Abstract
Purpose of review:
Anxiety disorders are some of the most common psychiatric diagnoses in children and adolescents, but attempts to improve outcome prediction and treatment have stalled. This review highlights recent findings on neural indices related to fear and anxiety that provide novel directions for attempts to create such improvements.
Recent findings:
Stimuli capable of provoking fear engage many brain regions, including the amygdala, medial prefrontal cortex, hippocampus, and bed nucleus of the stria terminalis. Studies in rodents suggest that sustained, low-level threats are particularly likely to engage the bed nucleus of the stria terminalis, which appears to malfunction in anxiety disorders. However, anxiety disorders, like most mental illnesses, appear less likely to arise from alterations in isolated brain regions than in distributed brain circuitry. Findings from large-scale studies of brain connectivity may reveal signs of such broadly distributed dysfunction, though available studies report small effect sizes. Finally we review novel approaches with promise for using such large-scale data to detect clinically relevant, broadly distributed circuitry dysfunction.
Summary:
Recent work maps neural circuitry related to fear and anxiety. This circuitry may malfunction in anxiety disorders. Integrating findings from animal studies, big datasets, and novel analytical approaches may generate clinically relevant insights based on this recent work.
Keywords: Neuroimaging, MRI, Fear, Anxiety, Childhood
Introduction
Anxiety disorders are among the most common psychiatric disorders with prevalence ranging from 10–14% during adulthood (1). The prevalence during childhood and adolescence might be even higher, with estimates ranging from 20–30% of at least one anxiety disorder during development (2,3). These data on prevalence across the lifespan resemble data for many mental disorders. Hence, like many mental illnesses, anxiety disorders represent developmental conditions, problems where persistent psychopathology in adults begins in children and adolescents (4).
This critical review unfolds in three steps. The review begins by describing evidence relating fear and anxiety to distinct neural mechanisms. This is followed by a description of two promising recent advances on translating findings from research in brain imaging to clinical practice. One advance involves the creation of big datasets, including longitudinal studies. The other involves the development of predictive models that aim at developing tools capable of translating research to clinically meaningful findings.
Classification of human responses to threat can adopt many approaches. One such approach is used in the RDoC (Research Domain Criteria) Negative Valence systems (5). As a framework for classification, RDoC attempts to bridge understanding of mental illness phenomenology with knowledge on brain-behavior relationships. The term “fear” has been used to refer to responses evoked in people by direct exposures to a threatening stimulus, whereas the term “anxiety” has been used to refer to responses evoked by the anticipation of such an exposure (4) (Figure 1). Following this distinction, acute threat (fear) and potential threat (anxiety) are considered two distinct constructs within the RDoC framework (5).
Figure 1 -. Schematic representation of brain regions involved in anxiety and fear.
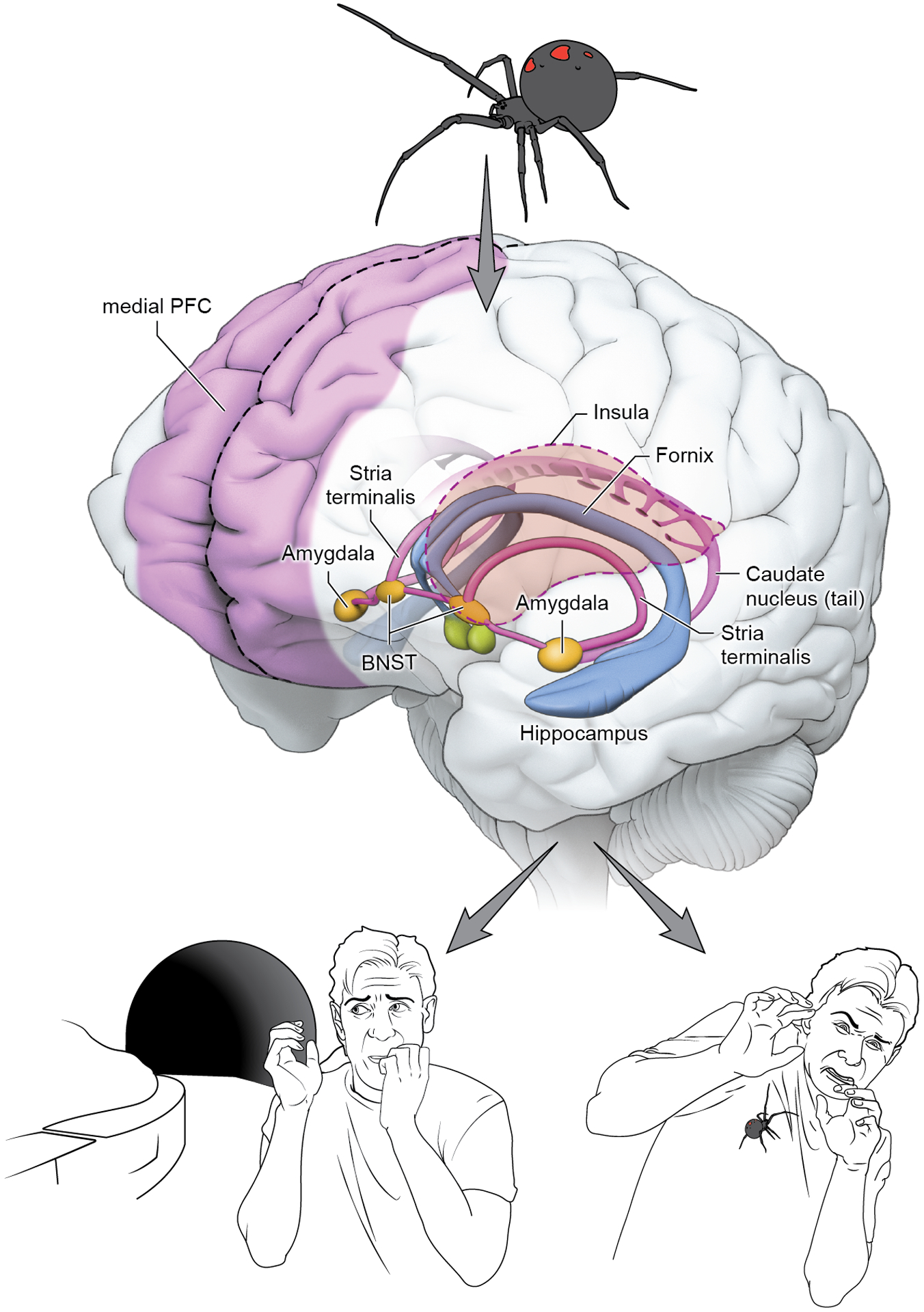
A threat stimulus is represented by the spider. The brain circuit involved in fear and anxiety is represented by the hippocampus, the amygdala, the stria terminalis and the frontal cortex. These regions are involved in both fear response after the immediate presence of the threat and in the anxious response of an individual facing uncertainty. BNST = Bed Nucleus of the Stria Terminalis.
Beyond RDoC, other approaches focus more narrowly on varieties of clinical problems. Such clinical classification schemes categorize anxiety disorders as pathological entities into multiple diagnostic entities based on phenomenology. Of the anxiety disorders, selective mutism (6) and separation anxiety have a typical onset earlier in childhood; specific phobia peaks at late-childhood and early adolescence and the remaining anxiety disorders onset are more common in adolescence and young adulthood (2,3). Comorbidity among anxiety disorders are common as well as the progression from one disorder to another (7). This makes it especially difficult to isolate each diagnostic category in research.
In child and adolescent anxiety, MRI studies have either relied on case-control design or correlational approaches, relating symptoms or diagnosis to brain-based measures. These two approaches are mutually informative. Dimensional approaches are useful because individual differences in the general tendency to experience fear and anxiety are continuously distributed in the population. Thus, anxiety disorders can be seen as lying on one end of the dimension, and subclinical symptoms among healthy subjects at another end. This dimensional approach is especially relevant to children, since fearful thoughts represent one component of normal development. Despite generating knowledge about the potential neurological mechanisms involved in fear and anxiety, there is a gap to fill that can relate research findings to differential diagnosis and clinical outcomes. This review focuses on novel MRI studies that investigate the underlying circuits related to childhood anxiety and recent advances in generating useful clinical application of neuroimaging biomarkers to patients. While the field remains years away from such application, recent findings provide a path for pursuing this long-term goal.
Neural circuits of Fear and Anxiety
Research in basic neuroscience can help focus the lens of imaging techniques on particular aspects of brain structure or function. Such basic research connects many brain regions to the mammalian response to threats, which are engaged when the organism detects stimuli capable of producing harm. Many of these brain regions are depicted in Figure 1. These regions include amygdala, stria terminalis (particularly the bed nucleus), prefrontal cortex, anterior cingulate cortex and hippocampus (8). Fear and anxiety, viewed as the physiological response to danger detection, is readily observed across species (9). The amygdala has been implicated in threat conditioning (10) and response (11), and more recently in valence and salience (12). The amygdala is a complex structure, with multiple subregions that regulate distinct phenomena. The basolateral amygdala integrates sensory information, and excites the central nucleus of the amygdala, which in turn projects to other regions triggering fear response. There is evidence that the bed nucleus of the stria terminalis (BNST) is another key region, being associated with sustained threat response, thus being central to responses in humans that can be characterized as provoking “anxiety” (13). Such recent work has pointed towards the existence of precisely functioning microcircuits. These circuits regulate distinct responses, such as approach or avoidance, to one or another stimulus from circuitry components that lie very close to one another.
Key sectors of the human neocortex possess no homologue with other species and, for those regions, the translation of animal findings to humans is difficult. Other structures do exhibit such homology, such as portions of the insula, including mid and posterior sections, which are implicated in the monitoring of internal stimuli and the regulation of response to aversive stimuli (14). In humans, portions of the medial PFC (15) and anterior cingulate cortices (16) function as part of the default mode network (DMN) (17), which relates to threat responding and anxiety. These regions have been implicated in cognitive processes such as episodic memory and self-representations. Animal models of anxiety in rodents associate portions of the prefrontal cortex, involved in working memory function, with afferent and efferent connections to the amygdala (9). The dorsolateral PFC (dlPFC) is thought to exert a fundamental role in maintenance of goal representation and motor plans for achieving such goals. These functions enable working memory in humans, and there is evidence of altered dlPFC activation in anxiety patients (18). The higher cognitive functions associated with frontal cortical regions are targeted by current therapeutic approaches for the treatment of anxiety disorders (for review see (19)).
Basic science research suggests that parallels are likely to exist in aspects of fear and anxiety among children, adolescents and adults. However, in humans, a robust body of evidence shows that the brain changes during development (20). Structural MRI shows that the cortex volume increases during early childhood, decreasing during late childhood and adolescence. Cortical thickness results point to a monotonic decline from childhood through adolescence (21). The hippocampus and amygdala volume appear to peak early in life, followed by a relatively stable period during adolescence and young adulthood (22). There is at least some evidence of difference in the slope (rate of change) of the right ventromedial PFC in youths with any anxiety compared to healthy volunteers (23).
Likewise, brain connectivity patterns appear to change during childhood and adolescence (24). Overall findings using different methodology show an increase in integration of different brain regions during this period (24,25). There is data suggesting that the within-subject connectivity is relatively stable over time in healthy subjects, including across adolescence (26). Interestingly, the reliability of connectome metrics does not appear to be stable over time, being less reliable in infancy and old age, than in adulthood (24). It is still unknown how subtle changes detectable at group levels are related to changes in subject specific patterns of connectivity over development (27). This opens a line of research for the use of brain connectivity in predictive work (see below).
How different stages of brain development relate to the emergence of symptoms is still unclear. There are, however, a few studies directly comparing childhood and adolescent anxiety to adult anxiety. In a study with 200 participants aged 8 to 50, Gold et. al. (28) showed group differences between anxious and healthy adults during threat appraisal in the vmPFC but not in youth. In the inferior temporal gyrus youths with anxiety showed greater activation during memory tasks, but not appraisal, while the opposite was seen in adults.
ENIGMA and other large scale initiatives
Research on genetics shows that understandings of mental illnesses benefit from large-scale research combining data across multiple research groups. This suggests the promise of creating similar approaches with imaging. The ENIGMA (Enhancing NeuroImaging Genetics through Meta Analysis) Consortium supports multi-group efforts that are generating valuable insights (29). These insights concern the nature of altered brain structure in several psychiatric disorders. The ENIGMA-Anxiety working group includes subgroups dedicated to specific disorders (Generalized Anxiety Disorder, Social Anxiety Disorder, Panic Disorder, Specific Phobia) (30). Unlike traditional meta-analyses, ENIGMA conducts preprocessing and analytical steps simultaneously across samples (31), to reduce bias arising from different preprocessing and quality assurance methods. Many ENIGMA working groups have further implemented mega-analysis methodology, whereby the individual participants’ data (IPD) are shared within the group. This allows for even further standardization of processing methods, as all data can be assessed by the team that is leading the analysis (31). The sharing of IPD helps improve the consistency of inclusion criteria, treatment of confounds and handling of missing data (32).
Recently finalized analyses by ENIGMA-Anxiety (Harrewjin, submitted) show no evidence of structural alteration in patients with GAD when compared with healthy subjects. The interaction between age by GAD was also non-significant. This is in line with previous work that showed no evidence of structural differences in anxiety disorders, dimming enthusiasm for attempts to find diagnosis-specific structural findings.
There is work in progress to conduct a similar mega-analysis with functional data. Resting-state fMRI (rs-fMRI) data from participant centers is being centralized for processing and analysis. The advantage of resting-state data as opposed to task fMRI is that it is collected somewhat similarly across all centers, with the research subject being asked to remain still and look at a fixation cross, or close their eyes during scanning. Rs-fMRI measures have been shown to be relatively stable over time and conditions (26). Although limited by relatively small sample sizes, previous work shows that anxiety patients exhibit alterations in within and between network connectivity in using rs-fMRI when compared with controls. A recent meta-analysis showed alterations in multiple networks: affective , salience , default mode and executive control (33).
Another promising avenue involves studies of prospective cohorts such as the Adolescent Brain Cognitive Development (ABCD) (34), Generation R (35) and the Brazilian High-Risk Cohort Study (BHRCS) (36). These studies aim to collect data from many subjects that includes neuroimaging measures and behavioral data from childhood through young adulthood. One difficulty in past work is that both brain measures and measures of psychopathology change during this period (Figure 2). Results from prospective studies will help identify how different symptom trajectories relate to differences in neuroimaging measures. A difficulty with large studies is that they require streamlined data acquisition that is feasible for many subjects across multiple centers. This limits the depth of coverage for particular dimensions (e.g.: anxiety).
Figure 2 -. Schematic representation of correlation vs prediction.
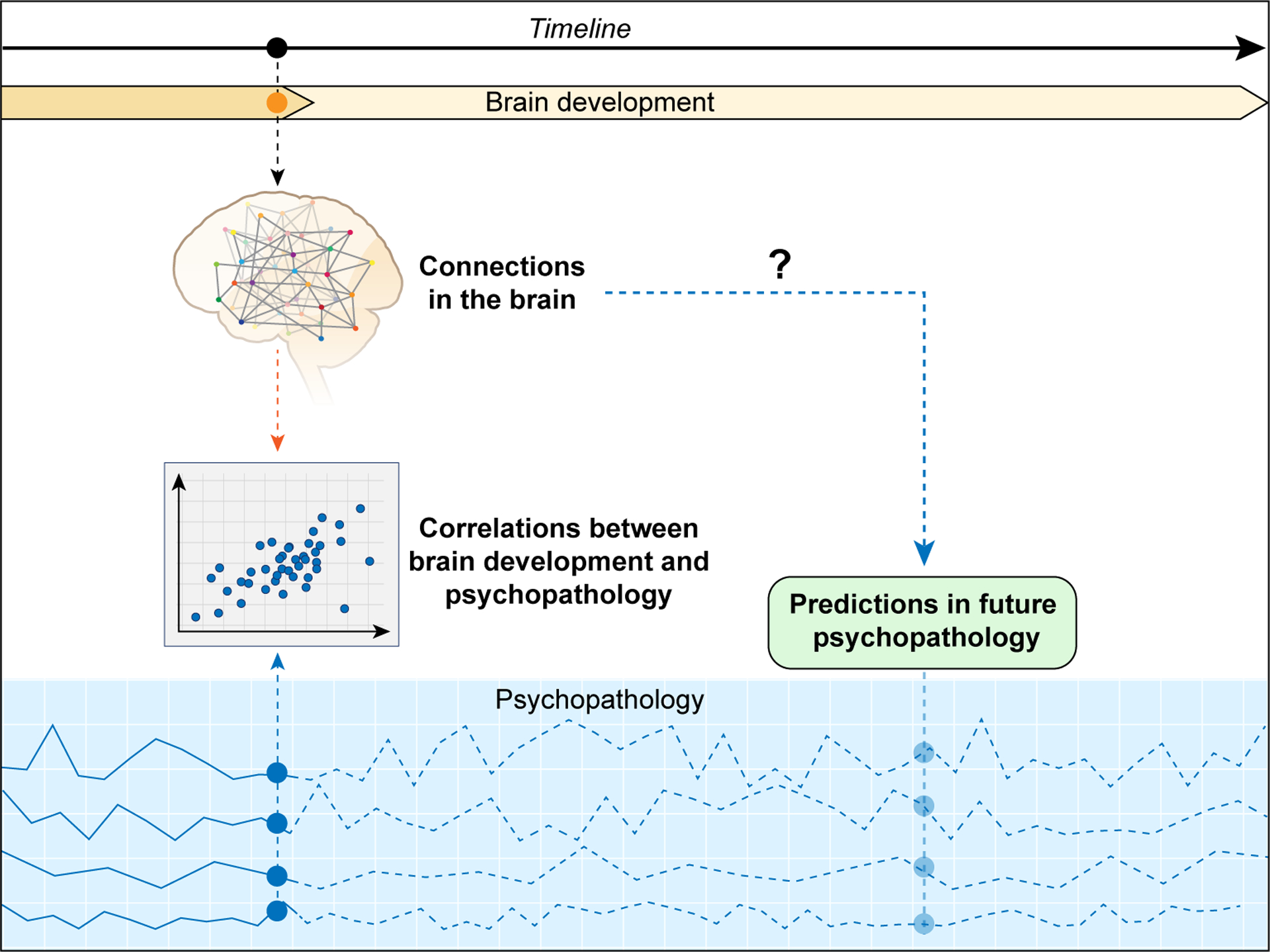
Cross-sectional studies are limited to correlating neuroimaging findings - in this case represented by brain connections - to concurrent symptoms. Psychopathology can present multiple dimensions - represented by the blue lines - with fluctuating time course. Predicting outcomes based on brain measures is still not possible. Cross-sectional designs do not allow for the full comprehension of the timing of brain differences found in anxiety. It is unclear if brain changes remain after symptom remission or worsening of symptoms and how brain functioning reflects shifts in diagnosis or the development of comorbidities; or even how underlying traits that confer risk to later development of anxiety disorders relates to the brain.
Translating findings to clinical practice: prediction
Large datasets support applications of prediction algorithms in neuroimaging research (37,38). Most knowledge comes from studies deploying classical statistical inference when quantifying associations between variables. However, the effect sizes in applications of this approach to imaging are frequently small, as noted in the material appearing above on structural findings (39). Associations between behavior and fMRI measures may reflect aggregate effects from small, diffuse effects as opposed to large localized effects (40). Small sample sizes can lead to underpowered studies and spurious associations. Likewise small effects can become detectable in large datasets, rendering statistically significant findings clinically irrelevant, or leading to the detection of unrelated noise as signal (41,42). Complicating this even further is the fact that many psychiatric disorders and symptoms have overlapping imaging findings.
Work that uses a predictive approach estimates an outcome using data from one or more imaging modality. Figure 2 illustrates some of the considerations that inform such predictive approaches. The available data is usually split between a training and a test dataset, with the training dataset being used to create (or train) the model, which is then used to make estimates of the variable of interest in the test dataset (which can be constituted of a single test subject). An additional dataset can be used to further assess model validity (43). This is particularly important when a cross-validation method is used. Many predictive methods have been applied in neuroimaging with no superiority of one approach over another (e.g., deep learning, support vector machines, support vector regression, random forests, clustering, linear discriminant analysis); some methods seek to combine multiple algorithms (44). Some of these methods provide models that produce difficult-to-interpret results. Neural networks and deep learning may rely on a series of non-linear relations that have been called ‘black box’ models. The interpretation of the effect of each parameter in the prediction model, and thus the biological meaning, might be more difficult to obtain (45,46). There are other approaches, however, that favor interpretability. One of such approaches, called connectome predictive modeling (CPM) uses a connectivity matrix (the correlation between brain regions one with another), which is then correlated with a measure of interest. The resulting correlations are then used to select the connections of the correlation matrix. The sum of the selected connections is then used in the model for prediction (47). CPM has been used in healthy volunteers to predict trait anxiety with promising results (48).
Much work is still needed before neuroimaging predictive models make the leap to become clinically significant. To date, work in the field has focused on predicting the category of diagnosis or a symptom or neurocognitive score. There has been insufficient work in clinically significant topics, such as treatment outcome. There is an inherent difficulty when trying to build predictive models for specific anxiety diagnosis in children. Anxiety diagnoses are frequently comorbid and it is unclear how to accommodate that in predictive models. Another difficulty is that prediction algorithms might not perform well on an imbalanced dataset (i.e.: many more healthy volunteers than patients) (49). This might make it particularly difficult to apply predictive models on infrequent diagnosis or symptoms, even with large datasets becoming available. One question that remains is if predictive algorithms will be able to work across different age ranges, ethnicities, culture and across all the slight phenotypic variations seen in clinical settings.
Conclusion
Recent work has been instrumental in helping unveil mechanisms associated with fear, anxiety and anxiety disorders. Ongoing collaborative research efforts such as ENIGMA and multi-site studies are underway and will help make it clearer how these disorders develop during childhood and adolescence. Hopefully the rapid growing field will help develop clinically useful markers of anxiety.
KEY POINTS:
Fear follows from immediate encounters with a threat, whereas anxiety arises when anticipating a potential forthcoming encounter.
Anxiety disorder symptoms are believed to relate to function in neural circuits involved in threat processing and response.
Rodent, non-human primate, and human studies implicate similar structures in the response to danger: amygdala, hippocampus, medial prefrontal cortex, and the bed nucleus of the stria terminalis.
Neuroimaging studies have shown small effect sizes for when comparing structure and function in particular brain regions among patients with anxiety and healthy volunteers.
Large datasets are becoming available and will allow for novel multivariate approaches for analyzing neuroimaging data that may detect clinically-relevant findings.
Acknowledgments
Financial support and sponsorship
Andre Zugman, Anderson M. Winkler and Daniel S. Pine are supported by NIH through ZIA-MH002781 and ZIA-MH002782.
Footnotes
Conflict of interest
The authors report no conflict of interest in the making of this article.
References
- 1.Polanczyk GV, Salum GA, Sugaya LS, et al. Annual Research Review: A meta-analysis of the worldwide prevalence of mental disorders in children and adolescents. J Child Psychol Psychiatry. 2015;56(3):345–65. [DOI] [PubMed] [Google Scholar]
- 2.Copeland WE, Angold A, Shanahan L, Costello EJ. Longitudinal patterns of anxiety from childhood to adulthood: the Great Smoky Mountains Study. J Am Acad Child Adolesc Psychiatry. 2014January;53(1):21–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ormel J, Raven D, Oort F van, et al. Mental health in Dutch adolescents: a TRAILS report on prevalence, severity, age of onset, continuity and co-morbidity of DSM disorders. Psychol Med. 2015January;45(2):345–60. [DOI] [PubMed] [Google Scholar]
- 4.Pine DS, Fox NA. Childhood Antecedents and Risk for Adult Mental Disorders. Annu Rev Psychol. 2015;66(1):459–85. [DOI] [PubMed] [Google Scholar]
- 5.Morris SE, Cuthbert BN. Research Domain Criteria: cognitive systems, neural circuits, and dimensions of behavior. Dialogues Clin Neurosci. 2012March;14(1):29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bergman RL, Piacentini J, Mccracken JT. Prevalence and Description of Selective Mutism in a School-Based Sample. J Am Acad Child Adolesc Psychiatry. 2002August1;41(8):938–46. [DOI] [PubMed] [Google Scholar]
- 7.Lamers F, van Oppen P, Comijs HC, et al. Comorbidity Patterns of Anxiety and Depressive Disorders in a Large Cohort Study: the Netherlands Study of Depression and Anxiety (NESDA). J Clin Psychiatry. 2011March15;72(03):341–8. [DOI] [PubMed] [Google Scholar]
- 8.Penninx BW, Pine DS, Holmes EA, Reif A. Anxiety disorders. The Lancet. 2021March6;397(10277):914–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Robinson OJ, Pike AC, Cornwell B, Grillon C. The translational neural circuitry of anxiety. J Neurol Neurosurg Psychiatry. 2019December1;90(12):1353–60. [DOI] [PubMed] [Google Scholar]
- 10.Krabbe S, Gründemann J, Lüthi A. Amygdala Inhibitory Circuits Regulate Associative Fear Conditioning. Biol Psychiatry. 2018May15;83(10):800–9. [DOI] [PubMed] [Google Scholar]
- 11.Janak PH, Tye KM. From circuits to behaviour in the amygdala. Nature. 2015January;517(7534):284–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kong M-S, Zweifel LS. Central amygdala circuits in valence and salience processing. Behav Brain Res. 2021May11;410:113355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fox AS, Shackman AJ. The central extended amygdala in fear and anxiety: Closing the gap between mechanistic and neuroimaging research. Neurosci Lett. 2019February6;693:58–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gogolla N The insular cortex. Curr Biol. 2017June19;27(12):R580–6. [DOI] [PubMed] [Google Scholar]
- 15.Adhikari A, Topiwala MA, Gordon JA. Synchronized Activity between the Ventral Hippocampus and the Medial Prefrontal Cortex during Anxiety. Neuron. 2010January28;65(2):257–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burkhouse KL, Kujawa A, Hosseini B, et al. Anterior cingulate activation to implicit threat before and after treatment for pediatric anxiety disorders. Prog Neuropsychopharmacol Biol Psychiatry. 2018June8;84(Pt A):250–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Raichle ME. The brain’s default mode network. Annu Rev Neurosci. 2015July8;38:433–47. [DOI] [PubMed] [Google Scholar]
- 18.**.Balderston NL, Flook E, Hsiung A, et al. Patients with anxiety disorders rely on bilateral dlPFC activation during verbal working memory. Soc Cogn Affect Neurosci. 2020December1;15(12):1288–98. [DOI] [PMC free article] [PubMed] [Google Scholar]; In this article the authors investigate dlPFC activation during a working memory task. They included 41 HV and 32 anxiety patients. This work is unique because the authors investigate working memory during safety and unpredictable shock. They found distinct activation patterns in the dlPFC of patients compared to controls.
- 19.Pine DS, Wise SP, Murray EA. Evolution, Emotion, and Episodic Engagement. Am J Psychiatry. 2021June33. doi: 10.1176/appi.ajp.2020.20081187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Backhausen LL, Herting MM, Tamnes CK, Vetter NC. Best Practices in Structural Neuroimaging of Neurodevelopmental Disorders. Neuropsychol Rev [Internet]. 2021April24; Available from: 10.1007/s11065-021-09496-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Frangou S, Modabbernia A, Williams SCR, et al. Cortical thickness across the lifespan: Data from 17,075 healthy individuals aged 3–90 years. Hum Brain Mapp. 2021:1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dima D, Modabbernia A, Papachristou E, et al. Subcortical volumes across the lifespan: Data from 18,605 healthy individuals aged 3–90 years. Hum Brain Mapp. 2021:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.**.Feurer C, Suor JH, Jimmy J, et al. Differences in cortical thinning across development among individuals with and without anxiety disorders. Depress Anxiety. 2021;38(3):372–81. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study investigated cortical thinning in subjects covering a large age range (ages 7–35). The group with anxiety showed a different rate of thinning in the vmPFC compared to controls. The results are a good example of how brain measurements, thus brain-behavior correlations might change during development.
- 24.Gozdas E, Holland SK, Altaye M. Developmental changes in functional brain networks from birth through adolescence. Hum Brain Mapp. 2018December23;40(5):1434–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kundu P, Benson BE, Rosen D, et al. The Integration of Functional Brain Activity from Adolescence to Adulthood. J Neurosci. 2018April4;38(14):3559–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Horien C, Shen X, Scheinost D, Constable RT. The individual functional connectome is unique and stable over months to years. NeuroImage. 2019April1;189:676–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Noble S, Scheinost D, Constable RT. A guide to the measurement and interpretation of fMRI test-retest reliability. Curr Opin Behav Sci. 2021August1;40:27–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.*.Gold AL, Abend R, Britton JC, et al. Age Differences in the Neural Correlates of Anxiety Disorders: An fMRI Study of Response to Learned Threat. Am J Psychiatry. 2020April7;177(5):454–63. [DOI] [PMC free article] [PubMed] [Google Scholar]; The authors of this study used an fMRI paradigm involving extinction recall to compare brain function in youths and adults. They found differences in activation moderated by attention and age in the inferior temporal gyrus and vmPFC. This highlights the need for more research investigating how anxiety-brain relations change across development.
- 29.Thompson PM, Jahanshad N, Ching CRK, et al. ENIGMA and global neuroscience: A decade of large-scale studies of the brain in health and disease across more than 40 countries. Transl Psychiatry. 2020March20;10(1):100–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bas-Hoogendam JM, Groenewold NA, Aghajani M, et al. ENIGMA-anxiety working group: Rationale for and organization of large-scale neuroimaging studies of anxiety disorders. Hum Brain Mapp. 2020July3;1–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zugman A, Harrewijn A, Cardinale EM, et al. Mega-analysis methods in ENIGMA: The experience of the generalized anxiety disorder working group. Hum Brain Mapp. 2020June29; 1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Riley RD, Lambert PC, Abo-Zaid G. Meta-analysis of individual participant data: rationale, conduct, and reporting. BMJ. 2010February5;340:c221. [DOI] [PubMed] [Google Scholar]
- 33.Xu J, Van Dam NT, Feng C, et al. Anxious brain networks: A coordinate-based activation likelihood estimation meta-analysis of resting-state functional connectivity studies in anxiety. Neurosci Biobehav Rev. 2019January1;96:21–30. [DOI] [PubMed] [Google Scholar]
- 34.Casey BJ, Cannonier T, Conley MI, et al. The Adolescent Brain Cognitive Development (ABCD) study: Imaging acquisition across 21 sites. Dev Cogn Neurosci. 2018August;32:43–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jaddoe VWV, Mackenbach JP, Moll HA, et al. The Generation R Study: Design and cohort profile. Eur J Epidemiol. 2006;21(6):475–84. [DOI] [PubMed] [Google Scholar]
- 36.Salum GA, Gadelha A, Pan PM, et al. High risk cohort study for psychiatric disorders in childhood: rationale, design, methods and preliminary results. Int J Methods Psychiatr Res. 2015;24(1):58–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Horien C, Constable RT, Ross DA. Imaging and Reimagining the Mind: fMRI and Psychiatric Illness. Biol Psychiatry. 2021May1;89(9):e45–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bzdok D, Engemann D, Thirion B. Inference and Prediction Diverge in Biomedicine. Patterns (N Y). 2020October8;1(8):100119–100119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Szucs D, Ioannidis JPA. Empirical assessment of published effect sizes and power in the recent cognitive neuroscience and psychology literature. PLOS Biol. 2017March2;15(3):e2000797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.*.Linke JO, Abend R, Kircanski K, et al. Shared and Anxiety-Specific Pediatric Psychopathology Dimensions Manifest Distributed Neural Correlates. Biol Psychiatry. 2021March15;89(6):579–87. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study aimed at using canonical correlation analysis to identify correlated latent dimensions of psychopathology and brain connectivity. Of the three canonical variates identified in both discovery and replication samples, two captured shared components of psychopathology and one was specific to anxiety. The associations with the brain consisted of multiple weak distributed associations.
- 41.Alfaro-Almagro F, McCarthy P, Afyouni S, et al. Confound modelling in UK Biobank brain imaging. NeuroImage. 2021January1;224:117002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Smith SM, Nichols TE. Statistical Challenges in “Big Data” Human Neuroimaging. Neuron. 2018January17;97(2):263–8. [DOI] [PubMed] [Google Scholar]
- 43.Varoquaux G, Raamana PR, Engemann DA, et al. Assessing and tuning brain decoders: Cross-validation, caveats, and guidelines. NeuroImage. 2017January15;145:166–79. [DOI] [PubMed] [Google Scholar]
- 44.Bzdok D, Nichols TE, Smith SM. Towards Algorithmic Analytics for Large-scale Datasets. Nat Mach Intell. 2019July;1(7):296–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen J, Li X, Calhoun VD, et al. Sparse deep neural networks on imaging genetics for schizophrenia case–control classification. Hum Brain Mapp. 2021;42(8):2556–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Scheinost D, Noble S, Horien C, et al. Ten simple rules for predictive modeling of individual differences in neuroimaging. NeuroImage. 2019June1;193:35–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shen X, Finn ES, Scheinost D, et al. Using connectome-based predictive modeling to predict individual behavior from brain connectivity. Nat Protoc. 2017March;12(3):506–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.**.Wang Z, Goerlich KS, Ai H, et al. Connectome-Based Predictive Modeling of Individual Anxiety. Cereb Cortex. 2021June1;31(6):3006–20. [DOI] [PubMed] [Google Scholar]; The authors have applied CPM on resting-state data to predict trait anxiety in 76 individuals. Connections between limbic and prefrontal regions were particularly important for prediction. Their findings generalized to a larger validation sample. These results show that connectome-based predictive modeling is a promising tool for studying anxiety.
- 49.Krawczyk B Learning from imbalanced data: open challenges and future directions. Prog Artif Intell. 2016November1;5(4):221–32. [Google Scholar]


