Abstract
Purpose
Different commercial human embryo culture mediums can alter embryo quality and change birthweight. One component that could be contributing to variations but is not widely investigated is human serum albumin (HSA). HSA plays a multitude of roles during embryo culture and is a carrier for molecules including lipids. It remains unclear if lipid composition of HSA varies among commercial products and its effects on embryo quality, implantation, and fetal outcomes are relatively unknown.
Methods
Utilizing a mouse model of embryo culture, we cultured zygotes until the blastocyst stage (72-h culture) in G1/G2 containing either Vitrolife HSA, Sage HSA, or Recombinant HSA at 10%. Blastocyst quality (development, total cell number, superoxide generation), blastocyst lipid content (neutral lipids, non-esterified fatty acids, phospholipids, and triglycerides), implantation, and fetal lengths and weights were assessed. Fatty acid quantification of HSA source was assessed by standard thin-layer chromatography.
Results
Sage HSA had the greatest fatty acid composition, with an eightfold increase in saturated fatty acids. This coincided with reduced blastocyst development, increased superoxide generation, neutral lipids and triglycerides levels of blastocysts, and decreased implantation rates (p < 0.05). Unexpectedly, while Recombinant HSA had the lowest overall lipids it had 70-fold increase in palmitoleic acid and the lowest fetal weights (p < 0.05).
Conclusion
Indicates the importance of a balance between different types/amount of lipids, and an “optimal ratio” required for embryo and fetal development. Therefore, the lipid content of HSA should be considered when choosing a suitable HSA source for use in clinical IVF.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10815-021-02255-5.
Keywords: In vitro fertilization, Assisted reproduction technology, Blastocyst, Fetal weight, Fatty acids
Introduction
Human embryo culture media have evolved from simple compositions originally used for somatic cell culture to the specialized formulations seen today [1–4]. This evolution has allowed for the clinical in vitro fertilization (IVF) laboratory to extend embryo culture until the blastocyst stage, improving pregnancy rates and fetal outcomes [4, 5]. Although commercial IVF culture mediums tend to have similar compositions of key nutrients, including amino acids and carbohydrates [6], reports have shown that embryos grown in different human commercial culture media can result in infants with altered birthweight, with size effects persisting until 2 years [7–10]. The reasons for these changes in birthweight are not yet known; however, variations in the concentrations of key nutrients (carbohydrates, amino acids, vitamins) across commercial products have been suggested as a possible reason [11]. One component of commercial culture media that could be contributing to the variations in birthweight but is not widely investigated is the source of albumin, through the addition of human serum albumin (HSA). Only one study to date has assessed the variations to birthweight due to changes in HSA source, finding birthweight z scores altered (z scores G1-PLUS.5 versus G1.5 = 0.28 ± 1.12 vs 0.09 ± 1.15, P = 0.04) from implanted embryos grown in culture media that already had HSA added (G1-PLUS.5) compared with the addition of HSA in the laboratory (G1.5 with the addition of Vitrolife HSA) [12].
HSA in embryo culture medium plays a multitude of roles, acting as a pH buffer, carrier of growth-promoting substances, a scavenger for toxins and metals, and has a functional role as a surfactant which facilitates embryo manipulation [13]. However, HSA used in embryo culture medium is human serum derived and therefore its composition is poorly defined due to its heterogeneous sources and variable biological properties. Albumin itself is a carrier for hormones, growth factors, metals, enzymes, and also energy substrates (citrate and fatty acids) and has significant variance in its binding capacity for small molecules including lipid residues [14–16]. The influence of lipid on the developing embryo is not well understood; however, human and animal oocytes exposed to high lipid environments as a result of obesity have impaired mitochondrial function, reduced oocyte number, and decreased fertilization rates, and subsequent poorer embryo development [17, 18]. Thus, differences in lipid compositions from a source such as HSA may have a similar impact on embryo metabolism and quality.
It remains unclear if different commercial HSA sources have varying degrees of residual lipids. Furthermore, the effects of using different HSA sources/manufacturers on pre-implantation embryo development, pregnancy establishment, and fetal outcomes are poorly understood. Therefore, the aims of our study were to (1) establish the extent of the variation in lipid composition between commonly used commercial HSA sources for human embryo culture and (2) determine the effects of different HSA sources on embryo quality and fetal development in a mouse model of human embryo culture.
Materials and methods
Animals
All experiments involving animals were conducted in accordance with ethics applications-M-2017-046 and M-2017-003, granted by the University of Adelaide Animal Ethics Committee. Mice were handled in accordance with the Australian Code of Practice for the Care and Use of Animals for Scientific Purposes. F1 (CBA/C57) female mice (aged 3–4 weeks) were housed in groups of five, and F1 (CBA/C57) males (aged 7–8 weeks) were housed individually. A total of 4–6 females and 4–6 males were used in each embryo culture replicate. Swiss females (aged 7–8 weeks) were recipients for embryo transfer and housed in groups of five. Mice were maintained on a 12 light:12 dark photoperiod cycle, fed standard chow (Specialty Feeds, Australia), and had access to water ad libitum.
Culture media
MOPS-G1-Plus handling medium (Vitrolife, Göteborg Sweden) was used for embryo manipulation. For the first 46h, embryos were randomly allocated and cultured in G1 supplemented with either Recombinant GMM albumin (Vitrolife), human serum albumin (HSA) (Vitrolife), or Sage human serum albumin (HSA) (Origio, Måløv, Denmark) at 10%, followed by the culture in G2-Plus (Vitrolife) to the blastocyst stage for a further 24 h in the same serum supplement.
Serum albumin lipid composition
The lipid quantification and fatty acid profile was assessed using standard thin-layer chromatography (FOODPlus Research Centre School of Agriculture, University of Adelaide). Neat 5-ml samples of Recombinant, Sage, and Vitrolife albumin (100mg/ml) were analyzed in five replicates from the same lot number. Fatty acids were extracted by chloroform/methanol (2:1, v/v), and separated into different lipid classes through gas chromatography techniques. The quantification of lipids was compared with commercial lipid standards (Nu-Chek Prep Inc., Elysian, MN, USA) using the Hewlett-Packard Chemstation data system [19]. FOODPlus Research Centre then provided the averaged concentration of each different class of lipid from the five replicates from the same lot number for each HSA source.
In vitro zygote collection and culture
Female mice were super ovulated with an intraperitoneal injection of 5IU of pregnant mare’s serum gonadotrophin (PMSG; Folligon, Intervet, Bendigo, Australia) and 5IU of human chorionic gonadotrophin (hCG; Chorulon, Intervet,) 48h apart, prior to mating with stud males overnight. Successful mating was determined by the presence of copulatory plug 20-h post hCG administration. Putative zygotes were retrieved from the ampullary region of the oviducts from mice. Zygotes collected from different female mice in each replicate experiment were pooled together and denuded of surrounding cumulus cells with 0.5mg/ml hyaluronidase (Sigma-Aldrich). Zygotes were washed in MOPS-G1 handling medium before being randomly assigned in groups of ten to 20-μl drop of G1 medium supplemented with the albumin sources stated above under paraffin oil in a controlled gas environment (6% CO2, 5% O2, and 89% N2) for 48h at 37°C. After 48h, embryos were washed and cultured in G2-Plus for an additional 48h. All culture dishes were prepared a minimum of 4h prior to culture to ensure gas and temperature equilibrium [20]. All HSA treatments were compared in all replicates and the same lot of G1 and G2, incubators, and laboratory equipment were keep consistent over the study period.
Embryo morphological assessment
Morphology was assessed on day 3 (48h of culture) and on day 4 (72h of culture) to assess “on time” blastocyst development (equivalent to in vivo blastocyst development). On day 3, embryos were categorized as delayed (<4 cells), 4 cells, 6 cells, or 8 cells with or without compaction. On day 4, embryos were categorized as delayed (≤8 cells), morula, or blastocyst, which were further categorized as either an early blastocyst (blastocoel cavity <2/3 of embryo), blastocyst (blastocoel cavity >2/3 of embryo), expanded blastocyst (blastocoel is full), or hatching blastocyst (blastocyst herniating through zona pellucida) [14].
Reactive oxygen species generation of blastocyst
Superoxide generation of the blastocyst was determined by the fluorescent probe MitoSOX Red (Thermofisher, Massachusetts, USA). Briefly, blastocysts on day 4 (72 h of embryo culture media) were incubated in 5μM of MitoSOX Red in G-MOPS (Vitrolife) at 37°C for 30 min [21]. Embryos were then counterstained with Hoescht (0.5mg/ml) (Life Technologies, USA) in G-MOPS for 5 min. Blastocyst were washed through G-MOPS and imaged using epiflorescent microscopy at an excitation wavelength of 488 and 520 nm. Fluorescence intensity was quantified on ImageJ software 1.48v. and was normalized to a fluorescent bead (Thermofisher) as an inter-replicate control. Nuclei were counted and used to quantify superoxide generation per cell.
Neutral lipid staining of blastocysts
Lipophilic dye BODIPY493/503 (Sigma-Aldrich) was used to stain intracellular neutral lipid (fatty acids and steryl esters) in embryos (n = 30 per group). Embryos in 1mg/ml of BODIPY diluted in PBS/PVP incubated for 1h at room temperature were counterstained in Hoescht (0.5mg/ml) (Life Technologies) diluted in PBS/PVP. Negative controls were stained in parallel using DMSO (1mg/ml) diluted in PBS/PVP. Embryos were mounted in glycerol loading medium on glass slides and imaged using epifluorescence. The average fluorescence emitted at excitation wavelength range 460–490nm was used to quantify total neutral lipid in ImageJ and normalized to a fluorescent bead (ThermoFisher) as an inter-replicate control. Nuclei (excitation wavelength range 330–400nm) were counted live and used to quantitate mean fluorescence per cell.
Differential lipid histochemical staining of blastocysts
Two stains previously validated in porcine embryos [22] were adapted and used to differentially identify specific lipid groups of embryos; 1% Nile Blue Sulfate (Sigma-Aldrich) diluted in water which stains non-esterified fatty acids red, and Sudan Black B (2.5mg/ml) diluted in ethanol (Sigma-Aldrich) which stains phospholipids grey. Blastocysts were immersed in Nile Blue Sulfate diluted in water and PBS/PVP (10mg/ml) (pH = 2) (Sigma-Aldrich) and differentiated in 1% acetic acid for 1min, whilst for Sudan Black B, method blastocysts were immersed in Sudan Black B solution for 30s. Slides were immediately rinsed in PBS/PVP, mounted in glycerol, and imaged under bright field. Captured images were split into red, green, blue (RGB) and grey (0.299R+0.587G+0.114B) color channels. The total area of the embryo was measured to determine the relative intensities for each stain, subtracting background staining in ImageJ.
Triglyceride uptake in blastocysts
Fluorescently labelled triglyceride analogue 1, 2-dioleoyl-3-[11-(dipyrrometheneboron difluoride) undecanoyl]-sn-glycerol (TopFluor, Avanti Lipids Polar, Alabama) was used to determine triglyceride uptake during embryo culture. Triglyceride tracer (50μM) was added to G1 media. Zygotes were cultured with the addition of equivalent pre-equilibrated triglyceride tracer solution for 72h. Blastocysts were imaged live using confocal microscopy at an excitation wavelength range 460–490nm and total embryo fluorescence was measured using ImageJ and normalized to a fluorescent bead (ThermoFisher) as an inter-replicate control.
Vitrification and warming of blastocysts
Cryopreservation and warming of blastocysts occurred from five replicate experiments per treatment group using the RapidVit-Blast and Rapid-Warm-Blast (Vitrolife) [23]. Cryopreserved blastocysts were used for embryo transfer experiments instead of fresh embryo transfer as this allowed optimization of the number of embryos required on each day of successful plugging and is also an appropriate model to use to mimic frozen embryo transfer in the human which is increasingly becoming the more common treatment type compared with fresh embryo transfer [24]. Briefly, blastocysts were transferred from G-MOPS Plus handling media to Vitri2 Blast Equilibration media for 2 min at 37°C, then into Vitri3 Blast media for 35–45 s at 37°C before being transferred onto a Rapid-I straw, sealed, and immediately submerged into liquid nitrogen. The warming of blastocysts the morning of embryo transfer surgeries involved removing the top of the Rapid-I and plunging the insert into Warm1 cleave, media followed by Warm2 Cleave, Warm3 Cleave, and Warm4 Cleave media for 30s, 1min, 2min, and 3–5min respectively. To ensure survival and re-expansion, blastocysts were then transferred into a pre-equilibrated G2-Plus culture dish (6% CO2, 37°C) 3–4h prior to embryo transfer with survival and re-expansion rates being >95% in each HSA group with no significant difference seen between HSA sources. This is typical of mouse blastocysts which have a high cryosurvival rate [25].
Embryo transfers and day 18 pregnancy outcomes
Pseudopregnancy was achieved by natural mating Swiss females with vasectomized adult F1 studs. On day 3.5 of pseudopregnancy, six expanded or hatching blastocysts from treatments allocated by permuted block randomization were surgically transferred into contralateral uterine horns (one treatment per uterine horn), indicating that each mother was gestating a fetus generated from two different treatment groups. Sixty embryos were transferred (six per uterine horn) from five replicate experiments into 10 different recipient mothers per HSA treatment for a total of 15 recipient mothers. Embryo transfer surgeries were performed using 2% Avertin (Sigma-Aldrich) anesthesia 0.2mg/kg body weight. On day 18 of pregnancy, total number of implantation sites, resorption sites, and viable fetuses were examined [23] and fetal and placental weights and lengths were measured [26].
Statistical analysis
All data are expressed as the mean ± SEM for continues data and percentages for proportional data. Blastocyst lipid content, cell numbers, and superoxide generation were analyzed using a univariate general linear model with replicate fitted as a covariate. Embryo development and pregnancy data were assessed by a binomial logistic regression adjusting for multiple comparisons. Fetal outcomes were analyzed by a univariate general linear model, with mother included as a random effect to control for variations to fetal outcomes induced by mother, and litter size was added as a fixed factor to control for differences between litter sizes. All statistical analysis was performed in SPSS software (version 24; SPSS, Inc.) with 80% power and a P value of < 0.05 deemed significant.
Results
Quantification and analysis of lipid composition profile of human serum albumin sources
Of the albumins tested, Sage HSA had the overall greatest fatty acid composition (Table 1). Trans-unsaturated fatty acids were the lowest in Vitrolife Recombinant albumin and Vitrolife HSA (Table 1). Saturated fatty acids were up to eight times higher in Sage HSA compared with the other two albumin sources (Table 1). Saturated fatty acids made up the greatest lipid fraction in the human albumin sources, with majority being palmitic acid (Table 1). Omega-9 oleic acid (18:1n-9) followed by omega-7 palmitoleic acid (16:1n-7) made up the highest monounsaturated fatty acid fractions for all albumin sources (Table 1). Unexpectedly, Recombinant albumin had ~70-fold more palmitoleic acid (16:1n-7) compared with Vitrolife HSA and ~sixfold more compared with Sage HSA (Table 1). Omega-6 linoleic acid (18:2n-6) and omega-3 alpha-linoleic acid (18:3n-3) were the most common polyunsaturated fatty acid fractions present across all serum albumin sources (Table 1).
Table 1.
The relative amounts of individual fatty acids in different human albumin sources used for pre-implantation embryo culture (mg/100g of albumin protein)
| RecombinantHSA | Vitrolife HSA | Sage HSA | |
|---|---|---|---|
| Total saturated | 1.52 | 2.95 | 11.76 |
| 13:0: Tridecanoic acid | − | 0.10 | 0.08 |
| 14:0: Myristic acid | 0.37 | 0.15 | 0.46 |
| 15:0: Pentadecanoic acid | 0.12 | 0.08 | 0.11 |
| 16:0: Palmitic acid | 0.82 | 1.90 | 8.27 |
| 17:0: Margaric acid | − | 0.01 | 0.02 |
| 18:0: Stearic acid | 0.18 | 0.66 | 2.71 |
| 20:0: Arachidic acid | 0.03 | 0.02 | 0.03 |
| 22:0: Behenic acid | − | 0.01 | − |
| 24:0: Lignoceric acid | − | 0.01 | 0.03 |
| Total trans | 0.09 | 0.05 | 0.62 |
| t16: | 0.05 | − | 0.03 |
| t18:1n-9: | 0.04 | 0.05 | 0.24 |
| t18:1n-7: | − | − | 0.35 |
| t18:2: | − | − | − |
| Total monounsaturated | 4.61 | 0.98 | 11.14 |
| Total omega-9 | 1.48 | 0.85 | 9.63 |
| 18:1n-9: Oleic acid | 1.43 | 0.78 | 9.53 |
| 20:1n-9: Eicosanoid acid | − | 0.02 | 0.07 |
| 22:1n-9: Docosenoic acid | 0.05 | 0.05 | 0.03 |
| Total omega-7 | 3.13 | 0.13 | 1.51 |
| 16:1n-7: Palmitoleic acid | 3.07 | 0.07 | 0.81 |
| 18:1n-7: Vaccenic acid | 0.06 | 0.06 | 0.69 |
| Total polyunsaturated | 0.22 | 1.14 | 8.72 |
| Total omega-3 | 0.03 | 0.11 | 0.61 |
| 18:3n-3: Alpha-linoleic acid | − | 0.03 | 0.36 |
| 20:5n-3: Eicosapentaenoic acid | 0.03 | 0.03 | 0.05 |
| 22:5n-3: Docosapentaenoic acid | – | 0.01 | 0.05 |
| 22:6n-3: Docosahexaenoic acid | – | 0.05 | 0.15 |
| Total omega-6 | 0.18 | 1.03 | 8.11 |
| 18:2n-6: Linoleic acid | 0.18 | 0.84 | 7.11 |
| 18:3n-6: Gamma-linoleic acid | − | 0.01 | 0.05 |
| 20:2n-6: Eicosadienoic acid | − | 0.01 | 0.08 |
| 20:3n-6: Dihomo-gamma-linolenic acid | − | 0.02 | 0.13 |
| 20:4n-6: Arachidonic acid | − | 0.15 | 0.71 |
| 22:4n-6+22:3n-3 | − | − | 0.03 |
5 replicate samples from the same lot number were measured and averaged to generate final values. Undetected levels reported as (−). HSA human serum albumin
Bolded values represent the summary values of each fatty acid subcategories
When comparing several different lots of Vitrolife HSA, while three out of the four lot numbers (23365, 19621, and 19949) displayed relatively similar levels of saturated, monounsaturated, and polyunsaturated fatty acids (Supplementary Table 1), one lot number (22762) had nearly a ~20–25% increase in fatty acid levels (Supplementary Table 1).
Embryo development, cell numbers, and superoxide generation
Sage HSA had significantly fewer embryos that were at the 8-cell stage at 48h of culture compared with both Recombinant HSA and Vitrolife HSA (P < 0.05, Table 2). This delay in embryo development was also present after 72 h of culture (on day 4), with Sage HSA having fewer numbers of blastocysts compared with both Recombinant HSA and Vitrolife HSA (P < 0.05, Table 2) and increased numbers of embryos at the early blastocyst stage compared with Recombinant HSA (P < 0.05).
Table 2.
Effect of human serum albumin on embryo development
| Recombinant HSA | Vitrolife HSA | Sage HSA | |
|---|---|---|---|
| 48-h culture (day 3) | |||
| < 8cell (delayed) (%) | 70.0 | 65.0 | 73.0 |
| 8 cell (%) | 17.0a | 19.0a | 12.0b |
| Compacting (%) | 8.0 | 12.0 | 12.0 |
| 72-h culture (day 4) | |||
| Morula (%) | 31.4 | 35.0 | 32.3 |
| Early blastocyst (%) | 10.2a | 13.2ab | 18.2b |
| Blastocyst (%) | 18.1a | 17.5a | 11.4b |
| Expanded and hatching blastocyst (%) | 15.7 | 17.8 | 16.2 |
Embryo development data is expressed as percentage of presumptive zygote that reached each development stages. N = 361, 355, and 347 embryos for Recombinant, Vitrolife, and Sage HSA respectively (15 replicate experiments). Different superscript letters denote significance between treatment groups within a row (P < 0.05). HSA human serum albumin
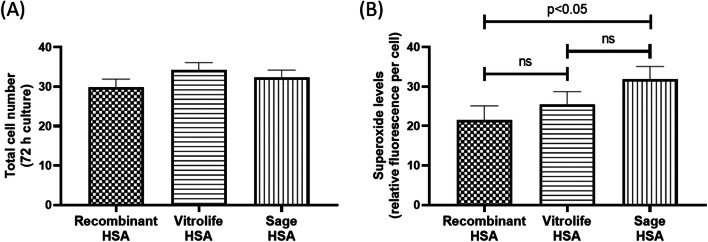
There was no difference in total cell numbers of blastocyst after 72h of culture between the different human albumin sources (P > 0.05, Fig. 1A), although embryos cultured with Vitrolife HSA had the highest average cell number (34.2 ± 1.9).
Fig. 1.
The effect of human serum albumin on blastocyst cell number and superoxide generation after 72 h in culture. A Total cell number and B superoxide generation. Cell number and superoxide generation are expressed as mean ± SEM and are representative of n ≥ 25 blastocysts per treatment group with 6 replicate experiments per measure
Sage HSA significantly increased superoxide generation in blastocysts after 72h of culture compared with Recombinant HSA (P < 0.05, Fig. 1B). Superoxide generation in blastocyst cultured in Vitrolife HSA was no different compared with the other two albumin supplements (P > 0.05, Fig. 1B).
Lipid content of blastocysts
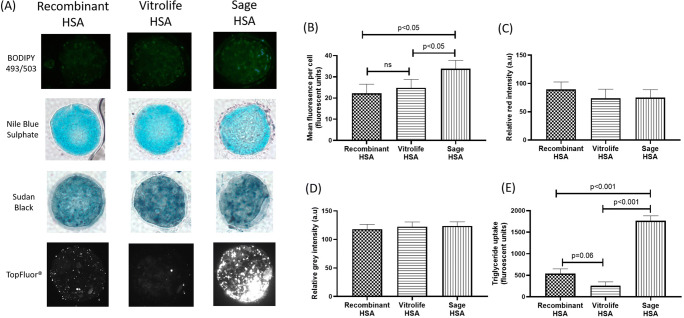
Blastocyst cultured in Sage HSA had increased neutral lipid content compared with blastocyst cultured in Recombinant and Vitrolife HSA (P < 0.05, Fig. 2B). There was no effect of albumin source on both non-esterified fatty acid and phospholipid content of blastocysts after 72 h of culture (P > 0.05, Fig. 2C, D). Blastocyst cultured in Vitrolife HSA had the lowest triglyceride up take compared with Sage HSA (P < 0.001, Fig. 2E) and Recombinant HSA (P= 0.06, Fig. 2E). Further, blastocyst cultured in Sage HSA had increased triglyceride up take compared with those blastocyst cultured in Recombinant HSA (P < 0.001, Fig. 2E).
Fig. 2.
The effect of human serum albumin on blastocyst lipid composition after 72 h in culture. A Representative images for each lipid dye. B Neutral lipid content. C Non-esterified fatty acid content. D Phospholipid content. E Triglyceride uptake. Data is expressed as mean ±SEM. Lipid composition is representative of n ≥ 20 per group with 4 replicate experiments per measure. Different superscript letters denote significance between treatments (P < 0.05). HSA, human serum albumin; a.u., arbitrary units
Implantation, pregnancy, and fetal outcomes
Embryos cultured in Sage HSA had a significant reduction in total implantations compared with both Recombinant HSA and Vitrolife HSA (P < 0.05, Table 3), and reduced total proportion of fetus compared with Vitrolife HSA (P < 0.05, Table 3). When fetuses were expressed as a proportion of implantations there were no differences between albumin sources (P > 0.05, Table 3).
Table 3.
The effect of serum albumin on implantation and fetal development
| RecombinantHSA | Vitrolife HSA | Sage HSA | |
|---|---|---|---|
| Pregnancy | |||
| Pregnancy rate/uterine horn transfer (%) | 90 (9/10) | 90 (9/10) | 90 (9/10) |
| Total implantations/embryo transferred (%) | 82a | 76a | 57b |
| Total fetus/embryo transferred (%) | 54ab | 56a | 35b |
| Fetus per implantation (%) | 62 | 76 | 70 |
| Fetal development | |||
| Fetal weight (mg) | 858 ± 28a | 941 ± 26b | 899 ± 33ab |
| Fetal length (mm) | 19.1 ± 0.4 | 19.6 ± 0.3 | 19.2 ± 0.4 |
| Placental weight (mg) | 108 ± 4a | 121 ± 4b | 114 ± 5ab |
| Fetal:placenta weight ratio | 8.26 ± 0.36 | 8.06 ± 0.35 | 8.09 ± 0.43 |
Results shown as percentages for dichotomous variables and mean ± SEM for continuous variables. N = 60 embryos transferred (six per uterine horn) from five replicate experiments into 10 recipient mothers per HSA treatment (15 recipient mothers total). Different superscript letters denote significance between treatment groups within a row (P < 0.05). HSA human serum albumin
Embryos cultured in Vitrolife HSA produced the biggest fetuses and placentas, which were significantly heavier than those fetus and placentas produced from embryos cultured in Recombinant HSA (P < 0.05, Table 3). There was no effect of albumin source on fetal length or fetal/placental weight ratios (P > 0.05, Table 3).
Discussion
Differences in human embryo culture media are considered one of the key factors in contributing to the reported variability in live birth rates between IVF clinics [6, 11, 27]. While different embryo culture media concentrations of amino acids and carbohydrates have been shown to influence both pregnancy rates and birthweights of infants [7–10, 28], less information is known about the effects of HSA source on embryo culture and fetal outcomes [12]. In this study, we have shown that embryo development, blastocyst reactive oxygen species (ROS) generation, lipid uptake, implantation rates, and fetal size are modified based on the HSA supplement used during embryo culture. Further, we have shown that lipid constitutes are highly variable among these HSA sources, which may in part explain the differences to embryo quality and fetal weights.
Supplementation of the embryo culture media with HSA containing high levels of residual lipids correlated with poorer pregnancy and fetal outcomes. For instance, Sage HSA which was found to have nearly 10-fold higher levels of saturated (primarily to palmitic acid), monounsaturated, omega-9s, and polyunsaturated fatty acids had the worst blastocyst development and quality, with increased superoxide generation, neutral lipid and cholesterol uptake, and reduced blastocyst implantation rates. This is not surprising given the literature showing that supplementing embryo culture media in vitro with elevated saturated fatty acids [29–31] or a maternal high fat diet high in saturated fatty acids [32] results in embryos with similar delays in development, increased ROS production, and reduced implantation rates. While Recombinant HSA contained some of the lowest concentrations of lipids, it did contain the highest concentration of palmitoleic acid and was the group that produced the smallest fetus, suggesting importance of a balance between different types of lipids in addition to total amount, and an “optimal ratio” required for embryo and fetal development. In complex biological systems, the ratio of different types of lipids plays a more important role than individual fatty acid levels in maintaining cellular processes [33]. Increased unsaturated fatty acids may compensate for the negative effects induced by saturated fatty acid as demonstrated in bovine oocytes and embryos [34, 35]. Oleic acid is the most common monounsaturated fatty acid reported in all albumin sources and has been shown to improve bovine blastocyst formation [36]. Equally, polyunsaturated fatty acids have a beneficial role in embryonic cell signaling and may influence gene expression of lipid metabolism, oxidation, and fatty acid synthesis [37–41]. Trans-unsaturated fatty acids have an unknown effect on embryo development but have been shown to interfere with the metabolism of polyunsaturated fatty acids in human pancreatic β-cells by interfering with their synthesis [42], which may decrease embryonic and fetal development. This indicates that the specific balance of residual lipids present in HSA source may be important for pre-implantation embryo development, implantation, and fetal growth.
Embryos exposed to the highest HSA fatty acid composition also had the highest uptake of cholesterol and neutral lipids. The formation of lipid droplets through pinocytosis in developing oocytes and embryos is largely dictated by lipid composition of their surrounding environment [43, 44]; therefore, any changes to lipid compositions due to HSA source can modify the amount of lipids incorporated into the developing embryo. Blastocyst can use both endogenous and exogenous fatty acids stored in lipid droplets as an energy source, through the lipid metabolic pathway β-oxidation [43, 44]; however, this pathway alone cannot sufficiently support metabolic requirements of the blastocyst [45]. Further, lipids also play a significant role in embryo intracellular signaling, through influencing gene expression by binding to nuclear receptors such as peroxisome proliferator-activated receptor (PPAR) and transcription factors including sterol-regulatory element binding protein (SREBP) and acyl-CoA synthetases which are involved in β-oxidation [46, 47]. Therefore, the signaling role of accumulated lipid, as those seen in blastocysts cultured in Sage HSA, is likely modifying key regulatory genes involved in β-oxidation, which could be contributing to the embryonic phenotypes reported.
While there are no studies that have clearly defined the complete lipid composition of mammalian oviduct or uterine fluid, follicular fluid lipid composition has been defined in large mammals including humans [48–50]. The follicular lipid composition is comparable to the most abundant lipid constituents in albumin sources reported in this study, including high levels of oleic acid, palmitic, stearic and; linoleic acid. The level of linoleic acid and α-linoleic acid (ω-6:ω-3) in uterine fluid can alter the production of molecular mediators critical for embryo implantation [51, 52] with increase in linoleic acid compared to α-linoleic acid in uterine fluid positively associated with increased pregnancy and implantation rates in women undergoing IVF [53]. Recombinant HSA and Vitrolife HSA which had the highest implantation rates also had the largest relative amount of linoleic acid compared with α-linoleic acid. Therefore, this could suggest a positive correlation between increased exposures of linoleic acid compared with α-linoleic acid in culture media and higher implantation rates.
The availability of lipids in culture media for uptake by the embryo depends on the composition of serum albumin, and specifically its purity and source [54]. Although albumin shares homologous functions between mammalian species, there are conformational differences between species, with human serum albumin containing fewer amino acid residues [55]. This results in the binding of several low molecular weight compounds including fatty acids in varying amounts [56, 57]. Variation in albumin lipid composition may also exist due to differences in the method of protein extraction and the extent of the purification. The extraction process often involves cold ethanol plasma fractionation and only specific purification of blood-borne viruses, such as hepatitis virus and immunodeficiency virus. The genetically engineered Recombinant albumin has high purity with minimal fatty acid compared to other sources [54]. However, unexpectantly, it contained increased levels of monounsaturated fatty acids especially omega-7 fatty acid compared with its non-genetically engineered counterpart. It is suggested that because Recombinant albumin is not exposed to blood nutrients, it may have an increased binding availability, acting as a “sponge” binding to molecules during engineering in yeast cells grown in medium [58]. However, as the exact production and purification of these protein sources are not well documented, it poses a great challenge in defining the exact lipid composition and acquiring a safe and consistent protein source for use in human embryo culture.
We also reported variations in the lipid composition of different lot numbers from the same HSA source, with considerable differences in levels of saturated, monounsaturated, and polyunsaturated fatty acids. This suggests that despite similar purification techniques, lot-to-lot variability still exists, thus ultimately changing the environment in which the pre-implantation embryo is exposed. For instance, a publication in humans found that different HSA sources either commercially added to G1.5 or added in the laboratory were able to modify the birthweights of subsequent babies (Z scores G1-PLUS.5 verse G1.5 = 0.28 ± 1.12 vs 0.09 ± 1.15, P = 0.04), even though both media and HSA solutions were purchased from the same company [12]. Therefore, even the slight changes in fatty acid composition of HSA lots even if they are coming from the same supplier have the potential to modify embryo and fetal outcomes.
Despite investigating lipids and their relationship with embryo development, blastocyst lipid uptake, and pregnancy and fetal outcomes, there are other factors bound to serum albumin which may differ between our HSA sources including embryo trophic growth factors, ions, and microRNA [15]. These factors could be contributing to the observed outcomes by influencing the expression of fatty acid transport genes and altering embryo lipid uptake. For example, granulocyte-macrophage colony-stimulating factor (GM-CSF) is one factor that can bind to albumin and has been shown to improve embryo development to blastocyst stage and increase improve implantation rates in humans [59]. Further work is required in this area to better understand the variability in HSA and which factors may have either a positive or negative effect on pre-implantation embryo development.
The use of different human serum albumin sources for supplementation in culture media exposes the pre-implantation embryo to varying levels and classes of lipids. This study highlights the potential effect of residual fatty acids in HSA on embryo health and quality and that different HSA sources can modify implantation rates and fetal outcomes. It remains unclear whether the reported impacts are solely due to differences in lipid constitutions in HSA sources or a combination of other contaminants in albumin, although it does suggest that the lipid content of HSA should be carefully considered when choosing a suitable HSA source for use in clinical IVF.
Supplementary Information
(DOCX 22 kb)
Acknowledgements
We would like to acknowledge Lauren Sandeman, Alex Penn and Bridget Arman for their technical assistance. The author group would also like to gratefully acknowledge Professor Michelle Lane, who sadly passed away in February 2020, for her advice on the study design.
Author contribution
DZ—conception and design, interpretation of data, revising, and approval of manuscript. LV—concept and design, acquisition, analysis and interpretation of data, and drafting, revising, and approval of manuscript. NOM—concept and design, analysis and interpretation of data, and drafting and approval of manuscript.
Funding
NOM is the recipient of an NHMRC Peter Doherty Early Career Research Fellowship.
Data availability
All data was originally generated. For access to data, please contact the corresponding author Associate Professor Deirdre Zander-Fox.
Declarations
Ethics approval
All experiments involving animals were conducted in accordance with ethics applications-M-2017-046 and M-2017-003, granted by the University of Adelaide Animal Ethics Committee.
Conflict of Interest
DZ and LV are paid employees of Monash IVF Group Lmt.
Footnotes
Lauren Villarosa is co-first author.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Biggers JD. Metabolism of mouse embryos. J Reprod Fertil Suppl. 1971;14:41–54. [PubMed] [Google Scholar]
- 2.Biggers JD, Stern S. Metabolism of the preimplantation mammalian embryo. Adv Reprod Physiol. 1973;6(0):1–59. [PubMed] [Google Scholar]
- 3.Wales RG. Biochemistry of the developing embryo. J Reprod Fertil Suppl. 1973;18:117–125. [PubMed] [Google Scholar]
- 4.Zander-Fox D, Lane M. The future of human embryo culture media - or have we reached the ceiling. In: Tamanda S, Takakuwa T, editors. The Human Embryo: IntechOpen; 2012.
- 5.Chronopoulou E, Harper JC. IVF culture media: past, present and future. Hum Reprod Update. 2015;21(1):39–55. doi: 10.1093/humupd/dmu040. [DOI] [PubMed] [Google Scholar]
- 6.Morbeck DE, Krisher RL, Herrick JR, Baumann NA, Matern D, Moyer T. Composition of commercial media used for human embryo culture. Fertil Steril. 2014;102(3):759–766. doi: 10.1016/j.fertnstert.2014.05.043. [DOI] [PubMed] [Google Scholar]
- 7.Dumoulin JC, Land JA, Van Montfoort AP, Nelissen EC, Coonen E, Derhaag JG, et al. Effect of in vitro culture of human embryos on birthweight of newborns. Human reproduction (Oxford, England) 2010;25(3):605–612. doi: 10.1093/humrep/dep456. [DOI] [PubMed] [Google Scholar]
- 8.Nelissen EC, Van Montfoort AP, Coonen E, Derhaag JG, Geraedts JP, Smits LJ, et al. Further evidence that culture media affect perinatal outcome: findings after transfer of fresh and cryopreserved embryos. Human Reproduction. 2012;27(7):1966–1976. doi: 10.1093/humrep/des145. [DOI] [PubMed] [Google Scholar]
- 9.Kleijkers SH, van Montfoort AP, Smits LJ, Viechtbauer W, Roseboom TJ, Nelissen EC, et al. IVF culture medium affects post-natal weight in humans during the first 2 years of life. Human reproduction. 2014;29(4):661–669. doi: 10.1093/humrep/deu025. [DOI] [PubMed] [Google Scholar]
- 10.Eskild A, Monkerud L, Tanbo T. Birthweight and placental weight; do changes in culture media used for IVF matter? Comparisons with spontaneous pregnancies in the corresponding time periods. Hum Reprod. 2013;28(12):3207–3214. doi: 10.1093/humrep/det376. [DOI] [PubMed] [Google Scholar]
- 11.Tarahomi M, Vaz FM, van Straalen JP, Schrauwen FAP, van Wely M, Hamer G, et al. The composition of human preimplantation embryo culture media and their stability during storage and culture. Hum Reprod. 2019;34(8):1450–1461. doi: 10.1093/humrep/dez102. [DOI] [PubMed] [Google Scholar]
- 12.Zhu J, Li M, Chen L, Liu P, Qiao J. The protein source in embryo culture media influences birthweight: a comparative study between G1 v5 and G1-PLUS v5. Hum Reprod. 2014;29(7):1387–1392. doi: 10.1093/humrep/deu103. [DOI] [PubMed] [Google Scholar]
- 13.Blake D, Svalander P, Jin M, Silversand C, Hamberger L. Protein supplementation of human IVF culture media. Journal of Assisted Reproduction and Genetics. 2002;19(3):137–143. doi: 10.1023/a:1014788821965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gardner DK, Lane M, Watson A, editors. A laboratory guide to the mammalian embryo. New York: Oxford University Press; 2004. [Google Scholar]
- 15.Morbeck DE, Paczkowski M, Fredrickson JR, Krisher RL, Hoff HS, Baumann NA, Moyer T, Matern D. Composition of protein supplements used for human embryo culture. Journal of Assisted Reproduction and Genetics. 2014;31(12):1703–1711. doi: 10.1007/s10815-014-0349-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bavister BD. Culture of preimplantation embryos: facts and artifacts. Hum Reprod Update. 1995;1(2):91–148. doi: 10.1093/humupd/1.2.91. [DOI] [PubMed] [Google Scholar]
- 17.Wu LL, Russell DL, Norman RJ, Robker RL. Endoplasmic reticulum (ER) stress in cumulus-oocyte complexes impairs pentraxin-3 secretion, mitochondrial membrane potential (DeltaPsi m), and embryo development. Molecular endocrinology. 2012;26(4):562–573. doi: 10.1210/me.2011-1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Metwally M, Cutting R, Tipton A, Skull J, Ledger WL, Li TC. Effect of increased body mass index on oocyte and embryo quality in IVF patients. Reprod Biomed Online. 2007;15(5):532–538. doi: 10.1016/S1472-6483(10)60385-9. [DOI] [PubMed] [Google Scholar]
- 19.Liu G, Mühlhäusler BS, Gibson RA. A method for long term stabilisation of long chain polyunsaturated fatty acids in dried blood spots and its clinical application. Prostaglandins, Leukotrienes and Essential Fatty Acids (PLEFA) 2014;91(6):251–260. doi: 10.1016/j.plefa.2014.09.009. [DOI] [PubMed] [Google Scholar]
- 20.Lane M, Mitchell M, Cashman KS, Feil D, Wakefield S, Zander-Fox DL. To QC or not to QC: the key to a consistent laboratory? Reprod Fertil Dev. 2008;20(1):23–32. doi: 10.1071/RD07161. [DOI] [PubMed] [Google Scholar]
- 21.Al-Zubaidi U, Adhikari D, Cinar O, Zhang QH, Yuen WS, Murphy MP, et al. Mitochondria-targeted therapeutics, MitoQ and BGP-15, reverse aging-associated meiotic spindle defects in mouse and human oocytes. Hum Reprod. 2021;36(3):771–784. doi: 10.1093/humrep/deaa300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Romek M, Gajda B, Krzysztofowicz E, Smorąg Z. Changes of lipid composition in non-cultured and cultured porcine embryos. Theriogenology. 2010;74(2):265–276. doi: 10.1016/j.theriogenology.2010.02.010. [DOI] [PubMed] [Google Scholar]
- 23.Zander-Fox DL, Fullston T, McPherson NO, Sandeman L, Kang WX, Good SB, et al. Reduction of mitochondrial function by FCCP during mouse cleavage stage embryo culture reduces birth weight and impairs the metabolic health of offspring. Biology of Reproduction. 2015;92(5):124. doi: 10.1095/biolreprod.114.123489. [DOI] [PubMed] [Google Scholar]
- 24.Newman J, Paul R, Chambers GM. Assisted reproductive technology in Australia and New Zealand. In: Unit SNPEaS, vol. 2020. Sydney: The University of New South Wales; 2018.
- 25.Truong TT, Gardner DK. Antioxidants increase blastocyst cryosurvival and viability post-vitrification. Hum Reprod. 2020;35(1):12–23. doi: 10.1093/humrep/dez243. [DOI] [PubMed] [Google Scholar]
- 26.Zander-Fox DL, Mitchell M, Thompson JG, Lane M. Alterations in mouse embryo intracellular pH by DMO during culture impair implantation and fetal growth. Reproductive BioMedicine Online. 2010;21(2):219–229. doi: 10.1016/j.rbmo.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 27.Harris K, Fitzgerald O, Paul RC, Macaldowie A, Lee E, Chambers GM. Assisted reproductive technology in Australia and New Zealand 2014. Sydney National Perinatal Epidemiology and Statistics Unit (NPESU), the University of New South Wales 2016
- 28.Gu F, Deng M, Gao J, Wang Z, Ding C, Xu Y, Zhou C. The effects of embryo culture media on the birthweight of singletons via fresh or frozen-thawed embryo transfer: a large-scale retrospective study. BMC Pregnancy Childbirth. 2016;16:270. doi: 10.1186/s12884-016-1077-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang X, Wu LL, Chura LR, Liang X, Lane M, Norman RJ, Robker RL. Exposure to lipid-rich follicular fluid is associated with endoplasmic reticulum stress and impaired oocyte maturation in cumulus-oocyte complexes. Fertil Steril. 2012;97:1438–1443. doi: 10.1016/j.fertnstert.2012.02.034. [DOI] [PubMed] [Google Scholar]
- 30.Bermejo-Alvarez P, Rosenfeld CS, Roberts RM. Effect of maternal obesity on estrous cyclicity, embryo development and blastocyst gene expression in a mouse model. Human Reproduction. 2012;27(12):3513–3522. doi: 10.1093/humrep/des327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Desmet KLJ, Van Hoeck V, Gagné D, Fournier E, Thakur A, O’Doherty AM, et al. Exposure of bovine oocytes and embryos to elevated non-esterified fatty acid concentrations: integration of epigenetic and transcriptomic signatures in resultant blastocysts. BMC Genomics. 2016;17(1):1004. doi: 10.1186/s12864-016-3366-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu LLY, Dunning KR, Yang X, Russell DL, Lane M, Norman RJ, Robker RL. High-Fat diet causes lipotoxicity responses in cumulus-oocyte complexes and decreased fertilization rates. Endocrinology. 2010;151:5438–5445. doi: 10.1210/en.2010-0551. [DOI] [PubMed] [Google Scholar]
- 33.Muro E, Atilla-Gokcumen GE, Eggert US. Lipids in cell biology: how can we understand them better? Molecular Biology of the Cell. 2014;25(12):1819–1823. doi: 10.1091/mbc.E13-09-0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aardema H, Vos PL, Lolicato F, Roelen BA, Knijn HM, Vaandrager AB, et al. Oleic acid prevents detrimental effects of saturated fatty acids on bovine oocyte developmental competence. Biol Reprod. 2011;85(1):62–69. doi: 10.1095/biolreprod.110.088815. [DOI] [PubMed] [Google Scholar]
- 35.Van Hoeck V, Sturmey RG, Bermejo-Alvarez P, Rizos D, Gutierrez-Adan A, Leese HJ, et al. Elevated non-esterified fatty acid concentrations during bovine oocyte maturation compromise early embryo physiology. PLoS One. 2011;6:e23183. doi: 10.1371/journal.pone.0023183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McKeegan PJ, Sturmey RG. The role of fatty acids in oocyte and early embryo development. Reproduction, Fertility and Development. 2011;24(1):59–67. doi: 10.1071/RD11907. [DOI] [PubMed] [Google Scholar]
- 37.Pawar A, Jump DB. Unsaturated fatty acid regulation of peroxisome proliferator-activated receptor α activity in rat primary hepatoctes. Journal of Biological Chemistry. 2003;278(38):35931–35939. doi: 10.1074/jbc.M306238200. [DOI] [PubMed] [Google Scholar]
- 38.Mater MK, Thelen AP, Pan DA, Jump DB. Sterol response element-binding protein 1c (SREBP1c) Is involved in the polyunsaturated fatty acid suppression of hepatic S14 gene transcription. Journal of Biological Chemistry. 1999;274(46):32725–32732. doi: 10.1074/jbc.274.46.32725. [DOI] [PubMed] [Google Scholar]
- 39.Schoonjans K, Watanabe M, Suzuki H, Mahfoudi A, Krey G, Wahli W, Grimaldi P, Staels B, Yamamoto T, Auwerx J. Induction of the acyl-coenzyme a synthetase gene by fibrates and fatty acids is mediated by a peroxisome proliferator response element in the C promoter. Journal of Biological Chemistry. 1995;270(33):19269–19276. doi: 10.1074/jbc.270.33.19269. [DOI] [PubMed] [Google Scholar]
- 40.Frohnert BI, Hui TY, Bernlohr DA. Identification of a functional peroxisome proliferator-responsive element in the murine fatty acid transport protein gene. Journal of Biological Chemistry. 1999;274(7):3970–3977. doi: 10.1074/jbc.274.7.3970. [DOI] [PubMed] [Google Scholar]
- 41.Brandt JM, Djouadi F, Kelly DP. Fatty acids activate transcription of the muscle carnitine palmitoyltransferase I gene in cardiac myocytes via the peroxisome proliferator-activated receptor α. Journal of Biological Chemistry. 1998;273(37):23786–23792. doi: 10.1074/jbc.273.37.23786. [DOI] [PubMed] [Google Scholar]
- 42.Furstova V, Kopska T, James RF, Kovar J. Comparison of the effect of individual saturated and unsaturated fatty acids on cell growth and death induction in the human pancreatic beta-cell line NES2Y. Life sciences. 2008;82(13-14):684–691. doi: 10.1016/j.lfs.2007.12.023. [DOI] [PubMed] [Google Scholar]
- 43.Dunning KR, Russell DL, Robker RL. Lipids and oocyte developmental competence: the role of fatty acids and beta-oxidation. Reproduction. 2014;148(1):R15–R27. doi: 10.1530/REP-13-0251. [DOI] [PubMed] [Google Scholar]
- 44.Bradley J, Swann K. Mitochondria and lipid metabolism in mammalian oocytes and early embryos. The International journal of developmental biology. 2019;63(3-4-5):93–103. doi: 10.1387/ijdb.180355ks. [DOI] [PubMed] [Google Scholar]
- 45.Kane MT. Fatty acids as energy sources for culture of one-cell rabbit ova to viable morulae. Biology of Reproduction. 1979;20(2):323–332. doi: 10.1095/biolreprod20.2.323. [DOI] [PubMed] [Google Scholar]
- 46.Sampath H, Ntambi JM. Polyunsaturated fatty acid regulation of genes of lipid metabolism. Annual review of nutrition. 2005;25:317–340. doi: 10.1146/annurev.nutr.25.051804.101917. [DOI] [PubMed] [Google Scholar]
- 47.Montjean D, Entezami F, Lichtblau I, Belloc S, Gurgan T, Menezo Y. Carnitine content in the follicular fluid and expression of the enzymes involved in beta oxidation in oocytes and cumulus cells. Journal of Assisted Reproduction and Genetics. 2012;29(11):1221–1225. doi: 10.1007/s10815-012-9855-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jungheim ES, Louden ED, Chi MMY, Frolova AI, Riley JK, Moley KH. Preimplantation exposure of mouse embryos to palmitic acid results in fetal growth restriction followed by catch-up growth in the offspring. Biology of Reproduction. 2011;85(4):678–683. doi: 10.1095/biolreprod.111.092148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Aardema H, Vos PLAM, Lolicato FAJ, Roelen B, Knijn H, Vaandrager A et al. Oleic acid prevents detrimental effects of saturated fatty acids on bovine oocyte Developmental Competence. 2011 [DOI] [PubMed]
- 50.Valckx SDM, Arias-Alvarez M, De Pauw I, Fievez V, Vlaeminck B, Fransen E, et al. Fatty acid composition of the follicular fluid of normal weight, overweight and obese women undergoing assisted reproductive treatment: a descriptive cross-sectional study. Reproductive Biology and Endocrinology. 2014;12(1):13. doi: 10.1186/1477-7827-12-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Salleh N. Diverse roles of prostaglandins in blastocyst implantation. The Scientific World Journal. 2014;2014:11. doi: 10.1155/2014/968141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Waclawik A, Kaczynski P, Jabbour HN. Autocrine and paracrine mechanisms of prostaglandin E2 action on trophoblast/conceptus cells through the prostaglandin E2 receptor (PTGER2) during implantation. Endocrinology. 2013;154(10):3864–3876. doi: 10.1210/en.2012-2271. [DOI] [PubMed] [Google Scholar]
- 53.Jungheim ES, Frolova AI, Jiang H, Riley JK. Relationship between serum polyunsaturated fatty acids and pregnancy in women undergoing in vitro fertilization. The Journal of Clinical Endocrinology & Metabolism. 2013;98(8):E1364–E13E8. doi: 10.1210/jc.2012-4115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lane M, Gardner DK. Embryo culture medium: which is the best? Best Practice & Research Clinical Obstetrics & Gynaecology. 2007;21(1):83–100. doi: 10.1016/j.bpobgyn.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 55.Spahr PF, Edsall JT. Amino acid composition of human and bovine serum mercaptalbumins. Journal of Biological Chemistry. 1964;239:850–854. doi: 10.1016/S0021-9258(18)51668-9. [DOI] [PubMed] [Google Scholar]
- 56.Steinhardt J, Krijn J, Leidy JG. Differences between bovine and human serum albumins. Binding isotherms, optical rotatory dispersion, viscosity, hydrogen ion titration, and fluorescence effects. Biochemistry. 1971;10(22):4005–4015. doi: 10.1021/bi00798a001. [DOI] [PubMed] [Google Scholar]
- 57.Taverna M, Marie A-L, Mira J-P, Guidet B. Specific antioxidant properties of human serum albumin. Annals of Intensive Care. 2013;3(1):4. doi: 10.1186/2110-5820-3-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fasano M, Curry S, Terreno E, Galliano M, Fanali G, Narciso P, Notari S, Ascenzi P. The extraordinary ligand binding property of human serum albumin. IUBMB life. 2006;57:787–796. doi: 10.1080/15216540500404093. [DOI] [PubMed] [Google Scholar]
- 59.Robertson SA. GM-CSF regulation of embryo development and pregnancy. Cytokine & Growth Factor Reviews. 2007;18(3):287–298. doi: 10.1016/j.cytogfr.2007.04.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 22 kb)
Data Availability Statement
All data was originally generated. For access to data, please contact the corresponding author Associate Professor Deirdre Zander-Fox.