Abstract
The skeletal system undergoes irreversible structural deterioration with aging, leading to increased fracture risk and detrimental changes in mobility, posture, and gait. This state of low bone mass and microarchitectural changes, diagnosed as osteoporosis, affects millions of individuals worldwide and has high clinical and economic burdens. Recently, pre-clinical studies have linked the onset of age-related bone loss with an accumulation of senescent cells in the bone microenvironment. These senescent cells appear to be causal to age-related bone loss, as targeted clearance of these cells leads to improved bone mass and microarchitecture in old mice. Additionally, other pathologies leading to bone loss that result from DNA damage, such as cancer treatments, have shown improvements after clearance of senescent cells. The development of new therapies that clear senescent cells, termed “senolytics”, is currently underway and may allow for the modulation of bone loss that results from states of high senescent cell burden, such as aging.
Keywords: Osteoporosis, cellular senescence, osteocytes, age-related bone loss
Graphical Abstract:
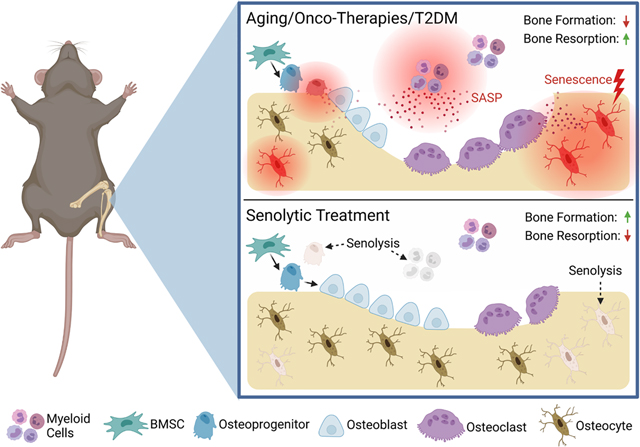
Alleviation of cellular senescence in the bone microenvironment with senolytic therapy. Aging, onco-therapies (radiation and chemotherapy), and Type 2 Diabetes Mellitis (T2DM) lead to increased inflammation in the bone microenvironment through the senescent-associated secretory phenotype (SASP) exhibited by senescent osteoprogenitors, osteocytes, and myeloid cells, among others. This state is associated with reduced bone formation and increased bone resorption, leading to reduced bone mass. Senolytic treatment targets and clears senescent cells, which alleviates SASP-mediated inflammation and allows for repopulation of non-senescent osteoblasts on the bone surface, leading to enhanced bone formation, reduced resorption, and improved bone mass.
Osteoporosis overview
Age-related bone loss and osteoporosis is a growing problem world-wide. In 2000, there were approximately 9 million osteoporotic fractures (including 1.6 million hip, 1.7 million forearm, and 1.4 million clinical vertebral fractures) (Johnell and Kanis, 2006) and it has been estimated that by 2050, the incidence of hip fractures will increase by 240% in women and 310% in men (Gullberg et al., 1997). It is often not well appreciated that the number of women sustaining a fracture per year is greater than the combined number of women experiencing incident breast cancer, myocardial infarction, or stroke (Cauley et al., 2008).
A number of drugs are currently approved specifically for the treatment of osteoporosis. These include estrogen, which is now rarely used for osteoporosis prevention or treatment due to concerns regarding breast cancer and cardiovascular disease risks (Rossouw et al., 2002); raloxifene (a selective estrogen receptor modulator); bisphosphonates (alendronate, risedronate, ibandronate, and zoledronic acid); a human monoclonal antibody to receptor activator of nuclear factor κ-B ligand (RANKL, denosumab); the parathyroid hormone (PTH)/PTH-related peptide (PTHrP) analogues, teriparatide and abaloparatide; and a human monoclonal antibody to sclerostin (Romosozumab) (Khosla and Hofbauer, 2017). Despite this, most patients with osteoporosis remain untreated. For example, among 22,598 patients with hip fracture, use of bisphosphonates declined from only 15% in 2004 to 3% in 2013 (Kim et al., 2016).
There are several reasons for this lack of appropriate treatment for osteoporosis. In part, this is due to largely misplaced concerns regarding very rare bisphosphonate-related side effects, including osteonecrosis of the jaw (ONJ) and atypical femur fractures (AFFs) (Khosla et al., 2017). In a broader context, however, is the issue of multiple co-morbidities of aging and the current approach of treating each co-morbidity separately. Thus, osteoporosis generally exists with additional co-morbidities, including cardiovascular disease, diabetes, rheumatoid arthritis, osteoarthritis, and other age-related diseases (Dennison et al., 2012). Treating each co-morbidity independently inevitably leads to the growing problem of polypharmacy. For example, in the United States it has been estimated that approximately 40% of adults over the age of 65 take 5 or more prescription medications, and approximately 70% take 5 or more over-the-counter and prescription medications (Levy, 2017).
The growing recognition that osteoporosis, along with other age-related co-morbidities, all share aging as a common risk factor has led to the “Geroscience Hypothesis” which postulates that targeting fundamental aging mechanisms will simultaneously delay the appearance or severity of multiple chronic age-associated diseases (Tchkonia et al., 2013). Additionally, the existence, or treatment, of other age-related pathologies may exacerbate bone loss (Mirza and Canalis, 2015). This lends further to the idea that diseases of aging are intertwined, rather than independent, and targeting aging mechanisms may alleviate the occurrence of both primary and secondary osteoporosis. It is in this context that cellular senescence, one of the key fundamental aging mechanisms, may be an important target to prevent and/or treat age-related osteoporosis as well as other skeletal pathologies (Khosla et al., 2020).
Background of senescence in bone
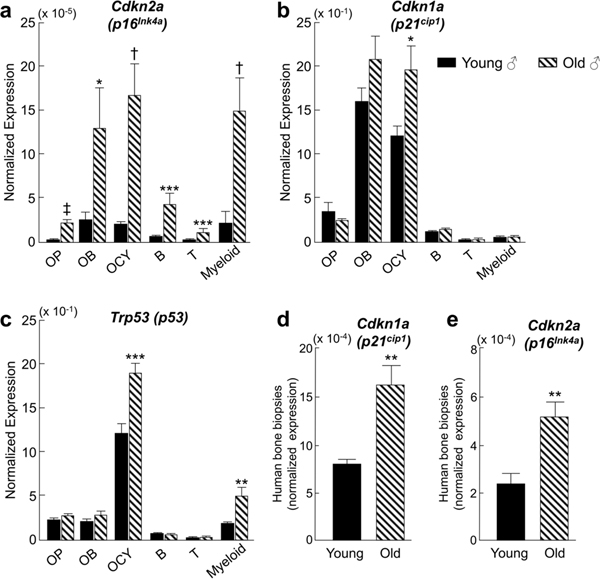
Senescence appears to be present in the bone and marrow cell compartment in an age-dependent and cell-specific manner. We identified senescent cells in the bone microenvironment with aging by examining the expression of key senescent genes (e.g. p16Ink4a, p21Cip1 and p53) in highly enriched populations of osteoblastic lineage cells and bone marrow hematopoietic cells in young (6-month) and old (24-month) mice (Farr et al., 2016). We found that p16Ink4a expression was increased with age in each of the cell populations, whereas increased p21Cip1 and p53 was found primarily in osteocytes and/or myeloid cells, respectively (Fig. 1a–c). This induction of osteocyte senescence was confirmed using both the senescence-associated distension of satellites (SADS) (Swanson et al., 2013) and telomere dysfunction-associated foci (TAF) (Wang et al., 2012) assays in old mice. The presence of senescent cells is accompanied by the development of the senescence-associated secretory phenotype (SASP). Measurement of an established panel of SASP gene markers demonstrated significant upregulation of these markers in both osteocytic and myeloid cell populations in bone. Interestingly, bone biopsies from older women (mean 78 years of age) also exhibited increased p16Ink4a, p21Cip1 (Fig. 1d, e), and SASP gene expression markers. These studies established that aging in both mice and humans is associated with increases in senescence markers in multiple cell types within the bone microenvironment.
Figure 1:
Cells in the bone microenvironment exhibit increased senescent marker expression with age. Bones from young (6-month) and old (24-month) C57BL/6N male mice were isolated and processed to obtain cell suspensions for RNA analysis. qPCR revealed significant upregulation of p16Ink4a (a), p21cip (b), and p53 (c) mRNA expression in subsets of bone and marrow cell populations with age. For human studies, cells were isolated from needle bone biopsies taken from the posterior iliac crest of 10 young (mean ± SD; 27 ± 3 years) and 10 old (mean ± SD; 78 ± 6 years) healthy female volunteers. qPCR analysis indicated significant upregulation of p16Ink4a (d) and p21cip (e) with aging. OP = osteoprogenitors (Lin-Lepr+), OB = osteoblasts (AP+/CD31/34/45/54−), OCY = osteocytes (liberase-digested vertebrae), B = B cells (CD19+), T = T cells (CD3ε+), Myeloid = (CD14+). Data are presented as mean ± SE. *p < 0.05; **p < 0.01; ***p < 0.001; †p < 0.0001; ‡p < 0.00001 using two-sample t-test. Data are from Farr et al. (Farr et al., 2016)
Other groups have also investigated the presence of senescent cells in the bone milieu. In a study by Piemontese et al. (Piemontese et al., 2017), it was demonstrated that aged mice exhibited increased cortical porosity and cortical thinning associated with increased osteoclasts and decreased osteoblasts. These phenotypes were associated with increased p16Ink4a expression in osteocyte-enriched bone samples. Additionally, aging led to increased osteocytic expression of γ-H2AX, GATA4, and p62 protein levels, all of which are implicated in senescence-associated responses to DNA damage. Furthermore, increased Rankl gene expression in osteocyte-enriched bone fractions and increased plasma RANKL protein levels from aged mice was observed, suggesting a possible link between osteocyte senescence and increased bone resorption. Studies from Kim et al. (Kim et al., 2017) demonstrated that Osterix-positive osteoprogenitors also exhibit features of senescence with aging, consistent with our observation that multiple cell types within the bone microenvironment acquire senescent characteristics.
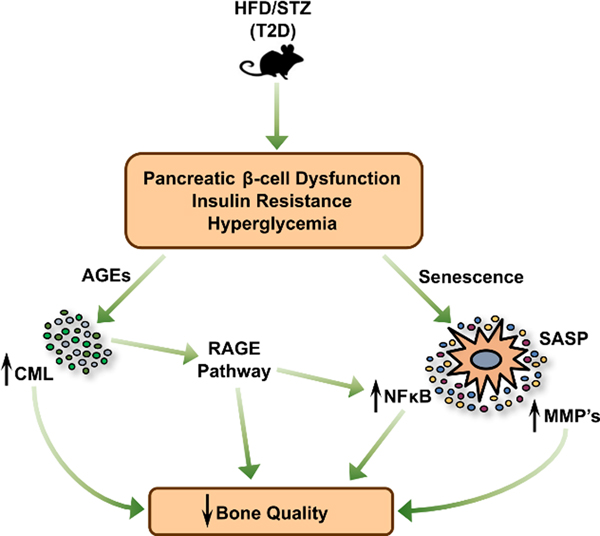
In addition to aging, the role of cellular senescence in the context of type 2 diabetes mellitus (T2DM) has been of scientific and clinical interest. It is well-known that T2DM is associated with multiple comorbidity risks in the aging population, with recent evidence also implicating bone as an additional tissue with substantial T2DM complications (Farr and Khosla, 2016). Although patients with T2DM often have increased bone mineral density (BMD) (Ma et al., 2012), they also exhibit deficits in bone material properties. These deficits appear to preferentially affect cortical bone mainly through increased cortical porosity and reductions in bone strength (Burghardt et al., 2010; Farr et al., 2014; Karim et al., 2018; Nilsson et al., 2017; Samelson et al., 2018; Yu et al., 2015). This is proposed to be through the accumulation of advanced glycation endproducts (AGEs), shown to lead to brittle bone due to reduced bone formation (Kume et al., 2005; Sanguineti et al., 2008) and abnormal collagen cross-linking (Tang et al., 2009), as well as subsequent activation of the AGE receptor (RAGE) signaling pathway in bone. In turn, RAGE signaling has been implicated in driving inflammation and senescence in multiple tissues (Liu et al., 2014; Shi et al., 2019), suggesting a possible link between diabetes and senescence. Therefore, our group recently investigated the effects of T2DM on bone quality, bone metabolism, and senescence using a mouse model system (Eckhardt et al., 2020) involving the use of high fat diet and streptozotocin (STZ) to induce classic features of T2DM in adult (post-pubertal) mice (Luo et al., 1998; Mu et al., 2009; Mu et al., 2006). We found that the induction of T2DM for 4 months decreased cortical volumetric BMD, reduced cortical thickness, and impaired bone material properties compared to non-diabetic control mice. These phenotypes were associated with increased bone resorption levels and an overall decrease in bone formation rates. Additionally, increased levels of the AGE Nε-(1-carboxymethyl)-l-lysine (CML) were found in both serum and bone of diabetic mice. Increases in the senescence markers p16Ink4a and p21Cip1, along with SASP markers predominantly related to NFκB signaling and matrix metalloproteinases (MMPs) were observed in osteocyte-enriched bone samples of T2DM mice. These increases in senescent biomarkers were also accompanied by increased SADS- and TAF-positive osteocytes in cortical bone. These findings support the hypothesis that T2DM can accelerate the senescent cell burden in the skeleton of young adult mice. Thus, it is possible that targeting senescent cells may serve as a therapy for T2DM-associated bone pathology. Based on these findings, Fig. 2 presents a proposed working model for the pathogenesis of skeletal fragility in T2DM, where increased RAGE signaling, accompanied by accelerated senescence which drives the diabetic SASP, together lead to increased inflammation and reduced bone quality.
Figure 2:
Proposed mechanism of senescence-mediated bone pathology in Type 2 Diabetes Mellitus (T2DM). Mice treated with a high-fat diet (HFD) and streptozotocin (STZ) develop classical symptoms of T2DM. In bone, increased advanced glycation endproducts (AGEs), such as CML, accumulate and contribute to AGE receptor (RAGE) pathway signaling. Additionally, accelerated senescence of osteocytes occurs in mice with T2DM. This is accompanied by a distinct senescence-associated secretory phenotype (SASP) consisting of upregulated NFκB- and matrix metalloproteinase (MMP)-pathway signaling. This senescent phenotype, together with AGEs accumulation and RAGE signaling, induce inflammation in the bone and are proposed to lead to reduced bone quality in T2DM. Reproduced from Eckhardt et al. (Eckhardt et al., 2020), with permission.
Senolytics for treatment of age-related bone loss
It has recently been demonstrated that removal of senescent cells has beneficial effects on the aged skeleton. This concept was first tested using the INK-ATTAC mouse model (p16INK4a – apoptosis through targeted activation of caspase) (Baker et al., 2016; Baker et al., 2011): a genetic model containing a “suicide” caspase transgene driven by the p16Ink4a promoter, which is activated in senescent cells. This allows for the selective clearance of p16Ink4a-positive cells through treatment with a synthetic compound (AP20187; AP), thereby clearing a subset of senescent cells without any known off-target effects on non-senescent cells. After AP treatment in aged (24-month-old) INK-ATTAC mice, in depth skeletal phenotyping revealed that genetic clearance of p16Ink4a-positive senescent cells had favorable effects on the skeleton. This was exemplified by the alleviation of trabecular and cortical bone loss. Further investigation revealed that mice displayed suppressed bone resorption (i.e., reduced osteoclast numbers) and increased osteoblast numbers on the endocortical surfaces of bone (Farr et al., 2017). As this genetic approach in INK-ATTAC mice is not one that is readily translatable to humans, comparable pharmacological studies in old mice were accomplished using senotherapeutics: treatments aimed at clearing or neutralizing the effects of senescent cells. More specifically, old mice were treated with drugs that either selectively kill senescent cells (“senolytics”), or drugs that inhibit the SASP (“senomorphics”). As an initial attempt to establish proof-of concept, old mice were treated intermittently with the first generation senolytic cocktail – dasatinib (D) plus quercetin (Q) – D+Q, which acts by inhibiting pro-survival mechanisms that senescent cells use to resist apoptosis (Zhu et al., 2015). Consistent with the genetic INK-ATTAC model, intermittent D+Q therapy eliminated senescent cells in bone and was sufficient to prevent age-related bone loss (Farr et al., 2017). These findings are supported by a recent study by Zhou et al. that uncovered that D+Q treatment can rescue the osteogenic potential of bone marrow stromal cells (BMSCs) isolated from old mice (Zhou et al., 2021). In addition to these senolytic approaches, old mice treated continuously (i.e., daily) with the senomorphic JAK inhibitor, ruxolitinib, were also protected against age-related bone loss predominantly mediated through anti-resorptive effects (Farr et al., 2017).
The use of either senolytic or senomorphic therapies appear to have comparable beneficial effects on age-related bone loss, although each treatment presents with its own limitations. One potential drawback of the senomorphic approach is the requirement for continuous dosing, whereas senolytic therapies are effective following intermittent “hit-and-run” treatments, which avoids potential off-target effects on non-senescent cells. One example of these effects was uncovered in a recent study by Sharma et al. (Sharma et al., 2020). This study found that daily treatment, rather than intermittent treatment, of old mice with another first-generation senolytic drug, ABT-263 (Navitoclax), actually caused the loss of trabecular bone, at least in part by impairing the functions of osteoprogenitors. Clearly, additional studies are needed to further establish the relative beneficial versus toxic effects of different senolytic compounds, and dosing regimens must be optimized to identify approaches that may prevent or delay age-related bone loss most effectively. Additionally, while these preclinical studies will be important, they may not necessarily translate to clinical applications until similar beneficial effects are observed in humans. Therefore, it will be important to conduct parallel clinical trials in humans to identify the optimal senolytic dosing regimens for the prevention or treatment of osteoporosis in the elderly population. Indeed, an initial clinical trial examining the effects of senolytic therapy on bone metabolism in older postmenopausal women is currently underway (ClinicalTrials.gov Identifier NCT04313634).
In addition to age-related bone loss, sex steroid deficiency (e.g., following the menopause) is another major cause of bone loss in humans (Riggs et al., 2002). In terms of the role of cellular senescence in mediating bone loss due to sex steroid deficiency, we should note that although others have found associations between estrogen and senescence in bone (Wei et al., 2021; Wu et al., 2020), we have tested and found that estrogen/androgen signaling and senescence pathways are independent of one another (Farr et al., 2019). Our work found that neither ovariectomy nor orchidectomy in mice led to any increase in senescence/SASP signaling. Additionally, estrogen treatment in elderly postmenopausal women did not lead to any changes in senescence markers in bone biopsies. Thus, additional studies are needed to address the possible role of cellular senescence in contributing to bone loss secondary to sex steroid deficiency.
Senolytics in bone diseases induced by cancer treatment
Along with age-related bone loss, bone pathologies resulting from cancer treatment appear to be attenuated following clearance of senescent cells. Cancer treatment often involves levels of radiation and chemotherapy that can lead to long-term effects on bone, such as premature bone loss and increased levels of senescent cells (Pacheco and Stock, 2013; Yaprak et al., 2018; Zhang et al., 2018). Focal radiation therapy (FRT) leads to DNA damage and cellular senescence in a myriad of cells of the bone microenvironment, including osteoblasts and osteocytes (Chandra et al., 2020). In an initial screen, individual senolytic treatments using Fisetin (a polyphenol shown to have senolytic properties (Yousefzadeh et al., 2018)), D, or Q did not alleviate radiation-induced bone loss in young adult mice. However, when treating with both D and Q, trabecular bone mass was preserved in irradiated femurs (Chandra et al., 2020). Additional analyses uncovered that D+Q treatment maintained functional osteoblasts and improved bone formation in irradiated bones. D+Q treatment reduced the senescent cell burden in the bone microenvironment after radiation, as observed by a reduction in TAF+ osteocytes, osteoblasts, and bone marrow cells, along with reduced expression of p21cip1, p16Ink4a, and numerous SASP markers (Chandra et al., 2020). Interestingly, the SASP profile in bone after radiation largely resembles that of aged bone, suggesting similar senescence mechanisms. This data reinforces the use of D+Q as an effective senolytic for bone tissue, and sheds light on its possible use for premature radiation-induced senescence in addition to chronic, accumulated senescence through aging.
Early data also suggests beneficial effects for senolytics in treating chemotherapy-induced bone loss. Typically used alongside radiation, chemotherapy is an effective treatment for targeting cancer cells, but also leads to bone loss, predicted to arise from systemic hormone imbalances (Cameron et al., 2010; Hadji et al., 2012; Saarto et al., 1997) and localized cellular senescence (Park et al., 2000; Robles and Adami, 1998; Wang et al., 1998). To test causality, Yao and colleagues cleared senescent cells in chemotherapy-treated mice and found beneficial effects (Yao et al., 2020). Mice treated with the chemotherapy drug doxorubicin (DOXO) exhibited upregulated expression of senescence markers in sorted bone resident cells (CD45-CD31−), and, to a lesser extent, in endothelial cells (CD45-CD31+), lymphocytes, and myeloid cells. Global clearance of p16Ink4a+ senescent cells using the INK-ATTAC mouse model alleviated chemotherapy-induced trabecular bone loss. This was shown to be through the reduction of osteoclasts and preservation of osteoblasts, leading to improved bone formation. Interestingly, senomorphic inhibition of the SASP using p38MAPK-MK2 pathway inhibitors also partially alleviated chemotherapy-induced bone loss, demonstrating another mechanism through which senescent cells can be targeted to prevent bone pathology.
Senolytics in bone regeneration
Depletion of senescent cells has also been found to have advantageous effects for bone regeneration. In a recent study, Honda et al. studied the accumulation of cells exhibiting stress-induced premature senescence (SIPS) during bone regeneration in a calvarial defect model in rats (Honda et al., 2020). Upon implanting lipopolysaccharide (LPS) sustained-release gelatin sponges (LS-G), they observed an increase in local inflammation and an accumulation of SIPS cells, which remained after the acute inflammatory response had subsided. Treatment of these animals with D+Q led to a reduction of SIPS in the bone defects, as measured by decreased expression of p21cip1, p16Ink4a, and SA-β-gal immunostaining. This effect was also accompanied by a dramatic upregulation of bone formation. Additionally, another cohort of rats were implanted with gelatin sponges that were chemically modified with epigallocatechin gallate (ECGC), a polyphenol isolated from green tea which has anti-inflammatory and antioxidant properties. These rats also displayed reductions in SIPS and had increased bone formation levels that were higher than D+Q-treated rats. This suggests that ECGC may have senotherapeutic properties advantageous for bone formation when administered locally. Overall, this study outlines an innovative use for senolytics to promote bone regeneration, which may be beneficial for the clinical healing of bone defects resulting from trauma, tumor resection, or other bone diseases. The discovery and application of novel senotherapeutics is a necessary arm of senolytic research in order to broaden approaches to clear or suppress the negative effects of senescent cells.
Future of senolytics in bone
The broader treatment of heterogeneous states of cellular senescence will be greatly facilitated by discovery of novel senolytics. As exemplified in the study by Chandra et al (Chandra et al., 2020), some senolytics that have beneficial effects in other tissues have no effect on radiation-induced bone loss (Fisetin), while others do (D+Q). This is likely due to the heterogeneity of senescent cells (Hernandez-Segura et al., 2017; Kirschner et al., 2020), which may lead to cell- and tissue-specific effects of different senolytic drugs. Therefore, the development of multiple senolytic approaches would allow for individualized approaches and broader efficacy for aged individuals. This workflow will rely on the following steps: (i) Identification of senolytics through in vitro screening, (ii) animal treatment with candidate drugs to evaluate senolytic efficacy and toxicity, and (iii) optimization of senolytic activity and reduction of toxicity to levels suitable for human trials. In addition to the effects reviewed above of the senolytic combination of D+Q on bone in pre-clinical models, a number of additional senolytic drugs are in development and are discussed briefly below.
One therapy undergoing this workflow is ABT-263, also known as Navitoclax: a BCL-2 inhibitor that has shown promising results as a senolytic in pre-clinical experiments. An early study by Zhu et al. identified ABT263 to have senolytic activity on cultured senescent cells from a number of tissues (Zhu et al., 2016). Additionally, Chang et al. identified that ABT-263 can clear senescent cells in bone marrow in both irradiated and aged mice (Chang et al., 2016). This led to an upregulation of clonogenicity of not only hematopoietic stem cells, but also muscle stem cells from neighboring tissue. Subsequently, Kim et al. showed that ABT-263 induced apoptosis in cultured BMSCs isolated from aged mice and led to a reduction in expression of SASP markers (Kim et al., 2017). Furthermore, another study screening candidate senolytics identified ABT-263 to have moderate selectivity for senescent human BMSCs (Grezella et al., 2018). However, this study also found that ABT-263 affects non-senescent cells, suggesting some toxicity or off-target effects. This has been reported by others, as ABT-263 treatment has been observed to have platelet toxicity and induce transient thrombocytopenia (Schoenwaelder et al., 2011). As mentioned above, cytotoxic effects of ABT-263 on bone tissue were observed in a recent study by Sharma and colleagues (Sharma et al., 2020). This study showed that ABT-263 treatment actually reduced bone mass, measured histologically, and led to impaired differentiation of osteoprogenitors isolated from these mice. As observed by others, in vitro treatment of BMSCs with ABT-263 led to clearance of senescent cells. However, the authors also observed cytotoxic effects, as well as reduced clonogenicity and impaired osteogenic capacity (Sharma et al., 2020). This is clear evidence that the current form of ABT-263 requires further refinement before proceeding to human trials as a candidate senolytic.
Due to the observed cytotoxic effects of ABT-263, several groups have chemically engineered optimized forms to reduce off-target effects and improve suitability as a potential senolytic. González-Gualda and colleagues engineered a “galacto-conjugated” form of Navitoclax that is selectively activated by the heightened SA-β-gal activity in senescent cells (Gonzalez-Gualda et al., 2020). This led to higher selectivity in the killing of senescent cells with limited apoptosis of non-senescent cells. In another study, He et al. used proteolysis-targeting chimera (PROTAC) technology to reduce thrombocytopenia caused by ABT-263 by essentially limiting its targeting of Bcl-xL in platelets, which is essential for their survival (He et al., 2020). This new compound, termed PZ15227, was shown to have higher senolytic ability than ABT-263 without causing thrombocytopenia in old mice. Additionally, treatment of BMSCs isolated from old mice with PZ15227 led to reduced senescence and improved osteogenesis. In contrast to the previously mentioned study by Sharma et al. (Sharma et al., 2020), this group also observed beneficial effects on osteogenesis of BMSCs after ABT-263 treatment, indicating some uncertainty as to its effects on osteoblast differentiation. Recently, it was shown that in vivo treatment of old mice with ABT-263, as well as the newly developed PZ15227, led to reduced protein expression of p16, γ-H2AX, and GATA4, as well as RANKL in bone tissue. As RANKL is known to be produced by osteoblasts and osteocytes, the reduction in RANKL suggests that senescent osteogenic cells are cleared with this treatment, which would presumably lead to reduced osteoclast-mediated resorption through interrupted RANKL-RANK signaling. However, the bone phenotype in these mice was not reported, so the senolytic effects of ABT-263 or PZ15227 on bone in vivo remain unclear. Thus, although there are reported negative effects of ABT-263 on bone tissue, the development of these optimized senolytics may have fewer toxic effects and could be used to treat skeletal pathologies if beneficial effects are found in future pre-clinical studies.
Fisetin, an antioxidant found to have senolytic effects in other tissues, may have positive effects in bone as well. This compound was originally identified in a screening of candidate senolytics that revealed reductions in senescence markers in multiple tissues and cell types after treatment with Fisetin (Yousefzadeh et al., 2018). Previous work in bone tissue uncovered a role for Fisetin in maintaining bone mass through the downregulation of osteoclastogenesis through the suppression of NFκB signaling (Leotoing et al., 2013). However, the mice in this study were only 8 weeks of age, leaving its role in age-related senescence unclear. As mentioned above, Fisetin had no observable effects in ameliorating radiation-induced bone loss (Chandra et al., 2020). Interestingly, a recent study using a mouse model of accelerated aging (Zmpste24−/−) demonstrated that Fisetin treatment led to improved BMD (Hambright et al., 2020), suggesting beneficial effects on age-related bone loss. Although compelling, the overall role of Fisetin in bone is somewhat unclear. The lack of effect in irradiated bones, along with the positive effects on bone in young mice suggest a senescence-independent effect. Additionally, as Fisetin was administered weekly in the studies showing beneficial effects, and every other week in the radiation study, more frequent dosing may be required for Fisetin to execute its senotherapeutic effects in bone. Further investigation into the role of Fisetin in age-related bone loss is required to uncover if Fisetin executes its beneficial skeletal effects through senescence-related or senescence-independent mechanisms, or perhaps both.
Another potential candidate senolytic for bone is the hormone irisin. In a preliminary study, Colaianni et al. demonstrated that irisin, a myokine released from skeletal muscle after physical exercise, regulates bone mass (Colaianni et al., 2015), and treatment with irisin prevents bone loss and muscle wasting after unloading (Colaianni et al., 2017). In humans, serum levels of irisin correlate positively with BMD and negatively with age (Colaianni et al., 2021). Additionally, irisin levels are lower in patients with osteopenia or osteoporosis (BMD T score ≤ −1) compared to healthy matched control subjects. Interestingly, this reduction in irisin levels is observed alongside an increase in p21cip1 mRNA expression in bone biopsies. Strikingly, when treated in culture with irisin, both mouse and human osteoblasts exhibited a reduction in p21cip1 expression. However, these data are quite preliminary, as the senescent phenotyping was limited, and no effects on other senescent or SASP genes was shown. Nevertheless, these findings provide intriguing evidence for irisin as a possible senolytic and reinforces the powerful role of crosstalk between muscle and bone during aging. As irisin already has other recently established roles in weight loss, glucose homeostasis, and thermoregulation (Bostrom et al., 2012; Calton et al., 2016; Maalouf and El Khoury, 2019; Polyzos et al., 2018; So et al., 2014), its use as an anti-aging treatment in elderly populations has tremendous potential. Table 1 summarizes current candidate senolytics/senomorphics that are being investigated for the treatment of age-related and other forms of bone loss.
Table 1:
Candidate senolytics/senomorphics for the treatment of bone loss
| Agent(s) | Type | Cellular Effects | Tissue-level effects | References |
|---|---|---|---|---|
| Dasatinib + Quercetin (D+Q) | Senolytic | ↓ Senescent osteocytes ↓ Senescent BMSCs ↓ Osteoclasts ↑ Osteoblasts |
↑ Femur cortical bone mass ↑ Femur/spine trabecular bone mass (aging & radiation) |
(Chandra et al., 2020; Farr et al., 2017; Zhou et al., 2021; Zhu et al., 2015) |
| Ruxolitinib (JAKi) | Senomorphic | ↓ Osteoclastogenesis | ↑ Femur/spine trabecular bone mass (aging) | (Farr et al., 2017; Xu et al., 2015) |
| Navitoclax (ABT- 263) | Senolytic | ↓ Senescent BMSCs & osteoprogenitors | ↓ Tibia trabecular bone mass (aging) | (Grezella et al., 2018; Kim et al., 2017; Zhu et al., 2016) |
| PZ15227 (modified ABT-263) | Senolytic | ↓ Senescent BMSCs, ↑ Osteogenesis |
Not reported | (He et al., 2020; Kim et al., 2020) |
| Fisetin | Senolytic | ↓ Osteoclastogenesis | ↑ BMD (accelerated aging mouse model) | (Hambright et al., 2020; Leotoing et al., 2013) |
| Irisin | Unknown | ↓ p21 expression in osteoblasts | ↑ BMD and cortical bone mass (young) Correlates with BMD (+) and age (−) in humans | (Colaianni et al., 2015; Colaianni et al., 2021; Colaianni et al., 2017) |
Potential role of senescent cells in tissue repair
Although much effort has focused on the removal of senescent cells to help prevent tissue dysfunction, cellular senescence and the SASP has also been shown to play important roles in other contexts. For example, one of the long-known beneficial roles of cellular senescence is as an anti-cancer mechanism to prevent malignant cell transformation by withdrawing pre-cancerous cells from the cell cycle (Sharpless et al., 2001). More recently, a surprising role of cellular senescence was revealed in the mammalian embryo as mice deficient in p21cip1 had defective embryonic development, tissue growth, and patterning (Storer et al., 2013). Furthermore, this p21cip1-mediated role of senescent cells in embryonic patterning was shown to mechanistically depend on the TGF-β and PI3K pathways (Munoz-Espin et al., 2013). After performing their physiological functions, these acute senescent cells are cleared by infiltrating macrophages (Munoz-Espin et al., 2013; Storer et al., 2013). Another important physiological process in which senescent cells play a crucial acute role is in the context of wound healing. In a study by Demaria et al., senescent endothelial cells and fibroblasts appeared at the site of a cutaneous skin wound where, via their SASP and more specifically platelet-derived growth factor AA (PDGF-AA), they enhanced myofibroblast differentiation to accelerate wound closure (Demaria et al., 2014). Acute senescence also has a role following wound closure where, for example, myofibroblasts can undergo cellular senescence to limit excessive fibrosis at the site of an injury or in response to trauma (Jun and Lau, 2010). Additionally, acutely senescent myofibroblasts can have a beneficial role in repairing damaged organs. In the liver, myofibroblasts are primarily derived from activated hepatic stellate cells that initially proliferate in response to organ damage. These myofibroblasts then develop a senescent phenotype to limit liver fibrosis, following which they are cleared by natural killer immune cells (Krizhanovsky et al., 2008). Similarly, premature senescence of myofibroblasts has been identified as an essential antifibrotic mechanism in the context of limiting myocardial fibrosis (Meyer et al., 2016). Thus, the senescence program is activated in these circumstances to curb the fibrogenic responses in liver damage and heart disease, thereby identifying cellular senescence as a potential mechanistic stimulator to limit excess fibrosis. Collectively, these beneficial effects of senescent cells in natural physiological processes may explain, at least in part, why the SASP evolved to promote organismal fitness earlier in life without regard for the antagonistic pleiotropic effects of senescence and the SASP later in life when it causes detrimental age-associated tissue and organ functional decline.
The role of senescent cells in wound healing may provide analogous insights into the role of senescence in fracture healing. Fractures in the elderly are associated with high morbidity and mortality (Cauley et al., 2000), while fracture healing is impaired with age due to a number of causes including, but not limited to, inflammation, reduced angiogenesis, and impaired differentiation of bone stem cells (Clark et al., 2017; Nieminen et al., 1981). However, the role of cellular senescence in this process is currently unclear. Perhaps similar to cutaneous wound healing, senescent cells may be beneficial in the repair of fractured bone tissue. On the other hand, bone healing is substantially different from skin healing, and clearance of senescent cells may, in fact, improve fracture healing and bone union. Future studies directed at understanding the role of senescent cells in bone healing may provide useful insights into the possible use of senolytic treatments for osteoporotic patients suffering from fragility fractures.
Conclusions
The use of senolytics for bone pathologies presents a new wave of promising therapies for osteoporosis and other bone diseases. Clearance of senescent cells has positive effects on bone at least in part through the clearance of senescent osteoprogenitors and osteocytes, although the role of other senescent cells (e.g., immune cells) in the bone microenvironment remains to be defined. Moreover, the systemic effects of senolytics on other organ systems may provide a more holistic approach to preventing fractures in the elderly; improvements in muscle function, cognition, and metabolism may reduce risks of falling due to sarcopenia, reduced mobility, and impaired balance. Although encouraging, there is still much to investigate with regards to the off-target effects of current candidate senolytics, which may be mitigated via hit-and-run administration, as well as potential unknown beneficial roles of senescent cells in bone physiology that may be negatively affected by senolytic treatment. Therefore, rigorous clinical trials will be necessary to evaluate efficacy and safety before widespread applications in humans for bone loss can be considered. Until then, the research field will continue to pursue this propitious avenue of senotherapeutics to uncover and optimize novel treatments that may modulate foundational aging mechanisms in bone.
Highlights:
Senescent cells accumulate in bone with aging and systemic pathologies
Clearance of senescent cells alleviates bone loss in old mice
Novel senolytics are being discovered and tested in bone
Acknowledgements
Supported by NIH grants P01 AG062413 (SK, DGM, JNF), R21 AG065868 (JNF, SK), R01 AG063707 (DGM) and R01 DK128552 (JNF). The graphical abstract was created with BioRender.com
Footnotes
Disclosure Summary: The authors have nothing to disclose
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- Baker DJ, Childs BG, Durik M, Wijers ME, Sieben CJ, Zhong J, Saltness RA, Jeganathan KB, Verzosa GC, Pezeshki A, Khazaie K, Miller JD, van Deursen JM, 2016. Naturally occurring p16(Ink4a)-positive cells shorten healthy lifespan. Nature 530, 184–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker DJ, Wijshake T, Tchkonia T, LeBrasseur NK, Childs BG, van de Sluis B, Kirkland JL, van Deursen JM, 2011. Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature 479, 232–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bostrom P, Wu J, Jedrychowski MP, Korde A, Ye L, Lo JC, Rasbach KA, Bostrom EA, Choi JH, Long JZ, Kajimura S, Zingaretti MC, Vind BF, Tu H, Cinti S, Hojlund K, Gygi SP, Spiegelman BM, 2012. A PGC1-alpha-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature 481, 463–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burghardt AJ, Issever AS, Schwartz AV, Davis KA, Masharani U, Majumdar S, Link TM, 2010. High-resolution peripheral quantitative computed tomographic imaging of cortical and trabecular bone microarchitecture in patients with type 2 diabetes mellitus. J Clin Endocrinol Metab 95, 5045–5055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calton EK, Soares MJ, James AP, Woodman RJ, 2016. The potential role of irisin in the thermoregulatory responses to mild cold exposure in adults. Am J Hum Biol 28, 699–704. [DOI] [PubMed] [Google Scholar]
- Cameron DA, Douglas S, Brown JE, Anderson RA, 2010. Bone mineral density loss during adjuvant chemotherapy in pre-menopausal women with early breast cancer: is it dependent on oestrogen deficiency? Breast Cancer Res Treat 123, 805–814. [DOI] [PubMed] [Google Scholar]
- Cauley JA, Thompson DE, Ensrud KC, Scott JC, Black D, 2000. Risk of mortality following clinical fractures. Osteoporos Int 11, 556–561. [DOI] [PubMed] [Google Scholar]
- Cauley JA, Wampler NS, Barnhart JM, Wu L, Allison M, Chen Z, Hendrix S, Robbins J, Jackson RD, Women’s Health Initiative Observational, S., 2008. Incidence of fractures compared to cardiovascular disease and breast cancer: the Women’s Health Initiative Observational Study. Osteoporos Int 19, 1717–1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandra A, Lagnado AB, Farr JN, Monroe DG, Park S, Hachfeld C, Tchkonia T, Kirkland JL, Khosla S, Passos JF, Pignolo RJ, 2020. Targeted Reduction of Senescent Cell Burden Alleviates Focal Radiotherapy-Related Bone Loss. J Bone Miner Res 35, 1119–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang J, Wang Y, Shao L, Laberge RM, Demaria M, Campisi J, Janakiraman K, Sharpless NE, Ding S, Feng W, Luo Y, Wang X, Aykin-Burns N, Krager K, Ponnappan U, Hauer-Jensen M, Meng A, Zhou D, 2016. Clearance of senescent cells by ABT263 rejuvenates aged hematopoietic stem cells in mice. Nat Med 22, 78–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark D, Nakamura M, Miclau T, Marcucio R, 2017. Effects of Aging on Fracture Healing. Curr Osteoporos Rep 15, 601–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colaianni G, Cuscito C, Mongelli T, Pignataro P, Buccoliero C, Liu P, Lu P, Sartini L, Di Comite M, Mori G, Di Benedetto A, Brunetti G, Yuen T, Sun L, Reseland JE, Colucci S, New MI, Zaidi M, Cinti S, Grano M, 2015. The myokine irisin increases cortical bone mass. Proc Natl Acad Sci U S A 112, 12157–12162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colaianni G, Errede M, Sanesi L, Notarnicola A, Celi M, Zerlotin R, Storlino G, Pignataro P, Oranger A, Pesce V, Tarantino U, Moretti B, Grano M, 2021. Irisin Correlates Positively With BMD in a Cohort of Older Adult Patients and Downregulates the Senescent Marker p21 in Osteoblasts. J Bone Miner Res 36, 305–314. [DOI] [PubMed] [Google Scholar]
- Colaianni G, Mongelli T, Cuscito C, Pignataro P, Lippo L, Spiro G, Notarnicola A, Severi I, Passeri G, Mori G, Brunetti G, Moretti B, Tarantino U, Colucci SC, Reseland JE, Vettor R, Cinti S, Grano M, 2017. Irisin prevents and restores bone loss and muscle atrophy in hind-limb suspended mice. Sci Rep 7, 2811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demaria M, Ohtani N, Youssef SA, Rodier F, Toussaint W, Mitchell JR, Laberge RM, Vijg J, Van Steeg H, Dolle ME, Hoeijmakers JH, de Bruin A, Hara E, Campisi J, 2014. An essential role for senescent cells in optimal wound healing through secretion of PDGF-AA. Dev Cell 31, 722–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennison EM, Compston JE, Flahive J, Siris ES, Gehlbach SH, Adachi JD, Boonen S, Chapurlat R, Diez-Perez A, Anderson FA Jr., Hooven FH, LaCroix AZ, Lindsay R, Netelenbos JC, Pfeilschifter J, Rossini M, Roux C, Saag KG, Sambrook P, Silverman S, Watts NB, Greenspan SL, Premaor M, Cooper C, Investigators G, 2012. Effect of co-morbidities on fracture risk: findings from the Global Longitudinal Study of Osteoporosis in Women (GLOW). Bone 50, 1288–1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckhardt BA, Rowsey JL, Thicke BS, Fraser DG, O’Grady KL, Bondar OP, Hines JM, Singh RJ, Thoreson AR, Rakshit K, Lagnado AB, Passos JF, Vella A, Matveyenko AV, Khosla S, Monroe DG, Farr JN, 2020. Accelerated osteocyte senescence and skeletal fragility in mice with type 2 diabetes. JCI Insight 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farr JN, Drake MT, Amin S, Melton LJ 3rd, McCready LK, Khosla S, 2014. In vivo assessment of bone quality in postmenopausal women with type 2 diabetes. J Bone Miner Res 29, 787–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farr JN, Fraser DG, Wang H, Jaehn K, Ogrodnik MB, Weivoda MM, Drake MT, Tchkonia T, LeBrasseur NK, Kirkland JL, Bonewald LF, Pignolo RJ, Monroe DG, Khosla S, 2016. Identification of Senescent Cells in the Bone Microenvironment. J Bone Miner Res 31, 1920–1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farr JN, Khosla S, 2016. Determinants of bone strength and quality in diabetes mellitus in humans. Bone 82, 28–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farr JN, Rowsey JL, Eckhardt BA, Thicke BS, Fraser DG, Tchkonia T, Kirkland JL, Monroe DG, Khosla S, 2019. Independent Roles of Estrogen Deficiency and Cellular Senescence in the Pathogenesis of Osteoporosis: Evidence in Young Adult Mice and Older Humans. J Bone Miner Res 34, 1407–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farr JN, Xu M, Weivoda MM, Monroe DG, Fraser DG, Onken JL, Negley BA, Sfeir JG, Ogrodnik MB, Hachfeld CM, LeBrasseur NK, Drake MT, Pignolo RJ, Pirtskhalava T, Tchkonia T, Oursler MJ, Kirkland JL, Khosla S, 2017. Targeting cellular senescence prevents age-related bone loss in mice. Nat Med 23, 1072–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Gualda E, Paez-Ribes M, Lozano-Torres B, Macias D, Wilson JR 3rd, Gonzalez-Lopez C, Ou HL, Miron-Barroso S, Zhang Z, Lerida-Viso A, Blandez JF, Bernardos A, Sancenon F, Rovira M, Fruk L, Martins CP, Serrano M, Doherty GJ, Martinez-Manez R, Munoz-Espin D, 2020. Galacto-conjugation of Navitoclax as an efficient strategy to increase senolytic specificity and reduce platelet toxicity. Aging Cell 19, e13142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grezella C, Fernandez-Rebollo E, Franzen J, Ventura Ferreira MS, Beier F, Wagner W, 2018. Effects of senolytic drugs on human mesenchymal stromal cells. Stem Cell Res Ther 9, 108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gullberg B, Johnell O, Kanis JA, 1997. World-wide projections for hip fracture. Osteoporos Int 7, 407–413. [DOI] [PubMed] [Google Scholar]
- Hadji P, Gnant M, Body JJ, Bundred NJ, Brufsky A, Coleman RE, Guise TA, Lipton A, Aapro MS, 2012. Cancer treatment-induced bone loss in premenopausal women: a need for therapeutic intervention? Cancer Treat Rev 38, 798–806. [DOI] [PubMed] [Google Scholar]
- Hambright S, Kawakami Y, Mu X, Gao X, Lu A, Kirkland J, Huard J, 2020. The senolytic drug fisetin mitigates age-related bone density loss in the progeroid mouse model Zmpste24−/−. The FASEB Journal 34, 1–1. [Google Scholar]
- He Y, Zhang X, Chang J, Kim HN, Zhang P, Wang Y, Khan S, Liu X, Zhang X, Lv D, Song L, Li W, Thummuri D, Yuan Y, Wiegand JS, Ortiz YT, Budamagunta V, Elisseeff JH, Campisi J, Almeida M, Zheng G, Zhou D, 2020. Using proteolysis-targeting chimera technology to reduce navitoclax platelet toxicity and improve its senolytic activity. Nat Commun 11, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez-Segura A, de Jong TV, Melov S, Guryev V, Campisi J, Demaria M, 2017. Unmasking Transcriptional Heterogeneity in Senescent Cells. Curr Biol 27, 2652–2660.e2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda Y, Huang A, Tanaka T, Han X, Gao B, Liu H, Wang X, Zhao J, Hashimoto Y, Yamamoto K, Matsumoto N, Baba S, Umeda M, 2020. Augmentation of Bone Regeneration by Depletion of Stress-Induced Senescent Cells Using Catechin and Senolytics. Int J Mol Sci 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnell O, Kanis JA, 2006. An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos Int 17, 1726–1733. [DOI] [PubMed] [Google Scholar]
- Jun JI, Lau LF, 2010. The matricellular protein CCN1 induces fibroblast senescence and restricts fibrosis in cutaneous wound healing. Nat Cell Biol 12, 676–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karim L, Moulton J, Van Vliet M, Velie K, Robbins A, Malekipour F, Abdeen A, Ayres D, Bouxsein ML, 2018. Bone microarchitecture, biomechanical properties, and advanced glycation end-products in the proximal femur of adults with type 2 diabetes. Bone 114, 32–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khosla S, Cauley JA, Compston J, Kiel DP, Rosen C, Saag KG, Shane E, 2017. Addressing the Crisis in the Treatment of Osteoporosis: A Path Forward. J Bone Miner Res 32, 424–430. [DOI] [PubMed] [Google Scholar]
- Khosla S, Farr JN, Tchkonia T, Kirkland JL, 2020. The role of cellular senescence in ageing and endocrine disease. Nat Rev Endocrinol 16, 263–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khosla S, Hofbauer LC, 2017. Osteoporosis treatment: recent developments and ongoing challenges. Lancet Diabetes Endocrinol 5, 898–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HN, Chang J, Shao L, Han L, Iyer S, Manolagas SC, O’Brien CA, Jilka RL, Zhou D, Almeida M, 2017. DNA damage and senescence in osteoprogenitors expressing Osx1 may cause their decrease with age. Aging Cell 16, 693–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HN, Xiong J, MacLeod RS, Iyer S, Fujiwara Y, Cawley KM, Han L, He Y, Thostenson JD, Ferreira E, Jilka RL, Zhou D, Almeida M, O’Brien CA, 2020. Osteocyte RANKL is required for cortical bone loss with age and is induced by senescence. JCI Insight 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SC, Kim DH, Mogun H, Eddings W, Polinski JM, Franklin JM, Solomon DH, 2016. Impact of the U.S. Food and Drug Administration’s Safety-Related Announcements on the Use of Bisphosphonates After Hip Fracture. J Bone Miner Res 31, 1536–1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschner K, Rattanavirotkul N, Quince MF, Chandra T, 2020. Functional heterogeneity in senescence. Biochem Soc Trans 48, 765–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krizhanovsky V, Yon M, Dickins RA, Hearn S, Simon J, Miething C, Yee H, Zender L, Lowe SW, 2008. Senescence of activated stellate cells limits liver fibrosis. Cell 134, 657–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kume S, Kato S, Yamagishi S, Inagaki Y, Ueda S, Arima N, Okawa T, Kojiro M, Nagata K, 2005. Advanced glycation end-products attenuate human mesenchymal stem cells and prevent cognate differentiation into adipose tissue, cartilage, and bone. J Bone Miner Res 20, 1647–1658. [DOI] [PubMed] [Google Scholar]
- Leotoing L, Wauquier F, Guicheux J, Miot-Noirault E, Wittrant Y, Coxam V, 2013. The polyphenol fisetin protects bone by repressing NF-kappaB and MKP-1-dependent signaling pathways in osteoclasts. PLoS One 8, e68388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy HB, 2017. Polypharmacy Reduction Strategies: Tips on Incorporating American Geriatrics Society Beers and Screening Tool of Older People’s Prescriptions Criteria. Clin Geriatr Med 33, 177–187. [DOI] [PubMed] [Google Scholar]
- Liu J, Huang K, Cai GY, Chen XM, Yang JR, Lin LR, Yang J, Huo BG, Zhan J, He YN, 2014. Receptor for advanced glycation end-products promotes premature senescence of proximal tubular epithelial cells via activation of endoplasmic reticulum stress-dependent p21 signaling. Cell Signal 26, 110–121. [DOI] [PubMed] [Google Scholar]
- Luo J, Quan J, Tsai J, Hobensack CK, Sullivan C, Hector R, Reaven GM, 1998. Nongenetic mouse models of non-insulin-dependent diabetes mellitus. Metabolism 47, 663–668. [DOI] [PubMed] [Google Scholar]
- Ma L, Oei L, Jiang L, Estrada K, Chen H, Wang Z, Yu Q, Zillikens MC, Gao X, Rivadeneira F, 2012. Association between bone mineral density and type 2 diabetes mellitus: a meta-analysis of observational studies. Eur J Epidemiol 27, 319–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maalouf GE, El Khoury D, 2019. Exercise-Induced Irisin, the Fat Browning Myokine, as a Potential Anticancer Agent. J Obes 2019, 6561726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer K, Hodwin B, Ramanujam D, Engelhardt S, Sarikas A, 2016. Essential Role for Premature Senescence of Myofibroblasts in Myocardial Fibrosis. J Am Coll Cardiol 67, 2018–2028. [DOI] [PubMed] [Google Scholar]
- Mirza F, Canalis E, 2015. Management of endocrine disease: Secondary osteoporosis: pathophysiology and management. Eur J Endocrinol 173, R131–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu J, Petrov A, Eiermann GJ, Woods J, Zhou YP, Li Z, Zycband E, Feng Y, Zhu L, Roy RS, Howard AD, Li C, Thornberry NA, Zhang BB, 2009. Inhibition of DPP-4 with sitagliptin improves glycemic control and restores islet cell mass and function in a rodent model of type 2 diabetes. Eur J Pharmacol 623, 148–154. [DOI] [PubMed] [Google Scholar]
- Mu J, Woods J, Zhou YP, Roy RS, Li Z, Zycband E, Feng Y, Zhu L, Li C, Howard AD, Moller DE, Thornberry NA, Zhang BB, 2006. Chronic inhibition of dipeptidyl peptidase-4 with a sitagliptin analog preserves pancreatic beta-cell mass and function in a rodent model of type 2 diabetes. Diabetes 55, 1695–1704. [DOI] [PubMed] [Google Scholar]
- Munoz-Espin D, Canamero M, Maraver A, Gomez-Lopez G, Contreras J, Murillo-Cuesta S, Rodriguez-Baeza A, Varela-Nieto I, Ruberte J, Collado M, Serrano M, 2013. Programmed cell senescence during mammalian embryonic development. Cell 155, 1104–1118. [DOI] [PubMed] [Google Scholar]
- Nieminen S, Nurmi M, Satokari K, 1981. Healing of femoral neck fractures; influence of fracture reduction and age. Ann Chir Gynaecol 70, 26–31. [PubMed] [Google Scholar]
- Nilsson AG, Sundh D, Johansson L, Nilsson M, Mellstrom D, Rudang R, Zoulakis M, Wallander M, Darelid A, Lorentzon M, 2017. Type 2 Diabetes Mellitus Is Associated With Better Bone Microarchitecture But Lower Bone Material Strength and Poorer Physical Function in Elderly Women: A Population-Based Study. J Bone Miner Res 32, 1062–1071. [DOI] [PubMed] [Google Scholar]
- Pacheco R, Stock H, 2013. Effects of radiation on bone. Curr Osteoporos Rep 11, 299–304. [DOI] [PubMed] [Google Scholar]
- Park JI, Jeong JS, Han JY, Kim DI, Gao YH, Park SC, Rodgers GP, Kim IH, 2000. Hydroxyurea induces a senescence-like change of K562 human erythroleukemia cell. J Cancer Res Clin Oncol 126, 455–460. [PubMed] [Google Scholar]
- Piemontese M, Almeida M, Robling AG, Kim HN, Xiong J, Thostenson JD, Weinstein RS, Manolagas SC, O’Brien CA, Jilka RL, 2017. Old age causes de novo intracortical bone remodeling and porosity in mice. JCI Insight 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polyzos SA, Anastasilakis AD, Efstathiadou ZA, Makras P, Perakakis N, Kountouras J, Mantzoros CS, 2018. Irisin in metabolic diseases. Endocrine 59, 260–274. [DOI] [PubMed] [Google Scholar]
- Riggs BL, Khosla S, Melton LJ 3rd, 2002. Sex steroids and the construction and conservation of the adult skeleton. Endocr Rev 23, 279–302. [DOI] [PubMed] [Google Scholar]
- Robles SJ, Adami GR, 1998. Agents that cause DNA double strand breaks lead to p16INK4a enrichment and the premature senescence of normal fibroblasts. Oncogene 16, 1113–1123. [DOI] [PubMed] [Google Scholar]
- Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SA, Howard BV, Johnson KC, Kotchen JM, Ockene J, Writing Group for the Women’s Health Initiative I, 2002. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women’s Health Initiative randomized controlled trial. JAMA 288, 321–333. [DOI] [PubMed] [Google Scholar]
- Saarto T, Blomqvist C, Valimaki M, Makela P, Sarna S, Elomaa I, 1997. Chemical castration induced by adjuvant cyclophosphamide, methotrexate, and fluorouracil chemotherapy causes rapid bone loss that is reduced by clodronate: a randomized study in premenopausal breast cancer patients. J Clin Oncol 15, 1341–1347. [DOI] [PubMed] [Google Scholar]
- Samelson EJ, Demissie S, Cupples LA, Zhang X, Xu H, Liu CT, Boyd SK, McLean RR, Broe KE, Kiel DP, Bouxsein ML, 2018. Diabetes and Deficits in Cortical Bone Density, Microarchitecture, and Bone Size: Framingham HR-pQCT Study. J Bone Miner Res 33, 54–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanguineti R, Storace D, Monacelli F, Federici A, Odetti P, 2008. Pentosidine effects on human osteoblasts in vitro. Ann N Y Acad Sci 1126, 166–172. [DOI] [PubMed] [Google Scholar]
- Schoenwaelder SM, Jarman KE, Gardiner EE, Hua M, Qiao J, White MJ, Josefsson EC, Alwis I, Ono A, Willcox A, Andrews RK, Mason KD, Salem HH, Huang DC, Kile BT, Roberts AW, Jackson SP, 2011. Bcl-xL-inhibitory BH3 mimetics can induce a transient thrombocytopathy that undermines the hemostatic function of platelets. Blood 118, 1663–1674. [DOI] [PubMed] [Google Scholar]
- Sharma AK, Roberts RL, Benson RD Jr., Pierce JL, Yu K, Hamrick MW, McGee-Lawrence ME, 2020. The Senolytic Drug Navitoclax (ABT-263) Causes Trabecular Bone Loss and Impaired Osteoprogenitor Function in Aged Mice. Front Cell Dev Biol 8, 354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpless NE, Bardeesy N, Lee KH, Carrasco D, Castrillon DH, Aguirre AJ, Wu EA, Horner JW, DePinho RA, 2001. Loss of p16Ink4a with retention of p19Arf predisposes mice to tumorigenesis. Nature 413, 86–91. [DOI] [PubMed] [Google Scholar]
- Shi M, Yang S, Zhu X, Sun D, Sun D, Jiang X, Zhang C, Wang L, 2019. The RAGE/STAT5/autophagy axis regulates senescence in mesangial cells. Cell Signal 62, 109334. [DOI] [PubMed] [Google Scholar]
- So B, Kim HJ, Kim J, Song W, 2014. Exercise-induced myokines in health and metabolic diseases. Integr Med Res 3, 172–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storer M, Mas A, Robert-Moreno A, Pecoraro M, Ortells MC, Di Giacomo V, Yosef R, Pilpel N, Krizhanovsky V, Sharpe J, Keyes WM, 2013. Senescence is a developmental mechanism that contributes to embryonic growth and patterning. Cell 155, 1119–1130. [DOI] [PubMed] [Google Scholar]
- Swanson EC, Manning B, Zhang H, Lawrence JB, 2013. Higher-order unfolding of satellite heterochromatin is a consistent and early event in cell senescence. J Cell Biol 203, 929–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang SY, Allen MR, Phipps R, Burr DB, Vashishth D, 2009. Changes in non-enzymatic glycation and its association with altered mechanical properties following 1-year treatment with risedronate or alendronate. Osteoporos Int 20, 887–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tchkonia T, Zhu Y, van Deursen J, Campisi J, Kirkland JL, 2013. Cellular senescence and the senescent secretory phenotype: therapeutic opportunities. J Clin Invest 123, 966–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Chen Q, Lee SH, Choi Y, Johnson FB, Pignolo RJ, 2012. Impairment of osteoblast differentiation due to proliferation-independent telomere dysfunction in mouse models of accelerated aging. Aging Cell 11, 704–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Wong SC, Pan J, Tsao SW, Fung KH, Kwong DL, Sham JS, Nicholls JM, 1998. Evidence of cisplatin-induced senescent-like growth arrest in nasopharyngeal carcinoma cells. Cancer Res 58, 5019–5022. [PubMed] [Google Scholar]
- Wei Y, Fu J, Wu W, Ma P, Ren L, Wu J, 2021. Estrogen prevents cellular senescence and bone loss through Usp10-dependent p53 degradation in osteocytes and osteoblasts: the role of estrogen in bone cell senescence. Cell Tissue Res. [DOI] [PubMed] [Google Scholar]
- Wu W, Fu J, Gu Y, Wei Y, Ma P, Wu J, 2020. JAK2/STAT3 regulates estrogen-related senescence of bone marrow stem cells. J Endocrinol 245, 141–153. [DOI] [PubMed] [Google Scholar]
- Xu M, Palmer AK, Ding H, Weivoda MM, Pirtskhalava T, White TA, Sepe A, Johnson KO, Stout MB, Giorgadze N, Jensen MD, LeBrasseur NK, Tchkonia T, Kirkland JL, 2015. Targeting senescent cells enhances adipogenesis and metabolic function in old age. Elife 4, e12997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Z, Murali B, Ren Q, Luo X, Faget DV, Cole T, Ricci B, Thotala D, Monahan J, van Deursen JM, Baker D, Faccio R, Schwarz JK, Stewart SA, 2020. Therapy-Induced Senescence Drives Bone Loss. Cancer Res 80, 1171–1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaprak G, Gemici C, Temizkan S, Ozdemir S, Dogan BC, Seseogullari OO, 2018. Osteoporosis development and vertebral fractures after abdominal irradiation in patients with gastric cancer. BMC Cancer 18, 972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yousefzadeh MJ, Zhu Y, McGowan SJ, Angelini L, Fuhrmann-Stroissnigg H, Xu M, Ling YY, Melos KI, Pirtskhalava T, Inman CL, McGuckian C, Wade EA, Kato JI, Grassi D, Wentworth M, Burd CE, Arriaga EA, Ladiges WL, Tchkonia T, Kirkland JL, Robbins PD, Niedernhofer LJ, 2018. Fisetin is a senotherapeutic that extends health and lifespan. EBioMedicine 36, 18–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu EW, Putman MS, Derrico N, Abrishamanian-Garcia G, Finkelstein JS, Bouxsein ML, 2015. Defects in cortical microarchitecture among African-American women with type 2 diabetes. Osteoporos Int 26, 673–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Qiu X, Xi K, Hu W, Pei H, Nie J, Wang Z, Ding J, Shang P, Li B, Zhou G, 2018. Therapeutic ionizing radiation induced bone loss: a review of in vivo and in vitro findings. Connect Tissue Res 59, 509–522. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Xin X, Wang L, Wang B, Chen L, Liu O, Rowe DW, Xu M, 2021. Senolytics improve bone forming potential of bone marrow mesenchymal stem cells from aged mice. NPJ Regen Med 6, 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Tchkonia T, Fuhrmann-Stroissnigg H, Dai HM, Ling YY, Stout MB, Pirtskhalava T, Giorgadze N, Johnson KO, Giles CB, Wren JD, Niedernhofer LJ, Robbins PD, Kirkland JL, 2016. Identification of a novel senolytic agent, navitoclax, targeting the Bcl-2 family of anti-apoptotic factors. Aging Cell 15, 428–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Tchkonia T, Pirtskhalava T, Gower AC, Ding H, Giorgadze N, Palmer AK, Ikeno Y, Hubbard GB, Lenburg M, O’Hara SP, LaRusso NF, Miller JD, Roos CM, Verzosa GC, LeBrasseur NK, Wren JD, Farr JN, Khosla S, Stout MB, McGowan SJ, Fuhrmann-Stroissnigg H, Gurkar AU, Zhao J, Colangelo D, Dorronsoro A, Ling YY, Barghouthy AS, Navarro DC, Sano T, Robbins PD, Niedernhofer LJ, Kirkland JL, 2015. The Achilles’ heel of senescent cells: from transcriptome to senolytic drugs. Aging Cell 14, 644–658. [DOI] [PMC free article] [PubMed] [Google Scholar]