Abstract
Extensive farming systems are characterized by seasons with different diet quality along the year, as pasture availability is strictly depending on climatic conditions. A number of problems for cattle may occur in each season. Tannins are natural polyphenolic compounds that can be integrated in cows’ diet to overcome these seasonal problems, but little is known about their effect on milk quality according to the season. This study was designed to assess the effects of 150 g/head × day of tannin extract supplementation on proximate composition, urea, colour, cheesemaking aptitude, antioxidant capacity, and fatty acid (FA) profile of cow milk, measured during the wet season (WS) and the dry season (DS) of Mediterranean climate. In WS, dietary tannins had marginal effect on milk quality. Conversely, in DS, the milk from cows eating tannins showed 10% lower urea and slight improvement in antioxidant capacity, measured with FRAP and TEAC assays. Also, tannin extract supplementation in DS reduced branched-chain FA concentration, C18:1 t10 to C18:1 t11 ratio and rumenic to linoleic acid ratio. Tannins effect on rumen metabolism was enhanced in the season in which green herbage was not available, probably because of the low protein content, and high acid detergent fibre and lignin contents in diet. Thus, the integration of tannin in the diet should be adapted to the season. This could have practical implications for a more conscious use of tannin-rich extracts, and other tannin sources such as agro-industrial by-products and forages.
Subject terms: Animal physiology, Fatty acids
Introduction
Extensive farming systems are characterized by a dietary imbalance along the year, as they are strictly depending on the climatic conditions1. In particular, the seasonal variations under Mediterranean climate cause the alternation of periods with different pasture availability, with implications on animal performance and product quality. For instance, dairy cows reared under traditional husbandry systems have higher milk yield, and protein and fat contents during the green season compared to the dry season2. In addition, grazing is reported to increase the contents of vitamins and aromatic compounds3, and the proportion of polyunsaturated fatty acids (PUFA) and conjugated linoleic acid4 in milk. On the other hand, young fresh herbage may cause an excess of degradable protein in the rumen with implications on protein metabolism efficiency and nitrogen excretion5.
To overcome these seasonal problems, farmers can adopt several strategies. For example, tannins are plant polyphenols used in ruminant farming as growth and health promoter. Many forages and agricultural by-products are naturally rich in tannins, especially in plant species characterizing marginal areas or dry habitats6, but tannins can be also added as dietary supplement for a better control of dose and quality. Thanks to their antimicrobial and protein binding activities, tannins are known to affect ruminal biohydrogenation (BH) and N metabolism, with potential positive consequences on milk quality and N emissions7.
However, the information available in literature does not clarify if and how the effects of dietary tannins might vary according to the season in extensive farming systems. In a recent study, a different response on in vitro rumen BH and fermentation was observed when tannin extracts were incubated with a green forage or a hay substrate8. Therefore, we hypothesized that a different effect of dietary tannins could be observed on cow milk quality when supplemented during the grazing season or the season in which diet is based on dry forages. Thus, the present study aimed to assess the effects of supplementing tannin extract to dairy cows grazing in two different seasons (spring and summer) under Mediterranean climate on the milk quality. We chose to fit into on-farm conditions to directly test the practical effects of dietary tannin extract.
The experimental design of studies focusing on dietary tannins generally provide for sampling at the end of the trial9–12. However, an earlier effect of dietary tannins could not be ruled out. Therefore, the present study was also designed to evaluate the effect of dietary tannins on milk quality from the beginning of the supplementation, throughout the experimental period, to have a deeper insight of the subject.
Methods
Experimental design, animals and diets
All the experiment was performed in accordance with relevant guidelines and regulations (following the ARRIVE guidelines13). All procedures were approved by the animal welfare committee (OPBA) of the University of Catania (UNCTCLE-0015295). The experimental design is detailed in a previous study12. Two experiments were performed in a commercial extensive farm located in an upland area of the Mediterranean island of Sicily, Italy (36° 57′ N, 14° 40′ E; altitude: 670 m). The first experiment was carried out during the wet season (WS), between March and April 2019, and the second one during the dry season (DS), in July 2019. In both experiments, 14 lactating dairy cows (Modicana breed) were divided into two groups (n = 7), namely control (CON) and tannin (TAN), balanced for average milk yield, and protein and fat contents recorded in the two days before the beginning of each trial, together with DIM, parity, and BCS, as reported in Menci et al.12. The groups were composed of different animals in WS and DS experiment. In WS, the cows were free to graze on 20 ha of spontaneous pasture. In DS, the cows were free to graze on 20 ha of dry stubble of an annual crop (vetch:oat:barley 40:40:20), and no fresh herbage was available. A detailed description of site, weather conditions, and pasture botanical composition is reported in Menci et al.12.
In both experiments, commercial pelleted concentrate was individually offered to cows in two equal meals just before milking, at a rate of 6.4 kg/head × day in WS and 9.6 kg/head × day in DS, following the farm routine. Pelleted concentrate was composed of: corn grain (420 g/kg), soybean meal CP 48% (250 g/kg), wheat middling (100 g/kg), corn flake (66 g/kg), carob germ (60 g/kg), carob pod (30 g/kg), beet pulp (30 g/kg), rumen protected fat (10 g/kg; Magnapac, Or Sell S.p.a.), Na2CO3 (10 g/kg), CaCO3 (10 g/kg), NaCl (8 g/kg), vitamins and minerals supplement (4 g/kg), urea (2 g/kg). Moreover, 2 kg/head of hay (vetch:oat:barley 40:40:20) was daily offered to cows in the same way as concentrate. The cows always completely consumed the offered concentrate and hay. The chemical composition of feedstuffs is shown in Table 1. In both WS and DS experiments, TAN cows daily received 150 g/head of a commercial tannin extract (Silvafeed ByProX; Silvateam), a mixture of chestnut (Castanea sativa Mill., 60%) and quebracho (Schinopsis lorentzii Engl., 40%) tannins, included in pelleted concentrate. Total phenolic compounds concentration in tannin extract was 688 g of tannic acid equivalents per kg of DM, with 90.2% of tannins, according to the method of Makkar et al.14. Basing on the potential intake capacity of experimental cows, estimated according to INRA method15, the tannin extract intake corresponded to 1% of estimated dry matter intake (DMI).
Table 1.
Chemical composition of feeds used in wet season (WS) and dry season (DS) experiments.
| Item | Concentrate | Hay | Pasture (only in WS) | Stubble (only in DS) |
|---|---|---|---|---|
| DM, g/kg | 889 | 833 | 186 | 876 |
| Chemical composition, g/kg DM | ||||
| CP | 200 | 79 | 222 | 69 |
| Ether extract | 36 | 12 | 28 | 11 |
| NDF | 179 | 708 | 415 | 672 |
| ADF | 80 | 460 | 269 | 472 |
| ADL | 22 | 62 | 38 | 76 |
| Ash | 51 | 66 | 94 | 67 |
| Phenolic compounds, g TAeqa/kg DM | ||||
| Phenols | 5.2 | 5.2 | 14.2 | 5.4 |
| Tannins | 3.9 | 1.6 | 4.7 | 1.7 |
| Protein fractionsb, g/100 g CP | ||||
| A | 15.1 | 37.3 | 37.1 | 22.3 |
| B1 | 7.9 | 10.8 | 7.6 | 18.2 |
| B2 | 59.6 | 12.2 | 21.4 | 20.3 |
| B3 | 12.9 | 29.5 | 28.2 | 26.0 |
| C | 4.5 | 10.1 | 5.8 | 13.1 |
| Fatty acids, g/100 g fatty acids | ||||
| C16:0 | 18.2 | 29.2 | 14.1 | 25.1 |
| C18:0 | 9.2 | 6.0 | 2.4 | 6.2 |
| C18:1 c9 | 16.8 | 9.4 | 3.0 | 6.5 |
| C18:2 c9c12 | 36.2 | 27.0 | 12.1 | 21.1 |
| C18:3 c9c12c15 | 1.8 | 16.0 | 52.2 | 21.6 |
aTAeq tannic acid equivalents.
bA NPN, B1 buffer-soluble true protein, B2 neutral detergent soluble protein, B3 acid detergent soluble protein, C acid detergent insoluble protein.
In both WS and DS, the feeding trial lasted 23 days and tannin extract supplementation started at morning milking of day 0. To ensure correct feeding, the farmer was the only person aware of the treatment groups allocation. Blinding was used in the next steps of experimental process.
Feedstuff sampling and analyses
During both experiments, samples of concentrates, hay, and pasture or dry stubble were collected weekly, vacuum-packed and stored at − 20 °C. The weekly subsamples were then pooled in order to get a representative sample for each feed.
Ether extract, CP, and ash were determined according to AOAC16 methods 920.39, 976.06, and 942.05, respectively. Protein fractions were calculated according to the Cornell Net Carbohydrate and Protein System, as modified by Licitra et al.17. The analyses of NDF, ADF, and ADL were performed following the method of Van Soest et al.18. Total phenolic compounds and total tannins were analysed according to the procedure of Makkar et al.14, as modified by Luciano et al.19. Fatty acid profile of feeds was determined through a one-step extraction-transesterification with chloroform and sulfuric acid (2% in methanol, vol/vol) as methylation reagent20. Gas-chromatograph (ThermoQuest) equipment and settings were the same as described by Natalello et al.21.
Milk sampling and analyses
Milk sampling was performed at the following days of trial: − 2, − 1, 1, 2, 3, 4, 5, 8, 11, 15, 18 and 23. Cows were individually milked twice a day (0700 h and 1700 h) with a milking machine (43 kPa vacuum, 60 pulsations/min). The milk of each cow was sampled individually. Each sampling day included the milk of two subsamples (250 mL) from two consecutive milkings: the evening milking and the following morning milking. The evening milking subsample was stored refrigerated until the next morning. To get a representative daily sample, the two subsamples were pooled according to the proportion between the milk amount recorded at the respective evening and morning milking. Analyses of proximate composition, somatic cells count (SCC), colour parameters, laboratory cheese yield (LCY), and milk coagulation properties (MCP) were immediately performed on fresh milk samples. The aliquots for antioxidant capacity assays and FA profile determination were stored at − 80 °C. Before freezing, sodium azide was added to the aliquots for FA profile determination, to a final concentration of 0.3 g/L.
Fat, lactose and protein contents in milk, and milk urea nitrogen (MUN) were analysed with a Milkoscan FT 1 (Foss, Hillerod), according to ISO 962222. On the same aliquot, SCC was determined by using a BacSomatic (Foss), according to ISO 13366-223.
Milk colour parameters were measured using a Minolta CM-2022 portable spectrophotometer (d/8° geometry) in the CIE L*a*b space (illuminant A, 10° standard observer). Measured parameters were lightness (L*), redness (a*), yellowness (b*), chroma (C*), hue angle (H*), and the reflectance spectra between 400 and 700 nm.
The LCY and laboratory dry matter cheese yield (LDMCY) were determined according to the method of Hurtaud et al.24, using a commercial liquid calf rennet (105 IMCU/mL, 80% chymosin and 20% pepsin; Biotec Fermenti S.r.l.). The MCP of milk were analysed using a formagraph (Maspres and Foss Italia), following the method of Zannoni and Annibaldi25. Determined parameters were clotting time (time needed for the beginning of coagulation), firming time (time needed to reach 20 mm of amplitude on the chart), and curd firmness (i.e., amplitude of the chart in mm) after 30 min and after two times clotting time. A detailed procedure for LCY, LDMCY, and MCP is reported in Menci et al.12.
The antioxidant capacity of the hydrophilic fraction of milk was assessed by ferric reducing antioxidant power (FRAP) and Trolox-equivalent antioxidant capacity (TEAC) assays. Milk was pre-treated before analyses, as follows. Defrosted samples were vortexed thoroughly and 100 µL of milk was transferred in a 1.5-mL tube with 900 µL of water and 200 µL of hexane. After centrifugation at 1500×g for 10 min at 4 °C, two aliquots of 50 µL and 20 µL of the lower phase were transferred in plastic tubes and analysed in duplicate for FRAP assay and TEAC assay, respectively. The FRAP assay was performed following Benzie and Strain26 method, with modifications. A solution 50:5:5:6 of pH 3.6 acetate buffer (300 mM sodium acetate trihydrate in 1.6% acetic acid), 0.01 M TPTZ [2,4,6-tris(2-pyridyl)-s-triazine] in 0.04 M hydrochloric acid, 0.02 M ferric chloride hexahydrate, and distilled water was made, and 1650 µL of this solution was added to samples. After incubation in water bath at 37 °C for 60 min, absorbance at 593 nm was read using a double beam UV/Vis spectrophotometer (UV-1601, Shimadzu Corporation). An external calibration curve was prepared using 1 mM ferrous sulphate heptahydrate, and FRAP values were expressed as mmol of Fe2+ equivalent per L of milk. The TEAC assay was performed according to Re et al.27, with some modifications. A stable radical solution 1:1 of 14 mM ABTS (2,2-azinobis-3-ethylbenzothiazoline-6-sulfonic acid) and 4.9 mM potassium persulfate was incubated in the dark at room temperature for 12–14 h and then diluted to an absorbance of 0.75 at 734 nm. After adding 2 mL of diluted radical solution, samples were incubated at 30 °C for 60 min and absorbance at 734 nm was read using UV-1601 spectrophotometer. The reduction of absorbance was compared to a blank and an external seven-points calibration curve was prepared using 2.5 mM Trolox solution. Results are expressed as mmol of Trolox equivalent per L of milk.
The FA profile of experimental milk was determined by gas chromatographic analysis of fatty acid methyl esters, after fat separation according to the method B described by Feng et al.28, with some modification. Briefly, the top fat-cake layer of 50-mL milk samples was removed after centrifugation at 13,000×g for 30 min at 4 °C. Fat was then transferred in a 2-mL tube, let melt at room temperature for 30 min and centrifuged at 19,300×g for 20 min. About 50 mg of the top lipid-layer was then transferred in a glass tube for transesterification, following the method described by Christie29, with modifications. Briefly, 1 mL of 0.5 N methanolic sodium methoxide was added, and samples were vortexed for 3 min. After a 5 min pause, 2 mL of hexane was added, and samples were vortexed for 30 s. The upper phase was then transferred in a 2-mL vial, a little spoon of sodium sulphate was added, and vials were then stored at − 20 °C. Gas-chromatograph (ThermoQuest) equipment and settings were the same as described by Natalello et al.21. Moreover, the separation of C18:1 t10 and C18:1 t11 was achieved by isothermal analysis at 165 °C.
Calculations and statistics
The reflectance spectrum at wavelengths between 530 and 450 nm was elaborated as done by Priolo et al.30 to calculate the integral value (I450–530). Before statistical analysis, SCC data was transformed to log10/mL to obtain normalized distribution.
All data from WS and DS trials were statistically elaborated separately using a mixed model ANOVA for repeated measures of IBM SPSS 21 For Analytics, with individual milk sample as statistical unit, using formula (1).
| 1 |
where yijkl is the observation, µ is the overall mean, Ti is the fixed effect of treatment (i = 1–2), Dj is the fixed effect of sampling day (j = 1–10), (D × T)ij is the interaction between diet and sampling time, Ck is the random effect of the cow nested within the treatment (k = 1–7), BXik is the covariate adjustment for each cow, and eijkl is the residual error. The milk sampled in the two days before the beginning of the trial (i.e., sampling days − 2 and − 1) was analysed and averaged to constitute the covariate for statistical elaboration. In addition, statistical elaboration was adjusted for a covariate composed of DIM. For individual FA, fat content was included as covariate in the statistical model. When the effect of the covariate had P ≤ 0.100, it was removed from the statistical model. Multiple comparisons among means were performed using the Tukey’s test and differences between treatment means were considered to be significant at P ≤ 0.050 and a trend towards significance at P ≤ 0.100. All the results showed in tables refer to estimated marginal means.
Results
WS experiment
Table 2 shows the results on proximate composition, physical parameters, and antioxidant capacity of WS milk. Dietary tannins did not affect (P > 0.100) milk yield, milk composition, and colour parameters. Likewise, milk cheesemaking parameters and antioxidant capacity did not differ (P > 0.100) between the two dietary groups.
Table 2.
Effect of dietary tannin extract on physicochemical properties of milk in wet season experiment.
| Itema | Treatmentb (T) | SEM | P valuec | |||
|---|---|---|---|---|---|---|
| CON | TAN | T | Day (D) | T × D | ||
| Milk yield, kg/day | 11.60 | 13.21 | 0.616 | ns | * | ns |
| ECM, kg/day | 12.84 | 14.51 | 0.643 | ns | † | ns |
| Fat, g/100 g | 3.908 | 3.971 | 0.087 | ns | ** | ns |
| Lactose, g/100 g | 4.584 | 4.611 | 0.029 | ns | ** | ns |
| Protein yield, g/day | 460 | 497 | 18.6 | ns | * | ns |
| Protein, g/100 g | 3.927 | 3.918 | 0.051 | ns | ns | ns |
| Casein, g/100 g | 3.042 | 3.037 | 0.047 | ns | ns | ns |
| Urea, mg/dL | 33.00 | 33.40 | 0.625 | ns | *** | † |
| SCC, log10/mL | 2.843 | 2.898 | 0.040 | ns | ns | ns |
| Colour parameters | ||||||
| L* | 70.00 | 70.32 | 0.295 | ns | *** | ns |
| a* | − 1.434 | − 1.491 | 0.056 | ns | ** | ns |
| b* | 3.59 | 3.14 | 0.237 | ns | *** | ns |
| C* | 3.96 | 3.65 | 0.204 | ns | *** | ns |
| H* | 115.2 | 120.0 | 2.24 | ns | *** | ns |
| I450–530 | − 264 | − 234 | 12.0 | ns | *** | ns |
| Cheesemaking properties | ||||||
| LCY, g/kg | 263.9 | 265.5 | 4.42 | ns | ns | ns |
| LDMCY, g/kg | 83.33 | 83.88 | 0.725 | ns | *** | ns |
| R, min:s | 24:15 | 24:23 | 0:42 | ns | *** | ns |
| K20, min:s | 5:33 | 5:10 | 0:20 | ns | ns | ns |
| A30, mm | 30.2 | 27.6 | 2.75 | ns | * | ns |
| A2R, mm | 44.9 | 45.2 | 1.00 | ns | ns | ns |
| Antioxidant capacity, mmol/L | ||||||
| FRAP | 4.90 | 4.80 | 0.130 | ns | *** | ns |
| TEAC | 10.89 | 9.87 | 0.427 | ns | *** | ns |
aL* lightness, a* redness, b* yellowness, C* chroma, H* hue angle, I450–530 integral value of the absorbance spectrum from 450 to 530 nm, LCY laboratory cheese yield, LDMCY laboratory dry matter cheese yield, R clotting time, K20 firming time, A30 curd firmness after 30 min, A2R curd firmness after two times R, FRAP ferric reducing antioxidant power, TEAC Trolox-equivalent antioxidant capacity, ECM energy corrected milk, SCC somatic cells count.
bCON control group, TAN group receiving 150 g/head × day of tannin extract.
cns ≥ 0.100; †< 0.100; *< 0.050; **< 0.010; ***< 0.001.
Concerning FA concentration (Table 3), no differences were found between dietary groups for almost all FA, but we observed a different kinetic of some FA along the trial. Tannin supplementation depressed (P = 0.021) the concentration of de novo FA with less than 16 C, particularly C8:0 (P = 0.033), C10:0 (P = 0.010), C12:0 (P = 0.030) and C14:0 (P = 0.082), but only in the first two days of treatment (Fig. 1). Moreover, a higher proportion of C18:1 c9 (P = 0.047), C18:1 t9 (P = 0.001), C18:1 c11 (P = 0.040) was observed in TAN milk in the first days of tannin administration, reflecting on total monounsaturated fatty acids (MUFA) concentration (P = 0.028; Fig. 2). On the other hand, FA profile of CON milk did not vary statistically throughout the observation period.
Table 3.
Effect of dietary tannin extract on fatty acid profile of milk in wet season experiment.
| Itema | Treatmentb (T) | SEM | P valuec | |||
|---|---|---|---|---|---|---|
| CON | TAN | T | Day (D) | T × D | ||
| C4:0 | 1.871 | 1.860 | 0.038 | ns | ns | ns |
| C6:0 | 1.643 | 1.626 | 0.031 | ns | ns | ns |
| C8:0 | 1.257 | 1.238 | 0.027 | ns | * | * |
| C10:0 | 3.218 | 3.127 | 0.078 | ns | *** | ** |
| C11:0 | 0.370 | 0.380 | 0.010 | ns | *** | ns |
| C12:0 | 3.943 | 3.823 | 0.098 | ns | *** | * |
| C13:0 | 0.255 | 0.250 | 0.008 | ns | *** | ns |
| C14:0 | 10.99 | 11.06 | 0.137 | ns | *** | † |
| C14:0 iso | 0.159 | 0.154 | 0.005 | ns | *** | ns |
| C14:1 c9 | 0.843 | 0.907 | 0.024 | ns | *** | ns |
| C15:0 | 1.324 | 1.304 | 0.020 | ns | *** | ns |
| C15:0 iso | 0.308 | 0.309 | 0.005 | ns | *** | ns |
| C15:0 anteiso | 0.648 | 0.638 | 0.014 | ns | *** | ns |
| C16:0 | 24.83 | 24.54 | 0.271 | ns | ns | ns |
| C16:0 iso | 0.325 | 0.320 | 0.008 | ns | ** | ns |
| C16:1 t9 | 0.157 | 0.158 | 0.007 | ns | ** | ns |
| C16:1 c9 | 1.223 | 1.214 | 0.024 | ns | *** | ns |
| C17:0 | 0.577 | 0.560 | 0.010 | ns | ** | ns |
| C17:0 iso | 0.412 | 0.419 | 0.009 | ns | ** | ns |
| C17:0 anteiso | 0.605 | 0.575 | 0.015 | ns | *** | ns |
| C18:0 | 10.18 | 9.73 | 0.155 | ns | *** | ns |
| C18:1 t6 + t7 + t8 | 0.186 | 0.188 | 0.005 | ns | * | ns |
| C18:1 t9 | 0.277 | 0.272 | 0.005 | ns | *** | *** |
| C18:1 t10 | 0.266 | 0.271 | 0.014 | ns | ns | ns |
| C18:1 t11 | 2.870 | 2.980 | 0.082 | ns | ns | ns |
| C18:1 c6 | 0.722 | 0.729 | 0.024 | ns | † | ns |
| C18:1 c9 | 19.86 | 20.24 | 0.329 | ns | † | * |
| C18:1 c11 | 0.523 | 0.527 | 0.012 | ns | *** | * |
| C18:1 c12 | 0.202 | 0.193 | 0.005 | ns | ** | ns |
| C18:1 c13 | 0.061 | 0.059 | 0.002 | ns | ns | ns |
| C18:1 c14 | 0.357 | 0.362 | 0.011 | ns | † | ns |
| C18:2 t8c13 | 0.268 | 0.260 | 0.010 | ns | *** | ns |
| C18:2 t9c13 | 0.501 | 0.498 | 0.016 | ns | ** | ns |
| C18:2 c9c12 (LA) | 2.306 | 2.389 | 0.061 | ns | *** | ns |
| C18:2 c9t11 (RA) | 1.444 | 1.523 | 0.047 | ns | ** | ns |
| C18:3 c9c12c15 | 1.114 | 1.134 | 0.025 | ns | *** | ns |
| C20:0 | 0.173 | 0.161 | 0.004 | ns | *** | ns |
| C20:3 n-6 | 0.099 | 0.093 | 0.004 | ns | * | ns |
| C20:4 n-6 | 0.152 | 0.155 | 0.005 | ns | *** | ns |
| C20:5 n-3 | 0.062 | 0.057 | 0.001 | ns | *** | ns |
| C22:0 | 0.129 | 0.122 | 0.003 | ns | *** | ns |
| C22:5 n-3 | 0.138 | 0.129 | 0.005 | ns | * | ns |
| C23:0 | 0.045 | 0.040 | 0.002 | ns | ** | ns |
| C24:0 | 0.091 | 0.087 | 0.003 | ns | *** | ns |
| Σ de novo FA < 16C | 22.97 | 22.70 | 0.368 | ns | *** | * |
| Σ SFA | 58.56 | 57.10 | 0.554 | ns | ns | ns |
| Σ MUFA | 27.27 | 28.04 | 0.397 | ns | ns | * |
| Σ PUFA | 6.24 | 6.43 | 0.119 | ns | † | ns |
| Σ OCFA | 2.937 | 2.922 | 0.042 | ns | *** | ns |
| Σ BCFA | 2.469 | 2.405 | 0.053 | ns | *** | ns |
| Σ iso-FA | 1.214 | 1.192 | 0.026 | ns | *** | ns |
| Σ anteiso-FA | 1.255 | 1.211 | 0.028 | ns | *** | ns |
| SFA/PUFA | 9.56 | 9.05 | 0.245 | ns | † | ns |
| n-6/n-3 PUFA | 2.017 | 2.073 | 0.046 | ns | *** | ns |
| C18:1 t10/t11 | 0.100 | 0.097 | 0.007 | ns | ns | ns |
| BHI | 7.76 | 7.96 | 0.194 | ns | * | ns |
| RA / LA | 0.649 | 0.660 | 0.022 | ns | *** | ns |
| DSI C14 | 0.072 | 0.076 | 0.002 | ns | *** | ns |
aDe novo FA < 16C sum of C4:0, C6:0, C8:0, C10:0, C12:0 and C14:0, SFA saturated fatty acids, MUFA monounsaturated fatty acids, PUFA polyunsaturated fatty acids, OCFA odd-chain fatty acids, BCFA branched-chain fatty acids, BHI biohydrogenation intermediates, DSI C14 desaturation index, calculated as C14:1 c9/(C14:0 + C14:1 c9).
bCON control group, TAN group receiving 150 g/head × day of tannin extract.
cns ≥ 0.100; †< 0.100; *< 0.050; **< 0.010; ***< 0.001.
Figure 1.
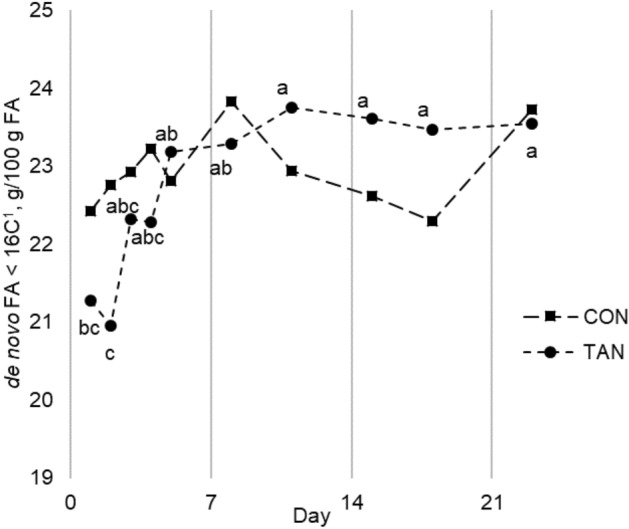
Concentration of de novo fatty acids (FA) in cow milk during wet season experiment. CON control group, TAN group receiving 150 g/head per day of tannin extract. 1De novo FA < 16C = sum of C4:0. C6:0. C8:0. C10:0. C12:0 and C14:0. a,b,cPoints with different letters differ at P < 0.050, within TAN group. There was no difference between points within CON group.
Figure 2.
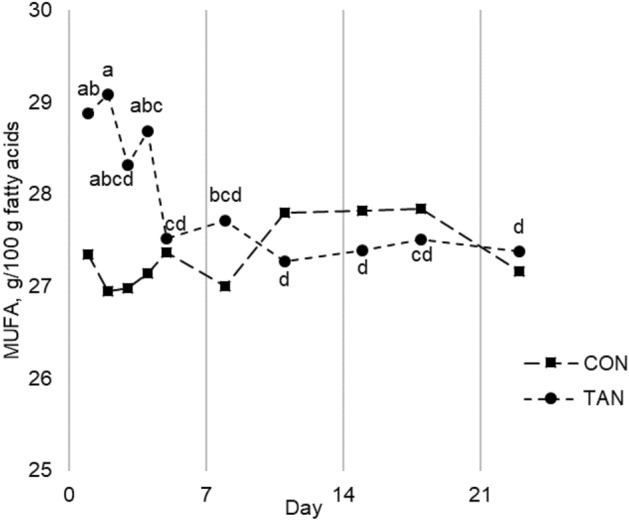
Sum of monounsaturated fatty acids (MUFA) concentrations in cow milk during wet season experiment. CON control group, TAN group receiving 150 g/head per day of tannin extract. a,b,c,dPoints with different letters differ at P < 0.050, within TAN group. There was no difference between points within CON group.
The sampling day effect was significant (P < 0.050) for almost all the parameters analysed.
DS experiment
Proximate composition, physical parameters, antioxidant capacity, and cheesemaking properties of milk in the DS experiment are shown in Table 4. Milk from TAN group had 10% lower (P = 0.042) urea nitrogen compared to CON group. Tannin extract supplementation tendentially increased FRAP (P = 0.056) and TEAC (P = 0.083) values in milk.
Table 4.
Effect of dietary tannin extract on physicochemical properties of milk in dry season experiment.
| Itema | Treatmentb (T) | SEM | P valuec | |||
|---|---|---|---|---|---|---|
| CON | TAN | T | Day (D) | T × D | ||
| Milk yield, kg/day | 12.58 | 12.99 | 0.115 | ns | *** | ns |
| ECM, kg/day | 12.98 | 13.51 | 0.173 | ns | *** | ns |
| Fat, g/100 g | 3.81 | 3.99 | 0.113 | ns | ns | ns |
| Lactose, g/100 g | 4.669 | 4.630 | 0.024 | ns | ns | ns |
| Protein yield, g/day | 409.8 | 416.4 | 3.03 | ns | * | ns |
| Protein, g/100 g | 3.386 | 3.353 | 0.016 | ns | † | ns |
| Casein, g/100 g | 2.569 | 2.533 | 0.014 | ns | * | ns |
| Urea, mg/dL | 29.24 | 26.53 | 0.533 | * | ** | ns |
| SCC, log10/mL | 2.963 | 3.014 | 0.038 | ns | *** | † |
| Colour parameters | ||||||
| L* | 67.32 | 68.04 | 0.547 | ns | ** | ns |
| a* | − 1.980 | − 1.907 | 0.062 | ns | *** | ns |
| b* | 0.32 | 0.64 | 0.171 | ns | *** | ns |
| C* | 2.51 | 2.59 | 0.100 | ns | * | ns |
| H* | 165.4 | 159.2 | 3.64 | ns | ** | ns |
| I450–530 | − 73.1 | − 86.9 | 7.26 | ns | *** | ns |
| Cheesemaking properties | ||||||
| LCY, g/kg | 240.0 | 245.0 | 2.86 | ns | *** | ns |
| LDMCY, g/kg | 73.63 | 73.96 | 0.580 | ns | ns | ns |
| R, min:s | 19:38 | 19:51 | 0:27 | ns | *** | ns |
| K20, min:s | 5:04 | 5:29 | 0:16 | ns | * | ns |
| A30, mm | 35.4 | 30.8 | 1.64 | ns | *** | ns |
| A2R, mm | 39.3 | 39.2 | 1.26 | ns | ns | ns |
| Antioxidant capacity, mmol/L | ||||||
| FRAP | 3.31 | 3.94 | 0.122 | † | *** | ns |
| TEAC | 8.82 | 10.21 | 0.365 | † | *** | ns |
aL* lightness, a* redness, b* yellowness, C* chroma, H* hue angle, I450–530 integral value of the absorbance spectrum from 450 to 530 nm, LCY laboratory cheese yield, LDMCY laboratory dry matter cheese yield, R clotting time, K20 firming time, A30 curd firmness after 30 min, A2R curd firmness after two times R, FRAP ferric reducing antioxidant power, TEAC Trolox-equivalent antioxidant capacity, ECM energy corrected milk, SCC somatic cells count.
bCON control group, TAN group receiving 150 g/head × day of tannin extract.
cns ≥ 0.100; †< 0.100; *< 0.050; **< 0.010; ***< 0.001.
The FA profile of DS milk is reported in Table 5. Dietary tannins decreased (P < 0.001) branched-chain fatty acids (BCFA), particularly C15:0 anteiso, C16:0 iso, and C17:0 iso (P = 0.009, P = 0.007, and P = 0.040, respectively). Milk from TAN group had tendentially lower (P = 0.063) C18:1 t10 concentration, resulting in tendentially lower (P = 0.093) C18:1 t10 to C18:1 t11 ratio (t10/t11). Also, C18:2 c9t11 concentration was lower (P = 0.043) and C18:2 c9c12 concentration tended to be higher (P = 0.057) in TAN milk. As a result, C18:2 c9t11 to C18:2 c9c12 ratio (RA/LA) was lower (P = 0.044) in milk from cows given tannin extract.
Table 5.
Effect of dietary tannin extract on fatty acid profile of milk in dry season experiment.
| Itema | Treatmentb (T) | SEM | P valuec | |||
|---|---|---|---|---|---|---|
| CON | TAN | T | Day (D) | T × D | ||
| C4:0 | 1.812 | 1.805 | 0.043 | ns | † | † |
| C6:0 | 1.529 | 1.454 | 0.025 | ns | *** | ns |
| C8:0 | 0.970 | 0.932 | 0.019 | ns | * | † |
| C10:0 | 2.099 | 2.093 | 0.048 | ns | † | ns |
| C11:0 | 0.256 | 0.238 | 0.006 | ns | ns | † |
| C12:0 | 2.468 | 2.475 | 0.054 | ns | † | ns |
| C13:0 | 0.154 | 0.148 | 0.003 | ns | ns | ns |
| C14:0 | 9.40 | 9.28 | 0.125 | ns | † | ns |
| C14:0 iso | 0.238 | 0.222 | 0.007 | ns | * | ns |
| C14:1 c9 | 0.794 | 0.727 | 0.015 | ns | *** | ** |
| C15:0 | 1.251 | 1.228 | 0.018 | ns | ** | ns |
| C15:0 iso | 0.420 | 0.399 | 0.007 | ns | ** | ns |
| C15:0 anteiso | 0.792 | 0.725 | 0.010 | ** | *** | ns |
| C16:0 | 26.15 | 26.30 | 0.147 | ns | ns | ns |
| C16:0 iso | 0.495 | 0.441 | 0.008 | ** | * | ns |
| C16:1 t9 | 0.080 | 0.078 | 0.003 | ns | *** | ns |
| C16:1 c9 | 1.437 | 1.483 | 0.022 | ns | *** | ns |
| C17:0 | 0.731 | 0.735 | 0.009 | ns | *** | ns |
| C17:0 iso | 0.488 | 0.457 | 0.006 | * | *** | ns |
| C17:0 anteiso | 0.735 | 0.734 | 0.004 | ns | *** | ns |
| C18:0 | 10.45 | 10.54 | 0.137 | ns | ** | ns |
| C18:1 t6 + t7 + t8 | 0.179 | 0.175 | 0.005 | ns | * | ns |
| C18:1 t9 | 0.327 | 0.315 | 0.004 | ns | † | ns |
| C18:1 t10 | 0.270 | 0.240 | 0.007 | † | *** | ns |
| C18:1 t11 | 1.653 | 1.603 | 0.020 | ns | *** | † |
| C18:1 c6 | 0.493 | 0.459 | 0.011 | ns | *** | ns |
| C18:1 c9 | 25.32 | 25.20 | 0.312 | ns | * | ns |
| C18:1 c11 | 0.622 | 0.649 | 0.008 | ns | * | * |
| C18:1 c12 | 0.249 | 0.242 | 0.008 | ns | ** | † |
| C18:1 c13 | 0.050 | 0.060 | 0.003 | ns | * | ns |
| C18:1 c14 | 0.230 | 0.221 | 0.005 | ns | *** | ns |
| C18:2 t8c13 | 0.169 | 0.153 | 0.004 | † | *** | ns |
| C18:2 t9c13 | 0.106 | 0.108 | 0.007 | ns | * | ns |
| C18:2 c9c12 (LA) | 2.632 | 2.699 | 0.058 | † | * | ns |
| C18:2 c9t11 (RA) | 0.851 | 0.791 | 0.011 | * | *** | ns |
| C18:3 c9c12c15 | 0.360 | 0.381 | 0.015 | ns | *** | ns |
| C20:0 | 0.241 | 0.248 | 0.003 | ns | ** | † |
| C20:3 n-6 | 0.100 | 0.104 | 0.002 | ns | *** | ns |
| C20:4 n-6 | 0.140 | 0.140 | 0.003 | ns | *** | ns |
| C20:5 n-3 | 0.075 | 0.082 | 0.002 | ns | ** | ns |
| C22:0 | 0.138 | 0.148 | 0.004 | ns | *** | ns |
| C22:5 n-3 | 0.102 | 0.105 | 0.003 | ns | *** | ns |
| C23:0 | 0.064 | 0.064 | 0.002 | ns | ** | ns |
| C24:0 | 0.041 | 0.059 | 0.003 | † | *** | ns |
| Σ de novo FA < 16C | 18.28 | 18.06 | 0.273 | ns | † | ns |
| Σ SFA | 55.13 | 55.52 | 0.336 | ns | ** | ns |
| Σ MUFA | 32.31 | 31.83 | 0.321 | ns | * | ns |
| Σ PUFA | 4.720 | 4.790 | 0.081 | ns | * | † |
| Σ OCFA | 2.515 | 2.440 | 0.035 | ns | *** | ns |
| Σ BCFA | 3.185 | 2.960 | 0.025 | *** | ns | ns |
| Σ iso-FA | 1.600 | 1.500 | 0.016 | ** | ns | ns |
| Σ anteiso-FA | 1.532 | 1.454 | 0.011 | ** | ** | ns |
| SFA/PUFA | 11.89 | 11.80 | 0.260 | ns | * | ns |
| n-6/n-3 PUFA | 5.75 | 5.55 | 0.101 | ns | *** | * |
| C18:1 t10/t11 | 0.172 | 0.156 | 0.004 | † | *** | ns |
| BHI | 5.297 | 5.175 | 0.059 | ns | *** | ns |
| RA/LA | 0.329 | 0.301 | 0.006 | * | *** | ns |
| DSI C14 | 0.075 | 0.075 | 0.001 | ns | * | ns |
aDe novo FA < 16C sum of C4:0, C6:0, C8:0, C10:0, C12:0 and C14:0, SFA saturated fatty acids, MUFA monounsaturated fatty acids, PUFA polyunsaturated fatty acids, OCFA odd-chain fatty acids, BCFA branched-chain fatty acids, BHI biohydrogenation intermediates, DSI C14 desaturation index, calculated as C14:1 c9/(C14:0 + C14:1 c9).
bCON control group, TAN group receiving 150 g/head × day of tannin extract.
cns ≥ 0.100; †< 0.100; *< 0.050; **< 0.010; ***< 0.001.
A significant interaction of dietary treatment with sampling day was found for few FA (P < 0.050), reflecting a different evolution of the concentration throughout the trial in the two groups. The concentration of C14:1 c9 in CON milk was higher at the 23rd day of trial than in the first four days, whereas in TAN milk there were no changes along time (P = 0.004). The concentration of C18:1 c11 in TAN milk after two weeks of treatment (i.e., at sampling day 15, 18 and 23) was higher than it was at the first sampling day, whereas no significative variation was observed in CON milk (P = 0.021). Finally, n-6 PUFA to n-3 PUFA ratio (n-6/n-3) increased, peaked at the 11th day and then decreased in both the experimental groups, but in CON milk the highest value differed only from the first five sampling days whereas in TAN milk the highest value differed from all but the fifth’s day observations (P = 0.033). Anyway, neither the concentrations of C14:1 c9 and C18:1 c11 nor n-6/n-3 statistically differed between CON and TAN milk on any of the sampling day.
The sampling day effect was significant (P < 0.050) for almost all the parameters analysed.
Discussion
The significance of sampling day effect found in this study was due to environmental factors falling outside our experimental design, so it will not be discussed further. Probably, the periodical monitoring of milk throughout the 23 days of trial, combined with the continuous free ranging of cows, made our experimental design sensitive to weather variations. For example, in July, during DS experiment, the temperature leap led to a range of average temperature between 19.5 and 32 °C.
Milk proximate composition
In the present study, the effect of dietary tannin supplementation on milk yield and its main constituents was not significant, regardless of the season. In the last decade, most scientific articles reported no improvement in milk yield, or fat and protein contents from cows eating different sources of tannin31–33. Henke et al.34 reported an increase in milk fat content in cows supplemented for 21 days with 3% DMI of quebracho tannin extract, but the effect was combined with a lower milk yield. Even, increasing doses of tannin have been reported to have a negative linear effect on cows' milk protein content35,36.
In addition, our results suggest that the lack of effectiveness of dietary tannins is constant from the 1st to the 23rd day of administration in dairy cows. Consistently, Denninger et al.37 did not observe any variations in cow milk yield, and fat and protein contents throughout 3 days of dietary Acacia mearnsii (De Wild) tannin extract supplementation (14.7 g of total tannins per kg of DM).
On the other hand, a reduction in MUN of TAN group was expected, as it is reported in several studies on dairy cows involving different tannin sources, such as quebracho34, bayberry, Acacia mangium Willd. and valonia32 or sainfoin (Onobrychis viciifolia Scop.)38. The reduction in MUN is potentially positive for the environment, as it is an indicator of urinary N excretion39. In extensive farming systems, this effect is desirable right in green season, as the high degradable protein content of herbage maximizes the N emission5. The lack of an effect on MUN in WS experiment may be due to the relatively low dose of tannin supplementation, as in the studies where a significant effect was reported cows ingested about 3% DMI of tannin32,34,38. Also, the plant species from which tannins are extracted are a well-known limit to studies comparison40. Aguerre et al.41 fed cows a tannin extract very similar to ours at increasing doses and no evident MUN reduction occurred at supplementations lower than 1.8% DMI. Anyway, a high intake of tannins could have detrimental consequences on animal performance35 and could be economically unpractical on commercial farm.
On the contrary, in DS experiment we observed a constant reduction in MUN. Probably, the dose of tannin extract we supplemented in cow’s diet was not enough to modulate ruminal N metabolism when CP intake is as high as it was in WS experiment. Aguerre et al.35 did not found any interaction between dietary treatment and CP intake on cow’s N partitioning when administrating 0.45% up to 1.8% DMI of quebracho-chestnut tannin mixture. However, the two dietary CP levels they compared were 15.3% and 16.6% DM, whereas in our study the estimated CP levels were 13.9% and 19.9% DM in DS and WS experiment, respectively.
Milk cheesemaking aptitude
In WS experiment, the lack of an effect on milk cheesemaking aptitude was not surprising, considering that fat, protein and casein contents, and even MUN were not affected by dietary tannin extract supplementation. This occurred also in DS experiment, where the reduction in MUN could have been linked with other parameters related to cheesemaking properties42. In accordance with our findings, Herremans et al.43 concluded in a meta-analysis study that dietary tannins do not have any effect on N use efficiency in dairy cattle, except for the reduction of urea emissions.
In a previous study from the same experiment, investigating the effect of dietary tannin extract on cheese quality12, we found no effects on cheesemaking aptitude after 23 days of dietary trial. With the findings of the present study, we can add that this lack of effect is consistent from the 1st to the 23rd day of administration.
Kalber et al.44 found that milk from cows eating buckwheat (Fagopyrum esculentum Moench) silage had a shorter clotting time compared to milk from cows eating chicory (Cichorium intybus L.) or ryegrass (Lolium multiflorum Lam.) silages. The three treatments significantly differed for condensed tannins intake, with 6.1 g/day for cows eating buckwheat and about 2.2 g/day for cows eating chicory or ryegrass, but these intake levels seem too low to confidently attribute them the observed effect. At the best of our knowledge, no other study is available for comparison, and we cannot speculate if dietary tannins at doses higher than 1% DMI could exert an effect on cow milk cheesemaking properties. Also, the few scientific articles investigating dietary tannins effect on clotting time of ewe milk are discordant, reporting no effect45 or even longer clotting and firming times46 with ewes eating 1.6% DMI of tannin extracts. Anyway, literature suggests that a plant specific effect may occur, and results may vary administrating different tannin sources.
Antioxidant capacity of milk hydrophilic fraction
In our study, we investigated both the reducing power and the radical scavenging capacity of skimmed milk. Avila et al.36 did not observe an improvement in milk reducing power when cows’ diet was supplemented with 5 up to 20 g/kg DM of A. mearnsii tannin extract. Unlike them, we analysed not-deproteinized milk, to also include the antioxidant activity of caseins and whey proteins47. Interestingly, although we observed no variation in protein and casein contents of milk, the dietary tannin extract supplementation tended to increase both the reducing power and the radical scavenging capacity of defatted milk in DS experiment. Although the antioxidant activity of polyphenolic compounds is well-known48, is not yet clear how they could improve the antioxidant status of animal products. Probably, tannins had an indirect effect preserving other antioxidants (e.g., vitamin E, vitamin C) during digestion, or low molecular-weight molecules derived from tannins digestion could have been absorbed in the intestine and therefore exerted their antioxidant activity in milk49. The lack of effect in WS experiment could be explained by the already relatively high TEAC and FRAP values. Probably, the diet of cows in WS experiment had an antioxidants content high enough to suffice for milk oxidative stability, without the contribution of supplementary tannins bioactivity. Indeed, grazing pasture is commonly reported to increase the content of antioxidants in milk3, and b* and I450–530 values we found in WS milk indicated a higher content of carotenoids50, compared to DS milk.
Milk fatty acid profile
An effect on microbial and rumen preformed FA concentrations in milk is generally expected with dietary tannins, because of their well-known activity against rumen BH51. This is in contrast with the results of WS experiment, were neither odd- and branched-chain FA nor the C18:1 and C18:2 isomers differed between CON and TAN milk. Probably, as suggested above, the tannin extract supplementation dose used in the present study was not enough to exert an effect on milk quality during the WS experiment.
Interestingly, the effect of dietary tannins on some MUFA and de novo FA concentrations right after the beginning of administration was never observed before. Recently, Denninger et al.37 investigated the effect of A. mearnsii tannin extract supplementation in the first 3 days of administration. They observed a decrease in microbial-derived FA in milk starting from the second day of trial, indicating a quite rapid effectiveness of dietary tannins against microbial rumen activity. Likewise, in WS experiment we observed an immediate response of milk FA profile to dietary tannins administration, even if concerning different FA compared to Denninger et al.37. As C18:1 c9 can undergo BH in the rumen52 and dietary supplementation of C18:1 c9 is reported to reduce mammary FA synthesis53, we hypothesized th at, in WS experiment, dietary tannins impaired the ruminal metabolism of C18:1 c9, with consequent increase in C18:1 c9 intestinal flow and reduction in de novo FA synthesis. Milk C18:1 c9 also results from the activity of mammary Δ9-desaturase, but we found no variation of desaturation index throughout the WS experiment. However, this effect against rumen BH vanished soon, probably indicating a rapid adaptation of ruminal microbiota to dietary tannins, as already suggested by Toral et al.54 observing ewe milk.
The different conditions of DS experiment modified the effect of dietary tannin extract on milk FA profile, compared to WS experiment. As recently reviewed by Frutos et al.51, a decrease of bacterial FA concentration, such as odd-chain fatty acids (OCFA) and BCFA, is often reported in studies investigating dietary tannins effect on rumen digesta. Interestingly, in DS experiment we observed a decrease in both main iso- and anteiso-FA in TAN milk, whereas milk’s OCFA did not varied between dietary treatments. Likewise, Denninger et al.37 reported a decrease in some BCFA in cow milk after A. mearnsii tannin extract feeding and no effect on milk’s OCFA. As changes of bacterial FA proportions in milk likely reflect shifts in rumen microbial community, and the bacterial FA synthesis is species-specific55, the effect observed could be explained by the different sensitivity of rumen microorganisms to tannins. Probably, in DS experiment, the ruminal microorganisms enriched in BCFA were more sensitive to tannins bioactivity than those enriched in OCFA. Indeed, cellulolytic and amylolytic bacteria are reported to be enriched in BCFA and OCFA, respectively55, and Díaz Carrasco et al.56 documented the ability of tannins to modify the cellulolytic:amylolytic bacteria balance in the rumen.
In DS experiment, the effect of dietary tannin extract on t10/t11 and RA/LA seems to indicate an impaired rumen BH. Indeed, C18:1 t10, C18:1 t11 and C18:2 c9t11 are not dietary FA and they form in the rumen by microorganism activity57. The reduced RA/LA may indicate a slowdown in the first steps of BH, where C18:2 c9c12 is isomerized to C18:2 c9t1158. A second source of milk C18:2 c9t11 is the mammary Δ9-desaturation of C18:1 t1159. However, an effect of dietary tannin extract on mammary Δ9-desaturase may be ruled out, as desaturation index did not differ between CON and TAN milk in DS experiment. Unfortunately, the extent of the modifications induced by dietary tannins on FA profile in our experiments is hardly relevant in terms of product healthiness.
Our study suggests a different effect of dietary tannins on cows' milk FA profile depending on the grazing season in the Mediterranean. This phenomenon is likely related to the different diet in WS and DS experiment, concerning the green herbage availability, the CP level, the forage to concentrate ratio, the different amount and composition of biohydrogenation precursors, or a combination of all these aspects. Similarly, Menci et al.8 found two different tannin extracts (quebracho vs chestnut + quebracho) to reduce iso-FA concentration and RA/LA in rumen digesta fermenting a hay substrate, whereas none of these effects was observed fermenting the corresponding green herbage. Different diets are reported to select different microbiota populations in the rumen60 and the specific microorganisms can show a different sensitivity to tannins bioactivity56. Therefore, the microbiota selected by the diet of DS experiment could have been more sensitive to dietary tannin supplementation. A second hypothesis is that the highly nutritious diet in WS experiment made the rumen microbiota resilient, whereas in DS experiment the microbiota could not adapt to tannin extract supplementation. Indeed, the variations observed in FA profile of DS milk were consistent throughout the whole trial. Anyway, as different rumen microorganisms show a different sensitivity to different kinds of tannin61, results may change by varying the source of tannin.
Conclusions
The dietary supplementation of tannin extract at the dose of 150 g/day in dairy cows showed different effects on milk quality according to the season under Mediterranean climate. No effect on milk quality was observed in WS, whereas in DS the milk from cows eating tannins showed lower BCFA concentration, C18:1 t10 to C18:1 t11 ratio, and rumenic to linoleic acid ratio. Also, tannin extract supplementation in DS reduced MUN and slight improved the antioxidant capacity of milk. Thus, tannin supplementation in grazing dairy cows was more effective during the dry season, when diet is low in CP and rich in ADF and ADL. Probably, the integration of tannin in the diet should be adapted to the CP or fibre intakes, or both, if the purpose is modifying milk quality. This could have practical implications for a more conscious use of tannin-rich extracts and also other tannin sources such as agro-industrial by-products and forages (especially those from dry areas). Further studies are needed to investigate the effects of longer supplementations or different doses and tannin sources. Finally, a deeper knowledge of the sensitivity of rumen microbiota to tannins could help to explain the variability in dietary tannins effectiveness.
Abbreviations
- ADF
Acid detergent fibre
- ADL
Acid detergent lignin
- BCFA
Branched-chain fatty acids
- BCS
Body condition score
- BH
Biohydrogenation
- CON
Control group
- CP
Crude protein
- DIM
Days in milk
- DMI
Dry matter intake
- DS
Dry season
- ECM
Energy corrected milk
- FA
Fatty acids
- FRAP
Ferric reducing antioxidant power
- I450–530
Integral value of the absorbance spectrum between 450 and 530 nm
- IMCU
International milk clotting units
- LCY
Laboratory cheese yield
- LDMCY
Laboratory dry matter cheese yield
- MCP
Milk coagulation properties
- MUFA
Monounsaturated fatty acids
- MUN
Milk urea nitrogen
- n-6/n-3
n-6 PUFA to n-3 PUFA ratio
- NDF
Neutral detergent fibre
- OCFA
Odd-chain fatty acids
- PUFA
Polyunsaturated fatty acids
- RA/LA
C18:2 c9t11 to C18:2 c9c12 ratio
- SCC
Somatic cells count
- t10/t11
C18:1 t10 to C18:1 t11 ratio
- TAN
Group receiving 150 g/head per day of tannin extract
- WS
Wet season
Author contributions
R.M. contributed to acquisition, analysis and interpretation of data, and he drafted the work. A.N. contributed to the design of the work, the acquisition and interpretation of data, and he revised the work. G.L., A.P., B.V., and M. Caccamo contributed to the conception, design and revision of the work. G.F. contributed to the acquisition of data. V.N. revised the work. M. Coppa contributed to the design of the work, the interpretation of data, and he revised the work. All authors read and approved the final manuscript.
Funding
Financial support for designing the study, sampling, and analyses was provided by transnational funding bodies, being partners of the H2020 ERA-net project—CORE Organic Cofund—and the cofund from the European Commission, under the project ProYoungStock “Promoting young stock and cow health and welfare by natural feeding systems”. Moreover, the University of Catania funded part of the research activities conducted (project “QUALIGEN”; Linea 2—Piano di Incentivi per la Ricerca di Ateneo 2020/2022; principal investigator: Giuseppe Luciano). Also, R. Menci was granted fellowship by Programma Operativo Nazionale Ricerca e Innovazione 2014–2020, “Dottorati Innovativi con caratterizzazione Industriale” Borsa di studio DOT1308937-1—CUP: E67I18001070006, PhD course in Agricultural, Food and Environmental Science of the University of Catania.
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ramírez-Rivera EJ, Rodríguez-Miranda J, Huerta-Mora IR, Cárdenas-Cágal A, Juárez-Barrientos JM. Tropical milk production systems and milk quality: A review. Trop. Anim. Health Prod. 2019;51:1295–1305. doi: 10.1007/s11250-019-01922-1. [DOI] [PubMed] [Google Scholar]
- 2.Licitra G, et al. Assessment of the dairy production needs of cattle owners in Southeastern Sicily. J. Dairy Sci. 1998;81:2510–2517. doi: 10.3168/jds.S0022-0302(98)70143-2. [DOI] [PubMed] [Google Scholar]
- 3.Prache S, Martin B, Coppa M. Review: Authentication of grass-fed meat and dairy product from cattle and sheep. Animal. 2020;14:854–863. doi: 10.1017/S1751731119002568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coppa M, et al. Forage system is the key driver of mountain milk specificity. J. Dairy Sci. 2019;102:10483–10499. doi: 10.3168/jds.2019-16726. [DOI] [PubMed] [Google Scholar]
- 5.Kingston-Smith AH, Theodorou MK. Post-ingestion metabolism of fresh forage. New Phytol. 2000;148:37–55. doi: 10.1046/j.1469-8137.2000.00733.x. [DOI] [PubMed] [Google Scholar]
- 6.Wanapat M, Kang S, Polyorach S. Development of feeding systems and strategies of supplementation to enhance rumen fermentation and ruminant production in the tropics. J. Anim. Sci. Biotechnol. 2013;4:32. doi: 10.1186/2049-1891-4-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Patra AK, Saxena J. Exploitation of dietary tannins to improve rumen metabolism and ruminant nutrition. J. Sci. Food Agric. 2011;91:24–37. doi: 10.1002/jsfa.4152. [DOI] [PubMed] [Google Scholar]
- 8.Menci R, et al. Effects of two tannin extracts at different doses in interaction with a green or dry forage substrate on in vitro rumen fermentation and biohydrogenation. Anim. Feed Sci. Technol. 2021;278:114977. doi: 10.1016/j.anifeedsci.2021.114977. [DOI] [Google Scholar]
- 9.Dschaak CM, et al. Effects of supplementing condensed tannin extract on intake, digestion, ruminal fermentation, and milk production of lactating dairy cows. J. Dairy Sci. 2011;94:2508–2519. doi: 10.3168/jds.2010-3818. [DOI] [PubMed] [Google Scholar]
- 10.Henke A, et al. Effect of dietary quebracho tannin extract on milk fatty acid composition in cows. J. Dairy Sci. 2017;100:6229–6238. doi: 10.3168/jds.2016-12149. [DOI] [PubMed] [Google Scholar]
- 11.Focant M, Froidmont E, Archambeau Q, Van Dang QC, Larondelle Y. The effect of oak tannin (Quercus robur) and hops (Humulus lupulus) on dietary nitrogen efficiency, methane emission, and milk fatty acid composition of dairy cows fed a low-protein diet including linseed. J. Dairy Sci. 2019;102:1144–1159. doi: 10.3168/jds.2018-15479. [DOI] [PubMed] [Google Scholar]
- 12.Menci R, et al. Cheese quality from cows given a tannin extract in 2 different grazing seasons. J. Dairy Sci. 2021;104:9543–9555. doi: 10.3168/jds.2021-20292. [DOI] [PubMed] [Google Scholar]
- 13.Percie du Sert N, et al. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. PLoS Biol. 2020;18:e3000410. doi: 10.1371/journal.pbio.3000410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Makkar HPS, Blümmel M, Borowy NK, Becker K. Gravimetric determination of tannins and their correlations with chemical and protein precipitation methods. J. Sci. Food Agric. 1993;61:161–165. doi: 10.1002/jsfa.2740610205. [DOI] [Google Scholar]
- 15.INRA (Institut National de la Recherche Agronomique) Alimentation des ruminants. Editions Quae; 2018. [Google Scholar]
- 16.AOAC . Official Method of Analysis. 16. AOAC International; 1995. [Google Scholar]
- 17.Licitra G, Hernandez TM, Van Soest PJ. Standardization of procedures for nitrogen fractionation of ruminant feeds. Anim. Feed Sci. Technol. 1996;57:347–358. doi: 10.1016/0377-8401(95)00837-3. [DOI] [Google Scholar]
- 18.Van Soest PJ, Robertson JB, Lewis BA. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991;74:3583–3597. doi: 10.3168/jds.S0022-0302(91)78551-2. [DOI] [PubMed] [Google Scholar]
- 19.Luciano G, et al. Feeding lambs with silage mixtures of grass, sainfoin and red clover improves meat oxidative stability under high oxidative challenge. Meat Sci. 2019;156:59–67. doi: 10.1016/j.meatsci.2019.05.020. [DOI] [PubMed] [Google Scholar]
- 20.Valenti B, et al. Dried tomato pomace supplementation to reduce lamb concentrate intake: Effects on growth performance and meat quality. Meat Sci. 2018;145:63–70. doi: 10.1016/j.meatsci.2018.06.009. [DOI] [PubMed] [Google Scholar]
- 21.Natalello A, et al. Effect of feeding pomegranate byproduct on fatty acid composition of ruminal digesta, liver, and muscle in lambs. J. Agric. Food Chem. 2019;67:4472–4482. doi: 10.1021/acs.jafc.9b00307. [DOI] [PubMed] [Google Scholar]
- 22.ISO. Milk and liquid milk products—Guidelines for the application of mid-infrared spectrometry. Method no. 9622. International Organization for Standardization. https://www.iso.org/standard/56874.html (2013).
- 23.ISO. Milk—Enumeration of somatic cells—Part 2: Guidance on the operation of fluoro-opto-electronic counters. Method no. 13366-2. International Organization for Standardization. https://www.iso.org/standard/40260.html (2006).
- 24.Hurtaud C, Rulquin H, Delaite M, Vérité R. Prediction of cheese yielding efficiency of individual milk of dairy cows. Correlation with coagulation parameters and laboratory curd yield. Ann. Zootech. 1995;44:385–398. doi: 10.1051/animres:19950405. [DOI] [Google Scholar]
- 25.Zannoni, M. & Annibaldi, S. Standardization of the renneting ability of milk by Formagraph. Pt. 1. Scienza e Tecnica Lattiero-Casearia (Italy) (1981).
- 26.Benzie IFF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of “Antioxidant Power”: The FRAP assay. Anal. Biochem. 1996;239:70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- 27.Re R, et al. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999;26:1231–1237. doi: 10.1016/S0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- 28.Feng S, Lock AL, Garnsworthy PC. Technical note: A rapid lipid separation method for determining fatty acid composition of milk. J. Dairy Sci. 2004;87:3785–3788. doi: 10.3168/jds.S0022-0302(04)73517-1. [DOI] [PubMed] [Google Scholar]
- 29.Christie WW. A simple procedure for rapid transmethylation of glycerolipids and cholesteryl esters. J. Lipid Res. 1982;23:1072–1075. doi: 10.1016/S0022-2275(20)38081-0. [DOI] [PubMed] [Google Scholar]
- 30.Priolo A, Prache S, Micol D, Agabriel J. Reflectance spectrum of adipose tissue to trace grass feeding in sheep: Influence of measurement site and shrinkage time after slaughter. J. Anim. Sci. 2002;80:886–891. doi: 10.2527/2002.804886x. [DOI] [PubMed] [Google Scholar]
- 31.Kälber T, Meier JS, Kreuzer M, Leiber F. Flowering catch crops used as forage plants for dairy cows: Influence on fatty acids and tocopherols in milk. J. Dairy Sci. 2011;94:1477–1489. doi: 10.3168/jds.2010-3708. [DOI] [PubMed] [Google Scholar]
- 32.Zhang J, et al. Effect of different tannin sources on nutrient intake, digestibility, performance, nitrogen utilization, and blood parameters in dairy cows. Animals. 2019;9:507. doi: 10.3390/ani9080507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kapp-Bitter AN, et al. Graded supplementation of chestnut tannins to dairy cows fed protein-rich spring pasture: Effects on indicators of protein utilization. J. Anim. Feed Sci. 2020;29:97–104. doi: 10.22358/jafs/121053/2020. [DOI] [Google Scholar]
- 34.Henke A, et al. Effect of dietary Quebracho tannin extract on feed intake, digestibility, excretion of urinary purine derivatives and milk production in dairy cows. Arch. Anim. Nutr. 2017;71:37–53. doi: 10.1080/1745039X.2016.1250541. [DOI] [PubMed] [Google Scholar]
- 35.Aguerre MJ, Capozzolo MC, Lencioni P, Cabral C, Wattiaux MA. Effect of quebracho-chestnut tannin extracts at 2 dietary crude protein levels on performance, rumen fermentation, and nitrogen partitioning in dairy cows. J. Dairy Sci. 2016;99:4476–4486. doi: 10.3168/jds.2015-10745. [DOI] [PubMed] [Google Scholar]
- 36.Avila AS, et al. Black wattle (Acacia mearnsii) condensed tannins as feed additives to lactating dairy cows. Animals. 2020;10:662. doi: 10.3390/ani10040662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Denninger TM, et al. Immediate effect of Acacia mearnsii tannins on methane emissions and milk fatty acid profiles of dairy cows. Anim. Feed Sci. Technol. 2020;261:114388. doi: 10.1016/j.anifeedsci.2019.114388. [DOI] [Google Scholar]
- 38.Girard M, et al. Ability of 3 tanniferous forage legumes to modify quality of milk and Gruyère-type cheese. J. Dairy Sci. 2016;99:205–220. doi: 10.3168/jds.2015-9952. [DOI] [PubMed] [Google Scholar]
- 39.Jonker JS, Kohn RA, Erdman RA. Using milk urea nitrogen to predict nitrogen excretion and utilization efficiency in lactating dairy cows1. J. Dairy Sci. 1998;81:2681–2692. doi: 10.3168/jds.S0022-0302(98)75825-4. [DOI] [PubMed] [Google Scholar]
- 40.Verma S, Taube F, Malisch CS. Examining the variables leading to apparent incongruity between antimethanogenic potential of tannins and their observed effects in ruminants—A review. Sustainability. 2021;13:2743. doi: 10.3390/su13052743. [DOI] [Google Scholar]
- 41.Aguerre MJ, Duval B, Powell JM, Vadas PA, Wattiaux MA. Effects of feeding a quebracho–chestnut tannin extract on lactating cow performance and nitrogen utilization efficiency. J. Dairy Sci. 2020;103:2264–2271. doi: 10.3168/jds.2019-17442. [DOI] [PubMed] [Google Scholar]
- 42.Martin B, Coulon JB, Chamba JF, Bugaud C. Effect of milk urea content on characteristics of matured Reblochon cheeses. Lait. 1997;77:505–514. doi: 10.1051/lait:1997436. [DOI] [Google Scholar]
- 43.Herremans S, Vanwindekens F, Decruyenaere V, Beckers Y, Froidmont E. Effect of dietary tannins on milk yield and composition, nitrogen partitioning and nitrogen use efficiency of lactating dairy cows: A meta-analysis. J. Anim. Physiol. Anim. Nutr. 2020;104:1209–1218. doi: 10.1111/jpn.13341. [DOI] [PubMed] [Google Scholar]
- 44.Kälber T, Kreuzer M, Leiber F. Effect of feeding buckwheat and chicory silages on fatty acid profile and cheese-making properties of milk from dairy cows. J. Dairy Res. 2013;80:81–88. doi: 10.1017/S0022029912000647. [DOI] [PubMed] [Google Scholar]
- 45.Buccioni A, et al. Milk fatty acid composition, rumen microbial population, and animal performances in response to diets rich in linoleic acid supplemented with chestnut or quebracho tannins in dairy ewes. J. Dairy Sci. 2015;98:1145–1156. doi: 10.3168/jds.2014-8651. [DOI] [PubMed] [Google Scholar]
- 46.Buccioni A, et al. Chestnut or quebracho tannins in the diet of grazing ewes supplemented with soybean oil: Effects on animal performances, blood parameters and fatty acid composition of plasma and milk lipids. Small Rumin. Res. 2017;153:23–30. doi: 10.1016/j.smallrumres.2017.05.006. [DOI] [Google Scholar]
- 47.Khan IT, et al. Antioxidant properties of Milk and dairy products: A comprehensive review of the current knowledge. Lipids Health Dis. 2019;18:41. doi: 10.1186/s12944-019-0969-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rice-Evans CA, Miller NJ, Paganga G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic. Biol. Med. 1996;20:933–956. doi: 10.1016/0891-5849(95)02227-9. [DOI] [PubMed] [Google Scholar]
- 49.Huang Q, Liu X, Zhao G, Hu T, Wang Y. Potential and challenges of tannins as an alternative to in-feed antibiotics for farm animal production. Anim. Nutr. 2018;4:137–150. doi: 10.1016/j.aninu.2017.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Prache S, Theriez M. Traceability of lamb production systems: Carotenoids in plasma and adipose tissue. Anim. Sci. 1999;69:29–36. doi: 10.1017/S1357729800051067. [DOI] [Google Scholar]
- 51.Frutos P, et al. Ability of tannins to modulate ruminal lipid metabolism and milk and meat fatty acid profiles. Anim. Feed Sci. Technol. 2020;269:114623. doi: 10.1016/j.anifeedsci.2020.114623. [DOI] [Google Scholar]
- 52.Mosley EE, Powell GL, Riley MB, Jenkins TC. Microbial biohydrogenation of oleic acid to trans isomers in vitro. J. Lipid Res. 2002;43:290–296. doi: 10.1016/S0022-2275(20)30171-1. [DOI] [PubMed] [Google Scholar]
- 53.Dorea JRR, Armentano LE. Effects of common dietary fatty acids on milk yield and concentrations of fat and fatty acids in dairy cattle. J. Anim. Prod. Sci. 2017;57:2224–2236. doi: 10.1071/AN17335. [DOI] [Google Scholar]
- 54.Toral PG, Hervás G, Belenguer A, Bichi E, Frutos P. Effect of the inclusion of quebracho tannins in a diet rich in linoleic acid on milk fatty acid composition in dairy ewes. J. Dairy Sci. 2013;96:431–439. doi: 10.3168/jds.2012-5622. [DOI] [PubMed] [Google Scholar]
- 55.Vlaeminck B, Fievez V, Cabrita ARJ, Fonseca AJM, Dewhurst RJ. Factors affecting odd- and branched-chain fatty acids in milk: A review. Anim. Feed Sci. Technol. 2006;131:389–417. doi: 10.1016/j.anifeedsci.2006.06.017. [DOI] [Google Scholar]
- 56.Díaz Carrasco JM, et al. Impact of chestnut and quebracho tannins on rumen microbiota of bovines. Biomed. Res. Int. 2017;2017:9610810. doi: 10.1155/2017/9610810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chilliard Y, et al. Diet, rumen biohydrogenation and nutritional quality of cow and goat milk fat. Eur. J. Lipid Sci. Technol. 2007;109:828–855. doi: 10.1002/ejlt.200700080. [DOI] [Google Scholar]
- 58.Lourenço M, Ramos-Morales E, Wallace RJ. The role of microbes in rumen lipolysis and biohydrogenation and their manipulation. Animal. 2010;4:1008–1023. doi: 10.1017/S175173111000042X. [DOI] [PubMed] [Google Scholar]
- 59.Palmquist, D. L., Lock, A. L., Shingfield, K. J. & Bauman, D. E. Biosynthesis of conjugated linoleic acid in ruminants and humans. Adv. Food Nutr. Res.50, 179–217. 10.1016/S1043-4526(05)50006-8 (2005). [DOI] [PubMed]
- 60.Belanche A, et al. Shifts in the rumen microbiota due to the type of carbohydrate and level of protein ingested by dairy cattle are associated with changes in rumen fermentation. J. Nutr. 2012;142:1684–1692. doi: 10.3945/jn.112.159574. [DOI] [PubMed] [Google Scholar]
- 61.Costa M, et al. Effects of condensed and hydrolyzable tannins on rumen metabolism with emphasis on the biohydrogenation of unsaturated fatty acids. J. Agric. Food Chem. 2018;66:3367–3377. doi: 10.1021/acs.jafc.7b04770. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.


