Description
A 33-year-old second para had an uneventful vaginal delivery of a female newborn (3240 g) in the 39th week of gestation. Oxytocin was given and 30 min after delivery, cord traction was performed to remove the placenta. The inverted uterus with the adherent placenta prolapsed (Fig. 1). The woman was immediately transferred into the operation room and manual replacement was attempted in general anaesthesia. Placenta could be separated, but laparotomy was necessary to correct the uterine fundus that was still inverted (Fig. 2). Only the tubal fimbriae were visible (Fig. 2a). The complete repositioning of the fundus was succeeded by manual pressure with the hand from vaginally. An iatrogenic lesion was repaired with two sutures (Fig. 3).
Fig. 1.
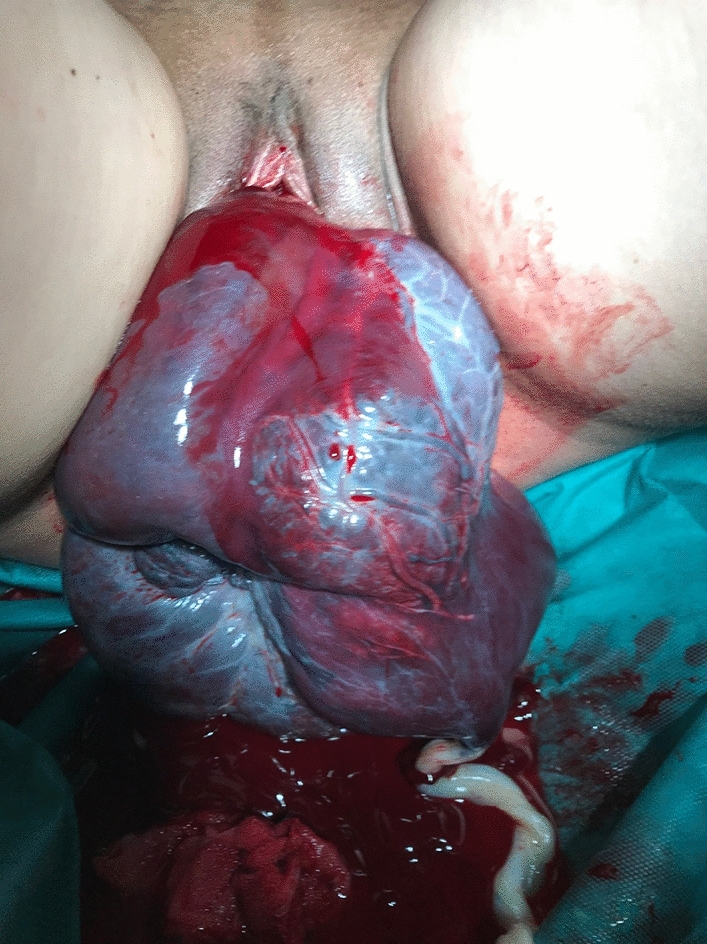
Inverted uterus with adherent placenta
Fig. 2.
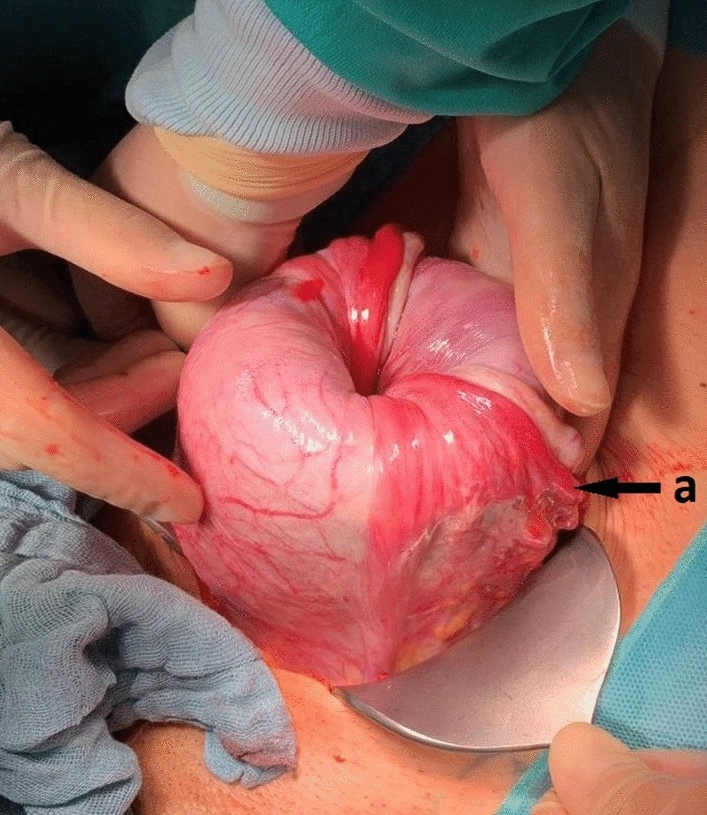
Repositioned uterus with still inverted fundus and visible fimbriae of the tube (a)
Fig. 3.
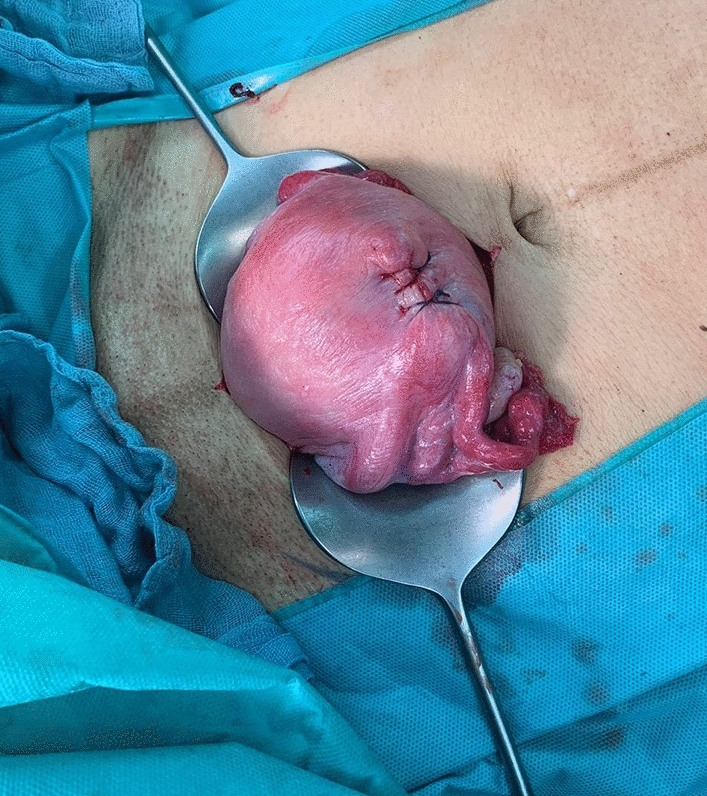
Normal configured uterus after complete repositioning and suturing
Postpartum haemorrhage with a total blood loss of 3000 millilitres was treated with oxytocin, prostaglandins, fluid and blood transfusion. One day after delivery, the uterus showed a normal configuration and was contracted well.
Author contributions
ADS: planned and wrote the article. BA: participated in treatment and follow-up of the patient. LW: participated in treatment and follow-up of the patient. HS: planned and reviewed the article, participated in treatment and follow-up of the patient.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Availability of data and materials
Not applicable.
Code availability
Not applicable.
Declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval and consent to participate
This was not a study and, therefore, no IRB approval was necessary. Patient written informed consent for the scientific use of her anonymized data was obtained as an institutional standard procedure. All procedures were in accordance with the Ethical Standards of the Responsible Committee on Human Experimentation (Institutional and National) and with the Helsinki Declaration of 1975 (in its most recently amended version).
Consent for publication
This article has the patient’s approval for imaging and publication.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.
Not applicable.


