Abstract
Oral cancer is one of the most common cancers in our population. These cancers are drained by the nodes located in the cervical region which are easily accessible for clinical examination. However, these cervical nodes may also be enlarged due to various other nonmalignant causes. Hence, accuracy of clinical examination and ultrasound screening for cervical lymph nodes is invaluable. The aims of this study are (1) to correlate the clinical, radiological, and pathological results of cervical lymph nodes in patients with oral malignancy and (2) to calculate the accuracy of clinical and radiological methods in detecting malignant cervical lymph nodes. A prospective observational study was undertaken from January 2016 to December 2016 amounting to a total of 76 patients. All patients diagnosed with squamous cell carcinoma of the oral cavity and having a palpable neck node(s), who were planned for surgery were included. Clinical examination, ultrasonographic (USG) screening of the neck, and the final histopathology reports were noted. The sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) for radiological screening by ultrasound were 90.5%, 90.9%, 79.2%, and 96.2%, respectively, and that for clinical examination were 61.9%, 69.1%, 43.3%, and 82.6%, respectively. Area under the curve (AUC) for ultrasound screening was 0.907, and the AUC for clinical examination was 0.655. Ultrasonography is a reliable, cost-effective imaging method in the assessment of malignant cervical nodes in patients with oral cancer, which is to be used along with clinical palpation for improving the accuracy of clinical staging and surgical planning preoperatively.
Keywords: Ultrasonography , Neck nodes, Neck dissection, Squamous cell carcinoma
Introduction
Oral cancer ranks among the top three types of cancer in the country and accounts for over 30% of all cancers. Almost 80,000 new cases are diagnosed annually, in the adult population with mean age of occurrence around 55 years. Oral cancers formed the majority of the head and neck cancers with a predilection for tongue, except in rural males, in whom the pharynx was the predominant subsite [1, 2]. Approximately 95% of all oral cancers are of the squamous cell variety [3]. Treatment modalities include surgery, radiotherapy (RT), chemotherapy, targeted therapy, and combinations thereof [4–7]. The extent of cancer at the time of diagnosis is a key factor which is used to plan treatment and to assess the chance of a successful outcome. Accurate clinical staging, hence, is important for patient counselling, treatment planning, and prognostication. Various staging systems are designed to compare similarly staged patients and provide useful information for treatment decisions as well as to rationally design clinical trials. They are also used to identify groups of patients for selective therapeutic interventions and comparisons of outcomes across populations. Location, as well as the extent of the primary tumor, is usually known with a sufficient degree of precision, by clinical and radiological examination. Most of the uncertainties about clinical staging are related to the regional lymph node status.
Disparities between pathological and clinical nodal staging data for head and neck carcinoma have been described in the literature by several authors [4–7]. It is well known that palpation is an inaccurate technique to stage cancer in the neck. The decreased reliability of palpation is because the nodes can be deeply seated, neck can be short, and fatty, and there are some very small nodes which are metastatic and not picked up by palpation. Clinical assessment by palpation has been shown to be 60–70% accurate, but the incorporation of radiological evaluation may improve the accuracy. In this study, we compared the efficacy of palpation and USG in the preoperative evaluation of cervical lymph node metastasis of patients with oral squamous cell carcinoma. Staging was done based on AJCC staging guidelines 7th edition.
Methodology
This study was approved by the Institutional Review Board, and patient confidentiality was maintained using identifiers, and a password-protected access to the data for a limited number of individuals was maintained to ensure protection of privacy.
This prospective observational study was conducted in the Surgical Oncology Department of Regional Cancer Centre, Trivandrum, from January 2016 to December 2016. All patients diagnosed with squamous cell carcinoma of the oral cavity with clinically palpable neck node(s) and planned for surgery were included in the study. Patients with a history of any previous neck surgeries, second malignancy, radiation to the neck, systemic chemotherapy, synchronous primary, nonsquamous cell histology, history of thyroid surgery, and patients unfit for general anesthesia were excluded. All these patients underwent a thorough physical examination and biopsy of the primary. Fine-needle aspiration cytology was not used to diagnose the suspicious nodes as malignant. Complete blood count; renal, liver, and pulmonary function tests; and chest radiography was done as part of routine workup for surgery.
Palpation was performed for all these patients by a single trained examiner who was blinded for the imaging investigations, and the details of examination were recorded in a prescribed format. Palpation of the neck was done thoroughly; first from the back, then from the front of the neck, after exposing the neck down to the level of the clavicle bilaterally to determine the presence or absence of enlarged cervical lymph node. The site, size, location, consistency, and mobility of each palpable node were noted. The node to be examined was localized; the mediolateral and anteroposterior dimensions were measured. The site was determined according to the description of level I–V. The size criteria to determine a node as metastatic with palpatory method was size > 10 mm, hard in consistency, and restricted mobility/fixity to the underlying or overlying structure.. Any node which satisfied these criteria was noted as clinical nodal metastasis. The primary tumor and lymph node status was classified according to the International TNM staging system (AJCC 7th edition).
Ultrasonogram (USG) of neck is routinely performed in our department for all cases of oral cancer planned for surgery. For the purpose of study, all scans were performed by a single radiologist who was blinded about palpation findings, using a linear probe of 9-MHz frequency (GE Health Care-Logiq E9). The radiologist examined the neck longitudinally and transversely in a continuous sweep technique from thoracic inlet and scalenus muscle to submental and parotid regions. The characteristics of node assessed for determination of metastasis were size, echogenicity (hyper-, iso-, or hypo-echoic or heterogeneous appearance), surface border (regular, irregular), shape (oblong, oval, or spherical), nodal hilum (present or absent), and nodal blood flow pattern. The findings were recorded in a prescribed format and radiological staging done according to the AJCC 7th edition.
Treatment plan was formulated as per the demand consisting of primary tumor excision, modified radical neck dissection if clinical and imaging wise there was a suspicious/malignant node, and supraomohyoid neck dissection if no nodes were detected on clinical and ultrasound examination and reconstruction of primary site if necessary. Subsequently excised neck specimens along with primary tumor were packed separately. Further neck dissection specimen was cut into levels I–V labelled appropriately and sent to a blinded pathologist. Tumor resection specimen was checked for dimensions, adequacy of margins, grade of malignancy, presence of high-risk features, and premalignancy in surrounding mucosa. All nodes in the neck dissection specimen were examined in multiple sections for metastatic deposits irrespective of the size of nodes. Immunohistochemistry was not utilized. The number and size of lymph nodes with metastatic deposits and presence of extracapsular spread (ECS) were noted down. ECS on clinical examination was determined if the node was fixed (underlying/overlying structure) or matted. Features suggestive of ECS on USG were perinodal edema, irregular margin, matting, and cystic appearance. Intraoperatively, the lymph nodes noted to have ECS were sent separately for histopathological examination.
Statistical Analysis
A data sheet was made using Microsoft Excel version 16, after which Statistical Package for Social Sciences (SPSS Inc. Released 2015, version 23.0, Chicago) was used to analyze the data collected. Categorical variables were described using frequencies and percentages. The findings of clinical examination and USG were compared with that of postoperative histopathological findings, and the results were evaluated statistically for sensitivity, specificity, positive predictive value, negative predictive value, and accuracy. For all tests, a two-sided P ≤ 0.05 was considered statistically significant. Receiver operating characteristics area under the curve (ROC-AUC) was generated and compared to identify the association between radiological and clinical nodal status.
Results
During the 12-month study period, we recruited 76 patients. There were 52 males (68.4%) and 24 females (31.6%). Majority were between 30 and 60 years (69.7%). The baseline characteristics of the patients are shown in Table 1. Of the patients, 64.5% had primary in tongue followed by 23.7% in buccal mucosa, 5.3% in floor of mouth and lower alveolus, and 1.3% in upper alveolus (Table 2). About 61.5% of males and 70.8% of females had primary in tongue.
Table 1.
Baseline characteristics (N = 76)
| Variable | Number | Percentage |
|---|---|---|
| Males | 52 | 68.4 |
| Females | 24 | 31.6 |
| Age < 60 years | 53 | 69.7 |
| Age > 60 years | 23 | 30.3 |
| Comorbidities | ||
| Diabetes mellitus | 36 | 47.4 |
| Hypertension | 35 | 46.1 |
| Chronic obstructive pulmonary disease | 8 | 10.5 |
| Cardiac disease | 16 | 21.1 |
| Thyroid dysfunction | 7 | 9.2 |
| Chronic smoking | 35 | 46.1 |
| Chewing tobacco (pan) | 43 | 56.6 |
| Chronic alcohol consumption | 25 | 32.8 |
Table 2.
Primary site of the tumor
| Primary site | Number | Percentage |
|---|---|---|
| Tongue | 49 | 64.5 |
| Buccal mucosa | 18 | 23.7 |
| Floor of the mouth | 4 | 5.2 |
| Upper alveolus | 1 | 5.2 |
| Lower alveolus | 4 | 1.3 |
Clinically around 12% were staged I, and pathologically, the figure increased to 20%. Clinically, most of the patients (74.6%) had stage II/III carcinomas which after pathological staging remained almost the same (73.4%) (Table 3). Table 4 shows the level of the node detected by each method by a blinded examiner, blinded radiologist, and a blinded pathologist. The diagnostic accuracy of clinical and radiological examination is shown in Table 5. The sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) for radiological screening by ultrasound were 90.5%, 90.9%, 79.2%, and 96.2%, respectively. The sensitivity, specificity, PPV, and NPV for clinical examination were 61.9%, 69.1%, 43.3%, and 82.6% respectively.
Table 3.
Tumor size and staging
| T1 | T2 | T3 | T4 | |
| Clinical stage | 12 (15.8) | 33 (43.4) | 24 (31.6) | 7 (9.2) |
| Pathological stage | 13 (17.11) | 46 (60.5) | 17 (22.4) | 0 |
| Stage 1 | Stage 2 | Stage 3 | Stage 4 | |
| Clinical stage | 9 (11.84) | 25 (32.89) | 32 (42.10) | 10 (13.16) |
| Pathological stage | 15 (19.74) | 35 (46.05) | 20 (26.32) | 6 (7.89) |
Table 4.
The level of nodes detected by each method separately by a blinded examiner, blinded radiologist, and blinded pathologist
| Characteristic | CE (clinical examination) | USG/radiological examination | PE (pathological examination) |
|---|---|---|---|
| Level IA | 18 | 20 | 19 |
| Level IB | 17 | 19 | 20 |
| Level II | 10 | 12 | 13 |
| Level III | 6 | 8 | 9 |
| Level IV | 5 | 6 | 8 |
| Level V | 2 | 4 | 4 |
| Total | 58 | 69 | 73 |
Table 5.
Diagnostic accuracy of clinical and radiological examination
| Variables | Sensitivity | Specificity | PPV | NPV | Accuracy |
|---|---|---|---|---|---|
| Radiological node | 90.5 | 90.9 | 79.2 | 96.2 | 31.6 |
| Clinical node | 61.9 | 69.1 | 43.3 | 82.6 | 39.5 |
Majority of the neck dissection specimens (65.7%) which did not have any palpable nodes in the neck correlated radiologically (N0 neck) and were proven by histopathology as no evidence of metastasis.
Twelve nodes were labelled as having extracapsular extension on clinical examination, and 14 nodes were reported as having ECS on ultrasonogram. On histopathological examination, all 14 nodes noted to have ECS on imaging were noted to have ECS.
Receiver operating characteristics (ROC) curve was generated to compare the performance of clinical and radiological (ultrasonography) screening accuracy (Fig. 1). The AUC for ultrasound screening was 0.907, and the AUC for clinical examination was 0.655.
Fig. 1.
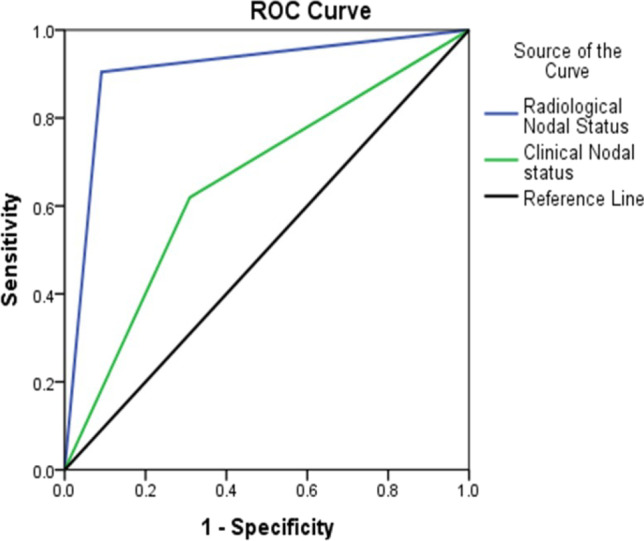
Receiver operating characteristics (ROC) curve
Discussion
Most of our patients were in the age group of 30 to 60 years. As expected, males were more affected with oral cancer as compared to females. Tongue carcinoma was the most common oral cancer in our study which is the most common site identified in the state as well [8].
Clinical and Radiological Examination
According to D G John et al., the sensitivity of clinical palpation was 61.9%, the specificity was 69.1%, while the accuracy was 39.5%. PPV for this method was 43.3% and NPV was 82.6%. Ultrasound did not add significantly to the information obtainable by simple neck palpation in this group of patients. These figures are in accordance with most reported series for the results of clinical palpation [9]. In our study, ultrasonography showed sensitivity of 90.5% and specificity of 90.9% with an accuracy of 31.6%. PPV for this modality was 79.2% and NPV ranged at 96.2%. The probable causes for a low specificity and sensitivity of clinical examination is probably due to a thick neck with a lot of adipose tissue and a very muscular, well-developed sterocleidomastoid muscle which can confound the palpatory findings in the neck.
Griffith et al. showed that the addition of neck ultrasonography resulted in a change in TNM staging in 18% of patients in their study. They suggested that ultrasonography is equally effective as CT in the detection of malignant lymph nodes in the neck. The more accurate preoperative staging with neck ultrasonography allows a more consistent selection of patients for different treatment protocols [10].
Safaan et al. reported a sensitivity of 71.43%, specificity of 75.86%, and an accuracy of 72.7% for palpation of lymph nodes and a sensitivity of 97.1%, specificity of 93%, and accuracy of 95.96% for ultrasound scanning [11]. Steinkamp et al. reported 95% accuracy of the L/S (long to short axis) ratio with ultrasonography. A ratio more than 2 is in favor of benign node, and a ratio less than 2 is in favor of metastatic node. The accuracy was 37% for the long axis method and 86% for the transverse diameter method alone [12]. They noted the decrease of false positive results from 63 to 5% using the L/S ratio. Sarvanan et al. have showed similar sensitivity (94.44%) and higher specificity (100%) than that reported by Steinkamp et al. [13]. In a more recent study, Sureshkannan et al. presented a sensitivity of 85.7% and a specificity of 90% for ultrasound scanning, while the clinical examination presented sensitivity 68.7% and specificity 87.5% [3]. Geetha et al. reported the sensitivity in detection of cervical lymph nodes to be 83% for palpation and 100% for ultrasound scanning [14]. Ultrasonography (USG) is deemed better than CT and MRI in many aspects including the affordability for the patients, availability, and no exposure to radiation. CT and MRI detect malignant nodes based on certain characteristics viz. size, shape, peripheral rim enhancement, and central necrosis, but USG can detect shape, echogenic pattern, peripheral vascularity, and calcification of lymph nodes which is important to differentiate malignant nodes from benign nodes [15]. The detection of nodes in the submental and submandibular regions is found to be superior with USG whereas CT and MRI studies have occasionally been impaired by artifacts from bones and dental amalgam restorations [16].
On contrast-enhanced CT, the reported sensitivity and specificity in the evaluation of metastatic cervical lymph nodes are 90.2% and 93.9%, respectively [10]. On high-resolution MRI, the sensitivity and specificity in assessing metastatic nodes are 86% and 94%, respectively [16].
Our study supports the fact [17] that extranodal extension can be detected accurately with an ultrasonogram (Table 6).
Table 6.
Extranodal extension detected by all 3 modalities
| Number | |
|---|---|
| Clinical examination | 12 |
| Ultrasonographic examination | 14 |
| Histopthological examination | 14 |
Conclusions
Ultrasonography is a safe and reliable imaging method in the assessment of malignant cervical nodes in patients with oral cancer, which can be used along with palpation for improving the accuracy of clinical staging preoperatively. Conventional ultrasound is the recommended imaging method for lymph node diseases with the advantages of high resolution, real-time evaluation, and relative low costs and should be the done before surgical planning as a node suggestive of metastasis would mandate for a comprehensive neck dissection whereas a N0 neck can be adequately treated with a selective neck dissection. As compared to the computed tomogram and PET CT, USG is much cheaper and does not require an expensive setup.
Declarations
Conflict of Interest
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Elango JK, Gangadharan P, Sumithra S, Kuriakose MA. Trends of head and neck cancers in urban and rural India. Asian Pac J Cancer Prev. 2006;7(1):108–112. [PubMed] [Google Scholar]
- 2.Misra S, Chaturvedi A, Misra NC. Management of gingivobuccal complex cancer. Ann R CollSurg Engl. 2008;90:546–553. doi: 10.1308/003588408X301136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sureshkannan P, Vijayprabhu John R. Role of ultrasound in detection of metastatic neck nodes in patients with oral cancer. Indian J Dent Res. 2011;22:419–23. doi: 10.4103/0970-9290.87064. [DOI] [PubMed] [Google Scholar]
- 4.Cmelak AJ. Current issues in combined modality therapy in locally advanced head and neck cancer. Crit Rev OncolHematol. 2012;84:261–273. doi: 10.1016/j.critrevonc.2012.04.004(ok). [DOI] [PubMed] [Google Scholar]
- 5.Kavanagh BD, Haffty BG, Tepper JE. Radiation oncology: a snapshot in time. J ClinOncol. 2014;32:2825–6. doi: 10.1200/JCO.2014.57.3071(0k). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pryor DI, Solomon B, Porceddu SV. The emerging era of personalized therapy in squamous cell carcinoma of the head and neck. Asia Pac J ClinOncol. 2011;7:236–51. doi: 10.1111/j.1743-7563.2011.01420.(ok). [DOI] [PubMed] [Google Scholar]
- 7.Thariat J, Hannoun-Levi JM, Sun Myint A, Vuong T, Gerard JP. Past, present, and future of radiotherapy for the benefit of patients. Nat Rev ClinOncol. 2013;10:52–60. doi: 10.1038/nrclinonc.2012.203(ok). [DOI] [PubMed] [Google Scholar]
- 8.Iype EM, Pandey M, Mathew A, Thomas G, Sebastian P, Nair MK. Oral cancer among patients under the age of 35 years. J Postgrad Med. 2001;47:171. [PubMed] [Google Scholar]
- 9.John DG, Anaes FC, Williams SR, Ahuja A, Evans R, To KF, et al. Palpation compared with ultrasound in the assessment of malignant cervical lymph nodes. J Otol Laryngol. 1993;107:821–3. doi: 10.1017/S002221510012451X. [DOI] [PubMed] [Google Scholar]
- 10.Griffith JF, Chan AC, Ahuja AT, Leung SF, Chow LT, Chung SC, et al. Neckultrasound in staging squamous oesophageal carcinoma - a high yield technique. ClinRadiol. 2000;55:696–701. doi: 10.1053/crad.2000.0502. [DOI] [PubMed] [Google Scholar]
- 11.Saafan ME, Elguindy AS, Abdel-Aziz MF, Abdel-Rahman Younes A, Albirmawy OA, et al. Assessment of cervical lymph nodes in squamous cell carcinoma of the head and neck. Surgery Curr Res. 2013;3:145. doi: 10.4172/2161-1076.1000145(correct). [DOI] [Google Scholar]
- 12.Steinkamp HJ, Cornehl M, Hosten N, Pegios W, Vogl T, et al. Cervical lymphadenopathy: ratio of long- to short-axis diameter as a predictor ofmalignancy. Br J Radiol. 1995;68:266–270. doi: 10.1259/0007-1285-68-807-266. [DOI] [PubMed] [Google Scholar]
- 13.Sarvanan K, Bapuraj JR, Sharma SC, RadotraBD Khandelwal N, et al. Computed tomography and ultrasonographic evaluation of metastatic cervical lymph nodes with surgico clinic pathologic correlation. J Laryngol Otol. 2002;116:194–199. doi: 10.1258/0022215021910519. [DOI] [PubMed] [Google Scholar]
- 14.Geetha NT, Hallur N, Goudar G, Sikkerimath BC, Gudi SS. Cervical lymph node metastasis in oral squamous carcinoma preoperative assessment and histopathology after neck dissection. J Maxillofac Oral Surg. 2010;9:42–47. doi: 10.1007/s12663-010-0013-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Esen G. Ultrasound of Superficial Lymph Nodes. Eur J Radiol. 2006;58:345–359. doi: 10.1016/j.ejrad.2005.12.039. [DOI] [PubMed] [Google Scholar]
- 16.Chikui T, Yonetsu K, Nakamura T. Multivariate feature analysis of sonographic findings of metastatic cervical Lymph Nodes: Contribution of Blood flow features revealed by power doppler sonography for predicting metastasis. Am J Neuroradiol. 2000;21:561–567. [PMC free article] [PubMed] [Google Scholar]
- 17.Qualliotine JR, Coquia SF, Hamper UM, Fakhry C. Association of ultrasound characteristics with extranodal extension in metastatic papillary thyroid carcinoma. JAMA Otolaryngol Head Neck Surg. 2016;142(3):263–269. doi: 10.1001/jamaoto.2015.3558. [DOI] [PubMed] [Google Scholar]


