Abstract
Objective:
We investigated the role of chronic stress burden on adiposity and adiposity-related inflammation with two hypotheses: (1) greater chronic stress is associated with higher central adiposity and selective accumulation of visceral adipose tissue (VAT) compared to subcutaneous adipose tissue (SAT); and (2) associations between VAT and inflammatory biomarkers are exacerbated when chronic stress is high.
Methods:
Data come from 1,809 participants included in a Multi-Ethnic Study of Atherosclerosis ancillary study of body composition and adiposity-related inflammation. Chronic psychosocial stress was measured with a 5-item version of the Chronic Stress Burden Scale. First, we tested associations between chronic stress (3-level categorical variable) and VAT, SAT, and VAT/SAT ratio. Second, we tested whether associations between VAT and inflammatory biomarkers varied by level of chronic stress.
Results:
Participants were approximately 65 years, 50% female, and 40.5% White, 25.6% Hispanic, 21.2% African American, and 12.8% Chinese American. About half of the sample reported little to no stress, and a quarter and a fifth of the sample reported medium and high levels of stress. Higher levels of chronic stress were associated with greater VAT and SAT, but not VAT/SAT ratio. Greater levels of VAT were associated with increased levels of adiposity-related inflammation in a graded pattern. These associations did not vary by stress level.
Conclusions:
Greater chronic stress burden is associated with both central and subcutaneous adiposity. We found no evidence that the associations between VAT and inflammatory biomarkers are exacerbated by chronic stress. Findings contribute to ongoing literature untangling pathways in which psychosocial stress contributes to adiposity-related inflammation.
Keywords: visceral adipose tissue, central adiposity, inflammation, chronic psychosocial stress
INTRODUCTION
In several epidemiologic studies, chronic psychosocial stress has been associated with low-grade systemic inflammation and is considered a cardiovascular disease risk factor. [1–4] One of the most studied potential mechanisms linking chronic stress to inflammatory biomarkers has been via accumulation of adipose tissue. Chronic stress may promote fat accumulation through several behavioral responses, including excess caloric intake, reduced physical activity and increased sedentary behavior.[5–9] In addition to these behavioral mechanisms, chronic stress is associated with dysregulation of the HPA axis. Increased production of cortisol and reduced glucocorticoid sensitivity has also been associated with increased adiposity and increased levels of circulating pro-inflammatory cytokines.[4, 10–12]
The existing literature in this area, however, is limited because the adiposity outcome is most frequently measured with body mass index (BMI, kg/m2) or, in fewer studies, waist circumference or waist-to-hip ratio.[5] These measures of adiposity are not able to distinguish between fat accumulated in specific depots of the abdominal region. For example, two individuals with the same measured waist circumference may have different amounts of adipose tissue stored directly under the skin (subcutaneous adipose tissue, SAT) and around organs in the abdominal cavity (visceral adipose tissue, VAT). This distinction is clinically relevant because VAT is more metabolically active than SAT, has more glucocorticoid receptors, and is independently associated with increased morbidity and mortality.[13, 14] Moreover, VAT is a well-established risk factor of metabolic and cardiovascular disease, [15–18] and contributes to the secretion of several adipokines including tumor necrosis factor alpha (TNF-a), interleukin-6 (IL-6), resistin and adiponectin. Dysregulation of these adipokines is associated with endothelial dysfunction, atherosclerosis, and low-grade chronic inflammation. [19–22]
There is some evidence to suggest that chronic hypersecretion of stress hormones affects fat distribution and promotes selective accumulation of VAT. [23–26] However, only a few studies have examined psychosocial factors in relation to CT-assessed visceral fat in human participants. Cross-sectional analyses from the Study of Women’s Health Across the Nation (SWAN) cohort suggest that experiences of discrimination [27], depressive symptoms, [28] and hostility [29] are associated with increased VAT. Longitudinal data collected from a small cohort of mothers of children with autism spectrum disorder show increased VAT associated with chronic caregiving stress.[30] Additional research studies, with larger sample sizes, longitudinal study designs, and specific measurement of chronic stress burden, are needed to further evaluate the hypothesis that exposure to chronic stress promotes selective accumulation of VAT.
In addition to this hypothesized relationship between chronic stress and VAT, chronic stress may also affect functioning of adipocytes. Previous animal research shows that chronic stress may increase secretion of pro-inflammatory cytokines such as TNF-alpha and Il-6[31, 32]. However, there is no evidence from human studies that the metabolic functioning of adipocytes differs under high stress conditions.
To fill these knowledge gaps, the current study aimed to a) investigate the role of chronic stress burden on adiposity and adiposity-related inflammation and b) test two hypotheses: (1) greater chronic stress burden is associated with higher central adiposity with selective accumulation of VAT compared to SAT; and (2) associations between VAT and inflammatory biomarkers will be exacerbated when chronic stress burden is high.
METHODS:
Study population
This study was based on participants from Multi Ethnic Study of Atherosclerosis (MESA); a longitudinal study of CVD progression and risk factors that has been previously described.[33] In brief, MESA recruited 6,814 men and women ages 46 to 84 from six US cities between 2000–2002. Eligible participants were free of known CVD at baseline and follow-up for cardiovascular events occurs annually.
During follow-up examinations occurring between July 2002 to January 2004 (exam 2) and January 2004 to September 2005 (exam 3), a randomly selected subsample of 2202 participants were invited to participate in an ancillary study of body composition and adiposity-related inflammation. Of those invited, 30 declined participation and 202 were excluded because they were pre-menopausal or underwent an abdominal CT scan in the prior 6 months, leaving a study sample of 1,970. An additional 10 participants were excluded due to incomplete visualization of the visceral cavity [34]. For the current analyses, we excluded participants with incomplete data for self-reported chronic stress burden (n=24), visceral adipose tissue (N=50), inflammatory biomarkers (n=85), or other model covariates (n=2). Hence, the analytic sample is the remaining 1,809 participants, representing 92.0% of the initial ancillary study sample.
The MESA and relevant ancillary study were approved by the institutional review board at each site. Written informed consent was obtained from all participants.
Measurement
The primary independent variable, chronic stress burden across important life domains, was reported at MESA exam 1 by participants via a 5-item version of the Chronic Stress Burden Scale [35, 36]. Participants reported if they experienced ongoing stress due to the following difficulties in the past year: health problem, health problem with someone close to them, job difficulties, financial strains, and difficulties in a relationship with someone else. For each difficulty experienced, participants further reported duration of stress (6+ months vs. less) and severity of the situation (not very stressful, moderately stressful, very stressful). Domains in which participants reported moderate to severe stress lasting over 6 months were summed to create a total stress score (total range 0 to 5, where higher scores indicate higher levels of stress). Consistent with prior research conducted with the MESA cohort, total chronic stress burden score was categorized as high (2 or more), medium (1), and low (0). [37, 38] Additionally, we elected to categorize chronic stress because the distribution of the total chronic stress burden score was highly skewed (Figure S1, Supplemental Digital Content).
Using standard protocols, visceral and subcutaneous adipose tissue (VAT, SAT) area and density measurements were based on images obtained by abdominal computed tomography (CT) at either MESA exam 2 (40% of sample) or exam 3 (60% of sample). Each participant was measured only once. Two sites used an Imatron C-150 electron-beam scanner, while three sites used multi-detector CT scanners. VAT and SAT were defined as fat tissue (identified as being between −190 and −30 Hounsfield units) within the visceral and subcutaneous tissue depots, respectively. Area for both depots (in cm2) was calculated by averaging fat area across 6 transverse slices. Image evaluators were blinded from other clinical data when interpreting the scan images.[16] The median time between exam 1 and CT scan was 3 years (range 1.1 to 4.8).
Fasting morning venous blood samples were taken at the same visit as the CT scan (exam 2 or 3). Participants were instructed to avoid strenuous activity and smoking for 12 hours prior to the blood draw. Samples were centrifuged, shipped overnight to the MESA central laboratory at the University of Vermont (Burlington, VT), and stored at −80 °C. From these, we evaluated six markers of inflammation: C-reactive protein (CRP), interleukin-6 (IL-6), tumor necrosis factor (TNF-alpha), resistin, leptin, and adiponectin. Specific methods and commercial assays used to measure levels of each inflammatory marker have been previously described. [39, 40] Inter-assay coefficient of variation (CV) for CRP was 3.6%, and for other adipokines the CV ranged between 6.0% and 13.0%. [41–43]
Demographic variables included age, sex, race/ethnicity and educational attainment; all of which were participant-reported at exam 1. Educational attainment was collapsed into three categories (high school or less, some college / technical school / association, bachelor’s degree or greater). Depressive symptoms were collected at exam 1 using the 20-item Center for Epidemiologic Studies Depression Scale (CES-D).[44] The range of possible scores was 0 to 42, with higher scores indicating more depressive symptoms. Self-reported lifestyle factors included smoking, physical activity (moderate to vigorous physical activity in Met-min per week) and sedentary behavior (Met-min per week) were collected at the same visit as the CT scan (exam 2 or 3). Data collected on smoking included self-reported smoking status (never, former, current), and pack-years of cigarette smoking for former and current smokers. Physical activity and sedentary behavior were self-reported using the MESA Typical Physical Activity Survey, which was a 28-item questionnaire of participation in specific activities during a typical week. The survey was adapted from the Cross-Cultural Activity Participation Study.[45] Participants reported frequency and duration spent engaging in select physical (household chores, dancing, sport activities, conditioning, etc.) and sedentary (TV, watching, knitting, recreational computer, etc.) activities. Responses were summed to compute minutes per week spent in moderate to vigorous physical activity and sedentary behavior.
Statistical Analysis
Data analysis was performed in SAS 9.4 (Cary, North Carolina). The distributions of all study variables were examined and all inflammatory biomarkers were log transformed to meet assumptions of normality because these are dependent variables in the subsequently described regression models [46]. The analytic dataset was described according to levels of chronic stress (high vs. med. vs. low) and by quartiles of VAT using descriptive statistics (mean ± SD, median (min, max) percentages, etc.). Group differences were assessed with chi-square (categorical data), independent samples t-tests (normally distributed continuous data), and Wilcoxon rank sum tests (skewed continuous data). For all analyses, statistical significance was determined if the two-sided p-value was less than 0.05.
First, we used sequentially adjusted linear regression models to assess associations between levels of chronic stress burden and VAT area (sq cm), SAT area (sq cm) and VAT/SAT ratio. Chronic stress burden was considered a 3-level categorical variable (reference level = low stress). Model 1 adjusted for age, sex, race/ethnicity, educational attainment, and height. In model 2, we added smoking status (current, former, never) and pack-years cigarette smoking to the covariates included in model 1. In model 3, we further added lifestyle variables including physical activity and sedentary behavior, as well as depressive symptoms. Covariates were added to models 2 and 3 separately due to concerns that they might be in the pathway from chronic stress to adipose tissue deposition. All models including adipose tissue variables adjust for participant height (in meters), which was measured at the same clinic visit. In post hoc analyses, we added a fourth model which additionally adjusted for body mass index (kg/m2) rather than participant height. Also in post hoc analyses, we assessed whether there was an interaction between race/ethnicity and chronic stress burden for the three outcomes: VAT area, SAT area and VAT/SAT. The data supported no evidence of an interaction (p=0.61, p=0.34, p=0.22, respectively) so analyses were not stratified by race/ethnicity. Additionally, we found no evidence to suggest that these associations varied by time (in years) between the baseline exam and exam 2/3 (p=0.53, p = 0.3, p=0.12), or by sex (p=0.26, p=0.38, p = 0.69)
Secondly, we evaluated the associations between chronic stress burden level and each inflammatory biomarker using generalized linear models. In post hoc analyses, we found evidence to suggest that the associations varied by time (in years) between baseline exam and exam2/3. Thus, we present associations for the full sample, and stratified by which exam (2 or 3) the inflammatory biomarkers were collected at.
Lastly, we evaluated whether chronic stress burden level was a modifier of the associations between VAT and inflammatory biomarkers. For these analyses, we considered VAT area as a categorical variable (quartile split) to assess the possibility of nonlinear associations. We used six linear regression models where the dependent variables were inflammatory biomarkers and the main independent variable was VAT quartile. We then used an interaction term (VAT area * chronic stress) to evaluate effect modification. Models were fully adjusted for age, sex, race/ethnicity, educational attainment, height, current smoking status and pack years, physical activity, and sedentary behavior. We created box plots using R Studio (V 1.2.5033) to visualize the relationships between VAT area and each biomarker stratified by level of chronic stress burden.
RESULTS:
Participants in the analytic sample were approximately 65 years of age, 50% female, and 40.5% White, 25.6% Hispanic, 21.2% African American, and 12.8% Chinese American. About half of the sample reported little to no chronic stress burden (i.e. score of zero), while about a quarter and a fifth of the sample reported medium and high levels of stress, respectively. When categorized into the aforementioned three levels, participants reporting the highest levels of chronic stress burden were younger and more likely to be female. About 29% of African American participants reported the highest levels of chronic stress burden, followed by 25% of White participants, 19% of Hispanic participants, and 14% of Chinese American participants. Also, participants with high chronic stress burden had higher educational attainment and slightly higher levels of sedentary behavior. Average income level, smoking status, and physical activity did not vary significantly by level of chronic stress burden in univariable analyses (Table 1).
Table 1.
Study Sample Characteristics in Full Sample and According to Levels of Chronic Stress, Multi-Ethnic Study of Atherosclerosis: Ancillary Study of Body Composition and Adiposity-Related Inflammation (Exam 2/3), United States
| Chronic Stress Level | ||||
|---|---|---|---|---|
| Low (N=918) | Med (N=478) | High (N=413) | P-Value | |
| Age in years, mean (sd) | 65.6 ± 9.6 | 64.6 ± 9.7 | 62.3 ± 9.1 | <0.001 |
| Sex | ||||
| Women, % | 414 (45.1) | 252 (52.7) | 243 (58.8) | <0.001 |
| Man, % | 504 (54.9) | 226 (47.3) | 170 (41.2) | |
| Race/Ethnicity | ||||
| White, % | 315 (34.3) | 237 (49.6) | 181 (43.8) | <0.001 |
| Chinese American, % | 165 (18.0) | 34 (7.1) | 32 (7.7) | |
| African American, % | 188 (20.5) | 85 (17.8) | 110 (26.6) | |
| Hispanic, % | 250 (27.2) | 122 (25.5) | 90 (21.8) | |
| Education Attainment | ||||
| High school or less, % | 368 (40.1) | 160 (33.5) | 115 (27.8) | <0.001 |
| Some college, technical school, associate, % | 239 (26.9) | 250 (27.2) | 133 (27.8) | |
| Bachelor’s degree or greater, % | 300 (32.7) | 185 (38.7) | 163 (39.5) | |
| Total Gross Family income | ||||
| <$25,000, % | 271 (30.8) | 116 (25.4) | 117 (30.0) | 0.174 |
| $25,000 to $49,000, % | 275 (31.3) | 133 (29.1) | 123 (31.5) | |
| $50,000 to %100,000, % | 201 (22.9) | 129 (28.2) | 96 (24.6) | |
| >$100,000 | 132 (15.0) | 79 (17.3) | 54 (13.8) | |
| Lifestyle | ||||
| Ever smoker, % | 484 (52.7) | 256 (53.6) | 237 (57.4) | 0.28 |
| Pack years smoked (former smokers) | 10.69 (20.24) | 12.13 (21.42) | 12.97 (22.69) | 0.157 |
| MVPA in MET, min/wk M-Su | 4785.46 (4461.89) | 4954.71 (4811.58) | 5225.53 (5033.59) | 0.283 |
| Sed Behavior, min/week | 1615.75 (1061.96) | 1670.33 (1069.72) | 1772.53 (1131.99) | 0.05 |
| Body mass index kg/m2 | 27.7 (4.6) | 28.2 (5.4) | 29.0 (5.4) | <0.01 |
| Obese, % | 26.8 (246) | 33.1 (158) | 36.6 (151) | <0.01 |
MVPA=Moderate-to-vigorous physical activity, WHO = World Health Organization
N (%) reported for categorical variables, Mean ± SD reported for continuous variables
P-value ascertained from a test of differences across groups. Analyses were chi-square (categorical data), t-tests (normally distributed continuous data), and Wilcoxon rank sum tests (skewed continuous data).
In univariable analyses, participants with more VAT were, on average, older, more likely to be male, more likely to be White, more likely to have high school or less education, more likely to be ever smokers and to have smoked more pack years and to spend more min/week in sedentary behavior (Table 2).
Table 2.
Study Sample Characteristics in Full Sample and According to Quartile of Visceral Adipose Tissue, Multi-Ethnic Study of Atherosclerosis: Ancillary Study of Body Composition and Adiposity-Related Inflammation (Exam 2/3), United States
| Quartile of Visceral Adipose Tissue (sq cm) | |||||
|---|---|---|---|---|---|
| Q1 (N=441) | Q2 (N=452) | Q3 (N=461) | Q4 (N=456) | P-Value | |
| Age in years, mean (sd) | 63.40 ± 9.79 | 63.53 ± 9.67 | 65.13 ± 9.27 | 66.34 ± 9.36 | <0.001 |
| Sex | |||||
| Woman, % | 297 (67.3) | 240 (53.2) | 214 (46.4) | 158 (34.6) | <0.001 |
| Man, % | 144 (32.7) | 211 (46.8) | 247 (53.6) | 298 (65.4) | |
| Race/Ethnicity | |||||
| White, % | 170 (38.5) | 143 (31.7) | 188 (40.8) | 232 (50.9) | <0.001 |
| Chinese American, % | 83 (18.8) | 79 (17.5) | 48 (10.4) | 21 (4.6) | |
| African American, % | 129 (29.3) | 114 (25.3) | 77 (16.7) | 63 (13.8) | |
| Hispanic, % | 59 (13.4) | 115 (25.5) | 148 (32.1) | 140 (30.7) | |
| Education Attainment | |||||
| High school or less, % | 124 (28.1) | 166 (36.8) | 168 (36.4) | 185 (40.6) | 0.006 |
| Some college, technical school, associate, % | 134 (30.4) | 121 (26.8) | 136 (29.5) | 127 (27.9) | |
| Bachelor’s degree or greater, % | 183 (41.5) | 164 (36.4) | 157 (34.1) | 144 (31.6) | |
| Total Gross Family income | |||||
| <$25,000, % | 104 (25.2) | 125 (29.1) | 132 (29.5) | 143 (32.8) | 0.247 |
| $25,000 to $49,000, % | 126 (30.6) | 136 (31.6) | 136 (30.4) | 133 (30.5) | |
| $50,000 to %100,000, % | 105 (25.5) | 111 (25.8) | 117 (26.1) | 93 (21.3) | |
| >$100,000 | 77 (18.7) | 58 (13.5) | 63 (14.1) | 67 (15.4) | |
| Lifestyle | |||||
| Ever smoker, % | 224 (50.8) | 211 (46.8) | 255 (55.3) | 287 (62.9) | <0.001 |
| Pack years smoked (former smokers) | 9.92 (18.40) | 9.69 (19.51) | 10.94 (20.90) | 15.74 (24.63) | <0.001 |
| MVPA in MET, min/wk M-Su | 4935.41 (4511.62) | 5091.00 (4775.31) | 5121.10 (4864.39) | 4574.46 (4594.89) | 0.272 |
| Sed Behavior, min/week | 1604.40 (1049.98) | 1589.39 (1011.22) | 1668.08 (1154.61) | 1799.51 (1093.54) | 0.014 |
MVPA=Moderate-to-vigorous physical activity
N (%) reported for categorical variables, Mean ± SD reported for continuous variables
P-value ascertained from a test of differences across groups. Analyses were chi-square (categorical data), t-tests (normally distributed continuous data), and Wilcoxon rank sum tests (skewed continuous data).
In sequentially adjusted regression models, greater levels of chronic stress burden was positively associated with more VAT and SAT. In the model adjusted for age, sex, race/ethnicity, educational attainment, and height (model 1), medium and high levels of chronic stress burden were associated with 3.2 (se=3.6) and 9.4 (se=3.9) cm2 higher VAT, compared to the low stress group. Similarly, medium and high levels of chronic stress burden were associated with 3.6 (se = 6.5) and 14.7 (se = 6.9) cm2 higher SAT. The test for trend was statistically significant for both depots (p<0.05). Further adjustment for smoking (model 2) and activity (model 3) slightly attenuated effect estimates from model 1. All effect estimates were attenuated towards the null after including body mass index in the model. Lastly, the mean VAT/SAT ratio was 0.61, and our data show no evidence of a meaningful association between chronic stress burden level and VAT/SAT ratio (Table 3).
Table 3.
Associations between levels of chronic psychological stress and VAT, SAT, and VAT/SAT ratio, Multi-Ethnic Study of Atherosclerosis: Ancillary Study of Body Composition and Adiposity-Related Inflammation (Exam 2/3), United States
| Model 1 | Model 2 | Model 3 | Model 4 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Visceral Adipose Tissue (VAT) | |||||||||
| Adj Mean (95%CI) | Beta (SE) | p-trend | Beta (SE) | p-trend | Beta (SE) | p-trend | Beta (SE) | p-trend | |
| Chronic Stress | |||||||||
| Low (0) | 140.1 (135.9, 144.4) | Ref | 0.02 | Ref | 0.02 | Ref | 0.03 | Ref | 0.49 |
| Med (1) | 143.3 (137.3, 149.4) | 3.2 (3.6) | 2.5 (3.7) | 3.1 (3.7) | 1.8 (2.8) | ||||
| High (2+) | 149.6 (143.1, 156.0) | 9.4 (3.9) | 9.5 (3.9) | 10.8 (4.1) | 3.7 (3.2) | ||||
| Subcutaneous Adipose Tissue (SAT) | |||||||||
| Adj Mean (95%CI) | Beta (SE) | p-trend | Beta (SE) | p-trend | Beta (SE) | p-trend | Beta (SE) | p-trend | |
| Chronic Stress | |||||||||
| Low (0) | 246.2 (238.7, 253.6) | Ref | 0.04 | Ref | 0.03 | Ref | 0.16 | Ref | 0.92 |
| Med (1) | 249.8 (239.1, 260.6) | 3.6 (6.5) | 3.0 (6.5) | 2.34 (6.6) | 1.23 (3.6) | ||||
| High (2+) | 260.9 (249.4, 272.4) | 14.7 (6.9) | 15.5 (7.0) | 13.9 (7.4) | −0.31 (4.1) | ||||
| VAT / SAT ratio | |||||||||
| Adj Mean (95%CI) | Beta (SE) | p-trend | Beta (SE) | p-trend | Beta (SE) | p-trend | Beta (SE) | p-trend | |
| Chronic Stress | |||||||||
| Low (0) | 0.61 (0.60, 0.64) | Ref | 0.46 | Ref | 0.56 | Ref | 0.38 | Ref | 0.32 |
| Med (1) | 0.61 (0.58, 0.64) | −0.02 (0.02) | −0.002 (0.02) | 0.003 (0.01) | 0.003 (0.01) | ||||
| High (2+) | 0.63 (0.60, 0.66) | 0.02 (0.02) | 0.01 (0.02) | 0.03 (0.02) | 0.03 (0.02) | ||||
Units for adjusted means (Adj Mean) and Beta estimates are Sq Cm
Model 1 adjusts for age, sex, race, education, and height
Model 2: Model 1 + smoking status (yes/no) and pack years smoked
Model 3: Model 2 + physical activity, sedentary behavior, depressive symptoms
Model 4: Model 3 + body mass index (height removed from model)
Categorical chronic stress variable was entered into linear regression model as continuous variable to evaluate p-trend.
After adjusting for age, sex, race/ethnicity, educational attainment, height, smoking status, pack years smoked, physical activity, and sedentary behavior, greater levels of VAT were significantly associated with reduced levels of adiponectin and increased levels of Il-6, leptin, resistin, TNF-a, and CRP (Table 4). For each outcome, there was a clear graded relationship between VAT area and level of the inflammatory variable (Table 4).
Table 4:
Cross-sectional associations between quartiles of VAT and inflammatory biomarkers. Multi-Ethnic Study of Atherosclerosis: Ancillary Study of Body Composition and Adiposity-Related Inflammation (Exam 2/3), United States
| lnIL6 | lnLeptin | Resistin | lnTNF-alpha | lnCRP | lnAdiponectin | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Beta (SE) | P-Val | Beta (SE) | P-Val | Beta (SE) | P-Val | Beta (SE) | P-Val | Beta (SE) | P | Beta (SE) | P | |
| VAT area | ||||||||||||
| Q1 | ref | <0.01 | Ref | ref | Ref | ref | ref | |||||
| Q2 | 0.17 (0.04) | 0.63 (0.05) | <0.01 | 0.04 (0.03) | 0.06 | 0.02 (0.04) | <0.01 | 0.34 (0.07) | <0.01 | −0.26 (0.03) | <0.01 | |
| Q3 | 0.30 (0.04) | 1.01 (0.06) | 0.06 (0.03) | 0.09 (0.04) | 0.56 (0.07) | −0.41 (0.03) | ||||||
| Q4 | 0.48 (0.04) | 1.38 (0.06) | 0.07 (0.03) | 0.12 (0.04) | 0.77 (0.07) | −0.50 (0.04) | ||||||
Models adjust for age, sex, race, education, height, smoking status (yes/no), pack years smoked, physical activity and sedentary behaviors
We found no statistically significant associations between level of chronic stress burden and the inflammatory biomarkers. As the level of chronic stress increased, lnLeptin increased slightly [med vs low: B = 0.06 (se = 0.05), high vs low: B = 0.12 (se = 0.06)], though these estimates were not statistically significant. The magnitude of associations between chronic stress burden and other inflammatory biomarkers were all close to zero, indicating null findings (Table 5). In post hoc analyses, we found evidence that associations between chronic stress and inflammatory markers may vary by follow-up time. As such, we present stratified analyses in Table 5. Similarly, we found evidence to suggest that these associations may vary by participant sex and thus present stratified analyses for all outcomes.
Table 5:
Associations between levels of chronic psychological stress and inflammatory biomarkers. Multi-Ethnic Study of Atherosclerosis: Ancillary Study of Body Composition and Adiposity-Related Inflammation (Exam 2/3), United States
| lnIL6 | lnLeptin | Resistin | lnTNF-alpha | lnCRP | lnAdiponectin | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Beta (SE) | P-Val | Beta (SE) | P-Val | Beta (SE) | P-Val | Beta (SE) | P-Val | Beta (SE) | P | Beta (SE) | P | |
| Full Sample | ||||||||||||
| Chronic Stress | ||||||||||||
| Low (0) | ref | 0.20 | Ref | 0.10 | ref | 0.73 | ref | 0.90 | ref | 0.81 | ref | 0.93 |
| Med (1) | −0.01 (0.03) | 0.06 (0.05) | 0.02 (0.02) | 0.01 (0.03) | −0.02 (0.06) | 0.01 (0.03) | ||||||
| High (2+) | 0.06 (0.04) | 0.12 (0.06) | 0.01 (0.02) | −0.01 (0.03) | −0.04 (0.06) | 0.002 (0.03) | ||||||
| Stratified by study visit | ||||||||||||
| Exam 2 (n= 704) | ||||||||||||
| Low (0) | ref | 0.60 | ref | 0.38 | ref | 0.28 | ref | 0.09 | ref | 0.20 | ref | 0.23 |
| Med (1) | 0.03 (0.05) | 0.10 (0.08) | 0.06 (0.04) | 0.01 (0.05) | −0.06 (0.09) | 0.06 (0.05) | ||||||
| High (2+) | −0.03 (0.06) | −0.03 (0.09) | 0.03 (0.04) | −0.11 (0.05) | −0.18 (0.10) | 0.08 (0.05) | ||||||
| Exam 3 (n= 1077) | ||||||||||||
| Low (0) | ref | 0.01 | ref | 0.02 | ref | 0.96 | ref | 0.42 | ref | 0.84 | ref | 0.51 |
| Med (1) | −0.03 (0.04) | 0.04 (0.07) | −0.01 (0.03) | 0.005 (0.04) | −0.003 (0.08) | −0.02 (0.04) | ||||||
| High (2+) | 0.12 (0.05) | 0.21 (0.07) | −0.01 (0.03) | 0.05 (0.04) | 0.04 (0.08) | −0.05 (0.04) | ||||||
| P-Interactiona | 0.08 | 0.11 | 0.75 | 0.03 | 0.20 | 0.41 | ||||||
| Stratified by sex | ||||||||||||
| Women | ||||||||||||
| Low (0) | ref | 0.05 | Ref | 0.41 | Ref | 0.74 | ref | 0.88 | ref | 0.35 | ref | 0.28 |
| Med (1) | −0.07 (0.05) | 0.03 (0.07) | −0.01 (0.04) | 0.01 (0.03) | −0.10 (0.09) | −0.03 (0.04) | ||||||
| High (2+) | 0.07 (0.05) | 0.09 (0.07) | −0.03 (0.05) | −0.004 (0.03) | 0.03 (0.09) | −0.07 (0.04) | ||||||
| Men | ||||||||||||
| Low (0) | ref | 0.34 | Ref | 0.16 | Ref | 0.88 | ref | 0.64 | ref | 0.12 | ref | 0.18 |
| Med (1) | 0.07 (0.05) | 0.11 (0.08) | 0.02 (0.04) | 0.02 (0.03) | 0.07 (0.08) | 0.04 (0.04) | ||||||
| High (2+) | 0.04 (0.05) | 0.16 (0.09) | 0.02 (0.05) | 0.03 (0.04) | −0.13 (0.09) | 0.09 (0.05) | ||||||
| P-Interactionb | 0.04 | 0.47 | 0.84 | 0.78 | 0.06 | 0.18 | ||||||
Models adjust for age, sex, race, education, height, smoking status (yes/no), pack years smoked, physical activity and sedentary behaviors
p-value for interaction between chronic stress * time between baseline exam and inflammatory biomarker data collection
p-value for interaction between chronic stress * sex
Finally, we tested whether associations between VAT and inflammatory biomarkers varied by levels of stress. We found no evidence of significant interactions. The p-values for interaction were all non-significant: adiponectin (p interact = 0.84), leptin (p interact = 0.35), resistin (p interact = 0.88), CRP (p interact = 0.96), TNF-a (p interact = 0.37), IL-6 (p interact = 0.27). Regardless of whether visceral fat was considered as a categorical or continuous variable, p-values for interaction remained statistically non-significant (Figure 1).
Figure 1.
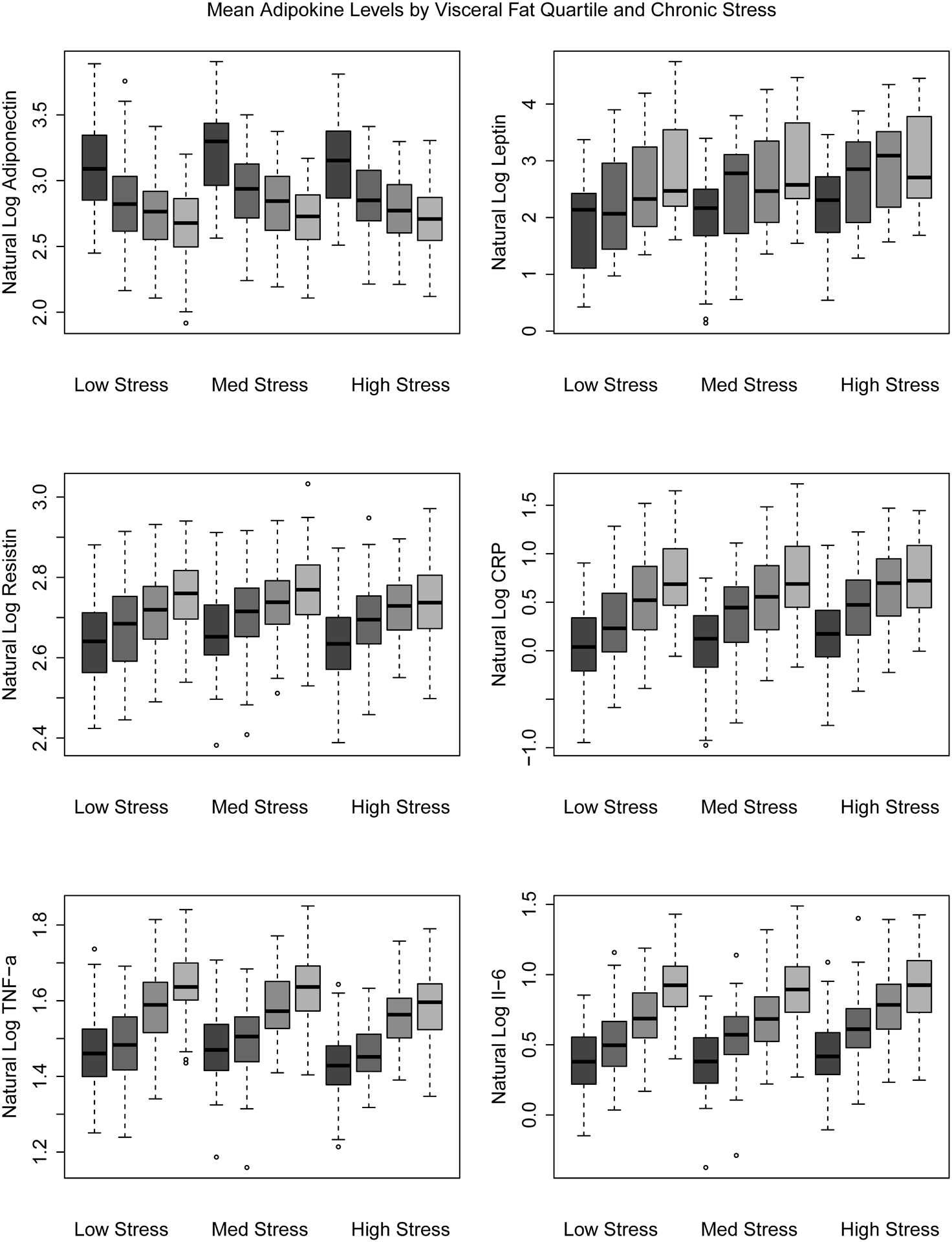
Associations Between Visceral Fat Quartile and Inflammatory Biomarkers Stratified by Level of Chronic Stress, Multi-Ethnic Study of Atherosclerosis: Ancillary Study of Body Composition and Adiposity-Related Inflammation (Exam 2/3), United States
DISCUSSION
This study examined two hypotheses concerning the relationship between chronic stress burden across important life domains and adiposity-related inflammation among US adults ages 46 to 84 years. We first tested the hypothesis that chronic stress burden is associated with increased adipose tissue and, specifically, increased VAT. In adjusted models, we found significant graded relationships between higher levels of chronic stress burden and greater quantity levels of both VAT and SAT. The effect magnitudes were slightly higher for SAT compared to VAT.
Conversely, the ratio of VAT/SAT was not meaningfully different by levels of chronic stress burden. Thus, we did not find evidence to support the hypothesis that high levels of chronic stress directly relate to specific accumulation of VAT, versus SAT. Instead, chronic stress burden related to increased fat accumulation in both depots. This is inconsistent with previous research demonstrating that elevated cortisol levels, which may result from psychological stress, lead to an exacerbation of the accumulation of intra-abdominal visceral fat, more so than fat in other depots.[22, 24] However, in our study, we used a self-reported measure of chronic stress burden, which may not correlate well with circulating cortisol levels. Previous studies that assess correlation between hair cortisol concentration and self-reported measures of chronic stress report weak correlations [47, 48]. In a substudy of MESA participants with measured stress hormones, chronic stress burden score was not associated with urinary stress hormone levels [10].
Also, high scores on the chronic stress burden scale may not reflect chronic activation of the HPA axis and there is likely individual variability in HPA axis functioning that may have influenced our findings. Additionally, constrained variability in the self-reported chronic stress score may have made it more difficult to detect small effect sizes. Disentangling the mechanisms in which chronic stress burden may contribute to increased total adiposity and depot-specific adiposity is an area for further research. This disentangling will require future studies to replicate analyses using different constructs of psychosocial stress. Although our findings may appear inconsistent with previous findings from the SWAN study suggesting specific effects of stress on visceral, but not subcutaneous fat, we note that the constructs used to measure psychosocial stress are different and study findings are not directly comparable.[27, 29] It is plausible that variables like discrimination[27] and hostility[29] effect adiposity through different mechanisms than the construct of chronic stress burden used in this study – which is a score of accumulated stressful life events. We recommend future studies replicate this work with more varied measurements of psychosocial stress.
The second hypothesis tested was that chronic stress burden modifies the relationship between VAT and inflammatory biomarkers. Greater levels of VAT were associated, in a graded pattern, with increases in all inflammatory biomarkers except adiponectin, which was inversely associated with VAT. In our examination of effect modification, we saw no meaningful differences in the VAT-inflammation relationship by chronic stress burden scale score..
We consider several limitations of this study. First, we only used one measure of chronic stress and it was self-reported. Chronic stress burden was collected at exam 1 and the measures of VAT and SAT, as well as the inflammatory biomarkers, were ascertained at exams 2 or 3, between two to four years later. Future studies can improve upon this work by analyzing relationships between time-varying exposure of chronic stress, adiposity, and inflammation. Second, the associations between VAT/SAT and inflammatory biomarkers were cross-sectional, so temporality cannot be established. Lastly, unmeasured and residual confounding are potential limitations in observational research.
Our study is strengthened by the inclusion of a diverse multi-ethnic cohort and CT measurement of depot-specific fat accumulation. Additionally, we were able to test two hypotheses regarding the relationship between chronic psychosocial stress on adiposity.
In summary, the evidence presented here support previous research suggesting chronic stress is associated with greater central adiposity (i.e. VAT) but not differentially. That is, chronic stress was associated with higher VAT and SAT. Lastly, we found no evidence to suggest that the associations between VAT and inflammatory biomarkers were exacerbated by chronic stress. In testing these two hypotheses, this work informs the ongoing literature investigating the complex pathways in which chronic psychosocial stress is a risk factor for CVD due to its potential effect on VAT and adiposity-related inflammation.
Supplementary Material
Conflicts of Interest and Source of Funding:
This research was supported by contracts 75N92020D00001, HHSN268201500003I, N01-HC-95159, 75N92020D00005, N01-HC-95160, 75N92020D00002, N01-HC-95161, 75N92020D00003, N01-HC-95162, 75N92020D00006, N01-HC-95163, 75N92020D00004, N01-HC-95164, 75N92020D00007, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168 and N01-HC-95169 from the National Heart, Lung, and Blood Institute, and by grants UL1-TR-000040, UL1-TR-001079, and UL1-TR-001420 from the National Center for Advancing Translational Sciences (NCATS). Reading of the CT scans was funded by NHLBI R01HL088541. The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org. E Delker was supported by NHBLI T32-HL079891-13
Authors report no conflicts of interest.
ACRONYMS USED IN TEXT
- VAT
visceral adipose tissue
- SAT
subcutaneous adipose tissue
- MESA
Multi-Ethnic Study of Atherosclerosis
- HPA
hypothalamic-pituitary adrenal
- TNF-a
tumor necrosis factor alpha
- IL-6
interleukin-6
- CRP
C-reactive protein
- CVD
cardiovascular disease
- CT
computed tomography
- SD
standard deviation
Contributor Information
Erin Delker, Joint Doctoral Program in Epidemiology, San Diego State University and University of California San Diego, San Diego CA, USA.
Bandar AlYami, School of Medicine, University of California San Diego, San Diego CA, USA.
Linda C. Gallo, Department of Psychology, San Diego State University, San Diego CA, USA.
John M Ruiz, Department of Psychology, University of Arizona, Tucson AZ, USA.
Moyses Szklo, Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore MD, USA.
Matthew A. Allison, Family Medicine and Public Health, University of California San Diego, San Diego CA, USA.
References
- 1.McEwen BS, Protective and damaging effects of stress mediators. N Engl J Med, 1998. 338(3): p. 171–9. [DOI] [PubMed] [Google Scholar]
- 2.Bairey Merz CN, Dwyer J, Nordstrom CK, Walton KG, Salerno JW, and Schneider RH, Psychosocial stress and cardiovascular disease: pathophysiological links. Behav Med, 2002. 27(4): p. 141–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ranjit N, Diez-Roux AV, Shea S, Cushman M, Seeman T, Jackson SA, and Ni H, Psychosocial factors and inflammation in the multi-ethnic study of atherosclerosis. Arch Intern Med, 2007. 167(2): p. 174–81. [DOI] [PubMed] [Google Scholar]
- 4.Wirtz PH and von Kanel R, Psychological Stress, Inflammation, and Coronary Heart Disease. Curr Cardiol Rep, 2017. 19(11): p. 111. [DOI] [PubMed] [Google Scholar]
- 5.Wardle J, Chida Y, Gibson EL, Whitaker KL, and Steptoe A, Stress and adiposity: a meta-analysis of longitudinal studies. Obesity (Silver Spring), 2011. 19(4): p. 771–8. [DOI] [PubMed] [Google Scholar]
- 6.Isasi CR, Parrinello CM, Jung MM, Carnethon MR, Birnbaum-Weitzman O, Espinoza RA, Penedo FJ, Perreira KM, Schneiderman N, Sotres-Alvarez D, Van Horn L, and Gallo LC, Psychosocial stress is associated with obesity and diet quality in Hispanic/Latino adults. Ann Epidemiol, 2015. 25(2): p. 84–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tomiyama A, Stress and Obesity. Annu. Rev. Psychol, 2019. 70:5.1–5.16. [DOI] [PubMed] [Google Scholar]
- 8.Cuevas AG, Chen R, Thurber KA, Slopen N, and Williams DR, Psychosocial Stress and Overweight and Obesity: Findings From the Chicago Community Adult Health Study. Ann Behav Med, 2019. 53(11): p. NP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ouakinin SRS, Barreira DP, and Gois CJ, Depression and Obesity: Integrating the Role of Stress, Neuroendocrine Dysfunction and Inflammatory Pathways. Front Endocrinol (Lausanne), 2018. 9: p. 431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Castro-Diehl C, Diez Roux AV, Seeman T, Shea S, Shrager S, and Tadros S, Associations of socioeconomic and psychosocial factors with urinary measures of cortisol and catecholamines in the Multi-Ethnic Study of Atherosclerosis (MESA). Psychoneuroendocrinology, 2014. 41: p. 132–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kahn SE, Hull RL, and Utzschneider KM, Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature, 2006. 444(7121): p. 840–6. [DOI] [PubMed] [Google Scholar]
- 12.Anagnostis P, Athyros VG, Tziomalos K, Karagiannis A, and Mikhailidis DP, Clinical review: The pathogenetic role of cortisol in the metabolic syndrome: a hypothesis. J Clin Endocrinol Metab, 2009. 94(8): p. 2692–701. [DOI] [PubMed] [Google Scholar]
- 13.Hamdy O, Porramatikul S, and Al-Ozairi E, Metabolic obesity: the paradox between visceral and subcutaneous fat. Curr Diabetes Rev, 2006. 2(4): p. 367–73. [DOI] [PubMed] [Google Scholar]
- 14.Ibrahim MM, Subcutaneous and visceral adipose tissue: structural and functional differences. Obes Rev, 2010. 11(1): p. 11–8. [DOI] [PubMed] [Google Scholar]
- 15.Shah RV, Murthy VL, Abbasi SA, Blankstein R, Kwong RY, Goldfine AB, Jerosch-Herold M, Lima JAC, Ding J, and Allison MA, Visceral Adiposity and the Risk of Metabolic Syndrome Across Body Mass Index: The MESA Study. JACC Cardiovasc Imaging, 2014. 7(12): p. 1221–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shah R, Allison M, Lima J, Abbasi S, Eisman A, Lai C, Jerosch-Herold M, Budoff M, and Murthy V, Abdominal fat radiodensity, quantity and cardiometabolic risk: the Multi-Ethnic Study of Atherosclerosis. Nutr Metab Cardiovasc Dis, 2016. 26(2): p. 114–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wajchenberg BL, Subcutaneous and visceral adipose tissue: their relation to the metabolic syndrome. Endocr Rev, 2000. 21(6): p. 697–738. [DOI] [PubMed] [Google Scholar]
- 18.Dutheil F, Gordon BA, Naughton G, Crendal E, Courteix D, Chaplais E, Thivel D, Lac G, and Benson AC, Cardiovascular risk of adipokines: a review. J Int Med Res, 2017: p. 300060517706578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Van Gaal LF, Mertens IL, and De Block CE, Mechanisms linking obesity with cardiovascular disease. Nature, 2006. 444(7121): p. 875–80. [DOI] [PubMed] [Google Scholar]
- 20.Kwon H and Pessin JE, Adipokines mediate inflammation and insulin resistance. Front Endocrinol (Lausanne), 2013. 4: p. 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dutheil F, Gordon BA, Naughton G, Crendal E, Courteix D, Chaplais E, Thivel D, Lac G, and Benson AC, Cardiovascular risk of adipokines: a review. J Int Med Res, 2018. 46(6): p. 2082–2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kloting N and Bluher M, Adipocyte dysfunction, inflammation and metabolic syndrome. Rev Endocr Metab Disord, 2014. 15(4): p. 277–87. [DOI] [PubMed] [Google Scholar]
- 23.Loucks EB, Sullivan LM, Hayes LJ, D’Agostino RB Sr., Larson MG, Vasan RS, Benjamin EJ, and Berkman LF, Association of educational level with inflammatory markers in the Framingham Offspring Study. Am J Epidemiol, 2006. 163(7): p. 622–8. [DOI] [PubMed] [Google Scholar]
- 24.Bjorntorp P, Do stress reactions cause abdominal obesity and comorbidities? Obes Rev, 2001. 2(2): p. 73–86. [DOI] [PubMed] [Google Scholar]
- 25.Marniemi J, Kronholm E, Aunola S, Toikka T, Mattlar CE, Koskenvuo M, and Ronnemaa T, Visceral fat and psychosocial stress in identical twins discordant for obesity. J Intern Med, 2002. 251(1): p. 35–43. [DOI] [PubMed] [Google Scholar]
- 26.Shively CA, Register TC, and Clarkson TB, Social stress, visceral obesity, and coronary artery atherosclerosis in female primates. Obesity (Silver Spring), 2009. 17(8): p. 1513–20. [DOI] [PubMed] [Google Scholar]
- 27.Lewis TT, Kravitz HM, Janssen I, and Powell LH, Self-reported experiences of discrimination and visceral fat in middle-aged African-American and Caucasian women. Am J Epidemiol, 2011. 173(11): p. 1223–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Everson-Rose SA, Lewis TT, Karavolos K, Dugan SA, Wesley D, and Powell LH, Depressive symptoms and increased visceral fat in middle-aged women. Psychosom Med, 2009. 71(4): p. 410–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lewis TT, Everson-Rose SA, Karavolos K, Janssen I, Wesley D, and Powell LH, Hostility is associated with visceral, but not subcutaneous, fat in middle-aged African American and white women. Psychosom Med, 2009. 71(7): p. 733–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mason AE, Schleicher S, Coccia M, Epel ES, and Aschbacher K, Chronic Stress and Impulsive Risk-Taking Predict Increases in Visceral Fat over 18 Months. Obesity (Silver Spring), 2018. 26(5): p. 869–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Karagiannides I, Golovatscka V, Bakirtzi K, Sideri A, Salas M, Stavrakis D, Polytarchou C, Iliopoulos D, Pothoulakis C, and Bradesi S, Chronic unpredictable stress regulates visceral adipocyte-mediated glucose metabolism and inflammatory circuits in male rats. Physiol Rep, 2014. 2(5): p. e00284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu YZ, Chen JK, Zhang Y, Wang X, Qu S, and Jiang CL, Chronic stress induces steatohepatitis while decreases visceral fat mass in mice. BMC Gastroenterol, 2014. 14: p. 106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, Greenland P, Jacob DR Jr., Kronmal R, Liu K, Nelson JC, O’Leary D, Saad MF, Shea S, Szklo M, and Tracy RP, Multi-Ethnic Study of Atherosclerosis: objectives and design. Am J Epidemiol, 2002. 156(9): p. 871–81. [DOI] [PubMed] [Google Scholar]
- 34.Forbang NI, Allison MA, Ix JH, Criqui MH, Vaidya D, Yeboah J, Duprez DA, and Jacobs DR Jr., Associations of body composition measures and C2, a marker for small artery elasticity: The MESA. Obesity (Silver Spring), 2015. 23(11): p. 2294–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ortiz MS, Myers HF, Dunkel Schetter C, Rodriguez CJ, and Seeman TE, Psychosocial Predictors of Metabolic Syndrome among Latino Groups in the Multi-Ethnic Study of Atherosclerosis (MESA). PLoS One, 2015. 10(4): p. e0124517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bromberger JT and Matthews KA, A longitudinal study of the effects of pessimism, trait anxiety, and life stress on depressive symptoms in middle-aged women. Psychol Aging, 1996. 11(2): p. 207–13. [DOI] [PubMed] [Google Scholar]
- 37.Kershaw KN, Lane-Cordova AD, Carnethon MR, Tindle HA, and Liu K, Chronic Stress and Endothelial Dysfunction: The Multi-Ethnic Study of Atherosclerosis (MESA). Am J Hypertens, 2017. 30(1): p. 75–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kershaw KN, Diez Roux AV, Bertoni A, Carnethon MR, Everson-Rose SA, and Liu K, Associations of chronic individual-level and neighbourhood-level stressors with incident coronary heart disease: the Multi-Ethnic Study of Atherosclerosis. J Epidemiol Community Health, 2015. 69(2): p. 136–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Allison MA, Bluemke DA, McClelland R, Cushman M, Criqui MH, Polak JF, and Lima JA, Relation of Leptin to Left Ventricular Hypertrophy (From the Multi-Ethnic Study of Atherosclerosis). Am J Cardiol, 2013. 112(5): p. 726–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Arnett DK, McClelland RL, Bank A, Bluemke DA, Cushman M, Szalai AJ, Jain N, Gomes AS, Heckbert SR, Hundley WG, and Lima JA, Biomarkers of inflammation and hemostasis associated with left ventricular mass: The Multiethnic Study of Atherosclerosis (MESA). Int J Mol Epidemiol Genet, 2011. 2(4): p. 391–400. [PMC free article] [PubMed] [Google Scholar]
- 41.Nettleton JA, Steffen LM, Mayer-Davis EJ, Jenny NS, Jiang R, Herrington DM, and Jacobs DR Jr., Dietary patterns are associated with biochemical markers of inflammation and endothelial activation in the Multi-Ethnic Study of Atherosclerosis (MESA). Am J Clin Nutr, 2006. 83(6): p. 1369–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rasmussen-Torvik LJ, Wassel CL, Ding J, Carr J, Cushman M, Jenny N, and Allison MA, Associations of body mass index and insulin resistance with leptin, adiponectin, and the leptin-to-adiponectin ratio across ethnic groups: the Multi-Ethnic Study of Atherosclerosis (MESA). Ann Epidemiol, 2012. 22(10): p. 705–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Harhay MO, Kizer JR, Criqui MH, Lima JA, Tracy R, Bluemke DA, and Kawut SM, Adipokines and the Right Ventricle: The MESA-RV Study. PLoS One, 2015. 10(9): p. e0136818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Radloff LS. The CES‐D Scale. A self‐report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 45.Ainsworth BE, Irwin ML, Addy CL, Whitt MC, and Stolarczyk LM, Moderate physical activity patterns of minority women: the Cross-Cultural Activity Participation Study. J Womens Health Gend Based Med, 1999. 8(6): p. 805–13. [DOI] [PubMed] [Google Scholar]
- 46.Afifi AA, Kotlerman JB, Ettner SL, and Cowan M, Methods for improving regression analysis for skewed continuous or counted responses. Annu Rev Public Health, 2007. 28: p. 95–111. [DOI] [PubMed] [Google Scholar]
- 47.Braig S, Grabher F, Ntomchukwu C, Reister F, Stalder T, Kirschbaum C, Rothenbacher D, and Genuneit J, The Association of Hair Cortisol with Self-Reported Chronic Psychosocial Stress and Symptoms of Anxiety and Depression in Women Shortly after Delivery. Paediatr Perinat Epidemiol, 2016. 30(2): p. 97–104. [DOI] [PubMed] [Google Scholar]
- 48.Wells S, Tremblay PF, Flynn A, Russell E, Kennedy J, Rehm J, Van Uum S, Koren G, and Graham K, Associations of hair cortisol concentration with self-reported measures of stress and mental health-related factors in a pooled database of diverse community samples. Stress, 2014. 17(4): p. 334–42. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


