Abstract
Objective:
The purpose of this study was to assess cerebral blood flow (CBF) and its association with self-reported symptoms in chronic traumatic brain injury (TBI).
Participants:
Sixteen participants with mild to severe TBI and persistent self-reported neurological symptoms, 6 to 72 months post-injury were included. For comparison, 16 age- and gender-matched healthy normal control participants were also included.
Main Measures:
Regional CBF and brain volume were assessed using pseudo-continuous Arterial Spin Labeling (PCASL) and T1-weighted data respectively. Cognitive function and self-reported symptoms were assessed in TBI participants using the national institutes of health (NIH) Toolbox Cognition Battery and Patient-Reported Outcome Measurement Information System respectively. Associations between CBF and cognitive function, symptoms were assessed.
Results:
Global CBF and regional brain volumes were similar between groups, but region of interest (ROI) analysis revealed lower CBF bilaterally in the thalamus, hippocampus, left caudate, and left amygdala in the TBI group. Voxel-wise analysis revealed that CBF in the hippocampus, parahippocampus, rostral anterior cingulate, inferior frontal gyrus, and other temporal regions were negatively associated with self-reported anger, anxiety, and depression symptoms. Furthermore, region of interest (ROI) analysis revealed that hippocampal and rostral anterior cingulate CBF were negatively associated with symptoms of fatigue, anxiety, depression, and sleep issues.
Conclusion:
Regional CBF deficit was observed in the group with chronic TBI compared to the normal control (NC) group despite similar volume of cerebral structures. The observed negative correlation between regional CBF and affective symptoms suggests that CBF-targeted intervention may potentially improve affective symptoms and quality of life after TBI, which needs to be assessed in future studies.
Keywords: chronic traumatic brain injury, cerebral blood flow, hippocampus, rostral anterior cingulate, affective symptoms, anxiety, depression, fatigue, sleep disturbance, sleep impairment
1. Introduction
Traumatic brain injury (TBI) has lifelong implications on brain function, however, long-term outcomes after TBI are unpredictable (Wilson et al., 2017). In addition to neuronal injury, cerebrovascular dysfunction, which includes hypo perfusion, impaired cerebral autoregulation, blood-brain barrier disruption, and edema formation, is well documented in the acute stage after TBI (Jullienne et al., 2016). These acute vascular injuries and cerebrovascular dysfunction may improve over time to an extent, but deficits in global or regional CBF have been observed in the chronic TBI stage (Gilkey et al., 1997; Haber et al., 2018; Kenney et al., 2016; Kim et al., 2010).
Regional CBF deficits may contribute to neurological and behavioral outcomes in the chronic stage after TBI. For example, participants with post-traumatic headaches had regional and hemispheric CBF asymmetries as measured by the “xenon (Xe) 133 inhalation imaging” (Gilkey et al., 1997). A single-photon emission computerized tomography (SPECT) study also suggested that lower frontal CBF correlated with worsening disinhibited behavior and lower CBF in thalamic regions was associated with cognitive impairment in individuals with severe TBI (Oder et al., 1992). However, these methods are expensive and require exposure to radioactive agents and radiation.
Regional CBF can be noninvasively assessed using Arterial Spin Labeling (ASL), an MRI- based technique that magnetically labels water molecules in the blood flowing into the brain instead of using an exogenous contrast agent (Alsop et al., 2015; Andre, 2015). Recent reports have demonstrated the usage of ASL-based CBF as a biomarker to predict cognitive function in the elderly (De Vis et al., 2018), assess brain function in athletes (Thomas et al., 2013), detect and monitor Alzheimer’s disease (AD) progression (Wang et al., 2013),(Chao et al., 2010), and assess brain function response to therapeutic intervention in early AD (Thomas et al., 2020). Regional and global alterations of CBF based on ASL have also been reported in animal models of TBI (Foley et al., 2013; Hayward et al., 2010; Hayward et al., 2011; Kochanek et al., 2002; Park et al., 2009; Shen et al., 2007; Steinman et al., 2019; Wei et al., 2009) and in individuals with TBI (Amyot et al., 2018; Haber et al., 2018; Kim et al., 2010; Lin et al., 2016; Stephens et al., 2018; Wang et al., 2016). Furthermore, regional CBF alterations during the acute and subacute stages were found to correlate with performance on neuropsychological tests and post-concussion symptoms after mild TBI (Lin et al., 2016; Stephens et al., 2018; Wang et al., 2016). Thus, ASL is a promising method to non-invasively measure CBF and may represent a sensitive imaging biomarker to plan future treatments and detect the effects of therapeutic interventions on brain function in chronic TBI. However, the association of ASL-based CBF with neurological and behavioral symptoms in the chronic stage is not well understood.
The purpose of this study was to assess regional CBF using the ASL technique in chronic TBI participants compared to age and gender-matched healthy normal control (NC) participants. We hypothesized that regional CBF deficits would be observed in TBI participants and that CBF deficits would be associated with TBI-related symptoms.
2. Results
2.1. Participant Characteristics
A total of 16 (9 female) participants with TBI (27 to 63 years old with a mean age of 48) were recruited (Table 1). Five participants had a history of moderate-to-severe TBI and 11 had mild TBI. None of the participants had surgeries for TBI during the acute stage. Twelve injuries were motor vehicle related, 2 due to falls, 1 due to assault, and 1 was sports-related. Age at injury ranged from 26 to 61 years old with a mean of 47.7. The time between the initial injury and the MRI scan ranged from 6 to 72 months, with a mean of 20 months. Four of the participants with TBI had abnormal head computerized tomography (CT) at the initial presentation, including subarachnoid hemorrhage, cerebral contusion, subdural hemorrhage, and skull fracture. Five had normal head CT, and the initial head CT findings were not available in the remaining 7 participants (Table 1). Duration of loss of consciousness was less than 30 minutes for 8 participants, ≥ 30 minutes for 4 particpants, and not known for 5 participants (Table 1). As part of the inclusion criteria, all participants reported symptoms on the RPQ. The mean RPQ score was 30, with a range between 7 and 51 (RPQ range is 0–64 with higher scores indicative of more severe symptoms). The most commonly reported symptoms were slow thinking process, forgetfulness, and being irritable. None of the participants with TBI had focal lesions visible on T2 FLAIR MRI at the time of the study.
Table 1.
TBI information, cognitive and psychological results
| Sample Size | 16 (9 females, 7 males) | |
| TBI Mechanism | RTA (12), Fall (2), Assault (1), Sport (1) | |
| TBI Severity | Mild (11), Moderate -Severe (5) | |
| Initial head CT | Normal (5), Abnormal (4), unknown (7) | |
| Duration of LOC | < 30 mins (8), ≥ 30 minutes (3), unknown (5) | |
| Age at injury (years) | 47.7 ± 12.3 | (26 – 61, 51) |
| Time since Injury (months) | 20 ± 16 | (6 – 72, 15) |
| RPQ | 30 ± 13 | (7 – 51, 34) |
| Cognition Measures (NIHTB-CB) | ||
| Total Composite Score | 49 ± 9 | (35 – 64, 49) |
| Fluid Composite Score | 45 ± 11 | (27 – 70, 43) |
| Attention - Flanker Inhibitory Control Attention | 44 ± 11 | (27 – 61, 44) |
| Working memory - List Sort Working Memory | 48 ± 9 | (30 – 62, 50) |
| Executive function - Dimensional Change Card Sort | 54 ± 13 | (30 – 75, 56) |
| Processing speed - Pattern Comparison | 41 ± 16 | (17 – 72, 37) |
| Episodic memory - Picture Sequence Memory | 46 ± 8 | (28 – 62, 45) |
| Crystallized Composite Score- Language | 53 ± 8 | (42 – 69, 54) |
| Picture Vocabulary | 51 ± 7 | (42 – 66, 52) |
| Oral Reading Recognition | 55 ± 10 | (37 – 71, 54) |
| Patient-Reported Outcome Measures (PROMIS) | ||
| Sleep Related Impairment | 54 ± 11 | (30 – 65, 58) |
| Sleep Disturbance | 52 ± 10 | (30 – 69, 54) |
| Fatigue | 56 ± 6 | (43 – 64, 57) |
| Pain Interference | 56 ± 7 | (39 – 65, 57) |
| Depression | 54 ± 8 | (39 – 70, 53) |
| Anxiety | 55 ± 8 | (37 – 65, 57) |
| Anger | 54 ± 9 | (36 – 66, 55) |
All data are presented in mean ± standard deviation (minimum – maximum, median). RTA: road traffic accidents which include motor vehicle accident, motorcycle collision, and motor vehicle pedestrian accident. LOC: Loss of consciousness. RPQ: Rivermead Post-Concussion Symptoms Questionnaire. Cognition measures and PROMIS data are presented as normalized T-scores (mean of 50, SD = 10) corrected for age, sex, education, and race/ethnicity in the general population.
The overall group NIHTB-CB total composite score, Fluid composite score, and crystallized composite score were within the normal range of the general population, suggesting relatively good recovery (Table 1).
Participants with TBI also completed the PROMIS measures. All PROMIS scores were within the average range (Table 1), although 56% of participants reported clinically significant symptoms in at least one PROMIS domain (T score >1 SD above the mean of the general population).
2.2. CBF in TBI
Whole-brain voxel-wise CBF maps revealed significantly lower CBF in the left thalamus (Figure 1a) in participants with TBI compared to the NC group. The reverse comparison at the same statistical significance threshold did not reveal any regions with increased CBF in the TBI group compared to the NC group. ROI analysis showed lower CBF bilaterally in the thalamus and hippocampus, left caudate, and amygdala in participants with TBI (Table 2). It has to be noted that voxel-wise analysis is less sensitive at detecting differences between groups due to comparison of multiple voxels across groups.
Figure 1.
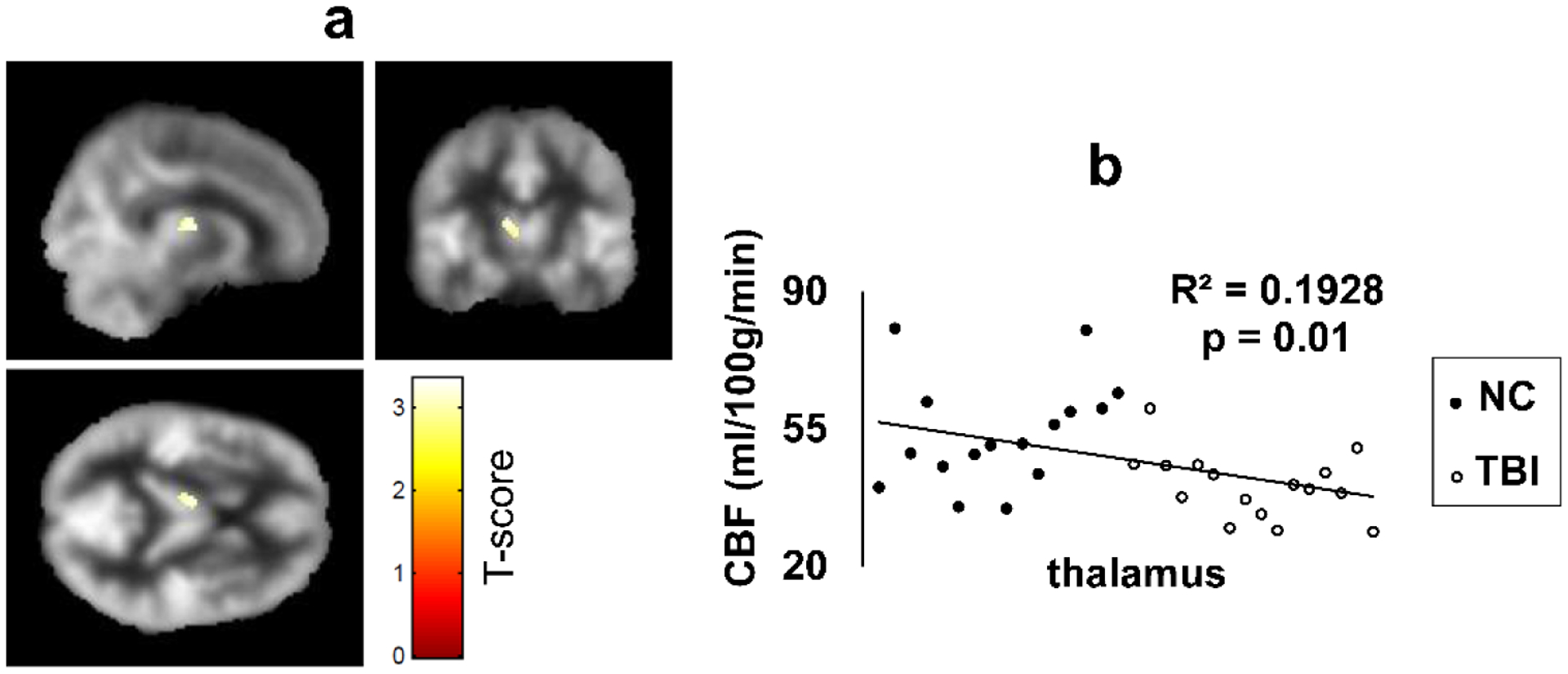
(a) Voxel-wise comparison (T-score map) of CBF between the TBI and NC group. Colored voxels indicate regions with statistically significant CBF deficit in the group with TBI compared to the NC group (p=0.005, uncorrected, minimum cluster size (k) = 50 voxels). Cross sectional view through the CBF deficit cluster in the Thalamus. Colored voxels are overlaid onto a group averaged CBF map from all participants in the TBI and NC groups.
(b) The scatter plot of CBF values from the Thalamus ROI for all subjects. CBF from NC are represented as filled circles and TBI as open circles. CBF: cerebral blood flow.
Table 2.
Demographics, CBF and brain volume ROI data from NC and TBI groups
| Variables | NC | TBI | p-value |
|---|---|---|---|
| N (women) | 16 (9) | 16 (9) | - |
| Age (years) | 47.3 ± 11.8 | 47.7 ± 12.3 | 0.930 |
| CBF (ml/100g/min) | |||
| Total cerebral cortex | 49.1 ± 7.9 | 50.6 ± 8.9 | 0.632 |
| Left hippocampus | 59.0 ± 10.8 | 45.8 ± 7.3 | 0.0003 |
| Left caudate | 37.5 ± 10.5 | 25.9 ± 7.6 | 0.001 |
| Right hippocampus | 59.3 ± 10.3 | 48.0 ± 7.4 | 0.001 |
| Left thalamus | 52.5 ± 12.4 | 39.3 ± 8.0 | 0.001 |
| Right thalamus | 54.4 ± 12.3 | 41.9 ± 9.8 | 0.003 |
| Left amygdala | 52.9 ± 10.5 | 43.3 ± 8.9 | 0.009 |
| Brain Volume (% WBV) | |||
| Total cerebral cortex | 27.3 ± 2.23 | 25.63 ± 2.81 | 0.23 |
| Left thalamus | 0.53 ± 0.06 | 0.53 ± 0.06 | 0.84 |
| Right thalamus | 0.43 ± 0.05 | 0.41 ± 0.04 | 0.43 |
| Left caudate | 0.20 ± 0.02 | 0.19 ± 0.02 | 0.20 |
| Left hippocampus | 0.25 ± 0.03 | 0.23 ± 0.03 | 0.19 |
| Right hippocampus | 0.25 ± 0.03 | 0.23 ± 0.02 | 0.05 |
| Left amygdala | 0.09 ± 0.01 | 0.09 ± 0.01 | 0.33 |
All data are presented as mean ± standard deviation
2.3. Correlation between regional CBF and cognitive and PROMIS measures
In the TBI group, there was no significant correlation between total cortical CBF and time since injury, RPQ, or cognitive performance. After controlling for age and sex, a significant negative correlation was observed between voxel-wise CBF in several regions and PROMIS measurements of affective symptoms. For example, participants with TBI who reported greater anger symptoms had lower CBF in the hippocampus, parahippocampus, and lentiform nucleus (Figure 2a). Participants with TBI that reported higher anxiety symptoms had lower CBF in the rostral anterior cingulate (BA 32), left hippocampus and parahippocampus (BA 28), right hippocampus and parahippocampus, bilateral inferior frontal gyrus (BA 13) (Figure 2b). Higher depression scores were also associated with lower CBF in the hippocampus, parahippocampus, and amygdala (Figure 2c). Of note, lower hippocampal CBF was associated with higher mood symptoms including anger, anxiety, and depression in participants with TBI.
Figure 2.
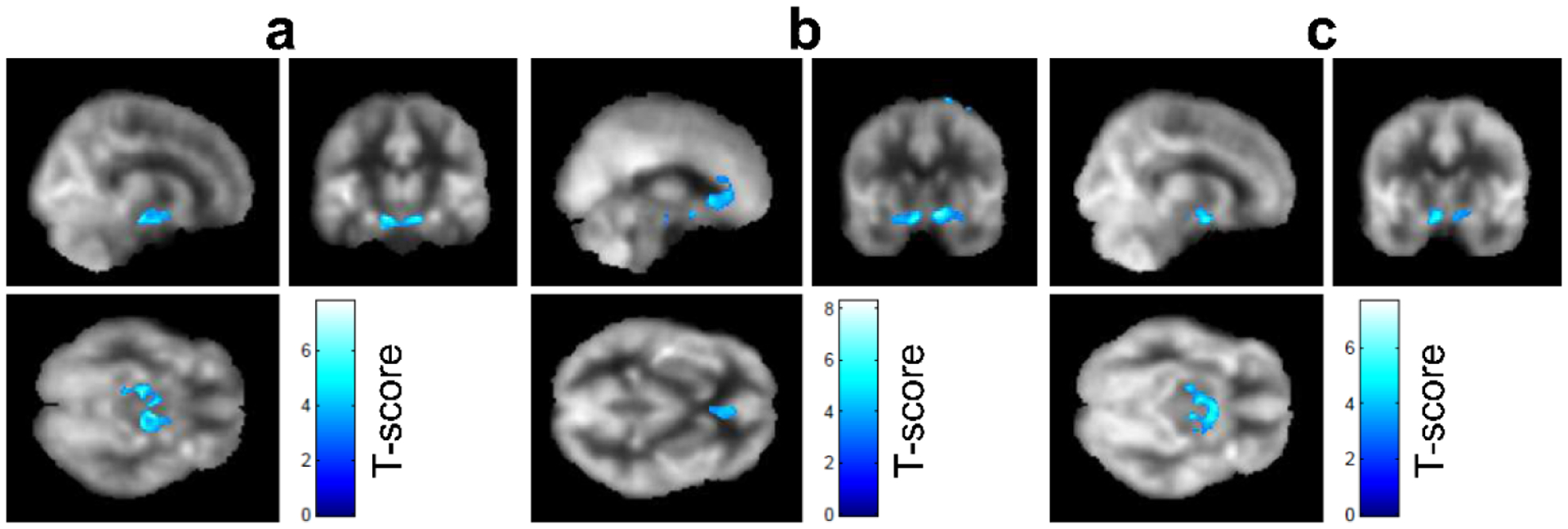
Voxel-wise negative correlation (T-score map) of CBF with TBI-related symptoms: (a) anger, (b) anxiety, and (c) depression after regressing out age and sex in the group with TBI. The colored voxels indicate regions that show statistically significant negative correlation (FWE p=0.05). Colored voxels are overlaid onto a group averaged CBF map from all TBI participants. CBF: cerebral blood flow; FWE: family-wise-error correction.
We then performed a Pearson correlation between regional CBF extracted from the bilateral hippocampus ROI and TBI-related symptom scores. A significant negative correlation was observed between hippocampal CBF and reported symptoms of fatigue (p=0.002), anxiety (p=0.01), depression (p=0.01), sleep impairment (p=0.008), (Figure 3 a - d) anger (p=0.07), and sleep disturbance (p=0.07).
Figure 3.
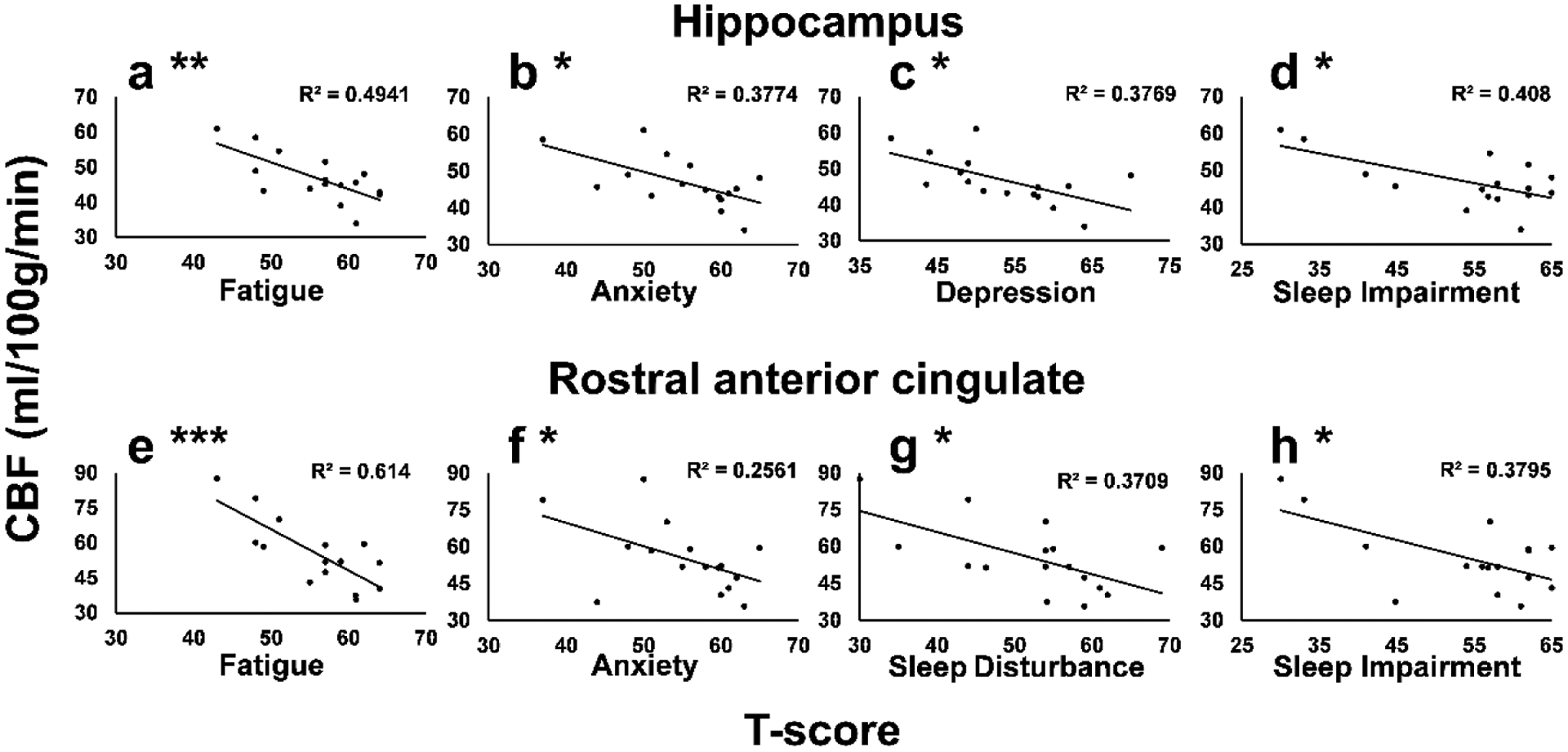
Negative correlation between hippocampus ROI CBF (a – d) and TBI-related symptoms of (a) Fatigue (p=0.002), (b) Anxiety (p=0.01), (c) Depression (p=0.01), and (d) Sleep Impairment (p=0.008) and negative correlation between rostral anterior cingulate cortex ROI CBF (e – h) and TBI-related symptoms of (e) Fatigue (p=0.0003), (f) Anxiety (p=0.045), (g) Sleep Disturbance (p=0.01), and (h) Sleep Impairment (p=0.01). TBI-related symptoms are reported as T-scores; significant correlations are denoted as: * p<0.05; ** p<0.005; *** p<0.0005
A significant negative correlation was also observed between CBF extracted from the rostral anterior cingulate ROI and self-reported symptoms of fatigue (p=0.0003), anxiety (p=0.045), sleep disturbance (p=0.01), and sleep impairment (p=0.01) (Figure 3 e - h).
2.4. Volumetric Changes in TBI
ROI analysis revealed that the right hippocampus volume was slightly lower in the group with TBI compared to the NC group (uncorrected p=0.05) (Table 2). Global brain volume and other ROI’s did not differ significantly between groups.
3. Discussion
This study demonstrated that 1) Participants with TBI and ongoing neurological symptoms had significantly lower CBF in the bilateral thalamus, bilateral hippocampus and parahippocampus, and the left caudate despite normal brain volume and cognitive performance. 2) Voxel-wise regression revealed that CBF deficits, mostly in the hippocampus and rostral anterior cingulate were associated with higher levels of self-reported symptoms of anger, anxiety, and depression. Notably, CBF in the hippocampus and rostral anterior cingulate was negatively correlated with most self-reported symptoms. Taken together, these findings provide additional evidence that regional CBF deficits exist in the chronic stage of TBI and that lower CBF in the hippocampus and rostral anterior cingulate may play a role in self-reported affective symptoms.
3.1. Negative correlations between CBF and behavioral symptoms
To our knowledge, this is the first study to report negative associations between regional CBF and self-reported affective symptoms using PROMIS. Significant negative correlations were observed between CBF in several regions and multiple self-reported symptoms. For example, CBF in the hippocampus was significantly negatively correlated with symptoms such as fatigue, anxiety, depression, and sleep impairment. Lower CBF in the rostral anterior cingulate cortex was significantly associated with more severe self-reported symptoms such as fatigue, anxiety, sleep disturbance, and sleep impairment. Our results suggest CBF deficits due to TBI persist in the chronic stage and may play a role in reported affective symptoms.
Several previous studies also reported negative associations between CBF and symptoms of headache and disinhibited behavior after TBI (Gilkey et al., 1997; Oder et al., 1992). Lin et al. reported that post-concussive symptoms of dizziness were negatively correlated with CBF in the bilateral frontal and left occipital cortex a month after mild TBI (Lin et al., 2016). Our findings do not replicate results of thalamus CBF being negatively associated with the cognitive performance that has been reported, however (Ge et al., 2009). The discrepancy may be due to TBI heterogeneity and the largely intact cognitive performances in our cohort. Of note, a causal relationship between regional CBF and neurological or behavioral symptoms cannot be addressed from this or previous cross-sectional studies, and it should be noted that mean scores across cognitive and emotional measures were within normal limits, which may have limited the magnitude of the correlations. Based on prior research, we speculate that microvascular injury from TBI may lead to regional cerebral hypo perfusion, which is further heightened in the chronic stage and may be linked with affective symptoms (Gilkey et al., 1997; Oder et al., 1992). Thus, regional CBF deficit may potentially be useful as a pharmacodynamics biomarker for perfusion targeted therapies to ameliorate chronic affective symptoms after TBI.
3.2. CBF deficits in TBI
Our finding of regional CBF deficits in participants with TBI is in alignment with the literature and further supports the idea that regional cerebral hypo perfusion may be a common phenomenon in individuals with chronic TBI who experience persistent neurobehavioral symptoms across the spectrum of TBI severity. We observed lower CBF bilaterally in the thalamus, hippocampus and parahippocampus, left caudate, and amygdala in the participants with TBI compared to the NC group. Subcortical structures such as the thalamus and the caudate are located centrally in the brain and can be affected by the shear stress and rotational forces resulting from TBI (Grossman and Inglese, 2016; Medana and Esiri, 2003). The thalamus is considered a central relay switch to the brain which has fiber projections to sensory, motor, and associative regions in the brain and is involved in the processing and transmission of information between these regions. It has also been reported that injury to the thalamus after TBI can lead to affective symptoms such as fatigue, insomnia, headache (Grossman and Inglese, 2016). CBF reductions in the thalamus have been reported in the chronic TBI stage. For example, decreased bilateral thalamus CBF was reported in participants with chronic mild TBI after an average of 26.5 months post injury (Ge et al., 2009). A cohort of 15 symptomatic sports-related concussion pediatric patients studied at a mean of 6 months after their concussion showed reduced CBF in the thalamus bilaterally compared to healthy age-matched controls (Bartnik-Olson et al., 2014). Reduction in CBF in the posterior cingulate cortices, the thalamus, and multiple locations in the frontal cortices has also been reported in participants with chronic moderate-to-severe TBI (Kim et al., 2010).
3.3. Relationship between CBF deficits and brain structural integrity
We observed regional CBF deficits in the chronic TBI group despite intact brain volume. This suggests that regional CBF deficits can exist independent of structural status after TBI and that vascular injuries are plausible without the presence of observable volumetric deficits in the chronic TBI stage and may be a biomarker for this stage of TBI. It is also possible that reversing chronic CBF deficits may need an intervention. Literature regarding the relationship between structural abnormalities and vascular function after TBI is inconclusive. Several studies suggested that regional hypo perfusion was seen around focal structural lesions (Amyot et al., 2018; Haber et al., 2018; Kim et al., 2010). Other studies demonstrated regional CBF deficits in normal-appearing regions in conventional FLAIR MRI. For example, mild TBI with normal-appearing FLAIR showed regional CBF reduction in the bilateral frontal and left occipital regions (Lin et al., 2016). In a fluid percussion injury animal model, both reductions of microvascular density and capillary diameter were observed not only in the original injury location but also diffusely in the ipsilateral hemisphere and the contralateral hippocampus with more severe injuries (Park et al., 2009). Also, the relationship between CBF and brain volume or white matter integrity may be dynamic and time-dependent as shown in a recent study in veterans with blast-exposed or moderate levels of combat exposure while on deployment (Clark et al., 2017). In this military cohort of mild to moderate TBI, reduced CBF was associated with poorer brain white matter microstructural integrity, which was observed in the more chronic phase (> 5 years), but not in the more recent phase (~ 3 years) (Clark et al., 2017). However, care should be taken in comparing studies of blunt injury versus blast injury.
3.4. Limitations
This pilot study is limited by the small sample size. Also, the heterogeneity of the TBI group with a relatively wide time range since the initial injury, as well as the varied severity of brain injury, may limit generalizability. Our findings should be validated in a more homogeneous population (i.e., mild injury without initial pathological findings on CT/MRI) to minimize the effect of other confounding factors. Even though cognitive function results were corrected for age, gender, education, race/ethnicity and that all composite cognitive function scores were in the normal range of the general population, it is to be noted that cognitive function testing was performed in the TBI group alone and not in the NC group. It is possible that observed CBF deficits in the TBI group compared to the NC group were due to differences in cognitive performances between groups and cannot be assessed as cognitive function data from the NC group was not evaluated. There are differences between voxel-wise and ROI analysis, which can be attributed to the voxel-wise analysis being less sensitive at detecting differences between groups, due to multiple voxels in the entire brain being compared across groups. However, it is to be noted that voxel-wise analysis were considered significant at a threshold of p=0.005 whereas ROI analysis were significant at the threshold of p=0.05, which may explain some of the differences between these results. Despite these limitations, this study has several strengths. This is one of the few studies to demonstrate regional CBF deficit in chronic TBI without significant brain structural abnormalities. Additionally, combining self-reported symptoms, cognitive testing using PROMIS and NIHTB-CB, and imaging markers strengthen our understanding of the TBI pathophysiology and its relationship to clinical outcome measures. Last, we are the first group to show a significant negative association between CBF and PROMIS-measured symptoms in participants with TBI.
4. Conclusions
CBF was lower bilaterally in subcortical regions such as the thalamus, hippocampus, parahippocampus, the left caudate, and left amygdala in this chronic TBI cohort without significant brain structural abnormalities when compared with a healthy control group. Furthermore, a decrease in regional CBF especially in the hippocampus and rostral anterior cingulate cortex was associated with higher self-reported symptoms including anxiety, anger, depression, fatigue, sleep disturbance, and impairment in the participants with TBI. Larger scale future perfusion studies with clinical outcome measures may lead to alternative treatment interventions to improve functional outcome and quality of life in chronic TBI.
5. Methods and Experimental Procedures
5.1. Participants
Participants with TBI were recruited from the trauma registry database of the Parkland Hospital Level 1 Trauma Center, prior observational TBI studies, and the TBI clinics at the UT Southwestern Medical Center and Parkland Hospital. Specific inclusion criteria were: 1) a history of TBI defined by the American Congress of Rehabilitation Medicine (ACRM) (Menon et al., 2010); 2) blunt mechanism of head injury without penetration; 3) persistent symptoms endorsed on the Rivermead Post-Concussion Symptoms Questionnaire (RPQ) which include headaches, dizziness, nausea and/or vomiting, noise or light sensitivity, sleep disturbance, fatigue, irritability and restlessness, forgetfulness, poor concentration, slow thinking process, and visual disturbance (Balalla et al., 2020; King et al., 1995); 4) 6 months to 6 years after initial TBI; 5) age between 18 to 65 years; and 6) fluent in English to complete neuropsychological measures. Exclusion criteria were: a history of multiple TBIs, substance abuse, any major psychiatric or other neurological disorders (e.g. stroke, dementia, etc.), taking medications that might have an effect on cognition or vascular reactivity, uncontrolled hypertension, diabetes mellitus, obesity with a body mass index (BMI) greater than or equal to 35 kg/m2, history of recurrent epilepsy, any other significant or unstable medical conditions, or contraindication for MRI scan (i.e. severe claustrophobia, a pacemaker or any metals in the body).
Mild TBI was defined as an initial Glasgow Coma Scale (GCS) in the range of 13 to 15, loss of consciousness (LOC) ≤ 30 minutes, and post-traumatic amnesia ≤ 24 hours. Moderate to severe TBI included abnormal acute head computed tomography plus initial GCS 3–12, LOC > 30 minutes, or PTA > 1 day (Department of Veterans Affairs, 2016) (Gerberding, 2003).
Screening procedures included a detailed medical history and medication questionnaire, a comprehensive physical examination, a 12-lead electrocardiogram, an echocardiography, and a carotid artery ultrasound to exclude severe cardiac conditions and severe carotid stenosis (> 50%).
Normal control (NC) participants were selected to pair with TBI participants as similar as possible in age and sex (potential covariates of cerebral perfusion). NC participants were randomly selected from an existing database containing de-identified data from our prior study “Arterial, aging, brain perfusion & exercise: impact on brain structure & function” (Xing et al., 2017). The same exclusion criteria applied to NC participants as TBI patients.
This HIPAA-compliant study was approved by the Institutional Review Boards at the UT Southwestern Medical Center, Parkland Hospital, and Texas Health Presbyterian Hospital Dallas and was performed following the guidelines of the Declaration of Helsinki and Belmont Report. All participants were granted informed consent.
5.2. Magnetic Resonance Imaging (MRI) acquisition and processing
MRI sequences including 3D magnetization-prepared-rapid-acquisition-of-gradient-echo (MPRAGE), T2 fluid-attenuated inversion recovery (FLAIR), and 2D pseudo-continuous ASL (PCASL) were acquired on a 3-Telsa scanner (Philips Medical System) with a body coil for radio frequency transmission and an 8-channel receive head coil with parallel imaging capability for signal reception.
5.2.1. T1-weighted high-resolution image acquisition and processing
T1-weighted high-resolution image was acquired using the MPRAGE sequence with following parameters: echo time (TE)/repetition time (TR) = 3.7/8.1 ms, flip angle = 12°, field of view = 256 mm × 204 mm, 160 1-mm slices, resolution = 1×1×1 mm3, SENSE factor = 2, and scan duration = 4 minutes.
Brain segmentation was performed using the FreeSurfer software (version 6.0, http://surfer.nmr.mgh.harvard.edu/) (Fischl, 2012). FreeSurfer data processing stream generates regional cortical and subcortical volumes, areas, and thicknesses (Han et al., 2006). Regional gray matter volumes were normalized by intracranial volume.
5.2.2. ASL acquisition and processing
ASL data were acquired at rest, using a 6-min 2D PCASL technique and employing a single-shot echo-planar-imaging (EPI) sequence (Alsop et al., 2015). Label duration was 1650 ms and the post-labeling delay was 1525 ms. The other ASL parameters were: TR/TE = 4260 /14 ms, pixel size = 3 × 3 mm2, slice thickness = 5 mm, field of view (FOV) = 240 mm × 240 mm, flip angle = 90°, 29 slices, 40 label/control pairs, no background suppression.
FMRIB Software Library (FSL, https://surfer.nmr.mgh.harvard.edu/fswiki) was used to preprocess the PCASL data from each subject. Rigid motion in the PCASL raw images was first corrected using MCFLIRT tool (Jenkinson et al., 2002). Then spatial smoothing was performed using a 3D Gaussian kernel with a 6-mm full width at half maximum. Finally, regional CBF was quantified in ml of blood /100 g of tissue /min based on the following PCASL equation: (Alsop et al., 2015)
where SASL is the signal difference between the control and label images from the PCASL acquisition. T1 of blood (T1blood) = 1650 ms, labeling efficiency (α) = 0.85, blood-brain partition coefficient of the whole brain (λ) = 0.9, w is the post-labeling delay time (1525 ms). M0 reflects the signal intensity of spins at equilibrium magnetization and was estimated by: (average of 40 control images)/(1-exp(−TR/T1tissue) with T1tissue = T1 of gray matter = 1421 ms (Zhu and Penn, 2005); and τ is the label duration (1650 ms). The CBF maps were co-registered to the T1-weighted MPRAGE image using the FLIRT tool in FSL and further normalized to the Montreal Neurological Institute (MNI) template using FNIRT to generate the CBF map in MNI space. Group-based, whole-brain, voxel-wise analysis of regional CBF was performed with Statistical Parametric Mapping (SPM 8) (Wellcome Department of Cognitive Neurology, UK) in Matlab (Mathworks, Natick, MA). Voxel-wise comparison of CBF maps between the TBI and NC groups was performed using SPM 8 (Friston, 1994). Region of interest (ROI) analysis was performed on regions segmented by Freesurfer (Fischl, 2012).
CBF quantification from white matter is not as reliable as compared to that from gray matter, given that white matter contains 70 to 75% less vasculature as present in gray matter (Lu et al., 2005). So, ROI analysis were focused on cortical and subcortical gray matter and did not include white matter regions.
5.2.3. FLAIR acquisition
To assess if focal lesions were present, Fluid-Attenuated-Inversion-Recovery (FLAIR) image was acquired. Imaging parameters of the sequence were as follows: TR/TE = 11,000/125 ms, inversion time (TI) = 2800 ms, FOV = 230 × 230 mm2, 64 slices, 2.5 mm thick, no gap, reconstruction matrix = 512, scan duration=3 min, 40 s.
5.3. Neuropsychological performance and patient-reported outcome measurements
Cognitive function was assessed in the TBI group by a trained research team member using the NIH Toolbox Cognition Battery (NIHTB-CB) iPad version (Gershon et al., 2013; Weintraub et al., 2013). Tests included the Flanker inhibitory control and attention test, list sort, picture sequence memory, dimensional change card sort (DCCS), and the pattern comparison processing speed test. These tests assessed attention, executive function, working memory, episodic memory, and processing speed. Language function was assessed with the picture vocabulary test and oral reading recognition. The NIHTB-CB Fluid Cognition composite score (including DCCS, Flanker, picture sequence memory, list sorting, and pattern comparison metrics) specifically assess cognitive function used in problem-solving, thinking and acting quickly, and encoding. The NIHTB-CB Crystalized Cognition composite score (including picture vocabulary and reading recognition measures) assesses verbal reasoning function. The total cognitive function composite score is a summary score of all NIHTB-CB measures. The NIH Toolbox generates corrected T-scores that are adjusted for age, sex, education, and race/ethnicity. T-scores have a mean of 50 and standard deviation (SD) of 10, with scores <40 considered impaired performance (Tulsky et al., 2017).
The Patient-Reported Outcome Measurement Information System (PROMIS) computer-adaptive tests were used to measure self-reported physical and mental health. These tests have been validated in the TBI population (Carlozzi et al., 2019a; Carlozzi et al., 2019b; DeWalt et al., 2007). The following PROMIS measures were administered: sleep disturbance, sleep-related impairment, fatigue, pain interference, depression, anxiety, and anger. These measures produce scores based on a population corrected T-score metric (i.e. M = 50, SD =10), where higher scores represent worse physical or mental health.
5.4. Statistics Analysis
Voxel-wise comparison of CBF maps between the TBI and NC groups was performed using two-sample t-test in SPM8 (Friston, 1994). Voxel-wise correlations of CBF maps with PROMIS measures were performed in the TBI group using multiple regression analysis in SPM 8. Age and sex were regressed out from the regression analysis. Voxels with a Family-Wise-Error (FWE) correction for multiple comparisons threshold of p < 0.05 were considered significant for this regression analysis. T-score maps were generated in SPM during statistical significance testing of voxel-wise comparison between the NC and TBI groups and for the regression analysis of voxel-wise CBF maps with PROMIS measures. Given the small sample size, we did not perform comparisons between different severities of TBI.
Two sample t-tests were performed to assess differences between TBI and NC groups in regional CBF and brain volume using SPSS 26.0 (SPSS, Inc. Chicago, IL). Given the exploratory nature of this study, correction for multiple comparisons was not performed for the voxel-wise and ROI analysis of comparison between NC and TBI groups. Voxel-wise group comparison analysis was considered significant at an uncorrected threshold of p=0.005. To make the voxel-wise group comparison analysis more stringent a contiguous significant voxel threshold of 50 voxels was applied to eliminate smaller clusters from falsely appearing significant.(Tamminga et al., 2012) This additional cluster threshold was not applied to voxel-wise regression analysis which survived a more stringent FWE correction for multiple comparisons threshold of p < 0.05.
We also performed a Pearson correlation between regional CBF extracted from the bilateral hippocampus, the rostral anterior cingulate ROI, and TBI-related symptom scores. Hippocampus, and rostral anterior cingulate ROIs were chosen for correlation analysis as these were two key regions that showed significant negative correlation between CBF and symptom scores in the voxel-wise regression analysis. Correlations were considered significant at p < 0.05 and were not corrected for multiple comparisons.
Hippocampal, anterior cingulate blood flow is associated with chronic TBI symptoms
Blood flow was negatively associated with symptoms of fatigue, anxiety, depression, sleep issues
Regional cerebral blood flow deficit was observed in the group with chronic TBI
Cerebral blood flow was assessed with pseudo-continuous Arterial Spin Labeling MRI
Acknowledgments
The authors thank our study participants, Justin Repshas and Marcel Turner for technical support, and Evie Kwei for editing assistance. We also thank the grant support from the Darrell K. Royal Research Fund, the Texas Institute for Brain Injury and Repair (TIBIR) at UT Southwestern Medical Center, and National Institutes of Health: R01HL102457.
Abbreviations:
- SPECT
single photon emission computerized tomography
- PCASL
pseudo-continuous arterial spin labeling
- RPQ
Rivermead post-concussion questionnaire
- GCS
Glasgow coma scale
- LOC
loss of consciousness
- MPRAGE
Magnetization prepared rapid acquisition-of gradient echo
- NIHTB-CB
NIH Toolbox-cognition battery
- PROMIS
Patient reported outcome measurement information system
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: None of the authors report any conflicts of interest.
References
- Alsop DC, et al. , 2015. Recommended implementation of arterial spin-labeled perfusion MRI for clinical applications: A consensus of the ISMRM perfusion study group and the European consortium for ASL in dementia. Magn Reson Med. 73, 102–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amyot F, et al. , 2018. Imaging of Cerebrovascular Function in Chronic Traumatic Brain Injury. J Neurotrauma. 35, 1116–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andre JB, 2015. Arterial Spin Labeling Magnetic Resonance Perfusion for Traumatic Brain Injury: Technical Challenges and Potentials. Top Magn Reson Imaging. 24, 275–87. [DOI] [PubMed] [Google Scholar]
- Balalla S, et al. , 2020. Is the Rivermead Post-Concussion Symptoms Questionnaire a Reliable and Valid Measure to Assess Long-Term Symptoms in Traumatic Brain Injury and Orthopedic Injury Patients? A Novel Investigation Using Rasch Analysis. Neurotrauma Reports. 1.1, 63–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartnik-Olson BL, et al. , 2014. Impaired neurovascular unit function contributes to persistent symptoms after concussion: a pilot study. J Neurotrauma. 31, 1497–506. [DOI] [PubMed] [Google Scholar]
- Carlozzi NE, et al. , 2019a. Understanding Health-related Quality of Life in Caregivers of Civilians and Service Members/Veterans With Traumatic Brain Injury: Establishing the Reliability and Validity of PROMIS Mental Health Measures. Arch Phys Med Rehabil. 100, S94–S101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlozzi NE, et al. , 2019b. Understanding Health-Related Quality of Life in Caregivers of Civilians and Service Members/Veterans With Traumatic Brain Injury: Establishing the Reliability and Validity of PROMIS Fatigue and Sleep Disturbance Item Banks. Arch Phys Med Rehabil. 100, S102–S109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao LL, et al. , 2010. ASL perfusion MRI predicts cognitive decline and conversion from MCI to dementia. Alzheimer Dis Assoc Disord. 24, 19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark AL, et al. , 2017. Dynamic association between perfusion and white matter integrity across time since injury in Veterans with history of TBI. Neuroimage Clin. 14, 308–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vis JB, et al. , 2018. Arterial-spin-labeling (ASL) perfusion MRI predicts cognitive function in elderly individuals: A 4-year longitudinal study. J Magn Reson Imaging. 48, 449–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Department of Veterans Affairs, D.o.D., 2016. VA/DoD Clinical Practice Guideline for the Management of Concussion-Mild Traumatic Brain Injury. Vol., ed.êds.
- DeWalt DA, et al. , 2007. Evaluation of item candidates: the PROMIS qualitative item review. Med Care. 45, S12–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, 2012. FreeSurfer. Neuroimage. 62, 774–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foley LM, et al. , 2013. MRI assessment of cerebral blood flow after experimental traumatic brain injury combined with hemorrhagic shock in mice. J Cereb Blood Flow Metab. 33, 129–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, 1994. Statistical parametric mapping. Functional neuroimaging: Technical foundations (p. 79–93). Academic Press. [Google Scholar]
- Ge Y, et al. , 2009. Assessment of thalamic perfusion in patients with mild traumatic brain injury by true FISP arterial spin labelling MR imaging at 3T. Brain Inj. 23, 666–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerberding J, 2003. Report to Congress on Mild Traumatic Brain Injury in the United States: Steps to Prevent a Serious Public Health Problem Centers for Disease Control and Prevention. 1–45. [Google Scholar]
- Gershon RC, et al. , 2013. IV. NIH Toolbox Cognition Battery (CB): measuring language (vocabulary comprehension and reading decoding). Monogr Soc Res Child Dev. 78, 49–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilkey SJ, et al. , 1997. Cerebral blood flow in chronic posttraumatic headache. Headache. 37, 583–7. [DOI] [PubMed] [Google Scholar]
- Grossman EJ, Inglese M, 2016. The Role of Thalamic Damage in Mild Traumatic Brain Injury. J Neurotrauma. 33, 163–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber M, et al. , 2018. Vascular Abnormalities within Normal Appearing Tissue in Chronic Traumatic Brain Injury. J Neurotrauma. 35, 2250–2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X, et al. , 2006. Reliability of MRI-derived measurements of human cerebral cortical thickness: the effects of field strength, scanner upgrade and manufacturer. Neuroimage. 32, 180–94. [DOI] [PubMed] [Google Scholar]
- Hayward NM, et al. , 2010. Association of chronic vascular changes with functional outcome after traumatic brain injury in rats. J Neurotrauma. 27, 2203–19. [DOI] [PubMed] [Google Scholar]
- Hayward NM, et al. , 2011. Magnetic resonance imaging of regional hemodynamic and cerebrovascular recovery after lateral fluid-percussion brain injury in rats. J Cereb Blood Flow Metab. 31, 166–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M, et al. , 2002. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 17, 825–41. [DOI] [PubMed] [Google Scholar]
- Jullienne A, et al. , 2016. Chronic cerebrovascular dysfunction after traumatic brain injury. J Neurosci Res. 94, 609–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenney K, et al. , 2016. Cerebral Vascular Injury in Traumatic Brain Injury. Exp Neurol. 275Pt 3, 353–66. [DOI] [PubMed] [Google Scholar]
- Kim J, et al. , 2010. Resting cerebral blood flow alterations in chronic traumatic brain injury: an arterial spin labeling perfusion FMRI study. J Neurotrauma. 27, 1399–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King NS, et al. , 1995. The Rivermead Post Concussion Symptoms Questionnaire: a measure of symptoms commonly experienced after head injury and its reliability. J Neurol. 242, 587–92. [DOI] [PubMed] [Google Scholar]
- Kochanek PM, et al. , 2002. Cerebral blood flow at one year after controlled cortical impact in rats: assessment by magnetic resonance imaging. J Neurotrauma. 19, 1029–37. [DOI] [PubMed] [Google Scholar]
- Lin CM, et al. , 2016. Arterial Spin Labeling Perfusion Study in the Patients with Subacute Mild Traumatic Brain Injury. PLoS One. 11, e0149109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H, et al. , 2005. Novel approach to the measurement of absolute cerebral blood volume using vascular-space-occupancy magnetic resonance imaging. Magn Reson Med. 54, 1403–11. [DOI] [PubMed] [Google Scholar]
- Medana IM, Esiri MM, 2003. Axonal damage: a key predictor of outcome in human CNS diseases. Brain. 126, 515–30. [DOI] [PubMed] [Google Scholar]
- Menon DK, et al. , 2010. Position statement: definition of traumatic brain injury. Arch Phys Med Rehabil. 91, 1637–40. [DOI] [PubMed] [Google Scholar]
- Oder W, et al. , 1992. Behavioural and psychosocial sequelae of severe closed head injury and regional cerebral blood flow: a SPECT study. J Neurol Neurosurg Psychiatry. 55, 475–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park E, et al. , 2009. An analysis of regional microvascular loss and recovery following two grades of fluid percussion trauma: a role for hypoxia-inducible factors in traumatic brain injury. J Cereb Blood Flow Metab. 29, 575–84. [DOI] [PubMed] [Google Scholar]
- Shen Y, et al. , 2007. In vivo measurement of tissue damage, oxygen saturation changes and blood flow changes after experimental traumatic brain injury in rats using susceptibility weighted imaging. Magn Reson Imaging. 25, 219–27. [DOI] [PubMed] [Google Scholar]
- Steinman J, et al. , 2019. Acute and chronic stage adaptations of vascular architecture and cerebral blood flow in a mouse model of TBI. Neuroimage. 202, 116101. [DOI] [PubMed] [Google Scholar]
- Stephens JA, et al. , 2018. Cerebral Blood Flow after Mild Traumatic Brain Injury: Associations between Symptoms and Post-Injury Perfusion. J Neurotrauma. 35, 241–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamminga CA, et al. , 2012. Hippocampal novelty activations in schizophrenia: disease and medication effects. Schizophr Res. 138, 157–63. [DOI] [PubMed] [Google Scholar]
- Thomas BP, et al. , 2013. Life-long aerobic exercise preserved baseline cerebral blood flow but reduced vascular reactivity to CO2. J Magn Reson Imaging. 38, 1177–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas BP, et al. , 2020. Brain Perfusion Change in Patients with Mild Cognitive Impairment After 12 Months of Aerobic Exercise Training. J Alzheimers Dis. 75, 617–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulsky DS, et al. , 2017. Using the NIH Toolbox Cognition Battery (NIHTB-CB) in individuals with traumatic brain injury. Rehabil Psychol. 62, 413–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, et al. , 2016. Cerebral Blood Flow Alterations in Acute Sport-Related Concussion. J Neurotrauma. 33, 1227–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, et al. , 2013. Arterial spin labeled MRI in prodromal Alzheimer’s disease: A multi-site study. Neuroimage Clin. 2, 630–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei EP, et al. , 2009. The long-term microvascular and behavioral consequences of experimental traumatic brain injury after hypothermic intervention. J Neurotrauma. 26, 527–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintraub S, et al. , 2013. Cognition assessment using the NIH Toolbox. Neurology. 80, S54–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson L, et al. , 2017. The chronic and evolving neurological consequences of traumatic brain injury. Lancet Neurol. 16, 813–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing CY, et al. , 2017. Arterial Pressure, Heart Rate, and Cerebral Hemodynamics Across the Adult Life Span. Hypertension. 69, 712–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu DC, Penn RD, 2005. Full-brain T1 mapping through inversion recovery fast spin echo imaging with time-efficient slice ordering. Magn Reson Med. 54, 725–31. [DOI] [PubMed] [Google Scholar]


