal edema and higher lymphatic vessel regeneration compared to VEGF-C or ADSCs only (P < 0.05) [15].
Clinical studies
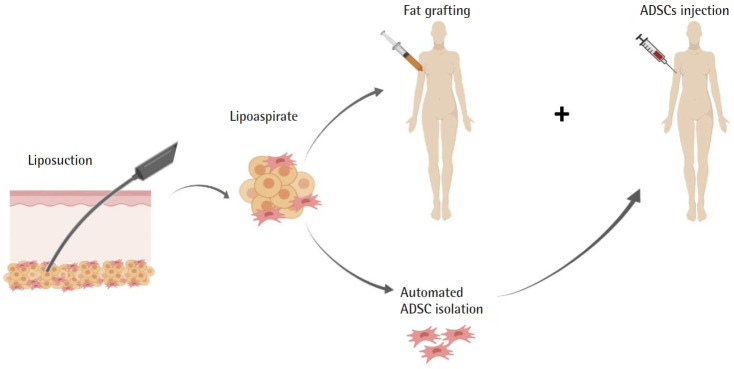
A small number of studies from the same group have explored the use of ADSCs for lymphatic regeneration in lymphedema patients (Table 3, Fig. 5). Toyserkani et al. [12-14] published a case report and a series of 10 breast cancer-related lymphedema patients who underwent: liposuction under general anesthesia, intraoperative axillar fat grafting (30 mL of lipoaspirate), and immediate-post-operative axillar injection of freshly isolated ADSCs (5.41 × 107± 0.97 × 107 cells). A case report of Toyserkani et al. [12] was a 48-year-old woman with breast cancer-related lymphedema in her arm who underwent their proposed treatment. They pointed out that, 4 months following the procedure, the patient’s lymphedema improved clinically, with a volume reduction of 292 mL in the affected limb (measured by dual-energy X-ray absorptiometry scan), representing 1 cm at the wrist/distal arm and 2 cm at the proximal arm. Moreover, the patient did not experience any adverse effects during the study follow-up time [12].
Table 3.
Summary of clinical studies
| Author (year) | Cases | ADSCs origin | Cultured | Additional agents | Route of delivery | Lymphangiogenesis or LE improvement |
|---|---|---|---|---|---|---|
| Toyserkani et al. (2016) [12] | 1 Patient; follow-up 4 months | Human | No | - | Injection | - |
| Toyserkani et al. (2017) [13] | 10 Patients; follow-up 6 months | Human | No | - | Injection | - |
| Toyserkani et al. (2019) [14] | 10 Patients; follow-up 12 months | Human | No | - | Injection | No |
ADSCs, adipose-derived stem cells; LE, lymphedema.
Fig. 5.
Clinical studies on the use of adipose-derived stem cells (ADSCs) for lymphatic regeneration. ADSC isolated from lipoaspirate was re-implanted in lymphedema patients.
Toyserkani et al. [13] conducted a pilot study in 10 patients to investigate the feasibility, safety, and efficacy of their treatment. Patients aged between 34 and 68 years had unilateral lymphedema stage I or II, were recurrence-free for a minimum of 1 year, had an American Society of Anesthesiologists status classification score 1 or 2, did not have diabetes mellitus, and were nonsmokers. A scar-releasing fat graft procedure accompanied ADSC injection. At 6 months of follow-up, they noted an improvement in patient-reported outcomes (P < 0.05), and half of the patients (5/10) reduced their use of conservative management. However, the overall volume reduction in the affected arm was small and not statistically significant. Therapy was well-tolerated, and no significant adverse effects were reported. At 12 months of follow-up, Toyserkani et al. [14] aimed to investigate lymph drainage through quantitative lymphoscintigraphy. They found no differences in the lymphoscintigraphic evaluation and affected arm volume. Although their results were limited due to a small number of patients and lack of control group, their patients presented with absence of any serious adverse events related to ADSC injection [14].
DISCUSSION
The literature on the pathogenesis and treatment of lymphedema has increased considerably over the years [26-28], but current options for treatment of lymphedema such as physiologic surgeries (lymphaticovenous bypass and lymph node transfer) still have unpredictable outcomes [29]. In this systematic literature review, we have shown the existence of promising evidence supporting the potential of ADSCs in lymphatic tissue engineering and regeneration. However, most of the studies were experimental (in vitro or in vivo on mice). The paracrine capability of ADSCs to secrete lymphangiogenic factors seems well established, but only a small number of publications noticed differentiation of those cells into LECs [15,16,18,19]. Studies provided empirical evidence supporting the feasibility to enhance ADSCs lymphangiogenic potential modulating lymphangiogenic cytokines/genes [15-19].
Most studies demonstrated favorable results for the potential use of ADSCs in lymphatic tissue engineering and regeneration. Ackermann et al. [25] noticed in their experiment that ADSCtreated mice had nearly the same density of lymphatic vessels compared to the control group. One possible explanation was the short follow-up period (14 days), which would not be enough to leverage ADSC antifibrotic potential. However, we noticed that two other studies conducted in similar animals (10-week-old adult male mice) were able to demonstrate the lymphangiogenic potential of ADSCs on day 14 [23,24]. The other explanation given was insufficient stimulation of ADSCs by VEGF-C, which seems more likely. Authors have proposed mechanisms to enhance the lymphangiogenic potential of ADSCs with promising results using enhanced culture media with VEGF-C or IL-7, cutaneous VEGF-C hydrogel, and viral transfection to increase expression of Prox-1.
Since lymphedema in developing countries is mainly secondary to oncologic surgical procedures, two important concerns of applying this therapy must be discussed. The first is patient safety, since lymphangiogenic growth factors are associated with tumor growth and metastasis [30]. Hayashida et al. [24] noticed in their experimental study that following injection of B16 melanoma cells, the group treated with ADSCs developed lymph node metastases faster than the control group. Although limited conclusions could be drawn through the 12-month-follow-up case series of 10 patients conducted by Toyserkani et al. [14], their study added valuable information regarding the safety of injecting ADSCs in breast cancer patients. The second concern is how well ADSCs could act in the setting of radiation injury (e.g., fibrosis, atrophy, and dry hypovascularized skin with impaired wound healing) [31]. Saijo et al. [21] investigated this, demonstrating that ADSCs were capable of lymphangiogenesis in vitro regardless of irradiation.
Other therapies have been proposed using non-autologous agents to control tissue inflammation or induce lymphangiogenesis [32]. Lymphangiogenesis has been observed through different mechanisms, mostly non-autologous agents such as anti-inflammatory agents targeting Th2-inflammatory responses and growth factors like VEGF-C [33,34]. Compared to them, the main advantage of cell therapies is the fact that they can be extracted from the patient’s own body. ADSCs are abundant and can be harvested by liposuction [10]. However, issues still need to be addressed before translating cell therapies to patients, such as determining the appropriate method of isolation and purification, its potential impact on tumor growth, and regulations by the US Food and Drug Administration [9].
We recognize several limitations to our study, common to systematic reviews. There is a potential for bias in interpreting the data reported in each study. Moreover, our search strategy did not include synonyms of ADSCs such as “adipose mesenchymal stem cells” or “stromal vascular fraction.” Despite these limitations, we feel that our study reports valuable pooled data, particularly regarding the use of ADSCs in lymphatic tissue engineering and regeneration, and proposes ways to enhance it, which can guide future studies to advance the field. Further studies with standardized protocols and long-term outcomes need to be performed to evaluate the effect of ADSCs in the treatment of lymphedema in DMEM (Gibco) supplemented with 2.5 mg/mL fungizone (life technologies) and 1% penicillin/streptomycin and washed several times.
CONCLUSION
Publications assessing the potential use of ADSCs in lymphatic tissue engineering and regeneration are still limited but demonstrate promising data. There seems to be general agreement that ADSCs could be useful in regenerative lymphangiogenesis and that its effect can be enhanced by adjusting lymphangiogenic cytokines/genes. Unparalleled advances in lymphedema treatment are imminent.
Footnotes
Conflict of interest
No potential conflict of interest relevant to this article was reported.
Author contribution
Conceptualization: all authors. Formal analysis: AJ Forte, D Boczar, R Sarabia-Estrada, T Aung, A Quiñones-Hinojosa. Funding acquisition: R Sarabia-Estrada. Methodology: AJ Forte, D Boczar, R Sarabia-Estrada, MT Huayllani, FR Avila, RA Torres, G Guliyeva, T Aung, A Quiñones-Hinojosa. Project administration: AJ Forte, A Quiñones-Hinojosa. Visualization: MT Huayllani, G Guliyeva. Writing - original draft: AJ Forte, D Boczar, MT Huayllani, FR Avila, RA Torres, G Guliyeva, A Quiñones-Hinojosa. Writing - review & editing: all authors.
REFERENCES
- 1.Lawenda BD, Mondry TE, Johnstone PA. Lymphedema: a primer on the identification and management of a chronic condition in oncologic treatment. CA Cancer J Clin. 2009;59:8–24. doi: 10.3322/caac.20001. [DOI] [PubMed] [Google Scholar]
- 2.Basta MN, Wu LC, Kanchwala SK, et al. Reliable prediction of postmastectomy lymphedema: the Risk Assessment Tool Evaluating Lymphedema. Am J Surg. 2017;213:1125–33. doi: 10.1016/j.amjsurg.2016.08.016. [DOI] [PubMed] [Google Scholar]
- 3.DiSipio T, Rye S, Newman B, et al. Incidence of unilateral arm lymphoedema after breast cancer: a systematic review and meta-analysis. Lancet Oncol. 2013;14:500–15. doi: 10.1016/S1470-2045(13)70076-7. [DOI] [PubMed] [Google Scholar]
- 4.McDuff SGR, Mina AI, Brunelle CL, et al. Timing of lymphedema after treatment for breast cancer: when are patients most at risk? Int J Radiat Oncol Biol Phys. 2019;103:62–70. doi: 10.1016/j.ijrobp.2018.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kayiran O, De La Cruz C, Tane K, et al. Lymphedema: from diagnosis to treatment. Turk J Surg. 2017;33:51–7. doi: 10.5152/turkjsurg.2017.3870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dayan JH, Ly CL, Kataru RP, et al. Lymphedema: pathogenesis and novel therapies. Annu Rev Med. 2018;69:263–76. doi: 10.1146/annurev-med-060116-022900. [DOI] [PubMed] [Google Scholar]
- 7.Breastcancer.org . Ardmore, PA: Breastcancer.org; Surgery for lymphedema [Internet] c2021 [cited 2021 Apr 28]. Available from: https://www.breastcancer.org/treatment/lymphedema/treatments/surgery. [Google Scholar]
- 8.Ceccarelli S, Pontecorvi P, Anastasiadou E, et al. Immunomodulatory effect of adipose-derived stem cells: the cutting edge of clinical application. Front Cell Dev Biol. 2020;8:236. doi: 10.3389/fcell.2020.00236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gir P, Oni G, Brown SA, et al. Human adipose stem cells: current clinical applications. Plast Reconstr Surg. 2012;129:1277–90. doi: 10.1097/PRS.0b013e31824ecae6. [DOI] [PubMed] [Google Scholar]
- 10.Toyserkani NM, Christensen ML, Sheikh SP, et al. Stem cells show promising results for lymphoedema treatment: a literature review. J Plast Surg Hand Surg. 2015;49:65–71. doi: 10.3109/2000656X.2014.964726. [DOI] [PubMed] [Google Scholar]
- 11.Toyserkani NM, Jorgensen MG, Tabatabaeifar S, et al. Concise review: a safety assessment of adipose-derived cell therapy in clinical trials. A systematic review of reported adverse events. Stem Cells Transl Med. 2017;6:1786–94. doi: 10.1002/sctm.17-0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Toyserkani NM, Jensen CH, Sheikh SP, et al. Cell-assisted lipotransfer using autologous adipose-derived stromal cells for alleviation of breast cancer-related lymphedema. Stem Cells Transl Med. 2016;5:857–9. doi: 10.5966/sctm.2015-0357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Toyserkani NM, Jensen CH, Andersen DC, et al. Treatment of breast cancer-related lymphedema with adipose-derived regenerative cells and fat grafts: a feasibility and safety study. Stem Cells Transl Med. 2017;6:1666–72. doi: 10.1002/sctm.17-0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Toyserkani NM, Jensen CH, Tabatabaeifar S, et al. Adipose-derived regenerative cells and fat grafting for treating breast cancer-related lymphedema: lymphoscintigraphic evaluation with 1 year of follow-up. J Plast Reconstr Aesthet Surg. 2019;72:71–7. doi: 10.1016/j.bjps.2018.09.007. [DOI] [PubMed] [Google Scholar]
- 15.Hwang JH, Kim IG, Lee JY, et al. Therapeutic lymphangiogenesis using stem cell and VEGF-C hydrogel. Biomaterials. 2011;32:4415–23. doi: 10.1016/j.biomaterials.2011.02.051. [DOI] [PubMed] [Google Scholar]
- 16.Yan A, Avraham T, Zampell JC, et al. Adipose-derived stem cells promote lymphangiogenesis in response to VEGF-C stimulation or TGF-β1 inhibition. Future Oncol. 2011;7:1457–73. doi: 10.2217/fon.11.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Strassburg S, Torio-Padron N, Finkenzeller G, et al. Adiposederived stem cells support lymphangiogenic parameters in vitro. J Cell Biochem. 2016;117:2620–9. doi: 10.1002/jcb.25557. [DOI] [PubMed] [Google Scholar]
- 18.Deng J, Dai T, Sun Y, et al. Overexpression of Prox1 induces the differentiation of human adipose-derived stem cells into lymphatic endothelial-like cells in vitro. Cell Reprogram. 2017;19:54–63. doi: 10.1089/cell.2016.0038. [DOI] [PubMed] [Google Scholar]
- 19.Sun Y, Lu B, Deng J, et al. IL-7 enhances the differentiation of adipose-derived stem cells toward lymphatic endothelial cells through AKT signaling. Cell Biol Int. 2019;43:394–401. doi: 10.1002/cbin.11093. [DOI] [PubMed] [Google Scholar]
- 20.Takeda K, Sowa Y, Nishino K, et al. Adipose-derived stem cells promote proliferation, migration, and tube formation of lymphatic endothelial cells in vitro by secreting lymphangiogenic factors. Ann Plast Surg. 2015;74:728–36. doi: 10.1097/SAP.0000000000000084. [DOI] [PubMed] [Google Scholar]
- 21.Saijo H, Suzuki K, Yoshimoto H, et al. Paracrine effects of adipose-derived stem cells promote lymphangiogenesis in irradiated lymphatic endothelial cells. Plast Reconstr Surg. 2019;143:1189e–1200e. doi: 10.1097/PRS.0000000000005669. [DOI] [PubMed] [Google Scholar]
- 22.Shimizu Y, Shibata R, Shintani S, et al. Therapeutic lymphangiogenesis with implantation of adipose-derived regenerative cells. J Am Heart Assoc. 2012;1:e000877. doi: 10.1161/JAHA.112.000877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yoshida S, Hamuy R, Hamada Y, et al. Adipose-derived stem cell transplantation for therapeutic lymphangiogenesis in a mouse secondary lymphedema model. Regen Med. 2015;10:549–62. doi: 10.2217/rme.15.24. [DOI] [PubMed] [Google Scholar]
- 24.Hayashida K, Yoshida S, Yoshimoto H, et al. Adipose-derived stem cells and vascularized lymph node transfers successfully treat mouse hindlimb secondary lymphedema by early reconnection of the lymphatic system and lymphangiogenesis. Plast Reconstr Surg. 2017;139:639–51. doi: 10.1097/PRS.0000000000003110. [DOI] [PubMed] [Google Scholar]
- 25.Ackermann M, Wettstein R, Senaldi C, et al. Impact of platelet rich plasma and adipose stem cells on lymphangiogenesis in a murine tail lymphedema model. Microvasc Res. 2015;102:78–85. doi: 10.1016/j.mvr.2015.09.001. [DOI] [PubMed] [Google Scholar]
- 26.Forte AJ, Boczar D, Huayllani MT, et al. Use of autologous blood components in lymphedema treatment: a systematic review. Cureus. 2019;11:e5638. doi: 10.7759/cureus.5638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Forte AJ, Boczar D, Huayllani MT, et al. Topical approach to delivering targeted therapies in lymphedema treatment: a systematic review. Cureus. 2019;11:e6269. doi: 10.7759/cureus.6269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Forte AJ, Huayllani MT, Boczar D, et al. Bioimpedance spectroscopy for assessment of breast cancer-related lymphedema: a systematic review. Plast Surg Nurs. 2020;40:86–90. doi: 10.1097/PSN.0000000000000306. [DOI] [PubMed] [Google Scholar]
- 29.Joseph WJ, Aschen S, Ghanta S, et al. Sterile inflammation after lymph node transfer improves lymphatic function and regeneration. Plast Reconstr Surg. 2014;134:60–8. doi: 10.1097/PRS.0000000000000286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kondo K, Kaneko T, Baba M, et al. VEGF-C and VEGF-A synergistically enhance lymph node metastasis of gastric cancer. Biol Pharm Bull. 2007;30:633–7. doi: 10.1248/bpb.30.633. [DOI] [PubMed] [Google Scholar]
- 31.Wu SH, Shirado T, Mashiko T, et al. Therapeutic effects of human adipose-derived products on impaired wound healing in irradiated tissue. Plast Reconstr Surg. 2018;142:383–91. doi: 10.1097/PRS.0000000000004609. [DOI] [PubMed] [Google Scholar]
- 32.Forte AJ, Boczar D, Huayllani MT, et al. Targeted therapies in surgical treatment of lymphedema: a systematic review. Cureus. 2019;11:e5397. doi: 10.7759/cureus.5397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zampell JC, Elhadad S, Avraham T, et al. Toll-like receptor deficiency worsens inflammation and lymphedema after lymphatic injury. Am J Physiol Cell Physiol. 2012;302:C709–19. doi: 10.1152/ajpcell.00284.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Avraham T, Zampell JC, Yan A, et al. Th2 differentiation is necessary for soft tissue fibrosis and lymphatic dysfunction resulting from lymphedema. FASEB J. 2013;27:1114–26. doi: 10.1096/fj.12-222695. [DOI] [PMC free article] [PubMed] [Google Scholar]