Abstract
The non-fullerene acceptors A1–A5 with diflourobenzene or quinoline core (bridge) unit, donor cyclopenta[1,2-b:3,4-b′]dithiophene unit and 2-(2-methylene-3-oxo-2,3-dihydro-1H-inden-1-ylidene)malononitrile as acceptor unit with additional phenyl, fulvene or thieno[3,2-d]pyrimidinyl 5-oxide groups have been designed through DFT calculations. The optimization of molecular geometries were performed with density functional theory (DFT) at B3LYP 6-31G (d,p) level of theory. The frontier molecular orbital (FMO) energies, band gap energies and dipole moments (ground and excited state) have been calculated to probe the photovoltaic properties. The band gap (1.42–2.01 eV) and dipole moment values (5.5–18. Debye) showed that these designed acceptors are good candidates for organic solar cells. Time-Dependent Density Functional Theory (TD-DFT) results showed λmax (wave length at maximum absorption) value (611–837 nm), oscillator strength (f) and excitation energies (1.50–2.02 eV) in gas phase and in CHCl3 solvent (1.48–1.89 eV) using integral equation formalism variant (IEFPCM) model. The λmax in CHCl3 showed marginal red shift for all designed acceptors compared with gas phase absorption. The partial density of states (PDOS) has been plotted by using multiwfn which showed that all the designed molecules have more electronic distribution at the donor moiety and lowest at the central bridge. The reorganization energies of electron (λe) (0.0007 eV to 0.017 eV), and the hole reorganization energy values (0.0003 eV to − 0.0403 eV) were smaller which suggested that higher charged motilities. The blends of acceptors A1–A5 with donor polymer D1 provided open circuit voltage (Voc) and ∆HOMO off-set of the HOMO of donor and acceptors. These blends showed 1.04 to 1.5 eV values of Voc and 0 to 0.38 eV ∆HOMO off set values of the donor–acceptor bends which indicate improved performance of the cell. Finally, the blend of D1–A4 was used for the study of distribution of HOMO and LUMO. The HOMO were found distributed on the donor polymer (D1) while the A4 acceptor was found with LUMO distribution. Based on λmax values, and band gap energies (Eg), excitation energies (Ex), reorganization energies; the A3 and A4 will prove good acceptor molecules for the development of organic solar cells.
Subject terms: Chemistry, Energy science and technology, Engineering, Materials science, Physics
Introduction
Presently, due to high demand of the energy and depletion of non-renewable resources, there has been great deal of focus on the alternate energy sources. The great advancement in the development of solar cells have been made1. Among various types of solar cells, the organic solar cells (OSCs) have received great attention due to their easy process-ability, low cost, tuning of the Highest Occupied Molecular Orbitals (HOMO) and Lowest Unoccupied Molecular Orbitals (LUMO). In organic solar cells, usually a polymer is used as donor material along with well-known fullerene as an acceptor to build heterojunction between donor and acceptor. The fullerene and its derivatives have been an immense choice for the chemists as small molecular acceptor (SMA) due to high power conversion efficiency (PCE), electron transport in three dimensions, high charge separation and low lying LUMO2. Therefore, lots of efforts have been devoted to develop OSCs with polymer-fullerene bulk heterojunctions (BHJ)3–5. The PCE of these OSCs with fullerene derivatives have exceeded 12%6–10. However, the disadvantages of the fullerenes such as fixed energy levels, solubility in organic solvents, poor processability, and poor light absorption above 600 nm created space for the development of alternative acceptor molecules11–15. The small organic molecular non-fullerene acceptors (FNAs) have been found an alternative to fullerene that removed the disadvantages associated with fullerene acceptor. These molecules were found to be easily fabricated through solution processed BHJ, and improved the film properties of the organic solar cells. The NFAs were designed with structural variation which considerably improved the PCE and film morphology of the OSCs. This structural variation also proved helpful in constructing the molecules with desirable HOMO and LUMO energies and optical band-gap energy. No doubt these small NFAs proved to be superior to the fullerene counterpart as these have removed the issues associated with fullerene derivatives, such as these have tunable frontier molecular orbitals (FMOs), good solubility in organic solvents, wide absorption band in visible region and transparency in the fabricated film14–16. A great deal of effort has been devoted in designing the NFAs for photovoltaic applications. The 3a,3b,6a,7a-tetrahydro-7H-cyclopenta[1,2-b:3,4-b′]dithiophene as donor moiety has been frequently used within the structure of NFAs due to its easy availability and good compatibility with a number of acceptor moieties. These properties of the donor moiety was found to be due to the efficient electron-donating nature of sulfur atom in acceptor–donor–acceptor (A–D–A) type structure of NFAs17–19. The fluorine substituted organic small molecules as photovoltaic materials offered promising results when these were fabricated in solar cells20.
The density function theory (DFT) has recently been used for the designing of photovoltaic materials. Isoindigo-dithiophenepyrrole based D–A oligomers were successfully designed by Ahmed et al. through DFT and time dependent density function theory (TD-DFT)21. Mehboob et al. designed acceptor molecules with benzodithenophene core and melanonitrile or dinitromethane electron–acceptor end groups. These designed molecules showed high charge mobility and low band-gap values at B3LYP level of theory with split valence 6-31 G(d,p) basis-set22. Farah et al. calculated the optical and electronic properties of newly designed dithieno[3,2-b:2′,3′-d]silole)2,6-diyl (DTS) based donor molecules through DFT and TD-DFT at CAM-B3LYP/6-31G (d) level of theory. FMOs energy, Voc, reorganization energies, excitation energy of the designed molecules were found better than the reference molecules23. Farhat et al. in 2020 used DFT at B3LYP with 6-31G(d,p) basis-set to calculate the λmax, reorganization energy (charge mobility), frontier molecular orbitals (FMOs) energy and open circuit voltage (Voc) of newly designed subphthalocyanine derived chromophores as donor materials. These designed donor molecules showed improved photovoltaic properties than the reference compounds24. Thus DFT with B3LYP theory level showed promising results for the evaluation of photovoltaic materials.
Li et al. in 2019 successfully synthesized NFAs with diflourophenylene as core structure, cyclopenta[2,1-b:3,4-b′]dithiophene donor and malanonitrile end-capped acceptor groups. The fabrication of these acceptors with the donor polymers provided good photovoltaic properties such as small Eloss and high PCE of above 10%25. By inspiring from the work of Li et al., acceptor molecules have been designed with diflourophenylene or quinoline core, cyclopenta[1,2-b:3,4-b′]dithiophene as donor unit and melanonitrile based acceptor units that provided enhanced photovoltaic properties through DFT calculation. These types of molecules were developed, and studied for photovoltaic properties. However, the power conversion efficiency of these molecules reached only 10%. The current work, herein, presents the designing of the NFA molecules with enhanced photovoltaic properties, and it is expected that these designed FNAs will improve the PCE of organic solar cell greater than 10% when will be fabricated in OSCs.
Computational detail
All the geometry optimizations were carried out by using Gaussian 09 package26 with Gaussview 5.027 for viewing the molecules. For geometry optimization, first of all the acceptor molecule A1 which has closed similarity with standard25 was optimized through DFT with a split valence basis set 6-31G (d,p) that is used at B3LYP28 and CAM-B3LYP28 levels of theory. It was noted that the B3LYP/6-31G (d,p) gave results of energy gap (Eg) close to the experimentally determined values for structurally similar NFA compound25. Therefore, B3LYP/6 31G (d,p) was used for the geometry optimization of designed acceptor molecules A1 to A5. The energies of the highest occupied molecular orbital (HOMO), lowest unoccupied molecular orbitals (LUMO) and band gap energies (EHOMO–ELUMO) were calculated at the same level of theory. The optoelectronic properties such as λmax, oscillation factor (f) and excitation energies were calculated with time dependent self-consistent field method (TD-SCF)29 at the same level of theory in gas phase and in CHCl3 using the integral equation formalism variant (IEFPCM)30. The properties of the designed molecules (A1–A5) were studied in the dissolved form in CHCl3. The solution processed fabrication of the solar cells for film formation is usually done with CHCl3 as the solvent of choice due to its good dissolution good properties. The density of states, dipole moments and reorganizational energies (λ) for electron and hole were calculated at the B3LYP 6-31 G (d,p). The Gaussam software31 was used for the simulation of the absorption spectra that were plotted by using MS excel. The reorganization energy can be associated with external sphere such as selection of medium or electron-transfer processes. However, the internal reorganizational energy is associated with the reorganization of geometric parameters. The internal reorganizational energies of electron (λe) and hole (λh) have only been focused and, thus calculated by using the following equations.
| 1 |
| 2 |
The Eo+ and Eo− correspond to the energies of cation and anion with neutral geometry; whereas, E+ and E− are the energies of cation and anion. The Eo is energy of the molecules at their neutral state. Density of the states (DOS) and partial densities of states (PDOS) have been extracted from the formatted check Gaussian files using multiwfn32.
Result and discussion
By taking inspiration from the work of Li et al., the small organic molecular NFAs A1–A5 have been designed with cyclopenta[1,2-b:3,4-b′]dithiophene donor moiety (Fig. 1) through DFT calculations. The A1 has close similarity with the small organic NFA developed by Li et al. except the cyclopenta[2,1-b:3,4-b′]dithiophene25 donor moiety was replaced with cyclopenta[1,2-b:3,4-b′]dithiophene. The acceptor moieties of the designed molecules A1–A3 have been derived from 2-(2-methylene-3-oxo-2,3-dihydro-1H-inden-1-ylidene)malononitrile, but A4 has fulvene group in addition to 2-(2-methylene-3-oxo-2,3-dihydro-1H-inden-1-ylidene)malononitrile end capped acceptor group. The A1–A4 molecules have diflourophenylene as bridge structure. The designed A5 molecule has qunioline core structure and the acceptor moiety contains thieno[3,2-d]pyrimidinyl 5-oxide incorporated in between donor cyclopenta[1,2-b:3,4-b′]dithiophene and end-capped cyclopenta[1,2-b:3,4-b′]dithiophene group (Fig. 1). The goal of the present work is to design NFA molecules with improved photovoltaic properties. The already developed NFAs showed only 10% PCE in organic solar cells, but the present work has focused the designing new NFAs which will show significantly improved photovoltaic properties such as low excitation energy, low optical band-gap, more absorption, high dipole moment and low reorganizational energies. These theoretically calculated parameters would lead to develop molecules with improved properties in a solar cell.
Figure 1.
The structure of designed small organic NFA molecules A1–A5; the topmost structure represents core and donor units with X as acceptor unit (A1–A4), the lowest A5 has core, donor and acceptor units.
Optimized geometries and frontier molecular orbitals (FMOs)
The study of the FMOs is useful for the optical and electronic properties of designed molecules A1–A5. These designed molecules were optimized by using density functional theory at B3LYP 6-31G (d,p).
Table 1 shows the HOMO, LUMO energies and band gap energies of A1 to A5 molecules. The A2 molecule has the lowest energy of the HOMO (− 5.15 eV) while the A4 has highest energy of HOMO (− 4.39 eV). The band gap energies (Eg) are considered as an important parameter as lower Eg values make excitation easier, thus improves the photoexcitation process. The designed molecules A3 and A4 have lowest Eg values (1.42 eV), A2 has highest Eg value (2.01 eV) among the designed acceptors, and the Eg of A1 and A5 were calculated to be 1.80 eV and 1.66 eV respectively. The ΔSCF based energy of HOMO–LUMO band-gap (Eg) of A4 was also calculated to make sure that the approximation of quasi-particle orbitals as their Kohn Sham values are valid for these systems. The energy of neutral, cationic and an anionic state of the A4 molecule were calculated which were followed by the calculation of the vertical ionization potential (IP) and electron affinity (EA). Finally, the difference of IP and EA provided ΔSCF based band gap energy (1.47 eV). The values of HOMO, LUMO and Eg with DFT/B3LYP and ΔSCF are close to each other for A4 molecule which approximates that the given basis set is valid and has provided good results for these molecules (Table 1).
Table1.
The calculated orbital and band gap energies of A1–A5 at B3LYP 6-31 G(d,p) in gas phase.
| Molecules | EHOMO (eV) | ELUMO (eV) | Eg (eV) |
|---|---|---|---|
| A1 | − 4.77 | − 2.97 | 1.80 |
| A2 | − 5.15 | − 3.14 | 2.01 |
| A3 | − 4.39 | − 2.97 | 1.42 |
| aA4 | − 4.42 | − 3.00 | 1.42 (1.47) |
| A5 | − 5.09 | − 3.43 | 1.66 |
aThe value Eg for A4 in parenthesis have been calculated by using ΔSCF/B3LYP.
The distribution of HOMO and LUMO in acceptor molecules gives information on the delocalization of electronic density. The HOMO describes the valence band, while the LUMO gives information about the conduction band of acceptor molecules. The band-gap energy is directly related to the structure of the molecule. The lowing of band-gap energy is related to the extended conjugation with more planar geometry of the molecule. The A3 and A4 molecules have planar geometry and extended conjugation due to phenyl or fulvene rings between donor and acceptor moieties, thus these molecules showed lowest Eg value (1.42 eV). However, the direct attachment of the acceptor moiety with the donor moiety in A1 and A2 showed increase Eg values (1.80 and 2.01 eV respectively) which can be associated with the reduced conjugation in the molecules. Finally, A5 which has quinoline bridge showed intermediate value of the Eg (1.66 eV). The A1–A4 molecules have cyclopenta[1,2-b:3,4-b′]dithiophene donor diflourophenylene as bridge structure. The designed A5 molecule has qunioline core structure, and the acceptor moiety contains thieno[3,2-d]pyrimidinyl 5-oxide incorporated in between donor cyclopenta[1,2-b:3,4-b′]dithiophene and end-capped cyclopenta[1,2-b:3,4-b′]dithiophene group.
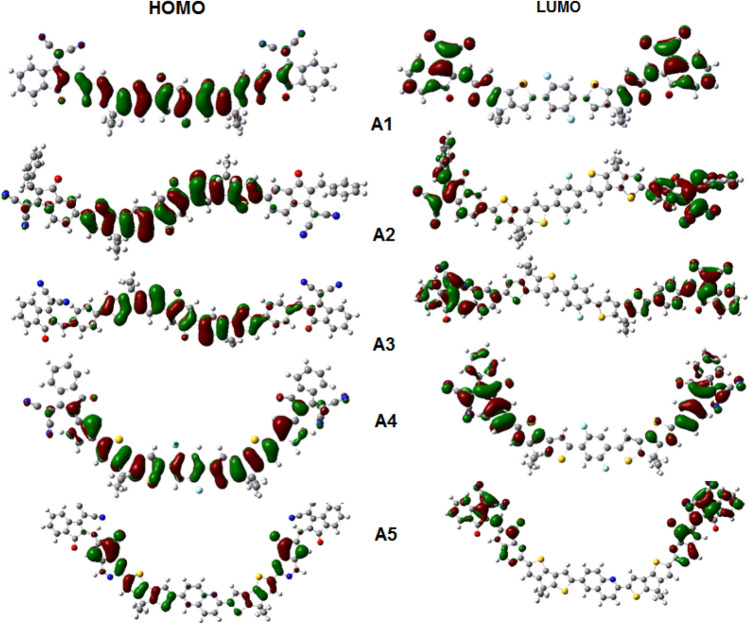
Figure 2 shows that the A1–A4 has their HOMO distributed over the bridge and donor moieties. It can be visualized that the A1–A4 has HOMO distribution on the bridge and donor units, while A5 has HOMO distribution on the donor moiety. The LUMO distribution for all the designed molecules has resided on the acceptor units.
Figure 2.
Distribution of HOMO and LUMO of the molecules A1–A5 at B3LYP 6-31 G (d,p).
The further confirmation of FMOs has been achieved through study of the partial density of the states (PDOS) analysis for all the designed A1-A5 molecules. The designed molecules were divided in to three fragments; acceptor which corresponds to the end-capped groups, donor fragment which consists of 7,7-dimethyl-7H-cyclopenta[1,2-b:3,4-b′]dithiophene and bridge structure which consist of difloropheylene for A1–A4 or quinoline unit for A5.
`
The distribution of total density of states is represented in Figure 3 by black line, donor units with blue lines, acceptor units with red lines and the bridge groups with green lines. The density distribution along negative x-axis values represent valence band (HOMO) and the distribution along positive x-axis value shows conduction band32. Figure 3 shows that the distribution of electron densities lies more on donor moieties and less on acceptor moieties; however, near the fermi energy, the acceptor unit (red curve) in all A1–A5 molecules have considerable contribution of density of states (DOS) in molecular orbitals. This trend suggests that these type of molecules can contribute effectively as acceptor molecules when fabricated in solar cells; and can, presumably, enhance the efficiency of the organic solar cell. The knowledge of the DOS is crucial for enhancing the organic solar cell efficiency. The DOS knowledge can lead to the better understanding of the carrier mobilities of the NFA molecules in a fabricated film33. The DOS of the blended film with HOMO (valence band) of donor and LUMO (conduction band) of the acceptor (NFAs in present case) molecules are considered. The DOS around the LUMO of NFAs are important, especially the PDOS on the acceptor moiety of FNAs. The more PDOS on the acceptor moiety suggests good electron affinity of the acceptor which can provide enhanced photovoltaic properties to the OSCs. All the designed molecules exhibit the more space of the PDOS on the conduction band (LUMO) than the valence band (HOMO) which suggest that these can accommodate excited electron efficiently34. The Bridge of all the designed molecules has lowest distribution. For A1, the donor and acceptor moieties have about equal distribution in the valence band region, whereas in conduction band region, the acceptor surpasses the donor moiety when moving to the positive on x-axis. Similar trend is also exhibited by the A5 molecule. The A2–A4 molecules exhibit more contribution of donor than acceptor units (Fig. 3).
Figure 3.
Total and partial density of states (PDOS) calculated at B3LYP/6-31G(d,p) for the designed molecules A1–A5.
Dipole moments of designed acceptor A1–A5 molecules
The dipole moments of the designed A1–A5 molecules with optimized geometry (µg) and the excited state (µe) were calculated at B3LYP 6-31G (d,p) level of theory in gas phase. Table 2 shows the µg, µe and µe− µg values. The dipole moment is an important parameter to evaluate the NFA molecules. The compounds with higher dipole moment can have higher charge separation. This higher charge separation results in the improved capability of the molecule to accommodate the excited electrons. The high dipole moment also favours the easy fabrication of the material film through solution based bulk-heterojunction due to their high solubility in organic solvents35. The higher values of µe have been calculated for A5 and A1 with 18.9 D and 13.8 D respectively. Similarly, the µg for A5 is 12.8 D and for A1 is 6.4 D respectively. The µe–µg for A1 is 7.4 D and 6.1 D for A5. The higher values of µe–µg show that the transition state has considerable polarity compared with ground state. The µe values for A2–A4 are 9.1, 7.7 and 8.7 D respectively, and their ground state (µg) values are in the range of 5.5–8.9 D. The µe–µg values for A2–A4 are 0.2, 1.8 and 3.2 D. The lowest value of µe–µg for A2 describes that the excited state has marginally higher polarity than the ground state geometry. Based on the dipole moment calculation, the A5 and A1 molecules are presumed to be more efficient for the fabrication through solution processed bulk heterojunction.
Table 2.
The ground state (µg) and excited state dipole moments (µe) of A1–A5 in gas phase.
| Compounds | µe | µg | µe–µg |
|---|---|---|---|
| A1 | 13.8 | 6.4 | 7.4 |
| A2 | 9.1 | 8.9 | 0.2 |
| A3 | 7.7 | 5.9 | 1.8 |
| A4 | 8.7 | 5.5 | 3.2 |
| A5 | 18.9 | 12.8 | 6.1 |
Photo-excitation of A1–A5 acceptor molecules
The photo-excitation (UV) studies were calculated at B3LYP 6-31G (d,p) with time-dependent density functional theory (TD-DFT). The optical properties were calculated in gas phase and in CHCl3 solvent (IEFPCM) for the designed A1–A5 molecules. Table 3 shows λmax, excitation energies (Ex) which correspond to the first excited state, oscillation factor (f) and percent contribution of HOMO to LUMO in gas phase. The value of λmax shows red shift in the order A4 > A5 > A1 > A3 > A2. The increase in the λmax can be attributed to the extended conjugation in a molecule. The extended conjugation, especially due to the bridge moiety of the small organic FNA molecules can enhance the efficiency of solar cells. The extended conjugation has been found to decrease the LUMO energy which results in the low band-gap and higher shift in λmax values36. The red shift in λmax value along with strong light absorption has resulted to lower the excitation energy, and ultimately reinforce the electron-acceptor property of the molecules37. The presence of fulvene extended conjugation in the A4 acceptor molecule and resulted in highest λmax value (810 nm). The quinoline core structure in A5 is responsible for the extended the conjugation which ultimately shifted λmax towards higher side (778 nm). A1, A2 and A3 have 670 nm, 631 nm and 645 nm λmax values. The Ex value provides useful information about the excitation of an electron from valence band (HOMO) to conduction band (LUMO). Lower value of Ex makes excitation easier. Table 3 shows that the order of Ex values is A2 > A1 > A5 > A3 = A4. The A2 has highest value of 1.92 eV, while A3 and A4 has the lowest value (1.39 eV). The acceptor molecules A1 and A5 have 1.72 and 1.62 eV of excitation energy values. Assignment of HOMO → LUMO is found in the order A1 (93%) = A3 (93%) > A4 (90%) > A5 (84%) > A2 (78%).
Table 3.
The calculated UV results of A1–A5 at TD-DFT/B3LYP 6-31G(d,p) in gas phase.
| Moleculesa | λmax (nm) | Ex (eV) | f | Assignment |
|---|---|---|---|---|
| A1 | 670 | 1.72 | 0.68 | H → L (93%) |
| A2 | 631 | 1.92 | 1.34 | H → L (78%) |
| A3 | 645 | 1.39 | 0.87 | H → L (93%) |
| A4 | 810 | 1.39 | 0.80 | H → L (90%) |
| A5 | 778 | 1.62 | 0.82 | H → L (84%) |
Table 4 shows the optoelectronic properties of the designed A1–A5 molecules in CHCl3 solvent with IEFPCM model. The λmax (nm) values are found in the order A4 > A5 > A3 > A1 > A2. The A4 shows highest value (847 nm), and A2 has lowest values (678 nm). The absorption values for A1, A3 and A5 are found to be 698, 706 and 789 nm. The excitation energy values for designed NFA molecules (A1–A5) which correspond to the first excited state have also been calculated in solvent CHCl3. The Ex values of A3 and A4 are 1.40 eV which are the lowest among all the designed acceptor molecules due to the extended conjugation with fulvene moiety is incorporated between donor and acceptor groups. The A2 has the highest Ex value (1.88 eV), while the A1 and A5 have 1.70 and 1.59 eV respectively. The oscillation factor (f) has value range from 0.77 to 1.09 for A1–A5. HOMO → LUMO contributions are highest for A1 with 97%, while A3 has 95% contribution. The A2, A4 and A5 have 85–90% contributions of HOMO → LUMO (Table 4). The A3 molecule has phenyl ring immersed between donor and acceptor moieties which increased the conjugation due to its planar structure and hence showed highest absorption value in gas and in CHCl3 solvent (with IEFPCM model) as compared to the other designed acceptor molecules. The extended conjugation and planarity are considered as important for increasing absorption of the radiations by the molecules37,38. All the designed acceptor molecules A1–A5 showed lower value of the excitation energies (Ex) than the ground state band-gap energies(Eg). This trend is consistent with previously reported data of the Ex and Eg values39.
Table 4.
The calculated UV results of A1–A5 at TD-DFT/B3LYP 6-31G(d,p) in CHCl3.
| Moleculesa | λmax (nm) | Ex (eV) | f | Assignment |
|---|---|---|---|---|
| A1 | 698 | 1.70 | 0.77 | H → L (97%) |
| A2 | 678 | 1.88 | 1.46 | H → L (85%) |
| A3 | 706 | 1.40 | 1.09 | H → L (95%) |
| A4 | 847 | 1.40 | 1.06 | H → L (90%) |
| A5 | 789 | 1.59 | 1.05 | H → L (87%) |
aValues are calculated in CHCl3 with IEFPCM model.
The A4 molecule has the highest λmax value in gas as well as in solution (CHCl3 solvent) phase. The A4 molecule contains fulvene moiety due to which this shifted the λmax to the higher side. This fulvene moiety is linear and an effective over-lap of the π-orbitals to its neighboring moieties rendered it to extend conjugation which ultimately resulted in absorption of UV in the higher wave-length range. The phenyl moiety in A2 and A3 could not enhance conjugation effectively as is evident from the λmax values of these molecules (A2–A3) compared with A1 in both gas phase and in CHCl3. This can be attributed to the aromaticity of the phenyl ring which may show reluctance to contribute in extending conjugation. Thus λmax values could not get considerable increase toward red-shift. However, the theinopyimidine oxide moiety showed considerable trend in extending conjugation in the designed molecule A5 and shifted the value of λmax towards longer wave-length (789 nm).
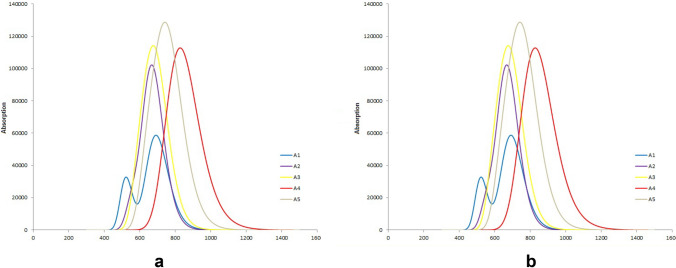
Figure 4 shows the absorption spectra of the A1–A5 molecules in gas phase and in CHCl3 solvent (IEFPCM model) with TD-DFT/B3LYP 6-31G (d,p). The similar trend can be found in gas phase (Fig. 4a) and in CHCl3 solvent (Fig. 4b) except λmax of all the molecules shows marginal red shift in CHCl3 solvent. The lowest absorption was observed for designed acceptor A1 in gas as well as in CHCl3. The highest absorption is given by A3 which can be attributed to the extended conjugation due to the presence of phenyl ring. The order of the absorption values for other acceptor molecules is A5 > A2 > A4 in gas phase and in CHCl3 (Fig. 4).
Figure 4.
The UV absorptions of A1–A5 at TD-DFT B3LYP 6-31 G(d,p): (a) in gas phase, (b) in CHCl3 (IEFPCM model).
Reorganizational energies of A1–A5 molecules
The reorganizational energies of the hole (λh) and electron (λe) for designed A1–A5 NFA molecules are given in Table 5. The reorganizational energy is generally divided into external reorganizational energy (λext.) and internal reorganizational energy (λint.). The λext results due to the external factors, such as environment, film morphology and temperature. The λext. is due to the intrinsic molecular structure and is only focused here. The reorganizational energy is inversely related to the mobility of electron and hole. When the reorganizational energy is lower, the mobility of ions will be higher40.
Table 5.
Reorganizational energies of A1–A5 at B3LYP 6-31G (d,p).
| Compounds | λe (eV) | λh (eV) |
|---|---|---|
| A1 | 0.0027 | 0.0099 |
| A2 | 0.0077 | 0.001 |
| A3 | 0.0007 | 0.0003 |
| A4 | 0.0059 | 0.0361 |
| A5 | 0.0117 | 0.0403 |
The A3 has the lowest reorganizational energies of electron (0.0007 eV) and hole (0.0003 eV), therefore, it offers the highest mobilities of electron and hole. The λe for the rest of molecules are in the order of A5 > A4 > A2 > A1and their mobility is in the A1 > A2 > A4 > A5 order. The order of the λh is A5 > A4 > A1 > A2 and their hole mobility order is A2 > A1 > A4 > A5. The electron and hole reorganization energies for a molecule are also different. The A1 and A5 has less electron reorganizations (λe) than the hole reorganization (λh). But A2 and A3 exhibited higher λe than corresponding λh (Table 5).
Open circuit voltage (Voc) and difference of HOMO of donor and acceptor
The open circuit voltage (Voc) is an important parameter in the fabrication of organic solar cells. When the solar cell is in zero current level, the maximum voltage drawn out of the cell is called Voc. The higher value of Voc indicates higher fill factor (FF) which is a key parameter in determining the efficiency of solar cells. The theoretical Voc value is calculated through the difference of HOMO of donor and LUMO of acceptor minus 0.3. The donor polymer D1 was used at B3LYP 6-31G (d,p) along with A1–A5 acceptors for the calculation of Voc through the following equation.
| 3 |
The theoretical calculation of Voc by subtracting the HOMO of donor from LUMO of acceptor and 0.3 can provide an estimation of the open circuit voltage when the designed acceptor is fabricated with some donor polymer (D1) through solution processed bulk heterojunction41,42. The highest Voc value of 1.5 eV is shown by the A1 and A3 with donor polymer D1. The blends of A2 and A4 with D1 provide 1.33 eV value of Voc. The lowest value of 1.04 eV was calculated for D1–A5 blend.
The difference of energies of HOMO of donor D1 and acceptors A1–A5 provide HOMO off-set values (∆HOMOD–A). The ∆HOMOD–A provides important information about the efficiency of the solar cell. It has been reported that lower ∆HOMOD–A value results in the enhanced solar cell efficiency25.
The D1–A1 blend shows zero ∆HOMOD–A off-set values, while the D1–A2 and D1–A4 blends provide − 0.38 eV and 0.35 eV respectively. 0.32 eV value is observed for D1–A5 (Fig. 5).
Figure 5.
Voc and ∆HOMO of A1–A5 with D1 polymer donor.
The complex of A4 with D1 has been used for studying charge transfer between donor and acceptor molecules (Fig. 6). Figure 7 shows that the HOMO are scattered on the donor polymer D1 while the LUMO are entirely distributed on the acceptor A4. The calculation of dipole moment provides useful information about the charge transfer as well as the electrostatic interaction between donor and acceptor units43,44.
Figure 6.
Optimized geometry of blend of D1–A4 at B3LYP 6-31G (d,p).
Figure 7.
HOMO and LUMO distribution on the blend of D1–A1.
Conclusion
The non-fullerene A1–A5 acceptor molecules have been designed successfully with diflourobenzene or quinoline bridge, cyclopenta[1,2-b:3,4-b′]dithiophene donor and 2-(2-methylene-3-oxo-2,3-dihydro-1H-inden-1-ylidene)malononitrile acceptor moiety which incorporates additional phenyl, fulvene or thieno[3,2-d]pyrimidine 5-oxide units through DFT calculations. For the illustration of the optoelectronic, photovoltaic and structure related properties; the EHOMO, ELUMO, Eg, λmax, Ex, and PDOS has been calculated. The designed non-fullerene acceptor A4 has shown lowest band gap energy (1.42 eV), as well as excitation energy in gas phase (1.39 eV) and in CHCl3 (1.40 eV). Thus A4 will, presumably, prove good candidate for future organic non-fullerene acceptors. The dipole moments of the designed acceptors A1–A5 at ground state (µg) and excited state (µe) have also been calculated; A5 showed highest dipole moment values (µe:18.6D; µe:12.8D). The reorganizational energies of electron (λe) and hole (λh) transport for A1–A5 are estimated. The A4 has the lowest λe (0.0007 eV) and λh (0.0003 eV) which exhibits its highest electron transport capability.
The open circuit voltage (Voc) values of the A1–A5 blends with D1 have been calculated by using HOMOD1—LUMOA1–A5—0.3 equation. Voc values are ranging from 1.04 to 1.50 eV. The Distribution of HOMO of donor and LUMO of acceptor has been studied for D1–A4 blend which has provided information that the HOMO are resided on the donor polymer (D1) and LUMO are scattered on acceptor A4. Thus keeping in view the optoelectronic, molecular orbital distribution and reorganization energies A3 and A4 molecules were found a very good acceptor which can be synthesized as non-fullerene acceptor for future organic solar cell. These acceptors are expected to provide improved photovoltaic properties to OSCs.
Author contributions
G.B. conceived the idea, performed the computations, wrote the manuscript, prepared the figures and supervised the overall research; L.G. wrote the manuscript and visualization; M.J. performed the computations, wrote the manuscript; perform the computations; M.A. performed the computations and edit the manuscript; W.A. conceived the idea, performed the computations, prepared the figures, wrote the manuscript and supervised the study; A.A. helped in calculations & wrote the manuscript; M.S. wrote the manuscript, helped in writing & editing; R.A. helped in editing & writing; D.H. helped in editing and computations. All authors analyzed numerical results and contributed to the preparation of the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Ghulam Bary, Email: ghulambary@gmail.com.
Waqar Ahmed, Email: waqar222us@gmail.com.
References
- 1.Mellit AB, Benghanem M. Practical Guide for Advanced Methods in Solar Photovoltaic Systems. Springer; 2020. [Google Scholar]
- 2.Sonar P, Lim JPF, Chan KL. Organic non-fullerene acceptors for organic photovoltaics. Energy Environ. Sci. 2011;4(5):1558–1574. doi: 10.1039/c0ee00668h. [DOI] [Google Scholar]
- 3.Yan J, Saunders BR. Third-generation solar cells: A review and comparison of polymer: Fullerene, hybrid polymer and perovskite solar cells. RSC Adv. 2014;4(82):43286–43314. doi: 10.1039/C4RA07064J. [DOI] [Google Scholar]
- 4.Bian L, Zhu E, Tang J, Tang W, Zhang F. Recent progress in the design of narrow bandgap conjugated polymers for high-efficiency organic solar cells. Prog. Polym. Sci. 2012;37(9):1292–1331. doi: 10.1016/j.progpolymsci.2012.03.001. [DOI] [Google Scholar]
- 5.Ameri T, Khoram P, Min J, Brabec CJ. Organic ternary solar cells: A review. Adv. Mater. 2013;25(31):4245–4266. doi: 10.1002/adma.201300623. [DOI] [PubMed] [Google Scholar]
- 6.Hedley GJ, Ward AJ, Alekseev A, Howells CT, Martins ER, Serrano LA, Cooke G, Ruseckas A, Samuel ID. Determining the optimum morphology in high-performance polymer-fullerene organic photovoltaic cells. Nat. Commun. 2013;4(1):1–10. doi: 10.1038/ncomms3867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liao S-F, Chen C-T, Chao C-Y. Isoindigo-dicyanobithiophene-based copolymer for high performance polymer–fullerene solar cells reaching 1.06 V open circuit voltage and 8.36% power conversion efficiency. ACS Macro Lett. 2017;6(9):969–974. doi: 10.1021/acsmacrolett.7b00547. [DOI] [PubMed] [Google Scholar]
- 8.Nam S, Seo J, Woo S, Kim WH, Kim H, Bradley DD, Kim Y. Inverted polymer fullerene solar cells exceeding 10% efficiency with poly (2-ethyl-2-oxazoline) nanodots on electron-collecting buffer layers. Nat. Commun. 2015;6(1):1–9. doi: 10.1038/ncomms9929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu X, Yu T, Bi Z, Ma W, Li Y, Peng Q. Realizing over 13% efficiency in green-solvent-processed nonfullerene organic solar cells enabled by 1, 3, 4-thiadiazole-based wide-bandgap copolymers. Adv. Mater. 2018;30(3):1703973. doi: 10.1002/adma.201703973. [DOI] [PubMed] [Google Scholar]
- 10.Kumari T, Lee SM, Kang S-H, Chen S, Yang C. Ternary solar cells with a mixed face-on and edge-on orientation enable an unprecedented efficiency of 12.1% Energy Environ. Sci. 2017;10(1):258–265. doi: 10.1039/C6EE02851A. [DOI] [Google Scholar]
- 11.Chen W, Yang X, Long G, Wan X, Chen Y, Zhang Q. A perylene diimide (PDI)-based small molecule with tetrahedral configuration as a non-fullerene acceptor for organic solar cells. J. Mater. Chem. 2015;3(18):4698–4705. [Google Scholar]
- 12.Wong HC, Li Z, Tan CH, Zhong H, Huang Z, Bronstein H, McCulloch I, Cabral JT, Durrant JR. Morphological stability and performance of polymer–fullerene solar cells under thermal stress: The impact of photoinduced PC60BM oligomerization. ACS Nano. 2014;8(2):1297–1308. doi: 10.1021/nn404687s. [DOI] [PubMed] [Google Scholar]
- 13.Lin Y, Zhan X. Oligomer molecules for efficient organic photovoltaics. Acc. Chem. Res. 2016;49(2):175–183. doi: 10.1021/acs.accounts.5b00363. [DOI] [PubMed] [Google Scholar]
- 14.Cheng P, Li G, Zhan X, Yang Y. Next-generation organic photovoltaics based on non-fullerene acceptors. Nat. Photonics. 2018;12(3):131–142. doi: 10.1038/s41566-018-0104-9. [DOI] [Google Scholar]
- 15.Yan C, Barlow S, Wang Z, Yan H, Jen AK-Y, Marder SR, Zhan X. Non-fullerene acceptors for organic solar cells. Nat. Rev. Mater. 2018;3(3):1–19. doi: 10.1038/natrevmats.2018.3. [DOI] [Google Scholar]
- 16.Hou J, Inganäs O, Friend RH, Gao F. Organic solar cells based on non-fullerene acceptors. Nat. Mater. 2018;17(2):119–128. doi: 10.1038/nmat5063. [DOI] [PubMed] [Google Scholar]
- 17.Wadsworth A, Moser M, Marks A, Little MS, Gasparini N, Brabec CJ, Baran D, McCulloch I. Critical review of the molecular design progress in non-fullerene electron acceptors towards commercially viable organic solar cells. Chem. Soc. Rev. 2019;48(6):1596–1625. doi: 10.1039/C7CS00892A. [DOI] [PubMed] [Google Scholar]
- 18.Willot P, De Cremer L, Koeckelberghs G. The use of cyclopenta [2, 1-b; 3, 4-b′] dithiophene analogues for the development of low-bandgap materials. Macromol. Chem. Phys. 2012;213(12):1216–1224. doi: 10.1002/macp.201200050. [DOI] [Google Scholar]
- 19.Wu J, Ma Y, Wu N, Lin Y, Lin J, Wang L, Ma C-Q. 2, 2-Dicyanovinyl-end-capped oligothiophenes as electron acceptor in solution processed bulk-heterojunction organic solar cells. Org. Electron. 2015;23:28–38. doi: 10.1016/j.orgel.2015.04.003. [DOI] [Google Scholar]
- 20.Leclerc N, Chávez P, Ibraikulov OA, Heiser T, Lévêque P. Impact of backbone fluorination on π-conjugated polymers in organic photovoltaic devices: A review. Polymers. 2016;8(1):11. doi: 10.3390/polym8010011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ahmed S, Kalita DJ. Charge transport in isoindigo-dithiophenepyrrole based DA type oligomers: A DFT/TD-DFT study for the fabrication of fullerene-free organic solar cells. J. Chem. Phys. 2018;149(23):234906. doi: 10.1063/1.5055306. [DOI] [PubMed] [Google Scholar]
- 22.Mehboob MY, Khan MU, Hussain R, Ayub K, Sattar A, Ahmad MK, Irshad Z, Adnan M. Designing of benzodithiophene core-based small molecular acceptors for efficient non-fullerene organic solar cells. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2020;244:118873. doi: 10.1016/j.saa.2020.118873. [DOI] [PubMed] [Google Scholar]
- 23.Manzoor F, Iqbal J, Zara Z, Eliasson B, Mahr MS, Ayub K. Theoretical calculations of the optical and electronic properties of dithienosilole-and dithiophene-based donor materials for organic solar cells. ChemistrySelect. 2018;3(5):1593–1601. doi: 10.1002/slct.201703086. [DOI] [Google Scholar]
- 24.Farhat A, Khera RA, Iqbal S, Iqbal J. Tuning the optoelectronic properties of Subphthalocyanine (SubPc) derivatives for photovoltaic applications. Opt. Mater. 2020;107:110154. doi: 10.1016/j.optmat.2020.110154. [DOI] [Google Scholar]
- 25.Li S, Zhan L, Sun C, Zhu H, Zhou G, Yang W, Shi M, Li C-Z, Hou J, Li Y. Highly efficient fullerene-free organic solar cells operate at near zero highest occupied molecular orbital offsets. J. Am. Chem. Soc. 2019;141(7):3073–3082. doi: 10.1021/jacs.8b12126. [DOI] [PubMed] [Google Scholar]
- 26.Frisch, M., Trucks, G., Schlegel, H., Scuseria, G., Robb, M., Cheeseman, J., Scalmani, G., Barone, V., Mennucci, B. & Petersson, G. Gaussian, Inc., Wallingford CT. Gaussian 09 (2009).
- 27.Dennington, R., Keith, T. & Millam, J. GaussView 5.0, Gaussian. Inc, Wallingford (2008).
- 28.Yanai T, Tew DP, Handy NC. A new hybrid exchange–correlation functional using the Coulomb-attenuating method (CAM-B3LYP) Chem. Phys. Lett. 2004;393(1–3):51–57. doi: 10.1016/j.cplett.2004.06.011. [DOI] [Google Scholar]
- 29.Improta R, Barone V. Absorption and fluorescence spectra of uracil in the gas phase and in aqueous solution: A TD-DFT quantum mechanical study. J. Am. Chem. Soc. 2004;126(44):14320–14321. doi: 10.1021/ja0460561. [DOI] [PubMed] [Google Scholar]
- 30.Klamt A, Moya C, Palomar J. A comprehensive comparison of the IEFPCM and SS(V)PE continuum solvation methods with the COSMO approach. J. Chem. Theory Comput. 2015;11(9):4220–4225. doi: 10.1021/acs.jctc.5b00601. [DOI] [PubMed] [Google Scholar]
- 31.O’boyle NM, Tenderholt AL, Langner KM. cclib: A library for package-independent computational chemistry algorithms. J. Comput. Chem. 2008;29(5):839–845. doi: 10.1002/jcc.20823. [DOI] [PubMed] [Google Scholar]
- 32.Lu T, Chen F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012;33(5):580–592. doi: 10.1002/jcc.22885. [DOI] [PubMed] [Google Scholar]
- 33.Fischer J, Ray D, Kleemann H, Pahner P, Schwarze M, Koerner C, Vandewal K, Leo K. Density of states determination in organic donor–acceptor blend layers enabled by molecular doping. J. Appl. Phys. 2015;117(24):245501. doi: 10.1063/1.4922587. [DOI] [Google Scholar]
- 34.Zhou Y, Long G, Li A, Gray-Weale A, Chen Y, Yan T. Towards predicting the power conversion efficiencies of organic solar cells from donor and acceptor molecule structures. J. Mater. Chem. 2018;6(13):3276–3287. [Google Scholar]
- 35.Irfan M, Iqbal J, Sadaf S, Eliasson B, Rana U, Khan UD, Ayub K. Design of donor–acceptor–donor (D–A–D) type small molecule donor materials with efficient photovoltaic parameters. Int. J. Quantum Chem. 2017;117:e25363. doi: 10.1002/qua.25363. [DOI] [Google Scholar]
- 36.Zhu J, Ke Z, Zhang Q, Wang J, Dai S, Wu Y, Xu Y, Lin Y, Ma W, You W. Naphthodithiophene-based nonfullerene acceptor for high-performance organic photovoltaics: Effect of extended conjugation. Adv. Mater. 2018;30(2):1704713. doi: 10.1002/adma.201704713. [DOI] [PubMed] [Google Scholar]
- 37.Liu D, Kan B, Ke X, Zheng N, Xie Z, Lu D, Liu Y. Extended conjugation length of nonfullerene acceptors with improved planarity via noncovalent interactions for high-performance organic solar cells. Adv. Energy Mater. 2018;8(26):1801618. doi: 10.1002/aenm.201801618. [DOI] [Google Scholar]
- 38.Tang A, Zhan C, Yao J, Zhou E. Design of diketopyrrolopyrrole (DPP)-based small molecules for organic-solar-cell applications. Adv. Mater. 2017;29(2):1600013. doi: 10.1002/adma.201600013. [DOI] [PubMed] [Google Scholar]
- 39.Bredas J-L. Mind the gap! Mater. Horiz. 2014;1(1):17–19. doi: 10.1039/C3MH00098B. [DOI] [Google Scholar]
- 40.Ans M, Manzoor F, Ayub K, Nawaz F, Iqbal J. Designing dithienothiophene (DTT)-based donor materials with efficient photovoltaic parameters for organic solar cells. J. Mol. Model. 2019;25(8):1–12. doi: 10.1007/s00894-019-4108-2. [DOI] [PubMed] [Google Scholar]
- 41.Brabec CJ, Cravino A, Meissner D, Sariciftci NS, Fromherz T, Rispens MT, Sanchez L, Hummelen JC. Origin of the open circuit voltage of plastic solar cells. Adv. Funct. Mater. 2001;11(5):374–380. doi: 10.1002/1616-3028(200110)11:5<374::AID-ADFM374>3.0.CO;2-W. [DOI] [Google Scholar]
- 42.Rauh D, Wagenpfahl A, Deibel C, Dyakonov V. Relation of open circuit voltage to charge carrier density in organic bulk heterojunction solar cells. Appl. Phys. Lett. 2011;98(13):69. doi: 10.1063/1.3566979. [DOI] [Google Scholar]
- 43.Marchiori C, Koehler M. Dipole assisted exciton dissociation at conjugated polymer/fullerene photovoltaic interfaces: A molecular study using density functional theory calculations. Synth. Met. 2010;160(7–8):643–650. doi: 10.1016/j.synthmet.2009.12.026. [DOI] [Google Scholar]
- 44.Koehler M, Santos M, Da Luz M. Positional disorder enhancement of exciton dissociation at donor∕ acceptor interface. J. Appl. Phys. 2006;99(5):053702. doi: 10.1063/1.2174118. [DOI] [Google Scholar]