Abstract
Objective:
Examine the association of implementing a revised analgesia-sedation protocol with midazolam usage in the pediatric intensive care unit (PICU).
Design:
A single center non-randomized before-after study.
Setting:
Pediatric intensive care unit at a quaternary pediatric hospital (Boston Children’s Hospital, Boston, MA).
Patients:
Children admitted to the PICU who were mechanically ventilated for greater than 24 hours. The pre-implementation cohort included 190 eligible patients admitted between July 29, 2017 – February 28, 2018, and the post-implementation cohort included 144 patients admitted between July 29, 2019 – February 28, 2020.
Interventions:
Implementation of a revised analgesia-sedation protocol.
Measurements and main results:
Our primary outcome, total dose of intravenous midazolam administered in mechanically ventilated patients up to day 14 of ventilation, decreased by 72% (95% confidence interval (CI) [61%, 80%], p-value<0.001) in the post-implementation cohort. Dexmedetomidine usage increased 230% (95% CI [145%, 344%]) in the post-implementation cohort. Opioid usage, our balancing metric, was not significantly different between the two cohorts. There were no significant differences in ventilator-free days, PICU length of stay, rate of unplanned extubations, failed extubations, cardiorespiratory arrest events, and 24-hour readmissions to the PICU.
Conclusions:
We successfully implemented an analgesia-sedation protocol that primarily uses dexmedetomidine and intermittent opioids, and it was associated with significant decrease in overall midazolam usage in mechanically ventilated patients in the PICU. The intervention was not associated with changes in opioid usage or prevalence of adverse events.
Keywords: sedation, midazolam, dexmedetomidine, pediatric intensive care, protocol, implementation
Introduction
Optimal analgesia and sedation strategies allow safe delivery of invasive therapies, such as mechanical ventilation. The goal is to effectively control pain and anxiety while avoiding unintended side effects of the drugs, such as delirium and increased ventilator dependence. For several decades, pediatric intensive care units (PICUs) have used continuous midazolam infusions to sedate mechanically ventilated patients.(1, 2) However, recent reports linking benzodiazepine exposure to increased risk of delirium have prompted a shift towards intermittent dosing rather than continuous benzodiazepine infusions, as well as the use of non-benzodiazepine agents in critically ill children.(3, 4) New sedation protocols focused on benzodiazepine-sparing strategies are now applied in the PICU and cardiac intensive care unit (CICU) settings in an effort to reduce overall benzodiazepine usage.(5, 6) Implementation of a nurse-led sedation protocol demonstrate decreased total days of benzodiazepine and opioid administration.(7) Furthermore, the impetus to reduce benzodiazepine usage in the pediatric population is also related to animal studies where benzodiazepines were linked to neurodegeneration in the developing rat and infant mouse brains. (8, 9) Additionally, prioritizing intermittent narcotic and sedative dosing over usage of continuous infusions in the pediatric CICU population was associated with decreased length of stay and similarly effective pain management.(3)
Decreased use of midazolam is met with resistance by clinicians due to concerns for ineffective sedation, lack of alternative sedative agents or their anticipated side effects.(5) Other considerations when shifting from midazolam infusions to alternative strategies include unintended increase in opioid usage or increase in rates of unplanned extubations. Importantly, sedation and analgesia are ubiquitous therapies in the PICU setting, and changes in practice require attention to the unit’s culture and attitudes.
We implemented a revised analgesia-sedation protocol and examined its association with midazolam usage in children receiving mechanical ventilation in a multidisciplinary PICU. Our goal was to implement a protocol that prioritizes dexmedetomidine infusion for sedation along with intermittent opioid dosing for analgesia. Since our primary goal with this intervention was to reduce midazolam usage, we assessed the success of this intervention by comparing the amount of midazolam administered in the post-intervention period versus the pre-intervention period as our main process measure. Our balancing metric was opioid usage, with the hypothesis that if the new protocol was not effective in achieving the desired analgesia and sedation, it might lead to unintended increase in the usage of opioids in the post-intervention period. Our outcome measures were change in ICU length of stay (LOS) and ventilator-free days up to 28 days (VFD28). (12) Our secondary outcome metrics were adverse events, including unplanned extubations, cardiac/respiratory arrest, 24-hour readmissions to the ICU, and clinically significant bradycardia or hypotension associated with dexmedetomidine use with the new protocol. We hypothesized that the protocol would be associated with a significant reduction in midazolam usage without a simultaneous unintended increase in opioid use or adverse events.
Materials and Methods
We conducted a single center non-randomized before-after study. The intervention consisted of development and implementation of a revised analgesia-sedation protocol. Data from each time period was collected retrospectively by chart review.
Protocol development
For over two decades, the PICU at Boston Children’s Hospital utilized a sedation-analgesia strategy in mechanically ventilated children that was based on midazolam and opioid infusions as the standard approach. Due to increasing concerns for midazolam’s role in contributing to PICU delirium as well as potential impact on neurocognitive development in the pediatric population, the PICU leadership prioritized this intervention to reduce reliance on midazolam infusions. We commissioned an interdisciplinary task force consisting of members of the PICU Quality Improvement (QI) Committee in 2017 with the objective of revising the existing sedation-analgesia protocol based on available evidence and best practice recommendations in the literature. The task force completed a systematic review of the literature and outlined the following principles for the new protocol: a) emphasize minimal effective dosing required to achieve optimal analgesia and sedation; b) re-invigorate the use of the State Behavioral Scale (SBS) scoring and age-appropriate pain assessment to guide titration of medications; c) discourage the use of midazolam infusions and instead prioritize dexmedetomidine for sedation and intermittent opioid for analgesia; and d) encourage intermittent medication dosing over infusions for other medications. After an iterative process over 5 months, the final protocol was developed and tested for usability. We incorporated feedback from a pilot testing phase and then finalized the protocol. The committee educated physician and nursing staff using virtual learning modules with a mandatory quiz on the contents of the revised protocol with a mandatory minimum score required for successful module completion. We disseminated the protocol using visual reminders, interactive discussions at multiple venues, dedicated conferences, and bedside one-on-one teaching. The final protocol was rolled out on July 15, 2019. After a 2 -week wash-in period, we began the post-implementation period on July 29, 2019.
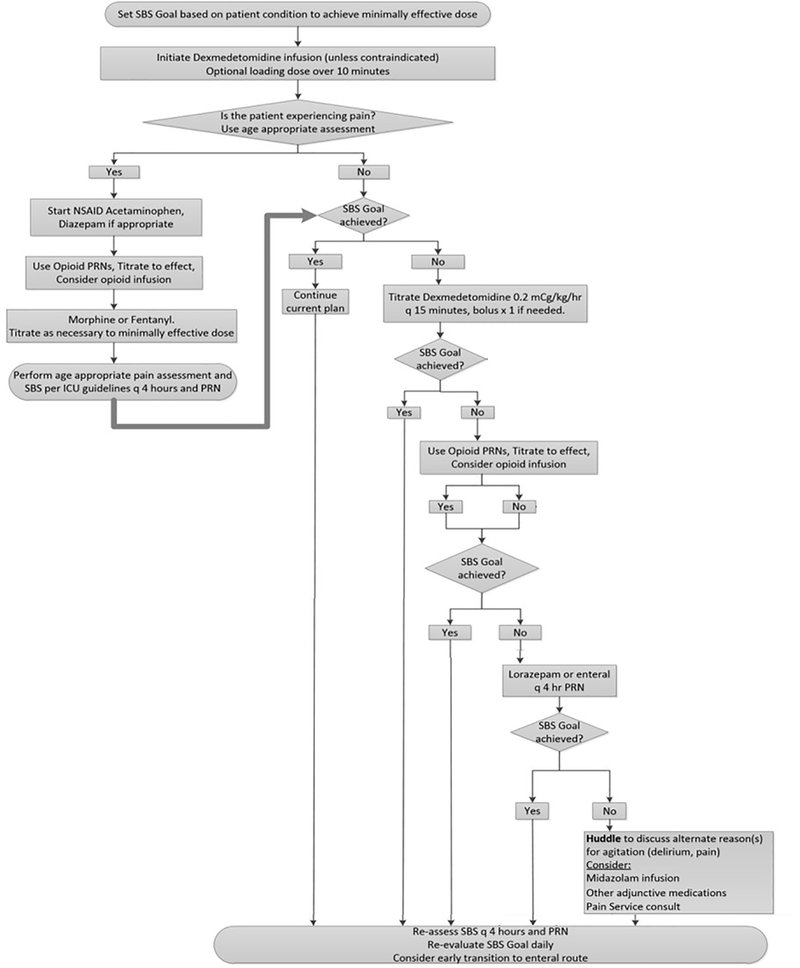
Figure 1 shows the analgesia-sedation protocol. The protocol is primarily driven by the SBS goal determined by the multidisciplinary team during daily rounds and the scoring is documented by the bedside nurse.(10) It begins with initiation of dexmedetomidine infusion for sedation with guidance for up-titration of the rate, as necessary, to reach goal SBS. The protocol emphasizes non-opioid analgesic strategies, encourages usage of intermittent opioids as necessary for analgesia, and suggests non-pharmacologic interventions for comfort. Age-appropriate pain assessments guide effective titration of analgesic medications. Opioid infusion may be initiated after maximizing dexmedetomidine infusion and intermittent opioid dosing. Benzodiazepine infusions are not recommended in the sedation protocol until intermittent medications and uptitration of other infusions are deemed inadequate. The protocol allows for deviation at any step based on clinician judgment. Since patients who are receiving neuromuscular blockade are not appropriate for SBS scoring, when chemical paralysis is used, the protocol recommends the clinical team to “assume pain present” (APP) and “assume agitation present” (AAP) and use clinical judgement to titrate infusions per the protocol.
Figure 1.
Flowchart of the analgesia-sedation protocol that was implemented as the intervention.
Data collection
This research project was approved by the Boston Children’s Hospital institutional review board (IRB) under protocol number P00032707, and informed consent was waived. Patients admitted to the PICU at Boston Children’s Hospital from July 29, 2017 through February 28, 2018 (pre-intervention cohort) and from July 29, 2019 through February 28, 2020 (post-intervention cohort) were eligible if they were mechanically ventilated for at least 24 hours. Exclusion criteria included documented allergy to dexmedetomidine, initiation of midazolam infusion prior to admission, rapid escalation of midazolam infusion for refractory status epilepticus, and presence of a tracheostomy. We included patients receiving venovenous or venoarterial extracorporeal membrane oxygenation (ECMO). A single investigator reviewed electronic medical records for collection of demographic data and clinical data, including age, weight upon admission, dates of admission and discharge from the hospital and PICU, and primary reason for admission. We obtained the Pediatric Index of Mortality 3 (PIM3) score from routinely collected quality improvement and administrative data. We also extracted administered medication, SBS score, pain score, and delirium score data from the institution’s data warehouse. We calculated midazolam and dexmedetomidine exposure as cumulative dose, including both intermittent boluses and continuous infusions, over the PICU stay up to 14 days. For opioid usage, all opioids were converted to morphine equivalents (assuming a 25% cross tolerance), then calculated as the cumulative dose analogously to the above.(9) Each patient’s cumulative dose was indexed to the patient’s admission weight and to the number of calendar days the patient was exposed to the medication (starting from the time mechanical ventilation was initiated). We defined clinically significant hypotension/bradycardia from dexmedetomidine as events that required atropine or initiation of a vasoactive infusion. After initiating dexmedetomidine, midazolam infusion was permitted as part of the protocol escalation outlined in Figure 1 in order to achieve the SBS goal.
Data analysis
Characteristics of the study population are summarized using descriptive statistics. Continuous variables are summarized by median (interquartile range) and categorical variables are summarized as frequency and percentage. For demographic data, we used the Mann-Whitney U test to compare continuous variables. We used Fisher’s exact test to compare proportions, and the Chi-square test of independence to compare categorical variables.
Our primary process variable was IV midazolam dose expressed in mg/kg/ICU day. We recorded the clinical outcome variables; PICU length of stay (LOS) and ventilator-free days up to 28 days (VFD28).(12) In addition, we recorded the following secondary variables: a) total opioid dose (mg/kg/day) of IV morphine equivalents; b) IV dexmedetomidine dose (mcg/kg/day); c) duration of infusions for all the aforementioned medications; and d) prevalence of the following adverse events: unplanned extubations, failed extubations (defined as re-intubation within 24 hours of prior extubation), cardiorespiratory arrest events, 24-hour readmissions to the PICU, and clinically significant bradycardia or hypotension (defined as the need for atropine, vasoactive medication or fluid bolus) associated with dexmedetomidine use.
The distribution of midazolam dosage (mg/kg/day), the primary process outcome, was positive continuous with exact zeros. Therefore, the primary outcome was modeled and analyzed using Tweedie regression with log link function and intervention group assignment (post-intervention) as the primary independent variable. Age, PIM3 score, and reason for admission, were the covariates in the model. We included analgesia-sedation dosing only up to 14 days because our protocol focused on initiation and initial titration of these medications. For secondary outcomes related to opioid and dexmedetomidine dose, we used Tweedie regression with the same covariates for analysis. Other secondary continuous outcomes (ICU LOS, VFD28) were analyzed using Mann–Whitney U test, and secondary proportion outcomes (adverse events as defined above) were analyzed using Fisher’s exact test. A p-value less than 0.05 was considered statistically significant. All data analysis and statistical tests were performed using R version 3.6.1 (R Foundation for Statistical Computing, Vienna, Austria).
We defined compliance with the protocol as the proportion of eligible patients in the post-intervention who were not initiated on a midazolam infusion as the first sedative infusion. Furthermore, since the protocol prioritizes starting dexmedetomidine before midazolam, we also analyzed compliance by examining the median (IQR) times from starting dexmedetomidine to starting midazolam infusions in the pre- and the post-intervention groups, for patients that received both infusions (Supplemental Figure 2).
Results
Based on the predefined inclusion and exclusion criteria, 190 cases in the pre-intervention group and 144 cases in the post-intervention group were eligible and were included in the study (Supplemental Figure 1). Demographic and clinical characteristics were similar between the two groups, although rate of tracheostomy placement during PICU stay was higher in the pre-intervention group (Table 1). The proportion of patients meeting study criteria in the pre-intervention group was lower than in the post-intervention group (8% vs. 11%, respectively). Approximately 10% of all PICU admissions and 49% and 50% of all mechanically ventilated patients met criteria for this study in the pre- and the post-intervention groups, respectively, and were included in the analyses. Compliance with the protocol was 94.4% in the post-intervention phase (Supplemental Table 2). We compared the time difference between the initiation of the two infusions by setting dexmedetomidine initiation as time 0. The median (IQR) times from starting dexmedetomidine to starting midazolam infusions were −21.35 hours (−73.81, −2.8) and 15.6 hours (−5.0, 78.1) in the pre- and post-intervention groups, respectively. This indicates compliance with the protocol by demonstrating that patients in the post-intervention period were initiated on dexmedetomidine prior to midazolam infusions. For both groups, in all patients in whom both infusions were used, dexmedetomidine was started first followed by addition of midazolam as an adjuvant and not a replacement.
Table 1.
Demographic and clinical data for patients in the pre-intervention and post-intervention groups. Data expressed as median (Q1, Q3) or n (%).
| Variable | Pre-intervention group (N=190) | Post-intervention group (N=144) |
|---|---|---|
| Age (years) | 3.9 (0.7, 10.6) | 4.1 (1.0, 12.6) |
| Weight (kg) | 13.9 (7.7, 31.2) | 16.2 (8.8, 36.2) |
| Sex (Female) | 75 (39.5) | 57 (39.6) |
| PIM 3 | −4.5 (−5.3, −3.3) | −4.4 (−5.3, −3.2) |
| Diagnostic category | ||
| Respiratory | 67 (35.3) | 56 (38.9) |
| Neurologic | 33 (17.4) | 19 (13.2) |
| Sepsis/shock | 19 (10) | 10 (6.9) |
| Elective surgical procedure | 64 (33.7) | 51 (35.4) |
| Others | 7 (3.7) | 8 (5.6) |
| Mortality | 12 (8.3) | 8 (5.6) |
| Tracheostomy during PICU stay | 15 (7.9) | 2 (1.4) |
| ECMO therapy | ||
| VV | 10 (5.3) | 2 (1.4) |
| VA | 1 (0.5) | 1 (0.7) |
PIM3 = pediatric index of mortality 3. ECMO = extracorporeal membrane oxygenation. VV = venovenous, VA = venoarterial.
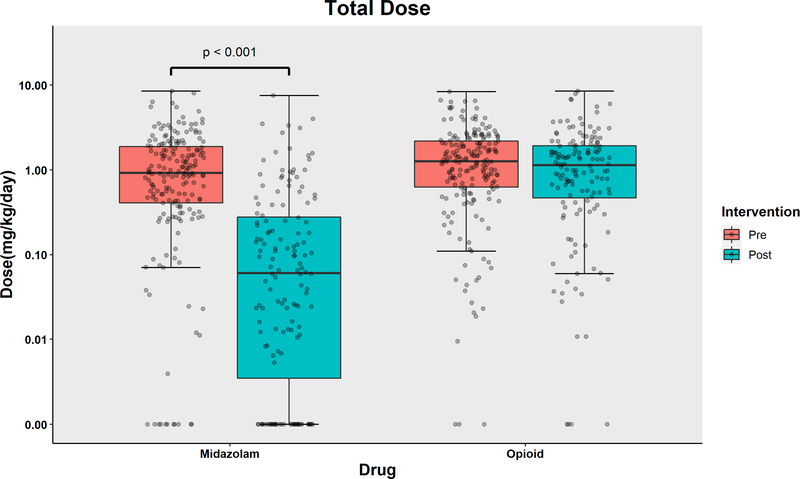
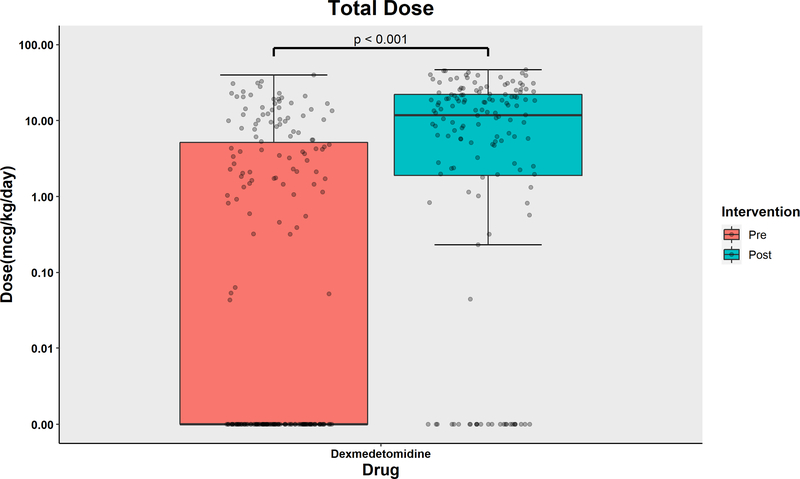
The distribution of the dose exposure of midazolam, opioids, and dexmedetomidine are displayed in Figure 2a and 2b, respectively. The data expressed in cumulative dose (mg over entire ICU stay) are shown in the Supplemental Figure 3. The median (IQR) dose exposure of midazolam (mg/kg/day) decreased from 0.92 (0.41, 1.89) in the pre-intervention group to 0.06 (0.00, 0.28) in the post-intervention group. Dexmedetomidine dose exposure (mcg/kg/day) increased from 0.00 (0.00, 5.17) to 11.76 (1.90, 22.12). Dexmedetomidine dose exposure increased significantly in the post-intervention period with an adjusted ratio of 3.39 (95% CI [2.53, 4.54]). Opioid dose exposure (mg/kg/day) in the pre- and post-intervention groups were 1.27 (0.63, 2.19) and 1.14 (0.47, 1.93), respectively, with no statistically significant difference and an adjusted ratio of 0.91 (95% CI [0.75, 1.11]). In summary, midazolam usage decreased by 72% (95% confidence interval (CI) [61%, 80%], p-value<0.001) in the post-implementation cohort. Dexmedetomidine usage increased 230% (95% CI [145%, 344%]) in the post-implementation cohort.
Figure 2a.
Boxplot with scatterplots comparing cumulative dose (in mg/kg/ICU day) of midazolam and opioid (morphine equivalents) between the pre-intervention and post-intervention groups.
Figure 2b.
Boxplot with scatterplots comparing cumulative dose (in mcg/kg/ICU day) of dexmedetomidine, between the pre-intervention and post-intervention groups.
In multivariable regression models for midazolam, opioid and dexmedetomidine dose exposure, the intervention, implementation of the new analgesia-sedation protocol, was associated with a statistically significant reduction in midazolam exposure and a statistically significant increase in dexmedetomidine exposure (Supplemental Table 1). In a subgroup analysis of patients who received neuromuscular blockade infusions, median midazolam usage was similarly decreased from 1.59 mg/kg/ICU day to 0.14 mg/kg/day between the pre- and post-intervention groups, respectively (point estimate of median of differences = −1.21, 95% CI [−1.57, −0.85], p-value < 0.001). Of note, there were patients who received neuromuscular blockade but were not exposed to a midazolam infusion in both the pre- and the post-intervention groups (7 out of 67 paralyzed patients in the pre-intervention group, 36 out of 63 in the post-intervention group).
The median (IQR) duration for infusion of midazolam decreased from 61.7 (21.2, 205.5) hours in the pre-intervention group to 0.00 (0.00, 6.72) hours in the post-intervention group. Total duration of dexmedetomidine increased while duration of opioid infusion was unchanged in the post-intervention group (Supplemental Figure 4). We calculated the percentage of patients on midazolam, opioid and dexmedetomidine infusion for the pre-intervention and post-intervention groups (Supplemental Table 2). Compared to the pre-intervention group, fewer patients in the post-intervention group were exposed to midazolam infusion (OR = 0.06, 95% CI [0.03, 0.11]), more were exposed to a dexmedetomidine infusion (OR = 4.81, 95% CI [2.83, 8.39]), and there was no statistically significant difference in exposure to an opioid infusion (OR = 0.61, 95% CI = [0.28, 1.30]). Additionally, in a time-to-event analysis, the probability of initiating a midazolam infusion (Supplemental Figure 5a) or a dexmedetomidine infusion (Supplemental Figure 5b) from time of initiation of mechanical ventilation was different between the pre- and the post-intervention groups. The probability of starting either infusion from time of mechanical ventilation increased over time. In a log-rank test, the probability of being on a midazolam infusion after initiation of mechanical ventilation is higher in the pre-intervention group and sustained over time (χ2 = 143, DOF = 1, p-value = < 0.001). The probability of being on a dexmedetomidine infusion after initiation of mechanical ventilation was higher in the post-intervention group and also sustained over time (χ2 = 82.9, DOF = 1, p-value = < 0.001), suggesting compliance with the protocol with prioritization of dexmedetomidine use and deprioritization of midazolam use. Lastly, lorazepam usage (in both enteral and IV formulations) in mg/kg/day of ICU stay was similar between both groups. However, the proportion of patients on lorazepam during their ICU stay increased in the post-intervention group compared to the pre-intervention group (Table 2).
Table 2.
Clinical outcomes during the pre-intervention and post-intervention period
Data expressed as median (Q1, Q3) or n (%).
| Variable | Pre-intervention group (N=190) | Post-intervention group (N=144) |
|---|---|---|
| Hospital LOS (days) | 25 (12, 45.7) | 20 (10, 34) |
| PICU LOS (days) | 9 (4, 16) | 8 (4, 16) |
| VFD28 (days) | 23.3 (15.9, 25.5) | 23.2 (16.5, 26) |
| Lorazepam usage (n) | 41 (21.5) | 65 (45.1) |
| Lorazepam (mg/kg/ICU day) | 0 (0, 0) | 0 (0, 0.019) |
| Adverse events | ||
| Unplanned extubation | 8 (4.2) | 2 (1.4) |
| Failed extubation | 11 (5.8) | 7 (4.9) |
| Cardiorespiratory arrest event | 6 (3.2) | 3 (2.1) |
| 24-hour readmission | 7 (3.7) | 1 (0.7) |
| Total | 32 (16.8) | 13 (9.0) |
LOS = length of stay, VFD28 = ventilator-free days up to 28 days.
After adjusting for multiple comparisons, we did not find significant differences between the pre-intervention and post-intervention groups for PICU LOS, hospital LOS, VFD-28, rate of unplanned extubation, failed extubation, cardiorespiratory arrest event during PICU admission, and readmission to the PICU within 24 hours of transferring out of the PICU (Table 2). We did not record any patients in the post-intervention group with clinically significant bradycardia or hypotension related to dexmedetomidine use (as defined in the Methods section).
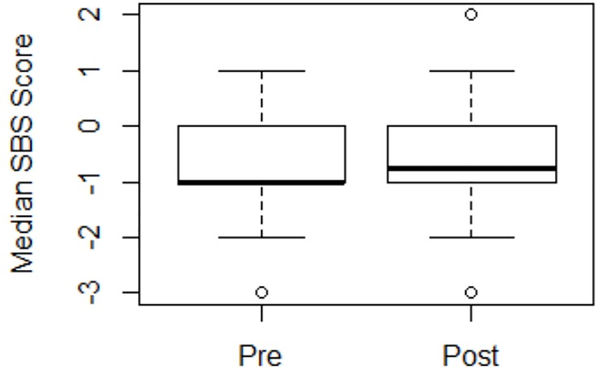
Median SBS scores recorded while on mechanical ventilation for each patient were similar in the pre- and post-intervention cohorts (Figure 3). There was no difference in the number of SBS scores recorded per patient per day of mechanical ventilation between the pre-intervention and post-intervention group, with median (IQR) 11 (8, 16) and 10 (7, 16) scores per patient per day, respectively (Supplemental Figure 6).
Figure 3.
Boxplot with scatterplot of the median SBS score recorded for patients in the pre- and post-intervention groups.
We used the Numerical Rating Scale (NRS) and Face, Legs, Activity, Crying, Consolability (FLACC) scales for pain scoring.(13 14) We analyzed the median pain score for patients that scored greater than 4, and the difference in medians between the pre- and the post-intervention groups. For the NRS and FLACC scores, the point estimate of the median (IQR) of differences was −0.343 (95% CI [−0.451, −0.191]) and 2.47 ×10−5 (95% CI [−0.014, 0.026]).
Discussion
Historically, we used benzodiazepine and opioid infusions for sedation and analgesia during mechanical ventilation in our PICU. There is emerging literature regarding potentially harmful effects of increased benzodiazepine exposure, including the prevalence of ICU delirium as well as negative effects on neurocognitive outcomes in the developing brain.(8, 9) Therefore, we aimed to reduce midazolam infusion based on the following key drivers: 1) benzodiazepine is associated with increased delirium and negative impact on neurocognitive development; 2) adequate sedation in mechanically ventilated patients may be achieved by using dexmedetomidine infusion and intermittent lorazepam; 3) a daily defined SBS goal for each patient and assessment of patient sedation adequacy by SBS scoring would drive standardized practice using a stepwise escalating protocol. In this manuscript, we have described our successful interdisciplinary QI effort of developing and implementing a revised stepwise approach that prioritizes dexmedetomidine infusion for sedation with intermittent opioids for pain management. This intervention was associated with a 72% reduction in overall IV midazolam usage, concurrent increase in dexmedetomidine use and lorazepam exposure, without significant increase in opioid usage or increase in the prevalence of adverse events. Additionally, the intervention was not associated with a change in sedation level and pain scores. The new protocol was associated with a successful transition from midazolam to dexmedetomidine as the sedative infusion of choice.
The results of our study, though comparable in some ways, have key differences compared to previous studies. Deeter et al. successfully demonstrated reduction in duration of benzodiazepine and opioid administration after implementation of a nursing-driven sedation protocol in a comparable patient population of mixed medical/surgical pediatric patients mechanically ventilated for greater than 48 hours.(7) The authors did not report the dosage of medications administered or changes in the usage of alternative sedative medications. Donnellan et al. described the impact of implementing a new guided comfort medication protocol in the postoperative population of pediatric CICU patients.(5) The authors reported decreased infusion rate of opioids, near elimination of benzodiazepine infusions, and no change in dexmedetomidine infusion rate. Their study only included the postoperative cardiac ICU (CICU) population and the cumulative dose of opioids in the post-intervention group was compared to a theoretical non-intervention group. As in our current study, they did not find any differences in CICU LOS, duration of mechanical ventilation and frequency of unplanned extubations after implementation of their intervention. Lastly, Yaghami et al. examined sustainability of an analgesia/sedation protocol but did not demonstrate protocol adherence over time.(19)
Our results must be interpreted in light of the limitations of the study. First, this is a retrospective study implemented as part of a QI paradigm and therefore is designed to examine association rather than causation. Furthermore, the two cohorts in our pre-post study were from two different time periods. Therefore, other factors within the PICU that can inadvertently affect the post-intervention cohort may not controlled for in the patient selection process. Though we could not account for all potential confounding factors, the baseline characteristics were similar between our two groups. Furthermore, as a pragmatic intervention in a general PICU population, our findings likely reflect real-world use of this bundled intervention. Additionally, our study included patients without tracheostomy and who are mechanically ventilated for greater than 24 hours in the PICU, which accounted for approximately 50% of all mechanically ventilated children admitted during this period. The choice of analgesia and sedative strategy is less important for patients who require less than 24 hours of mechanical ventilation. Future studies should examine changes in sedation practices for patients who receive non-invasive ventilation, as dexmedetomidine is known to be a safe and effective single sedative agent in this population.(15, 16) We have recently introduced delirium scoring in our PICU. Delirium scores were not available in the pre-intervention period and we are unable to compare the association of our intervention on the prevalence of delirium between the 2 groups. Although we had no direct pre-intervention comparator, we examined Cornell Assessment for Pediatric Delirium (CAPD) scores for the post-intervention group. A CAPD score > 8 is considered a positive screen.(17) In the post-intervention group, 76.6% of the patients had an average CAPD score >8 over the study period. This, our study included patients at risk of higher CAPD scores. Historical literature from other studies estimate prevalence of 30–40% in PICUs, although these study populations included patients who were not mechanically ventilated and excluded patients with developmental delay.(17) Additionally, as a participating site in an international point prevalence study of delirium in PICUs, overall point prevalence of delirium was 45% in 24 patients reported from our site, including non-mechanically ventilated patients.(18) Finally, our single center study was not designed or powered to detect significant differences in key patient-centered outcomes such as LOS and ventilator-free days.
Future directions include interval audits to examine sustainability and maintenance of the practice change that was achieved by this intervention. Improvements resulting from a QI intervention may deteriorate over time without routine monitoring and ongoing education to ensure sustained effectiveness.(18) Additionally, cost effectiveness is an important aspect of quality improvement and must be addressed in future studies. Lastly, benzodiazepines are associated with increased delirium in the PICU, which is an independent and strong risk factor for mortality.(19) Future studies must examine whether a benzodiazepine-sparing analgesia-sedation protocol intervention reduces the rate of delirium in the pediatric critically ill patient population. The impact of this strategy on patient-centered clinical outcomes must be examined in a large, multicenter trial designed and powered to address this question.
Conclusions
Implementation of an analgesia-sedation protocol that emphasizes dexmedetomidine and intermittent opioid as first-line strategy in the PICU was associated with significant reduction in total midazolam exposure in mechanically ventilated patients. The revised protocol implementation was not associated with increase in opioid usage or other adverse events. The impact of reduced midazolam exposure on delirium and other clinical outcomes must be examined in future studies.
Supplementary Material
Supplemental Figure 1. Flowchart showing the screening process and exclusion criteria.
Supplemental Figure 2. Histogram of the time difference between initiation of a dexmedetomidine infusion and midazolam infusion in the pre- and the post-intervention groups.
Supplemental Figure 3. Boxplot with scatterplots comparing cumulative dose (in mg) of midazolam, opioid (morphine equivalents) and dexmedetomidine, between the pre-intervention and post-intervention groups.
Supplemental Figure 4. Boxplot with scatterplots comparing duration of infusion (in hours) of midazolam, opioid, and dexmedetomidine, between the pre-intervention and post-intervention groups.
Supplemental Figure 5a. Time-to-event analysis of the probability of initiating a midazolam infusion between the pre- and the post-intervention groups. Time 0 = initiation of mechanical ventilation.
Supplemental Figure 5b. Time-to-event analysis of the probability of initiating a dexmedetomidine infusion between the pre- and the post-intervention groups. Time 0 = initiation of mechanical ventilation.
Supplemental Figure 6. Boxplots with scatterplots of the number of SBS scores recorded per patient per day for the pre-intervention and post-intervention cohorts.
Report in Context.
Emerging evidence has highlighted the detrimental side effects of midazolam as a sedative in patients in the pediatric intensive care unit.
A variety of barriers impede the change in practice from a benzodiazepine-focused sedation to an alternative strategy.
Dexmedetomidine has emerged as an alternative to benzodiazepine for sedation. However, concerns regarding its effectiveness and potential for adverse effects may preclude its use.
Past studies describing an alternative sedation strategy have not demonstrated sustained implementation or adequately quantified the reduction of midazolam exposure.
At the Bedside.
A new approach to sedation in pediatric intensive care units, replacing midazolam with dexmedetomidine as the primary sedative agent, can be successfully implemented using a stepwise escalation guideline driven by assessment of sedative scores.
The new approach can be successfully implemented and sustained with multidisciplinary stakeholder involvement and educational efforts.
Dexmedetomidine is a safe and effective sedative agent in pediatric patients who are mechanically ventilated.
Acknowledgements
The authors would like to acknowledge the support of the other members of the Analgesia-Sedation Task Force members: Leah Abecassis, RN; Kate Becla, RN; Tyler Blanchard, RN; Michelle Connors, RN; Gary Dhillon, MD; Diana Geisser, MD; Emily Hamilton RN, NP-C, Susan Hamilton, RN, MS; Amanda Harrington, MD; Olivia Hoffman, MD; Stephanie Larsen, RN; Kimberly LeBlanc, RN; Virginia Leon, RN; Liza Li, PharmD, BCPS; Thomas Mancuso, MD; Mary-Jeanne Manning, MSN, RN, BC-PNP, CCRN; Tara McGorman, RN; Allison Mello, BA; Mary O’Brien, RN, BSN, CCRN; Jordan Rettig, MD; Elizabeth Robertshaw, RN; Ethan Paul Schuler, DNP, RN, CPNP-PC/AC; Joana Shubert, RN; Deborah Sousa, RN; Elizabeth Tiemann, RN; and Tracy Walton, MSN, RN, CPNP. The project was supported by the Quality Improvement Committee and the Intensive Care Center for Outcomes Research & Evaluation (IC-CORE) in the Division of Critical Care Medicine. We acknowledge help from Michelle Lilley, RRT, and Shannon Manzi, PharmD, in data collection and compilation. We would like to extend a special acknowledgement to our late colleague, Dr. Craig Smallwood, PhD, RRT, for his assistance in the design and data analysis plan for this study.
Copyright form disclosure: Dr. Geva’s institution receivd funding from NICHD and NHLBI (K12 HD047349 and L40 HL133929), and he received support for article research from the NIH. Dr. Kleinman received funding from Burns White LLC. The remaining authors have disclosed that they do not have any potential conflicts of interest.
Footnotes
This work was performed within the Department of Anesthesiology, Critical Care and Pain Medicine at Boston Children’s Hospital in Boston, MA.
Article Tweet
Implementation of an analgesia-sedation protocol reduced midazolam usage without increasing the use of opioids in the #PedsICU at @BostonChildrensHospital. #ICUquality #QIstudy #ImplementationResearch
References
- 1.Rosen DA and Rosen KR: Midazolam for sedation in the paediatric intensive care unit. Intensive Care Med 1991; 17 Suppl 1:S15–19 [DOI] [PubMed] [Google Scholar]
- 2.Silvasi DL, Rosen DA and Rosen KR: Continuous intravenous midazolam infusion for sedation in the pediatric intensive care unit. Anesth Analg 1988; 67:286–288 [PubMed] [Google Scholar]
- 3.Penk JS, Lefaiver CA, Brady CM, et al. : Intermittent Versus Continuous and Intermittent Medications for Pain and Sedation After Pediatric Cardiothoracic Surgery; A Randomized Controlled Trial. Crit Care Med 2018; 46:123–129 [DOI] [PubMed] [Google Scholar]
- 4.Walker T and Kudchadkar SR: Pain and Sedation Management: 2018 Update for the Rogers’ Textbook of Pediatric Intensive Care. Pediatr Crit Care Med 2019; 20:54–61 [DOI] [PubMed] [Google Scholar]
- 5.Donnellan A, Sawyer J, Peach A, et al. : Reducing Exposure to Opioid and Benzodiazepine Medications for Pediatric Cardiac Intensive Care Patients: A Quality Improvement Project. Pediatr Crit Care Med 2019; 20:340–349 [DOI] [PubMed] [Google Scholar]
- 6.Smith HAB, Gangopadhyay M, Goben CM, et al. : Delirium and Benzodiazepines Associated With Prolonged ICU Stay in Critically Ill Infants and Young Children. Crit Care Med 2017; 45:1427–1435 [DOI] [PubMed] [Google Scholar]
- 7.Deeter KH, King MA, Ridling D, et al. : Successful implementation of a pediatric sedation protocol for mechanically ventilated patients. Crit Care Med 2011; 39:683–688 [DOI] [PubMed] [Google Scholar]
- 8.Young C, Jevtovic‐Todorovic V, Qin YQ, et al. : Potential of ketamine and midazolam, individually or in combination, to induce apoptotic neurodegeneration in the infant mouse brain. Br J Pharmacol 2005; 146: 189–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jevtovic-Todorovic V, Hartman RE, Izumi Y, et al. : Early exposure to common anesthetic agents causes widespread neurodegeneration in the developing rat brain and persistent learning deficits. J Neurosci 2003; 23: 876–882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Curley MA, Harris SK, Fraser KA, et al. : State Behavioral Scale: a sedation assessment instrument for infants and young children supported on mechanical ventilation. Pediatr Crit Care Med 2006; 7:107–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.ClinCalc LLC. Equivalent Opioid Calculator. Available at: https://clincalc.com/opioids/#.Accessed 2020
- 12.Yehya N, Harhay MO, Curley MAQ, et al. : Reappraisal of Ventilator-Free Days in Critical Care Research. Am J Respir Crit Care Med 2019; 200:828–836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Merkel SI, Voepel-Lewis T, Shayevitz JR, Malviya S. The FLACC: a behavioral scale for scoring postoperative pain in young children. Pediatr Nurs. 1997May-Jun;23(3):293–7. [PubMed] [Google Scholar]
- 14.Farrar JT, Young JP Jr, LaMoreaux L, Werth JL, Poole RM. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain. 2001November;94(2):149–58. doi: 10.1016/s0304-3959(01)00349-9. [DOI] [PubMed] [Google Scholar]
- 15.Shutes BL, Gee SW, Sargel CL, et al. : Dexmedetomidine as Single Continuous Sedative During Noninvasive Ventilation: Typical Usage, Hemodynamic Effects, and Withdrawal. Pediatr Crit Care Med 2018; 19:287–297 [DOI] [PubMed] [Google Scholar]
- 16.Venkatraman R, Hungerford JL, Hall MW, et al. : Dexmedetomidine for Sedation During Noninvasive Ventilation in Pediatric Patients. Pediatr Crit Care Med 2017; 18:831–837 [DOI] [PubMed] [Google Scholar]
- 17.Traube C, Silver G, Gerber LM, et al. : Delirium and Mortality in Critically Ill Children: Epidemiology and Outcomes of Pediatric Delirium. Crit Care Med 2017; 45:891–898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Traube C, Silver G, Reeder RW, Doyle H, Hegel E, Wolfe HA, Schneller C, Chung MG, Dervan LA, DiGennaro JL, Buttram SD, Kudchadkar SR, Madden K, Hartman ME, deAlmeida ML, Walson K, Ista E, Baarslag MA, Salonia R, Beca J, Long D, Kawai Y, Cheifetz IM, Gelvez J, Truemper EJ, Smith RL, Peters ME, O’Meara AM, Murphy S, Bokhary A, Greenwald BM, Bell MJ. Delirium in Critically Ill Children: An International Point Prevalence Study. Crit Care Med. 2017April;45(4):584–590. doi: 10.1097/CCM.0000000000002250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yaghmai BF, Di Gennaro JL, Irby GA, et al. : A Pediatric Sedation Protocol for Mechanically Ventilated Patients Requires Sustenance Beyond Implementation. Pediatr Crit Care Med 2016; 17:721–726 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Flowchart showing the screening process and exclusion criteria.
Supplemental Figure 2. Histogram of the time difference between initiation of a dexmedetomidine infusion and midazolam infusion in the pre- and the post-intervention groups.
Supplemental Figure 3. Boxplot with scatterplots comparing cumulative dose (in mg) of midazolam, opioid (morphine equivalents) and dexmedetomidine, between the pre-intervention and post-intervention groups.
Supplemental Figure 4. Boxplot with scatterplots comparing duration of infusion (in hours) of midazolam, opioid, and dexmedetomidine, between the pre-intervention and post-intervention groups.
Supplemental Figure 5a. Time-to-event analysis of the probability of initiating a midazolam infusion between the pre- and the post-intervention groups. Time 0 = initiation of mechanical ventilation.
Supplemental Figure 5b. Time-to-event analysis of the probability of initiating a dexmedetomidine infusion between the pre- and the post-intervention groups. Time 0 = initiation of mechanical ventilation.
Supplemental Figure 6. Boxplots with scatterplots of the number of SBS scores recorded per patient per day for the pre-intervention and post-intervention cohorts.