Abstract
Alterations to the mechanical properties of the microenvironment are a hallmark of cancer. Elevated mechanical stresses exist in many solid tumors and elicit responses from cancer cells. Uncontrolled growth in confined environments gives rise to elevated solid compressive stress on cancer cells. Recruitment of leaky blood vessels and an absence of functioning lymphatic vessels causes a rise in the interstitial fluid pressure. Here we review the role of the cancer cell cytoskeleton and the nucleus in mediating both the initial and adaptive cancer cell response to these two types of mechanical stresses. We review how these mechanical stresses alter cancer cell functions such as proliferation, apoptosis and migration.
Introduction
A hallmark of cancer is alterations to the mechanical properties of the tumor and its microenvironment (Nia, Munn, & Jain, 2020; Northey, Przybyla, & Weaver, 2017). Mechanical alterations include changes to the mechanical stiffness of the microenvironment as well as elevated mechanical stresses on tumor cells. Two types of mechanical stresses are particularly important in modulating cancer tissue and cell function in vivo - solid compressive stress and hydrostatic pressure (Davies & Tripathi, 1993; Wang & Li, 2010).
Compressive stresses build up on cancer cells in a growing, solid tumor due to mechanical resistance of the surrounding, confining environment to displacement (Figure 1a) (Jain, Martin, & Stylianopoulos, 2014). Solid compressive stress in tumors ranges from 0.7–75 mm Hg (0.1–10 kPa) for human tumors and 2–60 mm Hg (0.25–8 kPa) for murine tumors (Nia et al., 2016; Nia et al., 2020; Stylianopoulos, Munn, & Jain, 2018). Compressive stresses also build up on cancer cells migrating through narrow interstitial spaces of the tissue (Friedl & Alexander, 2011).
Figure 1. Build-up of solid compressive stress or hydrostatic pressure in tumors, and in vitro assays to study them.
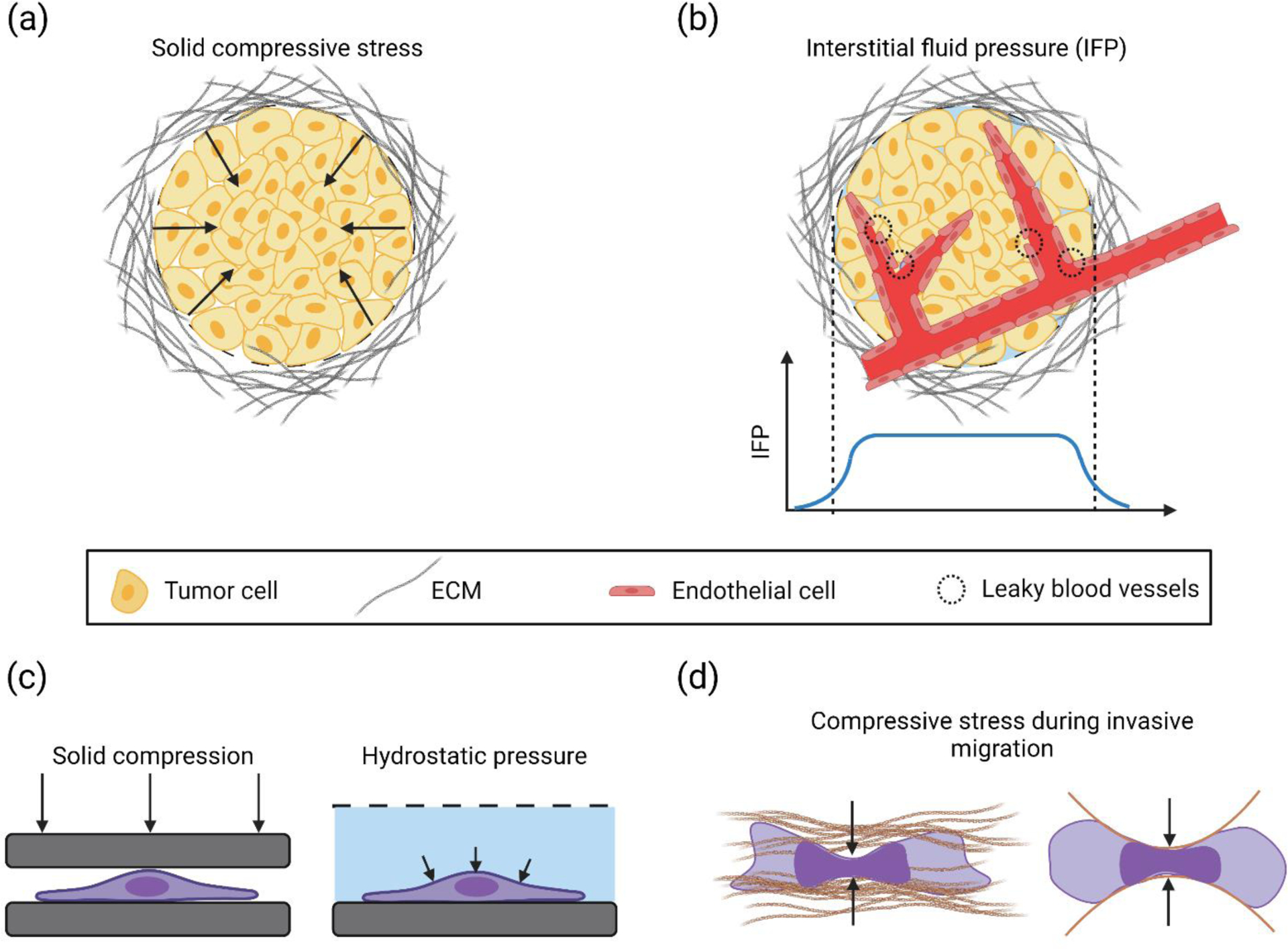
(a) A solid tumor mass surrounded by dense ECM. Overcrowding of cells in the tumor microenvironment due to abnormal cell proliferation displaces the surrounding ECM and causes a buildup of solid compressive stress (black arrows (b) Leaky/permeable blood vessels in the solid tumor cause plasma leakage which, combined with a lack of functioning lymphatic vessels, leads to elevated interstitial fluid pressure (IFP) in the bulk of the tumor, with a gradient near the tumor periphery. The IFP distribution in the tumor mass is shown by the blue curve. (c) In vitro approaches to apply solid compressive stress and hydrostatic pressure (black arrows) on cancer cells. (d) Solid compressive stress acts on an invading cancer cell in the confined environment of the extracellular matrix. Right image shows a schematic of a cancer cell migrating through microfabricated confining channels. Compressive stresses are indicated by solid black arrows.
Interstitial fluid pressure (IFP) is a marker of malignancy in a number of human cancers (Gutmann et al., 1992; Nathanson & Nelson, 1994). IFP is generated by accumulation of fluid in the growing tumor (Heldin, Rubin, Pietras, & Ostman, 2004). Proliferating tumor cells recruit thin-walled, leaky blood vessels to meet their high oxygen demand. Due to a lack of a functioning lymphatic system, build-up of fluid leaked from tumor capillaries results in elevated pressure in tumors (Figure 1b) (Ariffin, Forde, Jahangeer, Soden, & Hinchion, 2014; Stylianopoulos et al., 2018). Increased presence of interstitial fibroblasts can also contribute to increased IFP by contracting the extracellular matrix (Astafurov et al., 2014; Heldin et al., 2004). The interstitial fluid pressure (IFP) in human tumors ranges from ~5 mm Hg (~0.6 kPa) in brain tumors to ~40 mm Hg (~5.3 kPa) in ovarian and renal cell carcinomas (Jain, 2012) and the range is around 3–15 mm Hg (0.4–2 kPa) for murine tumors (Boucher, Baxter, & Jain, 1990; Sen et al., 2011).
The elevated solid stress and high IFP in a tumor can impact drug delivery. For example, accumulated solid compressive stress in tumors can be high enough to constrict blood vessels (Griffon-Etienne, Boucher, Brekken, Suit, & Jain, 1999; Padera et al., 2004; Stylianopoulos et al., 2012). The collapse of blood vessels can cause hypoxia (Chauhan et al., 2013; Stylianopoulos et al., 2012) and reduce the efficacy of therapeutic drug delivery (Jain, 2014; Munn & Jain, 2019). Elevated IFP in the tumor can inhibit convective transport of drugs to the tumor core (Jain & Baxter, 1988; Jain et al., 2014). The steep IFP gradient near the periphery of the tumor causes an outward flow of fluid from the interstitial space to the surrounding normal tissue, which can reduce the time of retention of drugs in the tumor (Jain, 2013).
Cancer cells in the tumor respond to elevated mechanical stresses, and these responses are important for cancer cell survival (Hope et al., 2021; Moose et al., 2020). Both solid compressive stress and IFP alter cancer cell behaviors such as proliferation, invasion and apoptosis, and are a factor in cancer progression (Jain et al., 2014; Northcott, Dean, Mouw, & Weaver, 2018; Provenzano & Hingorani, 2013). In this paper, we focus on cellular responses to these two types of mechanical stresses.
Cancer cell response to solid compressive stress
Methods to apply compressive stress
A typical approach to study the impact of solid compressive stress on the cytoskeleton in two-dimensional cultured cells is to confine cells through physical contact of the apical surface of the cell with another solid surface (Figure 1c). The compressing surface can be a soft flat surface like PDMS (He et al., 2018; Le Berre, Zlotek-Zlotkiewicz, Bonazzi, Lautenschlaeger, & Piel, 2014) or agarose (Aureille et al., 2019). Hard surfaces like a glass plate (Caille, Thoumine, Tardy, & Meister, 2002; Peeters, Oomens, Bouten, Bader, & Baaijens, 2005), a cantilever probe of other material, or ~5 micron-sized beads on an atomic force microscope (AFM) have also been used to indent the cell apex (Ofek, Wiltz, & Athanasiou, 2009). Limitations of such methods include the two-dimensional nature of cell culture, which typically involves flat cell and nuclear morphologies that are not typical of in vivo contexts. Yet, such 2D methods allow controlled probing of cells combined with high resolution imaging which has revealed significant information on the cellular response to mechanical stress. More physiologically relevant in vitro methods of compression include the application of compression to cell-containing 3D matrix gels (Boyle et al., 2020), the growth of tumor spheroids in confining gels (J. M. Tse et al., 2012) and osmotically driven collapse of the extracellular matrix to compress tumor spheroids (Dolega et al., 2021).
Effect of compressive stress on the cytoskeleton
While the methods to apply compressive stress differ in the spatial distribution of force applied (local versus entire cell, direct contact versus compression of cell containing gel) which can elicit differing responses from the cell, the cytoskeleton, and more recently, the nucleus, are consistently implicated in resisting solid compressive stresses as discussed below. The specific cytoskeletal components that are important in the cellular response may be cell-type dependent, and dependent on the magnitude of the applied stress and on the time/frequency of stress application.
The effect of an increase in the compressive stress on a cell can be understood with a simple force balance at a curved cellular interface. The main components of the force balance over a portion of a free (i.e. non-adherent), stationary cell interface, in the absence of extracellular mechanical stresses, are tension in the contractile, curved actomyosin cortex, which is balanced by the difference in the hydrostatic pressure across the cell membrane (Li et al., 2015). In a resting cell, the hydrostatic pressure difference across the membrane is primarily due to an osmotic pressure which exists because of a difference in the concentration of ions between the cytoplasm and extracellular space. The Law of Laplace applies at the curved interface:
| (1) |
where Pint is the internal pressure, Pext is the external pressure, 2H is the mean curvature, and T is the cortical tension.
An external compressive stress Pc applied by an AFM probe or by a confining barrier to such an interface will modify the above force equilibrium. Rapid adjustments can occur to the terms in equation (1), followed potentially by longer time adaptive changes. The modified equation is
| (2) |
where Pc is the new external pressure which is greater than Pext, Pnew is the new internal pressure, Tnew is the new tension and 2Hnew is the new curvature. Equation (2) can be considered to apply in the following two simple ways (or combinations of these two ways): if, at constant tension Tnew = T and curvature 2H = 2Hnew, the hydrostatic intracellular pressure Pint increases to Pnew, or if the cortical tension T decreases to Tnew and/or the curvature 2H reduces to 2Hnew at constant internal pressure Pint.
An instantaneous increase in the internal pressure Pint upon application of a compressive stress to cells is plausible given that water is incompressible. Such an increase in pressure is evident from the fact that application of confining compressive stress to rounded Hela-Kyoto cancer cells caused substantial blebbing of the plasma membrane (Lomakin et al., 2020). Increased intracellular pressure promotes bleb formation by causing membrane delamination from the actin cortex or causing local ruptures in the actin cortex (Charras & Paluch, 2008). To reduce blebbing, cells can adapt to the increased pressure by upregulating cortical actomyosin tension (Lomakin et al., 2020). As an example of cellular adaptation resulting in a potential decrease in cortical tension T, compression applied to HT1080 fibrosarcoma cells reduced RhoA activity through the activity of a membrane ion channel TRPV4 which is permeable to calcium ions (He et al., 2018); a reduction in RhoA activity should reduce cortical actomyosin tension.
The extent to which actomyosin networks remodel under confining compression differs between normal and cancer cells in 2D culture. For example, continuous compression (5.8 mm Hg or 0.77 kPa) of cultured 67NR breast cancer cells under a weight applied to an overlaid agarose gel, for example, oriented F-actin stress fibers perpendicular to adjacent vacant areas and caused longer filopodia to develop, while such effects were absent in non-cancerous MCF10A cells (J. M. Tse et al., 2012). Individual actin filaments in vitro have been reported to stiffen and resist confining compression (Greene, Anderson, Zeng, Zappone, & Israelachvili, 2009) although the extent to which this contributes to cellular responses to compression is unclear.
Because solid tumors are crowded environments, proliferating tumor cells must undergo rounding, assemble a mitotic spindle and perform cytokinesis against confining barriers. These changes in shape can only occur if cells exert outward pushing forces to deform the confining extracellular matrix. Rounding of cultured Hela-Kyoto tumor cells against a cantilever produces a force of ~ 60 nN, corresponding to a rounding pressure of ~ 0.14 nN/μm2(Stewart et al., 2011). Cells that are unable to push against confining barriers are unable to round up; these cells have an increased likelihood of entering apoptosis(Sorce et al., 2015). The rounding is driven by de-adhesion from the substrate, but may also be driven by an increase in intracellular osmotic pressure. Cell rounding may also involve a cytoskeletal stiffening mechanism in order to round up against the confining barrier and divide. For example, transient induction of oncogenic RasV12 stiffens MCF10A cells during mitotic rounding in an actomyosin-dependent manner, allowing them to undergo mitosis without chromosome segregation errors during cellular confinement by a stiff gel (Matthews et al., 2020).
Subsequent to rounding, spindle assembly causes an elongation of the rounded cell that also exerts an outward pushing force on the confining matrix. An elegant demonstration of the mechanism by which mitotic cancer cells push against the matrix was provided by Nam and Chaudhuri (Nam & Chaudhuri, 2018). Nam et al observed direct deformation of the surrounding, confining alginate matrix caused by single, mitotic MDA-MB-231 breast cancer cells. Laser ablation of microtubules in the mitotic spindle or inhibition of the actomyosin contractile ring that causes cytokinesis, relaxed some of the matrix deformation caused by mitosis. Further, spindles had a buckled appearance in confined mitotic cells. These experiments showed that at least part of the pushing force against confining barriers is due to mitotic spindle assembly and actomyosin contraction that splits the mitotic cell into daughter cells.
Cytoplasmic vimentin intermediate filament networks in cells can undergo extreme deformations (Hu et al., 2019) without damage and undergo strain hardening (Janmey, Euteneuer, Traub, & Schliwa, 1991). These properties of the vimentin network help protect mouse embryonic fibroblast (MEF) nuclei from rupture during migration through confined environments (Patteson et al., 2019), and help nuclei maintain mechanical homeostasis against local mechanical forces (Neelam et al., 2015). In epithelial cells, keratin networks may stiffen cells (Ma, Yamada, Wirtz, & Coulombe, 2001) and inhibit their migration (Seltmann, Fritsch, Käs, & Magin, 2013). More studies of the mechanics of intermediate filament networks in cancer cells are needed to understand their role in balancing and adapting to compressive stress applied to cells.
Effect of compressive stress on the nucleus
Owing to its size and stiffness, the nucleus is substantially compressed during cancer cell migration through narrow interstitial spaces typically present in tissue (Vortmeyer-Krause et al., 2020; Wolf et al., 2013). The resistance of the nuclear lamina to extension and the resistance of the nuclear volume to changes are key parameters that determine the mechanical response of the nucleus to such compressive stresses (Hobson et al., 2020; Lele, Dickinson, & Gundersen, 2018).
Mechanical cell compression can impact several nuclear structures in cancer cells. Compressive stress can cause the nuclear envelope to delaminate from the lamina to form a bleb, which can then rupture (Denais et al., 2016). The mechanical response of the nuclear envelope during this process is complex and not fully understood (Agrawal & Lele, 2019; Q. Zhang et al., 2019). Rupture causes an intermixing of the cytoplasm and the nucleus, exposing DNA to cytoplasmic DNAses such as TREX1 which can in turn cause DNA damage (Nader et al., 2020). Rupture of the nuclear envelope in cultured cancer cells can also occur in the absence of external cell compression, as a result of an increase in nuclear pressure due to compression by apical F-actin structures (Hatch & Hetzer, 2016). Mechanisms to repair envelope ruptures include early recruitment of the Barrier-to-Autointegration factor (BAF) to the site of envelope rupture (Halfmann et al., 2019), and repair through the recruitment of LEM domain proteins (Halfmann et al., 2019), and the ESCRT family of proteins (Raab et al., 2016).
Deformation of the nucleus during confined migration of MDA-MB-231 and BT-549 breast cancer cells or by mechanical compression of static cells can cause DNA damage in the S/G2 phase of the cell cycle without requiring mechanical rupture of the envelope (Shah et al., 2021). The DNA damage is likely due to a stalling of the DNA replication fork. Mechanical compression induces chromatin condensation in fibroblasts which correlate with changes in transcriptional response(Damodaran et al., 2018). Chromatin dilates when fibrobast nuclei change shape during cell migration from elongated shapes to circular shapes (Katiyar et al., 2019). These shape changes are also accompanied by an unfolding of the nuclear lamina.
Mechanical compression of the nucleus may cause mechanical adaptation of the cell. For example, mechanical confinement of Hela-Kyoto cancer cells stretches the nuclear envelope, and upregulation of actomyosin contractility which was attributed to signaling by stretch-sensitive nuclear envelope proteins (Lomakin et al., 2020).
Effect of solid compressive stress on cancer cell proliferation, apoptosis and migration
An early study by Jain and coworkers found that the final size of human cancer cell spheroids in agarose gels was lower at higher gel concentrations (Helmlinger, Netti, Lichtenbeld, Melder, & Jain, 1997). This suggested that at high gel concentrations, the tumor spheroid was unable to displace the mechanically resistant gel matrix beyond a certain size. At the cellular level, no measurable effects on cell proliferation rate were found, and a slight decrease in apoptotic rate was observed, which was consistent with the observed increased cell packing density in confined spheroids. In contrast, in a subsequent study, Munn, Jain and coworkers reported that cell proliferation was suppressed and apoptotic rates were increased in regions of high solid compressive stress in the spheroid (Cheng, Tse, Jain, & Munn, 2009). In both studies, care was taken to establish that the effect was indeed due to solid stress on cells, by ruling out changes in other factors such as gel toxicity, limitations of nutrients, growth factors or oxygen in gels at higher concentrations, or by showing similar results through direct mechanical compression of cells and spheroids (Cheng et al., 2009). The increased apoptotic rate under mechanical compression could be reduced by over-expression of Bcl-2, a protein which inhibits multiple caspases in the mitochondrial pathway.
The original approach by Jain and coworkers or variations on it have been used by others to study effects of solid compressive stress on multicellular tumor size. Solid compressive stress caused a decrease in tumor spheroid size formed by H4 and A172 brain cancer cell lines (Kalli & Stylianopoulos, 2018), breast cancer cell line BC52 (Delarue et al., 2014), mouse sarcoma cell lines AB6 and CT26 and the human colon carcinoma cell line HT29 (Delarue et al., 2014) (see Table 1). The mechanisms for these effects are not fully understood, but it is possible that some cancer cells may be arrested in mitosis. For example, confined HCT116 colorectal cancer cells are arrested in cancer spheroids due to perturbations of bipolar spindle assembly (Desmaison, Frongia, Grenier, Ducommun, & Lobjois, 2013). Overall, mechanical confinement of tumor spheroids causes an increase in compressive solid stress which inhibits cell proliferation and increases apoptosis (Table 1).
Table 1.
Survey of papers that reported cancer cell responses (proliferation, migration and apoptosis) to solid compressive stress or hydrostatic pressure. Model systems used, magnitude of stress and time of stress application are also included.
| Cancer Cell type | Type of model | Mechanical Stress | Stress parameters | Effect on | Ref. | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| 2D In vitro culture | 3D culture | Solid compressive stress | Hydrostatic pressure | Magnitude (mm Hg) | Duration | Proliferation | Migration | Apoptosis | ||
| H4 Brain tumor |
✓ | ✓ | 60 | 21 d | ↓ | (Kalli, Voutouri, et al., 2019) | ||||
| A172 Brain tumor |
✓ | ✓ | 26 | 21 d | ↓ | (Kalli, Voutouri, et al., 2019) | ||||
| MDA-MB 231 Breast cancer |
✓ | ✓ | 6 d | ↑ | (Chun Liu, Lewin Mejia, Chiang, Luker, & Luker, 2018) | |||||
| HCT116 Colorectal cancer |
✓ | ✓ | ↓ | No change | (Desmaison et al., 2013) | |||||
| HT29 Human colon carcinoma |
✓ | ✓ | 35 –75 | ↓ | No change | (Delarue et al., 2014) | ||||
| CT26 Mouse colon adenocarcinoma |
✓ | ✓ | 35 –75 | ↓ | (Delarue et al., 2014) | |||||
| BC52 Human breast cancer |
✓ | ✓ | 35 –75 | ↓ | (Delarue et al., 2014) | |||||
| AB6 Mouse sarcoma |
✓ | ✓ | 35 –75 | ↓ | (Delarue et al., 2014) | |||||
| LS174T Human colon adenocarcinoma |
✓ | ✓ | 45 –120 | 30 d | No change | ↓ | (Helmlinger et al., 1997) | |||
| 4T1, 67NR, MDA-MB 231 Breast cancer |
✓ | ✓ | 5.8 | 16 h | ↑ | (Janet M. Tse et al., 2012) | ||||
| MCF 10A, MCF7 Breast cancer |
✓ | ✓ | 5.8 | 16 h | ↓ | (Janet M. Tse et al., 2012) | ||||
| 67NR Breast cancer |
✓ | ✓ | > 5.8 –58 | 16 h | ↓ | ↑ | (Cheng et al., 2009; Janet M. Tse et al., 2012) | |||
| H4 Brain tumor (Glioma) |
✓ | ✓ | 4 | 16 h | ↑ | (Kalli, Voutouri, et al., 2019) | ||||
| A172 Brain tumor |
✓ | ✓ | 4 | 16 h | No change | (Kalli, Voutouri, et al., 2019) | ||||
| CFPAC-1 (Pancreatic cancer) | ✓ | ✓ | 1–6 | 6 h | ↑ | (Kalli et al., 2018) | ||||
| PaCa-2/BxPC-3 (Pancreatic cancer) |
✓ | ✓ | 4 | 16 h | ↑ | (Kalli, Minia, et al., 2019) | ||||
| hTRET-AM Ameloblastoma epithelial cells |
✓ | ✓ | 30 –90 | ↓ | ↑ | (Yang et al., 2018) | ||||
| CL1–5 & A549 Lung cancer cells |
✓ | ✓ | 0 –20 | 8 h | ↑ | ↑ | (Y. Kao, C. Lee, & P. Kuo, 2014; Kao et al., 2017) | |||
| SCC-4/SCC-9 Oral squamous carcinoma |
✓ | ✓ | 0 –30 | 24 h | ↑ | ↑ | (T. Yu et al., 2013) | |||
| U2OS Osteosarcoma |
✓ | ✓ | 0 –50 | 72 h | ↑ | ↓ | (DiResta et al., 2005; Nathan et al., 2005) | |||
| SaOS2 Osteosarcoma |
✓ | ✓ | 0 –50 | 72 h | ↓ | (DiResta et al., 2005) | ||||
| HOS Osteosarcoma |
✓ | ✓ | 0 –100 | 72 h | ↓ | ↑ | (DiResta et al., 2005; Nathan et al., 2005) | |||
| MCF7 Breast cancer |
✓ | ✓ | 0–100 | 72 h | ↓ | (DiResta et al., 2005) | ||||
| H1299 Lung carcinoma |
✓ | ✓ | 0–100 | 72 h | ↓ | (DiResta et al., 2005) | ||||
| A431/A 549 Epithelial tumor |
In vivo | ✓ | 10/5 | ↑ | (Hofmann et al., 2006) | |||||
In addition to modulating proliferation and apoptosis, solid compressive stress impacts cancer cell migration. Compressive stress applied to breast cancer cells 67NR, MDA-MB231 and 4T1, promoted the formation of leader cells that promote coordinated migration (Janet M. Tse et al., 2012). These effects depended on the cell type, as migration was actually impaired in non-cancerous MCF10A cells and in non-invasive MCF7 cells (Janet M. Tse et al., 2012). Migration also increased in glioma (H4) and pancreatic cancer cell lines CFPAC-1, PaCa-2 and BxPC-3 under compression (Kalli, Minia, et al., 2019; Kalli, Papageorgis, Gkretsi, & Stylianopoulos, 2018; Kalli, Voutouri, et al., 2019) (see Table 1). Cancer cell migration under confinement by solid interfaces formed by the tissue microenvironment requires the displacement of these surfaces by the migrating cell. This should place greater energy demands on cancer cells. Consistent with this, MDA-MB-231 breast cancer cells moving through confined spaces have been found to consume more energy for migration (Zanotelli et al., 2019). The energy demands scaled with cell stiffness and with matrix stiffness. Consistent with these findings, migration of different cells through confined environments correlates inversely with stiffness of the cell and nuclear volume (Lautscham et al., 2015) and the levels of nuclear lamins, lamin A/C and lamin B2 (Vortmeyer-Krause et al., 2020; Wolf et al., 2013).
The extent to which confinement in solid tissue microenvironments triggers shared pathways in invading cancer cells and cancer cells in solid tumors is presently unclear and deserves further investigation.
Cancer cell response to hydrostatic pressure
Methods to apply hydrostatic pressure to cells
To study the response of cancer cells to elevated IFP, in vitro studies have studied the impact of hydrostatic pressure on cancer cells in culture. The application of hydrostatic pressure to cells in vitro is simpler compared to cell confinement (Figure 1c), and is typically achieved by connecting an external liquid reservoir (Haberstroh, Kaefer, Retik, Freeman, & Bizios, 1999; Mandal, Shahidullah, & Delamere, 2010) or syringe pump (C. Liu et al., 2010; Daisuke Yoshino, Sato, & Sato, 2015) to the cell culture dish (Kao et al., 2017), or alternatively through applying pneumatic compression (S. Liu et al., 2019; Shang et al., 2021; Stover & Nagatomi, 2007; J. Yu et al., 2011). Limitations of pneumatic compression include potential increases in the dissolved concentration of gases and related changes in pH, while connections to closed liquid pump systems can result in longer-time decreases in dissolved gas concentrations. Ruling out such complications is important for reliable interpretation of results.
Effect of hydrostatic pressure on the cytoskeleton
Equation (2) can be similarly used to conceptualize the effect of external hydrostatic pressure; Pc can be interpreted as extracellular hydrostatic pressure in the equation. Instantaneous response to a step increase in hydrostatic pressure can be an increase in the intracellular pressure (Kao et al., 2017), while longer-time scale cellular adaptations could include a slower increase in the cell volume through changes to aquaporin 1 expression (Kao et al., 2017), which would result in a reduction in the curvature of the cell periphery and the reaching of a new equilibrium (equation (2)).
Cancer cell cytoskeletal responses to hydrostatic pressure are relatively under-studied in the literature. At least one study suggested that cancer cells respond to hydrostatic pressure differently from non-cancerous cells. Unlike normal bronchial epithelial cells, CL1–5 and A549 lung cancer cells responded to 20 mm Hg pressure applied with a syringe pump, by assembling F-actin containing filopodia (Kao et al., 2017). A large number of studies that applied pressure through an external liquid reservoir or pump, have examined microtubule behavior at pressures of the order of MPa (Gao et al., 2018; Nishiyama, 2017; Nishiyama, Kimura, Nishiyama, & Terazima, 2009; Nishiyama, Shimoda, Hasumi, Kimura, & Terazima, 2010), but these pressures are orders of magnitude larger than those prevalent in cancers in vivo (Stylianopoulos et al., 2018).
Effect of hydrostatic pressure on cancer cell migration and proliferation
The elevated pressure in solid tumors in vivo is spatially uniform through the majority of the tumor, and declines rapidly toward the periphery (Figure 1b) (Boucher et al., 1990). The decline in the pressure drives outwardly directed fluid flows in the peripheral region (Boucher et al., 1990). Fluid flows can exert shear stresses tangential to the cellular surfaces which can trigger molecular cellular responses that are distinct from responses to hydrostatic pressures which act normal to the cellular surface. There are at least two types of studies in the literature in the context of cancer cellular responses to fluid pressure: those that involved flows under pressure gradients imposed across cells embedded in 3D extracellular matrices (e.g. (Polacheck, Charest, & Kamm, 2011; Polacheck, German, Mammoto, Ingber, & Kamm, 2014; Tien, Truslow, & Nelson, 2012)), and studies in which a hydrostatic pressure was applied to cells in the absence of any flows (e.g. (Kao et al., 2017)). Here we focus specifically on papers where the cellular responses were solely due to hydrostatic pressure and not flow.
Application of hydrostatic pressure to cultured cancer cells alters their proliferation in a manner that depends on the magnitude of the pressure and on the cell type (DiResta et al., 2005). Pressures in the range of 100 mm Hg suppressed the proliferation of cultured osteosarcoma cancer cell lines SaOS2 and HOS, breast cancer cell line MCF7 and lung cancer cell line H1299 (DiResta et al., 2005). Conversely, lower pressure ranges of 0–50 mm Hg caused an increase in proliferation in some of these lines and a decrease in others (DiResta et al., 2005; Hofmann et al., 2006; Y. C. Kao, C. H. Lee, & P. L. Kuo, 2014; T. Yu et al., 2013). Pressure ranges of 0–30 mm Hg increased proliferation in oral squamous cell carcinoma cell lines SCC-4 and SCC-9 (T. Yu et al., 2013). Likewise, lung cancer cells CL1–5 proliferated more at elevated pressures in a similar pressure range (Kao et al., 2017).
The proliferation of the hTERT+-AM epithelial cell line, on the other hand, was suppressed in pressure ranges of 30 mm – 90 mm Hg (Yang et al., 2018). Finally, relieving the tumor IFP in nude mice caused a decrease in the proliferation of epidermal carcinoma A431/A549 cells in the tumor cortex, which may be due to a decrease in IFP-induced stretching of cells (Hofmann et al., 2006). Overall, these contrasting results suggest that hydrostatic pressure is clearly important in terms of its impact on tumor cell proliferation, but whether it is pro- or anti-proliferative depends on the pressure magnitude and on the specific tumor cell types. Hydrostatic pressure may also promote tumor cell proliferation indirectly by modulating the release of pro-proliferative molecules by other cell types (Sottnik, Dai, Zhang, Campbell, & Keller, 2015).
Elevated hydrostatic pressure has been reported to increase cell migration in a range of cancer cell types, over a broad range of pressures (0–90 mm Hg, see Table 1). Application of hydrostatic pressure to CL1–5 and A549 lung cancer cells caused an increase in cancer cell migration (Kao et al., 2017). Elevated hydrostatic pressure promoted migration and invasion of ameloblastoma cells by upregulating the expression of matrix metalloproteinases MMP-2 and MMP-9, which are targets of the Wnt signaling pathway (Yang et al., 2018). Pressure upregulated the expression of ~1800 genes in SCC-4 and SCC-9 oral squamous cell carcinoma cells (T. Yu et al., 2013) associated with metastasis, the Wnt pathway and cell adhesion pathways, consistent with the observed increase in cell migration.
Different non-cancerous tissue cells have been reported to respond to hydrostatic pressure, including human chondrocytes (Correia et al., 2012) and human endothelial cells(Shin, Bizios, & Gerritsen, 2003)(D. Yoshino & Sato, 2019)(Prystopiuk et al., 2018). In contrast with these and the above studies, one study reported no effects of hydrostatic pressure in the range of 100 mm Hg on the F-actin cytoskeleton, nor on cell functions like proliferation or apoptosis in endothelial cells or neuronal cells (Tworkoski, Glucksberg, & Johnson, 2018). Other studies have similarly raised uncertainty about whether there are any effects of hydrostatic pressure on cell function at all (Astafurov et al., 2014; Osborne et al., 2015). The reasons for the inconsistencies remain unclear.
Conclusions and future outlook
There is a growing body of evidence that solid compressive stresses and interstitial fluid pressure alter tumor cell behaviors like proliferation and invasion. The molecular mechanisms underlying these responses are not as well-understood. Studies so far suggest that response mechanisms are likely to be distinct depending on the cancer and cancer cell type, the type of mechanical stress, and the magnitude of stress.
Studies of cell responses to mechanical stresses have traditionally involved mechanical sensitization of cells over time scales of hours to a few days. Emerging evidence suggests however that cells may adapt to mechanical stimuli over periods of several days to weeks (reviewed in (Lele, Brock, & Peyton, 2020)). Pathways that mediate adaptation of cancer cells to mechanical stresses, such as Rho signaling, can protect cancer cells from therapy-induced death (Misek et al., 2020; Orgaz et al., 2020). Knowledge of the molecular mechanisms of long term adaptation of tumor cells (weeks to months) is crucial if clinical strategies that target cancer cellular adaptation pathways to mechanical changes in tumors (J. Zhang & Reinhart-King, 2020) are to become a reality.
Further, given that cancer cells in a tumor are genetically highly heterogeneous, it is possible that cancer cell responses to mechanical stresses depend on genetic heterogeneity. Mechanical stresses may act as agents of natural selection, causing evolution of cancer cell populations in the tumor. For example, we have shown that substrate stiffness can exert selection pressure on genetically variable fibroblast populations, resulting in the enrichment of specific genotypes over periods of weeks (Purkayastha et al., 2021). It is tempting to speculate that tumor mechanical stresses cause similar significant cancer cellular evolution, and that the resulting selected sub-populations are resistive to tumor therapies, or prone to higher invasion.
Acknowledgements
TPL acknowledges support from NIH U01 CA225566 and a CPRIT established investigator award grant # RR200043.
References:
- Agrawal A, & Lele TP (2019). Mechanics of nuclear membranes. J Cell Sci, 132(14). doi: 10.1242/jcs.229245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ariffin AB, Forde PF, Jahangeer S, Soden DM, & Hinchion J (2014). Releasing pressure in tumors: what do we know so far and where do we go from here? A review. Cancer Res, 74(10), 2655–2662. doi: 10.1158/0008-5472.CAN-13-3696 [DOI] [PubMed] [Google Scholar]
- Astafurov K, Dong CQ, Panagis L, Kamthan G, Ren L, Rozenboym A, … Danias J (2014). Complement expression in the retina is not influenced by short-term pressure elevation. Mol Vis, 20, 140–152. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/24505213 [PMC free article] [PubMed] [Google Scholar]
- Aureille J, Buffière-Ribot V, Harvey BE, Boyault C, Pernet L, Andersen T, … Guilluy C (2019). Nuclear envelope deformation controls cell cycle progression in response to mechanical force. EMBO Rep, 20(9), e48084. doi: 10.15252/embr.201948084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucher Y, Baxter LT, & Jain RK (1990). Interstitial pressure gradients in tissue-isolated and subcutaneous tumors: implications for therapy. Cancer Res, 50(15), 4478–4484. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/2369726 [PubMed] [Google Scholar]
- Boyle ST, Kular J, Nobis M, Ruszkiewicz A, Timpson P, & Samuel MS (2020). Acute compressive stress activates RHO/ROCK-mediated cellular processes. Small GTPases, 11(5), 354–370. doi: 10.1080/21541248.2017.1413496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caille N, Thoumine O, Tardy Y, & Meister JJ (2002). Contribution of the nucleus to the mechanical properties of endothelial cells. J Biomech, 35(2), 177–187. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/11784536 [DOI] [PubMed] [Google Scholar]
- Charras G, & Paluch E (2008). Blebs lead the way: how to migrate without lamellipodia. Nat Rev Mol Cell Biol, 9(9), 730–736. doi: 10.1038/nrm2453 [DOI] [PubMed] [Google Scholar]
- Chauhan VP, Martin JD, Liu H, Lacorre DA, Jain SR, Kozin SV, … Jain RK (2013). Angiotensin inhibition enhances drug delivery and potentiates chemotherapy by decompressing tumour blood vessels. Nat Commun, 4, 2516. doi: 10.1038/ncomms3516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng G, Tse J, Jain RK, & Munn LL (2009). Micro-Environmental Mechanical Stress Controls Tumor Spheroid Size and Morphology by Suppressing Proliferation and Inducing Apoptosis in Cancer Cells. PLOS ONE, 4(2), e4632. doi: 10.1371/journal.pone.0004632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correia C, Pereira AL, Duarte AR, Frias AM, Pedro AJ, Oliveira JT, … Reis RL (2012). Dynamic culturing of cartilage tissue: the significance of hydrostatic pressure. Tissue Eng Part A, 18(19–20), 1979–1991. doi: 10.1089/ten.TEA.2012.0083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damodaran K, Venkatachalapathy S, Alisafaei F, Radhakrishnan AV, Sharma Jokhun D, Shenoy VB, & Shivashankar GV (2018). Compressive force induces reversible chromatin condensation and cell geometry-dependent transcriptional response. Mol Biol Cell, 29(25), 3039–3051. doi: 10.1091/mbc.E18-04-0256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies PF, & Tripathi SC (1993). Mechanical stress mechanisms and the cell. An endothelial paradigm. Circ Res, 72(2), 239–245. doi: 10.1161/01.res.72.2.239 [DOI] [PubMed] [Google Scholar]
- Delarue M, Montel F, Vignjevic D, Prost J, Joanny J-F, & Cappello G (2014). Compressive Stress Inhibits Proliferation in Tumor Spheroids through a Volume Limitation. Biophysical Journal, 107(8), 1821–1828. doi: 10.1016/j.bpj.2014.08.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denais CM, Gilbert RM, Isermann P, McGregor AL, te Lindert M, Weigelin B, … Lammerding J (2016). Nuclear envelope rupture and repair during cancer cell migration. Science, 352(6283), 353–358. doi: 10.1126/science.aad7297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmaison A, Frongia C, Grenier K, Ducommun B, & Lobjois V (2013). Mechanical Stress Impairs Mitosis Progression in Multi-Cellular Tumor Spheroids. PLOS ONE, 8(12), e80447. doi: 10.1371/journal.pone.0080447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiResta GR, Nathan SS, Manoso MW, Casas-Ganem J, Wyatt C, Kubo T, … Healey JH (2005). Cell Proliferation of Cultured Human Cancer Cells are Affected by the Elevated Tumor Pressures that Exist In Vivo. Annals of Biomedical Engineering, 33(9), 1270–1280. doi: 10.1007/s10439-005-5732-9 [DOI] [PubMed] [Google Scholar]
- Dolega ME, Monnier S, Brunel B, Joanny JF, Recho P, & Cappello G (2021). Extracellular matrix in multicellular aggregates acts as a pressure sensor controlling cell proliferation and motility. Elife, 10. doi: 10.7554/eLife.63258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedl P, & Alexander S (2011). Cancer invasion and the microenvironment: plasticity and reciprocity. Cell, 147(5), 992–1009. doi: 10.1016/j.cell.2011.11.016 [DOI] [PubMed] [Google Scholar]
- Gao M, Berghaus M, Möbitz S, Schuabb V, Erwin N, Herzog M, … Winter R (2018). On the Origin of Microtubules’ High-Pressure Sensitivity. Biophys J, 114(5), 1080–1090. doi: 10.1016/j.bpj.2018.01.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene GW, Anderson TH, Zeng H, Zappone B, & Israelachvili JN (2009). Force amplification response of actin filaments under confined compression. Proc Natl Acad Sci U S A, 106(2), 445–449. doi: 10.1073/pnas.0812064106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffon-Etienne G, Boucher Y, Brekken C, Suit HD, & Jain RK (1999). Taxane-induced apoptosis decompresses blood vessels and lowers interstitial fluid pressure in solid tumors: clinical implications. Cancer Res, 59(15), 3776–3782. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/10446995 [PubMed] [Google Scholar]
- Gutmann R, Leunig M, Feyh J, Goetz AE, Messmer K, Kastenbauer E, & Jain RK (1992). Interstitial hypertension in head and neck tumors in patients: correlation with tumor size. Cancer Res, 52(7), 1993–1995. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/1551128 [PubMed] [Google Scholar]
- Haberstroh KM, Kaefer M, Retik AB, Freeman MR, & Bizios R (1999). The effects of sustained hydrostatic pressure on select bladder smooth muscle cell functions. J Urol, 162(6), 2114–2118. doi: 10.1016/s0022-5347(05)68136-0 [DOI] [PubMed] [Google Scholar]
- Halfmann CT, Sears RM, Katiyar A, Busselman BW, Aman LK, Zhang Q, … Roux KJ (2019). Repair of nuclear ruptures requires barrier-to-autointegration factor. J Cell Biol, 218(7), 2136–2149. doi: 10.1083/jcb.201901116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatch EM, & Hetzer MW (2016). Nuclear envelope rupture is induced by actin-based nucleus confinement. J Cell Biol, 215(1), 27–36. doi: 10.1083/jcb.201603053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He L, Tao J, Maity D, Si F, Wu Y, Wu T, … Sun SX (2018). Role of membrane-tension gated Ca. J Cell Sci, 131(4). doi: 10.1242/jcs.208470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldin CH, Rubin K, Pietras K, & Ostman A (2004). High interstitial fluid pressure - an obstacle in cancer therapy. Nat Rev Cancer, 4(10), 806–813. doi: 10.1038/nrc1456 [DOI] [PubMed] [Google Scholar]
- Helmlinger G, Netti PA, Lichtenbeld HC, Melder RJ, & Jain RK (1997). Solid stress inhibits the growth of multicellular tumor spheroids. Nature Biotechnology, 15(8), 778–783. doi: 10.1038/nbt0897-778 [DOI] [PubMed] [Google Scholar]
- Hobson CM, Kern M, O’Brien ET 3rd, Stephens AD, Falvo MR, & Superfine R (2020). Correlating nuclear morphology and external force with combined atomic force microscopy and light sheet imaging separates roles of chromatin and lamin A/C in nuclear mechanics. Mol Biol Cell, 31(16), 1788–1801. doi: 10.1091/mbc.E20-01-0073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann M, Guschel M, Bernd A, Bereiter-Hahn J, Kaufmann R, Tandi C, … Kippenberger S (2006). Lowering of tumor interstitial fluid pressure reduces tumor cell proliferation in a xenograft tumor model. Neoplasia (New York, N.Y.), 8(2), 89–95. doi: 10.1593/neo.05469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hope JM, Bersi MR, Dombroski JA, Clinch AB, Pereles RS, Merryman WD, & King MR (2021). Circulating prostate cancer cells have differential resistance to fluid shear stress-induced cell death. J Cell Sci, 134(4). doi: 10.1242/jcs.251470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J, Li Y, Hao Y, Zheng T, Gupta SK, Parada GA, … Guo M (2019). High stretchability, strength, and toughness of living cells enabled by hyperelastic vimentin intermediate filaments. Proc Natl Acad Sci U S A, 116(35), 17175–17180. doi: 10.1073/pnas.1903890116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain RK (2012). Delivery of molecular and cellular medicine to solid tumors. Adv Drug Deliv Rev, 64(Suppl), 353–365. doi: 10.1016/j.addr.2012.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain RK (2013). Normalizing tumor microenvironment to treat cancer: bench to bedside to biomarkers. J Clin Oncol, 31(17), 2205–2218. doi: 10.1200/JCO.2012.46.3653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain RK (2014). Antiangiogenesis strategies revisited: from starving tumors to alleviating hypoxia. Cancer Cell, 26(5), 605–622. doi: 10.1016/j.ccell.2014.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain RK, & Baxter LT (1988). Mechanisms of heterogeneous distribution of monoclonal antibodies and other macromolecules in tumors: significance of elevated interstitial pressure. Cancer Res, 48(24 Pt 1), 7022–7032. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/3191477 [PubMed] [Google Scholar]
- Jain RK, Martin JD, & Stylianopoulos T (2014). The role of mechanical forces in tumor growth and therapy. Annu Rev Biomed Eng, 16, 321–346. doi: 10.1146/annurev-bioeng-071813-105259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janmey PA, Euteneuer U, Traub P, & Schliwa M (1991). Viscoelastic properties of vimentin compared with other filamentous biopolymer networks. J Cell Biol, 113(1), 155–160. doi: 10.1083/jcb.113.1.155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalli M, Minia A, Pliaka V, Fotis C, Alexopoulos LG, & Stylianopoulos T (2019). Solid stress-induced migration is mediated by GDF15 through Akt pathway activation in pancreatic cancer cells. Scientific Reports, 9(1), 978. doi: 10.1038/s41598-018-37425-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalli M, Papageorgis P, Gkretsi V, & Stylianopoulos T (2018). Solid Stress Facilitates Fibroblasts Activation to Promote Pancreatic Cancer Cell Migration. Annals of Biomedical Engineering, 46(5), 657–669. doi: 10.1007/s10439-018-1997-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalli M, & Stylianopoulos T (2018). Defining the Role of Solid Stress and Matrix Stiffness in Cancer Cell Proliferation and Metastasis. Frontiers in Oncology, 8(55). doi: 10.3389/fonc.2018.00055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalli M, Voutouri C, Minia A, Pliaka V, Fotis C, Alexopoulos LG, & Stylianopoulos T (2019). Mechanical Compression Regulates Brain Cancer Cell Migration Through MEK1/Erk1 Pathway Activation and GDF15 Expression. Frontiers in Oncology, 9(992). doi: 10.3389/fonc.2019.00992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao Y, Lee C, & Kuo P (2014, 26–30 Aug. 2014). Increased hydrostatic pressure enhances motility of lung cancer cells. Paper presented at the 2014 36th Annual International Conference of the IEEE Engineering in Medicine and Biology Society. [DOI] [PubMed] [Google Scholar]
- Kao YC, Jheng JR, Pan HJ, Liao WY, Lee CH, & Kuo PL (2017). Elevated hydrostatic pressure enhances the motility and enlarges the size of the lung cancer cells through aquaporin upregulation mediated by caveolin-1 and ERK1/2 signaling. Oncogene, 36(6), 863–874. doi: 10.1038/onc.2016.255 [DOI] [PubMed] [Google Scholar]
- Kao YC, Lee CH, & Kuo PL (2014). Increased hydrostatic pressure enhances motility of lung cancer cells. Annu Int Conf IEEE Eng Med Biol Soc, 2014, 2928–2931. doi: 10.1109/EMBC.2014.6944236 [DOI] [PubMed] [Google Scholar]
- Katiyar A, Tocco VJ, Li Y, Aggarwal V, Tamashunas AC, Dickinson RB, & Lele TP (2019). Nuclear size changes caused by local motion of cell boundaries unfold the nuclear lamina and dilate chromatin and intranuclear bodies. Soft Matter, 15(45), 9310–9317. doi: 10.1039/c9sm01666j [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lautscham LA, Kammerer C, Lange JR, Kolb T, Mark C, Schilling A, … Fabry B (2015). Migration in Confined 3D Environments Is Determined by a Combination of Adhesiveness, Nuclear Volume, Contractility, and Cell Stiffness. Biophys J, 109(5), 900–913. doi: 10.1016/j.bpj.2015.07.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Berre M, Zlotek-Zlotkiewicz E, Bonazzi D, Lautenschlaeger F, & Piel M (2014). Methods for two-dimensional cell confinement. Methods Cell Biol, 121, 213–229. doi: 10.1016/B978-0-12-800281-0.00014-2 [DOI] [PubMed] [Google Scholar]
- Lele TP, Brock A, & Peyton SR (2020). Emerging Concepts and Tools in Cell Mechanomemory. Ann Biomed Eng, 48(7), 2103–2112. doi: 10.1007/s10439-019-02412-z [DOI] [PubMed] [Google Scholar]
- Lele TP, Dickinson RB, & Gundersen GG (2018). Mechanical principles of nuclear shaping and positioning. J Cell Biol, 217(10), 3330–3342. doi: 10.1083/jcb.201804052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Lovett D, Zhang Q, Neelam S, Kuchibhotla RA, Zhu R, … Dickinson RB (2015). Moving cell boundaries drive nuclear shaping during cell spreading. Biophysical Journal, 109(4), 670–686. doi: 10.1016/j.bpj.2015.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Lewin Mejia D, Chiang B, Luker KE, & Luker GD (2018). Hybrid collagen alginate hydrogel as a platform for 3D tumor spheroid invasion. Acta Biomaterialia, 75, 213–225. doi: 10.1016/j.actbio.2018.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Zhao Y, Cheung WY, Gandhi R, Wang L, & You L (2010). Effects of cyclic hydraulic pressure on osteocytes. Bone, 46(5), 1449–1456. doi: 10.1016/j.bone.2010.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Tao R, Wang M, Tian J, Genin GM, Lu TJ, & Xu F (2019). Regulation of Cell Behavior by Hydrostatic Pressure. Appl Mech Rev, 71(4), 0408031–4080313. doi: 10.1115/1.4043947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomakin AJ, Cattin CJ, Cuvelier D, Alraies Z, Molina M, Nader GPF, … Piel M (2020). The nucleus acts as a ruler tailoring cell responses to spatial constraints. Science, 370(6514). doi: 10.1126/science.aba2894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L, Yamada S, Wirtz D, & Coulombe PA (2001). A ‘hot-spot’ mutation alters the mechanical properties of keratin filament networks. Nat Cell Biol, 3(5), 503–506. doi: 10.1038/35074576 [DOI] [PubMed] [Google Scholar]
- Mandal A, Shahidullah M, & Delamere NA (2010). Hydrostatic pressure-induced release of stored calcium in cultured rat optic nerve head astrocytes. Invest Ophthalmol Vis Sci, 51(6), 3129–3138. doi: 10.1167/iovs.09-4614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews HK, Ganguli S, Plak K, Taubenberger AV, Win Z, Williamson M, … Baum B (2020). Oncogenic Signaling Alters Cell Shape and Mechanics to Facilitate Cell Division under Confinement. Dev Cell, 52(5), 563–573.e563. doi: 10.1016/j.devcel.2020.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misek SA, Appleton KM, Dexheimer TS, Lisabeth EM, Lo RS, Larsen SD, … Neubig RR (2020). Rho-mediated signaling promotes BRAF inhibitor resistance in de-differentiated melanoma cells. Oncogene, 39(7), 1466–1483. doi: 10.1038/s41388-019-1074-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moose DL, Krog BL, Kim TH, Zhao L, Williams-Perez S, Burke G, … Henry MD (2020). Cancer Cells Resist Mechanical Destruction in Circulation via RhoA/Actomyosin-Dependent Mechano-Adaptation. Cell Rep, 30(11), 3864–3874 e3866. doi: 10.1016/j.celrep.2020.02.080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munn LL, & Jain RK (2019). Vascular regulation of antitumor immunity. Science, 365(6453), 544–545. doi: 10.1126/science.aaw7875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nader GPF, Agüera-Gonzalez S, Routet F, Gratia M, Maurin M, Cancila V, … Piel M (2020). Compromised nuclear envelope integrity drives tumor cell invasion. bioRxiv, 2020.2005.2022.110122. doi: 10.1101/2020.05.22.110122 [DOI] [PubMed] [Google Scholar]
- Nam S, & Chaudhuri O (2018). Mitotic cells generate protrusive extracellular forces to divide in three-dimensional microenvironments. Nature Physics, 14(6), 621–628. doi: 10.1038/s41567-018-0092-1 [DOI] [Google Scholar]
- Nathan SS, DiResta GR, Casas-Ganem JE, Hoang BH, Sowers R, Yang R, … Healey JH (2005). Elevated physiologic tumor pressure promotes proliferation and chemosensitivity in human osteosarcoma. Clin Cancer Res, 11(6), 2389–2397. doi: 10.1158/1078-0432.CCR-04-2048 [DOI] [PubMed] [Google Scholar]
- Nathanson SD, & Nelson L (1994). Interstitial fluid pressure in breast cancer, benign breast conditions, and breast parenchyma. Ann Surg Oncol, 1(4), 333–338. doi: 10.1007/BF03187139 [DOI] [PubMed] [Google Scholar]
- Neelam S, Chancellor TJ, Li Y, Nickerson JA, Roux KJ, Dickinson RB, & Lele TP (2015). Direct force probe reveals the mechanics of nuclear homeostasis in the mammalian cell. Proc Natl Acad Sci U S A, 112(18), 5720–5725. doi: 10.1073/pnas.1502111112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nia HT, Liu H, Seano G, Datta M, Jones D, Rahbari N, … Jain RK (2016). Solid stress and elastic energy as measures of tumour mechanopathology. Nat Biomed Eng, 1. doi: 10.1038/s41551-016-0004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nia HT, Munn LL, & Jain RK (2020). Physical traits of cancer. Science, 370(6516). doi: 10.1126/science.aaz0868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiyama M (2017). High-pressure microscopy for tracking dynamic properties of molecular machines. Biophys Chem, 231, 71–78. doi: 10.1016/j.bpc.2017.03.010 [DOI] [PubMed] [Google Scholar]
- Nishiyama M, Kimura Y, Nishiyama Y, & Terazima M (2009). Pressure-induced changes in the structure and function of the kinesin-microtubule complex. Biophys J, 96(3), 1142–1150. doi: 10.1016/j.bpj.2008.10.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiyama M, Shimoda Y, Hasumi M, Kimura Y, & Terazima M (2010). Microtubule depolymerization at high pressure. Ann N Y Acad Sci, 1189, 86–90. doi: 10.1111/j.1749-6632.2009.05411.x [DOI] [PubMed] [Google Scholar]
- Northcott JM, Dean IS, Mouw JK, & Weaver VM (2018). Feeling Stress: The Mechanics of Cancer Progression and Aggression. Front Cell Dev Biol, 6, 17. doi: 10.3389/fcell.2018.00017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northey JJ, Przybyla L, & Weaver VM (2017). Tissue Force Programs Cell Fate and Tumor Aggression. Cancer Discov, 7(11), 1224–1237. doi: 10.1158/2159-8290.CD-16-0733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ofek G, Wiltz DC, & Athanasiou KA (2009). Contribution of the cytoskeleton to the compressive properties and recovery behavior of single cells. Biophys J, 97(7), 1873–1882. doi: 10.1016/j.bpj.2009.07.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orgaz JL, Crosas-Molist E, Sadok A, Perdrix-Rosell A, Maiques O, Rodriguez-Hernandez I, … Sanz-Moreno V (2020). Myosin II Reactivation and Cytoskeletal Remodeling as a Hallmark and a Vulnerability in Melanoma Therapy Resistance. Cancer Cell, 37(1), 85–103 e109. doi: 10.1016/j.ccell.2019.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne A, Aldarwesh A, Rhodes JD, Broadway DC, Everitt C, & Sanderson J (2015). Hydrostatic pressure does not cause detectable changes in survival of human retinal ganglion cells. PLoS One, 10(1), e0115591. doi: 10.1371/journal.pone.0115591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padera TP, Stoll BR, Tooredman JB, Capen D, di Tomaso E, & Jain RK (2004). Pathology: cancer cells compress intratumour vessels. Nature, 427(6976), 695. doi: 10.1038/427695a [DOI] [PubMed] [Google Scholar]
- Patteson AE, Vahabikashi A, Pogoda K, Adam SA, Mandal K, Kittisopikul M, … Janmey PA (2019). Vimentin protects cells against nuclear rupture and DNA damage during migration. J Cell Biol, 218(12), 4079–4092. doi: 10.1083/jcb.201902046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peeters EA, Oomens CW, Bouten CV, Bader DL, & Baaijens FP (2005). Mechanical and failure properties of single attached cells under compression. J Biomech, 38(8), 1685–1693. doi: 10.1016/j.jbiomech.2004.07.018 [DOI] [PubMed] [Google Scholar]
- Polacheck WJ, Charest JL, & Kamm RD (2011). Interstitial flow influences direction of tumor cell migration through competing mechanisms. Proc Natl Acad Sci U S A, 108(27), 11115–11120. doi: 10.1073/pnas.1103581108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polacheck WJ, German AE, Mammoto A, Ingber DE, & Kamm RD (2014). Mechanotransduction of fluid stresses governs 3D cell migration. Proc Natl Acad Sci U S A, 111(7), 2447–2452. doi: 10.1073/pnas.1316848111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provenzano PP, & Hingorani SR (2013). Hyaluronan, fluid pressure, and stromal resistance in pancreas cancer. Br J Cancer, 108(1), 1–8. doi: 10.1038/bjc.2012.569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prystopiuk V, Fels B, Simon CS, Liashkovich I, Pasrednik D, Kronlage C, … Fels J (2018). A two-phase response of endothelial cells to hydrostatic pressure. J Cell Sci, 131(12). doi: 10.1242/jcs.206920 [DOI] [PubMed] [Google Scholar]
- Purkayastha P, Pendyala K, Saxena AS, Hakimjavadi H, Chamala S, Dixit P, … Lele TP (2021). Reverse plasticity underlies rapid evolution by clonal selection within populations of fibroblasts propagated on a novel soft substrate. Mol Biol Evol. doi: 10.1093/molbev/msab102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raab M, Gentili M, de Belly H, Thiam HR, Vargas P, Jimenez AJ, … Piel M (2016). ESCRT III repairs nuclear envelope ruptures during cell migration to limit DNA damage and cell death. Science, 352(6283), 359–362. doi: 10.1126/science.aad7611 [DOI] [PubMed] [Google Scholar]
- Seltmann K, Fritsch AW, Käs JA, & Magin TM (2013). Keratins significantly contribute to cell stiffness and impact invasive behavior. Proc Natl Acad Sci U S A, 110(46), 18507–18512. doi: 10.1073/pnas.1310493110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen A, Capitano ML, Spernyak JA, Schueckler JT, Thomas S, Singh AK, … Repasky EA (2011). Mild elevation of body temperature reduces tumor interstitial fluid pressure and hypoxia and enhances efficacy of radiotherapy in murine tumor models. Cancer Res, 71(11), 3872–3880. doi: 10.1158/0008-5472.CAN-10-4482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah P, Hobson CM, Cheng S, Colville MJ, Paszek MJ, Superfine R, & Lammerding J (2021). Nuclear Deformation Causes DNA Damage by Increasing Replication Stress. Curr Biol, 31(4), 753–765 e756. doi: 10.1016/j.cub.2020.11.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang M, Kwon T, Hamel J-FP, Lim CT, Khoo BL, & Han J (2021). Investigating the influence of physiologically relevant hydrostatic pressure on CHO cell batch culture. Scientific Reports, 11(1), 162. doi: 10.1038/s41598-020-80576-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin HY, Bizios R, & Gerritsen ME (2003). Cyclic pressure modulates endothelial barrier function. Endothelium, 10(3), 179–187. doi: 10.1080/10623320390237883 [DOI] [PubMed] [Google Scholar]
- Sorce B, Escobedo C, Toyoda Y, Stewart MP, Cattin CJ, Newton R, … Muller DJ (2015). Mitotic cells contract actomyosin cortex and generate pressure to round against or escape epithelial confinement. Nat Commun, 6, 8872. doi: 10.1038/ncomms9872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sottnik JL, Dai J, Zhang H, Campbell B, & Keller ET (2015). Tumor-Induced Pressure in the Bone Microenvironment Causes Osteocytes to Promote the Growth of Prostate Cancer Bone Metastases. Cancer Research, 75(11), 2151. doi: 10.1158/0008-5472.CAN-14-2493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart MP, Helenius J, Toyoda Y, Ramanathan SP, Muller DJ, & Hyman AA (2011). Hydrostatic pressure and the actomyosin cortex drive mitotic cell rounding. Nature, 469(7329), 226–230. doi: 10.1038/nature09642 [DOI] [PubMed] [Google Scholar]
- Stover J, & Nagatomi J (2007). Cyclic pressure stimulates DNA synthesis through the PI3K/Akt signaling pathway in rat bladder smooth muscle cells. Ann Biomed Eng, 35(9), 1585–1594. doi: 10.1007/s10439-007-9331-9 [DOI] [PubMed] [Google Scholar]
- Stylianopoulos T, Martin JD, Chauhan VP, Jain SR, Diop-Frimpong B, Bardeesy N, … Jain RK (2012). Causes, consequences, and remedies for growth-induced solid stress in murine and human tumors. Proc Natl Acad Sci U S A, 109(38), 15101–15108. doi: 10.1073/pnas.1213353109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stylianopoulos T, Munn LL, & Jain RK (2018). Reengineering the Physical Microenvironment of Tumors to Improve Drug Delivery and Efficacy: From Mathematical Modeling to Bench to Bedside. Trends Cancer, 4(4), 292–319. doi: 10.1016/j.trecan.2018.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tien J, Truslow JG, & Nelson CM (2012). Modulation of invasive phenotype by interstitial pressure-driven convection in aggregates of human breast cancer cells. PLoS One, 7(9), e45191. doi: 10.1371/journal.pone.0045191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tse JM, Cheng G, Tyrrell JA, Wilcox-Adelman SA, Boucher Y, Jain RK, & Munn LL (2012). Mechanical compression drives cancer cells toward invasive phenotype. Proceedings of the National Academy of Sciences, 109(3), 911. doi: 10.1073/pnas.1118910109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tse JM, Cheng G, Tyrrell JA, Wilcox-Adelman SA, Boucher Y, Jain RK, & Munn LL (2012). Mechanical compression drives cancer cells toward invasive phenotype. Proc Natl Acad Sci U S A, 109(3), 911–916. doi: 10.1073/pnas.1118910109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tworkoski E, Glucksberg MR, & Johnson M (2018). The effect of the rate of hydrostatic pressure depressurization on cells in culture. PLoS One, 13(1), e0189890. doi: 10.1371/journal.pone.0189890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vortmeyer-Krause M, Lindert M. t., Riet J. t., Boekhorst V. t., Marke R, Perera R, … Wolf K (2020). Lamin B2 follows lamin A/C- mediated nuclear mechanics and cancer cell invasion efficacy. bioRxiv, 2020.2004.2007.028969. doi: 10.1101/2020.04.07.028969 [DOI] [Google Scholar]
- Wang JH, & Li B (2010). Mechanics rules cell biology. Sports Med Arthrosc Rehabil Ther Technol, 2, 16. doi: 10.1186/1758-2555-2-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf K, Te Lindert M, Krause M, Alexander S, Te Riet J, Willis AL, … Friedl P (2013). Physical limits of cell migration: control by ECM space and nuclear deformation and tuning by proteolysis and traction force. J Cell Biol, 201(7), 1069–1084. doi: 10.1083/jcb.201210152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Li K, Liang Q, Zheng G, Zhang S, Lao X, … Liao G (2018). Elevated hydrostatic pressure promotes ameloblastoma cell invasion through upregulation of MMP-2 and MMP-9 expression via Wnt/β-catenin signalling. Journal of Oral Pathology & Medicine, 47(9), 836–846. doi: 10.1111/jop.12761 [DOI] [PubMed] [Google Scholar]
- Yoshino D, Sato K, & Sato M (2015). Endothelial Cell Response Under Hydrostatic Pressure Condition Mimicking Pressure Therapy. Cellular and Molecular Bioengineering, 8(2), 296–303. doi: 10.1007/s12195-015-0385-8 [DOI] [Google Scholar]
- Yoshino D, & Sato M (2019). Early-stage dynamics in vascular endothelial cells exposed to hydrodynamic pressure. J Biomech Eng. doi: 10.1115/1.4044046 [DOI] [PubMed] [Google Scholar]
- Yu J, Zhong Y, Cheng Y, Shen X, Wang J, & Wei Y (2011). Effect of high hydrostatic pressure on the expression of glutamine synthetase in rat retinal Müller cells cultured in vitro. Exp Ther Med, 2(3), 513–516. doi: 10.3892/etm.2011.239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu T, Liu K, Wu Y, Fan J, Chen J, Li C, … Li L (2013). High interstitial fluid pressure promotes tumor cell proliferation and invasion in oral squamous cell carcinoma. Int J Mol Med, 32(5), 1093–1100. doi: 10.3892/ijmm.2013.1496 [DOI] [PubMed] [Google Scholar]
- Zanotelli MR, Rahman-Zaman A, VanderBurgh JA, Taufalele PV, Jain A, Erickson D, … Reinhart-King CA (2019). Energetic costs regulated by cell mechanics and confinement are predictive of migration path during decision-making. Nat Commun, 10(1), 4185. doi: 10.1038/s41467-019-12155-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, & Reinhart-King CA (2020). Targeting Tissue Stiffness in Metastasis: Mechanomedicine Improves Cancer Therapy. Cancer Cell, 37(6), 754–755. doi: 10.1016/j.ccell.2020.05.011 [DOI] [PubMed] [Google Scholar]
- Zhang Q, Tamashunas AC, Agrawal A, Torbati M, Katiyar A, Dickinson RB, … Lele TP (2019). Local, transient tensile stress on the nuclear membrane causes membrane rupture. Mol Biol Cell, 30(7), 899–906. doi: 10.1091/mbc.E18-09-0604 [DOI] [PMC free article] [PubMed] [Google Scholar]


