Abstract
The periaqueductal gray (PAG) is a complex mesencephalic structure involved in the integration and execution of active and passive self-protective behaviors against imminent threats, such as immobility or flight from a predator. PAG activity is also associated with the integration of responses against physical discomfort (e.g., anxiety, fear, pain, and disgust) which occurs prior an imminent attack, but also during withdrawal from drugs such as morphine and cocaine. The PAG sends and receives projections to and from other well-documented nuclei linked to the phenomenon of drug addiction including: (i) the ventral tegmental area; (ii) extended amygdala; (iii) medial prefrontal cortex; (iv) pontine nucleus; (v) bed nucleus of the stria terminalis; and (vi) hypothalamus. Preclinical models have suggested that the PAG contributes to the modulation of anxiety, fear, and nociception (all of which may produce physical discomfort) linked with chronic exposure to drugs of abuse. Withdrawal produced by the major pharmacological classes of drugs of abuse is mediated through actions that include participation of the PAG. In support of this, there is evidence of functional, pharmacological, molecular. And/or genetic alterations in the PAG during the impulsive/compulsive intake or withdrawal from a drug. Due to its small size, it is difficult to assess the anatomical participation of the PAG when using classical neuroimaging techniques, so its physiopathology in drug addiction has been underestimated and poorly documented. In this theoretical review, we discuss the involvement of the PAG in drug addiction mainly via its role as an integrator of responses to the physical discomfort associated with drug withdrawal.
Keywords: Periaqueductal gray, Reward circuit, Anti-reward circuit, Alcohol, Caffeine, Cannabis, Opioids, Stimulants
Introduction
The Diagnostic and Statistical Manual of Mental Disorders (DSM-5) uses the term "substance use disorders" to encompass 10 different classes of drugs of abuse: alcohol, caffeine, cannabis, hallucinogens, inhalants, opioids, sedatives (hypnotics or anxiolytics), stimulants, tobacco, and other (or unknown) substances [1]. All these drugs are similar in that they activate the reward circuit [2, 3] and, except for hallucinogens and inhalants, the rest induce withdrawal symptoms. The intake of a substance may result in a chronic addictive disorder if one of the following features is present: (i) compulsion to seek the substance; (ii) its uncontrolled intake; and (iii) the appearance of feelings of discomfort that include dysphoria, anxiety, pain, or irritability [4, 5]. During the establishment of drug addiction, the drug consumption behavior evolves differentially (i.e., not equally for every substance) from impulsiveness to compulsivity, especially with substances like alcohol [6]. The above suggests that drug addiction is not a simple sequence but instead, both impulsive and compulsive intake behaviors and the underlying biology are important [6]. The impulsive intake of substances is an early phase of the addiction cycle that generates a sense of pleasure and gratification via the brain reward system and the reinforcing effects of substance intake; whereas the compulsive intake is a late phase of the addiction cycle, in which the alleviation of physical discomfort, which may range from mild to severe distress, has been proposed to be elemental [2, 7]. Interestingly, specific environmental contexts such as visual and olfactory signals may promote craving and produce difficulties in controlling impulsive behaviors [8]. The establishment of a compulsivity intake behavior involves allostatic changes in the neurotransmission of the brain reward and anxiety systems such as alterations of dopaminergic function [4, 9–11]. Hence, drug addiction involves responses to psychiatric perturbations, stressful situations, and/or physiological challenges that are integrated to promote adaptation and coping and thus, homeostasis [12, 13]. Notably, compulsive behaviors induced by some drugs of abuse (alcohol, cocaine, morphine, and heroin) may develop before any evidence of dependency and tolerance [7, 14]; thus, neither dependence nor tolerance may solely explain the complexity of the drug addiction cycle.
Drug addiction can be cyclic and consists of at least three main stages: (i) binging/intoxication, in which there is a strong motivation to take drugs due to their reinforcing effects; (ii) withdrawal/negative affect, characterized by the presence of anxiety, irritability, widespread pain, dysphoria, and hyperkatifeia, among others; and (iii) anticipation/craving, that may drive to the binging stage again. Each of these stages encompasses brain circuits that are anatomically, neurophysiologically, and neurochemically specific [3, 15–19]. In animal models, addiction-like behaviors include a regular, predictable, and/or uninterrupted use of the substance [7].
The midbrain central gray, also referred to as the PAG or substantia grisea centralis, is a brainstem structure bordering the cerebral aqueduct [20]. The PAG, along with the amygdala, hypothalamus, and the bed nucleus of the stria terminalis (BNST), form the aversion system [21], which is activated during acute and chronic exposure to stressors [13] and is responsible for executing defensive behaviors against anxiety, pain, and fear, such as the consumption of a drug in order to avoid the discomfort associated with withdrawal of the drug [21–27]. During acute or prolonged withdrawal, the cerebral aversion system seems to be involved in the increased release of stress promotors such as glucocorticoids, corticotropin-releasing factor (CRF), norepinephrine, and dynorphin [17]. In normal conditions, factors such as neuropeptide Y (NPY), nociceptin, and endocannabinoids seem to be involved in maintaining a low stress response [17, 28].
Recently, based on anatomical and functional evidence, it has been proposed that the PAG plays a fundamental role in controlling motivated goal-directed behaviors of all types [29]. Therefore, in this review we aimed to discuss and propose the participation of the PAG in the pathophysiology of drug addiction.
Physiology of the Periaqueductal Gray
The PAG is a well-conserved midbrain area in chordate species [30, 31] and has a similar proportional size (up to 10% of the mesencephalon) in rodents, cats, and humans [32]. Its functions include food intake [29], pain modulation [33–35], anxiety/panic [23, 36], unconditioned, conditioned, as well as learned behaviors such as fear [37–39], vocalization [40], and sexual behavior via the integration of the lordosis reflex in coordination with the medullary reticular formation and the ventromedial nucleus of the hypothalamus [41] during mating stimulation [42], and the integration of autonomic responses [32, 43]. Furthermore, neurons in the PAG integrate negative phenomena such as anxiety, stress, and pain with the autonomic, neuroendocrine, and immune systems to facilitate responses to threat [44]. Pain, anxiety, and fear are normal emotions with great adaptive value in evolutionary selection in mammals [45]. Brain structures involved in processing pain, anxiety, and fear under natural circumstances are also related to the pain, anxiety, and fear associated with the intoxication/withdrawal induced by drugs of abuse [46–48]. While fear occurs in response to specific threats, the source of anxious behavior is usually undefined or unknown [45]. There is substantial evidence that the PAG is a key midbrain structure involved in the processing of pain, anxiety, and fear [49].
The PAG is partitioned into various structural subdivisions disposed like the neuroaxis itself: dorsomedial, dorsolateral (DL), lateral, and ventrolateral (VL). These subdivisions also project in other planes among themselves (anterior and posterior) with different participation around the integration of defensive behaviors such as pain, anxiety, and fear [32, 50, 51]. The dorsal and lateral PAG (D/L-PAG) play a role during combative defensive reactions and non-opioid-based analgesia [52, 53]. The D/L-PAG also receives inputs from the amygdala, the ventromedial hypothalamus, and the medial prefrontal cortex (mPFC) [54]; whereas the VL-PAG seems to be important for evoking passive behaviors and opioid analgesia via the anterior insula and the medial and dorsomedial PFC [50, 55]. Indeed, tonic immobility-elicited analgesia is mediated by mu opioid receptors in the VL-PAG via inhibition of the GABAergic projections to the rostral ventromedial medulla (RVM) and dorsal raphe (DR) [56]. On the other hand, glutamatergic neurons in the VL-PAG, when activated under physiological conditions, cause freezing behavior that is also regulated by mu opioid receptors [57]. Interestingly, hypofunction of glutamatergic transmission in the VL-PAG has been proposed to be associated with depressive-like behaviors [58].
Some studies have proposed the PAG as a structure that plays a role in the integration of other complex behaviors such as sadness, fury, pleasure, worry, and fear of painful stimuli through neuronal processes that involve the participation of other brain areas including the amygdala and hypothalamus [59–61]. In fact, one of the main functions of the PAG is related to the integration of peripheral and central afferent inputs, which in turn may result in homeostatic defensive reactions mainly via activation of the sympathetic autonomic nervous system (ANS) [59, 62], which modulates the activity of visceral tissues (e.g., blood flow and adrenaline release) via its monoaminergic receptors, α and β adrenoceptors. In this sense, the ANS prepares the organism for the classic fight or flight response by regulating visceral activity (cardiovascular, metabolic, and respiratory adaptations) through sympathetic fibers, which are finely modulated by monoaminergic receptors [63, 64]. Indeed, the interruption of sympathetic activity seems to involve both monoaminergic auto- and hetero-receptors [65, 66]. Interestingly, the endocannabinoid [67–69] and opioid systems [70] are also involved in the modulation of cardiovascular sympathetic and sensory drives.
Several preclinical and clinical studies have suggested that stressful experiences that occur throughout life may contribute crucially to the development and pathogenesis of several psychiatric disorders such as schizophrenia, mood disorders, anxiety, erroneous defensive responses, and affect reward-seeking [44, 71–73], all of which can promote drug abuse. Moreover, most of the symptoms of anxiety disorders are accompanied by activation of the hypothalamic-pituitary adrenal axis [71, 74, 75]. Due to similarities between the behavioral and autonomic responses induced by D-PAG stimulation and the semiology of panic attacks, it has been suggested that the D-PAG is deeply involved in the genesis of panic-related disorders in humans [76]. In support of this hypothesis, chemical or electrical stimulation of the D-PAG induces panic attacks in rodents [76]. Likewise, panic-like behaviors can be evoked by systemic cholecystokinin 2 (CCK2) receptor agonists, an effect that is prevented by intra-PAG microinjection of CCK antagonists [77–79]. Hence, the physiology of the PAG suggests that this structure plays a key role in the integration of actions primarily evoked to modulate discomfort (e.g., anxiety, fear, and painful situations that are commonly experienced during withdrawal in the addiction cycle [80–84]). Moreover, there is substantial evidence (see section 2) for the role of the pain circuit and its impact in producing negative reinforcement that may contribute to sustained habitual drug intake, as for the PAG-pain circuit and cocaine addiction) [5, 85, 86]. Importantly, the diminutive size and form of the PAG have made its functional analysis very challenging when using standard noninvasive magnetic resonance imaging (MRI) techniques [87]. Notably, with the advent of better imaging tools, this kind of structure will be better understood. In support of this notion, a very recent functional MRI (fMRI) study has proposed a cortex-PAG connection that appears to be essential in alcohol abuse [88].
The Link Between the PAG and the Reward Circuit
The possible participation of the PAG in the impulsive intake of substances of abuse should be related to its interaction with nuclei that are known to be involved in the reward, such as the nucleus accumbens (NAc), VTA, and hypothalamus [16], among others such as the substantia nigra [89–91]. The reward system is defined as a circuit whose activation leads to positive reinforcement with a positive hedonic overlay [19]. In such systems, chemical messengers including serotonin, cannabinoids, opioid peptides, enkephalin, and GABA are involved, directly or indirectly, in the actions that modulate the activation of the reward system via dopamine (DA) release in the NAc [89, 92, 93]. The PAG sends glutamatergic and GABAergic inputs directly to DA-containing and GABA-containing cells [94] in the VTA [95]. As the VTA is a key element in the reward system [96, 97], the PAG-VTA circuitry could also play an indirect role during the early phases of drug addiction [44]. Laurent et al. [98] found that activation of the GABAergic synapses between the VL-PAG and the VTA increases the immobility response that is blocked in the presence of opioids.
Another fundamental structure in the reward circuit is the NAc, which is vital for integrating reward, drug reinforcement, and motivated behavior [99, 100]. Moreover, this small nucleus is important for evaluating the cost-benefit decision-making in situations involving reward [99, 100]. It has been proposed that the NAc, together with the basolateral amygdala (BLA) and D-PAG, form a circuit in charge of responding to a prolonged stressful situation [101–103]. The NAc is anatomically separated into shell and core portions [104], both of which are involved in decision-making when seeking reward [100] and receive inputs from numerous areas such as the anterior cortex and the lateral hypothalamus (LH) [105]. The NAc shell increases the operant response to a reward-associated cue [106], participates in the expression of appetitive-type behaviors [107], and is more sensitive to the rewarding effects of cocaine and D1/D2 receptor agonists than the NAc core [108–110]. The NAc core is essential for the execution of Pavlovian behaviors since its inactivation causes indiscriminate responses during a cue-guided risk/reward paradigm in rats [100].
Another region important for the drug addiction cycle is the hypothalamus (conformed by lateral, medial lateral, medial periventricular, and medial zones), which is located ventrally in the brain and plays an important role in the regulation of the endocrine system [111]. The LH is involved in the control of feeding behavior [112]. Interestingly, the L-PAG [113] influences reward and reinforcement processes such as seeking appetitive reward by activation of the orexinergic cell group of the LH [114]. In addition, the LH projects to the ventrolateral neurons of the PAG and extended amygdala (EA) and receives afferent connections from the LH to modulate drug reward and neuro-adaptive changes that occur with chronic drug exposure [43, 115]. The ventromedial hypothalamus has reciprocal projections with the D-PAG that seem to be involved in the execution of panic-like behaviors [116]. The arcuate nucleus of the hypothalamus has NPY-containing projections towards the D- and V-PAG. Besides, the PAG is densely innervated by NPY fibers [117, 118] and contains very high levels of NPY [119, 120]. Among the NPY receptor subtypes identified so far (Y1–Y5), only two have been reported to have functional effects on the PAG: Y1 and Y2 [121]. These receptors have been shown to be involved in depression [122] and alcohol consumption [123]. Likewise, microinjection of NPY into the D-PAG and the VL-PAG has anxiolytic/analgesic effects [124] and may modulate alcohol intake [28].
The PFC can be divided into the mPFC, orbitofrontal cortex, ventrolateral PFC, dorsolateral PFC, and caudal PFC [125]. Cortical projections to the PAG originate primarily from the mPFC [126]. The interaction of the L- and D-PAG with the PFC involves executive functions [127]. The mPFC is mainly involved in cognitive functions [128], including reward-related activity [129]. The projections of the mPFC end in both the DL [130] and VL PAG [131] and it has been suggested that this mPFC-DL/VL projection participates in aversive and compulsive behavioral responses [132] as well as pain modulation [125, 133].
The Link Between the PAG and the Anti-reward Circuit
The anti-reward system includes the EA, which is composed of the central nucleus of the amygdala (CeA), BNST, and NAc [19]. The coordinated activity of these regions is involved in the integration of the negative affective states: anxiety, irritability, pain, and others [18]. It is important to note that the function of the above structures is not limited to the anti-reward system as they participate in other actions including yawning [134], impulsive behavior [135], and feeding behavior [136], as well as pain [137] and others.
The PAG provides inputs to the BNST [138, 139] which is a critical structure for stress, anxiety [140–142], and fear responses [143]; and it is enriched mainly in NPY and CRF neurons [144]. Release of the neurotransmitter NPY has anti-drinking effects mediated through the Y1 receptor, which inhibits BNST-CRF neurons [145], and is a key structure in the withdrawal/negative affect and anticipation/drug craving [82, 146, 147]. The BNST receives 50% of its DA contribution from the D- and V-PAG [139, 148] (Fig. 1), and this could be involved in pain regulation and anxiety [149]. Furthermore, it has been reported that the BNST and VL-PAG interaction is involved in the modulation of eating behavior through GABAergic pathways [136]. The amygdala is a connecting structure in the limbic system and can be divided into three groups: (i) the BLA, which includes the lateral, basal, basomedial and basoventral nuclei; (ii) the centromedial amygdala, which includes the CeA and medial nuclei (M) (the CeA nuclei have four subdivisions: the capsular, lateral (CeL), intermediate, and medial (CeM) subdivisions) [150, 151]; and (iii) the superficial or cortical-like region that includes the cortical nuclei and the nucleus of the lateral olfactory tract [152, 153]. The CeA is the main output area from the amygdala to the PAG at different points of the antero-posterior axis. For example, the CeM projects mainly to the rostral and caudal PAG, while the CeL projects only to the caudal PAG. Further, the D-PAG is selectively targeted by the CeM and the VL-PAG by the CeL [154]. Lesions in the BLA have been shown to block flight responses evoked by stimulation of the D-PAG [102]. In addition, it has been reported that the CeA/BLA–mPFC–VL-PAG circuit is involved in pain processing [133, 155], cataplexy [156], and pain symptoms correlated with depression [157].
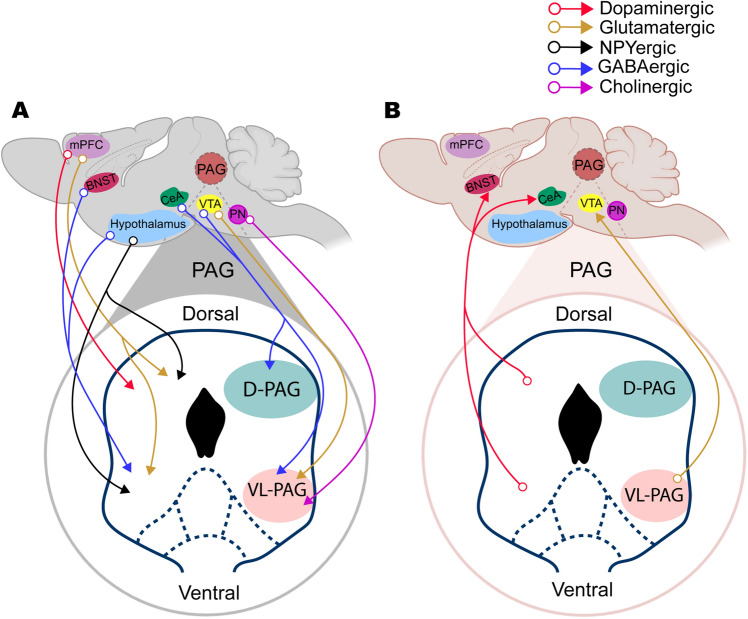
Fig. 1.
Interaction of the PAG with other brain nuclei potentially involved in drug addiction. A Inputs to the PAG from different nuclei. The dorsal and ventral PAG (orange lines) receive glutamatergic inputs from the mPFC; the D-PAG and VL-PAG receive GABAergic inputs (blue lines) from both CeA and VTA (blue lines); the VL-PAG receives GABAergic projections from both the BNST and the hypothalamus; the arcuate nucleus of the hypothalamus has NPYergic projections to the D- and V-PAG (black lines); the VTA projects to the VL-PAG via glutamatergic neurons (orange line); the Pn has a cholinergic projection to the VL-PAG (purple line). B Outputs from the PAG to different nuclei. The BNST and CEA receive dopaminergic inputs from both the dorsal and ventral PAG (red lines); the VL-PAG sends glutamatergic projections to the VTA (orange lines). BNST, bed nucleus of the stria terminalis; CeA, central nucleus of the amygdala; D-, L-PAG, dorsal and lateral periaqueductal gray; mPFC, medial prefrontal cortex; Pn, pontine nucleus; VL-PAG, ventrolateral periaqueductal gray; VTA, ventral tegmental area.
Chronic exogenous activation of the reward system may drive decreases of DA, serotonin, and opioid peptides and their actions in the ventral striatum-VTA as an adaptive response to lessen the effects of drugs. If this exogenous activation occurs, negative reinforcement such as intrusive thinking and anxiety may increase the probability of searching for drugs [11, 17, 158]. It has been suggested that these negative reinforcement actions are integrated by an anti-reward system [17] that would be complementary to the reward system to achieve coordinated activity under normal conditions, but that could also contribute to the seeking and binging of drugs during the drug addiction process. These reward and anti-reward circuits do not work independently; both are necessary for the establishment of addiction.
The PAG is involved in the execution of various emotional conditions/behaviors such as pain, anxiety, and fear; but it has been more recently addressed as a brain area participating in drug addiction. However, further understanding of its contribution during the negative affective state seen during drug-withdrawal is required [28, 85, 86, 159]. The PAG afferent and efferent nerve fibers with the nuclei involved in drug addiction are summarized in Fig. 1.
Links Between the PAG and Substances of Abuse and Appetitive/Consummatory Behaviors
As noted above, the PAG is a key integrating structure for executing defensive responses via opioid receptors [160]; and some groups have reported possible participation of the PAG in the expression of several signs of withdrawal from diverse drugs of abuse such as morphine [14, 80, 161, 162]. In fact, morphine injections into the PAG produce conditioned place preference, suggesting that the PAG may be involved in the reinforcing actions of opioids during the early (impulsive) phase [163]. Moreover, the PAG has been reported to mediate reward information that promotes food intake, whereas PAG inhibition has anorexic effects in hungry rats [164]. In the next section, we discuss the preclinical and clinical evidence available involving the role of PAG activity in the actions of the major pharmacological classes of drugs of abuse (Table 1). In addition, we extend the discussion to some appetitive consummatory behaviors.
Table 1.
Role of the PAG in the actions of the major pharmacological classes of drugs of abuse.
| Drug of abuse | Substance or receptor involved | PAG subdivision involved/outcome | References |
|---|---|---|---|
| Alcohol | NMDA or AMPA receptor antagonists | Intra-D-PAG injection of NMDA/AMPA receptor antagonists is anxiolytic and inhibits alcohol intake | [169] |
| carboxy-PTIO and L-NAME | NO inhibition in DL-PAG decreases the anxiogenic effects of alcohol withdrawal | [172] | |
| NPY-Y1 | Intra-D-PAG injection of NPY-Y1 is anxiolytic and reduces alcohol consumption | [28] | |
| Caffeine | Caffeine | fMRI shows that the anxiogenic actions of caffeine are associated with PAG activation | [179] |
| Cannabis | CB1 | Activation of CB1 in D-PAG has panicolytic-like effects, analgesia, anti-aversive effects, and prevention of hyperlocomotion | [190, 194–197] |
| Opioids | Morphine |
VL-PAG exhibits hyperexcitation of GABAergic neurons, astrocyte activation, and release of pro-inflammatory cytokines during opioid withdrawal |
[194, 197, 201, 202] |
| Sedatives |
Bicuculline or flumazenil (GABAA) |
Intra D-PAG injection of benzodiazepines has anti-panic effects | [203] |
| Stimulants | Cocaine | Cocaine withdrawal produces hyper-responsiveness of VL-PAG neurons | [204] |
| Cocaine | fMRI images show that PAG is involved in cocaine craving | [86] | |
| Tobacco | Nicotinic acetylcholine receptors | Anti-depressive effects of nicotine involve the NAc-DR-PAG circuit; its anxiolytic effects include actions on the DR and PAG | [205–207] |
Alcohol
Alcohol intake produces euphoria, disinhibition, anxiety-reduction, sedation, and hypnosis that are associated with its role as a positive allosteric modulator of the GABAA receptor [165]. Interestingly, the alcohol hangover involves severe physical discomfort which includes anxiety, pain (mainly headache), and nausea [166]. It is popularly believed that the physical discomfort induced by the alcohol hangover decreases with low alcohol consumption on the next day [167]. In fact, the alcohol hangover may slightly promote a faster recurrence of alcohol consumption [81]. The above suggest that, during alcohol relapse in some consumers, escaping from the physical discomfort induced by withdrawal may be a promoting stimulus. Remarkably, there is increased anxiety after alcohol consumption that can be explained by a decrease in the inhibitory control of GABA over the PAG, accompanied by an increase in the excitatory glutamatergic tone [168–170]. Microinjections of NMDA (N-methyl-D-aspartate) or AMPA (α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid) antagonists into the D-PAG during withdrawal decrease alcohol consumption [169]. Likewise, in brain slices from animals in a state of alcohol withdrawal, the glutamatergic transmission mediated by NMDA or AMPA receptors is altered in the PAG, as spontaneous excitatory postsynaptic potentials are decreased compared to controls [171]. Bonassoli et al. [172] injected carboxy-PTIO [a nitric oxide (NO) scavenger] or L-NAME, a nonselective NO synthase inhibitor, into the D-PAG and both drugs decreased the anxiogenic effects of alcohol withdrawal, suggesting that the NO pathway plays an important role during this stage [172]. In addition, it has been reported that during alcohol withdrawal, the blockade of NPY-Y1 receptors in the D-PAG induces anxiety and alcohol relapse [28]. Indeed, it has been reported that acute exposure to alcohol increases the activity of dopaminergic neurons in the VL-PAG, while chronic exposure to alcohol does not modify the activity of these neurons [173]. McClintick et al. [174] has suggested that early-age drinking of alcohol, specifically during adolescence, leads to changes in the expression of key genes in the PAG. They reported alterations in 1,670 of 12,123 detected genes in the PAG. The main decreased alterations caused by alcohol intake were in the GABAergic, serotoninergic, cholinergic, dopaminergic, and opioid systems, while the expression of hypocretin (orexin) neuropeptide precursor (Hcrt) increased in these neuronal populations [174]. Together, these changes may increase the susceptibility for developing anxiety, pain, and fear (i.e., physical discomfort) [174] which could facilitate compulsive alcohol intake.
Interestingly, alcohol intake is used by some patients as a pain reliever [175]. Egli et al. [176] have proposed a genetic and functional link between pathological pain and the development of alcohol dependence, as the neurocircuitry related to alcohol dependence is closely connected to the neurocircuitry for pain. For example, the PAG is not only involved in the direct spinothalamic processing of pain as well as in indirect processing via the amygdala tract [176]; but also, in the negative emotional states of drug addiction via its projections to the BNST and CeA (Fig. 1). In addition, Avegno et al. [155] reported that chronic alcohol exposure decreases GABAergic signaling from the CeA to the PAG and alters the melanocortin system in the CeA, phenomena that could be related to the hyperalgesia induced during alcohol withdrawal.
A recent fMRI study has suggested that the PAG is negatively involved in the physical and social pleasure expectancy in alcohol drinkers exposed to alcohol cues, whereas the medial orbitofrontal cortex (mOFC) is positively involved [177]. More recently, another fMRI study proposed that the connection between the mOFC and D-PAG is predominantly involved in alcohol abuse [88].
Hence, different neurotransmitters, neuropeptides, and gasotransmitters modulate the PAG during alcohol withdrawal, while alcohol exposure may induce functional, molecular, and genetic changes in the PAG, suggesting that this structure is a key modulator of alcohol intake behaviors. This idea has been recently confirmed using fMRI [88].
Caffeine
Caffeine is an extremely popular psychostimulant drug consumed all over the world in diverse presentations that include fresh or hot beverages, even mixed with cola-drinks and taurine. Exposure to caffeine increases DA in the NAc-shell [178], which explains its acute reinforcing effects. Caffeine also causes diastolic hypertension and exerts anxiogenic actions in humans [179]. It is popularly consumed early in the morning to generate a sensation of alertness (increased attention) and to avoid somnolence [180], actions that are associated with its anxiogenic effects. By fMRI, it has been shown that the anxiogenic action of caffeine involves activation of the PAG [179]. It is possible that this anxiogenic effect, induced via PAG activation, contributes to the desired effects in consumers (inhibition of somnolence and increased alertness and attention) [179]. However, it remains to be determined whether PAG inhibition also decreases caffeine intake.
Cannabis
Cannabinoids (phyto, endogenous, and synthetic) interact with type 1 (CB1) and 2 (CB2) cannabinoid receptors. Although the behavioral effects of cannabinoids have been classically associated with CB1 receptors mainly expressed in the brain [181], diverse studies have reported a potential role for CB2 [182]. Other putative receptors strongly associated with the endocannabinoid system (e.g., GPR12, GPR18, and GPR55) have also been associated with multiple cognitive processes such as learning and memory and food intake. [183–188].
Given that mainly glutamatergic and to a lesser extent GABAergic PAG projections to the VTA induce stimulation and DA release [94], substances that increase the activity of these neurons would be expected to trigger reinforcing actions through possible integration with the reward system. Interestingly, cannabinoids acting at an area that receives PAG inputs and projects PAG outputs (i.e., the tail of the VTA/rostromedial tegmental nucleus) may also facilitate CB1-mediated DA release from the VTA [189] to the NAc [92]. Casarotto et al. [190] reported that CB1 and transient receptor potential vanilloid 1 receptor (TRPV1; responsible for the painful sensation caused by capsaicin or heat) are expressed in the D-PAG and are frequently co-localized at the same synapses [190, 191]. In Casarotto’s study, stimulation of CB1 in the D-PAG had panicolytic actions, whereas stimulation of TRPV1 had the opposite effect [190]. The above opposing effects may explain, at least partially, why some consumers report a subjective feeling of tranquility and others develop panic attacks [192, 193], and both actions may involve the cannabinoid targets CB1 and TRPV1, respectively, in the PAG.
Exposure to stressful conditions or electrical stimulation of the PAG raises endocannabinoid levels [194, 195]. Moreover, CB1 receptor agonist injection and increased anandamide in the D-PAG alleviate physical discomfort by analgesia [195, 196], anxiolysis, and anti-aversive actions [194]. Other studies have indicated that the CB1 receptor agonist HU210, administered either systemically or specifically in the D-PAG, increases plasma corticosterone levels and prevents the hyperlocomotion induced by aversive stimuli [197]. CB1 receptors seem to be involved only in the anti-hyperlocomotion action of HU210, while the increase in plasma corticosterone levels is not blocked by rimonabant (a non-selective CB1 antagonist) [197]. Rimonabant per se increases corticosterone [197], suggesting a non-cannabinoid effect that remains to be elucidated. As GPR55 is a target for rimonabant [198] and is a receptor expressed in several areas (including the PAG) involved in learning, pain, and anxiety [183, 199, 200], its participation in the reward and anti-reward systems needs further analysis.
In summary, cannabinoids reach the PAG to produce analgesia, anxiolysis, fear inhibition, and anti-aversive affects (alleviation of physical discomfort). In addition, cannabinoids may modulate DA release in the NAc via activation of CB1 receptors in areas with reciprocal connectivity with PAG that regulate VTA neurons. All these actions may influence both the impulsive and compulsive behaviors of drug addiction.
Opioids
As a key region in the pain circuit, the PAG is known for its role in mediating negative emotions [208]. The PAG is rich in opioid receptors and enkephalins [55]. Consequently, opioid addiction (e.g., morphine) critically involves enkephalins and the PAG area. In support of this notion, several studies have shown that: (i) there are several alterations in the PAG during morphine withdrawal, including hyperactivation of GABAergic neurons in the VL-PAG and astrocyte activation, and the release of pro-inflammatory cytokines such as TNFα [201, 202]; (ii) intra-PAG administration of an enkephalin analog suppresses the signs of morphine withdrawal [209]; (iii) intra-PAG infusion of morphine produces physical dependence after a naloxone challenge (an opioid receptor antagonist) [14]; and (iv) intra-PAG injection of morphine produces conditioned place preference [163]. Thus, the PAG is important in both the physical dependence and reinforcing actions of opioids. The needed dose of morphine to produce conditioned place preference via PAG injections was ten-fold higher (i.e., 5 µg vs. 0.2 µg, respectively) than via VTA [163]. This observation perhaps explains why in previous reports, animals did not exert self-injections into the PAG, but do into the VTA [14].
An extensively studied (and thus, not further discussed here) link between analgesia and addiction involves the opioid system [210–212]. Indeed, the great risk in prescribing opioid analgesics is the rapid development of addiction [210, 212]. Both analgesia mediated by opioids [149] and by placebo require opioid receptors in the PAG [213, 214]. Intriguingly, morphine withdrawal produces extreme anxiety accompanied by a plethora of physically uncomfortable disturbances, phenomena linked to PAG sensitization [215]. It has been classically reported that painful stressors are strong factors in relapsing opioid-related addiction [216]. Moreover, chronic use of opioids drives hyperalgesia and negative emotional states which have been proposed to contribute to the compulsive phase of drug addiction [84].
Hence, PAG is a key region highly altered during opioid withdrawal and may be responsible for some of the compulsive actions in the drug addiction. But also, simple intra-PAG injections of opioids (e.g., morphine) produce reinforcing actions (e.g., conditioning place preference).
Sedatives, Hypnotics, or Anxiolytics
Electrical stimulation of D-PAG evokes fear, panic attacks, intrusive ideas about death, among other negative feelings in humans [37]. Notoriously, tThe panicolytic, but not the anxiolytic action of alprazolam (a benzodiazepine) is prevented by intra-D-PAG injection of bicuculline or flumazenil, suggesting the participation of GABAA receptors in this brain structure [203]. Moreover, a serotoninergic circuit integrating the DR nucleus and VL-PAG, and controlled by the BLA, has been proposed as a natural anti-panic system that is facilitated by chronic antidepressants [217]. Therefore, part of the alleviative actions of this kind of compounds may be directly and/or indirectly associated with its actions in the PAG.
Stimulants
Cocaine is a non-selective inhibitor of monoamine transporters; thus, this drug potentiates monoaminergic transmission (DA, serotonin, and norepinephrine) and increases DA levels in the NAc [218] and amygdala [219, 220]. Recently, Li et al. [149] characterized the DA/glutamate neurons in the VL-PAG that are involved in pain integration and opiate anti-nociception. The reciprocal PAG-EA connections [138, 139, 221] are dopaminergic and could be involved in the expression of withdrawal/negative affect symptoms of methamphetamine [222].
It is interesting to consider that social stress seems to strongly promote cocaine intake [223, 224]. In fact, stress induced by social defeat or cocaine intake produces Fos-like immunoreactive (Fos-LI) augments in PAG, DR and locus coeruleus [223]. These changes may be related to cocaine sensitization [224]. Prolonged use of cocaine attenuates the dopamine efflux in the NAc and promotes dose-dependent anxiety and anhedonia [204, 225]. Moreover, after a cocaine binge, the PAG neurons are hyper-responsive to tactile stimulation in rats [204]. In humans, Zhang et al. [85] reported alterations in functional connectivity between the PAG, hypothalamus, and D-mPFC in cocaine-dependent subjects compared to healthy controls. Furthermore, a higher activation of the PAG and connectivity of the PAG with the vmPFC was detected been reported during exposure to cocaine cues [86].
The PAG-vmPFC connectivity is correlated with tonic cocaine craving and biological sex differences have been confirmed by a slope test [86]. While PAG-vmPFC connectivity reflects tonic cocaine craving in men, the ventromedial PAG appears to play a role in influencing the PAG response to alleviate cocaine craving in women. These suggest that PAG may be a key structure involved in cocaine withdrawal and cocaine craving [86].
Self-administration of other stimulants such as methamphetamine produce biochemical adaptations in DA neurons in the PAG, but when methamphetamine-seeking is combined with exercise (e.g., wheel-running) there is a reduction in the biochemical alterations in the PAG and in the methamphetamine-seeking, suggesting that exercise plays a neuroprotective role via the prevention of PAG alterations [222]. For stimulants, the PAG seems to be involved in substance-seeking, while biochemical changes in DA neurons in the PAG may contribute to the consolidation of the drug addiction cycle.
Tobacco
Nicotine interacts with high affinity with the α4 subunit [226] of the nicotinic acetylcholine receptor (nAChR), which is ubiquitously expressed in the brain [227]. nAChRs are expressed in the mesocorticolimbic dopaminergic system, which is mainly implicated in the reinforcing action of tobacco consumption [228, 229]. The background of nicotine addiction has several components, including pharmacological, genetic, and environmental factors [230]. Pharmacologically, nicotine interacts as an agonist for the different subunits of nAChRs [226]. The nAChRs consist of an assembled complex with five subunits selected from nine α subunits (α2 to α10) and three β subunits (β2 to β4) [230].
Different combinations of these subunits produce the different subtypes of nAChR, the α4β2 subtype being the main receptor that mediates nicotine addiction [231, 232]. Activation of nAChRs modulates synaptic transmission, since it increases the probability of release of neurotransmitters, including DA, GABA, glycine, glutamate, norepinephrine, and ACh [233–236]. In fact, activation of nAChRs in midbrain DA neurons mediates DA release in the NAc shell [237] which explains (at least in part) the reinforcing actions of nicotine [238]. The ACh that endogenously regulates midbrain DA is released from the mesopontine nuclei in a circuit that involves PAG neurons [238]. The PAG receives cholinergic inputs from the pontine tegmentum [239] and they contribute to maintaining the tonic activity of its GABAergic neurons [233]. On the other hand, α7 nAChRs have been reported to be expressed in VL-PAG cholinergic neurons that project to the RVM [240, 241]. Activation of these cholinergic projections is involved in pain inhibition [233]. Interestingly, the VL-PAG neurons are also involved in opioid-mediated analgesia [62], which is mediated via inhibition of GABAergic neurons in the PAG [242]. The above dichotomy strongly supports the integrative role of the PAG in the physiology of pain and analgesia via stimulation and inhibition of different nuclei by its cholinergic and GABAergic projections, respectively [233, 241].
Some emotions, such as fear and anxiety induce functional changes in the PAG [32, 243]. Likewise, depression can modify the genetic profile of the VL-PAG via mechanisms that remain to be elucidated [244]. Interestingly, nicotine has been shown to have antidepressant effects in several animal models [245–248]. The link between the antidepressant effects induced by nicotine and the nAChRs in the PAG is unknown. However, depression is a sign of nicotine withdrawal [249]. A plausible explanation of this link may be the actions mediated by the important cholinergic control of serotoninergic neurons from the DR to the NAc [205]. It is important to note that DR neurons are under dual modulation by PAG DA/glutamate neurons with repercussions for anxiety and analgesia [207]. Moreover, nicotine induces anxiolytic effects via DR neurons [206].
Although the participation of the PAG in the reinforcing mechanisms of nicotine are obscure, like other main pharmacological classes of addictive drugs, its direct and/or indirect actions in the PAG drives pain inhibition and anxiolysis, which contribute to the alleviation of physical discomfort induced by several conditions including nicotine withdrawal.
PAG involvement in appetitive behaviors and food intake
PAG activation promotes food intake and reward processing [159], as it has extensive reciprocal connections with brain circuits that mediate appetitive processes and consummatory behaviors (prefrontal cortex, LH, BNST, amygdala, parabrachial nucleus, VTA, and DR) [29, 136, 250, 251]. The food reward depends on the AL, DL, and VL PAG [136, 164]. It has been reported that these columns of the PAG contain synaptic terminals that release relaxin and oxytocin [252], peptides with a modulatory effect on feeding [253, 254]. There is a reciprocal relationship between the DR and the PAG since they play roles in multiple behaviors such as food consumption, anxiety, withdrawal, and depression [29]. On the other hand, it has been shown that the suppression of GABAergic neuronal activity in the VL-PAG, either directly or by long-projection GABAergic inputs from the BNST or LH, is sufficient to induce feeding behavior and, on the contrary, activation of these cells suppresses this behavior [136].
A link between drug-seeking and food-seeking has been reported [255, 256]. DA, which is essential for activation of the reward system, is also key for activating the "motivation system" [255, 256]. An imbalance in the dopaminergic system has been associated with both the compulsion for a drug and the compulsion to eat food [256, 257]. Interestingly, both food intake and reward/aversion processes are under fine modulation by the endocannabinoid system and putative cannabinoid receptors [186, 187, 258–260]. Under these circumstances, the PAG might serve and participate as an interface between the endogenous opioid system and the hedonic aspects of food reward [261–264]. Actually, the PAG is required for the normal consumption of food [29, 136] and its participation in appetitive and consummatory behaviors was recently discussed by Silva and McNaughton in an excellent critical review [29]. Our view completely agrees with Silva and McNaughton about the key role of the PAG in motivated behaviors and we support their proposal with the inclusion of the drug addiction cycle among the phenomena in which the PAG is involved.
Conclusions
Especially due to its small size, anatomical location, and its indirect participation in multiple kinds of motivated behaviors, the contribution of the PAG in the consolidation of drug addiction remains elusive and underestimated. We propose that, mainly by promoting the alleviation of physical discomfort (mainly pain, anxiety, and fear) linked to substance-withdrawal, the PAG may contribute to the mechanisms underlying relapse (compulsive intake). However, the participation of the PAG in the initial phase (impulsive intake) of drug intake cannot be excluded and requires further investigation.
Acknowledgments
BAM-C and PB-I were supported by the Dirección General de Investigación y Posgrado from the Autonomous University of Aguascalientes. PB-I was supported by the PRODEP program and an early career research grant from the International Association for the Study of Pain. AM-P was supported by the “Instituto Politécnico Nacional” (SIP-IPN 20200241). PV-L was supported by a CONACyT Postdoctoral Fellowship (406562). Figure 1 was partially drawn using biorender.com. BAM-C, PB-I, PV-L and JC-R are current fellows of the “Sistema Nacional de Investigadores” from CONACYT. We would also like to thank Tarjani N. Shukla and Jamie K. Moy for editing this manuscript.
Conflict of interest
The authors declare no conflicts of interest.
Contributor Information
Paulino Barragán-Iglesias, Email: paulino.barragan@edu.uaa.mx.
Bruno A. Marichal-Cancino, Email: bruno.marichal@edu.uaa.mx
References
- 1.Association AAP. Diagnostic and statistical manual of mental disorders: DSM-V. 1994.
- 2.Horseman C, Meyer A. Neurobiology of Addiction. Clin Obstet Gynecol. 2019;62:118–127. doi: 10.1097/GRF.0000000000000416. [DOI] [PubMed] [Google Scholar]
- 3.Gardner EL. Addiction and brain reward and antireward pathways. Adv Psychosom Med. 2011;30:22–60. doi: 10.1159/000324065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koob GF. Addiction is a reward deficit and stress surfeit disorder. Front Psychiatry. 2013;4:72. doi: 10.3389/fpsyt.2013.00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ditre JW, Zale EL, LaRowe LR. A reciprocal model of pain and substance use: Transdiagnostic considerations, clinical implications, and future directions. Annu Rev Clin Psychol. 2019;15:503–528. doi: 10.1146/annurev-clinpsy-050718-095440. [DOI] [PubMed] [Google Scholar]
- 6.Lee RSC, Hoppenbrouwers S, Franken I. A systematic meta-review of impulsivity and compulsivity in addictive behaviors. Neuropsychol Rev. 2019;29:14–26. doi: 10.1007/s11065-019-09402-x. [DOI] [PubMed] [Google Scholar]
- 7.Wise RA, Koob GF. The development and maintenance of drug addiction. Neuropsychopharmacology. 2014;39:254–262. doi: 10.1038/npp.2013.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Volkow ND, Li TK. Drug addiction: The neurobiology of behaviour gone awry. Nat Rev Neurosci. 2004;5:963–970. doi: 10.1038/nrn1539. [DOI] [PubMed] [Google Scholar]
- 9.Becker HC. Influence of stress associated with chronic alcohol exposure on drinking. Neuropharmacology. 2017;122:115–126. doi: 10.1016/j.neuropharm.2017.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ahmed SH, Koob GF. Transition from moderate to excessive drug intake: Change in hedonic set point. Science. 1998;282:298–300. doi: 10.1126/science.282.5387.298. [DOI] [PubMed] [Google Scholar]
- 11.Volkow ND, Fowler JS, Wang GJ. The addicted human brain: Insights from imaging studies. J Clin Invest. 2003;111:1444–1451. doi: 10.1172/JCI18533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McEwen BS. Sex, stress and the Hippocampus: Allostasis, allostatic load and the aging process. Neurobiol Aging. 2002;23:921–939. doi: 10.1016/S0197-4580(02)00027-1. [DOI] [PubMed] [Google Scholar]
- 13.Koob GF, Schulkin J. Addiction and stress: An allostatic view. Neurosci Biobehav Rev. 2019;106:245–262. doi: 10.1016/j.neubiorev.2018.09.008. [DOI] [PubMed] [Google Scholar]
- 14.Bozarth MA, Wise RA. Anatomically distinct opiate receptor fields mediate reward and physical dependence. Science. 1984;224:516–517. doi: 10.1126/science.6324347. [DOI] [PubMed] [Google Scholar]
- 15.Koob GF. Drug addiction: Hyperkatifeia/negative reinforcement as a framework for medications development. Pharmacol Rev. 2021;73:163–201. doi: 10.1124/pharmrev.120.000083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koob GF. Negative reinforcement in drug addiction: The darkness within. Curr Opin Neurobiol. 2013;23:559–563. doi: 10.1016/j.conb.2013.03.011. [DOI] [PubMed] [Google Scholar]
- 18.Koob GF. A role for brain stress systems in addiction. Neuron. 2008;59:11–34. doi: 10.1016/j.neuron.2008.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koob GF, Le Moal M. Addiction and the brain antireward system. Annu Rev Psychol. 2008;59:29–53. doi: 10.1146/annurev.psych.59.103006.093548. [DOI] [PubMed] [Google Scholar]
- 20.Beitz AJ. Periaqueductal gray. In: Paxinos, G. (Ed.), The Rat Nervous System. 1994: 173–180.
- 21.Brandão ML, Troncoso AC, de Souza Silva MA, Huston JP. The relevance of neuronal substrates of defense in the midbrain tectum to anxiety and stress: Empirical and conceptual considerations. Eur J Pharmacol. 2003;463:225–233. doi: 10.1016/S0014-2999(03)01284-6. [DOI] [PubMed] [Google Scholar]
- 22.Graeff FG, Viana MB, Mora PO. Dual role of 5-HT in defense and anxiety. Neurosci Biobehav Rev. 1997;21:791–799. doi: 10.1016/S0149-7634(96)00059-0. [DOI] [PubMed] [Google Scholar]
- 23.Graeff FG. Serotonin, the periaqueductal gray and panic. Neurosci Biobehav Rev. 2004;28:239–259. doi: 10.1016/j.neubiorev.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 24.Blanchard DC, Canteras NS, Markham CM, Pentkowski NS, Blanchard RJ. Lesions of structures showing FOS expression to cat presentation: Effects on responsivity to a Cat, Cat odor, and nonpredator threat. Neurosci Biobehav Rev. 2005;29:1243–1253. doi: 10.1016/j.neubiorev.2005.04.019. [DOI] [PubMed] [Google Scholar]
- 25.Brandão ML, Borelli KG, Nobre MJ, Santos JM, Albrechet-Souza L, Oliveira AR, et al. Gabaergic regulation of the neural organization of fear in the midbrain tectum. Neurosci Biobehav Rev. 2005;29:1299–1311. doi: 10.1016/j.neubiorev.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 26.Brandão ML, Anseloni VZ, Pandóssio JE, de Araújo JE, Castilho VM. Neurochemical mechanisms of the defensive behavior in the dorsal midbrain. Neurosci Biobehav Rev. 1999;23:863–875. doi: 10.1016/S0149-7634(99)00038-X. [DOI] [PubMed] [Google Scholar]
- 27.Borelli KG, Brandão ML. Effects of ovine CRF injections into the dorsomedial, dorsolateral and lateral columns of the periaqueductal gray: A functional role for the dorsomedial column. Horm Behav. 2008;53:40–50. doi: 10.1016/j.yhbeh.2007.08.013. [DOI] [PubMed] [Google Scholar]
- 28.Vázquez-León P, Ramírez-San Juan E, Marichal-Cancino BA, Campos-Rodríguez C, Chávez-Reyes J, Miranda-Páez A. NPY-Y1 receptors in dorsal periaqueductal gray modulate anxiety, alcohol intake, and relapse in Wistar rats. Pharmacol Biochem Behav 2020, 199: 173071. [DOI] [PubMed]
- 29.Silva C, McNaughton N. Are periaqueductal gray and dorsal raphe the foundation of appetitive and aversive control? A comprehensive review. Prog Neurobiol. 2019;177:33–72. doi: 10.1016/j.pneurobio.2019.02.001. [DOI] [PubMed] [Google Scholar]
- 30.Kittelberger JM, Land BR, Bass AH. Midbrain periaqueductal gray and vocal patterning in a teleost fish. J Neurophysiol. 2006;96:71–85. doi: 10.1152/jn.00067.2006. [DOI] [PubMed] [Google Scholar]
- 31.Kingsbury MA, Kelly AM, Schrock SE, Goodson JL. Mammal-like organization of the avian midbrain central gray and a reappraisal of the intercollicular nucleus. PLoS One. 2011;6:e20720. doi: 10.1371/journal.pone.0020720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carrive P. The periaqueductal gray and defensive behavior: Functional representation and neuronal organization. Behav Brain Res. 1993;58:27–47. doi: 10.1016/0166-4328(93)90088-8. [DOI] [PubMed] [Google Scholar]
- 33.Reynolds DV. Surgery in the rat during electrical analgesia induced by focal brain stimulation. Science. 1969;164:444–445. doi: 10.1126/science.164.3878.444. [DOI] [PubMed] [Google Scholar]
- 34.Tracey I, Ploghaus A, Gati JS, Clare S, Smith S, Menon RS, et al. Imaging attentional modulation of pain in the periaqueductal gray in humans. J Neurosci. 2002;22:2748–2752. doi: 10.1523/JNEUROSCI.22-07-02748.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lueptow LM, Fakira AK, Bobeck EN. The contribution of the descending pain modulatory pathway in opioid tolerance. Front Neurosci. 2018;12:886. doi: 10.3389/fnins.2018.00886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Graeff FG. New perspective on the pathophysiology of panic: Merging serotonin and opioids in the periaqueductal gray. Revista Brasileira De Pesquisas Med E Biol. 2012;45:366–375. doi: 10.1590/S0100-879X2012007500036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nashold BS, Jr, Wilson WP, Slaughter DG. Sensations evoked by stimulation in the midbrain of man. J Neurosurg. 1969;30:14–24. doi: 10.3171/jns.1969.30.1.0014. [DOI] [PubMed] [Google Scholar]
- 38.Lowery-Gionta EG, DiBerto J, Mazzone CM, Kash TL. GABA neurons of the ventral periaqueductal gray area modulate behaviors associated with anxiety and conditioned fear. Brain Struct Funct. 2018;223:3787–3799. doi: 10.1007/s00429-018-1724-z. [DOI] [PubMed] [Google Scholar]
- 39.Mota-Ortiz SR, Sukikara MH, Bittencourt JC, Baldo MV, Elias CF, Felicio LF, et al. The periaqueductal gray as a critical site to mediate reward seeking during predatory hunting. Behav Brain Res. 2012;226:32–40. doi: 10.1016/j.bbr.2011.08.034. [DOI] [PubMed] [Google Scholar]
- 40.Leite-Panissi CR, Coimbra NC, Menescal-de-Oliveira L. The cholinergic stimulation of the central amygdala modifying the tonic immobility response and antinociception in Guinea pigs depends on the ventrolateral periaqueductal gray. Brain Res Bull. 2003;60:167–178. doi: 10.1016/S0361-9230(03)00031-5. [DOI] [PubMed] [Google Scholar]
- 41.Daniels D, Miselis RR, Flanagan-Cato LM. Central neuronal circuit innervating the lordosis-producing muscles defined by transneuronal transport of pseudorabies virus. J Neurosci. 1999;19:2823–2833. doi: 10.1523/JNEUROSCI.19-07-02823.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yamada S, Kawata M. Identification of neural cells activated by mating stimulus in the periaqueductal gray in female rats. Front Neurosci. 2014;8:421. doi: 10.3389/fnins.2014.00421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Behbehani MM. Functional characteristics of the midbrain periaqueductal gray. Prog Neurobiol. 1995;46:575–605. doi: 10.1016/0301-0082(95)00009-K. [DOI] [PubMed] [Google Scholar]
- 44.George DT, Ameli R, Koob GF. Periaqueductal gray sheds light on dark areas of psychopathology. Trends Neurosci. 2019;42:349–360. doi: 10.1016/j.tins.2019.03.004. [DOI] [PubMed] [Google Scholar]
- 45.Campos AC, Fogaça MV, Aguiar DC, Guimarães FS. Animal models of anxiety disorders and stress. Braz J Psychiatry. 2013;35(Suppl 2):S101–111. doi: 10.1590/1516-4446-2013-1139. [DOI] [PubMed] [Google Scholar]
- 46.Papagianni EP, Stevenson CW. Cannabinoid regulation of fear and anxiety: An update. Curr Psychiatry Rep. 2019;21:38. doi: 10.1007/s11920-019-1026-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Goode TD, Maren S. Common neurocircuitry mediating drug and fear relapse in preclinical models. Psychopharmacology (Berl) 2019;236:415–437. doi: 10.1007/s00213-018-5024-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Elman I, Borsook D, Volkow ND. Pain and suicidality: Insights from reward and addiction neuroscience. Prog Neurobiol. 2013;109:1–27. doi: 10.1016/j.pneurobio.2013.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vázquez-León P, Campos-Rodríguez C, Gonzalez-Pliego C, Miranda-Páez A. Differential effects of cholecystokinin (CCK-8) microinjection into the ventrolateral and dorsolateral periaqueductal gray on anxiety models in Wistar rats. Horm Behav. 2018;106:105–111. doi: 10.1016/j.yhbeh.2018.10.003. [DOI] [PubMed] [Google Scholar]
- 50.Bandler R, Keay KA. Columnar organization in the midbrain periaqueductal gray and the integration of emotional expression. Prog Brain Res. 1996;107:285–300. doi: 10.1016/S0079-6123(08)61871-3. [DOI] [PubMed] [Google Scholar]
- 51.Fanselow MS, Decola JP, de Oca BM, Landeira-Fernandez J. Ventral and dorsolateral regions of the midbrain periaqueductal gray (PAG) control different stages of defensive behavior: Dorsolateral PAG lesions enhance the defensive freezing produced by massed and immediate shock. Aggr Behav. 1995;21:63–77. doi: 10.1002/1098-2337(1995)21:1<63::AID-AB2480210109>3.0.CO;2-F. [DOI] [Google Scholar]
- 52.Monassi CR, Leite-Panissi CR, Menescal-de-Oliveira L. Ventrolateral periaqueductal gray matter and the control of tonic immobility. Brain Res Bull. 1999;50:201–208. doi: 10.1016/S0361-9230(99)00192-6. [DOI] [PubMed] [Google Scholar]
- 53.Monassi CR, Hoffmann A, Menescal-De-oliveira L. Participation of the periaqueductal gray matter in the modulation of tonic immobility in the Guinea pig. Braz J Med Biol Res. 1994;27:1243–1248. [PubMed] [Google Scholar]
- 54.Dampney RA, Furlong TM, Horiuchi J, Iigaya K. Role of dorsolateral periaqueductal grey in the coordinated regulation of cardiovascular and respiratory function. Auton Neurosci. 2013;175:17–25. doi: 10.1016/j.autneu.2012.12.008. [DOI] [PubMed] [Google Scholar]
- 55.Bandler R, Shipley MT. Columnar organization in the midbrain periaqueductal gray: Modules for emotional expression? Trends Neurosci. 1994;17:379–389. doi: 10.1016/0166-2236(94)90047-7. [DOI] [PubMed] [Google Scholar]
- 56.Miranda-Páez A, Zamudio S, Vázquez-León P, Campos-Rodríguez C, Ramírez-San Juan E. Involvement of opioid and GABA systems in the ventrolateral periaqueductal gray on analgesia associated with tonic immobility. Pharmacol Biochem Behav. 2016;142:72–78. doi: 10.1016/j.pbb.2016.01.002. [DOI] [PubMed] [Google Scholar]
- 57.Tovote P, Esposito MS, Botta P, Chaudun F, Fadok JP, Markovic M, et al. Midbrain circuits for defensive behaviour. Nature. 2016;534:206–212. doi: 10.1038/nature17996. [DOI] [PubMed] [Google Scholar]
- 58.Ho YC, Lin TB, Hsieh MC, Lai CY, Chou D, Chau YP, et al. Periaqueductal gray glutamatergic transmission governs chronic stress-induced depression. Neuropsychopharmacology. 2018;43:302–312. doi: 10.1038/npp.2017.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gross CT, Canteras NS. The many paths to fear. Nat Rev Neurosci. 2012;13:651–658. doi: 10.1038/nrn3301. [DOI] [PubMed] [Google Scholar]
- 60.Motta SC, Carobrez AP, Canteras NS. The periaqueductal gray and primal emotional processing critical to influence complex defensive responses, fear learning and reward seeking. Neurosci Biobehav Rev. 2017;76:39–47. doi: 10.1016/j.neubiorev.2016.10.012. [DOI] [PubMed] [Google Scholar]
- 61.Damasio AR, Grabowski TJ, Bechara A, Damasio H, Ponto LL, Parvizi J, et al. Subcortical and cortical brain activity during the feeling of self-generated emotions. Nat Neurosci. 2000;3:1049–1056. doi: 10.1038/79871. [DOI] [PubMed] [Google Scholar]
- 62.Linnman C, Moulton EA, Barmettler G, Becerra L, Borsook D. Neuroimaging of the periaqueductal gray: State of the field. Neuroimage. 2012;60:505–522. doi: 10.1016/j.neuroimage.2011.11.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Marichal-Cancino BA, González-Hernández A, Muñoz-Islas E, Villalón CM. Monoaminergic receptors as modulators of the perivascular sympathetic and sensory CGRPergic outflows. Curr Neuropharmacol. 2020;18:790–808. doi: 10.2174/1570159X18666200503223240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Escarcega González CE, González Hernández A, Villalón CM, Rodríguez MG, Marichal Cancino BA. Β-adrenoceptor blockade for infantile hemangioma therapy: Do β3-adrenoceptors play a role? J Vasc Res. 2018;55:159–168. doi: 10.1159/000489956. [DOI] [PubMed] [Google Scholar]
- 65.Altamirano-Espinoza AH, González-Hernández A, Manrique-Maldonado G, Marichal-Cancino BA, Ruiz-Salinas I, Villalón CM. The role of dopamine D2, but not D3 or D4, receptor subtypes, in quinpirole-induced inhibition of the cardioaccelerator sympathetic outflow in pithed rats. Br J Pharmacol. 2013;170:1102–1111. doi: 10.1111/bph.12358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Manrique-Maldonado G, González-Hernández A, Marichal-Cancino BA, Villamil-Hernández MT, del Mercado OA, Centurión D, et al. The dopamine receptors mediating inhibition of the sympathetic vasopressor outflow in pithed rats: Pharmacological correlation with the D(2) -like type. Basic Clin Pharmacol Toxicol. 2011;109:506–512. doi: 10.1111/j.1742-7843.2011.00762.x. [DOI] [PubMed] [Google Scholar]
- 67.Marichal-Cancino BA, González-Hernández A, MaassenVanDenBrink A, Ramírez-San Juan E, Villalón CM. Potential mechanisms involved in palmitoylethanolamide-induced vasodepressor effects in rats. J Vasc Res. 2020;57:152–163. doi: 10.1159/000506158. [DOI] [PubMed] [Google Scholar]
- 68.Marichal-Cancino BA, Manrique-Maldonado G, Altamirano-Espinoza AH, Ruiz-Salinas I, González-Hernández A, MaassenVanDenBrink A, et al. Analysis of anandamide- and lysophosphatidylinositol-induced inhibition of the vasopressor responses produced by sympathetic stimulation or noradrenaline in pithed rats. Eur J Pharmacol. 2013;721:168–177. doi: 10.1016/j.ejphar.2013.09.039. [DOI] [PubMed] [Google Scholar]
- 69.Marichal-Cancino BA, Altamirano-Espinoza AH, Manrique-Maldonado G, MaassenVanDenBrink A, Villalón CM. Role of pre-junctional CB1, but not CB2, TRPV1 or GPR55 receptors in anandamide-induced inhibition of the vasodepressor sensory CGRPergic outflow in pithed rats. Basic Clin Pharmacol Toxicol. 2014;114:240–247. doi: 10.1111/bcpt.12152. [DOI] [PubMed] [Google Scholar]
- 70.Wong TM, Shan J. Modulation of sympathetic actions on the heart by opioid receptor stimulation. J Biomed Sci. 2001;8:299–306. doi: 10.1007/BF02258370. [DOI] [PubMed] [Google Scholar]
- 71.Heim C, Nemeroff CB. The impact of early adverse experiences on brain systems involved in the pathophysiology of anxiety and affective disorders. Biol Psychiatry. 1999;46:1509–1522. doi: 10.1016/S0006-3223(99)00224-3. [DOI] [PubMed] [Google Scholar]
- 72.Wright KM, Jhou TC, Pimpinelli D, McDannald MA. Cue-inhibited ventrolateral periaqueductal gray neurons signal fear output and threat probability in male rats. Elife Sci 2019, 8: e50054. [DOI] [PMC free article] [PubMed]
- 73.Wright KM, McDannald MA. Ventrolateral periaqueductal gray neurons prioritize threat probability over fear output. Elife 2019, 8: e45013. [DOI] [PMC free article] [PubMed]
- 74.Risbrough VB, Stein MB. Role of corticotropin releasing factor in anxiety disorders: A translational research perspective. Horm Behav. 2006;50:550–561. doi: 10.1016/j.yhbeh.2006.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mathew SJ, Price RB, Charney DS. Recent advances in the neurobiology of anxiety disorders: Implications for novel therapeutics. Am J Med Genet C Semin Med Genet. 2008;148C:89–98. doi: 10.1002/ajmg.c.30172. [DOI] [PubMed] [Google Scholar]
- 76.Bertoglio LJ, de Bortoli VC, Zangrossi H., Jr Cholecystokinin-2 receptors modulate freezing and escape behaviors evoked by the electrical stimulation of the rat dorsolateral periaqueductal gray. Brain Res. 2007;1156:133–138. doi: 10.1016/j.brainres.2007.04.038. [DOI] [PubMed] [Google Scholar]
- 77.Bertoglio LJ, Zangrossi H., Jr Involvement of dorsolateral periaqueductal gray cholecystokinin-2 receptors in the regulation of a panic-related behavior in rats. Brain Res. 2005;1059:46–51. doi: 10.1016/j.brainres.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 78.Netto CF, Guimarães FS. Anxiogenic effect of cholecystokinin in the dorsal periaqueductal gray. Neuropsychopharmacology. 2004;29:101–107. doi: 10.1038/sj.npp.1300334. [DOI] [PubMed] [Google Scholar]
- 79.Zanoveli JM, Netto CF, Guimarães FS, Zangrossi H., Jr Systemic and intra-dorsal periaqueductal gray injections of cholecystokinin sulfated octapeptide (CCK-8s) induce a panic-like response in rats submitted to the elevated T-maze. Peptides. 2004;25:1935–1941. doi: 10.1016/j.peptides.2004.06.016. [DOI] [PubMed] [Google Scholar]
- 80.Chieng B, Christie MD. Local opioid withdrawal in rat single periaqueductal gray neurons in vitro. J Neurosci. 1996;16:7128–7136. doi: 10.1523/JNEUROSCI.16-22-07128.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Epler AJ, Tomko RL, Piasecki TM, Wood PK, Sher KJ, Shiffman S, et al. Does hangover influence the time to next drink? An investigation using ecological momentary assessment. Alcohol Clin Exp Res. 2014;38:1461–1469. doi: 10.1111/acer.12386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Erb S, Shaham Y, Stewart J. Stress-induced relapse to drug seeking in the rat: Role of the bed nucleus of the stria terminalis and amygdala. Stress. 2001;4:289–303. doi: 10.3109/10253890109014753. [DOI] [PubMed] [Google Scholar]
- 83.Fox HC, Bergquist KL, Hong KI, Sinha R. Stress-induced and alcohol cue-induced craving in recently abstinent alcohol-dependent individuals. Alcohol Clin Exp Res. 2007;31:395–403. doi: 10.1111/j.1530-0277.2006.00320.x. [DOI] [PubMed] [Google Scholar]
- 84.Koob GF. Neurobiology of opioid addiction: Opponent process, hyperkatifeia, and negative reinforcement. Biol Psychiatry. 2020;87:44–53. doi: 10.1016/j.biopsych.2019.05.023. [DOI] [PubMed] [Google Scholar]
- 85.Zhang S, Zhornitsky S, Wang WY, Le TM, Dhingra I, Chen Y, et al. Resting state hypothalamic and dorsomedial prefrontal cortical connectivity of the periaqueductal gray in cocaine addiction. Addict Biol. 2021;26:e12989. doi: 10.1111/adb.12989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhang S, Zhornitsky S, Wang WY, Dhingra I, Le TM, Li CR. Cue-elicited functional connectivity of the periaqueductal gray and tonic cocaine craving. Drug Alcohol Depend 2020, 216: 108240. [DOI] [PMC free article] [PubMed]
- 87.Satpute AB, Wager TD, Cohen-Adad J, Bianciardi M, Choi JK, Buhle JT, et al. Identification of discrete functional subregions of the human periaqueductal gray. Proc Natl Acad Sci USA. 2013;110:17101–17106. doi: 10.1073/pnas.1306095110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Jia TY, Xie C, Banaschewski T, Barker GJ, Bokde ALW, Büchel C, et al. Neural network involving medial orbitofrontal cortex and dorsal periaqueductal gray regulation in human alcohol abuse. Sci Adv 2021, 7: eabd4074. [DOI] [PMC free article] [PubMed]
- 89.Blum K, Oscar-Berman M, Stuller E, Miller D, Giordano J, Morse S, et al. Neurogenetics and nutrigenomics of neuro-nutrient therapy for reward deficiency syndrome (RDS): Clinical ramifications as a function of molecular neurobiological mechanisms. J Addict Res Ther. 2012;3:139. doi: 10.4172/2155-6105.1000139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhang Y, Larcher KM, Misic B, Dagher A. Anatomical and functional organization of the human substantia nigra and its connections. Elife 2017, 6: e26653. [DOI] [PMC free article] [PubMed]
- 91.Luo SX, Huang EJ. Dopaminergic neurons and brain reward pathways: From neurogenesis to circuit assembly. Am J Pathol. 2016;186:478–488. doi: 10.1016/j.ajpath.2015.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Cheer JF, Wassum KM, Heien ML, Phillips PE, Wightman RM. Cannabinoids enhance subsecond dopamine release in the nucleus accumbens of awake rats. J Neurosci. 2004;24:4393–4400. doi: 10.1523/JNEUROSCI.0529-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Schultz W. Recent advances in understanding the role of phasic dopamine activity. F1000Res 2019, 8: F1000FacultyRev–F1000Faculty1680. [DOI] [PMC free article] [PubMed]
- 94.Ntamati NR, Creed M, Achargui R, Lüscher C. Periaqueductal efferents to dopamine and GABA neurons of the VTA. PLoS One. 2018;13:e0190297. doi: 10.1371/journal.pone.0190297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Geisler S, Derst C, Veh RW, Zahm DS. Glutamatergic afferents of the ventral tegmental area in the rat. J Neurosci. 2007;27:5730–5743. doi: 10.1523/JNEUROSCI.0012-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Fields HL, Hjelmstad GO, Margolis EB, Nicola SM. Ventral tegmental area neurons in learned appetitive behavior and positive reinforcement. Annu Rev Neurosci. 2007;30:289–316. doi: 10.1146/annurev.neuro.30.051606.094341. [DOI] [PubMed] [Google Scholar]
- 97.Morales M, Margolis EB. Ventral tegmental area: Cellular heterogeneity, connectivity and behaviour. Nat Rev Neurosci. 2017;18:73–85. doi: 10.1038/nrn.2016.165. [DOI] [PubMed] [Google Scholar]
- 98.St Laurent R, Martinez Damonte V, Tsuda AC, Kauer JA. Periaqueductal gray and rostromedial tegmental inhibitory afferents to VTA have distinct synaptic plasticity and opiate sensitivity. Neuron. 2020;106:624–636.e4. doi: 10.1016/j.neuron.2020.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Olds J, Milner P. Positive reinforcement produced by electrical stimulation of septal area and other regions of rat brain. J Comp Physiol Psychol. 1954;47:419–427. doi: 10.1037/h0058775. [DOI] [PubMed] [Google Scholar]
- 100.Floresco SB, Montes DR, Tse MMT, van Holstein M. Differential contributions of nucleus accumbens subregions to cue-guided risk/reward decision making and implementation of conditional rules. J Neurosci. 2018;38:1901–1914. doi: 10.1523/JNEUROSCI.3191-17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Horovitz O, Richter-Levin G. Dorsal periaqueductal gray simultaneously modulates ventral subiculum induced-plasticity in the basolateral amygdala and the nucleus accumbens. Front Behav Neurosci. 2015;9:53. doi: 10.3389/fnbeh.2015.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kim EJ, Horovitz O, Pellman BA, Tan LM, Li QL, Richter-Levin G, et al. Dorsal periaqueductal gray-amygdala pathway conveys both innate and learned fear responses in rats. Proc Natl Acad Sci USA. 2013;110:14795–14800. doi: 10.1073/pnas.1310845110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Horovitz O, Richter-Levin A, Xu L, Jing L, Richter-Levin G. Periaqueductal Grey differential modulation of Nucleus Accumbens and Basolateral Amygdala plasticity under controllable and uncontrollable stress. Sci Rep. 2017;7:487. doi: 10.1038/s41598-017-00562-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Heimer L, Alheid GF, de Olmos JS, Groenewegen HJ, Haber SN, Harlan RE, et al. The accumbens: beyond the core-shell dichotomy. J Neuropsychiatry Clin Neurosci. 1997;9:354–381. doi: 10.1176/jnp.9.3.354. [DOI] [PubMed] [Google Scholar]
- 105.Li Z, Chen Z, Fan G, Li A, Yuan J, Xu T. Cell-type-specific afferent innervation of the nucleus accumbens core and shell. Front Neuroanat. 2018;12:84. doi: 10.3389/fnana.2018.00084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wyvell CL, Berridge KC. Intra-accumbens amphetamine increases the conditioned incentive salience of sucrose reward: Enhancement of reward “wanting” without enhanced “liking” or response reinforcement. J Neurosci. 2000;20:8122–8130. doi: 10.1523/JNEUROSCI.20-21-08122.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Taha SA, Fields HL. Inhibitions of nucleus accumbens neurons encode a gating signal for reward-directed behavior. J Neurosci. 2006;26:217–222. doi: 10.1523/JNEUROSCI.3227-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ikemoto S. Dopamine reward circuitry: Two projection systems from the ventral midbrain to the nucleus accumbens-olfactory tubercle complex. Brain Res Rev. 2007;56:27–78. doi: 10.1016/j.brainresrev.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ikemoto S. Brain reward circuitry beyond the mesolimbic dopamine system: A neurobiological theory. Neurosci Biobehav Rev. 2010;35:129–150. doi: 10.1016/j.neubiorev.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ikemoto S, Glazier BS, Murphy JM, McBride WJ. Role of dopamine D1 and D2 receptors in the nucleus accumbens in mediating reward. J Neurosci. 1997;17:8580–8587. doi: 10.1523/JNEUROSCI.17-21-08580.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Shahid Z SG. Physiology, Hypothalamus. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2020. https://www.ncbi.nlm.nih.gov/books/NBK535380/ 2018.
- 112.Reppucci CJ, Petrovich GD. Organization of connections between the amygdala, medial prefrontal cortex, and lateral hypothalamus: A single and double retrograde tracing study in rats. Brain Struct Funct. 2016;221:2937–2962. doi: 10.1007/s00429-015-1081-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Harris GC, Wimmer M, Aston-Jones G. A role for lateral hypothalamic orexin neurons in reward seeking. Nature. 2005;437:556–559. doi: 10.1038/nature04071. [DOI] [PubMed] [Google Scholar]
- 114.Han Y, Yuan K, Zheng YB, Lu L. Orexin receptor antagonists as emerging treatments for psychiatric disorders. Neurosci Bull. 2020;36:432–448. doi: 10.1007/s12264-019-00447-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Watkins SS, Koob GF, Markou A. Neural mechanisms underlying nicotine addiction: Acute positive reinforcement and withdrawal. Nicotine Tob Res. 2000;2:19–37. doi: 10.1080/14622200050011277. [DOI] [PubMed] [Google Scholar]
- 116.Ullah F, Dos Anjos-Garcia T, Mendes-Gomes J, Elias-Filho DH, Falconi-Sobrinho LL, Freitas RL, et al. Connexions between the dorsomedial division of the ventromedial hypothalamus and the dorsal periaqueductal grey matter are critical in the elaboration of hypothalamically mediated panic-like behaviour. Behav Brain Res. 2017;319:135–147. doi: 10.1016/j.bbr.2016.11.026. [DOI] [PubMed] [Google Scholar]
- 117.de Quidt ME, Emson PC. Distribution of neuropeptide Y-like immunoreactivity in the rat central nervous system——I. Radioimmunoassay and chromatographic characterisation. Neuroscience. 1986;18:527–543. doi: 10.1016/0306-4522(86)90056-4. [DOI] [PubMed] [Google Scholar]
- 118.Danger JM, Tonon MC, Jenks BG, Saint-Pierre S, Martel JC, Fasolo A, et al. Neuropeptide Y: Localization in the central nervous system and neuroendocrine functions. Fundam Clin Pharmacol. 1990;4:307–340. doi: 10.1111/j.1472-8206.1990.tb00497.x. [DOI] [PubMed] [Google Scholar]
- 119.de Quidt ME, Emson PC. Distribution of neuropeptide Y-like immunoreactivity in the rat central nervous system——II. Immunohistochemical analysis. Neuroscience. 1986;18:545–618. doi: 10.1016/0306-4522(86)90057-6. [DOI] [PubMed] [Google Scholar]
- 120.Allen YS, Adrian TE, Allen JM, Tatemoto K, Crow TJ, Bloom SR, et al. Neuropeptide Y distribution in the rat brain. Science. 1983;221:877–879. doi: 10.1126/science.6136091. [DOI] [PubMed] [Google Scholar]
- 121.Kask A, Harro J, von Hörsten S, Redrobe JP, Dumont Y, Quirion R. The neurocircuitry and receptor subtypes mediating anxiolytic-like effects of neuropeptide Y. Neurosci Biobehav Rev. 2002;26:259–283. doi: 10.1016/S0149-7634(01)00066-5. [DOI] [PubMed] [Google Scholar]
- 122.Morales-Medina JC, Dumont Y, Benoit CE, Bastianetto S, Flores G, Fournier A, et al. Role of neuropeptide Y Y1 and Y2 receptors on behavioral despair in a rat model of depression with co-morbid anxiety. Neuropharmacology. 2012;62:200–208. doi: 10.1016/j.neuropharm.2011.06.030. [DOI] [PubMed] [Google Scholar]
- 123.Gilpin NW, Misra K, Herman MA, Cruz MT, Koob GF, Roberto M. Neuropeptide Y opposes alcohol effects on gamma-aminobutyric acid release in amygdala and blocks the transition to alcohol dependence. Biol Psychiatry. 2011;69:1091–1099. doi: 10.1016/j.biopsych.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Vázquez-León P, Mendoza-Ruiz LG, Juan ER, Chamorro-Cevallos GA, Miranda-Páez A. Analgesic and anxiolytic effects of [Leu31, Pro34]-neuropeptide Y microinjected into the periaqueductal gray in rats. Neuropeptides. 2017;66:81–89. doi: 10.1016/j.npep.2017.10.001. [DOI] [PubMed] [Google Scholar]
- 125.Ong WY, Stohler CS, Herr DR. Role of the prefrontal cortex in pain processing. Mol Neurobiol. 2019;56:1137–1166. doi: 10.1007/s12035-018-1130-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Hardy SG, Leichnetz GR. Cortical projections to the periaqueductal gray in the monkey: A retrograde and orthograde horseradish peroxidase study. Neurosci Lett. 1981;22:97–101. doi: 10.1016/0304-3940(81)90070-7. [DOI] [PubMed] [Google Scholar]
- 127.Coulombe MA, Erpelding N, Kucyi A, Davis KD. Intrinsic functional connectivity of periaqueductal gray subregions in humans. Hum Brain Mapp. 2016;37:1514–1530. doi: 10.1002/hbm.23117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Goriounova NA, Mansvelder HD. Nicotine exposure during adolescence alters the rules for prefrontal cortical synaptic plasticity during adulthood. Front Synaptic Neurosci. 2012;4:3. doi: 10.3389/fnsyn.2012.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Amarante LM, Caetano MA, Laubach M. The Medial Frontal Cortex Generates Rhythmic Activity that Encodes Reward Value. bioRxiv 2017: 144550.
- 130.Vander Weele CM, Siciliano CA, Matthews GA, Namburi P, Izadmehr EM, Espinel IC, et al. Dopamine enhances signal-to-noise ratio in cortical-brainstem encoding of aversive stimuli. Nature. 2018;563:397–401. doi: 10.1038/s41586-018-0682-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Cheriyan J, Sheets PL. Altered Excitability and Local Connectivity of mPFC-PAG Neurons in a Mouse Model of Neuropathic Pain. J Neurosci. 2018;38:4829–4839. doi: 10.1523/JNEUROSCI.2731-17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Siciliano CA, Noamany H, Chang CJ, Brown AR, Chen XH, Leible D, et al. A cortical-brainstem circuit predicts and governs compulsive alcohol drinking. Science. 2019;366:1008–1012. doi: 10.1126/science.aay1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Huang JT, Gadotti VM, Chen LN, Souza IA, Huang S, Wang DC, et al. A neuronal circuit for activating descending modulation of neuropathic pain. Nat Neurosci. 2019;22:1659–1668. doi: 10.1038/s41593-019-0481-5. [DOI] [PubMed] [Google Scholar]
- 134.Kubota N, Amemiya S, Yanagita S, Nishijima T, Kita I. Central nucleus of the amygdala is involved in induction of yawning response in rats. Behav Brain Res 2019, 371: 111974. [DOI] [PubMed]
- 135.Kim B, Yoon S, Nakajima R, Lee HJ, Lim HJ, Lee YK, et al. Dopamine D2 receptor-mediated circuit from the central amygdala to the bed nucleus of the stria terminalis regulates impulsive behavior. Proc Natl Acad Sci USA. 2018;115:E10730–E10739. doi: 10.1073/pnas.1811664115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Hao SJ, Yang HB, Wang XM, He Y, Xu HF, Wu XT, et al. The lateral hypothalamic and BNST GABAergic projections to the anterior ventrolateral periaqueductal gray regulate feeding. Cell Rep. 2019;28:616–624.e5. doi: 10.1016/j.celrep.2019.06.051. [DOI] [PubMed] [Google Scholar]
- 137.Sardi NF, Lazzarim MK, Guilhen VA, Marcílio RS, Natume PS, Watanabe TC, et al. Chronic sleep restriction increases pain sensitivity over time in a periaqueductal gray and nucleus accumbens dependent manner. Neuropharmacology. 2018;139:52–60. doi: 10.1016/j.neuropharm.2018.06.022. [DOI] [PubMed] [Google Scholar]
- 138.Li YQ, Takada M, Shinonaga Y, Mizuno N. The sites of origin of dopaminergic afferent fibers to the lateral habenular nucleus in the rat. J Comp Neurol. 1993;333:118–133. doi: 10.1002/cne.903330110. [DOI] [PubMed] [Google Scholar]
- 139.Hasue RH, Shammah-Lagnado SJ. Origin of the dopaminergic innervation of the central extended amygdala and accumbens shell: A combined retrograde tracing and immunohistochemical study in the rat. J Comp Neurol. 2002;454:15–33. doi: 10.1002/cne.10420. [DOI] [PubMed] [Google Scholar]
- 140.Walker DL, Toufexis DJ, Davis M. Role of the bed nucleus of the stria terminalis versus the amygdala in fear, stress, and anxiety. Eur J Pharmacol. 2003;463:199–216. doi: 10.1016/S0014-2999(03)01282-2. [DOI] [PubMed] [Google Scholar]
- 141.Kash TL. The role of biogenic amine signaling in the bed nucleus of the stria terminals in alcohol abuse. Alcohol. 2012;46:303–308. doi: 10.1016/j.alcohol.2011.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Hammack SE, Guo JD, Hazra R, Dabrowska J, Myers KM, Rainnie DG. The response of neurons in the bed nucleus of the stria terminalis to serotonin: Implications for anxiety. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:1309–1320. doi: 10.1016/j.pnpbp.2009.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Walker DL, Miles LA, Davis M. Selective participation of the bed nucleus of the stria terminalis and CRF in sustained anxiety-like versus phasic fear-like responses. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:1291–1308. doi: 10.1016/j.pnpbp.2009.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Silberman Y, Winder DG. Emerging role for corticotropin releasing factor signaling in the bed nucleus of the stria terminalis at the intersection of stress and reward. Front Psychiatry. 2013;4:42. doi: 10.3389/fpsyt.2013.00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Pleil KE, Rinker JA, Lowery-Gionta EG, Mazzone CM, McCall NM, Kendra AM, et al. NPY signaling inhibits extended amygdala CRF neurons to suppress binge alcohol drinking. Nat Neurosci. 2015;18:545–552. doi: 10.1038/nn.3972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Buffalari DM, See RE. Inactivation of the bed nucleus of the stria terminalis in an animal model of relapse: Effects on conditioned cue-induced reinstatement and its enhancement by yohimbine. Psychopharmacology (Berl) 2011;213:19–27. doi: 10.1007/s00213-010-2008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Wenzel JM, Cotten SW, Dominguez HM, Lane JE, Shelton K, Su ZI, et al. Noradrenergic β-receptor antagonism within the central nucleus of the amygdala or bed nucleus of the stria terminalis attenuates the negative/anxiogenic effects of cocaine. J Neurosci. 2014;34:3467–3474. doi: 10.1523/JNEUROSCI.3861-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Meloni EG, Gerety LP, Knoll AT, Cohen BM, Carlezon WA., Jr Behavioral and anatomical interactions between dopamine and corticotropin-releasing factor in the rat. J Neurosci. 2006;26:3855–3863. doi: 10.1523/JNEUROSCI.4957-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Li C, Sugam JA, Lowery-Gionta EG, McElligott ZA, McCall NM, Lopez AJ, et al. Mu opioid receptor modulation of dopamine neurons in the periaqueductal gray/dorsal raphe: A role in regulation of pain. Neuropsychopharmacology. 2016;41:2122–2132. doi: 10.1038/npp.2016.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.McDonald AJ. Cytoarchitecture of the central amygdaloid nucleus of the rat. J Comp Neurol. 1982;208:401–418. doi: 10.1002/cne.902080409. [DOI] [PubMed] [Google Scholar]
- 151.Jolkkonen E, Pitkänen A. Intrinsic connections of the rat amygdaloid complex: Projections originating in the central nucleus. J Comp Neurol. 1998;395:53–72. doi: 10.1002/(SICI)1096-9861(19980525)395:1<53::AID-CNE5>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 152.Sah P, Faber ES, Lopez De Armentia M, Power J. The amygdaloid complex: Anatomy and physiology. Physiol Rev 2003, 83: 803–834. [DOI] [PubMed]
- 153.Amunts K, Kedo O, Kindler M, Pieperhoff P, Mohlberg H, Shah NJ, et al. Cytoarchitectonic mapping of the human amygdala, hippocampal region and entorhinal cortex: Intersubject variability and probability maps. Anat Embryol (Berl) 2005;210:343–352. doi: 10.1007/s00429-005-0025-5. [DOI] [PubMed] [Google Scholar]
- 154.Li JN, Sheets PL. The central amygdala to periaqueductal gray pathway comprises intrinsically distinct neurons differentially affected in a model of inflammatory pain. J Physiol. 2018;596:6289–6305. doi: 10.1113/JP276935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Avegno EM, Lobell TD, Itoga CA, Baynes BB, Whitaker AM, Weera MM, et al. Central amygdala circuits mediate hyperalgesia in alcohol-dependent rats. J Neurosci. 2018;38:7761–7773. doi: 10.1523/JNEUROSCI.0483-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Sun Y, Blanco-Centurion C, Zou BY, Bendell E, Shiromani PJ, Liu M. Amygdala GABA Neurons Project To vlPAG And mPFC. IBRO Rep. 2019;6:132–136. doi: 10.1016/j.ibror.2019.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Yin WW, Mei LS, Sun TT, Wang YP, Li J, Chen CM, et al. A central amygdala-ventrolateral periaqueductal gray matter pathway for pain in a mouse model of depression-like behavior. Anesthesiology. 2020;132:1175–1196. doi: 10.1097/ALN.0000000000003133. [DOI] [PubMed] [Google Scholar]
- 158.Carlezon WA, Jr, Thome J, Olson VG, Lane-Ladd SB, Brodkin ES, Hiroi N, et al. Regulation of cocaine reward by CREB. Science. 1998;282:2272–2275. doi: 10.1126/science.282.5397.2272. [DOI] [PubMed] [Google Scholar]
- 159.Lüthi A, Lüscher C. Pathological circuit function underlying addiction and anxiety disorders. Nat Neurosci. 2014;17:1635–1643. doi: 10.1038/nn.3849. [DOI] [PubMed] [Google Scholar]
- 160.Nobre MJ, Ribeiro dos Santos N, Aguiar MS, Brandão ML. Blockade of mu- and activation of kappa-opioid receptors in the dorsal periaqueductal gray matter produce defensive behavior in rats tested in the elevated plus-maze. Eur J Pharmacol 2000, 404: 145–151. [DOI] [PubMed]
- 161.Brandão ML. Involvement of opioid mechanisms in the dorsal periaqueductal gray in drug abuse. Rev Neurosci. 1993;4:397–405. doi: 10.1515/REVNEURO.1993.4.4.397. [DOI] [PubMed] [Google Scholar]
- 162.Maldonado R, Fournié-Zaluski MC, Roques BP. Attenuation of the morphine withdrawal syndrome by inhibition of catabolism of endogenous enkephalins in the periaqueductal gray matter. Naunyn Schmiedebergs Arch Pharmacol. 1992;345:466–472. doi: 10.1007/BF00176626. [DOI] [PubMed] [Google Scholar]
- 163.Olmstead MC, Franklin KB. The development of a conditioned place preference to morphine: Effects of microinjections into various CNS sites. Behav Neurosci. 1997;111:1324–1334. doi: 10.1037/0735-7044.111.6.1324. [DOI] [PubMed] [Google Scholar]
- 164.Tryon VL, Mizumori SJY. A novel role for the periaqueductal gray in consummatory behavior. Front Behav Neurosci. 2018;12:178. doi: 10.3389/fnbeh.2018.00178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Olsen RW. GABAA receptor: Positive and negative allosteric modulators. Neuropharmacology. 2018;136:10–22. doi: 10.1016/j.neuropharm.2018.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Slutske WS, Piasecki TM, Hunt-Carter EE. Development and initial validation of the Hangover Symptoms Scale: Prevalence and correlates of Hangover Symptoms in college students. Alcohol Clin Exp Res. 2003;27:1442–1450. doi: 10.1097/01.ALC.0000085585.81711.AE. [DOI] [PubMed] [Google Scholar]
- 167.Verster JC. The “hair of the dog”: A useful hangover remedy or a predictor of future problem drinking? Curr Drug Abuse Rev. 2009;2:1–4. doi: 10.2174/1874473710902010001. [DOI] [PubMed] [Google Scholar]
- 168.Eichenberger GC, Ribeiro SJ, Osaki MY, Maruoka RY, Resende GC, Castellan-Baldan L, et al. Neuroanatomical and psychopharmacological evidence for interaction between opioid and GABAergic neural pathways in the modulation of fear and defense elicited by electrical and chemical stimulation of the deep layers of the superior colliculus and dorsal periaqueductal gray matter. Neuropharmacology. 2002;42:48–59. doi: 10.1016/S0028-3908(01)00155-1. [DOI] [PubMed] [Google Scholar]
- 169.Ezequiel Leite L, Nobre MJ. The negative effects of alcohol hangover on high-anxiety phenotype rats are influenced by the glutamate receptors of the dorsal midbrain. Neuroscience. 2012;213:93–105. doi: 10.1016/j.neuroscience.2012.04.009. [DOI] [PubMed] [Google Scholar]
- 170.Koob GF. Theoretical frameworks and mechanistic aspects of alcohol addiction: Alcohol addiction as a reward deficit disorder. Curr Top Behav Neurosci. 2013;13:3–30. doi: 10.1007/978-3-642-28720-6_129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Long C, Yang L, Faingold CL, Steven Evans M. Excitatory amino acid receptor-mediated responses in periaqueductal gray neurons are increased during ethanol withdrawal. Neuropharmacology. 2007;52:802–811. doi: 10.1016/j.neuropharm.2006.09.019. [DOI] [PubMed] [Google Scholar]
- 172.Bonassoli VT, Contardi EB, Milani H, de Oliveira RM. Effects of nitric oxide synthase inhibition in the dorsolateral periaqueductal gray matter on ethanol withdrawal-induced anxiety-like behavior in rats. Psychopharmacology (Berl) 2013;228:487–498. doi: 10.1007/s00213-013-3049-1. [DOI] [PubMed] [Google Scholar]
- 173.Li C, McCall NM, Lopez AJ, Kash TL. Alcohol effects on synaptic transmission in periaqueductal gray dopamine neurons. Alcohol. 2013;47:279–287. doi: 10.1016/j.alcohol.2013.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.McClintick JN, McBride WJ, Bell RL, Ding ZM, Liu YL, Xuei XL, et al. Gene expression changes in glutamate and GABA-A receptors, neuropeptides, ion channels, and cholesterol synthesis in the periaqueductal gray following binge-like alcohol drinking by adolescent alcohol-preferring (P) rats. Alcohol Clin Exp Res. 2016;40:955–968. doi: 10.1111/acer.13056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Riley JL, King C. Self-report of alcohol use for pain in a multi-ethnic community sample. J Pain. 2009;10:944–952. doi: 10.1016/j.jpain.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Egli M, Koob GF, Edwards S. Alcohol dependence as a chronic pain disorder. Neurosci Biobehav Rev. 2012;36:2179–2192. doi: 10.1016/j.neubiorev.2012.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Le TM, Zhornitsky S, Zhang S, Li CR. Pain and reward circuits antagonistically modulate alcohol expectancy to regulate drinking. Transl Psychiatry. 2020;10:220. doi: 10.1038/s41398-020-00909-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Galvalisi M, Prieto JP, Martínez M, Abin-Carriquiry JA, Scorza C. Caffeine induces a stimulant effect and increases dopamine release in the nucleus accumbens shell through the pulmonary inhalation route of administration in rats. Neurotox Res. 2017;31:90–98. doi: 10.1007/s12640-016-9667-8. [DOI] [PubMed] [Google Scholar]
- 179.Smith JE, Lawrence AD, Diukova A, Wise RG, Rogers PJ. Storm in a coffee cup: Caffeine modifies brain activation to social signals of threat. Soc Cogn Affect Neurosci. 2012;7:831–840. doi: 10.1093/scan/nsr058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Bae JH, Park JH, Im SS, Song DK. Coffee and health. Integr Med Res. 2014;3:189–191. doi: 10.1016/j.imr.2014.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Koob GF, Buck CL, Cohen A, Edwards S, Park PE, Schlosburg JE, et al. Addiction as a stress surfeit disorder. Neuropharmacology 2014, 76 Pt B: 370–382. [DOI] [PMC free article] [PubMed]
- 182.Ignatowska-Jankowska BM, Muldoon PP, Lichtman AH, Damaj MI. The cannabinoid CB2 receptor is necessary for nicotine-conditioned place preference, but not other behavioral effects of nicotine in mice. Psychopharmacology (Berl) 2013;229:591–601. doi: 10.1007/s00213-013-3117-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Marichal-Cancino BA, Fajardo-Valdez A, Ruiz-Contreras AE, Mendez-Díaz M, Prospero-García O. Advances in the physiology of GPR55 in the central nervous system. Curr Neuropharmacol. 2017;15:771–778. doi: 10.2174/1570159X14666160729155441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Marichal-Cancino BA, Fajardo-Valdez A, Ruiz-Contreras AE, Méndez-Díaz M, Prospéro-García O. Possible role of hippocampal GPR55 in spatial learning and memory in rats. Acta Neurobiol Exp (Wars) 2018;78:41–50. [PubMed] [Google Scholar]
- 185.Marichal-Cancino BA, Sánchez-Fuentes A, Méndez-Díaz M, Ruiz-Contreras AE, Prospéro-García O. Blockade of GPR55 in the dorsolateral striatum impairs performance of rats in a T-maze paradigm. Behav Pharmacol. 2016;27:393–396. doi: 10.1097/FBP.0000000000000185. [DOI] [PubMed] [Google Scholar]
- 186.Ramírez-Orozco RE, García-Ruiz R, Morales P, Villalón CM, Villafán-Bernal JR, Marichal-Cancino BA. Potential metabolic and behavioural roles of the putative endocannabinoid receptors GPR18, GPR55 and GPR119 in feeding. Curr Neuropharmacol. 2019;17:947–960. doi: 10.2174/1570159X17666190118143014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Sánchez-Fuentes A, Marichal-Cancino BA, Méndez-Díaz M, Becerril-Meléndez AL, Ruiz-Contreras AE, Prospéro-Garcia O. mGluR1/5 activation in the lateral hypothalamus increases food intake via the endocannabinoid system. Neurosci Lett. 2016;631:104–108. doi: 10.1016/j.neulet.2016.08.020. [DOI] [PubMed] [Google Scholar]
- 188.Allende G, Chávez-Reyes J, Guerrero-Alba R, Vázquez-León P, Marichal-Cancino BA. Advances in neurobiology and pharmacology of GPR12. Front Pharmacol. 2020;11:628. doi: 10.3389/fphar.2020.00628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Barrot M, Sesack SR, Georges F, Pistis M, Hong S, Jhou TC. Braking dopamine systems: A new GABA master structure for mesolimbic and nigrostriatal functions. J Neurosci. 2012;32:14094–14101. doi: 10.1523/JNEUROSCI.3370-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Casarotto PC, Terzian AL, Aguiar DC, Zangrossi H, Guimarães FS, Wotjak CT, et al. Opposing roles for cannabinoid receptor type-1 (CB1) and transient receptor potential vanilloid type-1 channel (TRPV1) on the modulation of panic-like responses in rats. Neuropsychopharmacology. 2012;37:478–486. doi: 10.1038/npp.2011.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Cristino L, de Petrocellis L, Pryce G, Baker D, Guglielmotti V, di Marzo V. Immunohistochemical localization of cannabinoid type 1 and vanilloid transient receptor potential vanilloid type 1 receptors in the mouse brain. Neuroscience. 2006;139:1405–1415. doi: 10.1016/j.neuroscience.2006.02.074. [DOI] [PubMed] [Google Scholar]
- 192.Langs G, Fabisch H, Fabisch K, Zapotoczky H. Can Cannabis trigger recurrent panic attacks in susceptible patients? Eur Psychiatry. 1997;12:415–419. doi: 10.1016/S0924-9338(97)83568-7. [DOI] [PubMed] [Google Scholar]
- 193.de Mello Schier AR, de Oliveira Ribeiro NP, Coutinho DS, Machado S, Arias-Carrión O, Crippa JA, et al. Antidepressant-like and anxiolytic-like effects of cannabidiol: A chemical compound of Cannabis sativa. CNS Neurol Disord Drug Targets. 2014;13:953–960. doi: 10.2174/1871527313666140612114838. [DOI] [PubMed] [Google Scholar]
- 194.Moreira FA, Aguiar DC, Campos AC, Lisboa SF, Terzian AL, Resstel LB, et al. Antiaversive effects of cannabinoids: Is the periaqueductal gray involved? Neural Plast 2009, 2009: 625469. [DOI] [PMC free article] [PubMed]
- 195.Walker JM, Huang SM, Strangman NM, Tsou K, Sañudo-Peña MC. Pain modulation by release of the endogenous cannabinoid anandamide. PNAS. 1999;96:12198–12203. doi: 10.1073/pnas.96.21.12198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Martin WJ, Patrick SL, Coffin PO, Tsou K, Walker JM. An examination of the central sites of action of cannabinoid-induced antinociception in the rat. Life Sci. 1995;56:2103–2109. doi: 10.1016/0024-3205(95)00195-C. [DOI] [PubMed] [Google Scholar]
- 197.Finn DP, Jhaveri MD, Beckett SR, Kendall DA, Marsden CA, Chapman V. Cannabinoids modulate ultrasound-induced aversive responses in rats. Psychopharmacology (Berl) 2004;172:41–51. doi: 10.1007/s00213-003-1629-1. [DOI] [PubMed] [Google Scholar]
- 198.Henstridge CM, Balenga NA, Schröder R, Kargl JK, Platzer W, Martini L, et al. GPR55 ligands promote receptor coupling to multiple signalling pathways. Br J Pharmacol. 2010;160:604–614. doi: 10.1111/j.1476-5381.2009.00625.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Shi QX, Yang LK, Shi WL, Wang L, Zhou SM, Guan SY, et al. The novel cannabinoid receptor GPR55 mediates anxiolytic-like effects in the medial orbital cortex of mice with acute stress. Mol Brain. 2017;10:38. doi: 10.1186/s13041-017-0318-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Deliu E, Sperow M, Console-Bram L, Carter RL, Tilley DG, Kalamarides DJ, et al. The lysophosphatidylinositol receptor GPR55 modulates pain perception in the periaqueductal gray. Mol Pharmacol. 2015;88:265–272. doi: 10.1124/mol.115.099333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Chieng BC, Hallberg C, Nyberg FJ, Christie MJ. Enhanced c-Fos in periaqueductal grey GABAergic neurons during opioid withdrawal. Neuroreport. 2005;16:1279–1283. doi: 10.1097/01.wnr.0000175246.35837.5c. [DOI] [PubMed] [Google Scholar]
- 202.Hao S, Liu S, Zheng X, Zheng W, Ouyang H, Mata M, et al. The role of TNFα in the periaqueductal gray during naloxone-precipitated morphine withdrawal in rats. Neuropsychopharmacology. 2011;36:664–676. doi: 10.1038/npp.2010.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Frias AT, Fernandes GG, Zangrossi H., Jr GABAA/benzodiazepine receptors in the dorsal periaqueductal gray mediate the panicolytic but not the anxiolytic effect of alprazolam in rats. Behav Brain Res. 2019;364:99–105. doi: 10.1016/j.bbr.2019.02.016. [DOI] [PubMed] [Google Scholar]
- 204.Mutschler NH, Miczek KA, Hammer RP., Jr Reduction of zif268 messenger RNA expression during prolonged withdrawal following “binge” cocaine self-administration in rats. Neuroscience. 2000;100:531–538. doi: 10.1016/S0306-4522(00)00298-0. [DOI] [PubMed] [Google Scholar]
- 205.Chang B, Daniele CA, Gallagher K, Madonia M, Mitchum RD, Barrett L, et al. Nicotinic excitation of serotonergic projections from dorsal raphe to the nucleus accumbens. J Neurophysiol. 2011;106:801–808. doi: 10.1152/jn.00575.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Cheeta S, Irvine EE, Kenny PJ, File SE. The dorsal raphé nucleus is a crucial structure mediating nicotine's anxiolytic effects and the development of tolerance and withdrawal responses. Psychopharmacology (Berl) 2001;155:78–85. doi: 10.1007/s002130100681. [DOI] [PubMed] [Google Scholar]
- 207.Taylor NE, Pei JZ, Zhang J, Vlasov KY, Davis T, Taylor E, et al. The role of glutamatergic and dopaminergic neurons in the periaqueductal gray/dorsal raphe: Separating analgesia and anxiety. eNeuro 2019, 6: ENEURO.0018–ENEURO.0018.2019. [DOI] [PMC free article] [PubMed]
- 208.Buhle JT, Kober H, Ochsner KN, Mende-Siedlecki P, Weber J, Hughes BL, et al. Common representation of pain and negative emotion in the midbrain periaqueductal gray. Soc Cogn Affect Neurosci. 2013;8:609–616. doi: 10.1093/scan/nss038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Fukunaga Y, Kishioka S. Enkephalinergic neurons in the periaqueductal gray and morphine withdrawal. Jpn J Pharmacol. 2000;82:175–180. doi: 10.1254/jjp.82.175. [DOI] [PubMed] [Google Scholar]
- 210.Fields HL. The doctor's dilemma: Opiate analgesics and chronic pain. Neuron. 2011;69:591–594. doi: 10.1016/j.neuron.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Fields HL, Margolis EB. Understanding opioid reward. Trends Neurosci. 2015;38:217–225. doi: 10.1016/j.tins.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Volkow ND, McLellan AT. Opioid abuse in chronic pain——misconceptions and mitigation strategies. N Engl J Med. 2016;374:1253–1263. doi: 10.1056/NEJMra1507771. [DOI] [PubMed] [Google Scholar]
- 213.Eippert F, Bingel U, Schoell ED, Yacubian J, Klinger R, Lorenz J, et al. Activation of the opioidergic descending pain control system underlies placebo analgesia. Neuron. 2009;63:533–543. doi: 10.1016/j.neuron.2009.07.014. [DOI] [PubMed] [Google Scholar]
- 214.Bagley EE, Ingram SL. Endogenous opioid peptides in the descending pain modulatory circuit. Neuropharmacology 2020, 173: 108131. [DOI] [PMC free article] [PubMed]
- 215.Castilho VM, Borelli KG, Brandão ML, Nobre MJ. Anxiety-like symptoms induced by morphine withdrawal may be due to the sensitization of the dorsal periaqueductal grey. Physiol Behav. 2008;94:552–562. doi: 10.1016/j.physbeh.2008.03.014. [DOI] [PubMed] [Google Scholar]
- 216.Shaham Y, Rajabi H, Stewart J. Relapse to heroin-seeking in rats under opioid maintenance: The effects of stress, heroin priming, and withdrawal. J Neurosci. 1996;16:1957–1963. doi: 10.1523/JNEUROSCI.16-05-01957.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Paul ED, Johnson PL, Shekhar A, Lowry CA. The Deakin/Graeff hypothesis: Focus on serotonergic inhibition of panic. Neurosci Biobehav Rev. 2014;46(Pt 3):379–396. doi: 10.1016/j.neubiorev.2014.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Heinsbroek JA, Neuhofer DN, Griffin WC, Siegel GS, Bobadilla AC, Kupchik YM, et al. Loss of plasticity in the D2-accumbens pallidal pathway promotes cocaine seeking. J Neurosci. 2017;37:757–767. doi: 10.1523/JNEUROSCI.2659-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Hurd YL, Pontén M. Cocaine self-administration behavior can be reduced or potentiated by the addition of specific dopamine concentrations in the nucleus accumbens and amygdala using in vivo microdialysis. Behav Brain Res. 2000;116:177–186. doi: 10.1016/S0166-4328(00)00271-0. [DOI] [PubMed] [Google Scholar]
- 220.Kim ES, Lattal KM. Context-dependent and context-independent effects of D1 receptor antagonism in the basolateral and central amygdala during cocaine self-administration. eNeuro 2019, 6: ENEURO.0203–ENEURO.0219.2019. [DOI] [PMC free article] [PubMed]
- 221.Harris GC, Aston-Jones G. Involvement of D2 dopamine receptors in the nucleus accumbens in the opiate withdrawal syndrome. Nature. 1994;371:155–157. doi: 10.1038/371155a0. [DOI] [PubMed] [Google Scholar]
- 222.Sobieraj JC, Kim A, Fannon MJ, Mandyam CD. Chronic wheel running-induced reduction of extinction and reinstatement of methamphetamine seeking in methamphetamine dependent rats is associated with reduced number of periaqueductal gray dopamine neurons. Brain Struct Funct. 2016;221:261–276. doi: 10.1007/s00429-014-0905-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Nikulina EM, Marchand JE, Kream RM, Miczek KA. Behavioral sensitization to cocaine after a brief social stress is accompanied by changes in fos expression in the murine brainstem. Brain Res. 1998;810:200–210. doi: 10.1016/S0006-8993(98)00925-1. [DOI] [PubMed] [Google Scholar]
- 224.Miczek KA, Nikulina E, Kream RM, Carter G, Espejo EF. Behavioral sensitization to cocaine after a brief social defeat stress: C-fos expression in the PAG. Psychopharmacology (Berl) 1999;141:225–234. doi: 10.1007/s002130050829. [DOI] [PubMed] [Google Scholar]
- 225.Markou A, Koob GF. Postcocaine anhedonia. An animal model of cocaine withdrawal. Neuropsychopharmacology 1991, 4: 17–26. [PubMed]
- 226.Nirogi R, Mohammed AR, Shinde AK, Ravella SR, Bogaraju N, Subramanian R, et al. Discovery and Development of 3-(6-Chloropyridine-3-yloxymethyl)-2-azabicyclo[3.1.0]hexane Hydrochloride (SUVN-911): A Novel, Potent, Selective, and Orally Active Neuronal Nicotinic Acetylcholine α4β2 Receptor Antagonist for the Treatment of Depression. J Med Chem 2020, 63: 2833–2853. [DOI] [PubMed]
- 227.Gotti C, Zoli M, Clementi F. Brain nicotinic acetylcholine receptors: Native subtypes and their relevance. Trends Pharmacol Sci. 2006;27:482–491. doi: 10.1016/j.tips.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 228.Rose JE, Behm FM, Salley AN, Bates JE, Coleman RE, Hawk TC, et al. Regional brain activity correlates of nicotine dependence. Neuropsychopharmacology. 2007;32:2441–2452. doi: 10.1038/sj.npp.1301379. [DOI] [PubMed] [Google Scholar]
- 229.Corrigall WA, Coen KM, Adamson KL. Self-administered nicotine activates the mesolimbic dopamine system through the ventral tegmental area. Brain Res. 1994;653:278–284. doi: 10.1016/0006-8993(94)90401-4. [DOI] [PubMed] [Google Scholar]
- 230.Benowitz NL. Nicotine Addiction. N Engl J Med. 2010;362:2295–2303. doi: 10.1056/NEJMra0809890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Benowitz NL. Neurobiology of nicotine addiction: Implications for smoking cessation treatment. Am J Med. 2008;121:S3–S10. doi: 10.1016/j.amjmed.2008.01.015. [DOI] [PubMed] [Google Scholar]
- 232.Dani JA, De Biasi M. Cellular mechanisms of nicotine addiction. Pharmacol Biochem Behav. 2001;70:439–446. doi: 10.1016/S0091-3057(01)00652-9. [DOI] [PubMed] [Google Scholar]
- 233.Nakamura M, Jang IS. Presynaptic nicotinic acetylcholine receptors enhance GABAergic synaptic transmission in rat periaqueductal gray neurons. Eur J Pharmacol. 2010;640:178–184. doi: 10.1016/j.ejphar.2010.04.057. [DOI] [PubMed] [Google Scholar]
- 234.Genzen JR, McGehee DS. Short- and long-term enhancement of excitatory transmission in the spinal cord dorsal horn by nicotinic acetylcholine receptors. Proc Natl Acad Sci USA. 2003;100:6807–6812. doi: 10.1073/pnas.1131709100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Fu WM, Liou HC, Chen YH. Nerve terminal currents induced by autoreception of acetylcholine release. J Neurosci. 1998;18:9954–9961. doi: 10.1523/JNEUROSCI.18-23-09954.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Guo JZ, Tredway TL, Chiappinelli VA. Glutamate and GABA release are enhanced by different subtypes of presynaptic nicotinic receptors in the lateral geniculate nucleus. J Neurosci. 1998;18:1963–1969. doi: 10.1523/JNEUROSCI.18-06-01963.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Shin JH, Adrover MF, Alvarez VA. Distinctive modulation of dopamine release in the nucleus accumbens shell mediated by dopamine and acetylcholine receptors. J Neurosci. 2017;37:11166–11180. doi: 10.1523/JNEUROSCI.0596-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Xiao C, Zhou CY, Jiang JH, Yin C. Neural circuits and nicotinic acetylcholine receptors mediate the cholinergic regulation of midbrain dopaminergic neurons and nicotine dependence. Acta Pharmacol Sin. 2020;41:1–9. doi: 10.1038/s41401-019-0299-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Woolf NJ, Harrison JB, Buchwald JS. Cholinergic neurons of the feline pontomesencephalon. II. Ascending anatomical projections. Brain Res. 1990;520:55–72. doi: 10.1016/0006-8993(90)91691-9. [DOI] [PubMed] [Google Scholar]
- 240.Baddick CG, Marks MJ. An autoradiographic survey of mouse brain nicotinic acetylcholine receptors defined by null mutants. Biochem Pharmacol. 2011;82:828–841. doi: 10.1016/j.bcp.2011.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Umana IC, Daniele CA, Miller BA, Abburi C, Gallagher K, Brown MA, et al. Nicotinic modulation of descending pain control circuitry. Pain. 2017;158:1938–1950. doi: 10.1097/j.pain.0000000000000993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Depaulis A, Morgan MM, Liebeskind JC. GABAergic modulation of the analgesic effects of morphine microinjected in the ventral periaqueductal gray matter of the rat. Brain Res. 1987;436:223–228. doi: 10.1016/0006-8993(87)91665-9. [DOI] [PubMed] [Google Scholar]
- 243.Beitz AJ, Shepard RD. The midbrain periaqueductal gray in the rat. II. A Golgi analysis. J Comp Neurol 1985, 237: 460–475. [DOI] [PubMed]
- 244.Siuciak JA, Lewis DR, Wiegand SJ, Lindsay RM. Antidepressant-like effect of brain-derived neurotrophic factor (BDNF) Pharmacol Biochem Behav. 1997;56:131–137. doi: 10.1016/S0091-3057(96)00169-4. [DOI] [PubMed] [Google Scholar]
- 245.Semba J, Mataki C, Yamada S, Nankai M, Toru M. Antidepressantlike effects of chronic nicotine on learned helplessness paradigm in rats. Biol Psychiatry. 1998;43:389–391. doi: 10.1016/S0006-3223(97)00477-0. [DOI] [PubMed] [Google Scholar]
- 246.Tizabi Y, Overstreet DH, Rezvani AH, Louis VA, Clark E, Jr, Janowsky DS, et al. Antidepressant effects of nicotine in an animal model of depression. Psychopharmacology (Berl) 1999;142:193–199. doi: 10.1007/s002130050879. [DOI] [PubMed] [Google Scholar]
- 247.Tizabi Y, Rezvani AH, Russell LT, Tyler KY, Overstreet DH. Depressive characteristics of FSL rats: Involvement of central nicotinic receptors. Pharmacol Biochem Behav. 2000;66:73–77. doi: 10.1016/S0091-3057(00)00236-7. [DOI] [PubMed] [Google Scholar]
- 248.Ferguson SM, Brodkin JD, Lloyd GK, Menzaghi F. Antidepressant-like effects of the subtype-selective nicotinic acetylcholine receptor agonist, SIB-1508Y, in the learned helplessness rat model of depression. Psychopharmacology (Berl) 2000;152:295–303. doi: 10.1007/s002130000531. [DOI] [PubMed] [Google Scholar]
- 249.Covey LS, Glassman AH, Stetner F. Depression and depressive symptoms in smoking cessation. Compr Psychiatry. 1990;31:350–354. doi: 10.1016/0010-440X(90)90042-Q. [DOI] [PubMed] [Google Scholar]
- 250.Stachniak TJ, Ghosh A, Sternson SM. Chemogenetic synaptic silencing of neural circuits localizes a hypothalamus→midbrain pathway for feeding behavior. Neuron. 2014;82:797–808. doi: 10.1016/j.neuron.2014.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Nectow AR, Schneeberger M, Zhang HX, Field BC, Renier N, Azevedo E, et al. Identification of a brainstem circuit controlling feeding. Cell. 2017;170:429–442.e11. doi: 10.1016/j.cell.2017.06.045. [DOI] [PubMed] [Google Scholar]
- 252.Ma S, Bonaventure P, Ferraro T, Shen PJ, Burazin TC, Bathgate RA, et al. Relaxin-3 in GABA projection neurons of nucleus incertus suggests widespread influence on forebrain circuits via G-protein-coupled receptor-135 in the rat. Neuroscience. 2007;144:165–190. doi: 10.1016/j.neuroscience.2006.08.072. [DOI] [PubMed] [Google Scholar]
- 253.Smith CM, Ryan PJ, Hosken IT, Ma S, Gundlach AL. Relaxin-3 systems in the brain——the first 10 years. J Chem Neuroanat. 2011;42:262–275. doi: 10.1016/j.jchemneu.2011.05.013. [DOI] [PubMed] [Google Scholar]
- 254.Onaka T, Takayanagi Y. Role of oxytocin in the control of stress and food intake. J Neuroendocrinol. 2019;31:e12700. doi: 10.1111/jne.12700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Volkow ND, Baler RD. NOW vs LATER brain circuits: Implications for obesity and addiction. Trends Neurosci. 2015;38:345–352. doi: 10.1016/j.tins.2015.04.002. [DOI] [PubMed] [Google Scholar]
- 256.Volkow ND, Wise RA, Baler R. The dopamine motive system: Implications for drug and food addiction. Nat Rev Neurosci. 2017;18:741–752. doi: 10.1038/nrn.2017.130. [DOI] [PubMed] [Google Scholar]
- 257.Schultz W. Predictive reward signal of dopamine neurons. J Neurophysiol. 1998;80:1–27. doi: 10.1152/jn.1998.80.1.1. [DOI] [PubMed] [Google Scholar]
- 258.Deshmukh RR, Sharma PL. Stimulation of accumbens shell cannabinoid CB(1) receptors by noladin ether, a putative endocannabinoid, modulates food intake and dietary selection in rats. Pharmacol Res. 2012;66:276–282. doi: 10.1016/j.phrs.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 259.Guzmán-Rodríguez S, Chávez-Reyes J, Vázquez-León P, Soriano-Ursúa MA, Rosalez MN, Allende G, et al. 1-boc-piperidine-4-carboxaldehyde prevents binge-eating behaviour and anxiety in rats. Pharmacology. 2021;106:305–315. doi: 10.1159/000513376. [DOI] [PubMed] [Google Scholar]
- 260.Parsons LH, Hurd YL. Endocannabinoid signalling in reward and addiction. Nat Rev Neurosci. 2015;16:579–594. doi: 10.1038/nrn4004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Pomorska DK, do-Rego JC, do-Rego JL, Zubrzycka M, Janecka A. Opioid and cannabinoid system in food intake. Curr Pharm Des 2016, 22: 1361–1370. [DOI] [PubMed]
- 262.Kirkham TC, Williams CM. Synergistic efects of opioid and cannabinoid antagonists on food intake. Psychopharmacology (Berl) 2001;153:267–270. doi: 10.1007/s002130000596. [DOI] [PubMed] [Google Scholar]
- 263.Solinas M, Goldberg SR. Motivational effects of cannabinoids and opioids on food reinforcement depend on simultaneous activation of cannabinoid and opioid systems. Neuropsychopharmacology. 2005;30:2035–2045. doi: 10.1038/sj.npp.1300720. [DOI] [PubMed] [Google Scholar]
- 264.Befort K. Interactions of the opioid and cannabinoid systems in reward: Insights from knockout studies. Front Pharmacol. 2015;6:6. doi: 10.3389/fphar.2015.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]