Abstract
Although β-hairpins are widespread in proteins, there is still no universal tool to coax any small peptide to adopt a β-hairpin conformation, regardless of sequence. Here, we report that δ-linked γ(R)-methyl-ornithine (δMeOrn) provides an improved β-turn template for inducing a β-hairpin conformation in peptides. We have developed a synthesis of protected δMeOrn as a building block suitable for use in Fmoc-based solid-phase peptide synthesis. The synthesis begins with l-leucine and affords gram quantities of the Nα-Boc-Nδ-Fmoc-γ(R)-methyl-ornithine building block. X-ray crystallography confirms that the δMeOrn turn unit adopts a folded structure in a macrocyclic β-hairpin peptide. CD and NMR spectroscopic experiments allow comparison of the δMeOrn turn template to the δ-linked ornithine (δOrn) turn template that our laboratory has previously introduced and also to the popular d-Pro-Gly turn template. Collectively, these studies demonstrate that the folding of the δMeOrn turn template is substantially better than that of δOrn and is comparable to d-Pro-Gly.
Keywords: β-hairpin, β-turn, amino acid, peptidomimetics, peptides
Table of Contents Graphic
δ-Linked γ(R)-methyl-ornithine (δMeOrn) provides an improved β-turn template for inducing a β-hairpin conformation in peptides.

Introduction
Although almost three decades have elapsed since initial reports of small peptides that fold to form β-hairpins in aqueous solution, there is still no universal tool to coax any small peptide to adopt a β-hairpin conformation, regardless of sequence. β-Hairpins occur widely in folded peptides and proteins and are defined by two hydrogen-bonded antiparallel β-strands connected by a short loop. Even though β-hairpins are nearly ubiquitous within proteins, most β-hairpins will not retain a folded structure in aqueous solution when excised from the protein. Instead, most peptides that fold into β-hairpins in aqueous solution require relatively specific sequences for the β-strands and loop. One approach to stabilizing β-hairpin formation in peptides involves replacing the loop region with a turn template.1–8 The template mimics the loop and enforces proximity, hydrogen bonding, and an antiparallel alignment between the β-strands.
Early efforts to stabilize β-hairpins involved turn templates composed of either α-amino acids or other amino acid building blocks. A d-proline residue at the i+1 position proved particularly effective for driving the formation of a β-turn. Balaram and co-workers first observed the d-Pro-l-Pro motif acting as a turn in the X-ray crystal structure of pivaloyl-d-Pro-l-Pro-l-Ala-N-methylamide, and later reported the crystal structure of a designed octapeptide containing a d-Pro-Gly sequence that folds as a β-hairpin.9,10 Gellman and co-workers subsequently demonstrated that d-Pro-Gly is favorable for inducing a β-hairpin conformation in aqueous solution in several model peptide sequences.11–13 Not all turn templates are composed of α-amino acids. Kelly and co-workers demonstrated that a dibenzofuran amino acid can induce–a β-sheet structure in analogues of gramicidin S.14 A number of other cleverly designed templates based on aromatic and alkene scaffolds have also been described.15–18
Amongst the turn templates reported to date, the d-Pro-l-Pro and d-Pro-Gly turn templates have emerged as favorites for stabilizing useful β-hairpin peptides. Schneider and co-workers have developed a series of biomaterials based on MAX1, a β-hairpin peptide containing the d-Pro-l-Pro turn motif that self-assembles into a hydrogel.19 Further investigations of MAX1 have revealed antibacterial properties for non-sterile injections and lead to other analogues with useful attributes.20,21 Robinson and co-workers have applied the d-Pro-l-Pro turn to construct cyclic β-hairpin peptides inspired by natural antibiotics. These peptidomimetic antibiotics exhibit enhanced activity and have revealed lipopolysaccharide transport proteins as cellular targets in the outer membrane of Gram-negative bacteria.22–24 Wetzel and co-workers have used the d-Pro-Gly turn motif to create polyglutamine-containing β-hairpin peptides as chemical models for the aggregation of polyglutamine-containing proteins in Huntington’s disease.25–26
In 2003, our laboratory introduced δ-linked ornithine (δOrn) as a new turn-forming unit to induce a β-hairpin conformation in peptides.27 We have subsequently used the δOrn turn unit to stabilize a growing repertoire of macrocyclic β-hairpin peptides — containing sequences from Aβ,28–30 α-synuclein,31 IAPP,32 and β2-microglobulin33 — to provide insight into the structures of amyloid oligomers.34 In the current study, we set out to improve the δOrn turn. Here, we report that introduction of a single methyl group at the γ-position with R stereochemistry substantially improves folding of the δOrn turn unit.
Results and Discussion
Design of an improved δ-linked ornithine turn.
We hypothesized that the introduction of a methyl group into the side chain of ornithine, at the right position and with the right stereochemistry, might limit the number of unfolded conformers and thus favor a folded β-turn conformation. We used Monte Carlo Stochastic Dynamics (MC/SD) calculations to guide the design of a methylated δOrn turn with improved folding properties.35,36 We examined the equilibrium between the unfolded and folded states of the methylated ornithine derivatives shown in Figure 1 by MC/SD simulation in MacroModel using the MMFFs force field with GB/SA water solvation at 300 K. We explored the effect of methylation at the β-, γ-, and δ-positions and found that methylation at the γ-position with R stereochemistry shifts the equilibrium from 0.8% folded (for the unmethylated ornithine) to 20.3% folded — an enhancement of 2.08 kcal/mol (Figure S1). Molecular modeling studies suggest that other than enhanced folding, there should be little difference between the γ(R)-methyl-ornithine turn and the unmethylated ornithine turn, as the minimum-energy turn conformations (local minima) of the ornithine derivatives are virtually identical (Figure 2). These simulations provided us with the confidence to pursue the synthesis and study of turn formation by γ(R)-methyl-ornithine.
Figure 1.

Equilibrium between unfolded and folded conformations of methylated ornithine derivatives.
Figure 2.

Minimum-energy models (local minima) of ornithine and γ(R)-methyl-ornithine derivatives Ac-δOrn-NHMe (A) and Ac-δMeOrn-NHMe (B) in folded conformations.
Synthesis of orthogonally protected γ(R)-methyl-ornithine.
We have developed a synthesis of orthogonally protected γ(R)-methyl-ornithine as a building block suitable for use in standard Fmoc-based solid-phase peptide synthesis. The synthesis begins with l-leucine and relies on the recently reported finding by Renata and co-workers that l-leucine can be chemoenzymatically oxidized to 4-hydroxyleucine 2 with high stereoselectivity and that the resulting product can be isolated as the Boc-protected lactone 3.37

Ring opening of the lactone was achieved by treatment with N,O-dimethylhydroxylamine hydrochloride and trimethylaluminum to afford the Weinreb amide (Scheme 1). The liberated alcohol group was then converted to the corresponding mesylate using methanesulfonyl chloride, resulting in an overall two-step transformation with 71% yield of the mesylated intermediate 4. This procedure was previously reported by the Renata group en route to (2S,4R)-Nα-Boc-methylproline, but the intermediate 4 was not isolated.38
Scheme 1.

Synthesis of Nα-Boc-Nδ-Fmoc-γ(R)-methyl-ornithine (1) from Boc-protected lactone 3.
Displacement of the mesylate group with sodium azide afforded the Weinreb protected azidoleucine 5 in 85% yield. The reaction was run at 70 °C to increase solubility of sodium azide in dimethylformamide and facilitate SN2 displacement. Subsequent hydrolysis of the Weinreb amide with lithium hydroxide at 55 °C in a 1:1 mixture of water and THF furnished the Boc-azidoleucine intermediate 6 in a 93% yield. Reduction of the azide group to the corresponding amine was then carried out by catalytic hydrogenation with H2 and Pd/C, followed by Fmoc protection with Fmoc-OSu in a 1:1 mixture of water and THF to give Nα-Boc-Nδ-Fmoc-γ(R)-methyl-ornithine (1, Boc-MeOrn(Fmoc)-OH) in gram quantities and a yield of 57% over the two steps.
X-ray crystallographic structure of a macrocyclic β-hairpin peptide containing the δMeOrn turn.
To confirm the stereochemistry and conformation of the δMeOrn turn unit within a β-hairpin motif, we turned to X-ray crystallography. Our laboratory has previously reported the X-ray crystallographic structure of a β-hairpin peptide derived from β2-microglobulin (peptide 1a, PDB 4P4Z).33 Peptide 1a is a macrocyclic β-hairpin peptide containing two heptapeptide strands linked by two δOrn turn units. In the X-ray crystallographic structure of peptide 1a, only one of the two δOrn turn units (right side) adopts the characteristic hydrogen-bonded turn conformation shown in Figure 2A. The other δOrn turn unit (left side) does not adopt this hydrogen-bonded conformation. To visualize the δMeOrn turn unit and determine whether it adopts the hydrogen-bonded conformation hypothesized in Figure 2B, we prepared homologue 1b and studied its structure by X-ray crystallography (PDB 7LIB).

We crystallized peptide 1b using similar conditions to those used for peptide 1a and determined the X-ray crystallographic structure at 1.1 Å. In the X-ray crystallographic structure, peptide 1b adopts a well-defined macrocyclic β-hairpin conformation (Figure 3A). The δMeOrn turn unit adopts the hypothesized hydrogen-bonded conformation, with the γ(R)-methyl group clearly visible in the electron density map (Figure 3B). The conformation of the δMeOrn turn unit in peptide 1b is particularly noteworthy, since the corresponding δOrn turn unit in peptide 1a does not adopt this well-defined hydrogen-bonded conformation (Figure S2). The improved folding of this turn in peptide 1b corroborates the MC/SD prediction that the δMeOrn turn unit is superior to the δOrn turn unit.
Figure 3.

(A) X-ray crystallographic structure of peptide 1b. (B) 2FO-FC electron density map contoured at the 1 sigma level, showing the conformation of the δMeOrn turn unit and the stereochemistry of the γ-carbon.
Circular dichroism (CD) spectroscopic studies of a macrocyclic β-hairpin peptide containing the δMeOrn turn.
To investigate the propensity of the δMeOrn turn unit to induce β-hairpin formation in the solution state, we compared a macrocyclic β-hairpin peptide containing two δMeOrn turn units to a homologue containing two δOrn turn units by CD spectroscopy. Our laboratory has previously described the X-ray crystallographic structure of a β-hairpin peptide derived from the β-amyloid peptide Aβ (peptide 2a).28 Peptide 2a contains a heptapeptide strand derived from Aβ17–23 and a heptapeptide strand derived from Aβ30–36, which are linked by two δOrn turns to form a macrocycle. The CD spectrum of peptide 2a exhibits an extended region of negative ellipticity below 250 nm, with a minimum at 202 nm and an additional small minimum at 192 nm (Figure 4). These features reflect a predominance of random coil structure in aqueous solution, even though the peptide adopts a β-hairpin conformation in the crystal state.
Figure 4.

CD spectra of peptides 2a (blue) and 2b (red). CD spectra were acquired for each peptide at 150 μM in 10 mM phosphate buffer at pH 7.4; the ellipticity was normalized for the number of residues in each peptide.

Replacement of the two δOrn turn units with δMeOrn turn units affords peptide 2b. The CD spectrum of peptide 2b shows a well-defined minimum centered at 216 nm, with a maximum positive ellipticity at ca. 194 nm (Figure 4). These features suggest a predominance of β-sheet character. The striking difference between the CD spectra of peptides 2a and 2b provides additional evidence that the δMeOrn turn is more effective at inducing a β-hairpin conformation than the δOrn turn.
NMR and CD spectroscopic studies of β-hairpin peptides containing d-Pro-Gly, δOrn, and δMeOrn turns.
To further evaluate the propensity of the δMeOrn turn unit to induce β-hairpin formation in solution, we used NMR spectroscopy to compare a set of β-hairpin peptides containing d-Pro-Gly, δOrn, and δMeOrn turns. In 1998, Gellman and co-workers reported that a turn comprising d-Pro-Gly induces a β-hairpin conformation in peptide 3, a 12-residue linear peptide.12 We prepared peptides 4a and 4b as homologues of peptide 3 in which the d-Pro-Gly turn is replaced by δOrn and δMeOrn turn units. We used TOCSY and NOESY experiments in D2O and in 90:10 H2O:D2O to assign key NOE crosspeaks associated with β-hairpin folding for each of the three peptides (Figure 5).
Figure 5.

Chemical structures of peptide 3 and homologues 4a and 4b. Key NOEs associated with solution-state folding for each peptide are represented with double-headed arrows. Red arrows represent unambiguous NOEs. An orange arrow in peptide 3 and two orange arrows in peptide 4b represent NOEs in which overlap with other resonances preclude unambiguous assignment. The resonances of the α-protons of Lys9 and Glu4 are nearly coincident in peptide 4b, preventing identification of an NOE between these protons. NMR spectra were acquired for each peptide at 4.0 mM and 277 K in D2O or 90:10 H2O:D2O, with a buffer of 100 mM CD3COOD and 100 mM CD3COONa.
The NOEs represented in Figure 5 for peptides 3, 4a, and 4b suggest that all three peptides adopt a folded conformation in aqueous solution. Peptides 3 and 4b exhibit an extensive network of cross-strand NOEs associated with hydrogen-bonded β-hairpin formation. An expected NOE between the α-protons of Lys9 and Glu4 could not be observed in peptide 4b, because the α-proton resonances occur at very similar chemical shifts (Δδ = 0.03 ppm). Unlike peptides 3 and 4b, peptide 4a exhibits only three key cross-strand NOEs, which suggests that it is less well-folded than peptides 3 and 4b.
In addition to the cross-strand NOEs, each of the peptides also exhibits key NOEs associated with turn formation (Figure 5). Peptide 3 exhibits an NOE between the NH protons of Orn8 and Gly7. Peptide 4b exhibits NOEs between the NH proton of Orn8 and the δ-protons of δMeOrn. Peptide 4b also displays NOEs between the α-proton and δ-protons of δMeOrn. Peptide 4a only exhibits NOEs between the α-proton and δ-protons of δOrn. The diastereotopic δ-proton resonances of the δMeOrn turn in peptide 4b are separated by 0.72 ppm, reflecting the formation of a well-defined turn conformation, in which the pro-S δ-proton is proximal to the Val5 carbonyl group.39 In contrast, the diastereotopic δ-proton resonances of the δOrn turn in peptide 4a are only separated by 0.14 ppm, reflecting the formation of a significantly less well-defined turn structure.
To further compare the folding of peptides 3, 4a, and 4b, we examined the chemical shifts of the α-protons, which are widely used to probe the secondary structure of peptides and proteins.40–44 The chemical shifts of α-protons in a β-sheet are typically more downfield than those of a random coil or an α-helix. The differences between the α-proton chemical shifts of peptides 3, 4a, and 4b and those of a random coil are plotted in Figure 6. In this figure, residues 2–4 and 8–11 are grouped together, because they represent regions of the β-hairpin that can be compared in a meaningful fashion across peptides 3, 4a, and 4b. Residues 1, 5, and 12 are grouped separately, because they either terminate the β-hairpin are attached to the differing turn units.45,46
Figure 6.

Chemical shift differences between the α-protons of each residue in peptides 3 (black), 4a (blue), and 4b (red) and random coil values reported by Wüthrich.44 NMR spectra were acquired for each peptide at 4.0 mM and 277 K in D2O in a buffer of 100 mM CD3COOD and 100 mM CD3COONa with 0.06 mM DSA as a reference standard.47
The difference in α-proton chemical shifts relative to random coil values for residues 2–3 and 8–11 of peptide 4b are similar to those of peptide 3, with all but residue 11 shifted substantially downfield. This downfield shifting reflects β-hairpin formation and suggests that both peptides exhibit comparable degrees of β-hairpin folding. The α-protons of Leu11 in peptides 3 and 4b are shifted upfield by ca. 0.2 ppm, which may result from the proximity of the aromatic ring of Tyr2. The α-proton of Glu4 in peptide 3 is substantially downfield to that of peptide 4b (Δδ = 0.30 ppm), suggesting subtle differences in the folded β-hairpin conformations of the two peptides.
The chemical shifts of the α-protons of peptide 4a are similar to those observed for a random coil (Figure 6), suggesting that peptide 4a does not form a well-folded β-hairpin. This result seems to contradict the NOEs observed for peptide 4a (Figure 5) and may reflect that peptide 4a adopts an ensemble of poorly folded β-hairpin conformations, with significant cross-strand proximity between Leu11 and Tyr2 and between Lys9 and Glu4. Taken together, the NOE and chemical shift data suggest that peptide 4a exhibits substantially less β-hairpin formation than peptides 3 and 4b.
The CD spectrum of peptide 3 exhibits a well-defined negative ellipticity centered at 215 nm and a maximum positive ellipticity centered at 201 nm, reflecting a β-sheet-like conformation (Figure 7). The CD spectrum of peptide 4b has a broader minimum at ca. 205–220 nm and a second minimum centered at 196 nm, indicating a mixture of β-sheet and random coil conformations. The CD spectrum of peptide 4a displays a strong minimum at 197 nm, with a small inflection at ca. 217 nm, suggesting a predominance of random coil conformation. These observations corroborate the substantial improvement of the δMeOrn turn over the δOrn turn in inducing β-hairpin formation in peptides, while also suggesting that the d-Pro-Gly turn is superior to the δMeOrn turn.
Figure 7.

CD spectra of peptides 3 (black), 4a (blue), and 4b (red). CD spectra were acquired for each peptide at 100 μM in 10 mM phosphate buffer at pH 7.4; the ellipticity was normalized for the number of residues in each peptide.
NMR and CD spectroscopic studies of a second set of β-hairpin peptides.
We further investigated the effect of the δMeOrn turn with a second β-hairpin peptide reported by the Gellman group, peptide 5.13 We prepared peptides 6a and 6b as homologues of peptide 5 in which the d-Pro-Gly turn is replaced by δOrn and δMeOrn turn units. The NOEs represented in Figure 8 for peptides 5, 6a, and 6b suggest that all three peptides adopt a folded conformation in aqueous solution. All three peptides exhibit an extensive network of cross-strand NOEs associated with hydrogen-bonded β-hairpin formation. An expected NOE between the NH protons of Thr10 and Gln3 could not be observed in peptides 6a and 6b, because the NH proton resonances occur at very similar chemical shifts (Δδ = 0.03 ppm).
Figure 8.

Chemical structures of peptide 5 and homologues 6a and 6b. Key NOEs associated with solution-state folding for each peptide are shown with double-headed arrows. An orange arrow in peptides 6a and 6b represent NOEs in which overlap with other resonances preclude unambiguous assignment. The resonances of the NH protons of Thr10 and Gln3 are nearly coincident in peptides 6a and 6b, preventing identification of an NOE between these protons. NMR spectra were acquired for each peptide at 4.0 mM and 277 K in D2O or 90:10 H2O:D2O, with a buffer of 100 mM CD3COOD and 100 mM CD3COONa.
Peptides 5, 6a, and 6b each show NOE patterns associated with turn formation. Peptide 5 exhibits NOEs between the NH proton of Lys8 and both the NH proton of Gly7 and α-proton of d-Pro6 (Figure 8). Peptide 6a displays NOEs between the NH proton of Lys8 and the δ-protons of δOrn. Peptide 6a also displays NOEs between the α-proton and δ-protons of δOrn. Peptide 6b has the same pattern of NOEs associated with the δMeOrn turn. The diastereotopic δ-proton resonances of the δMeOrn turn in peptide 6b are separated by 0.89 ppm, indicating that the turn is well-folded, while the diastereotopic δ-proton resonances of the δOrn turn in peptide 6a are separated by 0.35 ppm, reflecting only moderate folding.39
The α-proton chemical shifts of peptides 5, 6a, and 6b suggest that peptides 6a and 6b fold in a similar fashion to each other but a different fashion from peptide 5 (Figure 9). In both peptides 6a and 6b, the α-protons of Trp2, Gln3, Lys8, Phe9, and Thr10 are shifted downfield, while the α-protons of Tyr4 and Val11 are shifted upfield. The downfield shifting for Trp2, Gln3, Lys8, Phe9, and Thr10 is greater in peptide 6b than in 6a, reflecting a greater degree of folding for peptide 6b. Peptide 5 exhibits a somewhat different pattern of chemical shifts, with the α-proton of Gln3 showing only slight downfield shifting, the α-proton of Phe9 showing slight upfield shifting, and the α-proton of Tyr4 showing substantial downfield shifting. These differences may result from subtle differences in the β-hairpin conformations of the peptides, with the magnetic anisotropies from the aromatic rings of Trp2, Tyr4, and Phe9 affecting the chemical shifts of many of the α-protons (Figure S3).
Figure 9.

Chemical shift differences between the α-protons of each residue in peptides 5 (black), 6a (blue), and 6b (red) and random coil values reported by Wüthrich.44 NMR spectra were acquired for each peptide at 4.0 mM and 277 K in D2O in a buffer of 100 mM CD3COOD and 100 mM CD3COONa with 0.06 mM DSA as a reference standard.47
The CD spectrum of peptide 5 has a maximum at 230 nm, a broad minimum at ca. 210–220 nm and a second more intense minimum at 200 nm, reflecting a mixture of β-sheet and random coil conformations (Figure 10). The CD spectrum of peptide 6b is similar to peptide 5, but with a more intense minimum at ca. 204–208 nm instead of ca. 210–220 nm. The combination of minima at 200 nm and at ca. 204–208 nm in peptide 6b suggest a mixture of β-sheet and random coil conformations, with the shifted minimum at ca. 204–208 nm reflecting significant contributions from the packing of the aromatic residues. The CD spectrum of peptide 6a exhibits a pair of small minima at 196 nm and 200 nm, reflecting a predominance of random coil conformations. These observations stand in agreement with our findings that the δMeOrn turn is more effective than the δOrn turn in inducing β-hairpin formation in peptides.
Figure 10.
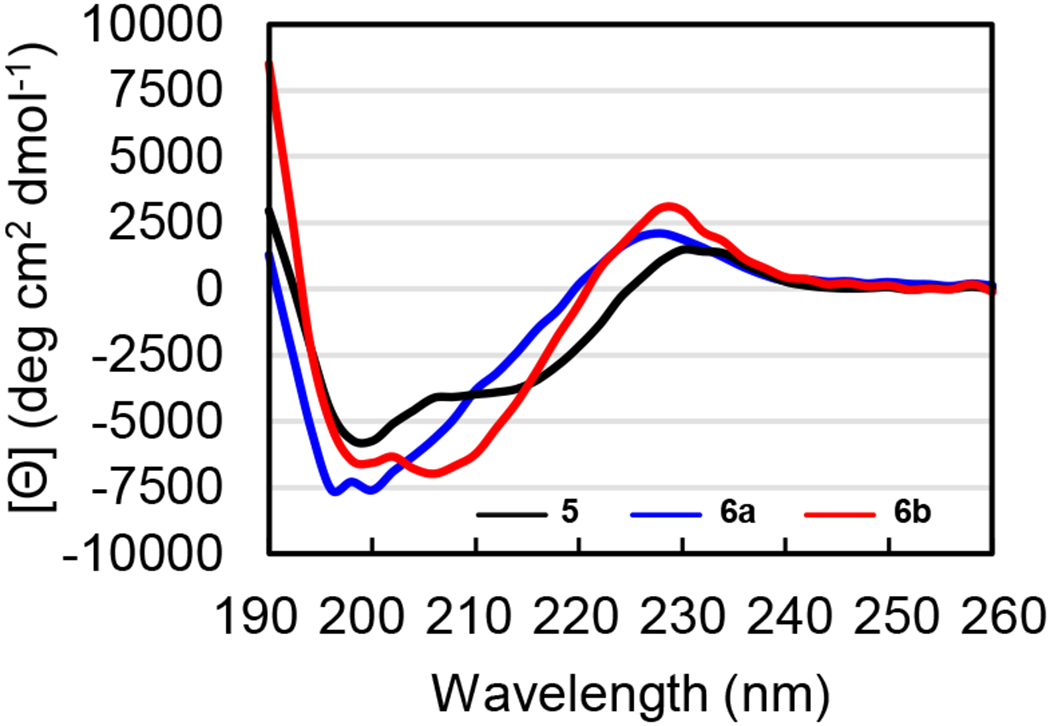
CD spectra of peptides 5 (black), 6a (blue), and 6b (red). CD spectra were acquired for each peptide at 100 μM in 10 mM phosphate buffer at pH 7.4; the ellipticity was normalized for the number of residues in each peptide.
Conclusion
Stereospecific incorporation of a single methyl group substantially enhances the propensity of δ-linked ornithine to induce β-hairpin formation in peptides. A recently reported chemoenzymatic hydroxylation of l-leucine selectively sets the R stereochemistry of the methyl group at the γ-position and enables the subsequent gram-scale synthesis of the protected γ(R)-methyl-ornithine derivative, Boc-MeOrn(Fmoc)-OH. This derivative bears orthogonal protecting groups compatible with solid-phase peptide synthesis and can be readily incorporated into different peptide sequences.
The X-ray crystallographic structure of peptide 1b unambiguously confirms the stereochemistry of the methyl group and the predicted hydrogen-bonded conformation of the δMeOrn turn. CD spectroscopic studies of peptides 2a and 2b reveal a distinct improvement in β-hairpin folding when both the δOrn turns are replaced with δMeOrn turns. NMR and CD spectroscopic studies of β-hairpin peptides 3–6 also indicate a substantial improvement in β-hairpin folding induced by δMeOrn. NMR and CD spectroscopic studies further indicate comparable β-hairpin folding induced by δMeOrn and d-Pro-Gly, with the latter perhaps being somewhat superior.
The improved β-hairpin formation provided by the δMeOrn turn places it in the same league as the popular d-Pro-l-Pro and d-Pro-Gly turns and opens the door to useful applications. We anticipate using it in our own laboratory to improve the mimicry of amyloid oligomers composed of β-hairpins.34 We also envision that the free α-amino group of the δMeOrn turn will offer advantages over the d-Pro-l-Pro and d-Pro-Gly turns in solubility and could also serve as a handle for further functionalization.30,38 Although there is still no universal tool to coax any small peptide — polyalanine, for example — to adopt a β-hairpin conformation, the δMeOrn turn represents a worthy addition to the toolbox of turn templates. MC/SD calculations predict that β(R),γ(R)-dimethyl-ornithine may be even better at inducing β-hairpin formation in peptides (Figure S1). We look forward to future synthetic advances or clever application of existing methodology that enable the facile preparation of derivatives of β(R),γ(R)-dimethyl-ornithine suitable for use in solid-phase peptide synthesis, so that this hypothesis can be tested.
Supplementary Material
Acknowledgements
We thank the National Institute of General Medical Sciences (NIGMS) for funding (GM097562). We thank Dr. Philip Dennison and the NMR Spectroscopy Facility in the UCI Department of Chemistry for assistance with NMR experiments. We thank Ben Katz and Dr. Felix Grun and at the UCI Mass Spectrometry facility, and Dr. Dmitry Fishman at the UCI Laser Spectroscopy Laboratories for their assistance and discussions. We thank Professor Hans Renata for providing the plasmid needed for expression of GriE, which is required for the chemoenzymatic synthesis of hydroxyleucine 2 and thus Boc-protected lactone 3. We also thank members of the Martin, Tsai, Weiss, Dong, and Jarvo laboratories for providing helpful advice and access to solvents and equipment. M.W. acknowledges the support from the Ministry of Science and Higher Education, Republic of Poland (Mobility Plus grant no. 1647/MOB/V/2017/0).
Footnotes
Supporting information for this article is given via a link at the end of the document
Conflicts of Interest
The authors declare no competing financial interest.
Crystallographic coordinates of peptide 1b were deposited into the Protein Data Bank (PDB) with code 7LIB.
References
- [1].Moriuchi T; Hirao T Chem. Soc. Rev 2004, 33 (5), 294–301. [DOI] [PubMed] [Google Scholar]
- [2].Khakshoor O; Nowick JS Curr. Opin. Chem. Biol 2008, 12 (6), 722–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Robinson JA Acc. Chem. Res 2008, 41 (10), 1278–1288. [DOI] [PubMed] [Google Scholar]
- [4].Marcelino AMC; Gierasch LM Biopolymers 2008, 89 (5), 380–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Fuller AA; Du D; Liu F; Davoren JE; Bhabha G; Kroon G; Case DA; Dyson HJ; Powers ET; Wipf P; Martin G; Kelly JW Proc. Natl. Acad. Sci. U. S. A 2009, 106 (27), 11067–11072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Nair RV; Baravkar SB; Ingole TS; Sanjayan GJ Chem. Commun 2014, 50 (90), 13874–13884. [DOI] [PubMed] [Google Scholar]
- [7].Horne WS; Grossmann TN Nat. Chem 2020, 12 (4), 331–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Lenci E; Trabocchi A Chem. Soc. Rev 2020, 49 (11), 3262–3277. [DOI] [PubMed] [Google Scholar]
- [9].Nair CM; Vijayan M; Venkatachalapathi YV Balaram P. J.C.S. Chem. Comm 1979, 1183–1184. [Google Scholar]
- [10].Karle IL; Awasthi SK; Balaram P Proc. Natl. Acad. Sci. U. S. A 1996, 93 (16), 8189–8193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Haque TS; Gellman SH J. Am. Chem. Soc 1997, 119 (9), 2303–2304. [Google Scholar]
- [12].Stanger HE; Gellman SH J. Am. Chem. Soc 1998, 120 (17), 4236–4237. [Google Scholar]
- [13].Espinosa JF; Gellman SH Angew. Chem. Int. Ed 2000, 11 (13), 2330–2333. [DOI] [PubMed] [Google Scholar]
- [14].Díaz H; Tsang KY; Choo D; Espina JR; Kelly JW J. Am. Chem. Soc 1993, 115 (9), 3790–3791. [Google Scholar]
- [15].Kemp DS; Li ZQ Tetrahedron Lett. 1995, 36 (24), 4175–4178. [Google Scholar]
- [16].Kemp DS; Li ZQ Tetrahedron Lett. 1995, 36 (24), 4179–4180. [Google Scholar]
- [17].Schopfer U; Stahl M; Brandl T; Hoffman RW Angew. Chem. Int. Ed 1997, 36 (16), 1745–1747. [Google Scholar]
- [18].Gardner RR; Liang GB; Gellman SH J. Am. Chem. Soc 1999, 121 (9), 1806–1816. [Google Scholar]
- [19].Schneider JP; Pochan DJ; Ozbas B; Rajagopal K; Pakstis L; Kretsinger J J. Am. Chem. Soc 2002, 124 (50), 15030–15037. [DOI] [PubMed] [Google Scholar]
- [20].Haines-Butterick L; Rajagopal K; Branco M; Salick D; Rughani R; Pilarz M; Lamm MS; Pochan DJ; Schneider JP Proc. Natl. Acad. Sci. U. S. A 2007, 104 (19), 7791–7796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Salick DA; Kretsinger JK; Pochan DJ; Schneider JP J. Am. Chem. Soc 2007, 129 (47), 14793–14799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Shankaramma SC; Athanassiou Z; Zerbe O; Moehle K; Mouton C; Bernardini F; Vrijbloed JW; Obrecht D; Robinson JA ChemBioChem 2002, 3 (11), 1126–1133. [DOI] [PubMed] [Google Scholar]
- [23].Srinivas N; Jetter P; Ueberbacher BJ; Werneburg M; Zerbe K; Steinmann J; Van Der Meijden B; Bernardini F; Lederer A; Dias RLA; Mission PE; Henze H; Zumbrunn J; Gombert FO; Obrecht D; Hunziker P; Schauer S; Ziegler U; Käch A; Eberl L; Riedel K; DeMarco SJ; Robinson JA Science. 2010, 327 (5968), 1010–1013. [DOI] [PubMed] [Google Scholar]
- [24].Andolina G; Bencze LC; Zerbe K; Müller M; Steinmann J; Kocherla H; Mondal M; Sobek J; Moehle K; Malojčić G; Wollscheid B; Robinson JA ACS Chem. Biol 2018, 13 (3), 666–675. [DOI] [PubMed] [Google Scholar]
- [25].Kar K; Hoop CL; Drombosky KW; Baker MA; Kodali R; Arduini I; Van Der Wel PCA; Horne WS; Wetzel R J. Mol. Biol 2013, 425 (7), 1183–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Kar K; Baker MA; Lengyel GA; Hoop CL; Kodali R; Byeon IJ; Horne WS; van der Wel PCA; Wetzel R J. Mol. Biol 2017, 429 (2), 308–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Brower JO; Nowick JS J. Am. Chem. Soc 2003, 125 (4), 876–877. [DOI] [PubMed] [Google Scholar]
- [28].Spencer RK; Li H; Nowick JS J. Am. Chem. Soc 2014, 136 (15), 5595–5598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Kreutzer AG; Hamza IL; Spencer RK; Nowick JS J. Am. Chem. Soc 2016, 138 (13), 4634–4642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Samdin TD; Wierzbicki M; Kreutzer AG; Howitz WJ; Valenzuela M; Smith A; Sahrai V; Truex NL; Klun M; Nowick JS J. Am. Chem. Soc 2020, 142 (26), 11593–11601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Salveson PJ; Spencer RK; Nowick JS J. Am. Chem. Soc 2016, 138 (13), 4458–4467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Wang Y; Kreutzer AG; Truex NL; Nowick JS J. Org. Chem 2017, 82 (15), 7905–7912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Spencer RK; Kreutzer AG; Salveson PJ; Li H; Nowick JS J. Am. Chem. Soc 2015, 137 (19), 6304–6311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Kreutzer AG; Nowick JS Acc. Chem. Res 2018, 51 (3), 706–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].McDonald QD; Still CW Tetrahedron Lett. 1992, 33 (50), 7747–7750. [Google Scholar]
- [36].McDonald QD; Still CW J. Am. Chem. Soc 1994, 116 (25), 11550–11553. [Google Scholar]
- [37].Zwick CR; Renata H J. Am. Chem. Soc 2018, 140 (3), 1165–1169. [DOI] [PubMed] [Google Scholar]
- [38].Zwick CR; Renata H J. Org. Chem 2018, 83 (14), 7407–7415. [DOI] [PubMed] [Google Scholar]
- [39].Woods RJ; Brower JO; Castellanos E; Hashemzadeh M; Khakshoor O; Russu WA; Nowick JS J. Am. Chem. Soc 2007, 129 (9), 2548–2558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Wishart DS; Sykes BD; Richards FM J. Mol. Biol 1991, 222 (2), 311–333. [DOI] [PubMed] [Google Scholar]
- [41].Wishart DS; Sykes BD; Richards FM Biochemistry 1992, 31 (6), 1647–1651. [DOI] [PubMed] [Google Scholar]
- [42].Wishart DS; Sykes BD Meth. Enzymol 1994, 239 (1982), 363–392. [DOI] [PubMed] [Google Scholar]
- [43].Wishart DS; Case DA Meth. Enzymol 2002, 338, 3–34. [DOI] [PubMed] [Google Scholar]
- [44].The random coil values that we used to calculate for the difference between observed α-proton chemical shifts in peptides 3–6b and those of a random coil are taken from:Wüthrich K. NMR of Proteins and Nucleic Acids, Wiley: New York, 1986. [Google Scholar]
- [45].Wishart DS; Bigam CG; Holm A; Hodges RS; Sykes BD 1H, 13C and 15N Random Coil NMR Chemical Shifts of the Common Amino Acids. I. Investigations of Nearest-Neighbor Effects. J. Biomol. NMR 1995, 5 (1), 67–81. [DOI] [PubMed] [Google Scholar]
- [46].Carlisle EA; Holder JL; Maranda AM; de Alwis AR; Selkie EL; McKay SL Effect of pH, Urea, Peptide Length, and Neighboring Amino Acids on Alanine α-Proton Random Coil Chemical Shifts. Biopolymers 2007, 85 (1), 72–80. [DOI] [PubMed] [Google Scholar]
- [47].Nowick JS; Khakshoor O; Hashemzadeh M; Brower JO Org. Lett 2003, 5 (19), 3511–3513. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


