Abstract
To investigate the efficacy and toxicities of CPX-351 outside a clinical trial, we analyzed 188 patients (median age 65 years, range 26–80) treated for therapy-related acute myeloid leukemia (t-AML, 29%) or AML with myelodysplasia-related changes (AML-MRC, 70%). Eighty-six percent received one, 14% two induction cycles, and 10% received consolidation (representing 22% of patients with CR/CRi) with CPX-351. Following induction, CR/CRi rate was 47% including 64% of patients with available information achieving measurable residual disease (MRD) negativity (<10−3) as measured by flow cytometry. After a median follow-up of 9.3 months, median overall survival (OS) was 21 months and 1-year OS rate 64%. In multivariate analysis, complex karyotype predicted lower response (p = 0.0001), while pretreatment with hypomethylating agents (p = 0.02) and adverse European LeukemiaNet 2017 genetic risk (p < 0.0001) were associated with lower OS. Allogeneic hematopoietic cell transplantation (allo-HCT) was performed in 116 patients (62%) resulting in promising outcome (median survival not reached, 1-year OS 73%), especially in MRD-negative patients (p = 0.048). With 69% of patients developing grade III/IV non-hematologic toxicity following induction and a day 30-mortality of 8% the safety profile was consistent with previous findings. These real-world data confirm CPX-351 as efficient treatment for these high-risk AML patients facilitating allo-HCT in many patients with promising outcome after transplantation.
Subject terms: Haematological cancer, Leukaemia
Introduction
Acute myeloid leukemia (AML) can arise de novo, as therapy-related complication following chemotherapy and/or ionizing radiation (t-AML) or from antecedent hematologic disorders [1]. The latter are also summarized as secondary AML (sAML) and account for approximately one quarter of all AML cases. AML with myelodysplasia-related changes (AML-MRC) is defined according to the WHO 2016 classification by the history of a myelodysplastic syndrome (MDS), signs of dysplasia, and/or MDS-related cytogenetic abnormalities. AML-MRC and sAML occur more frequently with advanced age and are associated with biologic properties such as adverse genetics and multidrug resistance phenotype, which contribute to poor outcome after conventional therapy [2–5]. While attempts to improve outcome after induction therapy by addition of other agents or intensification of post-remission therapy have generally failed, higher remission rates and longer overall survival (OS) compared to conventional cytarabine plus daunorubicin chemotherapy (7 + 3 regimen) were recently observed following CPX-351 (Jazz Pharmaceuticals, Palo Alto, CA), a liposomal encapsulation of cytarabine and daunorubicin at a fixed 5:1 synergistic molar ratio [6, 7]. The results from this phase-III trial, which investigated CPX-351 in 309 patients with AML-MRC or tAML aged 60–75 years, led to the approval of the drug combination by the health authorities in the USA 2017 and in Europe 2018 for adult patients with newly diagnosed AML-MRC or t-AML. Beside the aspect of age also several other issues, e.g. measurable residual disease (MRD), molecular subgroups, specific side effects as well as specific outcome parameters in the context of allogeneic hematopoietic cell transplantion (allo-HCT) were not addressed in the phase-III trial. Furthermore, in other settings data from real-world experiences with new therapies differed from clinical trial results [8, 9], suggesting that patients in clinical trials represent a selected cohort potentially limiting interpretation and translation of results to real-life patient care. Two recent retrospective analyses pointed towards some of these open questions [10, 11]. Therefore, aiming to address these open aspects and to provide more clinical data and experience for CPX-351, we performed a real-world analysis of consecutive newly diagnosed patients with AML, who were treated in-label with CPX-351 as first-line therapy.
Subjects and methods
Study design
For this retrospective analysis, we collected data from consecutive patients with newly diagnosed AML-MRC or t-AML, who were treated with CPX-351 according to the EMA label between 2018 and 2020 in 25 German centers participating in the Study Alliance Leukemia (SAL), German Cooperative Transplant Study Group, and the AML Study Group (AMLSG). Median number of patients included per center was 6 (range, 1–22 patients). Information about patient characteristics, treatment details including allo-HCT, and outcome were gathered using a specific, standardized data form sent to the participating centers. All patients in the participating centers treated with CPX-351 as first-line therapy for AML during this time period were reported. To ensure high data quality physicians’ review of data and personal requests at respective centers was performed for all patients. The study was approved by the ethics committee of the Heinrich-Heine-University, Duesseldorf (approval number: 2020-877) and all patients gave written informed consent for scientific use of their data. Data lock for the analysis was 1 November 2020.
Definitions and response criteria
AML subtypes were categorized according to the criteria of the WHO 2016 classification [1]. Response, relapse, and genetic risk categories were defined according to the 2017 European LeukemiaNet (ELN) recommendations [12]. For the definition of complete remission without minimal residual disease (CRMRD−) we used information of MRD results obtained by routine flow cytometry (FC)-based MRD monitoring performed at the participating site.
Conditioning intensity, the hematopoietic cell transplantation-specific comorbidity index (HCT-CI), and graft-versus-host disease (GvHD) were defined as previously described [13–16]. Non-hematologic toxicity was graded by the treating physician using the National Cancer Institute Common Toxicity Criteria (NCI CTC).
Statistical analyses
For categorical variables frequencies were displayed and differences were evaluated using cross-tabulation and Fisher’s exact t-test, whereas for continuous variables medians (range) were summarized and the Mann–Whitney test was used to detect differences. OS for the entire cohort was calculated as the time from the first day of treatment with CPX-351 to death from any cause or last follow-up in survivors. For the subgroup of patients undergoing allo-HCT OS was estimated as time between allo-HCT and death or date of last follow-up in surviving patients, while relapse-free survival (RFS) was calculated as time from allo-HCT until relapse or death without relapse censoring those patients, who had not relapsed until and were alive at date of last follow-up. Time-to-event curves were calculated by employing the Kaplan–Meier method and log-rank tests were applied for univariate comparisons.
Furthermore, for the transplant cohort cumulative incidence of relapse (CIR) and non-relapse mortality (NRM) were considered as competing risks and calculated using cumulative incidence (CI) estimates employing Gray test for univariate comparisons. Those parameters, which influenced OS of the entire cohort in univariate analysis with p < 0.10, were included into a multivariate analysis using a proportional hazard regression analysis (multiple Cox regression model). For factors associated with achievement of CR/CRi a multinominal logistic regression analysis was applied.
In all analyses, a p value <0.05 was considered to be significant. Statistical analyses were performed using GraphPad Prism® 7 (GraphPad Software Inc., La Jolla, USA) and IBM SPSS Statistics (SPSS Inc. Chicago, IL) as well as R 3.5.1.
Results
Patients characteristics
We analyzed data from 188 consecutive patients, who received CPX-351 induction chemotherapy as first-line therapy for AML between June 2018 and June 2020. Median age was 65 years (range 26–80 years) and 46 patients (24%) were <60 years. Most patients (82%) had a good performance status as indicated by a Karnofsky index of ≥80%, while the majority (82%) exhibited comorbidities as indicated by intermediate or high HCT-CI. A diagnosis of t-AML was present in 29% of patients, while 70% were diagnosed with AML-MRC. A total of 19 patients (10%) had been treated with hypomethylating agents (HMA) for anteceding MDS before initiation of CPX-351. Karyotype abnormalities were present in 65% of patients including 44 patients (25%) with a complex karyotype (CK). Considering the prognostically relevant mutations included in the 2017 ELN risk classification, ASXL1 (n = 31, 16%) was the most frequently mutated gene, followed by RUNX1 (n = 24, 13%), NPM1 (n = 18, 10%), TP53 (n = 14, 7%), and FLT3-ITD (n = 13, 7%). Accordingly, 7, 33, and 60% of patients belonged to the favorable, intermediate, and adverse ELN 2017 genetic risk category, respectively. Detailed patients characteristics are summarized in Table 1.
Table 1.
Patient characteristics (n = 188).
| Characteristics | n | % |
|---|---|---|
| Age, years (median, range) | 65 (26–80) | |
| Gender | ||
| Female | 70 | 37 |
| Male | 118 | 63 |
| Karnofsky (n = 165) | ||
| ≥80 | 135 | 82 |
| <80 | 30 | 18 |
| HCT-CI (n = 155) | ||
| Low | 29 | 18 |
| Intermediate | 55 | 35 |
| High | 74 | 47 |
| AML subtype (n = 186) | ||
| AML-MRC | 131 | 70 |
| t-AML | 53 | 29 |
| Othera | 2 | 1 |
| 2017 ELN genetic risk (n = 179) | ||
| Favorable | 12 | 7 |
| Intermediate | 59 | 33 |
| Adverse | 108 | 60 |
| Karyotype (n = 179) | ||
| Normal | 63 | 35 |
| Abnormal | 116 | 65 |
| Complex | 44 | 25 |
| Non-complex | 72 | 40 |
| Molecular genetics | ||
| NPM1/n.d. | 18/11 | 10/6 |
| FLT3-ITD/n.d. | 13/13 | 7/7 |
| ASXL1/n.d. | 31/24 | 16/13 |
| TP53/n.d. | 14/26 | 7/14 |
| RUNX1/n.d. | 24/20 | 13/11 |
| BM blast count at diagnosis (median, range) | 38 (7–99) | |
| PB blast count at diagnosis (median, range) | 10 (0–92) | |
| WBC at diagnosis, ×103/µl (median, range) | 3.8 (0.6–330) | |
| No treatment prior CPX | 169 | 90 |
| Pretreatment with HMA | 19 | 10 |
AML acute myeloid leukemia, AML-MRC AML with myelodysplasia-related changes, BM bone marrow, ELN European Leukemia Net, HCT-CI hematopoietic cell transplantation-specific comorbidity index, HMA hypomethylating agents, n.d. not done, n number, PB peripheral blood, t-AML therapy-related AML, WBC white blood cells.
Numbers in parentheses display patients with available information.
aSecondary AML evolving from systemic mastocytosis (n = 1) and blastic plasmacytoid dendritic cell neoplasm (BPDCN) with antecedent history of chronic myelomonocytic leukemia (CMML) (n = 1).
Treatment
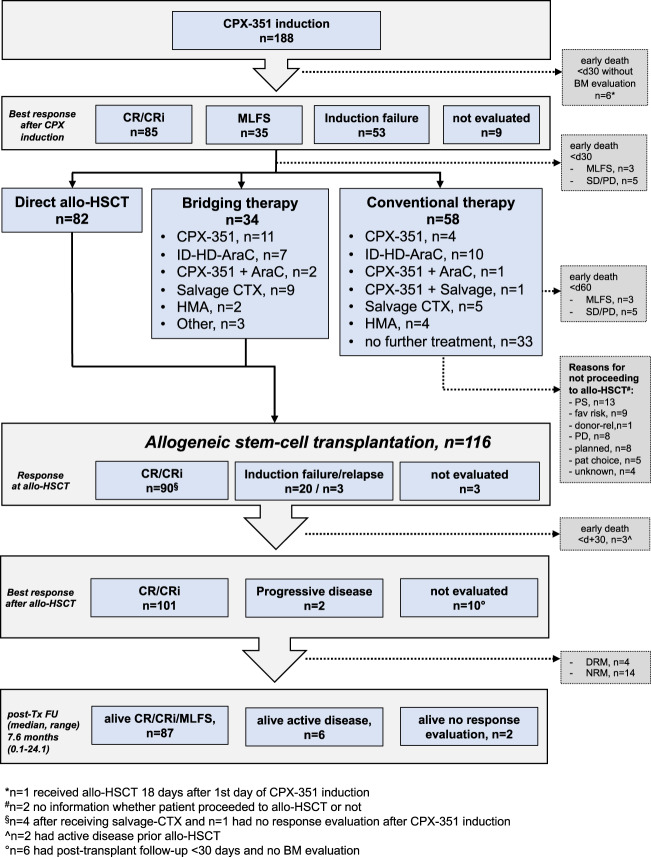
One-hundred and sixty-two patients (86%) received one cycle of CPX-351 induction, while 26 patients (14%) received two induction cycles (Table 2). Of these, 116 (62%) underwent allo-HCT, which was performed without further therapy in 82 patients. In the remaining 34 patients at least one cycle of intermittent therapy consisting of either CPX-351 and/or AraC consolidation, HMA or salvage therapy was performed (Fig. 1). In 58 patients (31%), who were alive beyond day +30 after induction, no allo-HCT was performed. Reasons for not proceeding to allo-HCT were mainly related to performance status, favorable disease risk, progressive disease, or patients’ decision (Fig. 1). Of these non-transplanted patients, 25 patients received at least one other line of conventional therapy consisting of CPX-351 and/or AraC consolidation, HMA or salvage therapy, while in the remaining 33 patients no further therapy was performed (Fig. 1). Overall, at least one cycle of CPX-351 consolidation therapy was administered in 19 patients (10%) of patients, which corresponds to 22% of patients with CR/CRi.
Table 2.
Treatment characteristics, response, and toxicity.
| Characteristics | n | % |
|---|---|---|
| Follow-up, months (median, range) | 9.3 (0.2–26.1) | |
| No. of induction cycles with CPX (median, range) | 1 (1–2) | |
| ANC recoverya in patients with CR/CRi after CPX-351 induction (n = 83) | ||
| Yes | 79 | 95 |
| No | 4 | 5 |
| Time, days (median, range) | 33 (6–99) | |
| Platelet recoveryb in patients with CR/CRi after CPX-351 induction (n = 83) | ||
| Yes | 76 | 92 |
| No | 7 | 8 |
| Time, days (median, range) | 30 (7–77) | |
| Response after induction (n = 179) | ||
| CR/CRi | 85 | 47 |
| MLFS | 35 | 20 |
| Refractory disease | 53 | 30 |
| Early death (<30 days) without response | 6 | 3 |
| Evaluation | ||
| MRD available in case of CR/CRi | 36 | 42c |
| MRD negative | 23 | 64d |
| Grade III/IV non-hematologic toxicities (n = 188) | ||
| Infection | 41 | 22 |
| GI (mucositis, nausea, vomiting) | 7 | 4 |
| Bleeding | 7 | 4 |
| Renal failure | 5 | 3 |
| Febrile neutropenia | 28 | 15 |
| Pneumonia | 42 | 22 |
| Mortality on d30 after CPX-351 induction (n = 176)e | ||
| Alive | 162 | 92 |
| Dead | 14 | 8 |
ANC absolute neutrophile count, CR complete remission, CRi complete remission with incomplete hematologic recovery, d day, GI gastrointestinal, MLFS morphologic leukemia-free state, MRD minimal residual disease, n number, SD stable disease, PD progressive disease, n.d. not done.
Numbers in parentheses display patients with available information.
aDefined as ANC > 500/µl.
bDefined as platelet count >50,000/µl.
cOf the 85 patients with CR/CRi.
dOf the 36 patients with available MRD results.
ePatients proceeding to allo-HSCT before d30 following CPX-351 induction have been excluded.
Fig. 1. Flow chart depicting treatment, response, and outcome of the study population.
Allo-HSCT allogeneic hematopoietic stem cell transplantation, BM bone marrow, CR complete remission, CRi complete remission with incomplete hematologic recovery, CTX chemotherapy, DRM disease-related mortality, d day, fav favorable, FU follow-up, HD-AraC high-dose cytarabine, ID-AraC intermediate dose cytarabin, MLFS morphologic leukemia-free state, NRM non-relapse mortality, pat patient, PD progressive disease, PS performance status, rel related, SD stable disease, Tx transplant.
Response after induction
Following CPX-351- induction 47% of evaluable patients achieved CR/CRi (n = 85) and 20% (n = 35) morphologic leukemia-free state (MLFS), while 30% of evaluable patients (n = 53) did not respond. Among patients with CR/CRi (n = 85) after CPX-351 induction data on MRD measured by flow cytometry were available for 36 patients (42%) demonstrating MRD negativity in 64% of the patients (n = 23) (Table 2). CR/CRi rate in patients with complex karyotype (n = 44) and in those with TP53 mutation (n = 14) were 33% and 54%, respectively.
Given the limited data in patients treated with CPX-351 so far [6, 10, 11], we aimed to identify predictors for achievement of CR/CRi. In univariate analyses, we observed higher CR/CRi rates in female patients, in those without complex karyotype and in those patients without HMA pretreament (Supplementary Table 1). In multivariate analysis, only a non-complex karyotype (p = 0.0001) predicted for a higher CR/CRi rate (Table 3).
Table 3.
Outcome after induction with CPX-351, multivariate analysis.
| Variable | Overall survival | Response rate | ||||
|---|---|---|---|---|---|---|
| P | HR | P | HR | |||
| Prior treatment with HMA | ||||||
| Yes | 0.02 | 2.4 [1.1–5.3] | n.s. | |||
| No | ||||||
| ELN risk stratification | ||||||
| Adverse | <0.0001 | 4.2 [1.9–8.9] | – | |||
| Favorable/intermediate | ||||||
| Karyotype | ||||||
| Complex | – | 0.0001 | 4.3 [1.9–9.2] | |||
| Not complex | ||||||
| NPM1 | ||||||
| wt | n.s. | – | ||||
| mut | ||||||
| TP53 | ||||||
| mut | n.s. | – | ||||
| wt | ||||||
| Age at diagnosis (median) | ||||||
| ≥65 | n.s. | – | ||||
| <65 | ||||||
| Gender | ||||||
| Female | – | n.s. | ||||
| Male | ||||||
ELN European Leukemia Net, HMA hypomethylating agents, HR hazard ratio, mut mutated, n.s. not significant, P p value, wt wild type.
Safety
In patients with CR/CRi recovery to an absolute neutrophil count (ANC) ≥ 500/µl and platelet count ≥50.000/µl was observed in 95% and 92% of patients, respectively (Table 2). Median time to ANC and platelet recovery was 33 days (range: 6–99 days) and 30 days (range: 7–77 days), respectively.
Regarding non-hematologic toxicity, adverse events (AE) ≥ grade III were reported in 130 patients (69%). As indicated in Table 2, these were mainly related to infectious complications, while gastrointestinal side effects and bleeding occurred rather infrequently. Of note, patients with leukocyte counts >20 G/L at diagnosis had a significantly higher frequency of grade III/IV AE as compared to those with leukocytes <20 G/L (74% vs. 50%, p = 0.01). The 30-day early death rate was 8% in the entire cohort and was significantly higher in patients ≥ 65 years (11% vs. 3%, p = 0.047).
Overall survival of the entire cohort
With a median follow-up (FU) of 9.3 months (range: 0.2–26.1 months) median OS of the entire cohort was 21 months and estimated 1-year OS was 64% (95% CI 55–72%, Fig. 2). In univariate analysis, pretreatment with HMA, the presence of an abnormal or complex karyotype, adverse ELN genetic risk, age ≥65 years, MRD positivity after induction, and not undergoing allo-HCT were associated with inferior OS (Supplementary Table 2). In multivariate analysis, pretreatment with HMA (p = 0.02) and adverse ELN genetic risk (p < 0.0001) retained their negative impact on OS (Table 3).
Fig. 2. Overall (OS) of the entire cohort.
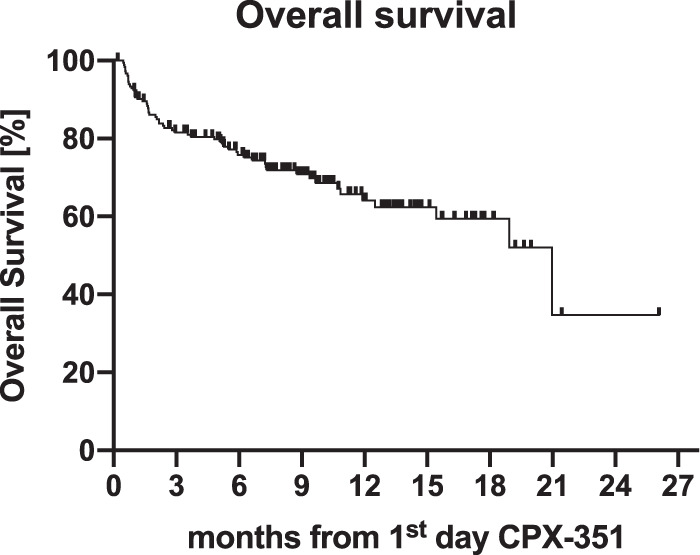
After a median follow-up of 9.3 months (range: 0.2–26.1 months) median OS of the entire cohort (n = 188) was 21 months and estimated 2-year OS was 35%. OS for the entire cohort was calculated as the time from the first day of treatment with CPX-351 to death from any cause or last follow-up in survivors.
Allo-HCT
One-hundred and sixteen (62%) patients underwent allo-HCT after CPX-351 induction with a median time from start of induction to transplantion of 70 days (range: 11–215 days). At the time of transplant 20% (n = 23) of patients had active disease (>5% BM blasts, induction failure n = 20, relapse n = 3), while 80% (n = 90) were in first remission (Fig. 1). The latter included 26 of the 35 patients initially categorized as MLFS at first remission control, which were classified as CRi (n = 16) or CR (n = 10) at the time of transplant. Among patients with CR/CRi at transplant flow-cytometry-measured MRD status was available in 36 patients with 23 patients (64%) exhibiting MRD negativity at the time of transplant. Detailed information on transplant characteristics are summarized in Table 4 and Supplementary Tables 3 and 4. The majority of patients (n = 95, 82%) received a graft from an unrelated donor following a reduced-intensity conditioning (RIC), which was used in all patients except for one.
Table 4.
Transplant characteristics, toxicity and outcome.
| Characteristics | n | % |
|---|---|---|
| Time between 1st day of CPX-351 and allo-HCT, days (median, range) | 70 (11–215) | |
| Median age at transplant | 64 (24–79) | |
| HLA-matching (n = 116) | ||
| MUD | 74 | 64 |
| MRD | 13 | 11 |
| MMUD | 21 | 18 |
| Haploidentical | 8 | 7 |
| Donor gender (n = 112) | ||
| Male | 78 | 70 |
| Female | 34 | 30 |
| CMV-status (donor/recipient) (n = 111) | ||
| pos/pos | 44 | 40 |
| pos/neg | 7 | 6 |
| neg/neg | 35 | 32 |
| neg/pos | 25 | 22 |
| Conditioning intensity (n = 116) | ||
| Reduced intensity | 115 | 99 |
| Standard dose | 1 | 1 |
| Stem cell source (n = 113) | ||
| PBSC | 110 | 97 |
| BM | 3 | 3 |
| In vivo T cell depletion (n = 110) | ||
| Yes | 69 | 63 |
| No | 41 | 37 |
| Engraftment (n = 114) | ||
| No | 2 | 2 |
| Yes | 112 | 98 |
| ANC recovery after allo-HCT (n = 101) | ||
| Yes | 100 | 99 |
| No | 1 | 1 |
| Time, days (median, range) | 16 (7–70) | |
| Platelet recovery after allo-HCT (n = 101) | ||
| Yes | 96 | 96 |
| No | 5 | 5 |
| Time, days (median, range) | 15 (9–45) | |
| Acute GvHD (n = 113) | ||
| Yes | 56 | 50 |
| No | 57 | 50 |
| ≥Grade III | 15 | 13 |
| Chronic GvHD (n = 110) | ||
| Yes | 23 | 21 |
| No | 87 | 79 |
| Severe | 4 | 4 |
| Grade III/IV non-hematologic toxicities (n = 116) | ||
| Infection | 36 | 31 |
| GI (mucositis, nausea, vomiting) | 11 | 9 |
| Bleeding | 2 | 2 |
| Renal failure | 8 | 7 |
| Febrile neutropenia | 2 | 2 |
| SOS | 1 | 1 |
| Relapse after allo-HCT | ||
| Yes | 11 | 11 |
| Time between allo-HCT and relapse, days (median, range) | 82 (33–549) | |
| No | 90 | 89 |
| Posttransplant day + 100 mortality (n = 116) | ||
| Dead | 8 | 7 |
| Alive | 95 | 82 |
| Alive, evaluated <day +100 | 13 | 11 |
| Posttransplant follow-up, months (median, range) | 7.6 (0.1–24.1) | |
ANC absolute neutrophile count, Allo-HCT allogeneic hematopoietic cell transplantation, BM bone marrow, CMV cytomegalovirus, GI gastrointestinal, GvHD graft-versus-host disease, HLA human leukocyte antigen, MMUD mismatched unrelated donor, MRD matched related donor, MUD matched unrelated donor, neg negative, PBSC peripheral blood stem cells, pos positive, SOS sinusoidal obstruction syndrome.
Numbers in parentheses display patients with available information.
A primary graft failure was observed in two patients (2%). Median time to ANC and platelet recovery was 16 days (range: 7–70 days) and 15 days (range: 9–45 days), respectively. A sinusoidal obstruction syndrome (SOS) was reported in one patient (1%). Acute GvHD (aGvHD) occurred in 50% of patients and aGVHD ≥grade III was observed in 13% of patients, while chronic GvHD (cGvHD) was seen in 21% of patients including 4% with severe cGvHD. In evaluable patients, day 100 mortality was 7%.
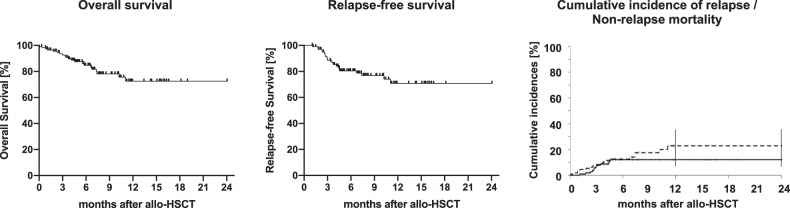
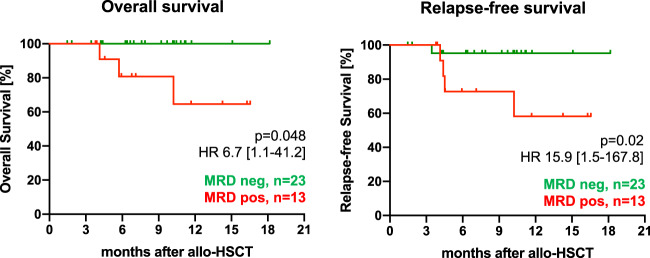
With a median FU of 7.6 months (range: 0.1–24.1 months) 1-year OS, RFS, CIR, and NRM probabilities 73%, 71%, 23%, and 12%, respectively (Fig. 3). In a next step, similar to the entire cohort, we aimed to identify predictors for outcome after allo-HCT. In univariate analyses, only MRD positivity prior to transplant was associated with shorter OS (Fig. 4), while diagnosis of t-AML and active disease at time of transplant adversely impacted RFS (Supplementary Table 5). A complex karyotype, previous treatment with HMA, and a mismatched donor were associated with a higher relapse incidence in univariate analyses (Supplementary Table 5). Due to the low number of events no parameter associated with NRM could be ascertained. Together with the limited availability of information regarding some parameters in all patients this also impeded multivariate analyses.
Fig. 3. Overall (OS), relapse-free survival (RFS), cumulative incidences of relapse (CIR), and non-relapse mortality (NRM) of the transplant cohort.
With a median FU of 7.6 months (range: 0.1–24.1 months) estimated 2-year OS, RFS, CIR, and NRM probabilities of the entire cohort were 73%, 71%, 23%, and 12%, respectively. OS was estimated as time between allo-HSCT and death or date of last follow-up in surviving patients, while RFS was calculated as time from allo-HSCT until relapse or death without relapse censoring those patients, who had not relapsed until and were alive at date of last follow-up. CIR and NRM were considered as competing risks and calculated using cumulative incidence (CI) estimates employing Gray test for univariate comparisons.
Fig. 4. Posttransplant outcome of patients depending on pre-transplant MRD status assessed by flow cytometry (FC).
Among patients with CR/CRi (n = 85) after CPX-351-based induction data on MRD estimated by FC were available for 36 patients (42%) representing MRD negativity in 64% of the patients (n = 23). The figure represents outcome in terms of overall and relapse-free survival for patients with pre-transplant CRMRD− (n = 23, green line) and CRMRD+ (n = 13, orange line).
Discussion
Here, we report data from 188 patients treated in-label with CPX-351 as first-line treatment for the diagnosis of AML. This patient number, which represents to the best of our knowledge the largest cohort outside a clinical trial reported so far, enabled us to provide sufficient data on response and outcome, course of treatment including allo-HCT and to identify predictors for response and survival.
First, our results show that in a real-life setting CPX-351 is used in patients exhibiting high-risk characteristics, which are in many aspects comparable to those in the phase-III trial, but also to those reported in two other real-world data sets of 103 and 71 patients [6, 10, 11]. This applies to the median age, performance status, comorbidities, frequency of AML subtypes, BM blast count, cytogenetics, molecular abnormalities, and NCCN/ELN risk categorization. Indeed, similar to the other cohorts [6, 10, 11] secondary-type mutations [17] in genes like ASXL1, RUNX1, and TP53 mutations represented the most common alterations, but most interestingly neither influenced response or survival following CPX-351 treatment. This is in contrast to the pivotal and also the French study, in which at least TP53 mutations predicted for inferior response. Still, it has to be taken into account that molecular data could not be comprehensively analyzed in all patients. The higher frequency of patients with HMA pretreatment in the prospective trial compared to the three real-world series might be related to the approval of HMA also for low-risk MDS in the US.
Despite many similarities in patients characteristics the treatment course substantially differed between our cohort, the population in the prospective trial, and the two real-world series [6, 10, 11]. Potentially due to the trial design a higher proportion of patients in the prospective study (31% compared to 5–14%) received a second induction with CPX-351. In contrast, in our cohort a lower frequency of patients received at least one CPX-351 consolidation (10%) compared to the three other populations (ranging from 32 to 46%). This is probably a consequence of a much higher rate of patients undergoing allo-HCT in our cohort (62% vs. 27–35%), with the majority proceeding directly to transplant after induction. The latter might be related to a higher frequency of rapidly available donors and/or a general treatment philosophy to proceed to transplant in as many patients as possible. Additionally, since a similar frequency of 20 patients in our cohort received conventional intermediate/high-dose cytarabine for consolidation, the low rate of CPX-351 consolidation might also be related in parts to a greater familiarness of physicians with conventional cytarabine consolidation.
Following induction, we observed an identical CR/CRi rate of 47% like in the prospective trial, while CR/CRi rates were 70 and 59% in the Italian and French patients [6, 10, 11]. Response assessment was not performed at a pre-defined time point in the retrospective analyses. Of note, from 35 patients initially classified as MLFS, 26 turned into CR/CRi at a later point. Thus, from these data a CR/CRi rate of 47% can realistically be expected after CPX-351 induction. If even a higher CR/CRi might be reached, will be prospectively addressed in the ongoing AMLSG 30-18 trial (NCT03897127).
The survival benefit of CPX-351 in the phase-III trial in all patients and those achieving CR/CRi but not undergoing allo-HCT as well as the likelihood of proceeding to allo-HCT suggest the potential for achievement of deeper responses with CPX-351 [6, 18]. While the impact of MRD was not prospectively addressed in this study, we observed MRD negativity detected by MFC in 64% of CR/CRi patients after induction. This appears not only similar to a rate of MRD negativity at the 10−3 sensitivity threshold (assessed with MFC or molecular methods) in 57% of patients of the French series [10], but also seems to be higher than the rate of MRD negativity observed after conventional 3 + 7 induction in such a high-risk population [19]. Accounting for the retrospective character and the limited patient number with available MRD information, the impact of MRD requires prospective and standardized investigation as envisaged, for example, in the AMLSG 30-18 trial.
The median survival of 21 and 16.1 months in our and the French cohort and the 1-year OS rate of 73% support the impression from the prospective trial that CPX-351 confers a survival benefit in such a high-risk population [6, 18]. Still, we have to acknowledge the short median follow-up of 9.3 months of our patients, which is comparable with the French (8.6 months) and Italian (11 months) cohorts, but definitively represents a limitation of our analysis.
Besides its efficacy in terms of remission induction, a favorable toxicity profile and a high rate of allo-HCT with favorable outcome after transplant contributed to the survival benefit in the phase-III trial. The low frequency of grade III/IV adverse events, especially gastrointestinal, expectable times to neutrophil and platelet recovery without excess of severe infectious complications and a day 30 mortality of 8% observed in our analysis confirm the favorable toxicity profile of CPX-351. This probably also contributed to the high rate of patients proceeding to allo-HCT with a RIC-based transplant. Indeed, we observed engraftment in all but two patients, hematopoietic recovery in the vast majority within an appropriate time, only one SOS and a low day 100 mortality of 7%. Together with a low relapse rate, especially in those patients transplanted in MRD-negative CR, this translated into a promising survival after transplant as already previously reported [6, 10, 11]. Finally, the opportunity of a growing number of treatment alternatives for patients with high-risk AML such as venetoclax [20] or glasdegib [21] renders the choice of optimal treatment more complex. Aiming to support physicians in this process, we identified previous HMA treatment and 2017 ELN adverse-risk categorization as negative predictors for survival and a complex karyotype for response in multivariate analysis. While in the prospective trial patients without HMA pretreatment also had also a survival benefit, this could not be supported by the results from the French and Italian consortia [6, 10, 11]. In the French cohort TP53 and PTPN11 mutations were associated with lower response rates, while splicosome mutations correlated with higher OS. In the Italian cohort no predictor for response could be ascertained, and performance of allo-HCT was the only factor associated with better survival. Therefore, the predictive and prognostic impact of patient- and disease-related factors including molecular characteristics in the context of CPX-351 treatment remains still elusive [22] and requires prospective investigation optimally within a randomized trial.
In summary, our data show that under real-world circumstances CPX-351 is an efficient and clinically relevant treatment option for patients with AML-MRC and t-AML. Given the promising outcome following transplant, a combined approach applying an effective induction with CPX-351 and inducing an MRD-negative CR followed by the subsequent allo-HCT without further delay [23] may constitute the treatment approach with the highest probability of cure for this high-risk population.
Supplementary information
Acknowledgements
This work was supported by a restricted grant of JAZZ Pharmaceuticals.
Author contributions
Study conception and design and collection and assembly of data: CR, FS, TSc, and JMM. Provision of patient data: all authors. Data analysis and interpretation: CR, FS, UG, ML, LH, TSa, and JMM. Manuscript writing: CR, FS, HD, KG, TSc, and JMM. Final approval of the manuscript: all authors.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Competing interests
T Schroeder: advisory boards, lecture fees from JAZZ Pharmaceuticals Germany. JMM: personal fee, advisory board from JAZZ Pharmaceuticals Germany. HD: Advisory Board with honoraria: AbbVie, Agios, Amgen, Astellas, Astex Pharmaceuticals, AstraZeneca, Berlin-Chemie, BMS, Celgene, GEMoaB, Gilead, Helsinn, Janssen, Jazz, Novartis, Oxford Biomedica, Roche; clinical research funding: Agios, Amgen, Astellas, Bristol Myers Squibb, Celgene, Jazz Pharmaceuticals, Novartis, Pfizer. KG: Advisory Board with honoraria: AbbVie, JAZZ, BMS/Celgene Deutschland GmbH, Alexion; research funding: BMS/Celgene. T Sauer: Advisory Board with honoraria: AbbVie, Takeda, Astellas. CR: financial travel support: JAZZ Pharmeceuticals and BMS/Celgene Deutschland GmbH; lecture fees: BMS/Celgene Deutschland GmbH. GK: received lecture fees from BMS/Celgene Deutschland GmbH, Novartis, Jazz Pharmaceuticals and Janssen-Cilag GmbH. FS: advisory board with honoraria: JAZZ Pharmaceuticals, AbbVie, medac GmbH. Ulrich Germing: research funding: BMS/Celgene Deutschland GmbH, Novartis.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Christina Rautenberg, Friedrich Stölzel, Thomas Schroeder, Jan Moritz Middeke.
Contributor Information
Thomas Schroeder, Email: Thomas.schroeder@uk-essen.de.
Jan Moritz Middeke, Email: Janmoritz.middeke@ukdd.de.
Supplementary information
The online version contains supplementary material available at 10.1038/s41408-021-00558-5.
References
- 1.Arber DA, Orazi A, Hasserjian R, Thiele J, Borowitz MJ, Le Beau MM, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127:2391–405. doi: 10.1182/blood-2016-03-643544. [DOI] [PubMed] [Google Scholar]
- 2.Nagel G, Weber D, Fromm E, Erhardt S, Lübbert M, Fiedler W, et al. German-Austrian AML Study Group (AMLSG) Epidemiological, genetic, and clinical characterization by age of newly diagnosed acute myeloid leukemia based on an academic population-based registry study (AMLSG BiO) Ann Hematol. 2017;96:1993–2003. doi: 10.1007/s00277-017-3150-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hulegårdh E, Nilsson C, Lazarevic V, Garelius H, Antunovic P, Rangert Derolf Å, et al. Characterization and prognostic features of secondary acute myeloid leukemia in a population-based setting: a report from the Swedish Acute Leukemia Registry. Am J Hematol. 2015;90:208–14. doi: 10.1002/ajh.23908. [DOI] [PubMed] [Google Scholar]
- 4.Kayser S, Döhner K, Krauter J, Köhne CH, Horst HA, Held G, et al. The impact of therapy-related acute myeloid leukemia (AML) on outcome in 2853 adult patients with newly diagnosed AML. Blood. 2011;117:2137–45. doi: 10.1182/blood-2010-08-301713. [DOI] [PubMed] [Google Scholar]
- 5.Granfeldt Østgård LS, Medeiros BC, Sengeløv H, Nørgaard M, Andersen MK, Dufva IH, et al. Epidemiology and clinical significance of secondary and therapy-related acute myeloid leukemia: a national population-based cohort study. J Clin Oncol. 2015;33:3641–9. doi: 10.1200/JCO.2014.60.0890. [DOI] [PubMed] [Google Scholar]
- 6.Lancet JE, Uy GL, Cortes JE, Newell LF, Lin TL, Ritchie EK, et al. CPX-351 (cytarabine and daunorubicin) liposome for injection versus conventional cytarabine plus daunorubicin in older patients with newly diagnosed secondary acute myeloid leukemia. J Clin Oncol. 2018;36:2684–92. doi: 10.1200/JCO.2017.77.6112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mayer LD, Tardi P, Louie AC. CPX-351: a nanoscale liposomal co-formulation of daunorubicin and cytarabine with unique biodistribution and tumor cell uptake properties. Int J Nanomed. 2019;14:3819–30. doi: 10.2147/IJN.S139450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Winters AC, Gutman JA, Purev E, Nakic M, Tobin J, Chase S, et al. Real-world experience of venetoclax with azacitidine for untreated patients with acute myeloid leukemia. Blood Adv. 2019;3:2911–9. doi: 10.1182/bloodadvances.2019000243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Juliusson G, Lazarevic V, Hörstedt AS, Hagberg O, Höglund M, Swedish Acute Leukemia Registry G. Acute myeloid leukemia in the real world: why population-based registries are needed. Blood. 2012;119:3890–9. doi: 10.1182/blood-2011-12-379008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chiche E, Rahmé R, Bertoli S, Dumas PY, Micol JB, Hicheri Y, et al. Real-life experience with CPX-351 and impact on the outcome of high-risk AML patients: a multicentric French cohort. Blood Adv. 2021;5:176–84. doi: 10.1182/bloodadvances.2020003159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guolo F, Fianchi L, Minetto P, Clavio M, Gottardi M, Galimberti S, et al. CPX-351 treatment in secondary acute myeloblastic leukemia is effective and improves the feasibility of allogeneic stem cell transplantation: results of the Italian compassionate use program. Blood Cancer J. 2020;10:96.. doi: 10.1038/s41408-020-00361-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Döhner H, Estey E, Grimwade D, Amadori S, Appelbaum FR, Büchner T, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood. 2017;129:424–47. doi: 10.1182/blood-2016-08-733196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bacigalupo A, Ballen K, Rizzo D, Giralt S, Lazarus H, Ho V, et al. Defining the intensity of conditioning regimens: working definitions. Biol Blood Marrow Transplant. 2009;15:1628–33. doi: 10.1016/j.bbmt.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Glucksberg H, Storb R, Fefer A, Buckner CD, Neiman PE, Clift RA, et al. Clinical manifestations of graft-versus-host disease in human recipients of marrow from HL-A-matched sibling donors. Transplantation. 1974;18:295–304. doi: 10.1097/00007890-197410000-00001. [DOI] [PubMed] [Google Scholar]
- 15.Filipovich AH, Weisdorf D, Pavletic S, Socie G, Wingard JR, Lee SJ, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplant. 2005;11:945–56. doi: 10.1016/j.bbmt.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 16.Sorror ML, Sandmaier BM, Storer BE, Maris MB, Baron F, Maloney DG, et al. Comorbidity and disease status–based risk stratification of outcomes among patients with acute myeloid leukemia or myelodysplasia receiving allogeneic hematopoietic cell transplantation. J Clin. Oncol. 2007;25:4246–54. doi: 10.1200/JCO.2006.09.7865. [DOI] [PubMed] [Google Scholar]
- 17.Lindsley RC, Mar BG, Mazzola E, Grauman PV, Shareef S, Allen SL, et al. Acute myeloid leukemia ontogeny is defined by distinct somatic mutations. Blood. 2015;125:1367–76. doi: 10.1182/blood-2014-11-610543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin TL, Rizzieri DA, Ryan DH, Schiller GJ, Kolitz JE, Uy GL, et al. Older adults with newly diagnosed high-risk/secondary AML who achieved remission with CPX-351: phase 3 post hoc analyses. Blood Adv. 2021;5:1719–28. doi: 10.1182/bloodadvances.2020003510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Paiva B, Vidriales MB, Sempere A, Tarín F, Colado E, Benavente C. et al. PETHEMA (Programa para el Estudio de la Terapéutica en Hemopatías Malignas) Cooperative Study Group. Impact of measurable residual disease by decentralized flow cytometry: a PETHEMA real-world study in 1076 patients with acute myeloid leukemia. Leukemia. 2021;35:2358–70. 10.1038/s41375-021-01126-3. [DOI] [PubMed]
- 20.DiNardo CD, Jonas BA, Pullarkat V, Thirman MJ, Garcia JS, Wei AH, et al. Azacitidine and Venetoclax in previously untreated acute myeloid leukemia. N. Engl J Med. 2020;383:617–29. doi: 10.1056/NEJMoa2012971. [DOI] [PubMed] [Google Scholar]
- 21.Cortes JE, Heidel FH, Hellmann A, Fiedler W, Smith BD, Robak T, et al. Randomized comparison of low dose cytarabine with or without glasdegib in patients with newly diagnosed acute myeloid leukemia or high-risk myelodysplastic syndrome. Leukemia. 2019;33:379–89. doi: 10.1038/s41375-018-0312-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lindsley RC, Gibson CJ, Murdock HM, Stone RM, Cortes JE, Uy GL, et al. Genetic characteristics and outcomes by mutation status in a Phase 3 study of CPX-351 versus 7+3 in older adults with newly diagnosed, high-risk/secondary acute myeloid leukemia (AML) Blood. 2019;134:15–15. doi: 10.1182/blood-2019-124500. [DOI] [Google Scholar]
- 23.Stölzel F, Platzbecker U, Mohr B, Röllig C, Middeke JM, Thiede C, et al. Early intervention with allogeneic hematopoietic cell transplantation during chemotherapy-induced aplasia in patients with high-risk acute myeloid leukemia. Leukemia. 2013;27:2068–72. doi: 10.1038/leu.2013.142. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.