Abstract
Purpose of review
Both social and genetic factors are associated with health outcomes in systemic lupus erythematosus (SLE), thus playing a role in its health disparities. Despite the growing list of social and genetic factors associated with SLE outcomes, studies integrating sociocultural and individual determinants of health to understand health disparities in SLE are lacking. We review the contributions of different social and genetic factors to the disparities in SLE, and propose a socioecological model to integrate and examine the complex interactions between individual and social factors in SLE outcomes.
Recent findings
Multiple studies collecting comprehensive social data and biospecimens from diverse populations are underway, which will contribute to the elucidation of the interplay and underlying mechanisms by which positive and negative social determinants of health influence epigenomic variation, and how the resulting biological changes may contribute to the lupus health disparities.
Summary
There is growing awareness of the need to integrate genomic and health disparities research to understand how social exposures affect disease outcomes. Understanding the contributions of these factors to the SLE health disparity will inform the development of interventions to eliminate risk exposures and close the health disparity gap.
Keywords: health disparities, systemic lupus erythematosus, social factors, genetic factors
INTRODUCTION
Health disparities in systemic lupus erythematosus (SLE, or lupus) are well established and supported by decades of evidence. As recently reviewed [1**, 2*], there are marked demographic differences in the incidence, prevalence, and disease outcomes of SLE. For example, women are 8-10 times more likely than men to develop lupus; relative to European American, African Americans are 3-4 times more likely to develop lupus, suffer from remarkably higher disease severity and death rates, and are more likely to suffer from multiple comorbidities such as depression, cardiovascular disease, diabetes, and worse health-related quality of life. SLE is among the leading causes of death in young females (highest for African American and Hispanic females) [3], underscoring its impact as an important public health issue.
Despite the disproportional impact of SLE on minority racial and ethnic communities, the factors underlying these health disparities remain elusive. The causal mechanisms underlying SLE risk and outcomes among and within ethnic groups are complex, involving biological, sociocultural, physical, and other environmental exposures. However, most SLE research to date has focused on biological mechanisms while ignoring the effects of social exposures. Similarly, health disparities research has focused primarily on the influence of socioeconomic determinants on outcomes without considering the biological mechanisms involved. Furthermore, studies of sociocultural determinants are sparse in SLE. This has resulted in a knowledge gap regarding the interactions between individual and social factors that contribute to disparities in SLE outcomes. We will herein review the contributions of different social factors and genetic factors to the health disparities in SLE. We propose a socioecological model of SLE outcomes that emphasizes the importance of integrating sociocultural and individual determinants to understand and address health disparities in SLE. We will summarize emerging studies poised to elucidate the mechanisms linking physical and social environments with differential gene expression and health disparities. Given our focus on integrating genetics into health disparities research, we start by discussing the importance of acknowledging the broader social context of health disparities.
RACE, ETHNICITY, AND ANCESTRY
Defining race, ethnicity, and ancestry and using these concepts in biomedical research has wide-ranging implications for how the research is translated into clinical care, reported in the media, incorporated into public understanding, and implemented in public policy [4**]. Race and ethnicity are self-ascribed or socially ascribed identities and are often “assigned” by police, hospital staff, or others on the basis of physical characteristics; these concepts have no genetic or biological basis [5]. Ancestry is generally used to imply one’s genetic origins. As reviewed elsewhere [4**], often these concepts are conflated in scientific literature, implying that racial groups map to discrete genetic groups, and conveying that health inequities are caused by genetic factors rather than structural racism. These misconceptions can lead to the biological reification of social categories and be used to fuel racism and discrimination [4**]. This conflation can also lead to results with poor scientific validity [4**]. It is thus essential to explicitly distinguish between variables that derive from non-genetic, reported information, versus genetically inferred information.
Although race and ethnicity are often correlated with genetic ancestry, the sociocultural and genetic information the former and later capture, respectively, are different information. The use of race and ethnicity in biomedical research and clinical practice is an imperfect proxy for important epidemiologic information, including social determinants of health such as racism and discrimination, economic stability, healthcare access and quality, education access, and environmental exposures. These social and environmental determinants are differentially experienced across racial/ethnic groups due to historical and contemporary discriminatory policies and practice, resulting in health disparities across groups and geography. As for most conditions, the role of social and physical environmental factors on SLE outcomes is poorly understood.
Mixing the concepts of race and genetic ancestry is especially problematic in admixed populations who are often assumed as homogeneous when they are, in fact, extremely heterogeneous [4**]. For example, individuals who self-report as Hispanic/Latino have diverse cultural backgrounds as well as varying proportions of genetic ancestry from Africa, America, and Europe. Similarly for individuals who self-report as Black/African American: their mean sub-Saharan African ancestry varies between 10-20% in Central and South America to about 75% in the United States and British Caribbean, but can vary from 2% to 100% among different individuals [6]. The heterogeneity of African Americans is well exemplified by our genetic studies in Gullah African Americans, a culturally distinctive group of African Americans living in the Sea Islands along the coast of the southeastern United States, from North Carolina to Florida. Despite their unique culture that retains deep African features, our results are consistent with historical data [7], confirming that the Gullah have complex African ancestry and reduced European admixture, and are a mixture of numerous people from different genetic, ethnic, and linguistic currents who formed their own culture and language [8]. This heterogeneity underscores the need to investigate within-group ethnic differences, which are greatly underexplored.
SOCIAL FACTORS
As recently reviewed [1**, 2*, 9*], history of trauma is associated with increased risk of incident SLE, and multiple socioeconomic and psychosocial stressors negatively affect SLE outcomes. These include low household income, poverty, unemployment, food insecurity, housing inability, medical care insecurity, exposure to violence, exposure to adverse childhood experiences, physical victimization, unfair treatment, perceived stress, depression, racial discrimination, and vicarious racism [1**, 2*, 9*]. It is noteworthy that those who are poor with SLE are estimated to live 14 fewer years than their nonpoor counterparts [9*]. Recently, Spears et al. [10] added anticipatory racism stress to this list of social stressors associated with poor disease outcomes.
Notably, African American women are more likely to experience these stressors [11]. African American women report racial discrimination as a particularly salient and chronic stressor over their life course, distinct from other forms of unfair treatment [12]. The health consequences of racial discrimination, whether structural (e.g. chronic poverty, poor infrastructure), institutional (e.g. educational institutions and employment discrimination), or individual (e.g. interpersonal discriminatory acts), are evidenced by poorer health for African American women across socioeconomic strata, including higher rates of cardiovascular, metabolic, immune, and endocrine chronic conditions [13].
Despite stressors co-existing in areas of concentrated poverty, protective factors may buffer the negative impacts of stressors [14]. For example, protective parenting behavior buffers the impact of racial discrimination on depression among Black Youth [15]. Resilience is traditionally conceptualized based on personal traits that include not only the individual, but also the role of family, community, physical, and social ecology [16]. The compensatory model of resilience postulates that resilience resources may neutralize exposure to a social risk factor given a specific outcome [17]. Social support might have a positive impact in SLE [9*]. For example, several peer support programs designed to enhance social support and provide health education among African American and Latino patients with SLE have decreased depression and anxiety, and resulted in improved outcomes [9*]. Amongst other outcomes, the Georgians Organized Against Lupus (GOAL) research cohort showed that a self-management program benefited low-income African American women with SLE, and revealed a significant association between organ damage and depression in African American women, with social support being protective of depression [2*]. In addition, exiting poverty can mitigate the strong effect of living in concentrated poverty on SLE damage [9*]. A large prospective study has shown that a combination of healthy lifestyle behaviors based on alcohol consumption, body mass index, smoking, diet, and exercise, could reduce the risk of incident SLE to half [18]. Collectively, these data suggest that peer-support, self-management, and programs to alleviate poverty and support healthy lifestyle behaviors can help improve SLE outcomes.
Most studies to date have focused on social risk factors, and there is a paucity of research investigating protective social factors on SLE. Studies integrating multiple positive and negative social determinants of health will allow a thorough understanding of how protective factors buffer the effects of risk factors on SLE outcomes, and of the contributions of these factors to the SLE health disparity.
GENETIC AND EPIGENETIC FACTORS
Human genetic variation changes gradually according to geographical gradients, so alleles that are common in one population might be rare in another, geographically distant group. Differences in disease risk allele frequency in populations might be underlying some of the health disparities. Many genetic loci are associated with increased risk of SLE; Lanata and colleagues [19*] have recently reviewed the genetic risk factors for SLE that vary among populations. For example, two apolipoprotein L1 (APOL1) alleles confer a substantially increased risk of kidney disease in African ancestry individuals [20]. Although a large proportion of the ethnic disparity in end-stage renal disease (ESRD) in African Americans with lupus nephritis is attributed to the APOL1 risk alleles [21], once these risk alleles are accounted for the ethic disparity in SLE-ESRD is nearly absent. This suggests that non-genetic factors can be leveraged to reduce the development of APOL1-associated kidney disease in genetically susceptible individuals [22]. Integrating genetic and non-genetic factors could be a powerful way to reduce health disparities by more sharply identifying residual disparities and leveraging actionable social factors.
Although candidate genes or polygenic scores explain part of the variation in health outcomes, social determinants of health such as economic inequality generally explain considerably more variation [23**]. This suggests that social factors have biological consequences, with epigenetics potentially playing a role in linking individual and contextual factors with health outcomes across the life course [23**]. Despite the role of genetic factors in SLE, health disparities are typically due to social and structural determinants of health. Adverse experiences might influence SLE through epigenetic changes. Epigenetic marks such as DNA methylation impact gene expression and can govern cell function and physiological response to social exposures. Variation in DNA methylation in multiple blood cell subsets is associated with SLE. The role of genetic and epigenetic factors in health disparities observed in SLE and other rheumatic diseases has been recently summarized [19*]. Although DNA methylation varies between populations [24–31], and this variation is partially explained by their distinct genetic ancestry, environmental factors not captured by ancestry are significant contributors to variation in DNA methylation [26]. This supports the notion that an interaction between social, genetic and epigenetic factors underlies the health disparity in SLE.
In addition to their association with disease status, DNA methylation levels are also associated with psychosocial factors such as socioeconomic status [32, 33], poverty [34], general perceived stress [35], and childhood stress and maltreatment [36, 37]. A DNA methylation biomarker for accelerated aging is associated with adverse environmental exposures, including low socioeconomic status, stress, and childhood adversity [38]. A DNA methylation biomarker of mortality risk is associated with neighborhood disadvantage [39]. The field of social epigenetics aims to elucidate the pathways linking the physical, built, and social environments with differential gene expression and health disparities. Most studies to date have focused on socioeconomic status and early-life adversity, followed by social exposures [23**]. Given its relative infancy, the interpretation of results from these social epigenetic studies remains challenging: the majority lacked diversity and included individuals from North America and Western Europe; there was substantial variation in cell and tissue types, in different epigenetic measurements, and in the age of the study participants [23**]. Future social epigenetics research including larger, representative groups, and well-defined social factors is poised to unravel the biological consequences of social exposures on gene expression, disease etiology, and health inequities.
EMERGING STUDIES INTEGRATING GENETIC AND SOCIAL FACTORS
The socioecological model of health asserts that health is affected by the interaction between the characteristics of the individual, the community, and the environment that includes the physical, social, and political components. We propose a conceptual framework based on the socioecological model that emphasizes the importance of integrating societal, community, interpersonal, and individual determinants to understand and address health disparities in SLE (Fig. 1) [40, 41]. Social determinants of health span the socioeconomic (employment, income, housing and food security), community (family and social support), neighborhood and physical environment (access to food and housing, crime and violence, safety, transportation, air and water quality), and the health care system (access, quality). Individual determinants include genetic (sex chromosomes, DNA, epigenetic, and gene expression variation) and behavioral factors (diet, smoking, alcohol use, physical and mental health). Since exposures and experiences vary across individuals from different populations, locations, and cultures it is critical to study population differences in lupus health disparities within the sociocultural context. This need is further underscored by both the paucity of disadvantaged communities in research, and the heterogeneity of racial/ethnic groups.
Figure 1. Simplified socioecological model of lupus outcomes.
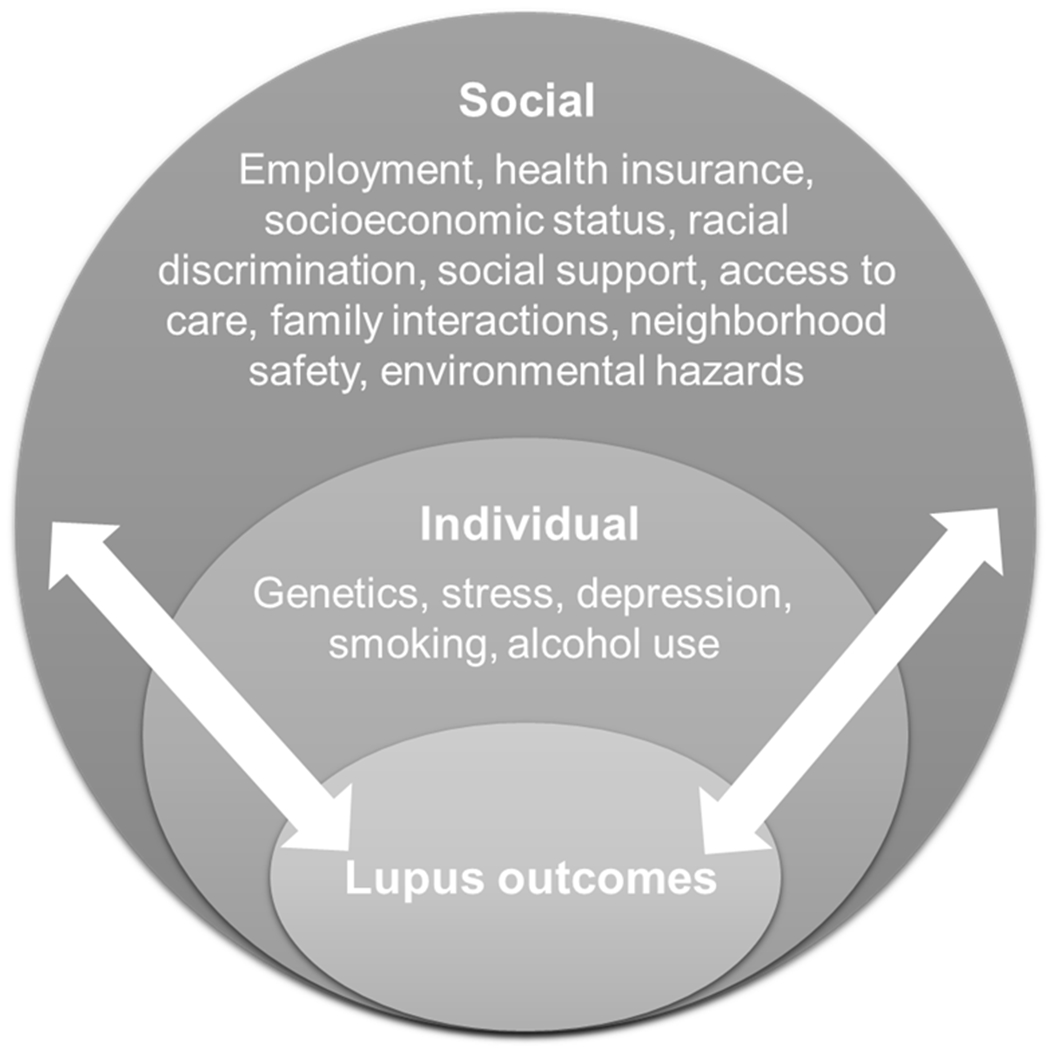
Socioecological factors that contribute to lupus disparities at the individual and social levels (including interpersonal, community and broader societal levels) are shown. As denoted by the lateral arrows, factors at each level interact to contribute to lupus disparities.
As reviewed above, both socioeconomic and psychosocial factors, as well as genetic factors are associated with poorer health outcomes in African American and other racial/ethnic minority patients with SLE. However, these groups are underrepresented in research, and the role of both individual and sociocultural determinants of health in heath disparities in SLE are poorly understood. Several research cohorts in the U.S. have been collecting data and biospecimens from racially and ethnically diverse populations to allow investigation of how various risk factors interact to influence SLE. These include the LUMINA (Lupus in Minorities: Nature vs. nurture), the Georgians Organized Against Lupus (GOAL), the Michigan Lupus Epidemiology & Surveillance (MILES), and the California Lupus Epidemiology Study (CLUES) cohorts [42]. The clinical, sociodemographic, psychosocial, and health services data collected from the patients from different racial/ethnic communities, together with genetic and other biologic material, is expected to provide a more comprehensive understanding of the reasons why disadvantaged groups experience disparities in SLE burden and outcomes, which will aid in the development of interventions to eliminate or mitigate SLE disparities.
Currently, it is not known how social or environmental experiences influence disease outcomes. Although studies linking specific experiences or behaviors to epigenetic changes in SLE are lacking, mounting evidence across several traits suggests that epigenetic mechanisms may provide a causal link between social adversity and health disparity [1**]. In reponse to the increasing awareness for the need for social epigenomic research (e.g. PAR-19-372), the goal of a recently funded project titled Social Factors, Epigenomics, and Lupus in African American Women (SELA) is to identify epigenetic changes by which positive and negative social factors affect gene function, and thereby influence lupus in African American women. Innovative aspects of this study include the focus on culturally distinct Gullah and non-Gullah African American women, the community partnership, and the integrative analysis of multiple individual and social factors, including risk and protective social effects. The identification of epigenetic mechanisms by which adverse and protective factors affect gene function and thereby influence SLE may inform the development of psychosocial interventions that prevent or mitigate risk exposures, and services or interventions that promote positive exposures. Development of these novel treatments and preventative interventions, as informed by the results of this study, is paramount to the closure of the health disparities gap.
Finally, future studies ought to include and analyze the role of metagenomic variation and intestinal barrier permeability on SLE disparities. Associations of microbiota dysbiosis, intestinal permeability, and intestinal inflammation with several autoimmune diseases have been reported [43]. Interestingly, social stress is a well-described intestinal disrupting factor [43]. Hence, studies are needed to understand the role of intestinal barrier disruption, intestinal inflammation, gut dysbiosis, and their interplay with other individual and social factors in SLE disparities.
CONCLUSION
The role for both genetic and social determinants of health on SLE disparities is well documented. However, knowledge of how physical and social exposures influence differential gene expression and disease outcomes is lacking, disadvantaged communities are poorly represented in research, racial/ethnic groups are heterogeneous and their within-group disparities unexplored, many studies mix biological with socially-constructed ethnoracial categories, the mechanisms by which adverse and protective social factors synergistically modulate disease outcomes are not understood, and comprehensive studies integrating multiple individual and social factors haven’t been published. Nevertheless, emerging studies in SLE have been collecting extensive genetic and social data, and are poised to elucidate how risk and protective factors from multiple levels of the social environment interact and influence SLE outcomes through epigenomic variation.
Results from these studies are expected to elucidate how risk factors affect SLE, how they can be mitigated in patients with SLE, inform the development of targets for interventions to minimize adverse stressors, improve outcomes for vulnerable patients with SLE, and minimize SLE disparities.
KEY POINTS.
The mechanisms underlying SLE disparities are complex and poorly understood, involving biological, sociocultural, physical, and other environmental exposures.
We propose a socioecological model to examine the complex interactions between individual (including genetic) and social factors that contribute to disparities in SLE outcomes.
Ongoing mechanistic studies integrating multiple individual with positive and negative social determinants of health will elucidate how protective social factors buffer the effects of risk factors on SLE outcomes, and the contributions of these factors to the SLE health disparity.
Understanding the effects of positive and negative social environments on SLE through epigenomic changes can inform the development of services or interventions that promote positive and mitigate negative exposures, helping close the health disparity gap.
Acknowledgements
This study was supported by the US National Institute of Arthritis and Musculoskeletal and Skin Diseases and the National Institute on Minority Health and Health Disparities of the National Institutes of Health (NIH) under Awards Number P30 AR072582 and R01 MD015395-01A1. The content is solely the responsibility of the author and does not necessarily represent the official views of the National Institutes of Health.
Funding:
P30 AR072582, R01 MD015395-01A1.
Footnotes
Conflicts of interest
There are no conflicts of interest.
References
- 1.**.Peschken CA. Health Disparities in Systemic Lupus Erythematosus. Rheum Dis Clin North Am. 2020;46(4):673–83. [DOI] [PubMed] [Google Scholar]; This comprehensive review delineates all the modifiable, ethnic disparities in SLE, pointing out how mistrust, poor communication, racism, geography, and hazardous environmental exposures contribute to worse disease outcomes.
- 2.*.Lim SS, Drenkard C. Understanding Lupus Disparities Through a Social Determinants of Health Framework: The Georgians Organized Against Lupus Research Cohort. Rheum Dis Clin North Am. 2020;46(4):613–21. [DOI] [PubMed] [Google Scholar]; This article explains the need to include social determinants of health in health disparities reseach in SLE, summarizing the major results of the GOAL cohort.
- 3.Yen EY, Singh RR. Brief Report: Lupus-An Unrecognized Leading Cause of Death in Young Females: A Population-Based Study Using Nationwide Death Certificates, 2000–2015. Arthritis & rheumatology. 2018;70(8):1251–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.**.Khan A, Gogarten SM, McHugh C, et al. Recommendations on the use and reporting of race, ethnicity, and ancestry in genetic research: experiences from the NHLBI Trans-Omics for Precision Medicine (TOPMed) program. arXiv:210807858 [q-bioOT] [Internet]. 2021. Available from: https://arxiv.org/abs/2108.07858. [Google Scholar]; This commentary, based on the experiences of the TOPMed program, clearly describes the challenges and delineates specific recommendations on the use and reporting of race, ethnicity, and ancestry in human genomic research.
- 5.Borrell LN, Elhawary JR, Fuentes-Afflick E, et al. Race and Genetic Ancestry in Medicine - A Time for Reckoning with Racism. N Engl J Med. 2021;384(5):474–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fortes-Lima C, Verdu P. Anthropological genetics perspectives on the transatlantic slave trade. Hum Mol Genet. 2021;30(R1):R79–R87. [DOI] [PubMed] [Google Scholar]
- 7.Pollitzer WS. The Gullah people and their African heritage. Athens, Georgia, USA: The University of Georgia Press; 1999; 1999. [Google Scholar]
- 8.Zimmerman KD, Schurr TG, Chen WM, et al. Genetic landscape of Gullah African Americans. American journal of physical anthropology. 2021;175(4):905–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.*.DeQuattro K, Yelin E. Socioeconomic Status, Health Care, and Outcomes in Systemic Lupus Erythematosus. Rheum Dis Clin North Am. 2020;46(4):639–49. [DOI] [PubMed] [Google Scholar]; This article provides an overview of the relationship between socioeconomic status and SLE, summarizing interventions that minimize disparities in SLE.
- 10.Spears EC, Allen AM, Chung KW, et al. Anticipatory racism stress, smoking and disease activity: the Black women’s experiences living with lupus (BeWELL) study. J Behav Med. 2021. [DOI] [PubMed] [Google Scholar]
- 11.Chae DH, Martz CD, Fuller-Rowell TE, et al. Racial Discrimination, Disease Activity, and Organ Damage: The Black Women’s Experiences Living With Lupus (BeWELL) Study. Am J Epidemiol. 2019;188(8):1434–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee DB, Peckins MK, Miller AL, et al. Pathways from racial discrimination to cortisol/DHEA imbalance: protective role of religious involvement. Ethnicity & health. 2018:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goosby BJ, Heidbrink C. Transgenerational Consequences of Racial Discrimination for African American Health. Sociol Compass. 2013;7(8):630–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zimmerman MA, Stoddard SA, Eisman AB, et al. Adolescent Resilience: Promotive Factors That Inform Prevention. Child Dev Perspect. 2013;7(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lei MK, Lavner JA, Carter SE, et al. Protective parenting behavior buffers the impact of racial discrimination on depression among Black youth. J Fam Psychol. 2021;35(4):457–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Spence ND, Wells S, Graham K, et al. Racial Discrimination, Cultural Resilience, and Stress. Can J Psychiatry. 2016;61(5):298–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garmezy N, Masten AS, Tellegen A. The study of stress and competence in children: a building block for developmental psychopathology. Child development. 1984;55(1):97–111. [PubMed] [Google Scholar]
- 18.Choi MY, Hahn J, Malspeis S, et al. A Combination of Healthy Lifestyle Behaviors Reduces Risk of Incident Systemic Lupus Erythematosus. Arthritis & rheumatology. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.*.Lanata CM, Blazer A, Criswell LA. The Contribution of Genetics and Epigenetics to Our Understanding of Health Disparities in Rheumatic Diseases. Rheum Dis Clin North Am. 2021;47(1):65–81. [DOI] [PubMed] [Google Scholar]; This comprehesive review summarizes the role of genetics and epigenetics in the health disparities observed in rheumatic diseases among patients of different ethnicities. It describes the role of population genetics in shaping genetic and epigenetic disease risk.
- 20.Nadkarni GN, Gignoux CR, Sorokin EP, et al. Worldwide Frequencies of APOL1 Renal Risk Variants. N Engl J Med. 2018;379(26):2571–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Freedman BI, Langefeld CD, Andringa KK, et al. End-stage renal disease in African Americans with lupus nephritis is associated with APOL1. Arthritis & rheumatology. 2014;66(2):390–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Langefeld CD, Comeau ME, Ng MCY, et al. Genome-wide association studies suggest that APOL1-environment interactions more likely trigger kidney disease in African Americans with nondiabetic nephropathy than strong APOL1-second gene interactions. Kidney Int. 2018;94(3):599–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.**.Evans L, Engelman M, Mikulas A, et al. How are social determinants of health integrated into epigenetic research? A systematic review. Soc Sci Med. 2021;273:113738. [DOI] [PMC free article] [PubMed] [Google Scholar]; This systematic review of the literature on social epigenetics identifies studies examining the impact of social determinants of health on DNA methylation outcomes, summarizes the social determinants of health most studied, identifies current challenges, and proposes future directos for social epigenetic research.
- 24.Michels KB, Binder AM, Dedeurwaerder S, et al. Recommendations for the design and analysis of epigenome-wide association studies. Nature methods. 2013;10(10):949–55. [DOI] [PubMed] [Google Scholar]
- 25.Barfield RT, Almli LM, Kilaru V, et al. Accounting for population stratification in DNA methylation studies. Genetic epidemiology. 2014;38(3):231–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Galanter JM, Gignoux CR, Oh SS, et al. Differential methylation between ethnic sub-groups reflects the effect of genetic ancestry and environmental exposures. eLife. 2017;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Husquin LT, Rotival M, Fagny M, et al. Exploring the genetic basis of human population differences in DNA methylation and their causal impact on immune gene regulation. Genome biology. 2018;19(1):222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Quach H, Rotival M, Pothlichet J, et al. Genetic Adaptation and Neandertal Admixture Shaped the Immune System of Human Populations. Cell. 2016;167(3):643–56 e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gopalan S, Carja O, Fagny M, et al. Trends in DNA Methylation with Age Replicate Across Diverse Human Populations. Genetics. 2017;206(3):1659–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fagny M, Patin E, MacIsaac JL, et al. The epigenomic landscape of African rainforest hunter-gatherers and farmers. Nature communications. 2015;6:10047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Heyn H, Moran S, Hernando-Herraez I, et al. DNA methylation contributes to natural human variation. Genome research. 2013;23(9):1363–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lam LL, Emberly E, Fraser HB, et al. Factors underlying variable DNA methylation in a human community cohort. Proc Natl Acad Sci U S A. 2012;109Suppl 2:17253–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Borghol N, Suderman M, McArdle W, et al. Associations with early-life socio-economic position in adult DNA methylation. International journal of epidemiology. 2012;41(1):62–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McDade TW, Ryan CP, Jones MJ, et al. Genome-wide analysis of DNA methylation in relation to socioeconomic status during development and early adulthood. American journal of physical anthropology. 2019;169(1):3–11. [DOI] [PubMed] [Google Scholar]
- 35.Vidal AC, Benjamin Neelon SE, Liu Y, et al. Maternal stress, preterm birth, and DNA methylation at imprint regulatory sequences in humans. Genet Epigenet. 2014;6:37–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van der Knaap LJ, Riese H, Hudziak JJ, et al. Adverse life events and allele-specific methylation of the serotonin transporter gene (SLC6A4) in adolescents: the TRAILS study. Psychosom Med. 2015;77(3):246–55. [DOI] [PubMed] [Google Scholar]
- 37.Mehta D, Klengel T, Conneely KN, et al. Childhood maltreatment is associated with distinct genomic and epigenetic profiles in posttraumatic stress disorder. Proc Natl Acad Sci U S A. 2013;110(20):8302–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dhingra R, Nwanaji-Enwerem JC, Samet M, et al. DNA Methylation Age-Environmental Influences, Health Impacts, and Its Role in Environmental Epidemiology. Curr Environ Health Rep. 2018;5(3):317–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ward-Caviness CK, Pu S, Martin CL, et al. Epigenetic predictors of all-cause mortality are associated with objective measures of neighborhood disadvantage in an urban population. Clinical epigenetics. 2020;12(1):44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Alvidrez J, Castille D, Laude-Sharp M, et al. The National Institute on Minority Health and Health Disparities Research Framework. Am J Public Health. 2019;109(S1):S16–S20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bronfenbrenner U Toward an experimental ecology of human development. American Psychologist. 1977;32(7):513–31. [Google Scholar]
- 42.Drenkard C, Lim SS. Update on lupus epidemiology: advancing health disparities research through the study of minority populations. Curr Opin Rheumatol. 2019;31(6):689–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ilchmann-Diounou H, Menard S. Psychological Stress, Intestinal Barrier Dysfunctions, and Autoimmune Disorders: An Overview. Frontiers in immunology. 2020;11:1823. [DOI] [PMC free article] [PubMed] [Google Scholar]


