Abstract
Purpose of review:
Clinicians treating end-stage kidney disease (ESKD) and kidney transplant (KT) patients face unique challenges in their care due to the high burden of frailty in these patients. Frailty has gained significant attention by medical and surgical specialties for risk stratification in the past decades. This review highlights the importance of measuring frailty in KT candidates and recipients.
Recent findings:
Emerging data support that frailty is present even at younger ages among patients undergoing dialysis, transplant evaluation, or transplantation. It is estimated that 18.8% of younger (18–64 years) candidates, 25.2% of older (≥65 years) candidates, 14.3% of younger recipients and 20.8% of older recipients are frail. Additionally, frailty is dynamic and subject to change pre- and post-transplantation. While many patients and clinicians are aware of the importance of measuring frailty, further studies addressing the need for interventions to reduce frailty burden are needed.
Summary:
Frailty is independently associated with many adverse outcomes in ESKD and KT populations. Given the growing number of ESKD and KT patients, it is pivotal to expand the utility of frailty measurement in clinical practice, recognize the burden of frailty, and identify appropriate interventions to mitigate the adverse effects of frailty.
Keywords: frailty, end-stage kidney disease, kidney transplantation
Introduction
Frailty, characterized as a biologic syndrome of decreased physiologic reserve and resistance to stressors, manifests across multiple physiologic systems resulting in elevated vulnerability to adverse outcomes.(1) In general surgery patients, frailty has been found associated with postoperative complications,(2–4) length of stay,(3) discharge to a skilled or assisted-living facility after previously living at home,(3) and even mortality.(5) There has been a growing interest in using frailty for risk stratification, especially in older patients with end-stage kidney disease (ESKD).
Frailty measurements used to largely rely on subjective or simple measures, such as age, comorbidity history, and individual clinician’s judgment, which at times are inaccurate. Recently, there are a number of tools having been developed for frailty assessment. One of the most widely accepted tool for frailty assessment in ESKD patients is the Physical Frailty Phenotype (PFP), which includes 5 components (Table 1).(6) Frailty status is usually classified as: frail (≥3 components present), prefrail/intermediately frail (2 components present), and nonfrail (0–1 component present).(7) Another tool is the clinical frailty scale, which measures frailty severity on a 7-point scale from very fit (1 point) to severely frail (7 points).(8) This measurement builds upon clinical assessments for mobility, comorbidities, cognitive impairment, history of falls, and independence with activities of daily living. However, this measure is time-consuming and subjective. The Short Physical Performance Battery (SPPB) test is an objective measurement of lower extremity function, combining walk speed, balance, and chair stands in the construct.(9) It was first identified and validated in community-dwelling older adults and has been studied in kidney transplantation. As found in a cohort study of KT patients, the SPPB test can distinguish a gradient of risk for mortality and nursing home admission, even in patients who report almost no disability.(10) While these tools can capture some level of vulnerability, the PFP is the most used comprehensive measurement of frailty in KT patients.
Table 1:
Criteria Used to Define Physical Frailty Phenotype by Fried et al. [6]
| Component | Measurement |
|---|---|
| Shrinking | “In the last year, have you lost more than 10 pounds unintentionally (i.e., not due to dieting or exercise)?” If yes, then frail for shrinking. At follow-up, weight loss is calculated as: (weight in previous year – current measured weight) / (weight in previous year). If weight loss ≥ 0.05 and the subject does not report that he/she was trying to lose weight (i.e., unintentional weight loss of at least 5% of previous year’s body weight), then frail for shrinking. |
| Exhaustion | Using the Center for Epidemiological Studies – Depression (CES-D) Scale, the following two statements are read. I felt that everything I did was an effort. (a) I could not get going. (b) The question is asked “How often in the last week did you feel this way?” 0 = rarely or none of the time (<1 day), 1 = some or a little of the time (1–2 days), 2 = a moderate amount of the time (3–4 days), or 3 = most of the time. Subjects answering “2” or “3” to either of these questions are categorized as frail for exhaustion. |
| Low Physical Activity | Based on the short version of the Minnesota Leisure Time Activity questionnaire, asking about walking, chores (moderately strenuous), mowing the lawn, raking, gardening, hiking, jogging, biking, exercise cycling, dancing, aerobics, bowling, golf, singles tennis, doubles tennis, racquetball, calisthenics, swimming. Kcals per week expended are calculated using standardized algorithm. This variable is stratified by gender. • Male: Kcals per week <383 are frail for physical activity • Female: Kcals per week <270 are frail for physical activity |
| Slowness | The slowest 20% of population is defined as frail for slowness, based on time to walk 15 feet. This variable is stratified by gender and height (i.e., gender-specific cutoff a medium height). |
| Weakness | The lowest 20% of population is defined as frail for weakness, based on maximal grip strength (kilograms) in the dominant hand (3 measures averaged) measured by a Jamar hand-held dynamometer. This variable is stratified by gender and body mass index (BMI) quartiles. |
Measuring frailty assists clinicians in identifying the most vulnerable patients and allocating resources and tools to mitigate adverse outcomes. This is particularly important for ESKD patients, who are at elevated risks of subsequent morbidity and adverse outcomes. For example, frailty assessment may benefit ESKD patients by delivering personalized care and appropriate interventions that reduce the risks of long-term adverse outcomes. In a cohort study of 324 adult hemodialysis patients from 27 free standing dialysis centers, 34.0% of participants were frail and 37.7% were intermediately frail in the first month of dialysis initiation.(11) A further analysis of this cohort found the prevalence of frailty was 71.4% for older patients and 47.3% for younger patients, which suggests a very high burden of frailty across the age spectrum of hemodialysis patients.(12) Additionally, hemodialysis patients who are frail are also at high risk of cognitive impairment, which links two important gerontologic constructs.(11) This presents a greater risk of mortality and hospitalization in dialysis patients being frail and cognitively impaired.
Frailty research also plays a key role in clinical practice for kidney transplant (KT) patients. Existing studies focused on estimating the frailty burden of patients seeking for KT and how their frailty status at admission for KT impacts their long-term post-KT outcomes. This review synthesizes recently published literature on burden, risk factors, and sequelae of frailty among KT patients. While many findings regarding frailty in adult KT populations arose from one 2-center prospective cohort study (the Frailty Assessment in Renal Disease [FAIR] Cohorts) from our group, we also highlight important works from other cohorts.
Burden of frailty in KT candidates and recipients
In the United States, frailty prevalence is estimated to be 16.4% in KT candidates and 14.3% in KT recipients.(13) Although frailty burden increases with age, there is a substantial burden in younger candidates and recipients. In the FAIR cohort of 4,304 KT candidates and 1,396 KT recipients, frailty prevalence was 25.2% in older candidates while 18.8% in younger candidates, 20.8% in older recipients while 14.3% in younger recipients.(12) In addition, frailty burden differs by donor type: it is estimated that 8.2% of living donor kidney transplant (LDKT) recipients while17.8% of deceased donor kidney transplant (DDKT) recipients were frail.(13) Moreover, there is consensus among clinicians that frailty is more common in ESKD patients, while less consensus among ESKD patients. In a 2-round Delphi study involving 460 ESKD patients, of whom 20% were identified as frail using the PFP, only 6% of patients self-identified as frail.(14) In consideration of the high burden of frailty among KT candidates and recipients and the lack of self-identification of frailty status, frailty screening should be implemented early at KT evaluation for patients across all age groups to identify patients at high risk for poor outcomes and to improve patient counselling and resource allocation.
Risk factors for frailty among KT candidates and recipients
Several studies have demonstrated that advanced age is associated with frailty in KT candidates and recipients.(15–19) Independent of age, female sex, non-Hispanic Black race, undergoing pre-KT dialysis, higher comorbidity burden are also risk factors of frailty but only among KT candidates.(12) There are also modifiable risk factors of frailty, such as obesity and low physical activity.(20)
Frailty Prior to KT
In the KT candidates cohort of FAIR, frail patients were almost half (48%) as likely to be listed for kidney transplantation and were transplanted 32% less frequently, compared to their nonfrail counterparts, independent of demographic factors including age.(17) Frail candidates had 1.70-fold higher risk of waitlist mortality, even after accounting for age, sex, body mass index (BMI), race, ethnicity, blood type, cause of ESKD, and dialysis vintage.(17) Therefore, it is important to identify frail individuals at evaluation for KT, or even early at the time of ESKD diagnosis in order to enhance the efforts to mitigate the impacts of frailty on these patients’ outcomes. Another study using the FAIR cohort reported that 54% of candidates had SPPB impairment; impaired patients had lower likelihood of being listed for KT and, once listed, a 1.6-fold higher risk of waitlist mortality.(21)
Frailty transitions between evaluation and KT
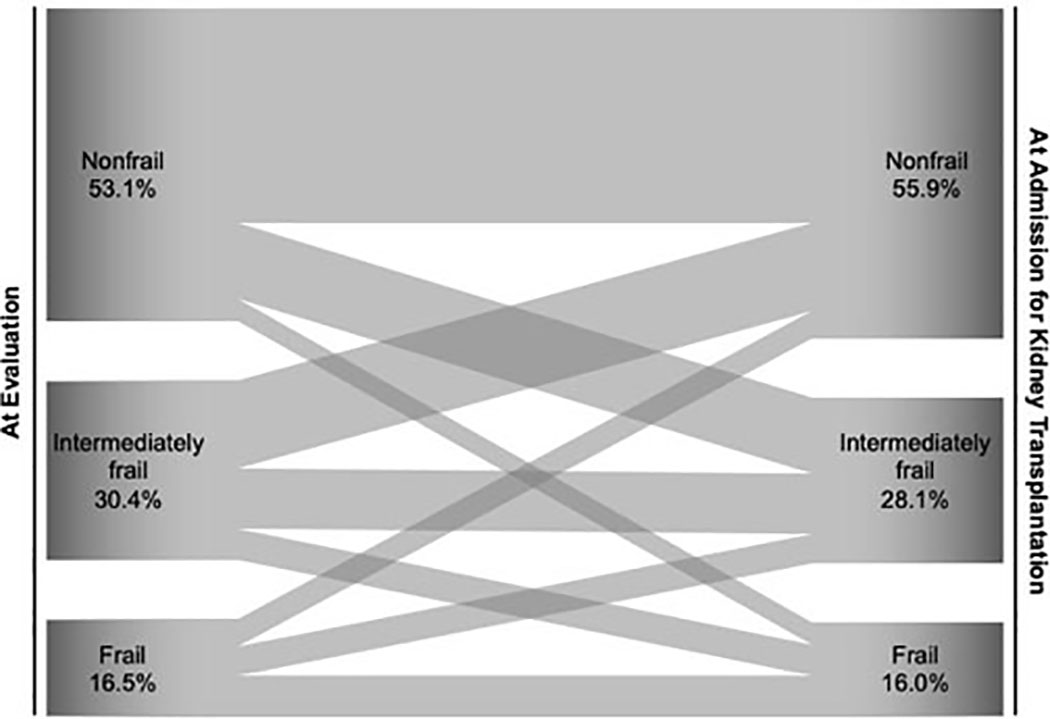
Chu et al. examined the patterns of frailty transitions from evaluation to KT admission using a cohort of KT candidates with frailty measures at both evaluation and KT. Between evaluation and KT, 22.0% of candidates became more frail, while 24.4% became less frail (Figure 1).(22) This study found that Black patients were more likely to experience frail-to-nonfrail transition, while diabetic candidates tended to remain stably frail; candidates who became more frail at KT had higher risk of post-KT mortality and length of stay ≥2 weeks.(22) This research highlights the dynamic nature of frailty among patients in the KT settings and its associations with multiple risk factors and adverse post-KT outcomes. Therefore, it may be helpful to monitor patient’s frailty status between evaluation and KT to inform clinical decision-making.
Figure 1: Change in 3-Category Frailty between Time of Evaluation and Admission for Kidney Transplantation by Chu et al. [22].
The Sankey diagram displays proportions (width of the arrows) of patients who were nonfrail, intermediately frail, and frail at time of evaluation that flowed into nonfrail, intermediately frail, or frail status at admission for kidney transplantation. The figure was created using data presented by Chu et al. [22] in a cohort of 569 adult kidney transplant patients, for whom frailty was measured both at time of evaluation and admission for transplantation.
Frailty and post-KT adverse outcomes
Frail patients, identified using PFP, are generally more vulnerable than nonfrail patients, as a result of dysregulated stress response systems, including chronic inflammation, altered glucose metabolism, and decreased mitochondrial energy production. (23–26) Therefore, KT patients who are frail likely have a different post-operative course, and clinicians should be aware of their prolonged recovery with possible complications.
Many studies have suggested that frail KT patients are more likely to have worse clinical outcomes due to their high vulnerability to surgical and immunologic stressors of KT than those who are nonfrail in both the short and the long term.(20) Preoperative frailty is independently associated with almost 2-fold higher risk of delayed graft function (27) and 2.05-fold higher risk of post-KT delirium.(28) Frail KT recipients also have 1.61-fold higher likelihood to experience early hospital readmission within 30 days of discharge from KT than their nonfrail counterparts.(29) Additionally, KT recipients having SPPB impairment tend to have a longer hospital length of stay following KT.(10) In the long term, frailty is associated with a 2.2-fold higher risk of post-KT mortality, while even intermediately frail is associated with a 1.5-fold higher risk of mortality, after accounting for recipient, donor, and transplant factors.(30) Similarly, SPPB impairment is associated with a 2.3-fold higher risk of post-KT mortality.(10) These findings not only reflect that screening for frail patients earlier in the process can assist in risk stratification for KT patients, but also highlight the importance of effective interventions to improve patient outcomes and to reduce the costs in the healthcare system.
In addition to studies focusing on the overall frailty status, there is emerging evidence showing that individual components of the PFP are associated with adverse outcomes after KT. In the FAIR cohort of KT recipients, the most common pattern of frailty was low grip strength, low physical activity, and slowed gait speed, while two patterns (exhaustion and slowed gait speed; and low grip strength, exhaustion, and slowed gait speed) of the 5 components were associated with 2.4-fold and 2.6-fold higher mortality risk after KT, respectively.(16) A subsequent analysis using the FAIR cohort of KT recipients sheds light on post-KT health outcomes of a single component of PFP – unintentional weight loss. This study found that unintentional weight loss is an independent risk factor for adverse outcomes post-KT: patients with pre-KT unintentional weight loss had 1.80-fold higher risk of all-cause graft loss and 1.72-fold higher risk of all-cause mortality.(31)
Post-KT changes in frailty
KT recipients are confronted with surgical and immunologic stressors while experience restored kidney function, which leads to the dynamic changes in frailty among KT patients.(20) In the FAIR cohort of 349 KT recipients, frailty initially worsened by 1 month, returned to baseline by 2 months, and then improved by 3 months post-KT.(32) Another study of KT recipients in the Netherlands used the Groningen Frailty Indicator to evaluate the changes in frailty status after KT in the longer term: during the first 3 years of follow-up, almost one-fifth of nonfrail patients became frail, while cognition and psychosocial functioning domains appeared to be the main contributors to the transition.(33)
How to improve outcomes in frail KT patients
KT candidates usually experience a profound loss of functional capacity due to the combination of aging, chronic conditions, and higher risk of frailty together with the stress of undergoing dialysis.(34) Prehabilitation, the process of enhancing patient pre-operative functional capacity to improve tolerance for the upcoming physiologic stressor,(35) can help improve post-KT outcomes for KT candidates. In a pilot study of prehabilitation, a diverse group of KT candidates was recruited and participated in weekly individualized physical therapy sessions. After 2 months of prehabilitation, there was a 64% increase (p=0.004) in objectively measured physical activity, and prehabilitation was associated with a 31% decreased length of stay, compared to age, sex, and race matched controls, (36) yet these preliminary findings need to be confirmed in a larger randomized controlled trial before this intervention could be expanded to routine clinical practice.
According to a meta-analysis of 41 trials in choric kidney disease, ESKD, or KT patients, regular exercise training for at least 8 weeks is associated with improved aerobic capacity, muscular functioning, cardiovascular function, walking capacity, and health-related quality of life.(37) This finding suggests that appropriate exercise rehabilitation may be an effective part of care for KT patients, while further research on longer term outcomes is needed.
Customizing the measurement of frailty to biomarkers
Although chronic inflammation is considered as one of the hallmarks of frailty,(23, 25) inflammatory biomarkers are not directly incorporated in any conventional frailty measures. Yet, many studies have reported that frailty, in community-dwelling older adults, is associated with elevated markers of systemic inflammation, including interleukin-6 (IL-6), soluble tumor necrosis factor-α receptor-1 (sTNFR1), and C-reactive protein (CRP).(38–44) Among hemodialysis patients, increased IL-6 levels were also found associated with an increased PFP score.(34) In the FAIR cohort of 1,975 KT candidates, frailty was associated with heightened markers of inflammation among KT candidates; further, IL-6, CRP, and the inflammatory index improved waitlist mortality prediction.(45) This suggests that inflammatory markers should be measured along with frailty status during the evaluation process, because knowing whether a patient is frail and whether this patient have elevated inflammatory markers can potentially provide a more comprehensive evaluation and a more accurate prediction of the risk of mortality while on the waitlist.
In a recent prospective cohort study of 1,154 KT candidates and recipients using the FAIR data, Haugen et al. proposed and validated a set of inflammatory-frailty indices for KT that includes one inflammatory biomarker (IL-6, tumor necrosis factor alpha [TNFα], CRP) with traditional physical phenotype components. The results showed that IL6-frailty was associated with an increased risk of post-KT mortality, although it did not improve risk prediction for post-KT mortality.(46) Identification of frail patients with IL6-frailty captures a larger proportion of the population and could be useful to help identify KT candidates who would benefit from intervention, but this study suggests that measuring the 5 PFP components is sufficient for clinical use. It is also possible that inflammatory markers would be useful in specific sub-populations that are yet to be identified.
Clinical utility of measuring frailty during KT evaluation
According to a projection of ESKD incidence and prevalence, the burden of ESKD will increase in the US population through 2030, specifically 11–18% increase in crude incidence rate from 2015 to 2030 and 29–68% increase in incidence during the same period.(47) In face of the increasingly substantial burden of ESKD, we expect that there will be a greater number of, not only KT recipients but particularly, older recipients in the near future. It is therefore imperative that we focus our efforts to mitigate the impacts of frailty on ESKD and KT populations.
Conclusion
This review highlights the importance of measuring frailty for KT candidates and recipients, regardless of age. Emerging data support the use of PFP or an objective measure of vulnerability such as SPPB for routine use in clinical practice for KT patients. However, further research is needed to identify appropriate interventions to reduce the burden and sequelae of frailty among KT candidates and recipients.
Key points.
Frailty is common in patients with end-stage kidney disease even in the select group of patients undergoing kidney transplantation.
Frailty burden is high across the age spectrum, suggesting the need to screen frailty regardless of age.
Measuring frailty at kidney transplant evaluation may contribute to the improvement of risk prediction and the identification of patients who can benefit from prehabilitation prior to kidney transplantation.
Financial support and sponsorship
Dr. McAdams-DeMarco was supported by R01DK120518 from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), R01DK114074 from the NIDDK, R01AG055781 from the National Institute of Aging (NIA).
Footnotes
Conflicts of Interest
There are no conflicts of interest.
REFERENCES
- 1.Hoogendijk EO, Afilalo J, Ensrud KE, et al. Frailty: implications for clinical practice and public health. Lancet. 2019;394(10206):1365–75. [DOI] [PubMed] [Google Scholar]
- 2.Robinson TN, Wu DS, Pointer L, et al. Simple frailty score predicts postoperative complications across surgical specialties. Am J Surg. 2013;206(4):544–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Makary MA, Segev DL, Pronovost PJ, et al. Frailty as a predictor of surgical outcomes in older patients. J Am Coll Surg. 2010;210(6):901–8. [DOI] [PubMed] [Google Scholar]
- 4.Revenig LM, Canter DJ, Taylor MD, et al. Too frail for surgery? Initial results of a large multidisciplinary prospective study examining preoperative variables predictive of poor surgical outcomes. J Am Coll Surg. 2013;217(4):665–70.e1. [DOI] [PubMed] [Google Scholar]
- 5.Englesbe MJ, Patel SP, He K, et al. Sarcopenia and mortality after liver transplantation. J Am Coll Surg. 2010;211(2):271–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146–56. [DOI] [PubMed] [Google Scholar]
- 7.McAdams-DeMarco MA, Law A, Salter ML, et al. Frailty as a novel predictor of mortality and hospitalization in individuals of all ages undergoing hemodialysis. J Am Geriatr Soc. 2013;61(6):896–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rockwood K, Song X, MacKnight C, et al. A global clinical measure of fitness and frailty in elderly people. Cmaj. 2005;173(5):489–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49(2):M85–94. [DOI] [PubMed] [Google Scholar]
- 10.Nastasi AJ, McAdams-DeMarco MA, Schrack J, et al. Pre-Kidney Transplant Lower Extremity Impairment and Post-Kidney Transplant Mortality. Am J Transplant. 2018;18(1):189–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McAdams-DeMarco MA, Tan J, Salter ML, et al. Frailty and Cognitive Function in Incident Hemodialysis Patients. Clin J Am Soc Nephrol. 2015;10(12):2181–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chu NM, Chen X, Norman SP, et al. Frailty Prevalence in Younger End-Stage Kidney Disease Patients Undergoing Dialysis and Transplantation. Am J Nephrol. 2020;51(7):501–10. * This publication highlights the burden of frailty among younger dialysis patients, kidney transplant candidates and kidney transplant recipients.
- 13.Haugen CE, Thomas AG, Chu NM, et al. Prevalence of frailty among kidney transplant candidates and recipients in the United States: Estimates from a National Registry and Multicenter Cohort Study. Am J Transplant. 2020;20(4):1170–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Van Pilsum Rasmussen S, Konel J, Warsame F, et al. Engaging clinicians and patients to assess and improve frailty measurement in adults with end stage renal disease. BMC Nephrol. 2018;19(1):8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bandeen-Roche K, Seplaki CL, Huang J, et al. Frailty in Older Adults: A Nationally Representative Profile in the United States. J Gerontol A Biol Sci Med Sci. 2015;70(11):1427–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McAdams-DeMarco MA, Ying H, Olorundare I, et al. Individual Frailty Components and Mortality in Kidney Transplant Recipients. Transplantation. 2017;101(9):2126–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haugen CE, Chu NM, Ying H, et al. Frailty and Access to Kidney Transplantation. Clin J Am Soc Nephrol. 2019;14(4):576–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lorenz EC, Cosio FG, Bernard SL, et al. The Relationship Between Frailty and Decreased Physical Performance With Death on the Kidney Transplant Waiting List. Prog Transplant. 2019;29(2):108–14. [DOI] [PubMed] [Google Scholar]
- 19.Pérez Fernández M, Martínez Miguel P, Ying H, et al. Comorbidity, Frailty, and Waitlist Mortality among Kidney Transplant Candidates of All Ages. Am J Nephrol. 2019;49(2):103–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harhay MN, Rao MK, Woodside KJ, et al. An overview of frailty in kidney transplantation: measurement, management and future considerations. Nephrol Dial Transplant. 2020;35(7):1099–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haugen CE, Agoons D, Chu NM, et al. Physical Impairment and Access to Kidney Transplantation. Transplantation. 2020;104(2):367–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chu NM, Deng A, Ying H, et al. Dynamic Frailty Before Kidney Transplantation: Time of Measurement Matters. Transplantation. 2019;103(8):1700–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yao X, Li H, Leng SX. Inflammation and immune system alterations in frailty. Clin Geriatr Med. 2011;27(1):79–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fried LP, Xue QL, Cappola AR, et al. Nonlinear multisystem physiological dysregulation associated with frailty in older women: implications for etiology and treatment. J Gerontol A Biol Sci Med Sci. 2009;64(10):1049–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Walston JD. Connecting Age-Related Biological Decline to Frailty and Late-Life Vulnerability. Nestle Nutr Inst Workshop Ser. 2015;83:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kalyani RR, Varadhan R, Weiss CO, et al. Frailty status and altered glucose-insulin dynamics. J Gerontol A Biol Sci Med Sci. 2012;67(12):1300–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Garonzik-Wang JM, Govindan P, Grinnan JW, et al. Frailty and delayed graft function in kidney transplant recipients. Arch Surg. 2012;147(2):190–3. [DOI] [PubMed] [Google Scholar]
- 28.Haugen CE, Mountford A, Warsame F, et al. Incidence, Risk Factors, and Sequelae of Post-kidney Transplant Delirium. J Am Soc Nephrol. 2018;29(6):1752–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McAdams-DeMarco MA, Law A, Salter ML, et al. Frailty and early hospital readmission after kidney transplantation. Am J Transplant. 2013;13(8):2091–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McAdams-DeMarco MA, Law A, King E, et al. Frailty and mortality in kidney transplant recipients. Am J Transplant. 2015;15(1):149–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Harhay MN, Chen X, Chu NM, et al. Pre-Kidney Transplant Unintentional Weight Loss Leads to Worse Post-Kidney Transplant Outcomes. Nephrol Dial Transplant. 2021. ** This study identifies unintentional but not intentional weight loss as a predictor of adverse outcomes after kidney transplantation.
- 32.McAdams-DeMarco MA, Isaacs K, Darko L, et al. Changes in Frailty After Kidney Transplantation. J Am Geriatr Soc. 2015;63(10):2152–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Quint EE, Schopmeyer L, Banning LBD, et al. Transitions in frailty state after kidney transplantation. Langenbecks Arch Surg. 2020;405(6):843–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Johansen KL, Dalrymple LS, Delgado C, et al. Factors Associated with Frailty and Its Trajectory among Patients on Hemodialysis. Clin J Am Soc Nephrol. 2017;12(7):1100–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cabilan CJ, Hines S, Munday J. The Impact of Prehabilitation on Postoperative Functional Status, Healthcare Utilization, Pain, and Quality of Life: A Systematic Review. Orthop Nurs. 2016;35(4):224–37. [DOI] [PubMed] [Google Scholar]
- 36.McAdams-DeMarco MA, Ying H, Van Pilsum Rasmussen S, et al. Prehabilitation prior to kidney transplantation: Results from a pilot study. Clin Transplant. 2019;33(1):e13450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Heiwe S, Jacobson SH. Exercise training in adults with CKD: a systematic review and meta-analysis. Am J Kidney Dis. 2014;64(3):383–93. [DOI] [PubMed] [Google Scholar]
- 38.Collerton J, Martin-Ruiz C, Davies K, et al. Frailty and the role of inflammation, immunosenescence and cellular ageing in the very old: cross-sectional findings from the Newcastle 85+ Study. Mech Ageing Dev. 2012;133(6):456–66. [DOI] [PubMed] [Google Scholar]
- 39.Barzilay JI, Blaum C, Moore T, et al. Insulin resistance and inflammation as precursors of frailty: the Cardiovascular Health Study. Arch Intern Med. 2007;167(7):635–41. [DOI] [PubMed] [Google Scholar]
- 40.Leng S, Chaves P, Koenig K, Walston J. Serum interleukin-6 and hemoglobin as physiological correlates in the geriatric syndrome of frailty: a pilot study. J Am Geriatr Soc. 2002;50(7):1268–71. [DOI] [PubMed] [Google Scholar]
- 41.Varadhan R, Yao W, Matteini A, et al. Simple biologically informed inflammatory index of two serum cytokines predicts 10 year all-cause mortality in older adults. J Gerontol A Biol Sci Med Sci. 2014;69(2):165–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chang SS, Weiss CO, Xue QL, Fried LP. Patterns of comorbid inflammatory diseases in frail older women: the Women’s Health and Aging Studies I and II. J Gerontol A Biol Sci Med Sci. 2010;65(4):407–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Leng SX, Tian X, Matteini A, et al. IL-6-independent association of elevated serum neopterin levels with prevalent frailty in community-dwelling older adults. Age Ageing. 2011;40(4):475–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Leng SX, Xue QL, Tian J, et al. Inflammation and frailty in older women. J Am Geriatr Soc. 2007;55(6):864–71. [DOI] [PubMed] [Google Scholar]
- 45.McAdams-DeMarco MA, Ying H, Thomas AG, et al. Frailty, Inflammatory Markers, and Waitlist Mortality Among Patients With End-stage Renal Disease in a Prospective Cohort Study. Transplantation. 2018;102(10):1740–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Haugen CE, Gross A, Chu NM, et al. Development and Validation of an Inflammatory-Frailty Index for Kidney Transplantation. J Gerontol A Biol Sci Med Sci. 2021;76(3):470–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McCullough KP, Morgenstern H, Saran R, et al. Projecting ESRD Incidence and Prevalence in the United States through 2030. J Am Soc Nephrol. 2019;30(1):127–35. [DOI] [PMC free article] [PubMed] [Google Scholar]