Abstract
Objectives
The current study was aimed at examining SARS-CoV-2 immune responses following two doses of Comirnaty® COVID-19 vaccine among elderly people in nursing homes.
Methods
A prospective cohort study in a representative sample from nursing homes in Valencia (n = 881; males: 271, females 610; median age, 86 years) recruited residents using a random one-stage cluster sampling approach. A lateral flow immunochromatography device (LFIC) (OnSite COVID-19 IgG/IgM Rapid Test; CTK BIOTECH, Poway, CA, USA) was used as the front-line test for detecting SARS-CoV-2-Spike (S)-specific antibodies in whole blood obtained using a fingerstick. Residents returning negative LFIC results underwent venipuncture and testing for presence of SARS-CoV-2-S-reactive antibodies and T cells using the Roche Elecsys® Anti-SARS-CoV-2 S (Roche Diagnostics, Pleasanton, CA, USA), the LIAISON® SARS-CoV-2 TrimericS IgG assay (Diasorin S.p.A, Saluggia, Italy) and by flow cytometry, respectively.
Results
The SARS-CoV-2-S antibody detection rate in nursing home residents was 99.6% (283/284) and 98.3% (587/597) for SARS-CoV-2 recovered and naïve residents, respectively, within a median of 99 days (range 17–125 days) after full vaccination. Three out of five residents lacking SARS-CoV-2-S antibodies had detectable S-reactive CD8+ and/or CD4+ T cells. In addition, 50/50 and 40/50 participants with detectable SARS-CoV-2 antibodies also had SARS-CoV-2-S-reactive interferon-γ-producing CD4+ and CD8+ T cells, respectively.
Discussion
The Comirnaty® COVID-19 vaccine is highly immunogenic in nursing home residents.
Keywords: Comirnaty®COVID-19 vaccine, Nursing home residents, SARS-CoV-2, SARS-CoV-2-S antibodies
Introduction
Older people have been prioritized for vaccination against SARS-CoV-2 because of their increased risk of severe COVID-19 [1]. Deployment of SARS-CoV-2 messenger RNA (mRNA) vaccines in nursing homes has resulted in a dramatic decrease in the incidence of SARS-CoV-2 infection and most notably of COVID-19 [2,3]. Although this effect is explained mainly by vaccine coverage among residents and staff, strict non-pharmacological infection control measures also contributed to prevent infection both of unvaccinated residents and those with suboptimal or undetectable SARS-CoV-2-specific immune response to the vaccine.
There is limited information as to the immunogenicity of SARS-CoV-2 mRNA vaccines in elderly people with comorbidities and frailty [[4], [5], [6]]. Data from the Comirnaty® COVID-19 vaccine (Pfizer-BNT162b2) phase I trial pointed to lower antibody responses in older people than in younger participants [7].
By March 2021, the Valencian Community (VC) had set up a COVID-19 vaccine research programme (ProVaVac) (Decree 10/2021 of 16 March) which among other assignments was tasked with evaluating the immunogenicity of SARS-CoV-2 mRNA vaccines among nursing home residents. The results of this investigation are reported herein.
Material and methods
Participants and study design
We conducted a prospective cohort study in a representative sample of VC nursing homes to examine SARS-CoV-2 immune responses following two doses of Comirnaty® COVID-19 vaccine. As of February 2021, the region is served by a total of 327 nursing homes, attending 22 044 residents. The electronic Valencia Health System Integrated Databases (VID) [8] were queried for sampling and to obtain sociodemographic data and SARS-CoV-2 infection status of participants prior to or after full-dose vaccination. The “Monitoring of antibody response following SARS-CoV-2 vaccination in nursing homes of the Valencian Community” programme was carried out under the epidemiological surveillance competences of the Valencia Government Health Department (Law 16/2003/May 28 on Cohesion and Quality of the National Health System, and Law 10/2014/December 29 on Public Health of the Valencian Community), without requiring informed consent or ethics approval by an institutional review board. Likewise, according to local law and regulations, the publication of the data is exempt from the approval of a research ethics committee. Personal data from nursing homes and residents were processed in accordance with European data protection regulations.
Testing strategy and immunoassays employed
Participants were scheduled to be tested for presence of SARS-CoV-2-Spike (S)-specific antibodies in whole blood obtained by fingerstick at the respective nursing home at least 15 days after complete vaccination. No pre-vaccination specimens were available for analyses. The OnSite COVID-19 IgG/IgM Rapid Test (CTK BIOTECH, Poway, CA, USA), a lateral flow immunochromatographic assay (LFIC) for detection of anti-SARS-CoV-2-S IgG and IgM antibodies with an specificity of 99% [9] was used for the purpose. Specifically, this LFIC detects antibodies binding to the subunit 1 (S1) of the S protein, which contains a receptor binding domain that recognizes and binds to the host receptor angiotensin-converting enzyme 2. The IgG line intensity was scored visually using a 4-level scale as previously reported [10]: 0, negative result; 1+, intensity of test band lower than control band; 2+, intensity of test band equal to control line; 3+, intensity of test band greater than control line. Experienced nurses at each sampling site were trained for qualitative and quantitative interpretation of LFIC results.
Participants returning negative results by LFIC immediately underwent venipuncture for further testing. Blood specimens were scheduled for collection in sodium heparin tubes (Becton Dickinson UK Ltd, Oxford, UK) to further assess B and T-cell responses. SARS-CoV-2-S antibodies were quantitated by Roche Elecsys® Anti-SARS-CoV-2 S (Roche Diagnostics, Pleasanton, CA, USA), which is calibrated with the first WHO International Standard and Reference Panel for anti-SARS-CoV-2 antibody [11], and by the chemiluminescent immunoassay (CLIA) LIAISON® SARS-CoV-2 TrimericS IgG assay (DiaSorin S.p.A, Saluggia, Italy). Specimens were assayed at the Microbiology Service of the Hospital Clínico Universitario of Valencia (HCU) in singlets within 24 hr of collection.
Available whole-blood specimens from participants testing negative for SARS-CoV-2 antibodies by all immunoassays (LFIC, ECLIA and CLIA) were also assayed (at HCU) for quantitation of SARS-CoV-2-S-reactive interferon (IFN) γ-producing-CD8+ and CD4+ T cells at HCU by flow cytometry for ICS (BD Fastimmune, Becton Dickinson and Company-Biosciences, San Jose, CA) as previously described [6,12]. Likewise, randomly selected whole blood specimens from residents (n = 50) testing LFIC negative/ECLIA positive were processed for T-cell immunity analysis.
Statistical methods
Frequency comparisons for categorical variables were carried out using the Fisher exact test. Differences between medians were compared using the Mann–Whitney U test. Two-sided exact p values were reported. The Spearman rank test was used for correlation analyses between continuous variables. A p value < 0.05 was considered statistically significant. The analyses were performed using SPSS version 20.0 (SPSS, Chicago, IL, USA).
Results
One small nursing home (n = 12) failed to provide blood specimens from residents testing negative by LFIC and was excluded; in turn, 36 randomly selected individuals from the additional nursing home incorporated for potential losses were included, leaving a total of 881 residents (males: 271, females: 610; median age, 86 years; range 32–101 years) from 13 clusters for analysis. Out of 881 residents, 284 (32%) had evidence of SARS-CoV-2 infection prior to undergoing immunological testing. In detail, 232 residents were diagnosed with SARS-CoV-2 infection (220 by RT-PCR) at a median of 51 days (range 4–319) prior to receipt of the first dose and 49 residents between the first and the second vaccine doses (in all cases by RT-PCR at a median of 25 days prior to receipt of the second dose; range 10–47). All these 49 residents had tested negative by RT-PCR in nasopharyngeal specimens before completing the vaccination schedule. In turn, three residents were diagnosed within 5 days after the second dose.
The main results of our analyses are depicted in Fig. 1 . LFIC was performed at a median of 99 days after the second vaccine dose (range 17–125 days). In total, 734 out of 881 residents (83%) tested positive for SARS-CoV-2 IgG, and 227/881 (25.7%) for IgMs (Table 1 ). No residents tested positive for IgMs and negative for IgGs. Positive LFIC IgG results were overall significantly more frequent (p < 0.001) in SARS-CoV-2-recovered than in SARS-CoV-2-naïve residents.
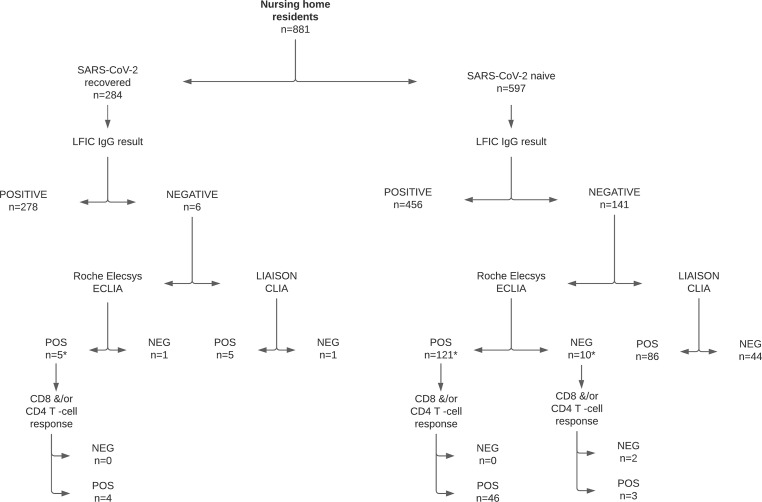
Fig. 1.
Flow chart depicting the testing strategy and most relevant data on the SARS-CoV-2-S- antibody and T-cell responses in nursing home residents recruited the current study. For recruitment, a random one-stage cluster sampling was carried out assuming a negative antibody detection rate of 10%, precision of 5% and CI of 95%, corrected for a design effect (Deff) of 5 (rho: 0.06), resulting in a sample size of 13 clusters (nursing homes) with 857 residents of nine different Health Departments in the provinces of Valencia, Castellón and Alicante. This sample was expanded by one additional nursing home (total no. Residents, 180) to compensate for possible losses if necessary. The electronic Valencia Health System Integrated Databases (VID) [8] were queried for assessing SARS-CoV-2 infection status of participants prior to full vaccination; in this context, according to public health policies in place in the Valencian Community within the study period, residents suspected of having COVID-19 were tested within 24 hr after the onset of symptoms for the presence of SARS-CoV-2 RNA in the upper respiratory tract by RT-PCR. In addition, systematic testing of asymptomatic residents was triggered by the occurrence of symptomatic cases and also conducted within 48 hr of the diagnosis of the index case. Participants testing negative by lateral flow immunochromatography (LFIC) were further tested by the Roche Elecsys® Anti-SARS-CoV-2 S (Roche Diagnostics, Pleasanton, CA, USA and by the chemiluminescent immunoassay (CLIA) LIAISON® SARS-CoV-2 TrimericS IgG assay (DiaSorin S.p.A, Saluggia, Italy). SARS-CoV-2-S-reactive IFN-γ-producing-CD8+ and CD4+ T cells were enumerated in whole blood by flow cytometry as previously described [[6], [12]]. The asterisk symbols indicate that whole-blood specimens either were not available or were not processed from all participants; in fact, a total of 5 subjects lacking antibody responses could be examined, whereas, a total of 50 individuals among those with detectable antibody responses were randomly selected for T-cell immunity testing.
Table 1.
Detection of SARS-CoV-2-S-reactive antibodies by lateral flow immunochromatography among nursing home residents following full-dose vaccination with Comirnaty®
| SARS-CoV-2 infection status (n) | IgM |
IgG |
||
|---|---|---|---|---|
| Positive (%) | Negative (%) | Positive (%) | Negative (%) | |
| All participants (881) | 227 (25.7) | 654 (74.0) | 734 (83.0) | 147 (16.7) |
| SARS-CoV-2-naïve (597) | 60 (10.0) | 537 (89.9) | 456 (76.4) | 141 (23.6) |
| SARS-CoV-2-convalescent/recovered (284) | 167 (58.8) | 117 (41.2) | 278 (97.9) | 6 (2.1) |
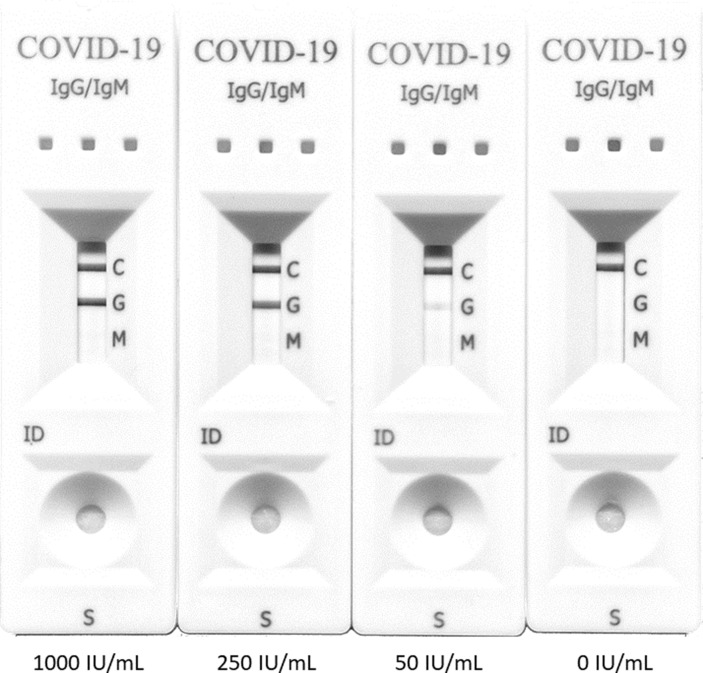
Experiments performed using plasma specimens with known SARS-CoV-2-S-reactive antibody levels (0, 50, 250 and 1000 IU/mL), as quantitated by the Roche ECLIA, revealed that IgG test band intensity increased in parallel with antibody levels (Fig. 2 ). IgG LFIC results were thus graded 0−3+, as detailed in Materials and Methods.
Fig. 2.
Lateral flow immunochromatography assay (LFIC) IgG line intensity according to SARS-CoV-2-S-reactive antibody levels as quantitated by the Roche Elecsys® Anti-SARS-CoV-2 S immunoassay. Line intensity was scored visually using a 4-level scale. From right to left 0, undetectable; 1+, weak positive result (intensity of test band lower than control band); 2+, positive result (intensity of test band equal to control line); 3+, strong positive result (intensity of test band greater than control line).
Overall, as shown in Table 2 , the percentage of SARS-CoV-2-naïve residents with undetectable IgG responses (grade 0) increased over time after receiving the second vaccine dose, which was apparently not the case in SARS-CoV-2-recovered participants (p < 0.01). The percentage of residents displaying strong IgG responses (arbitrarily defined as those ≥2+) was significantly higher (p < 0.01) across most testing time frames in SARS-CoV-2-recovered than naïve residents. Strikingly, of subjects tested ≥90 days after the second vaccine dose, 84 out of 90 (93.3%) of SARS-CoV-2-recovered had strong IgG responses, in contrast with just 33.6% (135/401) of naïve (p < 0.001).
Table 2.
SARS-CoV-2 IgG responses as determined by lateral flow immunochromatography following full-dose vaccination with Comirnaty® in SARS-CoV-2-naïve or recovered nursing home residents
| Gradinga/Timing of specimen collection after second vaccine dose | Study group |
|
|---|---|---|
| SARS-CoV-2-naïve n (%) | SARS-CoV-2-recovered n (%) | |
| 0 | ||
| 12−29 days | 3/52 (5.8%) | 0/2 (0%) |
| 30−59 days | 4/28 (14.3%) | 0/13 (0%) |
| 60−89 days | 14/104 (13.5%) | 4/177 (2.3%) |
| ≥90 days | 119/401 (29.7%) | 2/90 (2.2%) |
| 1+ | ||
| 12−29 days | 1/52 (1.9%) | 0/2 (0%) |
| 30−59 days | 11/28 (39.3%) | 1/13 (7.7%) |
| 60−89 days | 42/104 (40.4%) | 20/177 (11.3%) |
| ≥90 days | 147/401 (36.7%) | 4/90 (4.4%) |
| 2+ | ||
| 12−29 days | 13/52 (25%) | 1/2 (50%) |
| 30−59 days | 12/28 (42.9%) | 8/13 (61.5%) |
| 60–89 days | 35/104 (33.7%) | 92/177 (52%) |
| ≥90 days | 120/401 (29.9%) | 49/90 (54.4%) |
| 3+ | ||
| 12−29 days | 35/52 (67.3%) | 1/2 (50%) |
| 30−59 days | 1/28 (3.6%) | 4/13 (30.8%) |
| 60−89 days | 13/104 (12.5%) | 61/177 (34.5%) |
| ≥90 days | 15/401 (3.7%) | 35/90 (38.9%) |
IgG line intensity was scored visually using a 4-level scale: 0, negative result; 1+, weak positive result (intensity of test band lower than control band); 2+, positive result (intensity of test band equal to control line); 3+, strong positive result (intensity of test band greater than control line).
To determine whether the strength of SARS-CoV-2-S1 IgG responses after full vaccination in SARS-CoV-2 recovered residents (n = 284) was dependent upon the timeframe at which natural infection was diagnosed relative to the time of vaccination, we arbitrarily defined the following categories: ≤30 days, 30–60 days, 61–90 days and ≥90. The data are shown in Table S1. Although the frequency of residents displaying strong antibody responses (as defined herein, ≥2+) was comparable (p ≥ 0.5) across the different study subgroups, very high levels of SARS-CoV-2-S1 IgG (3+) were seen less frequently (p < 0.001) among residents who had acquired SARS-CoV-2 infection more than 90 days since receipt of the first vaccine dose and in those who presumably contracted it between the first and the second vaccine doses.
A total of 147 out of 881 residents (16.6%) returned negative results by LFIC. These had been sampled at a median of 109 days (range 17–125 days) after receipt of the second vaccine dose. When tested by the Roche ECLIA assay, 136 out of the 147 residents (92.5%) returned positive results (median 52 IU/mL; range 1.4–250 IU/mL), whereas only 91 out of 137 (66.4%) tested positive by DiaSorin trimeric-S antibody assay. None of the specimens analysed tested negative by the Roche ECLIA then positive by the DiaSorin CLIA. Antibody levels measured by the assays were strongly correlated (rho, 0.83, 95% CI, 0.78–0.88; p < 0.001).
Of the 11 residents testing negative by Roche ECLIA assay, ten had no evidence of prior SARS-CoV-2 infection. One resident had a positive RT-PCR result in NP 35 days prior to receiving the first vaccine dose.
Overall, therefore, the rate of SARS-CoV-2 antibody detection following the second vaccine dose was 98.7% (870/881), with a slightly higher figure for SARS-CoV-2-recovered (283/284; 99.6%) than for naïve (587/597; 98.3%) residents.
Finally, out of the 11 residents testing negative by LFIC and by the Roche ECLIA assay five had available specimens for T-cell immunological analyses, which were collected 34, 67, 94, 99 and 107 days after the second vaccine dose. Three out of five residents (60%) had SARS-CoV-2-S-reactive IFN-γ-producing CD4+ T cells (0.47%, 0.12% and 0.02%), but none had CD8+ T cells. A total of 50 randomly selected residents with detectable antibody responses were also examined for the presence of SARS-CoV-2-S-reactive T cells by a median of 99 days (range 19–115 days) after completion of the vaccination schedule. All subjects had SARS-CoV-2-S-reactive IFN-γ CD4+ T cells, whereas 80% (40/50) had detectable CD8+ T-cell responses (Table 3 ).
Table 3.
Detection of SARS-CoV-2-S-reactive T cells by flow cytometry among nursing home residents with detectable SARS-CoV-2-S antibodies following full-dose vaccination with Comirnaty®
| SARS-CoV-2 infection status (n) | CD4+ T cells detectable responses/median frequency (range) | CD8+ T cells detectable responses/median frequency (range) |
|---|---|---|
| SARS-CoV-2-naïve (46) | 46/0.91% (0.02–4.80) | 37/0.22% (0–5.67) |
| SARS-CoV-2-convalescent/recovered (4) | 4/0.50% (0.02–1.07) | 3/0.33% (0–1.50) |
Discussion
Herein, using a two-step serological testing strategy of on-site LFIC (specificity of 99% [9]) followed by highly sensitive ECLIA/CLIA for LFIC-negative specimens, we showed that over 98% of a large cohort of nursing home residents at VC (n = 881) had detectable SARS-CoV-2-S-reactive antibodies through day 125 (median 99 days) after full-dose vaccination with the Comirnaty® COVID-19 vaccine, regardless of SARS-CoV-2 infection status prior to vaccination. To our knowledge, no previous study has assessed antibody response elicited after completion of SARS-CoV-2 mRNA vaccination within this timeframe and in this population. Nevertheless, recent non-peer-reviewed research [6] found a comparable detection rate as measured by the Roche ECLIA of SARS-CoV-2-S-reactive antibodies by 2–3 weeks after the second vaccine dose across nursing home residents (95.2%) and younger healthy controls (94.4%).
The decision to carry out an LFIC test as a front-line testing assay was made to restrict venipuncture to those returning negative results by LFIC, given the extreme frailty of many recruited residents. Although LFICs are designed to be interpreted qualitatively (positive/negative result), in line with a previous report [9], we showed that the intensity of IgG test line reactivity increased in parallel to antibody levels measured by the Roche ECLIA, which in turn strongly correlate with neutralizing antibody titres [13,14]. Using this semiquantitative approach, we observed that the antibody response elicited by the vaccine appeared stronger over time and more durable in SARS-CoV-2-recovered than in naïve nursing home residents, in line with previous findings [6]. Stronger and more durable antibody response to vaccination elicited in recovered compared with naïve residents should be taken into account when interpreting breakthrough infection in vaccinated elderly individuals, and when considering the need for future booster vaccine doses.
Interestingly, among SARS-CoV-2-recovered residents, documentation of very strong (3+) post-vaccination antibody responses was dependent upon the time elapsed since acquisition of SARS-CoV-2 infection; in fact, residents having contracted the infection more than 90 days prior to receipt of the first vaccine dose displayed a significantly lower rate than those infected less than 90 days until vaccination. Unfortunately, the lack of information regarding the antibody response of participants prior to vaccination precluded drawing conclusions as to the potential impact of this variable on the magnitude of the booster vaccine effect.
Also of interest was the fact that very strong responses (3+) were unfrequently observed in those who presumably contracted SARS-CoV-2 infection between the first and the second vaccine dose. Since 90% of these individuals exhibited strong responses (2+), we speculate that peak antibody levels could have been reached after the time at which immunological analyses were carried out.
SARS-CoV-2-S antibodies were lacking in 11 individuals, of whom ten had no record of previous SARS-CoV-2 infection; nevertheless, three out of five residents with available specimens (60%) were capable of mounting SARS-CoV-2-S-reactive IFN-γ CD4+ T-cell responses, suggesting that undetectable antibody response after complete vaccination does not necessarily reflect vaccine immunogenicity failure. Whether individuals lacking both B- and T-cell detectable responses would benefit from receiving a third vaccine dose, either homologous or heterologous, remains to be investigated.
Remarkably, 100% and 80% of residents (46 SARS-CoV-2 naïve and four recovered) with measurable antibody responses and examined for T-cell responses (n = 50) had detectable SARS-CoV-2-S-reactive CD4+ and CD8+ T cells, respectively. This is in contrast to data reported in a recent study [6] that failed to show either detectable IFN-γ CD8+, CD4+ T-cell responses or both in around 35% of nursing home residents, whereas these were documented in all healthy and younger controls. Nevertheless, in that study post-vaccination specimens were drawn at a median of 17.5 days (range 14–35 days), whereas in the current study they were collected at later times (median 99 days; range 17–125 days). Taken collectively, these data are consistent with elderly people displaying delayed kinetics of primarily elicited or boosted post-vaccination T-cell responses compared with younger controls.
Interestingly, our data suggested that the choice of SARS-CoV-2-S immunoassay for evaluating antibody responses elicited by the Comirnaty® CIVOD-19 vaccine is important. In effect, among residents testing negative by LFIC, the Roche ECLIA assay returned more positive results than the Diasorin CLIA, which may reflect between-assay differences in analytical sensitivity but could also be related to the nature of the binding antigen in the assays (RBD vs. trimeric S protein, respectively).
The current study has several limitations. First, use of an LFIC assay for serological testing in most residents precluded precise quantitation of antibody levels. Moreover, the possibility that some of the positive LFIC results were false-positive ones (specificity, 99%) cannot be ruled out as these were not confirmed by ECLIA or CLIA. Second, no control group was included. Third, no pre-vaccination specimens were available for analyses; this precluded assessing the booster effect in those with prior SARS-CoV-2 infection. Fourth, some residences submitted red-top tubes for serum collection instead, which prevented T-cell immunity analyses. Fifth, testing for presence of SARS-CoV-2-S-reactive T cells with functional specificities other than IFN-γ production was not conducted. Sixth, we cannot rule out that some participants herein categorized as SARS-CoV2 naïve had indeed experienced asymptomatic or paucisymtomatic SARS-CoV-2 infection that were missed. Nonetheless, the sample size stands out as a major strength of this study.
In summary, the data presented herein revealed that most nursing home residents have achieved SARS-CoV-2 antibodies by a median of 3 months after full-dose vaccination with the Comirnaty® COVID-19 vaccine. The findings also suggest that antibody levels elicited by the vaccine may decrease over time, most notably among SARS-CoV-2-naïve residents. Likewise, de novo development or maintenance of pre-existing SARS-CoV-2-S-reactive T-cell responses can be documented in most nursing home residents. Our results may have important implications in the design of public health policies to combat SARS-CoV-2 infection among this highly vulnerable population.
Transparency declaration
The authors declare no conflicts of interest. This work received no public or private funds.
Author contributions
E.A., J.S.B., S.P., D.S., H.V., E.G., R.L., M.J.A., J.S.-P. and J.D.: Methodology and data validation. J.S.B., S.P., and D.N.: Conceptualization and data analysis. D.N.: writing the original draft. All authors reviewed the original draft.
Acknowledgements
We are grateful to the Vice-presidency and Ministry of Equality and Inclusive Policies of the Valencia Community, the Corporate Association of Residences and Services for People with Dependency of the Valencian Community (AERTE), the Valencia Health System nursing home departmental committees, and the staff and residents of the participant nursing homes for their collaboration in developing the ProVaVac programme. We are also grateful to Ana Berenguer, General Director of Analysis and Public Policies of the Presidency of the Generalitat. Ignacio Torres (Río Hortega Contract; CM20/00090), Eliseo Albert (Juan Rodés Contract; JR20/00011) and Estela Giménez (Juan Rodés Contract, JR18/00053) hold contracts funded by the Health Institute Carlos III (co-financed by the European Regional Development Fund, ERDF/FEDER).
Members of the Valencian vaccine research programme (ProVaVac) study group: Burgos JS (General Directorate of Research and Healthcare Supervision, Department of Health, Valencia Government, Valencia, Spain); Meneu de Guillerna R (Vice-President Foundation Research Institute in Public Services, Valencia, Spain); Vanaclocha Luna H (General Directorate of Public Health, Department of Health, Valencia Government, Valencia, Spain); Burks DJ (The Prince Felipe Research Center-CIPF-, Valencia, Spain; Cervantes A (INCLIVA Health Research Institute, Valencia, Spain); Comas I (Biomedicine Institute of Valencia, Spanish Research Council (CSIC); Díez-Domingo J (Foundation for the promotion of health and biomedical research of the Valencian Community-FISABIO-, Valencia, Spain); Peiro S (Foundation for the promotion of health and biomedical research of the Valencian Community-FISABIO-, Valencia, Spain); González-Candelas F (CIBER in Epidemiology and Public Health, Spain; Joint Research Unit “Infection and Public Health” FISABIO-University of Valencia, Valencia, Spain; Institute for Integrative Systems Biology (I2SysBio), CSIC-University of Valencia, Valencia, Spain); Ferrer Albiach C (Fundación Hospital Provincial de Castelló); Hernández-Aguado I (University Miguel Hernández, Alicante, Spain); Oliver Ramírez N (DataPop Alliance); Sánchez-Payá J (Preventive Medicine Service, Alicante General and University Hospital, Alicante, Spain; Alicante Institute of Health and Biomedical Research (ISABIAL), Alicante, Spain; Vento Torres M (Instituto de Investigación Sanitaria La Fe); Zapater Latorre E (Fundación Hospital General Universitario de València); Navarro D (Microbiology Service, Clinic University Hospital, INCLIVA Health Research Institute, Valencia, Spain; Department of Microbiology, School of Medicine, University of Valencia, Valencia, Spain).
Editor: M. Cevik
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cmi.2021.09.031.
Contributor Information
Valencian Vaccine Research Programme (ProVaVac) Study Group:
J.S. Burgos, R. Meneu de Guillerna, H. Vanaclocha Luna, D.J. Burks, A. Cervantes, I. Comas, J. Díez-Domingo, S. Peiro, F. González-Candelas, C. Ferrer Albiach, I. Hernández-Aguado, N. Oliver Ramírez, J. Sánchez-Payá, M. Vento Torres, E. Zapater Latorre, and D. Navarro
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Soiza R.L., Scicluna C., Thomson E.C. Efficacy and safety of COVID-19 vaccines in older people. Age Ageing. 2021;50:279–283. doi: 10.1093/ageing/afaa274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pilishvili T., Fleming-Dutra K.E., Farrar J.L., Gierke R., Mohr N.M., Talan D.A., et al. Interim estimates of vaccine effectiveness of Pfizer-BioNTech and Moderna COVID-19 vaccines among health care personnel – 33 U.S. sites, January-March 2021. MMWR Morb Mortal Wkly Rep. 2021;70:753–758. doi: 10.15585/mmwr.mm7020e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.White E.M., Yang X., Blackman C., Feifer R.A., Gravenstein S., Mor V. Incident SARS-CoV-2 infection among mRNA-vaccinated and unvaccinated nursing home residents. N Engl J Med. 2021;385:474–476. doi: 10.1056/NEJMc2104849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brockman M.A., Mwimanzi F., Sang Y., Ng K., Agafitei O., Ennis S., et al. Weak humoral immune reactivity among residents of long-term care facilities following one dose of the BNT162b2 mRNA COVID-19 vaccine. medRxiv. 2021 doi: 10.1101/2021.03.17.21253773. [DOI] [Google Scholar]
- 5.Collier D.A., Ferreira I., Datir R., Datir R., Meng B., Bergamaschi L., et al. Age-related heterogeneity in neutralising antibody responses to SARS-CoV-2 following BNT162b2 vaccination. Nature. 2021;596:417–422. [Google Scholar]
- 6.Torres I., Albert E., Giménez E., Alcaraz M.J., Botija P., Amat P., et al. B and T cell immune responses elicited by 1 the BNT162b2 (Pfizer–BioNTech) COVID-19 vaccine in nursing home residents. Clin Microbiol Infect. 2021 doi: 10.1016/j.cmi.2021.06.013. S1198-743X(21)00332-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Walsh E.E., Frenck R.W., Jr., Falsey A.R., Kitchin N., Absalon J., Gurtman A., et al. Safety and immunogenicity of two RNA-based Covid-19 vaccine candidates. N Engl J Med. 2020;383:2439–2450. doi: 10.1056/NEJMoa2027906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.García-Sempere A., Orrico-Sánchez A., Muñoz-Quiles C., Hurtado I., Peiró S., Sanfélix-Gimeno G., et al. Data resource profile: the Valencia health System integrated database (VID) Int J Epidemiol. 2020;49:740–741e. doi: 10.1093/ije/dyz266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lagerqvist N., Maleki K.T., Verner-Carlsson J., Olausson M., Dillner J., Wigren Byström J., et al. Evaluation of 11 SARS-CoV-2 antibody tests by using samples from patients with defined IgG antibody titers. Sci Rep. 2021;11:7614. doi: 10.1038/s41598-021-87289-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Valdivia A., Torres I., Latorre V., Albert E., Gozalbo-Rovira R., Alcaraz M.J., et al. Suitability of two rapid lateral flow immunochromatographic assays for predicting SARS-CoV-2 neutralizing activity of sera. J Med Virol. 2021;93:2301–2306. doi: 10.1002/jmv.26697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mattiuzzo G., Bentley E.M., Hassall M. 2020. Establishment of the WHO International standard and reference panel for anti-SARS-CoV-2 antibody.https://www.who.int/publications/m/item/WHO-BS-2020.2403 WHO/BS/2020. [Google Scholar]
- 12.Giménez E., Albert E., Torres I., Remigia M.J., Alcaraz M.J., Galindo M.J., et al. SARS-CoV-2-reactive interferon-gamma-producing CD8+ T cells in patients hospitalized with coronavirus disease 2019. J Med Virol. 2021;93:375–382. doi: 10.1002/jmv.26213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Higgins V., Fabros A., Kulasingam V. Quantitative measurement of anti-SARS-CoV-2 antibodies: analytical and clinical evaluation. J Clin Microbiol. 2021;59 doi: 10.1128/JCM.03149-20. e03149–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Poljak M., Oštrbenk Valenčak A., Štamol T., Seme K. Head-to-head comparison of two rapid high-throughput automated electrochemiluminescence immunoassays targeting total antibodies to the SARS-CoV-2 nucleoprotein and spike protein receptor binding domain. J Clin Virol. 2021;137:104784. doi: 10.1016/j.jcv.2021.104784. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.