Abstract
Introduction
Androgen deprivation therapy (ADT) is a well-established treatment for metastatic hormone-sensitive prostate cancer (mHSPC). It includes either bilateral orchiectomy or medical castration in form of luteinizing hormone-releasing hormone (LHRH) agonist or antagonist. We conducted this study to compare surgical and medical castration in terms of time to progression (TTP) to castration resistant prostate cancer.
Methods
Patients with mHSPC underwent either bilateral orchidectomy or medical castration by either LHRH agonist or by antagonist from November 2016 to May 2018 in our institution. Initial PSA and baseline imaging either magnetic resonance imaging (MRI) or positron emission tomography-computed tomography (PET CT) finding were recorded. Serum PSA, testosterone, and FSH were repeated every 3 months till 1 year. All enrolled patients were followed up with a bone scan/MRI/ PET CT at 6 months and 12 months. End point of study was progression of disease and death of patient.
Results
Mean nadir PSA (ng/ml) after treatment was 4.7 and 9.8 in surgical and medical group respectively, whereas mean time to the nadir PSA was 8.7 and 8.8 respectively with no statistically significant difference. Mean TTP was 13.9 months in bilateral orchidectomy group and 13.8 months in medical castration group (chi-square 0.003, p value 0.958).
Conclusion
There was no significant difference in time to progression between bilateral orchidectomy and medical castration. Considering nadir PSA level, better quality of life, patient compliance, reduced hospital visit, and decrease in cost of treatment, bilateral orchidectomy may be a better treatment option especially in developing countries.
Keywords: Metastatic, Hormone sensitive, Prostate cancer, Bilateral orchidectomy, Medical castration
Introduction
Prostate cancer is the most common cancer and leading cause of death in men in the USA [1]. Asian continent has a relatively high rate of advanced and metastatic prostate cancer at detection in comparison with rates in the USA and Europe, which is mostly explained by the lack of mass screening [2].
Contemporary research has led to the development of multiple active treatment modalities for men with metastatic disease with the goals of prolonging survival, minimizing complications, and maintaining quality of life. Combining androgen deprivation therapy (ADT) with any of docetaxel, abiraterone acetate, enzalutamide, or apalutamide have demonstrated significant overall survival (OS) benefits as compared with ADT alone [3]. ADT is a well-established treatment modality and has to be continued throughout the life of the patient. It includes either bilateral orchiectomy or medical castration in form of luteinizing hormone-releasing hormone (LHRH) agonist or antagonist and requires lifelong [4].
In developing countries, a large number of patients with metastatic prostate cancer are devoid of treatment because of overall high cost of treatment. Bilateral orchidectomy is a simple, cost-effective method of rapidly decreasing serum testosterone levels with additional benefits of rapid palliation of symptoms and elimination of patient compliance issues [5].
There are several reports comparing the castration level and the sustainability of LHRH agonist and antagonist [6]. However, there are few reports comparing efficacy of surgical and medical castration and the prognosis between these two groups in relation to their effectiveness over serum prostate-specific antigen (PSA), testosterone, and follicle stimulating hormone (FSH).
As part of a comprehensive study of the evidence on the relative effectiveness of methods of androgen suppression as primary treatment for metastatic prostate cancer, we conducted this study that compares surgical and medical castration in terms of time to progression (TTP) to castration resistant prostate cancer (CRPC) and determine whether there is a difference in outcome.
Methods
This prospective, observational study was conducted in a single multidisciplinary hospital and referral center in India from November 2016 to May 2018.
The primary objective was to determine the effectiveness of surgical and medical castration as judged by serum PSA response. Secondary objectives were to determine the effectiveness of surgical and medical castration as judged by serum testosterone and FSH levels.
An inclusion criterion was biopsy proven adenocarcinoma prostate with metastasis on bone scan or magnetic resonance imaging (MRI) or positron emission tomography-computed tomography (PET CT). Patients with history of radical prostatectomy, radical radiotherapy to prostate, prior exposure to hormonal therapy, radiotherapy or chemotherapy, hepatic, cardiac or renal dysfunction, other pre-existing malignancy, were excluded. Informed consent was taken from all patients. This study was approved by the institute ethics committee.
Detailed history and physical examination was performed. Initial/baseline PSA (iPSA) and baseline imaging (MRI/PET CT) finding were recorded.
All patients enrolled in this study were stratified in 2 groups. Group 1: surgical castration by bilateral orchidectomy; group 2: receiving medical castration by either LHRH antagonist (loading dose of Inj. Degarelix 240 mg subcutaneously for the first month and then 80 mg monthly) or by LHRH agonist (Inj. Leuprolide 22.5 depot every three monthly).
Serum PSA by immunometric assay (normal value 0–4.0 ng/ml), serum testosterone by chemiluminescent assay (normal value 280–800 ng/dL), serum FSH by immunoradiometric assay (normal value 1.3–19.3 mIU/ml) were measured as baseline (first visit). Serum PSA, testosterone, and FSH were repeated every 3 months till 1 year. All laboratory assessments were performed at our institution. All enrolled patients were followed up with a bone scan or cross sectional imaging study (MRI/ PET CT) at 6 and 12 months.
End points of study were progression of disease and death of patient. Progression was defined as either two consecutive rises (> 1 week apart) in total PSA after a nadir PSA despite castrate level of testosterone or progression of osseous lesions: progression or appearance of two or more lesions on bone scan or soft tissue lesions using Response Evaluation Criteria in Solid Tumors (RECIST). The date of progression was defined as the point halfway between the nadir date and the first rise of PSA.
Sample Size Calculation
We calculated sample size using time to progression (TTP) to CRPC. Considering mean TTP to CRPC 32.8 ± 9.8 months for surgical group and 27.3 ± 8.2 months for medical group with 30.0% coefficient of variation, sample size for 95% confidence level and 80% power worked out 43 per group. Assuming the lost to follow-up upto 20%, a sample size of 52 per group was recommended.
Statistical Analysis Plan
Descriptive analysis of quantitative data was expressed as means and standard deviation. Qualitative data was presented as absolute numbers and proportions. Continuous variables were analyzed using the independent Student’s t test and categorical variables were analyzed using chi-square. Mann–Whitney U test was applied for testing of difference of median value between the groups. Time to progression survival curves were obtained by the Kaplan–Meier method for surgical and medical groups and compared for their significance. The log rank test was used to assess differences between the groups. P value < 0.05 is considered statistically significant. SPSS software, version 24.0 was used for statistical analysis.
Results
A total of 105 consecutive men with metastatic hormone-sensitive prostate cancer (mHSPC) underwent different modalities of ADT. All of these patients had a minimum follow up of 12 months with a mean follow-up of 14.2 ± 0.3 months. Out of these 105 patients, 7 patients died during the follow-up time period and were excluded from the analysis, cause of death being unknown.
Mean age of all patients who underwent ADT was 71.7 ± 8.5 (range 45–88) years and median iPSA was 86 (range 6.5–5638) ng/ml. Mean serum testosterone was 353.6 ± 131.5 (90–920) ng/dl and mean serum FSH was 9.03 ± 3.6 (3.2–17.3) mIU/ml. In our study, surgical castration (bilateral orchidectomy) was performed in 51 patients (48.6%). Medical castration in form of LHRH agonists was given in 34 patients (32.4%) and LHRH antagonist in 20 patients (19.0%). Baseline patient characteristics were comparable in both groups with no significant difference (Table 1).
Table 1.
Comparison of bilateral orchidectomy and medical castration (LHRH agonist and antagonist)
| Androgen deprivation therapy (n = 105) |
p-value | ||
|---|---|---|---|
| Bilateral Orchidectomy (n = 51) | Medical Castration (n = 54) | ||
| Mean age ± SD (years) | 71.5 ± 8.1 | 71.8 ± 8.9 | 0.847 |
| Mean BMI ± SD (kg/m2) | 26.3 ± 3.5 | 27.2 ± 3.8 | 0.219 |
| Mean baseline PSA ± SD (ng/ml) | 424.0 ± 1114.0 | 287.4 ± 683.3 | 0.448 |
| Mean Gleason score | 8 ± 0.9 | 8 ± 0.9 | 0.848 |
| Low volume disease | 18 (35.3%) | 19 (35.2%) | > 0.05 |
| High volume disease | 33 (64.7%) | 35 (64.8%) | > 0.05 |
| Mean nadir PSA ± SD (ng/ml) | 4.7 ± 17.2 | 9.8 ± 42.5 | 0.401 |
| Median nadir PSA (ng/ml) | 0.6 | 0.4 | 0.401 |
| Mean time to nadir PSA ± SD (months) | 8.7 ± 3.8 | 8.8 ± 3.7 | 0.869 |
| Median time to nadir PSA (months) | 9.0 | 9.0 | 0.869 |
| Mean time to progression ± SD (months) | 13.9 | 13.8 | 0.958 |
LHRH luteinizing hormone releasing hormone (LHRH) agonist, BMI Body mass index, SD Standard deviation, PSA Prostate specific antigen
The mean nadir PSA (ng/ ml) after treatment was 4.7 ± 17.2 and 9.8 ± 42.5 in surgical and medical group respectively, whereas the mean time to the nadir PSA was 8.71 ± 3.8 and 8.83 ± 3.7, respectively. There was no statistically significant difference between the two groups in relation to nadir PSA and time to nadir PSA.
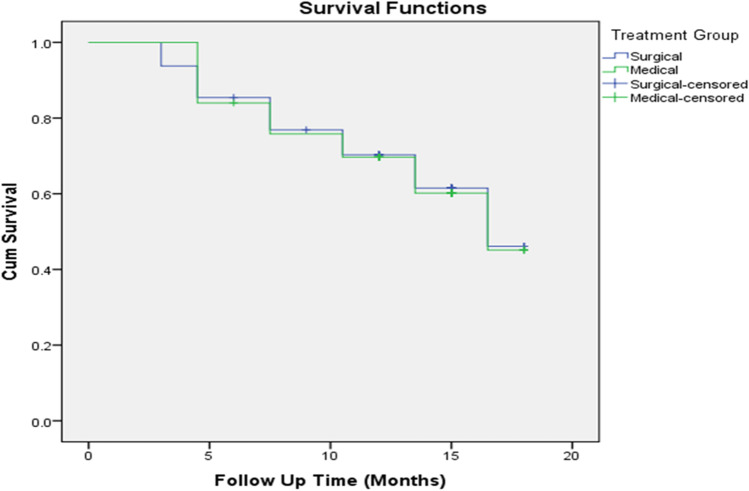
Mean TTP was 13.9 months with SE 0.795 (95% CI 12.36–15.48) in patients who underwent bilateral orchidectomy and 13.8 months with SE 0.768 (95% CI 12.36–15.37) in patients who received medical therapy (chi-square 0.003, p value 0.958) (Fig. 1). In the medical arm sub group, we found mean TTP in antagonist arm was 14.5 months with SE 1.155 (95% CI 12.234–16.763) and in agonist arm was 11.9 months with SE 0.752 (95% CI 10.51–13.46) with no statistically significant difference (chi-square 0.400, p value 0.819).
Fig. 1.
Kaplan Meier survival curve showing time to progression in patients of bilateral orchidectomy and medical castration
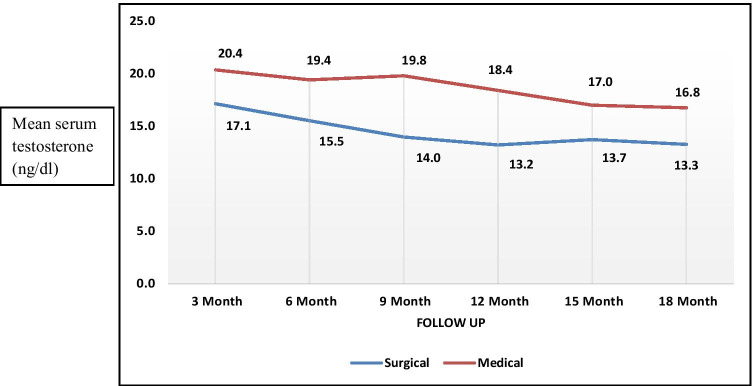
In our study, the mean testicular suppression between surgical and medical groups at defined follow-up periods was calculated. The mean testicular suppression in surgical arm was consistently < 20 ng/ml, but there was no statistically significant difference between two arms at defined follow up of 3 months interval (p value > 0.05) (Fig. 2).
Fig. 2.
Correlation of testosterone suppression between bilateral orchidectomy and medical castration (p – value: > 0.05)
In bilateral orchidectomy group, we noticed mean FSH level rising very high with levels of 56.2 mIU/ml and 72.10 mIU/ml at 3 and 15 months, respectively. In contrary to this, mean FSH was drastically suppressed in medical group. Mean FSH level at 3 and 15 months of agonist group were 1.2 ± 0.7 mIU/ml and 3.1 ± 0.9 mIU/ml respectively and that of antagonist group were 1.1 ± 0.8 mIU/ml and 2.4 ± 0.9 mIU/ml, respectively, (p value > 0.05). Mean FSH level in men treated with LHRH antagonist is lower than those treated with LHRH agonist.
Discussion
Huggins et al. first described the role of hormonal deprivation in cases of metastatic prostate cancer in 1941 by doing surgical castration and this remained the only treatment for several decades [7].
In late 1980s, with advent of LHRH agonist and antagonist, this practice has changed and led to a surge in medical castration and cost of treatment has gone up [4]. Direct treatment cost of medical castration is 6–20 times more as compared to surgical castration [8]. Sun et al. did not find a financial advantage to orchiectomy ($9726.98) as compared to medical castration ($8478.46) at 1 year after diagnosis of metastatic prostate cancer in Surveillance, Epidemiology, and End Results (SEER) program; however, most patients’ expenditures exceed $40,000 within 5 years in medical castration group [9].
Nadir PSA means the minimum PSA level achieved after any modality of treatment for prostate cancer (CaP). In our study, the nadir PSA 4.7 ng/ml and 9.9 ng/ml in surgical and medical group, respectively, whereas the time to the nadir PSA was 8.7 and 8.8 months, respectively. Demir et al. also found no significant difference between surgical and medical arms in relation to time to nadir PSA [10].
In our study, mean time to progression was 13.9 months and 13.8 months in patients with surgical and medical castration respectively without statistically significant difference (p value 0.958). There are many studies and meta-analysis comparing medical and surgical therapy in metastatic CaP where no significant differences in progression related outcomes was found [10–12]. In an Indian study of 60 patients comparing surgical and medical treatment in metastatic CaP, mean TTP was 20.8 months and 19.7 months in surgical and medical arms, respectively [11]. Lin et al. found improved tumor progression-free survival and biochemical failure rates after 12 months for patients in the surgical castration group in 121 consecutive patients. They compared Reijke scores among the patients and observed that surgical castration for patients with poor prognostic scores resulted in better disease-free survival and PSA normalization rates [13].
There are no studies comparing bilateral orchidectomy with LHRH antagonist, however there were quite a few studies comparing agonist and antagonist [6, 14]. Poppel et al. in their review article concluded time to PSA failure to be significantly longer with Degarelix as compared to Leuprolide (17.1 months vs. 10.1 months, p value < 0.05). They also gave a possible explanation that LHRH agonists are associated with testosterone microsurges in the start and thereafter, and small increases in testosterone levels might be associated with faster progression and gave a possible conclusion that the lack of microsurges with Degarelix contributes to the improvement in progression observed [6]. Though we had found better time to progression in antagonist group (14.5 months) as compared to agonist (11.9 months), it was not statistically significant. It may be due to small number of patients in this subgroup. Moreover, all the studies in literature comparing agonist and antagonist had a shorter follow-up period similar to our study.
In our study, we considered < 50 ng/dl as the threshold for castration and found all patients in surgical arm and all but 2 patients in medical arm to achieve castration. Those 2 patients failed to achieve castration level of testosterone even after 6 months of therapy. We also noticed a greater suppression of testosterone in surgical arm throughout the follow up period compared with medical group though it was statistically insignificant. This is consistent with the findings of other studies [15, 16].
In our study, we found greater impact of LHRH antagonist over agonist in suppressing serum FSH level. Mean FSH level at 3 and 15 months of agonist group were 1.2 mIU/ml and 3.1 mIU/ml respectively, and that of antagonist group were 1.1 mIU/ml and 2.4 mIU/ml, respectively. In literature also, there was a similar trend, where LHRH antagonists were causing more intense FSH suppression. Crawford et al. concluded that Degarelix caused more intense and rapid suppression of FSH in comparison to Leuprolide and thus assessed the role of FSH in PSA failure. He proposed that patients on Degarelix had a lower risk of PSA failure compared with Leuprolide, and the risk of PSA failure decreased in patients who switched from Leuprolide to Degarelix and attributed the role of FSH in it [17].
However, in the surgical arm, there was an opposite trend noticed in present study with mean FSH level rising very high with levels of 56.2 mIU/ml and 72.1 mIU/ml at 3 and 15 months, respectively. Hence, the role of FSH in predicting progression needs further study and bilateral orchidectomy should also be taken into consideration while assessing the role of FSH.
The majority of metastatic hormone-sensitive prostate cancer patients respond to ADT, but most of these patients ultimately develop CRPC within 1–3 years [18].
As per new concept of treatment of mHSPC, combining ADT with any of docetaxel (CHAARTED, GETUG-AFU and STAMPEDE-C arm trial), abiraterone acetate (LATTITUDE and STAMPEDE trial), enzalutamide (ENZAMET trial), or apalutamide (TITAN trial) have demonstrated significant overall survival (OS) benefits as compared with ADT alone. In a systemic review and network meta-analysis, Sathianathen et al. concluded that upfront combination therapies are the new standard of care for patients with mHSPC instead of ADT alone. ADT alone will likely to be used only in limited conditions or when patient cannot afford it [3].
In developing country like India, where large number of patients belongs to low-middle socioeconomic condition and they cannot afford the cost of upfront combination treatment modalities. Because of financial burden, bilateral orchidectomy is better management of these patients and should be preferred ADT along with other upfront modality of treatment and this can decrease overall cost of the treatment. Counseling of the patients is important to decide the type of ADT. If patient accept, bilateral orchidectomy is recommended.
This present study had some limitation. This was a single-center, non-randomized study so there may be chance of selection bias. The size of the study population was small along with short follow-up. Furthermore side effect profiles of the two modalities of treatment were not considered. Multicentric, prospective studies with long-term follow-up are needed to further delineate the natural history of metastatic CaP in Indian population so that optimal cost effective therapies could be devised for maximum benefit.
Conclusion
No significant difference in time to progression between bilateral orchidectomy and medical castration corroborated that both are equally effective in metastatic hormone-sensitive prostate cancer. Considering the nadir PSA level, better quality of life, patient compliance, reduced hospital visit, and decrease in the cost of treatment, bilateral orchidectomy may be a better treatment option for these patients, especially in developing countries.
Declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Cullen J, Elsamanoudi S, Brassell SA, Chen Y, Colombo M, Srivastava A, et al. The burden of prostate cancer in Asian nations. J Carcinog. 2012;11:7. doi: 10.4103/1477-3163.94025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sathianathen NJ, Koschel S, Thangasamy IA, Teh J, Alghazo O, Butcher G, et al. Indirect comparisons of efficacy between combination approaches in metastatic hormone-sensitive prostate cancer: a systematic review and network meta-analysis. Eur Urol. 2020;77(3):365–372. doi: 10.1016/j.eururo.2019.09.004. [DOI] [PubMed] [Google Scholar]
- 4.Merseburger AS, Alcaraz A, von Klot CA. Androgen deprivation therapy as backbone therapy in the management of prostate cancer. Onco Targets Ther. 2016;9:7263–7274. doi: 10.2147/OTT.S117176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garje R, Chennamadhavuni A, Mott SL, Chambers IM, Gellhaus P, Zakharia Y, et al. Utilization and outcomes of surgical castration in comparison to medical castration in metastatic prostate cancer. Clin Genitourin Cancer. 2020;18(2):e157–e166. doi: 10.1016/j.clgc.2019.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Poppel HV, Klotz L. Gonadotropin-releasing hormone: An update review of the antagonists versus agonists. Int J Urol. 2012;19(7):594–601. doi: 10.1111/j.1442-2042.2012.02997.x. [DOI] [PubMed] [Google Scholar]
- 7.Huggins C, Stevens RE, Hodges CV. Studies on prostatic cancer: II. The effects of castration on advanced carcinoma of the prostate gland. Arch Surg. 1941;43:209–223. doi: 10.1001/archsurg.1941.01210140043004. [DOI] [Google Scholar]
- 8.Henry MA, Leung A, Filson CP. Cost considerations for systemic therapy for patients with advanced genitourinary malignancies. Cancer. 2018;124:2897–2905. doi: 10.1002/cncr.31355. [DOI] [PubMed] [Google Scholar]
- 9.Sun M, Choueiri TK, Hamnvik OP, Preston MA, Velasco GD, Jiang W, et al. Comparison of gonadotropin-releasing hormone agonists and orchiectomy: effects of androgen-deprivation therapy. JAMA Oncol. 2016;2:500–507. doi: 10.1001/jamaoncol.2015.4917. [DOI] [PubMed] [Google Scholar]
- 10.Demir A, Cecen K, Karadag MA, Kocaaslan R, Turkeri L. The course of metastatic prostate cancer under treatment. Springerplus. 2014;3:725. doi: 10.1186/2193-1801-3-725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Purushothaman A, Kumar G, Gangadharan P, Roshni PR. A comparison of leuprolide acetate versus bilateral orchiectomy for patients with metastatic prostate cancer. Asian J Pharm Clin Res. 2016;9:51–54. [Google Scholar]
- 12.Seidenfeld J, Samson DJ, Hasselblad V, Aronson N, Albertsen PC, Bennett CL, et al. Single-therapy androgen suppression in men with advanced prostate cancer: a systematic review and meta-analysis. Ann Intern Med. 2000;132(7):566–577. doi: 10.7326/0003-4819-132-7-200004040-00009. [DOI] [PubMed] [Google Scholar]
- 13.Lin YH, Chen CL. A comparison of androgen deprivation therapy versus surgical castration for patients with advanced prostatic carcinoma. Acta Pharmacol Sin. 2011;32:537–542. doi: 10.1038/aps.2010.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iversen P, Damber JE, Malmberg A, Persson BE, Klotz L. Degarelix monotherapy compared with luteinizing hormone-releasing hormone (LHRH) agonists plus anti-androgen flare protection in advanced prostate cancer: an analysis of two randomized controlled trials. Ther Adv Urol. 2016;8(2):75–82. doi: 10.1177/1756287215621471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morote J, Orsola A, Planas J, Trilla E, Raventós CX, Cecchini L, et al. Redefining clinically significant castration levels in patients with prostate cancer receiving continuous androgen deprivation therapy. J Urol. 2007;178:1290–1295. doi: 10.1016/j.juro.2007.05.129. [DOI] [PubMed] [Google Scholar]
- 16.Perachino M, Cavalli V, Monferrato C. Testosterone (T) level correlates with survival in pts with advanced prostate cancer (APC): the lower is really the better. J Urol. 2008;179:179–180. doi: 10.1016/S0022-5347(08)60520-0. [DOI] [Google Scholar]
- 17.Crawford ED, Rove KO, Schally AV, Rick FG, Block NL, Thomas JR, et al. The role of the FSH system in the development and progression of prostate cancer. Am J Hematol Oncol. 2014;10(6):5–13. [Google Scholar]
- 18.Sweeney CJ, Chen YH, Carducci M, Liu G, Jarrard DF, Eisenberger M, et al. Chemohormonal therapy versus hormonal therapy for hormone naive newly metastatic prostate cancer: ECOG-led randomized trial. Ann Oncol. 2014;25(Suppl4):iv256. doi: 10.1093/annonc/mdu336.4. [DOI] [Google Scholar]