Abstract
The putative de novo methyltransferases, Dnmt3a and Dnmt3b, were reported to have weak methyltransferase activity in methylating the 3′ long terminal repeat of Moloney murine leukemia virus in vitro. The activity of these enzymes was evaluated in vivo, using a stable episomal system that employs plasmids as targets for DNA methylation in human cells. De novo methylation of a subset of the CpG sites on the stable episomes is detected in human cells overexpressing the murine Dnmt3a or Dnmt3b1 protein. This de novo methylation activity is abolished when the cysteine in the P-C motif, which is the catalytic site of cytosine methyltransferases, is replaced by a serine. The pattern of methylation on the episome is nonrandom, and different regions of the episome are methylated to different extents. Furthermore, Dnmt3a also methylates the sequence methylated by Dnmt3a on the stable episome in the corresponding chromosomal target. Overexpression of human DNMT1 or murine Dnmt3b does not lead to the same pattern or degree of de novo methylation on the episome as overexpression of murine Dnmt3a. This finding suggests that these three enzymes may have different targets or requirements, despite the fact that weak de novo methyltransferase activity has been demonstrated in vitro for all three enzymes. It is also noteworthy that both Dnmt3a and Dnmt3b proteins coat the metaphase chromosomes while displaying a more uniform pattern in the nucleus. This is the first evidence that Dnmt3a and Dnmt3b have de novo methyltransferase function in vivo and the first indication that the Dnmt3a and Dnmt3b proteins may have preferred target sites.
CpG methylation has been associated with reduced transcription (1, 13, 23), decreased DNase I sensitivity (14), and decreased site-specific recombination (8). Some of the effects of CpG methylation on chromatin structure are mediated by binding of a methylation binding protein (MeCP2) to methylated CpGs and recruitment of histone deacetylase (12, 19). CpG methylation is tightly regulated during replication and differentiation of somatic cells. A maintenance methyltransferase (designated DNA methyltransferase 1 [DNMT1] in humans and Dnmt1 in mice) functions during DNA replication to preserve the methylated pattern of preexisting methylated region (16). In general, genes lose their CpG methylation within the promoter when they become activated, whereas genes acquire CpG methylation after they are no longer transcribed (for reviews, see references 2 and 22). It has been demonstrated recently that protein binding can lead to demethylation through a replication-dependent process within the specific binding sites (11, 17). However, it remains unclear how regions of DNA acquire methylation in somatic cells (de novo methylation) or over what time intervals these changes occur, although many sequences have been reported to acquire methylation during development or the neoplastic process (25, 26).
De novo methylation is thought to establish the genomic CpG methylation pattern shortly after implantation in mammals (for a review, see reference 33). It is also believed to play an important role in gene regulation, X inactivation, genomic imprinting, and methylation of endogenous retroviruses and transposable elements (for a review, see reference 28). Many recent reports indicate that alteration of DNA methylation occurs in various tumors (25, 26). De novo methylation activity remains in mouse embryonic stem (ES) cells with homozygous deletion of the maintenance methyltransferase gene (Dnmt1) (15). Based on this finding, the search for other DNMTs has been intense. Three genes, Dnmt2, Dnmt3a, and Dnmt3b, have been reported to have homology with the methyltransferase motif (21, 34). There are several alternatively spliced products of Dnmt3b, and the largest peptide is the product of Dnmt3b1. Despite having homology to the methyltransferase motif, Dnmt2 has not been demonstrated to have methyltransferase activity (20). A weak de novo methyltransferase activity was detected by incubating a DNA fragment multiple times with crude cell extract from insect cells overexpressing Dnmt3a or Dnmt3b1 (21). The de novo methyltransferase activity of Dnmt3a and Dnmt3b1 is shown to be lower than that of Dnmt1 in this assay (21), although Dnmt1 has a much stronger affinity for hemimethylated DNA. De novo methylation is frequently found in tumors, although DNMT1 and DNMT3a overexpression has been reported to be much less frequent than DNMT3b overexpression in tumors (24). Therefore, it remains unclear what role these DNMTs play in de novo methylation in vivo.
It was reported that Dnmt3a and Dnmt3b are barely detectable in differentiated cells and adult tissues (21). It has also been shown that DNMT1 is expressed at a higher level than DNMT3a or DNMT3b in most human tissues (24). In many previous experiments, the methylation status of an episomal plasmid was maintained faithfully, most likely by DNMT1, in 293/EBNA1 cells over many weeks (9–11). However, de novo methylation of either an entirely unmethylated episome or the methylation-free region of a patch-methylated episome has not been observed (9, 10). It is clear that the de novo methyltransferase activity of DNMT1 does not result in de novo methylation of unmethylated sequences on the episome in 293/EBNA1 cells. Furthermore, if DNMT3a and DNMT3b are expressed at any appreciable level in 293/EBNA1 cells, they do not functionally de novo methylate the episome. These previous observations suggest that it is possible to use this oriP-based episomal system to investigate whether Dnmt3a and Dnmt3b have detectable de novo methyltransferase activity in vivo.
In this study, I found that mouse Dnmt3a methylates some regions of the episome in human cells, and some specific CpG sites on the episome become methylated in human cells overexpressing murine Dnmt3b protein. When the methyltransferase catalytic site of Dnmt3a and Dnmt3b is mutated, the de novo methylation activity of these proteins is abolished in vivo. This clearly demonstrates the de novo methylation function of Dnmt3a and Dnmt3b in vivo. Furthermore, these proteins appear to be different from DNMT1 in site preference and activity. I also found that Dnmt3a methylates the same sequence on the episome and in the chromosome in human cells. The findings in this study provide the first clear evidence of de novo methyltransferase function of Dnmt3a and Dnmt3b in vivo and open new experimental approaches in the understanding of how and where Dnmt3a and Dnmt3b act in human cells.
MATERIALS AND METHODS
Plasmids.
pMT3aMyc and pMT3bMyc were constructed by cloning the mouse Dnmt3a and Dnmt3b1 cDNAs, respectively, in frame into pCDNA3Myc. pCDNA3Myc contains a 12-amino-acid Myc tag downstream from the multiple cloning site of the pCDNA3 vector (Invitrogen). The mouse Dnmt3a and Dnmt3b1 coding sequences were amplified from the translational start site to one amino acid upstream of the stop codon from the mouse Dnmt3a cDNA clone (pMT3A) and Dnmt3b1 cDNA clone (pMT3B), respectively (generous gift from En Li, Massachusetts General Hospital). The primers were designed such that after an AseI/BamHI double digest, the coding sequence of Dnmt3a can be ligated into the expression vector in frame with the 12-amino-acid Myc tag on the vector. Primers for pMT3aMyc construction were 5′-CGCGGATCCTGCCCAGCAATGCCCTCCAG (forward) and 5′-GTGCACGCGTTCACACAAGCAAAATATTCCTTCAGC (reverse). Primers for pMT3bMyc construction were 5′-CGCGGATCCAGGAAACAATGAAGGGAGACAG (forward) and 5′-GTGCACGCGTATTCACAGGCAAAGTAGTCCTTC (reverse).
A single replacement of cysteine at position 706 of Dnmt3a and position 657 of Dnmt3b1 by a serine was made to abolish the cytosine methyltransferase catalytic site. Mutant plasmid pMT3aMut was constructed by amplifying sequences upstream and downstream of the catalytic site separately, using the same primers for pMT3aMyc and two mutant primers with the base substitution and 20 bases of overlap. The mutant primer for the mutated site and the upstream sequence is 5′-CAATGGAGAGGTCATTGGAGGGACTGCC, and the mutant primer for the mutated site and the downstream sequence is 5′-GGCAGTCCCTCCAATGACCT (the mutated site is underlined). Mutant plasmid pMT3bMut was constructed similarly to pMT3aMut by using the primers 5′-GGAAGCCCATCCAATGATCT and CGTTAGAGAGATCATTGGATGGGCTTCC (the mutated site is underlined). After the upstream and downstream sequences were amplified separately, the products were gel purified, mixed, and amplified again, using the same forward and reverse primers for wild-type plasmid construction. The final PCR products were digested and cloned into the pCDNA3myc vector as described above. The mutation was confirmed by sequencing both strands of the insert, and the expression of full-length proteins was confirmed by immunostaining of cells after transient transfection.
Several plasmids were used as the assay plasmids in transfection studies. pCLH22 (15) has oriP, the EBNA1 gene, a luciferase reporter gene driven by the Rous sarcoma virus long terminal repeat (RSV LTR), a hygromycin resistance gene driven by the herpes simplex virus thymidine kinase gene promoter, and the necessary prokaryotic replication and selection sequences. pCLH22 was generated by inserting the RSV LTR and luciferase gene between oriP and the hygromycin resistance gene on p220.2 (3), such that the only difference between these two plasmids is the lack of the RSV LTR and the luciferase gene on p220.2. p22ΔEBNA1 is pCLH22 with the EBNA1 sequence deleted by a ClaI/NsiI double digest. Cytomegalovirus (CMV) promoter-containing DNMT1 expression vector pCMV-HMT, containing the cDNA of human DNA methyltransferase from nucleotides 315 to 5054 (EMBL accession no. X63692), was obtained from S. Baylin’s laboratory (Johns Hopkins University). This vector was constructed prior to the knowledge of further 5′ sequences of DNMT1 (32) and therefore contains the short form of DNMT1; the short form of DNMT1 cDNA used here has been shown to have the same activity as the full-length DNMT1 (32). This expression vector has been characterized and used for integration experiments previously (27).
Cell lines and transfection.
The 293/EBNA1 cell line has been described previously (9). The 3a and 3b cell lines were generated by cotransfection of linearized pMT3aMyc and pMT3bMyc, respectively, with a puromycin expression vector at a ratio of 10:1 into 293/EBNA1 cells. Twelve independent puromycin-resistant cell clones were isolated and expanded from each transfection. Each cell clone was given a unique identification by appending a hyphenated number to the 3a or 3b prefix; for example, 3a-5 is a cell clone expressing the Dnmt3a protein. Expression of the Myc-tagged proteins was examined by immunofluorescent staining and Western blotting using a monoclonal anti-Myc antibody. Throughout this study, the calcium phosphate transfection method (9, 29) was used. All transfections were done in duplicate or triplicate for each experiment, and all experiments were performed multiple times.
Episome recovery and analysis.
Each time the transfected cells reached confluence, 2.5% of the cells were replated into a 100-mm-diameter plate, and the remaining cells were harvested for plasmid DNA extraction by the Hirt method (7). All transfection experiments were carried out without any selection for the episomal plasmid.
DNA harvested from each transfection was digested with restriction enzymes to determine the methylation status. The digested DNA was fractionated on 1% agarose gels, Southern transferred onto nylon membranes, and probed with either the entire plasmid or a specific fragment of the plasmid. The Southern blots were analyzed with a phosphorimager (GS525; Bio-Rad).
Genomic DNA extraction and analysis.
High-molecular-weight DNA was harvested from 293/EBNA1 cells, two Dnmt3a-expressing cell clones (3a-5 and 3a-11), and two Dnmt3a-expressing cell clones (3b-9 and 3b-11), using the standard proteinase K-phenol-chloroform method. Approximately 5 μg of DNA was double digested with HindIII and HhaI. The digested DNA was fractionated on a 0.8% agarose gel and Southern transferred onto a nylon membrane. The Southern filter was probed with the EBNA1 DNA fragment, and the Southern blot was analyzed with a phosphorimager.
Immunostaining and Western blotting.
The cells were seeded onto 22- by 22-cm coverslips 2 days before immunostaining, which was carried out according to the standard protocol (5). In brief, the cells were washed with phosphate-buffered saline (PBS) and fixed with 1.5% paraformaldehyde in PBS before permeabilization with a buffer containing 0.25% gelatin, 0.01% saponin, and 0.1% NP-40 in PBS (buffer A). After being permeabilized, the cells were incubated with a monoclonal anti-Myc antibody in buffer A without NP-40 for 1 h. The cells were then washed with buffer A without NP-40, and the final detection step was done by incubating the cells with fluorescein isothiocyanate-conjugated goat anti-mouse immunoglobulin G (Boehringer Mannheim Biochemicals). The staining was viewed with an Olympus BX60 epifluorescence microscope.
Crude extracts from 5 × 106 293/EBNA1, 3a-5, 3a-11, 3b-9, and 3b-11 cells were prepared by lysing the cells in 150 μl of lysis buffer containing 50 mM Tris (pH 8.0), 150 mM NaCl, 1% NP-40, 0.5% deoxycholate, 0.1% sodium dodecyl sulfate (SDS), and protease inhibitors. After the debri was pelleted by centrifugation, 50 μl of 4× reducing loading buffer was added to the lysate; 20 μl of the lysate from each cell line was heated at 55°C for 15 min before loaded onto an SDS–7% polyacrylamide gel with a positive control. Western blotting was done according to the standard protocol (4), using the anti-Myc monoclonal antibody and enhanced chemiluminescence nonradioactive detection system.
RESULTS
Overexpression of murine Dnmt3a and Dnmt3b in human cells.
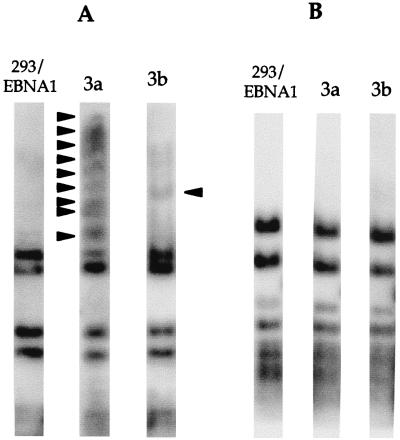
It was of interest to overexpress the Dnmt3a and Dnmt3b1 proteins to determine whether they could methylate DNA in vivo and to determine their specificities. The 293/EBNA1 cells were cotransfected with a puromycin expression vector and either pMT3aMyc or pMT3bMyc; 12 and 18 puromycin-resistant cell clones were isolated from cells transfected with pMT3aMyc and pMT3bMyc, respectively, 10 days after selection. Expression of the Myc-tagged Dnmt3a and Dnmt3b1 proteins was examined by immunofluorescent staining using a monoclonal anti-Myc antibody (data not shown). Nine and four clones were identified as Dnmt3a and Dnmt3b positive, respectively. It is noteworthy that the integrated Dnmt3b was expressed at a much lower level in all clones than the integrated Dnmt3a, although they were expressed equally well in transient transfections (Fig. 1A). Both proteins coated the metaphase chromosomes while displaying a more uniform pattern in the nucleus (Fig. 1A). Two independent cell clones, 3a-5 and 3a-11, for Dnmt3a protein, and two independent cell clones, 3b-9, and 3b-11, for Dnmt3b1 protein were further analyzed and used for transfection studies. Expression of the tagged protein was further confirmed by Western blotting using the monoclonal anti-Myc antibody (Figure 1B). These results demonstrate the expression of tagged proteins in these cells.
FIG. 1.
Dnmt3a and Dnmt3b expression in 293/EBNA1 cells. (A) Immunofluorescent staining of 293/EBNA1 cells transiently transfected with pMT3aMyc, pMT3bMyc, pMT3aMut, or pMT3bMut. Arrowheads indicate cells with no Myc-tagged protein expression. (B) Western blot of Myc-tagged Dnmt3a and Dnmt3b protein harvested from 3a-5, 3a-11, and 3b-11 cell clones. The solid arrowhead indicates the Dnmt3a protein, and the open arrowhead indicates the Dnmt3b protein.
De novo methyltransfase activity of Dnmt3a and Dnmt3b in vivo.
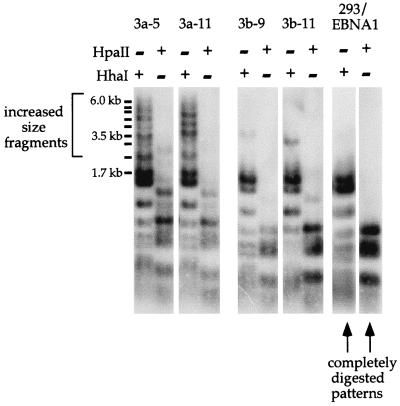
Plasmid pCLH22 was transfected into 293/EBNA1, 3a-5, 3a-11, 3b-9, and 3b-11 cells and harvested 10 days after transfection. The HhaI and HpaII single-digested plasmid DNA harvested from transfected 293/EBNA1 cells showed the completely digested pattern (Fig. 2). However, several larger fragments were detected in the HhaI and HpaII single-digested plasmid DNA, especially in the HhaI-digested DNA harvested from the transfected 3a-5 and 3a-11 cells (Fig. 2). There is a single fragment of increased size in the HhaI-digested plasmid and two fragments of increased size in the HpaII-digested plasmid harvested from the transfected 3b-9 and 3b-11 cells (Fig. 2). Observations for each protein were the same in both cell clones; therefore, this result is not the consequence of disruption of any specific endogenous gene by Dnmt3a or Dnmt3b expression vector integration. It is important to note that not all plasmids become methylated at these HhaI sites, because some completely digested fragments can be detected. Several HhaI sites in an extended region must acquire methylation to give rise to these rather large HhaI fragments (from 2.3 to 6 kb). However, not all HhaI sites on the plasmid become methylated, as indicated by the fact that no HhaI fragment larger than 6 kb (the entire plasmid is 12.1 kb) and no uncut molecules were detected. These findings clearly indicate that a fraction of the plasmids become methylated at some of the HhaI and HpaII sites when either Dnmt3a or Dnmt3b is overexpressed in 293/EBNA1 cells. The fact that the 3b cells appear to have a weaker activity may be due to the lower expression or different specificity of the protein. These observations were reproducible and consistent in over 20 transfection experiments. This analysis suggests that Dnmt3a and Dnmt3b can lead to de novo methylation of episomal plasmid with some specificity in vivo.
FIG. 2.
De novo methylation of assay plasmid pCLH22 by Dnmt3a and Dnmt3b. Shown is a Southern blot of pCLH22 DNA harvested 10 days after transfection from 293/EBNA, 3a, and 3b cells. The DNA was digested with restriction enzyme HhaI or HpaII, as indicated above each lane. The probe used is the entire plasmid pCLH22. 3a-5 and 3a-11 are cell clones with stably integrated pMT3aMyc, and 3b-9 and 3b-11 are cell clones that harbor stably integrated pMT3bMyc. Plasmid DNA harvested from 293/EBNA1 cells shows the complete digestion pattern, and plasmid DNA harvested from the 3a and 3b cell clones show some fragments of increased size.
Point mutations of Dnmt3a and Dnmt3b ablate the in vivo activity of these two enzymes.
It is possible that the de novo methylation of the episome observed was an indirect effect of Dnmt3a or Dnmt3b because both proteins showed only weak de novo methyltransferase activity in vitro. To rule out this possibility, point mutations were generated within the methyltransferase catalytic domain of Dnmt3a and Dnmt3b. These mutated expression vectors can be tested in the same experimental system for de novo methyltransferase activity in vivo. It is known that a cysteine-to-serine alteration in the P-C motif of methyltransferases destroys the catalytic activity without compromising other functions of these proteins (18, 30, 31). A single replacement of cysteine by serine at position 706 of Dnmt3a protein and position 657 of Dnmt3b1 protein was made to alter this known catalytic site of cytosine methylases. The mutation was confirmed by sequencing of both strands of the plasmids after construction, and the expression of full-length protein was detected by immunostaining of 293/EBNA1 cells after transient transfection (Fig. 1A) as described above.
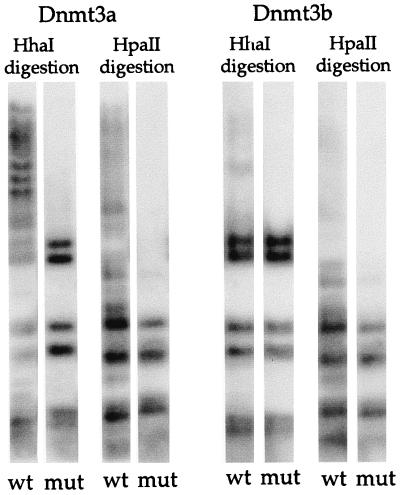
Plasmid pMT3aMut or pMT3bMut was cotransfected with the assay plasmid, pCLH22 or p220.2, into 293/EBNA1 cells for de novo methylation activity. The only difference between p220.2 and pCLH22 is that the former lacks the RSV LTR and luciferase gene. pMT3aMyc or pMT3bMyc was cotransfected with the assay plasmid into 293/EBNA1 cells as a positive control, and the assay plasmid was transfected alone into 293/EBNA1 cells as a negative control. The assay plasmid harvested from the negative control transfection (without cotransfection with Dnmt3a or Dnmt3b expression vector) was completely digested by HhaI or HpaII enzyme as illustrated above (data not shown). There were no increased-size HhaI fragments in the assay plasmid cotransfected with pMT3aMut or pMT3bMut, whereas increased-size HhaI fragments were observed in the positive control (Fig. 3). This finding clearly demonstrates that both Dnmt3a and Dnmt3b possess methyltransferase activity and are capable of de novo methylation of the stable episome in vivo.
FIG. 3.
Catalytic site mutants do not methylate the assay plasmid. Shown is a Southern blot of HhaI- or HpaII-digested plasmid DNA harvested 8 days after transfection. Plasmid expressing wild-type (wt) Dnmt3a, mutant (mut) Dnmt3a, wild-type Dnmt3b, or mutant Dnmt3b was cotransfected with assay plasmid p220.2. The p220.2 DNA harvested showed a complete HhaI and HpaII digestion pattern when cotransfected with either pMT3aMut or pMT3bMut.
De novo methylatransferase activity of Dnmt3a and Dnmt3b is distinct from that of DNMT1 in vivo.
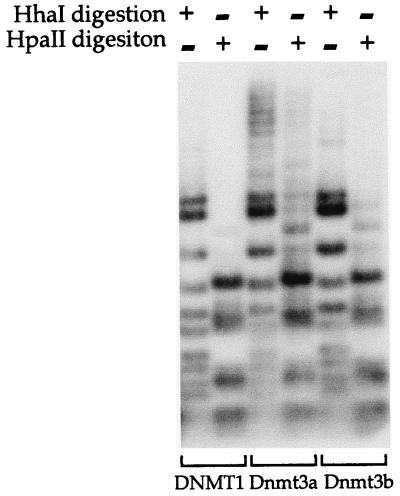
It has been demonstrated that Dnmt1 has a higher de novo methyltransferase activity than Dnmt3a and Dnmt3b1 in vitro (21). Although episome methylation by endogenous DNMT1 has not been observed in previous studies (9–11), overexpression of DNMT1 may lead to episome methylation. A DNMT1 expression vector, pCMV-HMT, with the CMV promoter driving the human DNMT1 cDNA, was used in the following experiments. This expression vector was chosen because it has been characterized previously (27). Note that the CMV promoter was used in the Dnmt3a and Dnmt3b expression vectors as well. The expression vector for DNMT1, Dnmt3a, or Dnmt3b was cotransfected with an assay plasmid, pCLH22 or p220.2, into 293/EBNA1 cells. Plasmid DNA harvested 12 days after transfection showed no altered-size HhaI or HpaII fragments when DNMT1 was cotransfected with the assay plasmid (Fig. 4). After long exposure, two very faint altered-size bands were observed in p220.2 DNA digested with each enzyme, and these bands were also visible in pCLH22 DNA after much longer exposure (data not shown). These altered-size fragments may be the result of DNMT1 de novo methylation activity when DNMT1 was overexpressed in the cells. Similar to the findings described above, many HhaI and HpaII fragments of increased size were detected when Dnmt3a was cotransfected with the assay plasmid, and a few HhaI and HpaII fragments of increased size were observed when Dnmt3b was cotransfected with the assay plasmid (Fig. 4). This observation indicates that overexpression of human DNMT1 does not lead to de novo methylation of the episome to the same extent as overexpression of either the murine Dnmt3a or Dnmt3b. Taken together with previous observations that endogenous DNMT1 does not lead to methylation of the episome over many weeks after transfection (9, 10), the findings here suggest that these three proteins may have different targets or requirements for their de novo methylation activity.
FIG. 4.
DNMT1, Dnmt3a, and Dnmt3b have distinct de novo methyltransferase activities in vivo. An expression vector for human DNMT1, murine Dnmt3a, or Dnmt3b was cotransfected into 293/EBNA1 cells with assay plasmid pCLH22. pCLH22 DNA was harvested 12 days after transfection and digested with restriction enzyme HhaI or HpaII, as indicated above each lane. The increased-size HhaI and HpaII fragments detected in pCLH22 DNA cotransfected with either the Dnmt3a or the Dnmt3b expression vector are the same as observed in Fig. 2. A complete digestion pattern is observed in pCLH22 DNA cotransfected with the DNMT1 expression vector, where only two very faint bands of increased size are detected in the HhaI- or HpaII-digested DNA after long exposure.
Targets of Dnmt3a and Dnmt3b de novo methylation activity on the episome are not random.
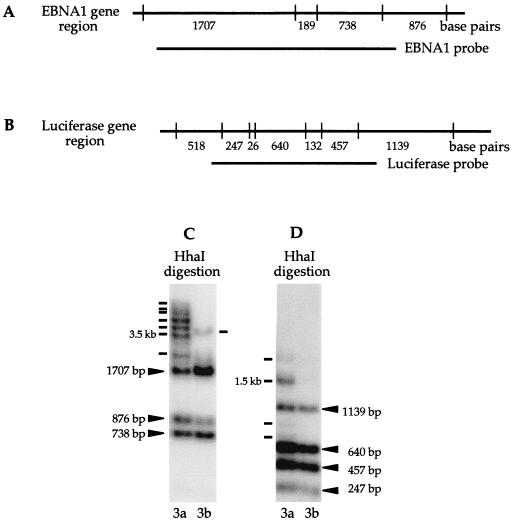
If the de novo methylation activity of Dnmt3a and Dnmt3b methylates CpG sequence randomly, any CpG sequence on the episome should be equally likely to become methylated. It is possible that some of the HhaI sites on the plasmid are too close together and fragments generated by random methylation are too small to be detected on the Southern blot when the entire plasmid is used as a probe. However, methylation of one or two HhaI sites in several regions of pCLH22 can result in fragments larger than the largest completely digested HhaI fragment of 1.7 kb. These regions include the EBNA1 gene, oriP, and the luciferase gene. The oriP region is known to undergo demethylation when the plasmid is methylated in vitro before transfection (11), and therefore it is not likely to become methylated. The EBNA1 and luciferase genes both of which are transcribed in human cells, flank the oriP region. Therefore, these two regions should be targeted similarly by the DNMTs if these enzymes target CpG sites randomly or if some feature of open chromatin structure (either due to transcription or near the replication origin) is the only requirement. To investigate whether this is the case, a Southern blot with HhaI-digested plasmid DNA harvested from 3a and 3b cells was hybridized sequentially with probes containing the entire plasmid, the EBNA1 gene, and the luciferase gene. This allows determination of whether all or some of the HhaI fragments of increased size contain either of these regions in the pCLH22 transfection experiments. To ensure complete stripping of the probe after each hybridization, the Southern blot was stripped with boiling water and stored for 2 months before the next hybridization.
With the EBNA1 probe, the completely digested bands containing any portions of the EBNA1 region were detected in DNA harvested from both 3a and 3b cells (Fig. 5A and C). In addition, most, if not all, of the increased-size HhaI fragments detected previously with the entire plasmid as the probe also hybridized to the EBNA1 probe (Fig. 2 and 5C). If the entire EBNA1 region is methylated while two flanking HhaI sites remained unmethylated and digestible by HhaI, a 3.5-kb HhaI fragment can be generated (Fig. 5A). Some of the HhaI sites within the EBNA1 region are clearly not methylated on a fraction of the plasmids, because a fragment smaller than 3.5 kb is detected in plasmid DNA harvested from both 3a and 3b cells (Fig. 5C). Although a 3.5-kb HhaI fragment was detected in the DNA harvested from 3a cells with the EBNA1 probe, several larger fragments were also clearly visible (Fig. 5C). It is also noteworthy that these increased-size HhaI fragments detected in the plasmid DNA harvested from 3a and 3b cells are different. These observations indicate that the HhaI fragments of increased size from pCLH22 frequently involve the EBNA1 region and extend beyond the EBNA1 region. It is also likely that Dnmt3a and Dnmt3b are dissimilar in their site preferences although both proteins target the same region of the assay plasmid.
FIG. 5.
Methylation of a specific region of the episome. (A) Illustration of HhaI sites and HhaI fragment sizes within and adjacent to the EBNA1 region. (B) Illustration of HhaI sites and fragment sizes in the luciferase gene. (C) Southern blot of HhaI digestion of pCLH22 DNA harvested from transfected 3a-5 and 3b-11 cells 10 days after transfection. The ENBA1 region-specific probe used for hybridization is as indicated in panel A. All increased-size HhaI and HpaII fragments observed in Fig. 2 are detected with this probe, as indicated with solid lines. (D) The same Southern blot in panel C, hybridized with a probe containing the luciferase coding region as indicated in panel B. The HhaI fragments derived from complete digestion are indicated with arrowheads labeled with fragment sizes. Four additional HhaI fragments were observed in the DNA harvested from 3a cells, as indicated with lines. Two of these fragments are smaller and two are larger than the largest completely digested band of 1.1 kb.
The Southern blot used above was stripped and then stored for 4 weeks before being hybridized to a probe containing only the coding region of the luciferase gene (Fig. 5B). Smaller altered-size HhaI fragments were detected in the plasmid DNA harvested from 3a cells, and no altered-size HhaI fragment was detected in the plasmid DNA harvested from 3b cells (Fig. 5C). If the entire luciferase gene region was methylated while the two flanking HhaI sites remained unmethylated and digestible by HhaI, a 3.1-kb HhaI fragment could be generated (Fig. 5B). However, the largest HhaI fragment was less than 3.1 kb in this hybridization, which indicates that de novo methylation occurring in the luciferase gene region does not extend much beyond the luciferase gene. Observations based on these sequential hybridizations indicate that the EBNA1 gene is more frequently part of the methylated regions than the luciferase gene and strongly suggest that the targets of de novo methylation on the episome are consistent and nonrandom.
EBNA1 is a preferred initiation site of Dnmt3a and Dnmt3b de novo methyltransferase activity.
Although the EBNA1 region is frequently involved in the de novo methylated regions on the episome, it is unclear whether methylation initiates in the EBNA1 region and extends into adjacent regions or if it initiates outside the EBNA1 region and extends into the EBNA1 gene. To investigate this, a plasmid lacking either the EBNA1 gene or the luciferase gene was assayed for de novo methylation by Dnmt3a or Dnmt3b. A plasmid without the oriP region cannot replicate in human cells and therefore was not tested. Plasmids p22ΔEBNA1 and p220.2 (see Materials and Methods for construction) were used for transfections into 3a and 3b as described above. Patterns of increased-size HhaI fragments similar to those observed in the pCLH22 DNA harvested from 3a and 3b cells (Fig. 2) were detected in the p220.2 DNA harvested from these cells (Fig. 6A). In contrast, no HhaI fragment of increased size was observed in the p22ΔEBNA1 DNA harvested from the 3a and 3b cells (Fig. 6B). If methylation is initiated outside the EBNA1 region and then spreads into the EBNA1 gene, the remaining region of methylation should persist when the 2.5-kb region harboring the EBNA1 gene is excised from the plasmid. If so, the increased-size HhaI fragments up to 3.5 kb in length should be detected (the largest increased-size HhaI fragment of 6 kb after subtracting the 2.5-kb excised segment). Experiments using p22ΔEBNA1 demonstrate that this is not the case. These findings suggest that the presence of the luciferase gene is not essential for the initiation of de novo methylation on the plasmid and has no effect on methylation of the EBNA1 region. Also, EBNA1 may be the preferred initiation site of Dnmt3a and Dnmt3b de novo methylation activity and from which methylation may spread into adjacent regions.
FIG. 6.
Lack of methylation of plasmid without the EBNA1 sequence. (A) Southern blot of HhaI-digested p220.2 (lacking the luciferase gene) DNA harvested 10 days after transfection. Arrowheads indicate increased-size DNA fragments detected in 3a and 3b cells. (B) Southern blot of HhaI digestion of p22ΔEBNA1 DNA harvested 10 days after transfection. There is no detectable HhaI fragment of increased size in any cells transfected with p22ΔEBNA1. The entire plasmid is used as the probe in both panels.
Integrated EBNA1 is also a target of the Dnmt3a de novo methylation activity.
The 293/EBNA1 cell line was originally generated by integrating the EBNA1 gene (driven by the CMV promoter) into the 293 embryonic kidney carcinoma cell line (9). All cell lines derived from 293/EBNA1, including all 3a and 3b cell clones, contain integrated EBNA1 at the same chromosomal site. The methylation status of the integrated EBNA1 should be essentially identical in all of these cell lines and therefore serve as an ideal test for whether Dnmt3a or Dnmt3b alters it. It is uncertain whether Dnmt3b will lead to methylation of the chromosomal EBNA1 sequence because its methyltransferase activity is barely detectable on pCLH22 or p220.2 in vivo. However, if EBNA1 is a target of Dnmt3a, integrated EBNA1 should become methylated just like the EBNA1 gene on the episome.
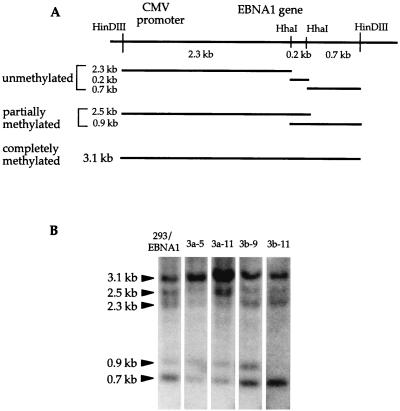
To investigate the methylation status of the EBNA1 gene in these cell lines, genomic DNA was harvested, HindIII/HhaI double digested, fractionated on a 0.8% agarose gel, Southern transferred, and probed with the EBNA1 coding region. There is a HindIII site just upstream of the CMV promoter and another HindIII site just downstream of the EBNA1 sequence (Fig. 7A). Therefore, the HindIII digest would release the CMV-EBNA1 segment from the integrated construct, regardless of the copy number and how the concatemer was formed. There are two HhaI sites within the EBNA1 sequence and none in the CMV promoter. If both HhaI sites were methylated in a given copy of CMV-EBNA1, a 3.1-kb HindIII/HindIII fragment would be generated by the digestion. Various-sized fragments would be generated by methylation at different sites, as illustrated in Fig. 7A. A partial digestion pattern is expected because multiple copies of the CMV-EBNA construct were integrated, and different patterns of methylation can occur on different segments of the integrated array. If de novo methyltransferase activity leads to increased methylation of the integrated EBNA1, increased amounts of the 3.1-, 2.5-, and 0.9-kb bands and decreased amounts of the 2.3- and the 0.7-kb bands should be observed.
FIG. 7.
Methylation of the EBNA1 sequence in the chromosome. (A) Illustration of HindIII and HhaI sites within the HindIII-to-HindIII fragment from the CMV-EBNA1 construct inserted into 293 cells. DNA fragments generated from the integrated construct by a HindIII/HhaI double digestion of unmethylated, partially methylated, and completely methylated sequences are also illustrated. (B) Southern blot of HindIII/HhaI double-digested genomic DNA harvested from 293/EBNA1, 3a-5, 3a-11, 3b-9, and 3b-11 cells. The probe used is the same EBNA1 fragment as indicated in Fig. 5A. Compared with 293/EBNA1, 3b-9, and 3b-11 cells, there is a decreasing amount of DNA in the smaller fragments and increasing amount of DNA in the 3.1-kb band in the DNA extracted from 3a-5 and 3a-11 cells. Lack of fragments larger than 3.1 kb indicates complete digestion of the DNA by HindIII. Probing of the same blot by the puromycin sequence also indicates complete digestion by HhaI (data not shown).
The intensities of the 2.3- and 0.7-kb fragments are reduced in the 3a-5 and 3a-11 genomic DNA, while the 3.1-kb band is stronger than bands from the DNA from 293/EBNA1 or 3b cells (Fig. 7B). Quantitation of the radioactivity in the 3.1-kb band divided by the total radioactivity in all five bands in each lane can be used to assess methylation in the EBNA1 region. This quantitation is done within each lane; therefore, any loading difference between lanes is irrelevant. This analysis revealed that 30% of the DNA is methylated at both HhaI sites in the EBNA1 region in 293/EBNA1 cells (30% of total radioactivity in the lane is in the 3.1-kb band). Six cell clones generated for an unrelated integration experiment using the 293/EBNA1 cells were also examined for EBNA1 gene methylation. An average of 24.8% of the DNAs from these six clones is methylated at both HhaI sites in the EBNA1 region (data not shown); 42 and 51% of the DNAs are methylated at these two sites in the 3a-5 and 3a-11 cells. There is no appreciable change of the fraction of radioactivity in the 3.1-kb fragment in the 3b-9 (28%) and 3b-11 (27%) cells, most likely due to the lack of significant methylation activity at these HhaI sites as demonstrated with the episome. This observation indicates that overexpression of Dnmt3a can lead to increased methylation at the two HhaI sites within the integrated EBNA1 gene and suggests that some sequence specificity may exist, based on the fact that EBNA1 is a target of de novo methylation regardless of whether it is on an episome or integrated into a chromosome.
DISCUSSION
There are several major novel findings in this study. First, overexpression of murine Dnmt3a or Dnmt3b leads to de novo methylation of episomes in human cells. Second, this de novo methylation is the direct result of the methyltransferase functions of Dnmt3a and Dnmt3b. Third, DNMT1, Dnmt3a, and Dnmt3b appear to have distinct methylation specificities in vivo. Fourth, the de novo methylation activity of Dnmt3a and Dnmt3b proteins does not methylate all CpG sites but, rather, has preferences. Fifth, Dnmt3a can lead to de novo methylation of the same sequence on the episome as in the chromosome.
The tagged Dnmt3a and Dnmt3b proteins are expected to be expressed at similar levels because the two expression vectors are essentially identical. As expected, the transiently transfected Dnmt3a and Dnmt3b proteins are expressed at similar levels, as detected by immunofluorescent staining of the proteins. However, expression of the integrated Dnmt3b is much lower than that of integrated Dnmt3a, as indicated by both immunofluorescent staining of the protein (data not shown) and Western blotting (Fig. 1). This indicates that Dnmt3b may be tightly regulated by the cells, and higher long-term expression may lead to negative selection. It is noteworthy that both Dnmt3a and Dnmt3b proteins coat the metaphase chromosomes; this suggests that they either are DNA binding proteins or associate with other proteins that interact with DNA.
This study provides the first evidence that Dnmt3a and Dnmt3b can lead to DNA methylation in vivo. When Dnmt3a or Dnmt3b is overexpressed in 293/EBNA1 cells, de novo methylation of the assay plasmid is observed. Although Dnmt3a and Dnmt3b have conserved methyltransferase domains and possess methyltransferase activity in vitro, one can argue that the in vivo activity observed is the result of another de novo methyltransferase activated by Dnmt3a or Dnmt3b. This possibility is ruled out by the experiments using the mutant Dnmt3a and Dnmt3b with the cysteine in the catalytic site of cytosine methyltransferases replaced by a serine. The assay plasmid remains unmethylated at all HhaI and HpaII sites in experiments in which a mutant Dnmt3a or mutant Dnmt3b expression vector is cotransfected. It is clear that the de novo methylation of the assay plasmid is a direct result of Dnmt3a and Dnmt3b methyltransferases activity in vivo. This is the first evidence that Dnmt3a and Dnmt3b function as de novo methyltransferases in vivo.
Dnmt3b showed a much lower activity than Dnmt3a for de novo methylation in cells transiently or stably expressing the protein. As discussed above, transiently transfected Dnmt3a and Dnmt3b expression levels are comparable. Therefore, it is likely that either Dnmt3b is a weaker methyltransferase than Dnmt3a or Dnmt3b acts on substrates different than the assay plasmids tested. In all experiments, Dnmt3a and Dnmt3b do not lead to methylation of all episomes (some completely digested bands are observed). One possibility is that de novo methylation by these two enzymes is random, and therefore some completely digested bands can always be detected from different molecules. However, de novo methylation of the plasmid does not appear to be random, as described in Results. It is more likely that Dnmt3a and Dnmt3b expression levels are reduced or accessory factors are expressed at a lower level in some cells. Therefore, the assay plasmid in these cells does not become methylated and gives rise to the completely digested HhaI or HpaII fragments. This suggests that the expression level of these two proteins may be important in their targeting.
De novo methylation by Dnmt3a and Dnmt3b can occur through several possible pathways. (i) De novo methylation could initiate at a specific site, and the methyltransferase could track along the DNA (in a processive manner) and methylate other CpG sites on the plasmid until the enzyme falls off, perhaps at preferred exit sites. (ii) There are multiple target sites for de novo methylation on the plasmid, and accessibility to the site dictates how frequent methylation is initiated at the site. (iii) De novo methylation has no sequence specificity; however, the methyltransferases must compete with transcriptional machinery for access to the DNA. Therefore, regions with stronger transcription are less accessible to the methyltransferases and acquire methylation less frequently. It has been demonstrated that CpG methylation sites exist upstream of the H19 gene at a very low frequency in the homozygote Dnmt1 knockout ES cells, and the neighboring CpG sites become methylated more frequently in the rescued ES cells (28). Therefore, one could propose that endogenous DNMT1 can spread methylation into adjacent DNA after Dnmt3a or Dnmt3b initiates de novo methylation at a specific site. However, this is highly unlikely (at least on the episome) because it has been clearly documented in a previous study (10) that focal methylation does not spread into adjacent DNA on the episome in the same cell line used in this study. Therefore, it is not likely that DNMT1 is responsible for spreading methylation from a specific site on the episome that becomes de novo methylated by Dnmt3a or Dnmt3b. In this study, most of the increased-size HhaI fragments contain the EBNA1 region, but only one fragment contains the luciferase gene region. Also, plasmids lacking the EBNA1 gene do not become methylated to the same extent as plasmids harboring the EBNA1 gene. Moreover, the integrated EBNA1 gene becomes more methylated in 3a-5 and 3a-11 cells than in the parental cell line, 293/EBNA1. There may be sequence-mediated features within the EBNA1 gene region that determine this specificity because the integrated EBNA1 gene is driven by the much stronger human CMV promoter, and the EBNA1 gene is surrounded by different sequences on the episome and in the chromosome. These findings suggest that the EBNA1 gene is the preferred target or the initiation site on the plasmid for de novo methylation by Dnmt3a or Dnmt3b. No uncut molecule is detected when assay plasmid is digested with either HhaI or HpaII, and the largest HhaI fragments observed are approximately 6 and 3.5 kb in pCLH22 (a 12.1-kb plasmid) harvested from 3a and 3b cells, respectively. This indicates that every molecule has at least one, and most likely more than one, CpG site that is not methylated by Dnmt3a or Dnmt3b and can be digested by HhaI or HpaII. Multiple increased-size HhaI fragments containing the EBNA1 region were detected in the plasmid DNA harvested from 3a cells, indicating that de novo methylation takes place through various distances from the EBNA1 region on different molecules. This suggests that Dnmt3a can fall off at different sites on different molecules. This study provides the first evidence that de novo methylation activity of Dnmt3a and Dnmt3b has some specificity and favors the first two possible pathways discussed above.
The specificity of target site selection by the DNMT may be due to some virus-specific signal or unique sequence feature (including sequence-mediated feature) in the EBNA1 gene. I am currently designing new experiments utilizing this episomal system to define the specificity and requirements of the de novo methylation activity as well as to understand the pathway of the de novo methylation. This study demonstrates the de novo methylation activity of Dnmt3a and Dnmt3b in vivo, demonstrates some specificity of this activity, and demonstrates that the de novo methylation activity can act on the same sequence on the episome and in the chromosome. The biochemical assay of Dnmt3a and Dnmt3b proteins has been difficult because of the weak in vitro activity of these two proteins; possibly other factors are required to enhance this activity. Therefore, the observation of de novo methylation activity in vivo using the episomal system opens up the possibility of answering many questions. Using this in vivo assay system, we can address the following questions: what is the signal for de novo methylation targeting; how fast can de novo methylation occur; what endogenous sequence can be affected by the de novo methylation activity; and what other factors may play a role in Dnmt3a and Dnmt3b activity?
ACKNOWLEDGMENTS
I thank M. R. Lieber, B. Tracy, and D. Van Den Berg for critical reading of the manuscript. I thank E. Li for his generous gift of the Dnmt3a and Dnmt3b cDNA clones. I also thank T. Bestor for suggesting the Dnmt3a and Dnmt3b mutant experiment.
This work was supported by NIH grant GM54781 and the Aresty endowment fund.
REFERENCES
- 1.Bird A P. The essentials of DNA methylation. Cell. 1992;70:5–8. doi: 10.1016/0092-8674(92)90526-i. [DOI] [PubMed] [Google Scholar]
- 2.Cedar H. DNA methylation and gene activity. Cell. 1988;53:3–4. doi: 10.1016/0092-8674(88)90479-5. [DOI] [PubMed] [Google Scholar]
- 3.DuBridge R B, Tang P, Hsia H C, Leong P-M, Miller J H, Calos M P. Analysis of mutation in human cells by using an Epstein-Barr virus shuttle vector. Mol Cell Biol. 1987;7:379–387. doi: 10.1128/mcb.7.1.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grawunder U, Schatz D G, Leu T M J, Rolink A, Melchers F. The half-life of RAG-1 protein in precursor B cells is increased in the absence of RAG-2 expression. J Exp Med. 1996;183:1731–1737. doi: 10.1084/jem.183.4.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grawunder U, Finnie N, Jackson S P, Riwar B, Jessberger R. Expression of DNA-dependent protein kinase holoenzyme upon induction of lymphocyte differentiation and V(D)J recombination. Eur J Biochem. 1996;241:931–940. doi: 10.1111/j.1432-1033.1996.00931.x. [DOI] [PubMed] [Google Scholar]
- 6.Hasse A, Schulz W A. Enhancement of reporter gene de novo methylation by DNA fragments from the alpha-fetoprotein control region. J Biol Chem. 1994;269:1821–1826. [PubMed] [Google Scholar]
- 7.Hirt B. Selective extraction of polyoma DNA from infected mouse cultures. J Mol Biol. 1967;26:365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- 8.Hsieh C-L, Lieber M R. CpG methylated minichromosomes become inaccessible for V(D)J recombination after undergoing replication. EMBO J. 1992;11:315–325. doi: 10.1002/j.1460-2075.1992.tb05054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hsieh C-L. Dependence of transcriptional repression on CpG methylation density. Mol Cell Biol. 1994;14:5487–5494. doi: 10.1128/mcb.14.8.5487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hsieh C-L. Stability of patch methylation and its impact in regions of transcriptional initiation and elongation. Mol Cell Biol. 1997;17:5897–5904. doi: 10.1128/mcb.17.10.5897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hsieh C-L. Evidence that protein binding specifies sites of DNA demethylation. Mol Cell Biol. 1999;19:46–56. doi: 10.1128/mcb.19.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jones P L, Veenstra G J, Wade P A, Vermaak D, Kass S U, Landsberger N, Strouboulis J, Wolffe A P. Methylated DNA and MeCP2 recruit histone deacetylase to repress transcription. Nat Genet. 1998;19:187–191. doi: 10.1038/561. [DOI] [PubMed] [Google Scholar]
- 13.Kass S U, Pruss D, Wolffe A P. How does DNA methylation repress transcription? Trends Genet. 1997;13:444–449. doi: 10.1016/s0168-9525(97)01268-7. [DOI] [PubMed] [Google Scholar]
- 14.Keshet I, Lieman-Hurwitz J, Cedar H. DNA methylation affects the formation of active chromatin. Cell. 1986;44:535–543. doi: 10.1016/0092-8674(86)90263-1. [DOI] [PubMed] [Google Scholar]
- 15.Lei H, Oh S P, Okano M, Juttermann R, Goss K A, Jaenisch R, Li E. De novo DNA cytosine methyltransferase activities in mouse embryonic stem cells. Development. 1996;122:3195–3205. doi: 10.1242/dev.122.10.3195. [DOI] [PubMed] [Google Scholar]
- 16.Leohnardt H, Bestor T H. Structure, function and regulation of mammalian DNA methyltransferase. In: Jost J P, Saluz H P, editors. DNA methylation: molecular biology and biological significance. Basel, Switzerland: Birkhauser Verlag; 1993. pp. 109–119. [DOI] [PubMed] [Google Scholar]
- 17.Matsuo K, Silke J, Georgiev O, Marti P, Giovannini N, Rungger D. An embryonic demethylation mechanism involving binding of transcription factors to replicating DNA. EMBO J. 1998;17:1446–1453. doi: 10.1093/emboj/17.5.1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mi S, Roberts R J. The DNA binding affinity of HhaI methyltase is increased by a single amino acid substitution in the catalytic center. Nucleic Acids Res. 1993;21:2459–2464. doi: 10.1093/nar/21.10.2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nan X, Ng H H, Johnson C A, Laherty C D, Turner B M, Eisenman R N, Bird A. Transcriptional repression by the methyl-CpG-binding protein MeCP2 involves a histone deacetylase complex. Nature. 1998;393:386–389. doi: 10.1038/30764. [DOI] [PubMed] [Google Scholar]
- 20.Okano M, Xie S, Li E. Dnmt2 is not required for de novo and maintenance methylation of viral DNA in embryonic stem cells. Nucleic Acids Res. 1998;26:2536–2540. doi: 10.1093/nar/26.11.2536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Okano M, Xie S, Li E. Cloning and characterization of a family of novel mammalian DNA (cytosine-5) methyltransferases. Nat Genet. 1998;19:219–220. doi: 10.1038/890. [DOI] [PubMed] [Google Scholar]
- 22.Razin A, Kafri T. DNA methylation from embryo to adult. Prog Nucleic Acid Res Mol Biol. 1994;48:53–81. doi: 10.1016/s0079-6603(08)60853-3. [DOI] [PubMed] [Google Scholar]
- 23.Razin A. CpG methylation, chromatin structure and gene silencing—a three-way connection. EMBO J. 1998;17:4905–4908. doi: 10.1093/emboj/17.17.4905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Robertson K D, Uzvolgyi E, Liang G, Talmadge C, Sumegi J, Gonzales F A, Jones P A. The human DNA methyltransferases (DNMTs) 1, 3a and 3b: coordinate mRNA expression in normal tissues and overexpression in tumors. Nucleic Acids Res. 1999;27:2291–2298. doi: 10.1093/nar/27.11.2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schmutte C, Jones P A. Involvement of DNA methylation in human carcinogenesis. Biol Chem. 1998;379:377–388. doi: 10.1515/bchm.1998.379.4-5.377. [DOI] [PubMed] [Google Scholar]
- 26.Schulz W A. DNA methylation in urological malignancies. Int J Oncol. 1998;13:151–167. [PubMed] [Google Scholar]
- 27.Vertino P M, Yen R W C, Gao J, Baylin S B. De novo methylation of Cpt island sequences in human fibroblasts overexpressing DNA (cytosine-5)-methyltransferase. Mol Cell Biol. 1996;16:4555–4565. doi: 10.1128/mcb.16.8.4555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Warnecke P M, Biniszkiewicz D, Jaenisch R, Frommer M, Clark S J. Sequence-specific methylation of the mouse H19 gene in embryonic cells deficient in the Dnmt-1 gene. Dev Genet. 1998;22:111–121. doi: 10.1002/(SICI)1520-6408(1998)22:2<111::AID-DVG1>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 29.Wigler M, Sweet R, Sim G K, Wold B, Pellicer A, Lacy E, Maniatis T, Silverstein S, Axel R. Transformation of mammalian cells with genes from prokaryotes and eukaryotes. Cell. 1979;16:777–785. doi: 10.1016/0092-8674(79)90093-x. [DOI] [PubMed] [Google Scholar]
- 30.Wilke K, Rauhut E, Noyer-Weidner M, Lauster R, Pawlek B, Behrens B, Trautner T A. Sequential order of target-recognizing domains in multispecific DNA-methyltransferases. EMBO J. 1988;7:2601–2609. doi: 10.1002/j.1460-2075.1988.tb03110.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wyszynski M W, Gabbara S, Bhagwat A S. Substitutions of a cysteine conserved among DNA cytosine methylases result in a variety of phenotypes. Nucleic Acids Res. 1992;20:319–326. doi: 10.1093/nar/20.2.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yoder J A, Yen R W C, Vertino P M, Bestor T H, Baylin S B. New 5′ regions of the murine and human genes for DNA (cytosine-5)-methyltransferase. Biol Chem. 1996;271:31092–31097. doi: 10.1074/jbc.271.49.31092. [DOI] [PubMed] [Google Scholar]
- 33.Yoder J A, Walsh C P, Bestor T H. Cytosine methylation and the ecology of intragenomic parasites. Trends Genet. 1997;13:335–340. doi: 10.1016/s0168-9525(97)01181-5. [DOI] [PubMed] [Google Scholar]
- 34.Yoder J A, Bestor T H. A candidate mammalian DNA methyltransferase related to pmt1p of fission yeast. Hum Mol Genet. 1998;7:279–284. doi: 10.1093/hmg/7.2.279. [DOI] [PubMed] [Google Scholar]