Abstract
Objectives:
Phase II drug metabolism is poorly studied in advanced age and older adults may exhibit significant variability in their expression of phase II enzymes. We hypothesized that age-related changes to epigenetic regulation of genes involved in phase II drug metabolism may contribute to these effects.
Methods:
We examined published epigenome-wide studies of human blood and identified the SULT1A1 and UGT1A6 genes as the top loci showing epigenetic changes with age. To assess possible functional alterations with age in the liver, we assayed DNA methylation (5mC) and histone acetylation changes around the mouse homologs Sult1a1 and Ugt1a6 in liver tissue from mice aged 4-32 months.
Results:
Our sample shows significant loss of 5mC at Sult1a1 (β=−1.08, 95%CI [−1.8, −0.2], SE=0.38, p=0.011), mirroring the loss of 5mC with age observed in human blood DNA at the same locus. We also detected increased histone 3 lysine 9 acetylation (H3K9ac) with age at Sult1a1 (β= 0.11, 95%CI [0.002,0.22], SE=0.05, p=0.04), but no change to histone 3 lysine 27 acetylation (H3K27ac). Sult1a1 gene expression is significantly positively associated with H3K9ac levels, accounting for 23% of the variation in expression. We did not detect any significant effects at Ugt1a6.
Conclusions:
Sult1a1 expression is under epigenetic influence in normal aging and this influence is more pronounced for H3K9ac than DNA methylation or H3K27ac in this study. More generally, our findings support the relevance of epigenetics in regulating key drug metabolizing pathways. In future, epigenetic biomarkers could prove useful to inform dosing in older adults.
Keywords: DNA Methylation, Acetylation, Histones, Epigenome, Protein Processing, Post-Translational, Liver, Gene Expression, Biomarkers, Aging
Introduction
Human life expectancy has more than doubled in the last two centuries [1] and by 2030, it is estimated that the US population aged 65+ will constitute one fifth of the total population [2]. However, current trends towards increasing longevity do not mean universal good health into old age. Older adults are still at greatly increased risk for many diseases and, as a result, typically take several concurrent medications [3,4]. According to a 2006 survey, 40% of persons aged 65+ were taking five to nine medications, while almost one fifth (18%) were taking 10 or more [5]. The incidence of adverse drug reactions (ADRs) increases as people live longer, have greater numbers of chronic conditions, and take more medications [4,6,7]. Ultimately, older adults are almost seven times as likely as younger persons to have ADRs that require hospitalization [8]. On the other hand, up to 80% of ADRs in older patients are dose related and therefore avoidable [9]. This suggests that biomarkers to aid clinical dosing decisions could be profoundly beneficial for geriatric healthcare.
The DNA sequence of our individual genomes does not change throughout our lifespan, except for random somatic mutations, so DNA sequence variants have little utility as biomarkers for tracking age-related changes [10]. However, the epigenome does change in profound ways with age [11]. The epigenome refers to reversible chemical marks made to chromatin, the complex of DNA and protein in the cell nucleus, and these marks regulate chromatin accessibility and gene expression. Some epigenetic marks typically reduce gene expression, such as the addition of a methyl group to the carbon 5 position of cytosine nucleotides (5mC, or DNA methylation). Others typically increase gene expression, such as the addition of acetyl groups to the 9th or 27th lysine residues of Histone 3 (H3K9ac, H3K27ac) [12-14]. Extensive changes to 5mC and histone marks occur over the lifespan [15,16]. While the genome-wide distribution of histone marks in aging has not been well characterized to date, changes to 5mC are not purely random and tend to occur at specific genes and genomic regions [11,17-20]. As such, several authors have suggested that these changes could occur at genes regulating drug metabolism, and therefore affect rates of drug metabolism in older adults [10,21,22].
Previously, we reported that age-related epigenetic changes at the cytochrome P450 2E1 (Cyp2e1) gene showed significant changes with age that were associated with CYP2E1 function in mouse liver [23]. We now turn our attention to genes encoding phase II (conjugation) drug metabolizing enzymes. There is substantial evidence for epigenetic variation affecting expression of phase II drug metabolism genes, albeit not in the context of aging. For example, 5mC levels at genes encoding several glutathione S-transferases (GSTs) and UDP-glucuronosyltransferases (UGTs) are associated with expression levels and clinical outcomes, with most attention to date in the area of oncology [22,24]. This suggested that an investigation into age-related epigenetic change at these genes could be fruitful. We identified sulfotransferase family 1A member 1 (SULT1A1) and UDP glucuronosyltransferase family 1 member A6 (UGT1A6) as the phase II genes showing best evidence for age-associated differentially methylated regions (a-DMRs) in human blood studies. To assess if these a-DMRs affected expression in liver, we assayed tissue from mice aged under controlled conditions because of the high degree of experimental control afforded. In humans, comorbid disease states, drug treatment history and other environmental factors could confound our results [9,25,26]. Furthermore, there is substantial evidence of cross-tissue and cross-species consistency in age-related epigenetic changes [11,18] and the mouse genomic regions investigated in this study show clear homology with their human counterparts [27,28]. In addition to assessing 5mC, we assayed H3K9ac and H3K27ac. Typically, 5mC represses transcription whereas H3K9ac and H3K27ac are associated with active gene promoters and enhancers [29]. Our goal was to evaluate the role of histone acetylation in addition to 5mC on the expression of phase II drug metabolism genes in normal aging.
Methods
Additional details are provided in the Supplementary Material.
Samples:
We obtained postmortem liver tissue of 20 male CB6F1 mice from the National Institute on Aging (NIA) rodent tissue bank. Each of the four age groups (4, 18, 24, and 32 months) comprised five subjects. We used the AllPrep DNA/RNA kit (Qiagen, Hilden, Germany) to extract DNA and RNA from the same liver samples. Quantity and purity of nucleic acids were assessed using a Nanodrop spectrometer (ThermoFisher, Waltham, MA).
Selection of genomic regions of interest:
We obtained results for published microarray epigenome-wide association studies (EWAS) of aging in human blood DNA that used the Illumina Infinium arrays [30-33]. Results from these studies were contrasted these with lists of genes involved in ADME (absorption, distribution, metabolism, and excretion) processes from the pharmaADME consortium (pharmaADME.org), counting the number of studies in which each gene showed a significant a-DMR. The mouse regions homologous to the human findings were identified via UCSC Genome Browser. Genomic coordinates of all regions investigated in mouse are provided in Supplementary Table S1. Using publicly available data (GEO accession numbers GSM1000153, GSM1000140), we identified regions around our target genes with high levels of histone 3 lysine 9 acetylation (H3K9ac) and histone 3 lysine 27 acetylation (H3K27ac).
Bisulfite conversion and High-Resolution Melt (HRM) analysis:
200ng of liver genomic DNA per sample was treated with sodium bisulfite using the EZ DNA Methylation kit (Zymo Research, Irvine, CA). DNA methylation levels were assayed using high resolution melt (HRM) assays with MeltDoctor reagents on a Quantstudio 3 real time PCR instrument (Applied Biosystems, Foster City, CA). Primer sequences are provided in Supplementary Table S1. A standard curve was generated from six 5mC standards of known percentage methylation (0, 5, 25, 50, 75, and 100%) that were obtained from EpigenDx (Hopkinton, MA) and were included on each assay plate. Samples were amplified by qPCR as follows: 10min hold at 95°C followed by 45 cycles of: 15sec at 95°C, 60sec at 60°C or 55°C for Sult1a1 or Ugt1a6 respectively, followed by a melt curve stage. In each reaction we used 20ng bisulfite-converted DNA, 0.2μM forward and 0.2μM reverse primer (Supplementary Table S1). All DNA samples and standards were run in triplicate. Net Temperature Shift values [34] of the liver DNA samples were interpolated on the standard curve to estimate their 5mC percentage.
Gene expression analysis by reverse transcription – quantitative PCR (RT-qPCR):
We measured the gene expression Sult1a1, and both isoforms of Ugt1a6 (Ugt1a6a and Ugt1a6b), as they both expressed from the same locus and the regulatory region investigated could affect either one of them. For each sample, we reversed transcribed 1μg of total liver mRNA using the iScript kit (Bio-Rad, Hercules, CA). We amplified the aliquots of cDNA in triplicate using TaqMan master mix (Applied Biosystems) and TaqMan Sult1a1 or Ugt1a6a/b Mouse Gene Expression Assay (Mm01132072_m1, Mm01967851_s1, Mm03032310_s1, ThermoFisher). We ran the qPCR using the following conditions: 2 min hold at 50 °C then 10min at 95 °C, followed by 40 cycles of 15 sec at 95°C and 60 sec at 60°C. Murine Gapdh expression was the endogenous control (Mm99999915_g1, ThermoFisher). We determined the quantification cycles (Cq) using the Relative Quantification application on the ThermoFisher Cloud and calculated the normalized quantification cycles (ΔCq) by subtracting mean Gapdh Cq from the mean Cq of the target genes.
Chromatin Immunoprecipitation Quantitative Polymerase Chain Reaction (ChIP-qPCR):
We used the TruChIP tissue kit (Covaris, Woburn, MA) to process 80 mg of mouse liver per sample. This involved initial fragmentation on dry ice, followed by fixation in 1% formaldehyde for 2 min, freezing in liquid N2, mechanical pulverization and cell lysis. Chromatin was sheared on a Covaris M220 for 8min (75 peak impulse power, 10% duty factor, 200 cycles per burst, 7°C set point temperature). Two percent of sheared chromatin per IP was retained as input control. Each ChIP used 2μg sheared chromatin and was incubated overnight (16 hours) at 4°C with either 5μg of anti-H3K9ac (39137, Active Motif, Carlsbad, CA), or 5μg of anti-H3K27ac (39133, Active Motif). We also used a separate control ChIP for each sample using 5μg of Rabbit IgG (ab171870, Abcam). After antibody incubation, we added the ChIP mixtures to Dynabeads Protein G (ThermoFisher) for 4 hours at 4°C before washing and elution at 65°C for 1 hour. We incubated the elutated DNA with RNAse followed by Proteinase K and DNA was purified using QIAquick spin columns (Qiagen).
We assayed each ChIP DNA sample in triplicate using PowerUp SYBR Green qPCR master mix (Applied Biosystems) and 0.2 μM of forward and reverse primers (see Supplementary Table S1 for primer sequences). We used the following cycling conditions: 2min at 50°C, 2min at 95°C followed by 45 cycles of: 15sec at 95°C, 30sec at 58 °C or 60°C for Sult1a1 or Ugt1a6 respectively and 1min at 72°C, followed by melt curve stage. For each sample, the mean threshold cycle (Cq) was normalized to the dilution factor (2%=1/50) corrected Cq value (Log2 (50) =5.6438) of the input control to obtain ΔCq. Percentage of input was calculated by multiplying 100 by 2ΔCq.
Data Analysis:
We used linear regression to test the relationship between age and levels of 5mC, H3K9ac or H3K27ac. We used R version 3.6.1 (www.r-project.org) with α=0.05.
Results
Gene and region selection
We reviewed four EWAS of human blood DNA (Table 1) and the top two phase II drug metabolism genes showing significant findings across studies were sulfotransferase family 1A member 1 (SULT1A1) and UDP glucuronosyltransferase family 1 member A6 (UGT1A6). Each of these genes showed age-related epigenetic changes in two studies. The a-DMR in human blood DNA at SULT1A1 corresponded to a mouse region approximately 600bp upstream of the Sult1a1 transcription start site. The UGT1 locus is complex and encodes several genes. However, the human a-DMR corresponded to a mouse region that overlapped specifically an exon present only in the Ugt1a6a and Ugt1a6b transcripts (see Supplementary Table S1).
Table 1.
Review of Illumina microarray EWAS studies of aging in human blood. We extracted results from these studies for genes encoding phase II drug metabolizing enzymes and those genes with at least one positive finding are shown in the Table. “X” indicates the study where the positive result was detected.
| Heyn et al. [30] | Reynolds et al. [32] | Martilla et al. [33] | Steegenga et al. [31] | Total | |
|---|---|---|---|---|---|
| 5mC * | 5mC * | 5mC and exp. * | 5mC and exp. * | ||
| SULT1A1 | X | X | 2 | ||
| UGT1A6 | X | X | 2 | ||
| UGT2B15 | X | 1 | |||
| GSTT1 | 1 | ||||
| SULT2B1 | X | 1 | |||
| UGT1A4 | X | 1 | |||
| UGT1A5 | X | 1 |
The type of study is given as 5mC for genome-wide DNA methylation analysis and “exp” is marked where the study also analyzed genome-wide gene expression in aging.
5mC and gene expression:
We extracted DNA and RNA from our liver samples from aged mice and obtained an average 260/280 value of 1.94 [1.8-2.1] for DNA and 2.05 [1.94-2.1] for RNA. We first assayed 5mC at the mouse regions homologous to the human a-DMRs using high resolution melt (HRM) analysis. This revealed that 5mC decreases significantly with age at Sult1a1 (β=−1.08, 95%CI [−1.8, −0.2], SE=0.38, p=0.011) (Figure 1). The observed Sult1a1 promoter 5mC decrease with age translates to a 24% decrease in the 32 months group versus the 4 months group. However, there was no significant change at Ugt1a6 (Supplementary Figure S1).
Figure 1.
Box plots of hepatic DNA methylation (5mC) and gene expression levels at Sult1a1 by age. Superimposed regression line (blue) of age associated changes of (a) Percentage 5mC at Sult1a1 promoter (b) gene expression levels of Sult1a1 mRNA measured as ΔCq or fold change relative to endogenous control gene (Gapdh) expression . For each age, n=5 subjects were assayed. Data represent median (middle hinge), 25% (lower hinge) and 75% (upper hinge) quantile. Data points beyond upper or lower 1.5 * Inter Quantile Range are represented as individual black dots.
TaqMan real time PCR analysis showed that Sult1a1 gene expression did not change significantly with chronological age, although we observed a non-significant upward trend with increasing age (Figure 1). We were unable to detect any significant change with age at either the Ugt1a6a or Ugt1a6b transcripts (Supplementary Figure S1).
Histone acetylation analysis of Sult1a1 regulatory region:
Our prior study of Cyp2e1 showed a stronger relationship between CYP2E1 function and H3K9ac than for 5mC [23]. We hypothesized that we would see a similar pattern at Sult1a1. We identified a region of high H3K9ac and H3K27ac adjacent to the Sult1a1 transcription start site (Figure 2) and tested this using chromatin immunoprecipitation (ChIP). We found that H3K9ac increased significantly with age at Sult1a1 (β= 0.11, 95%CI [0.002,0.22], SE=0.05, p=0.04). The observed change with age at Sult1a1 corresponds to an average increase of 0.11% in H3K9ac per month. However, H3K27ac levels did not change with age at Sult1a1 (Figure 3). For completeness, we also tested H3K9ac and H3K27ac at Ugt1a6. Consistent with our other findings for this locus, we did not observe any age-related change for these histone modifications at Ugt1a6 (Supplementary Figure S1).
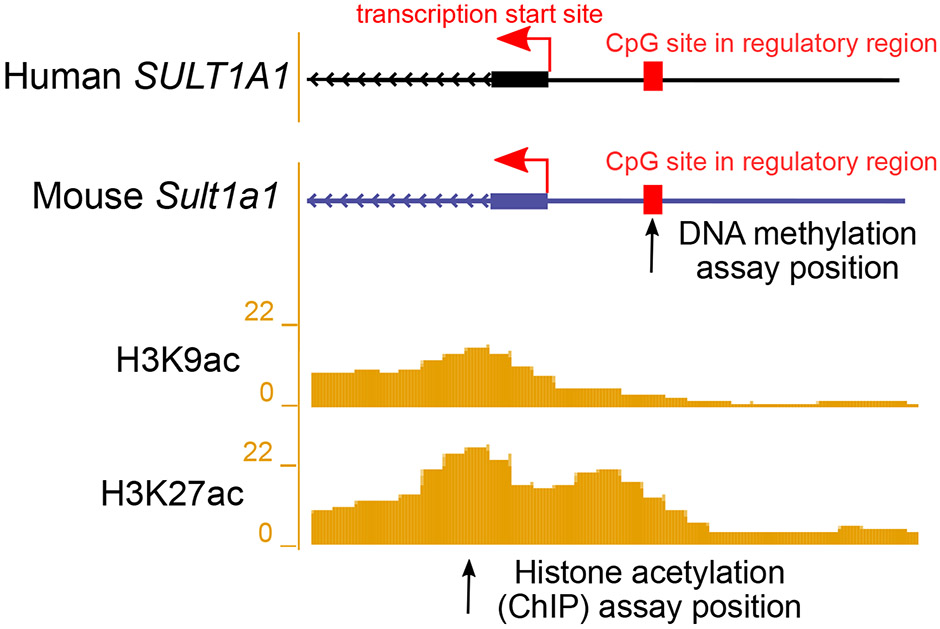
Figure 2.
Illustration showing the transcription start site (TSS) and upstream regulatory regions of the human SULT1A1 and the homologous mouse Sult1a1 gene. DNA methylation (5mC) was assayed at the regulatory region in mouse at the position marked by the arrow. Reference histone 3 lysine 9 acetylation (H3K9ac) and histone 3 lysine 27 acetylation (H3K27ac) in 8-week-old mouse livers are from the ENCODE/LICR track in UCSC Genome Browser. A locus with high liver histone acetylation levels was chosen for analysis using ChIP-qPCR in the current study, at the position marked by the arrow. For exact assay coordinates, see Supplementary Table S1.
Figure 3.
Box plots of histone H3 lysine 27 acetylation (H3K27ac) and histone H3 lysine 9 acetylation (H3K9ac) levels at Sult1a1. For each age, n=5 subjects were assayed using ChIP-qPCR data (n=5 per age). The first two panels show the percentage of input obtained for ChIP with H3K27ac and H3K9ac antibodies respectively, while IgG percentage of input shows a low background noise signal. Data represent median (middle hinge), 25% (lower hinge) and 75% (upper hinge) quantile. Data points beyond upper or lower 1.5 * Inter Quantile Range are represented as individual black dots.
Sult1a1 epigenetics and expression:
As outlined above, 5mC and H3K9ac levels at Sult1a1 changed with age. The overall effect direction of reduced 5mC and increased H3K9ac is consistent with greater chromatin accessibility and increased gene expression. Sult1a1 gene expression was not significantly associated with chronological age, but it did show a non-significant upward trend with age. We noticed that aging led to increased variation in expression at Sult1a1, as seen in Figure 3 when comparing the range of observed values in the oldest (32 month) group to the younger groups. We hypothesized that the increase in variation makes a clear-cut aging association difficult to detect, but that the functional relationship between epigenetic state and gene expression is consistent. Therefore, to determine the relationship between epigenetic marks and Sult1a1 gene expression, we tested the degree of association between 5mC and H3K9ac at Sult1a1 with its gene expression. As shown in Figure 4, H3K9ac changes at Sult1a1 are significantly associated with its gene expression (β=0.02, 95%CI [0.004,0.04], SE=0.008, p=0.018) and explain 23% of the variation in the latter (Adj.R2=0.23). However, 5mC at the Sult1a1 promoter was not significantly associated with its gene expression (p=0.35). This suggests that H3K9ac is a relatively robust independent predictor of Sult1a1 gene expression, more so than 5mC or chronological age.
Figure 4.
Linear regression plot of percentage of hepatic H3K9ac levels and corresponding Sult1a1 gene expression levels (n=20). Gene expression is expressed as ΔCq or fold change relative to endogenous control gene (Gapdh) expression. Superimposed regression line (blue) is shown with reported Adjusted R2 and p-value.
Discussion
Drug absorption, distribution, metabolism, and excretion are crucial processes for ensuring safe and effective use of medications. Many factors, including genetic variants, the microbiome, environmental factors such as diet and drug-drug interactions, influence the expression and activity of drug metabolizing enzymes, which ultimately lead to inter-individual variations in drug response. In recent years, there has been a growing interest in the role of epigenetics in the regulation of drug metabolizing enzymes [22,35,36]. In this study, we investigated how age-related changes to three epigenetic marks at phase II drug metabolism genes affect hepatic gene expression in a mouse model. Overall, we demonstrated that levels of 5mC (a suppressing epigenetic mark) were reduced with age while levels of H3K9ac (an activating epigenetic mark) increased with age at Sult1a1 in mouse liver. Strikingly, H3K9ac levels could explain almost a quarter of the variation in Sult1a1 gene expression, while chronological age and 5mC levels were not significantly associated, indicating that histone acetylation levels may have a strong influence on Sult1a1 activity in normal aging.
Prior work has shown that some, but not all, epigenetic aging effects are consistent across tissues and species [18,37,38]. Here, our findings in mice support prior observations in human blood. In particular, Reynolds et al. (2014) [32] reported hypomethylation with age at human SULT1A1 in blood DNA. In the current study, we assayed the mouse genomic region homologous to the Reynolds et al. finding and showed that it exhibited hypomethylation with age in mouse liver too, thereby demonstrating consistency of the effect across tissues and species. The fact that we have demonstrated a hepatic functional correlate in mouse of an epigenetic change detected in human blood DNA suggests that further work in evaluating human SULT1A1 epigenetic states as potential sulfonation biomarkers in aging could be warranted.
A prior study by [39] found a significant increase in mouse hepatic Sult1a1 expression with increasing age (27 months compared to 3 months). This suggests that Sult1a1 gene expression does increase with age but may require a larger sample size than used here to be reliably detectable. In our study, we observed increased variation in Sult1a1 gene expression in the oldest age group, which could make a consistent change with age harder to detect. Considering 5mC levels, these did significantly change with age but were not tightly coupled to expression levels and we did not detect a significant relationship between 5mC and expression in our sample. It is also possible that a larger sample size could detect a significant relationship between these variables. Another factor to consider is that bisulfite conversion of DNA as used here cannot distinguish between 5mC and 5-hydroxymethylcytosine (5hmC). 5hmC is relatively abundant in the liver and we previously showed that 5hmC levels change in mouse liver tissue with age [23]. Discrimination between 5mC and 5hmC could yield a clearer perspective on Sult1a1 expression in aging. On the other hand, age-related change in H3K9ac levels were relatively tightly coupled to Sult1a1 expression. Therefore, we conclude that H3K9ac is a significant driver of expression in aging and the degree of its change with age is more closely related to expression than chronological age itself.
SULT1A1 activity has been studied in development from early gestation to young adulthood and no significant changes were observed in early life, in contrast to many other drug metabolizing enzymes [40]. However, current understanding of phase II metabolism in old age is limited [41]. SULT1A1 comprises the majority, 53%, of the total hepatic sulfotransferase [42]. Substrates of SULT1A1 include acetaminophen, tamoxifen, levodopa, and estrogen replacement therapies [43,44] which are all drugs indicated for the treatment of conditions frequent in older individuals that may have detrimental and unpredictable toxicities. Acetaminophen (paracetamol) is one of the most commonly used analgesics in older individuals and its pharmacokinetics and effects can be influenced by changes in physiology with aging [45]. Tamoxifen is essential for its anti-estrogen activity in the treatment of breast cancer and SULT1A1 catalyzes the conversion of tamoxifen metabolites into excretable forms [46]. SULT1A1 variants may be associated with tamoxifen metabolite levels in breast cancer patients [47]. Furthermore, Singh et al. (2020) [48] found SULT1A1 to be up-regulated in tamoxifen-resistant breast cancer, while CRISPR-Cas9-mediated deletion of SULT1A1 increased sensitivity to the active metabolite 4-hydroxytamoxifen. These findings suggest that epigenetic aging affects on SULT1A1 expression may also impact tamoxifen response, but this will require further research. Considering estrogen-replacement therapy, prior studies have associated genetic sequence variants at SULT1A1 with estrogen response [49,50]. Therefore, it is conceivable that age-related functional epigenetic changes at SULT1A1 may similarly affect response to estrogen-replacement therapy.
Interestingly, SULT1A1 has been associated with longevity in humans and mice. Coughtrie et al. (1999) [51] originally reported an excess of SULT1A1*1 homozygotes in older populations relative to younger populations. More recently, McDaid et al. (2017) [52] conducted a large-scale genome-wide study using UK Biobank data and found evidence for single nucleotide polymorphisms proximal to SULT1A1 being associated with longevity in humans. Moreover, they also reported increased Sult1a1 expression levels in mice undergoing caloric restriction, a dietary intervention known to prolong lifespan. More detailed investigations of the relationship between SULT1A1 epigenetics, aging and longevity seem warranted.
In contrast to our findings with Sult1a1, we were unable to find any significant changes at Ugt1a6, despite there being a clear mouse homologue to the nucleotide sequence of the human a-DMR. The UGT1A locus is complex in both humans and mice, with some exons common to all UGT1A transcripts flanked by exons that encode the N-terminal of the different UGT1A proteins. There are tissue and species differences in UGT1A regulation [53], so although we identified the mouse homologous region to the human a-DMR in blood, this may not be conserved in liver due to the complexity of the locus. Moreover, while we assayed both Ugt1a6a and Ugt1a6b as the transcripts most proximal to the putative a-DMR, it is possible that long range regulatory effects link this region to other UGT1A genes. A more comprehensive understanding will come from epigenome-wide technologies that can assay epigenetic changes with age across the entire UGT1A region in liver.
Conclusion
Histone acetylation levels, specifically H3K9ac, are strongly associated with Sult1a1 hepatic expression in normal aging. A limitation of our study is that we could not directly manipulate epigenetic levels in our liver samples, so the relationship between epigenetics and gene expression is correlational, not causal. Nevertheless, the fact that H3K9ac levels explained almost a quarter of the variance in hepatic Sult1a1 gene expression suggests that further study of pharmacoepigenetic biomarkers may be fruitful.
Supplementary Material
Acknowledgements
We are grateful to the staff at the NIA Rodent Tissue Bank for providing us with the samples to carry out this study. We are gratfeul for support from the US National Institute on Aging, National Institutes of Health grant R15AG061649 to JLM and for a graduate studentship from Virginia Commonwealth University School of Pharmacy to MMK. Mohamad M. Kronfol completed this study in partial fulfillment of the doctoral requirements in Pharmaceutical Sciences at Virginia Commonwealth University. An earlier version of this report was released as a preprint on bioRxiv: doi.org/10.1101/2020.09.17.300657.
Footnotes
Statement of conflicts of interest
The authors declare no material or financial conflicts of interest.
References
- 1.Riley J Rising Life Expectancy: A Global History. Cambridge University Press; 2001. [Google Scholar]
- 2.US Census Bureau. 2017 National Population Projections Tables: Main Series [Internet]. The United States Census Bureau. 2017. [cited 2021 Jan 13]. Available from: https://www.census.gov/data/tables/2017/demo/popproj/2017-summary-tables.html [Google Scholar]
- 3.Chang AY, Skirbekk VF, Tyrovolas S, Kassebaum NJ, Dieleman JL. Measuring population ageing: an analysis of the Global Burden of Disease Study 2017. Lancet Public Health. 2019;4(3):e159–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McLean AJ, Le Couteur DG. Aging biology and geriatric clinical pharmacology. Pharmacol Rev. 2004;56(2):163–84. [DOI] [PubMed] [Google Scholar]
- 5.Slone Epidemiology Center. Patterns of medication use in the United States - A report from the Slone Survey [Internet]. Boston University; 2006. Available from: http://www.bu.edu/slone/files/2012/11/SloneSurveyReport2006.pdf [Google Scholar]
- 6.ElDesoky ES. Pharmacokinetic-pharmacodynamic crisis in the elderly. Am J Ther. 2007;14(5):488–98. [DOI] [PubMed] [Google Scholar]
- 7.Budnitz DS, Lovegrove MC, Shehab N, Richards CL. Emergency hospitalizations for adverse drug events in older Americans. N Engl J Med. 2011;365(21):2002–12. [DOI] [PubMed] [Google Scholar]
- 8.Budnitz DS, Pollock DA, Weidenbach KN, Mendelsohn AB, Schroeder TJ, Annest JL. National surveillance of emergency department visits for outpatient adverse drug events. JAMA;296(15):1858–66. [DOI] [PubMed] [Google Scholar]
- 9.Routledge PA, O’Mahony MS, Woodhouse KW. Adverse drug reactions in elderly patients. British journal of clinical pharmacology. 2004;57(2):121–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kronfol MM, Dozmorov MG, Huang R, Slattum PW, McClay JL. The role of epigenomics in personalized medicine. Expert Review of Precision Medicine and Drug Development. 2017;2(1):33–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Horvath S, Raj K. DNA methylation-based biomarkers and the epigenetic clock theory of ageing. Nature Reviews Genetics. 2018;19(6):371–84. [DOI] [PubMed] [Google Scholar]
- 12.Jones PA. Functions of DNA methylation: islands, start sites, gene bodies and beyond. Nat Rev Genet. 2012;13(7):484–92. [DOI] [PubMed] [Google Scholar]
- 13.Tessarz P, Kouzarides T. Histone core modifications regulating nucleosome structure and dynamics. Nat Rev Mol Cell Biol. 2014;15(11):703–8. [DOI] [PubMed] [Google Scholar]
- 14.Fenley AT, Anandakrishnan R, Kidane YH, Onufriev AV. Modulation of nucleosomal DNA accessibility via charge-altering post-translational modifications in histone core. Epigenetics Chromatin. 2018;11(1):11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Benayoun BA, Pollina EA, Brunet A. Epigenetic regulation of ageing: linking environmental inputs to genomic stability. Nat Rev Mol Cell Biol. 2015;16(10):593–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang W, Qu J, Liu G-H, Belmonte JCI. The ageing epigenome and its rejuvenation. Nat Rev Mol Cell Biol. 2020;21(3):137–50. [DOI] [PubMed] [Google Scholar]
- 17.Hannum G, Guinney J, Zhao L, Zhang L, Hughes G, Sadda S, et al. Genome-wide Methylation Profiles Reveal Quantitative Views of Human Aging Rates. Molecular Cell. 2013;49(2):359–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Horvath S DNA methylation age of human tissues and cell types. Genome Biol. 2013;14(10):R115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McClay JL, Aberg KA, Clark SL, Nerella S, Kumar G, Xie LY, et al. A methylome-wide study of aging using massively parallel sequencing of the methyl-CpG-enriched genomic fraction from blood in over 700 subjects. Hum Mol Genet. 2014;23(5):1175–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meer MV, Podolskiy DI, Tyshkovskiy A, Gladyshev VN. A whole lifespan mouse multi-tissue DNA methylation clock. eLife. 2018;7:e40675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seripa D, Panza F, Daragjati J, Paroni G, Pilotto A. Measuring pharmacogenetics in special groups: geriatrics. Expert Opin Drug Metab Toxicol. 2015;11(7):1073–88. [DOI] [PubMed] [Google Scholar]
- 22.Fisel P, Schaeffeler E, Schwab M. DNA Methylation of ADME Genes. Clin Pharmacol Ther. 2016;99(5):512–27. [DOI] [PubMed] [Google Scholar]
- 23.Kronfol MM, Jahr FM, Dozmorov MG, Phansalkar PS, Xie LY, Aberg KA, et al. DNA methylation and histone acetylation changes to cytochrome P450 2E1 regulation in normal aging and impact on rates of drug metabolism in the liver. GeroScience. 2020; 42(3):819–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang J, Yu L, Jiang H, Zheng X, Zeng S. Epigenetic Regulation of Differentially Expressed Drug-Metabolizing Enzymes in Cancer. Drug Metab Dispos. 2020;48(9):759–68. [DOI] [PubMed] [Google Scholar]
- 25.Kinirons MT, O’Mahony MS. Drug metabolism and ageing. Br J Clin Pharmacol. 2004;57(5):540–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Feinberg AP. Phenotypic plasticity and the epigenetics of human disease. Nature. 2007;447(7143):433–40. [DOI] [PubMed] [Google Scholar]
- 27.Sakakibara Y, Suiko M, Pai TG, Nakayama T, Takami Y, Katafuchi J, et al. Highly conserved mouse and human brain sulfotransferases: molecular cloning, expression, and functional characterization. Gene. 2002;285(1–2):39–47. [DOI] [PubMed] [Google Scholar]
- 28.Strassburg CP, Lankisch TO, Manns MP, Ehmer U. Family 1 uridine-5′-diphosphate glucuronosyltransferases (UGT1A): from Gilbert’s syndrome to genetic organization and variability. Arch Toxicol. 2008;82(7):415–33. [DOI] [PubMed] [Google Scholar]
- 29.Ernst J, Kheradpour P, Mikkelsen TS, Shoresh N, Ward LD, Epstein CB, et al. Mapping and analysis of chromatin state dynamics in nine human cell types. Nature. 2011;473(7345):43–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Heyn H, Li N, Ferreira HJ, Moran S, Pisano DG, Gomez A, et al. Distinct DNA methylomes of newborns and centenarians. Proc Natl Acad Sci U S A. 2012;109(26):10522–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Steegenga WT, Boekschoten MV, Lute C, Hooiveld GJ, de Groot PJ, Morris TJ, et al. Genome-wide age-related changes in DNA methylation and gene expression in human PBMCs. AGE. 2014;36(3):9648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reynolds LM, Taylor JR, Ding J, Lohman K, Johnson C, Siscovick D, et al. Age-related variations in the methylome associated with gene expression in human monocytes and T cells. Nat. Commun 2014;5:5366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marttila S, Kananen L, Häyrynen S, Jylhävä J, Nevalainen T, Hervonen A, et al. Ageing-associated changes in the human DNA methylome: genomic locations and effects on gene expression. BMC Genomics. 2015;16:179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Newman M, Blyth BJ, Hussey DJ, Jardine D, Sykes PJ, Ormsby RJ. Sensitive quantitative analysis of murine LINE1 DNA methylation using high resolution melt analysis. Epigenetics. 2012;7(1):92–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gomez A, Ingelman-Sundberg M. Pharmacoepigenetics: its role in interindividual differences in drug response. Clin Pharmacol Ther. 2009;85(4):426–30. [DOI] [PubMed] [Google Scholar]
- 36.Lauschke VM, Barragan I, Ingelman-Sundberg M. Pharmacoepigenetics and Toxicoepigenetics: Novel Mechanistic Insights and Therapeutic Opportunities. Annual Review of Pharmacology and Toxicology. 2018;58(1):161–85. [DOI] [PubMed] [Google Scholar]
- 37.Bell CG, Lowe R, Adams PD, Baccarelli AA, Beck S, Bell JT, et al. DNA methylation aging clocks: challenges and recommendations. Genome Biology. 2019;20(1):249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang T, Ma J, Hogan AN, Fong S, Licon K, Tsui B, et al. Quantitative Translation of Dog-to-Human Aging by Conserved Remodeling of the DNA Methylome. Cell Syst. 2020;11(2):176–185.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fu ZD, Csanaky IL, Klaassen CD. Effects of Aging on mRNA Profiles for Drug-Metabolizing Enzymes and Transporters in Livers of Male and Female Mice. Drug Metab Dispos. 2012;40(6):1216–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Duanmu Z, Weckle A, Koukouritaki SB, Hines RN, Falany JL, Falany CN, et al. Developmental expression of aryl, estrogen, and hydroxysteroid sulfotransferases in pre- and postnatal human liver. J Pharmacol Exp Ther. 2006;316(3):1310–7. [DOI] [PubMed] [Google Scholar]
- 41.McLachlan AJ, Pont LG. Drug Metabolism in Older People—A Key Consideration in Achieving Optimal Outcomes With Medicines. J Gerontol A Biol Sci Med Sci. 2012;67A(2):175–80. [DOI] [PubMed] [Google Scholar]
- 42.Riches Z, Stanley EL, Bloomer JC, Coughtrie MWH. Quantitative evaluation of the expression and activity of five major sulfotransferases (SULTs) in human tissues: the SULT “pie.” Drug Metab Dispos. 2009;37(11):2255–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Glatt H, Boeing H, Engelke CEH, Ma L, Kuhlow A, Pabel U, et al. Human cytosolic sulphotransferases: genetics, characteristics, toxicological aspects. Mutat. Res 2001;482(1):27–40. [DOI] [PubMed] [Google Scholar]
- 44.Marto N, Morello J, Monteiro EC, Pereira SA. Implications of sulfotransferase activity in interindividual variability in drug response: clinical perspective on current knowledge. Drug Metab. Rev 2017;49(3):357–71. [DOI] [PubMed] [Google Scholar]
- 45.Mian P, Allegaert K, Spriet I, Tibboel D, Petrovic M. Paracetamol in Older People: Towards Evidence-Based Dosing? Drugs Aging. 2018;35(7):603–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brauch H, Mürdter TE, Eichelbaum M, Schwab M. Pharmacogenomics of Tamoxifen Therapy. Clin. Chem 2009;55(10):1770–82. [DOI] [PubMed] [Google Scholar]
- 47.Sanchez-Spitman AB, Dezentjé VO, Swen JJ, Moes DJ a. R, Gelderblom H, Guchelaar H-J. Genetic polymorphisms of 3’-untranslated region of SULT1A1 and their impact on tamoxifen metabolism and efficacy. Breast Cancer Res Treat. 2018;172(2):401–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Singh M, Zhou X, Chen X, Santos GS, Peuget S, Cheng Q, et al. Identification and targeting of selective vulnerability rendered by tamoxifen resistance. Breast Cancer Res. 2020;22(1):80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rebbeck TR, Troxel AB, Wang Y, Walker AH, Panossian S, Gallagher S, et al. Estrogen Sulfation Genes, Hormone Replacement Therapy, and Endometrial Cancer Risk. JNCI: Journal of the National Cancer Institute. 2006;98(18):1311–20. [DOI] [PubMed] [Google Scholar]
- 50.Hildebrandt M, Adjei A, Weinshilboum R, Johnson JA, Berlin DS, Klein TE, et al. Very important pharmacogene summary: sulfotransferase 1A1. Pharmacogenet Genomics. 2009;19(6):404–6. [DOI] [PubMed] [Google Scholar]
- 51.Coughtrie MW, Gilissen RA, Shek B, Strange RC, Fryer AA, Jones PW, et al. Phenol sulphotransferase SULT1A1 polymorphism: molecular diagnosis and allele frequencies in Caucasian and African populations. Biochem J. 1999;337 (Pt 1):45–9. [PMC free article] [PubMed] [Google Scholar]
- 52.McDaid AF, Joshi PK, Porcu E, Komljenovic A, Li H, Sorrentino V, et al. Bayesian association scan reveals loci associated with human lifespan and linked biomarkers. Nat. Commun 2017;8(1):15842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shiratani H, Katoh M, Nakajima M, Yokoi T. Species differences in UDP-glucuronosyltransferase activities in mice and rats. Drug Metab Dispos. 2008;36(9):1745–52. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.