Abstract
The origin of SARS-CoV-2 is still the subject of a controversial debate. The natural origin theory is confronted to the laboratory leak theory. The latter is composite and comprises contradictory theories, one being the leak of a naturally occurring virus and the other the leak of a genetically engineered virus. The laboratory leak theory is essentially based on a publication by Rahalkar and Bahulikar in 2020 linking SARS-CoV-2 to the Mojiang mine incident in 2012 during which six miners fell sick and three died. We analyzed the clinical reports. The diagnosis is not that of COVID-19 or SARS. SARS-CoV-2 was not present in the Mojiang mine. We also bring arguments against the laboratory leak narrative.
Keywords: COVID-19, Origin of SARS-CoV-2, Mojiang mine, Clinical diagnosis
1. Main
The origin of SARS-CoV-2 is the subject of a strongly debated controversy between the natural origin and the laboratory accidental leak hypotheses. The latter covers two excluding hypotheses: the accidental release of a natural virus or that of an engineered virus. The laboratory accident theory was dismissed by the report of the WHO inspectors who deemed it “very unlikely” (WHO, 2020) but was recently reactivated following President Biden's call for a report from the US intelligence services on the virus origin. Nevertheless, these US intelligence reports could not bring either any evidence of a laboratory escape. Bloom and colleagues published in Science a call for another WHO-led investigation taking into account the laboratory accident theory (Bloom et al., 2021) arguing that not enough space was given to this hypothesis in the initial WHO report. The laboratory leak narrative is mostly based on arguments initially developed by Rahalkar and Bahulikar (2020) and relayed by others, very often by so-called “independent scientists” or journalists (Latham and Wilson, 2020; Speciale, 2021; Segreto and Deigin, 2021; Segreto et al., 2021; Sirotkin and Sirotkin, 2020; Relman, 2020). One specific narrative states that the Wuhan Institute of Virology (WIV) team led by Dr. Shi Zheng Li visited the Mojiang mine in 2012 following an accident involving six miners and that they collected SARS-CoV-2 from this mine. Rahalkar and Bahulikar, and followers, make a clear link between the Mojiang mine incident, WIV and SARS-CoV-2. Here, we show, based on the clinical reports, that the Mojiang miners did not developed COVID-19 or even SARS and were not infected by SARS-CoV-2. We thus dismiss the Mojiang mine as the origin of SARS-CoV-2. Dismissing the Mojiang mine theory leaves the laboratory leak narrative without any scientific support thus making it simply an opinion-based narrative.
The Mojiang mine incident. Six cases of severe pneumonia with 50% lethality which occurred in 2012 were described in a Master thesis from Kunming Medical University (Yunnan, China). In a retrospective analysis of the clinical and radiological data of this report, Rahalkar and Bahulikar found clues to the laboratory origin of SARS-CoV-2 in this event (Rahalkar and Bahulikar, 2020). We propose a more balanced interpretation of this case series and highlight major discrepancies with COVID-19 (Table 1 ). Clinically, the SARS-CoV-2 pneumonia typically associates dry cough with dry crackles, whereas productive cough, colored mucus and moist crackles or normal auscultation were mainly reported in the six cases. Hemoptysis occurred in three cases, whereas it is unusual in COVID-19 patients, including critical ones (Wei et al., 2020). In contrary to SARS-CoV-2 infected cases, none in the series had digestive signs and case 4 had mixed meningitis associated. On radiological examination, if some cases presented signs compatible with COVID-19, most of them developed CT-scan aspects which are not typical of COVID-19. Typical COVID-19 radiological features are CT patterns of acute viral pneumonia including bilateral peripheral ground-glass opacities, crazy paving, air space consolidation, vascular enlargement, and reticulations. Importantly, mediastinal lymphadenopathies that were found in all cases, and pleural effusions or thickening reported in 4/6 cases, are extremely rare in COVID-19 patients with 0.03 combined proportions reported by meta-analysis (Long et al., 2020; Yang et al., 2020). Radiological features of the two moderate cases 5 and 6 associated inflamed lymph nodes in mediastinum with chestnut shaped nodules in both lungs without any patterns of COVID-19. Cases 2 and 3 developed parenchymal emphysema with bulla, whereas SARS-CoV-2 infection outcome corresponds to fibrosis with traction bronchiectasis (Yang et al., 220). Certainly, thrombotic complications and secondary co-infections are evocative but they are not specific of severe SARS-CoV-2 infection. Moreover, the pulmonary embolism suspected in two cases was never formally confirmed with blood vessels imaging. Elevated D-dimer is associated with many illnesses and thus is not specific for vein thrombosis (Brill-Edwards and Lee, 1999). Notably, case 6 who displayed a favorable outcome without thrombotic event had a significant rise in D-dimer. A high level of serum amyloid A protein is not specific of a viral disease and an increase of up to 1000-fold can occur within the first 24–48 h of any acute inflammatory phase response. The C-reactive protein may remain low in fungal and toxic diseases. Treatments were wide-spectrum antibacterial, antiviral, antifungal, anticoagulant, and anti-inflammatory drugs and thus are not informative. All cases were administrated methylprednisolone. Apparently, no specific air-borne isolation or hygiene precautions, notably during invasive ventilation, were implemented in hospital. Similar diseases only occurred in co-exposed miners (no familial or nosocomial secondary cases were reported) which makes less likely the involvement of the highly contagious SARS-CoV-2. Cases 5 and 6 who presented the mildest illnesses, had the shortest time of exposure, which is in favor of a dose-dependent pathogenesis. In terms of professional exposure, other etiologies are compatible with these clinical and radiological patterns: viruses, histoplasmosis and mineral or chemical pneumonitis (https://www.pneumotox.com/). Although never confirmed, fungal infection was considered in the clinical reports by the experts from the Respiratory department of The First Affiliated Hospital at Sun Yat-Sen University who were involved in the clinical discussion. They hypothesized among other things, a “great possibility for fungi infection”. Moreover, incubation, clinical and radiological features of all cases, and more particularly of case 4, are compatible with hypersensitivity pneumonitis following environmental exposure to rapidly-growing non-tuberculous mycobacteria which may also be cured by corticosteroids (Ratnatunga et al., 2020). Furthermore, SARS-CoV serology was reported negative in case 3 clinical file. Four cases out of the six, including the three patients who died, have been tested for SARS-CoV and were negative (Zhou et al., 2020a). Since then, cases samples have also been tested for the presence of SARS-CoV-2 with negative results (Zhou et al., 2020a). Finally, another team of scientists from Beijing and Puer also explored the Mojiang mine and collected samples from bats, shrews and rats from which they found a novel henipavirus-like but no coronavirus (Wu et al., 2014; Stone, 2014).
Table 1.
Features reported in the analysis by Li Xu of six miners with severe pneumonia cases who presented at the first affiliated Hospital of Kunming in China in April–May 2012.
| Cases | Underlying diseases | Work in mine | Symptom's onset | Hospital admission | Clinical presentation | Thoracic CT-scan | Biological results | Outcome | Treatments |
|---|---|---|---|---|---|---|---|---|---|
| Case 1, male, 63 y | Suspicion of cancer but no confirmation | 02-16/04 | 16/04 | 26/04 (D10) | Dry cough, high grade fever, headache, dizziness, ear congestion, insomnia and loss of appetite, dry crackles | 25/04 Extensive and patchy consolidated exudate bilaterally, elevated bronchovascular shadows and lung markings, no pleural effusion, some nodules in different sizes, parts calcified, mediastinal lymph node enlargement,partially calcified. 30/04 Pleural thickening and pleural effusion in both lungs 06/05 Fluid in the left side of the pleural cavity to be evacuated. Pulmonary thromboembolism suspicion never confirmed |
25/04 CRP 20.3 mg/L, ferritin 484.86 27/04 sputum and blood cultures negative 06/05 D-dimer 7.2 μg/mL with rise in PCT and CPR 07/05 blood culture and sputum Acinetobacter baumannii Tumor Protein positive |
06/05 Severe ascites 07/05 (D21) Death |
Methylprednisolone Meronem Vancomycin Voriconazole Acyclovir Pleural draining |
| Case 2, male, 42 + 5 colleagues | Chronic hepatitis B | 02 to 16/04 | 11/04 | 25/04 (D14) | Fever, dyspnea, rusty-colored mucus with blood clots, hypotension 98/55, moist crackles | 25/04 bilateral pneumonia, limited emphysema in bottom of left lung and bulla in right lung 30/04 Mediastinal lymph nodes enlargement 29/05 Interstitial opacities and exudation in both lungs, pericardial effusion, multiples shadows of nodules spread across 23/05 Mediastinal lymph nodes inflamed, chestnut shaped nodules in both lungs 05/06 Deep vein thrombosis at the right side of the first rib |
26/04 CRP 117 mg/L and decreasing CRP with time despite worsening (23/05 CRP 23.5 mg/L) 26/04 BNP 33.44 pg/mL 02/05 D-dimer 4.4 μg/mL (the highest rate) Positive HBsAg and HbcAb and PCR EBV |
12/06 (D63) Death | Transfusion Methylprednisolone Ganciclovir Meropenem Voriconazole Sulfamethoxazole |
| Case 3, male, 45 y | Bowel obstruction surgery 1985 | 02 to 16/04 | 13/04 | 27/04 (D14) | Productive cough with yellow and greenish mucus, blood, fever, shortness of breath, headache, soreness in limbs, cyanosis, slightly moist crackles in lower right lung, no dry crackle from either lung |
25/04 Septal thickening, multiples nodules and floccular exudate, multiple inflamed lymph nodes in mediastinum 26/05 Partial pulmonary emphysema 07/06 fluid in the left and right side, small mediastinal lymph nodes 18/06 Interstitial fibrosis, with emphysema 08/07 multiple mediastinal lymph nodesinflamed, web-like shadow |
Complete blood test SARS-CoV negative 03/07 Gram + and gram – bacteria in sputum: Acinetobacter baumanii, Stenotrophomonas 11/08 Acinetobacter baumanii in blood |
02/06 Noninvasive ventilation 08/08 Invasive ventilation 13/08 (D120) Death |
Methylprednisolone Cefixime Piperacillin/tazobactam Vancomycin Cefoperazon/tazobactam Meropenem Levofloxacin Fosfomycin Tygecycline Caspofungin Fluconazole Micafungin Ganciclovir Oseltamivir Thymosin |
| Case 4, male, 46 y+ 5 colleagues | None | 02-16/04 | 16/04 | 26/04 (D10) | Productive cough and hemoptysis, photophobia, rough sound from lungs, moist crackles, cyanosis, Babinski on both sides |
29/04 multiple patchy opacity and exudative consolidation, pleural effusion in both lungs 03/05 Increase in lung marking with thickening 07/05 Possible bilateral pulmonary embolism 18/05 Right consolidation exudation 29/05 Sub-pleural aerial cavity 12/06 Right pleurisy to be evacuated (28/06 3.1 cm) 06/07 Multiple big inflamed lymph nodules in mediastinum 18/08 Air bronchogram in the large consolidation exudation in the right lung, multiple lymph nodes in mediastinum |
HIV and hepatitis virus tests negative PCR HSV, CMV, HPV negative 26/04 D-dimer 8.9 μg/mL 17/05 PCT 24.05 ng/mL 28/06: Lymphocytic exudative pleural effusion with giant cells (86%) and adenosine deaminase 16.8 U/L 29/06 and 02/07 CSF: neutrophils and then mixed cell reaction 18/05 Acinetobacter baumanii in mucus 28/05 Acinetobacter baumanii + E coli in mucus 02/07 Klebsiella pneumoniae in blood 12/08 CRP 90 mg/L |
29/05 Tracheotomy 06/07 Extubating 10/09 (D144) Recovery |
Methylprednisolone Prednisone Aciclovir Ganciclovir Voriconazole Itraconazole Fluconazole Caspofungin Moxifloxacin Meropenem Linezolid Cefoperazone sulbactam Piperacillin tazobactam Levofloxacin Heparin Warfarin Haloperidol Thymosin 27/06 Pleural draining |
| Case 5, male, 30 y | None | 22-26/04 | 27/04 | 02/05 (D5) | Cough with white slimy mucus, fever, chest tightness, shortness of breath, headache, soreness in limbs, sweating, dizziness, loss of strength, paroxysmal dyspnea at night and edema, little moist crackles sound |
28/04 Bilateral multiple chestnut shaped nodules, multiple inflamed big lymph nodes in mediastinum 13/05 Decrease in nodules and lymph nodes |
02/05 CRP 21.3 mg/L 07/05 PCT 0.75 mg/L 09/05 SAA 44.10 ng/L |
28/05 (D30) Recovery | Sulbencillin Fluconazole Methylprednisolone Prednisolone Thymosin |
| Case 6, male, 32 y | Inhalation of much irritating gas | 22-26/04 | 22/04 | 26/04 | Cough with white and slimy mucus, fever, difficulty in breathing, rough sound without crackle in lungs |
26/04 Bilateral multiple chestnut shaped nodules, increase in lung marking with thickening, multiple inflamed big lymph nodes in mediastinum 29/04 Thickening on the left back side of the pulmonary pleurae 07/05 Local emphysema and bullae |
27/04 CRP 34.2 mg/L, SAA 79 ng/L 18/05 D-Dimer 3.9 μg/mL, PCT 0.04 ng/mL, SAA 230 ng/L |
28/05 (D35) Recovery | Ganciclovir Piperacillline tazobactam Methylprednisolone |
Data inconsistent with COVID-19 are shown in italic bold. Dates are DD/MM in 2012. Abbreviations: D = delay in days after onset of symptoms; y = age in years; CMV = cytomegalovirus; CRP = C-reactive protein, CSF = cerebrospinal fluid; CT = computed tomography; EBV = Epstein-Barr virus; PCT = procalcitonin; SAA = serum amyloid A.
Infection of Wuhan Institute of Virology (WIV) staff members in the Mojiang mine. Another narrative about the origin of COVID-19 is that staff members of WIV were infected by SARS-CoV-2 while visiting the Mojiang mine. In addition to the results described above, one must consider that SARS-CoV-2 was never found in this mine (Wu et al., 2014; Ge et al., 2016) and that WIV staff members have been tested for SARS-CoV-2 and were reported negative (Cohen, 2020). One must also wonder why a virus which killed more than 4 million and infected more than 200 million in 18 months did not cause any illness in 7 years from 2012 to 2019. The WIV team was not the only one to have visited the Mojiang mine and considering the high transmissibility of SARS-CoV-2 it is highly surprising that no cases were recorded at that time.
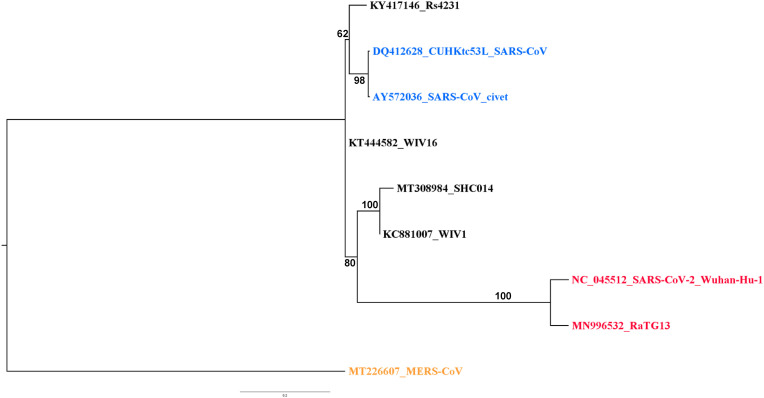
Engineering of SARS-CoV-2 from RaTG13. RaTG13 is not a virus but only a sequence generated by metagenomics (Ge et al., 2016; Zhou et al., 2020b). Therefore, there is no evidence that this sequence corresponds to any real and viable virus or even that all reads are coming from the same virus. The RaTG13 sequence might also be a chimera with fragments coming from different viruses. RaTG13 has never been isolated as a virus and replicated in cell cultures. It has no physical existence and thus cannot leak from a laboratory. Furthermore, considering the very high number of mutations separating RaTG13 from SARS-CoV-2 and their phylogenetic distance, RaTG13 can hardly be considered a progenitor of SARS-CoV-2 even if it corresponded to a real virus. The suggested engineering of SARS-CoV-2 for gain of function through in vitro synthesis from the RaTG13 sequence is a narrative making no sense from an operational standpoint. Engineering a complete virus is beyond current technical possibilities. There are too many subtle sequences, interactions and functions to master that we still do not understand. Building de novo a fully viable virus is far more complicated than expressing a single gene and is still not possible. It is possible to make a synthetic sequence from a well-known and validated sequence coming for an isolated and cultivated virus but this is not the case of RaTG13. There is no solid ground for trying to engineer a virus after the RaTG13 sequence and why should a laboratory invest all resources to engineer de novo a virus for which there is no evidence that it is viable and can be cultivated, a mandatory condition for gain of function experiments. The furin activation site in SARS-CoV-2 has also been mentioned as a proof of genetic engineering. However, furin activation sites are naturally occurring in different viruses, including coronaviruses, and thus cannot be a proof of genetic engineering (Frutos et al., 2021). WIV has conducted gain-of-function experiments but it was in the framework of an official and publicly available NIH grant (https://www.documentcloud.org/documents/21055989-understanding-risk-bat-coronavirus-emergence-grant-notice). Results were published in peer-reviewed journals (Menachery et al., 2015). The experiments conducted within the framework of this NIH grant involved several bat SARS-CoV-like viruses, i.e. WV1, WV16, SHC014 and Rs4231. The objective was to assess potential changes in pathogenicity to ACE2-humanized mice when swapping the spike protein on a WV1 background. All spike proteins tested were genetically distant from those from SARS-CoV-2 and RaTG13 (Fig. 1). The swapping of spike proteins only led to slight variations. The maximum effect, a 20% weight loss, was observed with the spike protein of SHC014. Other recombinants did not yield measurable effects. This work showed that the consequences of gain-of-function experiments on SARS-CoV-like viruses were extremely limited and certainly not to the magnitude of an epidemic as imagined by tenants of a laboratory accident. Furthermore, these experiments were conducted on viruses phylogenetically distant from SARS-CoV-2 and RaTG13 and no gain-of-function experiment was done on either SARS-CoV-2 or RaTG13 (Cohen, 2020). Not only the engineering of SARS-CoV-2 is merely a narrative but technical evidence indicate that no such engineering could generate a pandemic virus. There is today no evidence and no rationale to support this laboratory engineering narrative.
Fig. 1.
Phylogenetic analysis of the spike genes used for genetic engineering. The genes used for genetic engineering are those described in the NIH Grant 1RO1 Al 110,964–01 (https://www.documentcloud.org/documents/21055989-understanding-risk-bat-coronavirus-emergence-grant-notice), i.e. WV1, WV16, SHC014 and Rs4231. The spike protein gene of MERS-CoV was used as outgroup to root the tree. Sequences were aligned using MUSCLE in the SeaView package (Gouy et al., 2010). The phylogenetic tree was built using the maximum likelihood method with GTR-G-I evolutionary model and 500 bootstrap repeats.
As a conclusion, there is no evidence to support the Mojiang mine origin of SARS-CoV-2 and any of the laboratory leak theories. These are narratives expressing differing and also contradictory opinions. If the virus is engineered, it cannot be the accidental leak of a natural virus and vice-versa. These narratives are not evidence-based scientific conclusions. They are also built on the weaknesses of the “Spillover” theory and the absence of reservoirs and intermediaries in the wild. In a time of geopolitical conflicts characterized by hidden agendas, false information and manipulations, it is essential to rely only on scientific and evidence-based conclusions and to avoid opinion-based narratives.
Ethic
This is a retrospective analysis of previously published data. No primary clinical data were used.
Conflicts of interest
During the period 2005–2010, CD was the representative of the French Ministry of Research assisting the Chinese Ministry of Health for the building of the P4 laboratory facility in Wuhan.
The opinions expressed in this article are those of the authors and not those of the Ministry of Armies. The authors declare that there is no commercial conflict of interest.
Authors contribution
All authors participated to the design and writing of the article. EJ did the clinical diagnosis. CB did the radiographic analysis.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Bloom J.D., Chan Y.A., Baric R.S., Bjorkman P.J., Cobey S., Deverman B.E., et al. Investigate the origin of COVID-19. Science. 2021;372:694. doi: 10.1126/science.abj0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brill-Edwards P., Lee A. D-dimer testing in the diagnosis of acute venous thromboembolism. Thromb. Haemostasis. 1999;82:688–694. [PubMed] [Google Scholar]
- Cohen J. Wuhan coronavirus hunter Shi Zhengli speaks out. Science. 2020;369:487–488. doi: 10.1126/science.369.6503.487. [DOI] [PubMed] [Google Scholar]
- Frutos R., Gavotte L., Devaux C.A. Understanding the origin of COVID-19 requires to change the paradigm on zoonotic emergence from the spillover model to the circulation model. Infect. Genet. Evol. 2021:104812. doi: 10.1016/j.meegid.2021.104812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge X.Y., Wang N., Zhang W., Hu B., Li B., Zhang Y.Z., et al. Coexistence of multiple coronaviruses in several bat colonies in an abandoned mineshaft. Virol. Sin. 2016;31:31–40. doi: 10.1007/s12250-016-3713-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouy M., Guindon S., Gascuel O. SeaView version 4: a multiplatform graphical user interface for sequence alignment and phylogenetic tree building. Mol. Biol. Evol. 2010;27:221–224. doi: 10.1093/molbev/msp259. [DOI] [PubMed] [Google Scholar]
- Latham J., Wilson A. A proposed origin for SARS-CoV-2 and the COVID-19 pandemic. Independent Science News. 2020;15 [Google Scholar]
- Long C.-J., Fang P., Song T.J., Zhang J.C., Yang Q. Imaging features of the initial chest thin-section CT scans from 110 patients after admission with suspected or confirmed diagnosis of COVID-19. BMC Med. Imag. 2020;20:64. doi: 10.1186/s12880-020-00464-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menachery V.D., Yount B.L., Debbink K., Agnihothram S., Gralinski L.E., Plante J.A., et al. A SARS-like cluster of circulating bat coronaviruses shows potential for human emergence. Nat. Med. 2015;21:1508–1513. doi: 10.1038/nm.3985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahalkar M.C., Bahulikar R.A. Lethal pneumonia cases in Mojiang miners (2012) and the mineshaft could provide important clues to the origin of SARS-CoV-2. Frontiers in Public Health. 2020;8:638. doi: 10.3389/fpubh.2020.581569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratnatunga C.N., Lutzky V.P., Kupz A., Doolan D.L., Reid D.W., Field M., et al. The rise of non-tuberculosis mycobacterial lung disease. Front. Immunol. 2020;11:303. doi: 10.3389/fimmu.2020.00303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Relman D.A. Opinion: to stop the next pandemic, we need to unravel the origins of COVID-19. Proc. Natl. Acad. Sci. Unit. States Am. 2020;117:29246–29248. doi: 10.1073/pnas.2021133117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segreto R., Deigin Y. The genetic structure of SARS‐CoV‐2 does not rule out a laboratory origin: SARS‐COV‐2 chimeric structure and furin cleavage site might be the result of genetic manipulation. Bioessays. 2021;43:2000240. doi: 10.1002/bies.202000240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segreto R., Deigin Y., McCairn K., Sousa A., Sirotkin D., Sirotkin K., et al. Should we discount the laboratory origin of COVID-19? Environ. Chem. Lett. 2021;19:2743–2757. doi: 10.1007/s10311-021-01211-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirotkin K., Sirotkin D. Might SARS‐CoV‐2 have arisen via serial passage through an animal host or cell culture? A potential explanation for much of the novel coronavirus' distinctive genome. Bioessays. 2020;42:2000091. doi: 10.1002/bies.202000091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speciale A.C. Commentary: lethal pneumonia cases in Mojiang miners (2012) and the mineshaft could provide important clues to the origin of SARS-CoV-2. Frontiers in Public Health. 2021:869. doi: 10.3389/fpubh.2021.702199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone A. 2014. A New Killer Virus in China? Science.https://www.sciencemag.org/news/2014/03/new-killer-virus-china Available online at: [Google Scholar]
- Wei Y., Zeng W., Huang X., Li J., Qiu X., Li H., et al. Clinical characteristics of 276 hospitalized patients with coronavirus disease 2019 in Zengdu District, Hubei Province: a single-center descriptive study. BMC Infect. Dis. 2020;20:549. doi: 10.1186/s12879-020-05252-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO 2020. https://www.who.int/docs/default-source/coronaviruse/who-china-joint-mission-on-covid-19-final-report.pdf
- Wu Z., Yang L., Yang F., Ren X., Jiang J., Dong J., et al. Novel henipa-like virus, Mojiang paramyxovirus, in rats, China, 2012. Emerg. Infect. Dis. 2014;20:1064–1066. doi: 10.3201/eid2006.131022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H., Lan Y., Yao X., Lin S., Xie B. The chest CT features of coronavirus disease 2019 (COVID-19) in China: a meta-analysis of 19 retrospective studies. Virol. J. 2020;17:159. doi: 10.1186/s12985-020-01432-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou P., Yang X.L., Wang X.G., Hu B., Zhang L., Zhang W., et al. Addendum: a pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;588:E6. doi: 10.1038/s41586-020-2951-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou P., Yang X.L., Wang X.G., Hu B., Zhang L., Zhang W., et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]