Abstract
Clinical trials including an analytical treatment interruption (ATI) are vital for evaluating the efficacy of novel strategies for HIV remissions. We briefly describe an interactive tool for predicting viral rebound timing in ATI trials and the impact of post-treatment controller (PTC) definitions on PTC frequency estimates. A 4-week viral load threshold of 1,000 cps/mL provides both high specificity and sensitivity for PTC detection. PTC frequency varies greatly based on the definition of a PTC.
Analytical treatment interruption (ATI) is an essential strategy to determine the effectiveness of HIV cure strategies. Although previous work has demonstrated that ATI does not lead to an increase in the reservoir size [1,2], minimizing unnecessary prolonged exposure to viremia is an important consideration for all studies. Historically, there has been little concordance in antiretroviral therapy (ART)-restart criteria among ATI studies and our understanding of how different ART-restart criteria influence viral rebound dynamics remains incomplete [3]. The interactive viral rebound calculator (http://jonathanlilab.bwh.harvard.edu/rebound-calc/) was created as a pooled analysis of plasma viral loads (pVLs) of >700 participants from 12 ATI trials to predict HIV rebound after stopping ART [4].
The tool allows the user to set the ART-restart criteria to predict the percentage of 1) all participants, 2) post-treatment non-controllers (NCs), 3) and post-treatment controllers (PTCs) that would remain off therapy from week 1 through week 48. The interactive tool also allows the user to set an absolute pVL threshold or a multiweek threshold (e.g., pVL>1,000 for a duration of 4 weeks) as well as customize results based on: the timing of ART initiation, frequency of pVL measurements, ART regimens, therapeutic intervention arms, and PTC frequency (the default is the frequency identified in the CHAMP study of post-treatment controllers (PTCs) based on the criteria: pVL<400 cps/mL at ≥2/3 time points for ≥24 weeks post-ATI [3]). We also assessed how varying the threshold of suppressed time points and pVLs affected the frequency of PTC identification.
During ATI, investigators aim to balance safety issues of prolonged viremia with characterizing the effect of cure interventions such as: time to viral rebound, HIV viral set point and identification of PTCs. Although the time to viral rebound and set point data are easily quantifiable, PTC frequency calculations remain elusive as ART is often restarted before confirming controller status. Here, we compared the impact of several commonly used threshold pVL ART restart criteria (1,000 pVL, 1,000 pVL for 2 weeks, 1,000 pVL for 4 weeks [5], and 50,000 pVL for 4 weeks) on the ability of an ATI trial to detect PTCs as defined by the CHAMP definition [4]. The calculator applies the user’s ART restart criteria to the dataset containing the 700+ participants pVL data to estimate the proportion of participants experiencing viral rebound and remaining off ART after treatment discontinuation (see Supplemental methods). In the CHAMP study, PTCs frequently had an early viral load peak before subsequent viral control off ART. Some of these PTCs may be missed depending on the ART restart criteria, which would have mandated the resumption of ART prior to demonstrating their natural ability to suppress virus. Our calculator predicted that these criteria would fail to identify 47%, 18%, 0%, and 0% of PTCs, respectively, due to premature ART restart. Of the four criteria, the 1,000 pVL for 1-week criterion had high specificity (99%), but low sensitivity (53%), while the 50,000 pVL for 4-week criterion had low specificity (12%), but high sensitivity (100%). The 1,000 pVL for 4-weeks criterion achieved a balance with 90% specificity and 100% sensitivity for identifying PTCs.
The definition of posttreatment control remains fluid and not yet standardized within the field. In addition to the calculator’s ability to predict the number of CHAMP-defined PTCs identified by each ART restart criteria, we also evaluated five alternative PTC definitions, each changing one aspect of the CHAMP criteria (Supplemental Table 1): 1) VL suppression for 100% of timepoints; 2); VL suppression for 90% of timepoints; 3) VL threshold of 200 cps/mL; 4) VL threshold of 1,000 cps/mL; 5) Suppression for 48 weeks (Figure 1). Significantly fewer PTCS were identified in both the chronic and early-treated arms in definition 1, (100% suppression ≤ 400 pVL, p = .04 and .01, respectively) and significantly more PTCs were identified in the chronic-treated arm in definition 4 (≥2/3 suppression ≤ 1,000 pVL, p = .03). PTCs were more frequently identified in early-treated participants compared with chronic-treated participants in every iteration except for the final case, suppression for 48 weeks. Importantly, key characteristics (pre-ART VL, CD4 decline, baseline CD4+ count, peak VL, and peak VL week) remained comparable for the PTCs regardless of the specific PTC definition used (Supplemental Table 2).
Figure 1. Effect of post-treatment controller definitions on estimated frequency of control.
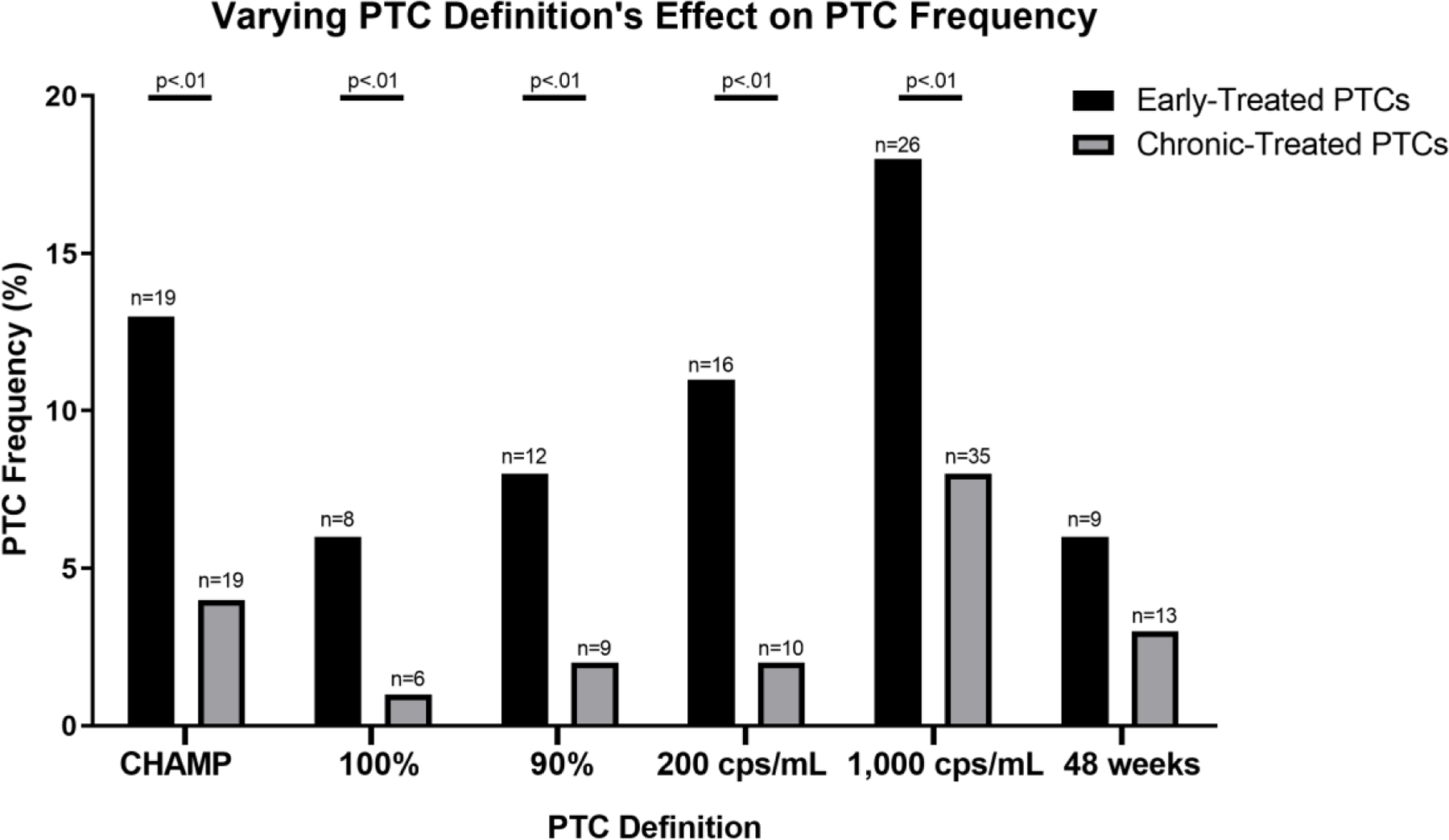
“CHAMP” refers to the post-treatment controller (PTC) criteria used in the CHAMP study: pVL<400 cps/mL at ≥2/3 time points for ≥24 weeks post-ATI. “100%” criteria = pVL<400 cps/mL at 100% of time points for ≥24 weeks post-ATI. “90%” criteria = pVL<400 cps/mL at 90% of time points for ≥24 weeks post-ATI. “200 cps/mL” criteria = pVL<200 cps/mL at ≥2/3 of time points for ≥24 weeks post-ATI. “1,000 cps/mL” criteria = pVL<1,000 cps/mL at ≥2/3 of time points for ≥24 weeks post-ATI. “48 weeks” criteria = pVL<200 cps/mL at ≥2/3 of time points for ≥48 weeks post-ATI. Frequencies were compared using Fishers exact test. n, refers to the number of PTCs identified in each condition.
One limitation of this analysis was the heterogeneity in frequency of viral load measurements during the ATI among studies, with some studies using weekly viral load monitoring, but other studies using less frequent monitoring. We also excluded participants on non-nucleoside reverse transcriptase inhibitor (NNRTI)-based therapy as it has been shown to impact viral rebound timing, likely due to the prolonged half-life of NNRTIs [6–8].
In summary, the results provide insights on the chances of identifying PTCs given different ART restart criteria and demonstrate that the expected frequency of post-treatment control is highly dependent on the viral load definitions used. The online calculator provides an interactive tool for estimating viral rebound outcomes and for supporting the design of ATI trials.
Supplementary Material
Acknowledgements
We thank the participants, investigators, and site staff for all of the included studies.
Funding Statement
This work was supported in part by the Harvard University Center for AIDS Research (to Drs. Li and Gandhi, NIAID 5P30AI060354-08); National Institutes of Health (NIH) grants UM1 AI068634 (Statistical and Data Management Center of the AIDS Clinical Trials Group), UM1 AI068636 (AIDS Clinical Trials Group), subcontract from UM1 AI106701 to the Harvard Virology Support Laboratory (to Dr. Li).
Footnotes
A list of the CHAMP study team contributors is provided in the Supplementary Appendix
Conflicts of Interest
There are no conflicts of interest.
REFERENCES
- 1.Clarridge KE, Blazkova J, Einkauf K, Petrone M, Refsland EW, Justement JS, et al. Effect of analytical treatment interruption and reinitiation of antiretroviral therapy on HIV reservoirs and immunologic parameters in infected individuals. PLoS Pathogens 2018; 14:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Strongin Z, Sharaf R, VanBelzen DJ, Jacobson JM, Connick E, Volberding P, et al. Effect of Short-Term Antiretroviral Therapy Interruption on Levels of Integrated HIV DNA. Journal of Virology 2018; 92:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pinkevych M, Cromer D, Tolstrup M, Grimm AJ, Cooper DA, Lewin SR, et al. HIV Reactivation from Latency after Treatment Interruption Occurs on Average Every 5–8 Days—Implications for HIV Remission. PLOS Pathogens 2015; 11:e1005000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Namazi G, Fajnzylber JM, Aga E, Bosch RJ, Acosta EP, Sharaf R, et al. The control of HIV after antiretroviral medication pause (CHAMP) study: Posttreatment controllers identified from 14 clinical studies. Journal of Infectious Diseases 2018; 218. doi: 10.1093/infdis/jiy479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Julg B, Dee L, Ananworanich J, Barouch DH, Bar K, Caskey M, et al. Recommendations for analytical antiretroviral treatment interruptions in HIV research trials—report of a consensus meeting. The Lancet HIV 2019; 6:e259–e268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hare CB, Mellors J, Krambrink A, Su Z, Skiest D, Margolis DM, et al. Detection of Nonnucleoside Reverse‐Transcriptase Inhibitor–Resistant HIV‐1 after Discontinuation of Virologically Suppressive Antiretroviral Therapy. Clinical Infectious Diseases 2008; 47:421–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Parienti JJ, Das-Douglas M, Massari V, Guzman D, Deeks SG, Verdon R, et al. Not all missed doses are the same: Sustained NNRTI treatment interruptions predict HIV rebound at low-to-moderate adherence levels. PLoS ONE 2008; 3:5–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mackie NE, Fidler S, Tamm N, Clarke JR, Back D, Weber JN, et al. Clinical implications of stopping nevirapine-based antiretroviral therapy: Relative pharmacokinetics and avoidance of drug resistance. HIV Medicine 2004; 5:180–184. [DOI] [PubMed] [Google Scholar]
- 9.Henry K, Katzenstein D, Cherng DW, Valdez H, Powderly W, Vargas MB, et al. A pilot study evaluating time to CD4 T-cell count <350 cells/mm(3) after treatment interruption following antiretroviral therapy +/− interleukin 2: results of ACTG A5102. J Acquir Immune Defic Syndr 2006; 42:140–148. [DOI] [PubMed] [Google Scholar]
- 10.Sneller MC, Justement JS, Gittens KR, Petrone ME, Clarridge KE, Proschan MA, et al. A randomized controlled safety/efficacy trial of therapeutic vaccination in HIV-infected individuals who initiated antiretroviral therapy early in infection. Sci Transl Med 2017; 9. doi: 10.1126/scitranslmed.aan8848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gandhi RT, O’Neill D, Bosch RJ, Chan ES, Bucy RP, Shopis J, et al. A randomized therapeutic vaccine trial of canarypox-HIV-pulsed dendritic cells vs. canarypox-HIV alone in HIV-1-infected patients on antiretroviral therapy. Vaccine 2009; 27:6088–6094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kilby JM, Bucy RP, Mildvan D, Fischl M, Santana-Bagur J, Lennox J, et al. A randomized, partially blinded phase 2 trial of antiretroviral therapy, HIV-specific immunizations, and interleukin-2 cycles to promote efficient control of viral replication (ACTG A5024). J Infect Dis 2006; 194:1672–1676. [DOI] [PubMed] [Google Scholar]
- 13.Schooley RT, Spritzler J, Wang H, Lederman MM, Havlir D, Kuritzkes DR, et al. AIDS clinical trials group 5197: a placebo-controlled trial of immunization of HIV-1-infected persons with a replication-deficient adenovirus type 5 vaccine expressing the HIV-1 core protein. J Infect Dis 2010; 202:705–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Volberding P, Demeter L, Bosch RJ, Aga E, Pettinelli C, Hirsch M, et al. Antiretroviral therapy in acute and recent HIV infection: a prospective multicenter stratified trial of intentionally interrupted treatment. AIDS 2009; 23:1987–1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stekler JD, Wellman R, Holte S, Maenza J, Stevens CE, Corey L, et al. Are there benefits to starting antiretroviral therapy during primary HIV infection? Conclusions from the Seattle Primary Infection Cohort vary by control group. Int J STD AIDS 2012; 23:201–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jacobson JM, Pat Bucy R, Spritzler J, Saag MS, Eron JJ, Coombs RW, et al. Evidence that intermittent structured treatment interruption, but not immunization with ALVAC-HIV vCP1452, promotes host control of HIV replication: the results of AIDS Clinical Trials Group 5068. J Infect Dis 2006; 194:623–632. [DOI] [PubMed] [Google Scholar]
- 17.Skiest DJ, Su Z, Havlir DV, Robertson KR, Coombs RW, Cain P, et al. Interruption of Antiretroviral Treatment in HIVInfected Patients with Preserved Immune Function Is Associated with a Low Rate of Clinical Progression: A Prospective Study by AIDS Clinical Trials Group 5170. The Journal of Infectious Diseases 2007; 195:1426–1436. [DOI] [PubMed] [Google Scholar]
- 18.Gianella S, Anderson CM, Richman DD, Smith DM, Little SJ. No Evidence of Post Treatment Control after Early Initiation of Antiretroviral Therapy in the San Diego Primary Infection Cohort. AIDS 2015; 29:2093–2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rosenberg ES, Graham BS, Chan ES, Bosch RJ, Stocker V, Maenza J, et al. Safety and immunogenicity of therapeutic DNA vaccination in individuals treated with antiretroviral therapy during acute/early HIV-1 infection. PLoS One 2010; 5:e10555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jain V, Liegler T, Vittinghoff E, Hartogensis W, Bacchetti P, Poole L, et al. Transmitted Drug Resistance in Persons with Acute/Early HIV-1 in San Francisco, 2002–2009. PLOS ONE 2010; 5:e15510. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


