Abstract
Background
Mammalian spermatogenesis is responsible for male fertility and is supported by the self-renewal and differentiation of spermatogonial stem cells (SSCs). Sertoli cells provide a supportive microenvironment for SSCs, in part by the production of stem cell factor (SCF), which is a potent regulator of spermatogonia proliferation and survival.
Methods
We investigated the novel role of β-estradiol in modulating the proliferation and apoptosis of fetal SSCs via the regulation of SCF secretion in Sertoli cells isolated from human fetal testes. The proliferation of SSCs in the co-culture system was determined by colony formation and BrdU incorporation assays. TUNEL assay was used to measure SSC apoptosis in co-culture in response to treatment with control, β-estradiol, or the combination of β-estradiol and the estrogen receptor inhibitor ICI 182780.
Results
In the system with purified human fetal Sertoli cells (MIS+/c-Kit−/AP−), β-estradiol upregulated the production of SCF in a dose- and time-dependent manner. In the co-culture system of primary human fetal SSCs (c-Kit+/SSEA-4+/Oct-4+/AP+) and Sertoli cells (MIS+), β-estradiol markedly increased the proliferation of SSCs. Moreover, SSC apoptosis was significantly inhibited by β-estradiol and was completely reversed by the combination of β-estradiol and ICI 182780.
Conclusion
Here we report, for the first time, that β-estradiol can induce the increase of SCF expression in human fetal Sertoli cells and regulates the growth and survival of human fetal SSCs. These novel findings provide new perspectives on the current understanding of the role of estrogen in human spermatogenesis.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10815-021-02240-y.
Keywords: Human fetal testis, Sertoli cells, Stem cell factor, Spermatogonial stem cells, β-estradiol
Introduction
Spermatogonial stem cells (SSCs) are defined by their capacity for self-renewal and differentiation and are crucial for spermatogenesis and male fertility [1]. SSCs are primarily located in the stem cell niche [2], a specialized microenvironment in the basal compartment of seminiferous tubules, where they are in close contact with Sertoli cells, peritubular myoid cells, secreted soluble factors, and extracellular matrix [3]. Sertoli cells are columnar-shaped epithelial cells that play important roles in supporting the biological functions of SSCs. Key evidence comes from a previous study that demonstrated that transplantation of normal Sertoli cells into seminiferous tubules successfully restored spermatogenesis in infertile mouse testes in which the infertility was caused by a defect in Sertoli cells [4]. Sertoli cells not only provide nutritional support but also produce growth factors, including stem cell factor (SCF) and activin, which stimulate the differentiation of SSCs, and glial cell–derived neurotrophic factor (GDNF) and fibroblast growth factor 2 (FGF2), which promote the self-renewal of SSCs [2, 5, 6]. Specifically, it has been shown that depletion of SCF alone greatly suppressed the proliferation of germ cells in rat testes, which could be rescued by supplementation of exogenous SCF [7]; this finding indicates that SCF plays an indispensable role in regulating the proliferation of undifferentiated SSCs. However, the regulatory mechanism of Sertoli cell–induced SCF secretion remains largely unknown, especially in the fetal testes. Aggregation of fetal Sertoli cells initiates testes cord formation, and germ cells are subsequently encased within the testes cords [8]. These fetal germ cells are characterized by the expression of alkaline phosphatase, and the pluripotency markers, NANOG, Oct4, and SSEA4 [9, 10]. Fetal Sertoli cells are required to maintain testicular differentiation and coordinate the proliferation of all other cell types within the testes, including the germ cells [11]. This prompts us to explore the factors that affect the SCF level in Sertoli cells and consequently modulate the growth and survival of SSCs.
Accumulating evidence has shown that estrogen plays a vital role in spermatogenesis and male fertility [12]. Indeed, targeted disruption of aromatase [13] or estrogen receptor (ER) [14] in animal models has been shown to significantly suppress spermatogenesis and sperm functions. In the testis, aromatase in Leydig cells induces androgens to synthesize estrogens, which subsequently bind to high-affinity ERs and transcriptionally regulate downstream estrogen-responsive genes [15]. There are two isoforms of ER: ERα and ERβ. Although they have high sequence homology, they are encoded by different genes on different chromosomes and are involved in distinct biological processes [16]. Both ERα and ERβ are expressed in the mammalian fetal testis with distinct distribution profiles [17, 18]. However, in the human fetal testis, ERβ is primarily located in the fetal germ cells, Sertoli cells, and Leydig cells, especially between 13 and 24 weeks of fetal age, while ERα is mostly absent [19, 20]. Our preliminary studies confirmed the existence of ERβ mRNA in the human fetal testis, the expression of which decreased with increasing fetal age. We have also demonstrated that the ability of SCF to stimulate the proliferation of human gonocytes decreases with increasing fetal age [21]. Given these previous findings, we hypothesized that estrogen binds to ERβ in Sertoli cells to stimulate SCF secretion, which in turn activates c-Kit signaling in SSCs and regulates the growth and survival of SSCs in the human fetal testis.
In this study, using β-estradiol as a tool, we sought to explore the effects of estrogens on SCF expression in a purified human fetal Sertoli cell system at both the transcriptional and translational level. We further established a unique co-culture system with primary fetal SSCs, identified using the markers SSEA4, Oct4, c-Kit, and AP, and Sertoli cells isolated from human fetal testes, using the marker MIS, and investigated whether estrogens regulate the proliferation and apoptosis of SSCs [8, 9, 21, 22]. Our results show, for the first time, the involvement of estrogens in the regulation of SCF-mediated growth and survival of SSCs in the human fetal testis.
Materials and methods
Collection of fetal testes
Sixteen human fetuses, between 16 and 28 weeks, were collected from pregnant women who underwent medical abortions in the Obstetrics and Gynecology Clinic of Changsha Maternity and Child Health Hospital. All the women provided informed consent. The integrity and morphology of the embryos were evaluated, and there were no obvious defects associated with testes development. The testes were taken from fetuses of 16–18 weeks (n = 7), 20–22 weeks (n = 7), and 24–28 weeks (n = 2) of gestation. The gestational age was determined based on ultrasonography. This study was performed in accordance with the national guidelines (Review of the Guidance on the Research Use of Fetuses and Fetal Material 1989). We used samples that were 16–28 weeks old in the Sertoli cell culture experiment and samples that were 16–18 weeks and 20–22 weeks old in the Sertoli and SSCs co-culture experiment.
Ex vivo culture of human fetal Sertoli cells and fetal SSCs
The testes were aseptically removed from fetuses (n = 16), washed 3 times in Dulbecco’s Modified Eagle Medium (DMEM; GIBCO, Life Technologies, New York, NY, USA), trimmed of the testicular capsules under a stereomicroscope, and minced with fine scissors before two-step enzymatic digestion. The decapsulated testes were then digested in Hank’s buffered salt solution (HBSS; GIBCO) containing 500-ng/ml DNase I and 500-ng/ml collagenase IV (Sigma Aldrich, St. Louis, MO, USA) at 37°C by shaking in a water bath for 15 min. After unit gravity sedimentation for 5 min, the interstitial cell–enriched supernatant was discarded. The remaining tissues were digested in DMEM containing 0.25% trypsin and 500-ng/ml DNase I at 37°C for 5 min. The reaction was quenched by the addition of DMEM supplemented with 10% fetal bovine serum (FBS; GIBCO), followed by centrifugation at 300 × g for 5 min at 4°C. A differential adherence method was used to obtain a purified cell suspension that mainly contained germ and Sertoli cells, while the majority of interstitial and myoblast-like cells were removed. Briefly, following two-step enzymatic digestion, the solutions were incubated at 37°C and 5% CO2 for 4 h. Following incubation, the solutions were gently transferred to a large culture dish, and the culture medium, which contained mostly germ and Sertoli cells, was collected after 3 h [23, 24]. For separation and culture of human fetal Sertoli cells, the cell suspension was filtered through a 40-μm cell strainer (BD Falcon, BD Biosciences, San Jose, CA, USA) and grown in DMEM supplemented with 1% MEM nonessential amino acid solution, 50-μM 2-mercaptoethanol, 2-mM L-glutamine, 1-mM sodium pyruvate (GIBCO), 10-ng/ml basic fibroblast growth factor (bFGF; R&D Systems, Minneapolis, MN, USA), 2.5-ng/ml mouse epidermal growth factor (EGF; Invitrogen, Carlsbad, CA, USA), and 100-ng/ml β-estradiol (E2; Sigma Aldrich) in a humidified atmosphere of 5% CO2. For co-culture of human fetal Sertoli cells and fetal SSCs, the suspension was filtered through a 50-μm cell strainer (BD Falcon) and grown in DMEM (high glucose) supplemented with 0.2% bovine serum albumin (BSA; Sigma Aldrich), 2-mM L-glutamine, 0.1-mM 2-mercaptoethanol, 1% MEM nonessential amino acid solution, 100-ng/ml SCF (R&D Systems), 10-ng/ml bFGF, and 103-U/ml leukemia inhibitory factor (LIF; R&D Systems) in a humidified atmosphere of 5% CO2.
Immunocytochemical staining
Immunocytochemical staining, including immunofluorescence staining, was performed on primary Sertoli cells and fetal SSCs after 3 passages. Cells were fixed with 4% paraformaldehyde and permeabilized with 0.1% Triton X-100 (Sigma). After blocking (1% BSA and 10% goat serum [Maixin Biotech, Fuzhou, China]) in phosphate-buffered saline (PBS; GIBCO) at room temperature for 30 min, the cells were incubated with primary antibodies at a dilution of 1:100 overnight at 4°C. The primary antibodies used in this study were as follows: mouse anti-human Mullerian inhibiting substance (MIS) monoclonal antibody (R&D Systems), rabbit anti-human c-Kit monoclonal antibody (Agilent Dako, Santa Clara, CA, USA), goat anti-human SCF polyclonal antibody (R&D Systems), goat anti-human ERβ polyclonal antibody, mouse anti-human stage-specific embryonic antigen-4 (SSEA4) monoclonal antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA), and mouse anti-human octamer-binding transcription factor 4 (Oct-4) monoclonal antibody (Santa Cruz). After washing with PBS for 5 min (three times), the cells were incubated with FITC-conjugated or rhodamine-conjugated secondary antibodies at a dilution of 1:500 for 1 h at room temperature. The slides were counterstained with 4,6-diamidino-2-phenylindole (DAPI; Sigma) for nuclear staining and were analyzed under a Nikon fluorescence microscope (Nikon 80i, Nikon, Tokyo, Japan). In addition, cellular alkaline phosphatase (AP) activity was detected via the BCIP/NBT Kit (GIBCO) according to the manufacturer’s instructions. For each experiment, at least 20 randomly selected fields (40 × magnification) were captured and analyzed for each sample.
Quantitative reverse transcription polymerase chain reaction (RT-PCR)
Total RNAs were extracted using TRI reagent (Sigma), followed by the First Strand cDNA Synthesis Kit for RT-PCR (Sigma) according to the manufacturer’s protocols. Quantitative RT-PCR was performed with SYBR® Green PCR Supermixes (Bio-Rad Laboratories, Hercules, CA, USA) at 95°C for 2 min, followed by 30 cycles of 94°C for 30 s, 60°C for 30 s, and 72°C for 30 s. The primers used were as follows: SCF, ACTAGTGACTGTGTGCTCTC (forward), ACTAGTTACCAGCCACTGTG (reverse); and β-actin (housekeeping gene), CGCACCACTGGCATTGTCAT (forward), TTCTCCTTGATGTCACGCAC (reverse).
BrdU cell proliferation assay
The DNA replication activity of primary human fetal SSCs co-cultured with Sertoli cells was analyzed with 5-bromodeoxyuridine (BrdU) labeling and detection. Cells were incubated with 10-μM BrdU (Sigma) overnight, fixed in 4% paraformaldehyde, and stored in 70% alcohol at 4°C. BrdU-labeled cells were then denatured in HCl (2 M) for 10 min, followed by immediate neutralization with sodium borate buffer (0.1 M, pH 8.5) for 20 min at room temperature. Cells were subsequently blocked with 5% BSA and 1% Triton X-100 in PBS to prevent unspecific binding, before incubating with mouse anti-human BrdU monoclonal antibody (1:500; Sigma) overnight at 4°C. Following incubation, the cells were incubated with FITC-conjugated anti-mouse antibody (1:100; Santa Cruz) at room temperature for 40 min. Nuclear DNA was labeled by 300-nM DAPI for 5 min at room temperature before visualization under a Nikon fluorescence microscope. The proliferation index of fetal SSCs was determined by the percentage of BrdU-positive nuclei/DAPI-labeled total nuclei within the fetal SSC colonies.
TUNEL apoptosis assay
Primary human fetal SSCs co-cultured with Sertoli cells were treated with 100-ng/ml E2 for 7 days. On day 7, E2 was removed, and the cells were further cultured with a medium supplemented with vehicle control, 100-ng/ml E2, or 100-ng/ml E2 + 10−7 M ICI 182780 (Sigma), a specific ER inhibitor, for 1, 3, or 5 days. To determine the percentage of apoptotic cells, we applied the terminal deoxynucleotidyl transferase–mediated dUTP nick-end labeling (TUNEL) technique using the In Situ Cell Death Detection Kit (Roche Applied Sciences) following the manufacturer’s instructions. Briefly, the cells were mounted on glass slides, washed with PBS, air-dried, fixed in fresh 4% paraformaldehyde for 1 h at room temperature, incubated in 0.1% Triton X-100 for 10 min on ice for membrane permeabilization, and covered with TUNEL reaction mixture for 1 h at 37°C in the dark. Cells were then counterstained with DAPI for quantification of the cell number before being analyzed under a Nikon fluorescence microscope. The percentage of TUNEL-positive nuclei/DAPI-labeled total nuclei within the fetal SSC colonies was designated as the index of apoptosis.
Statistical analysis
All quantitative data are presented as the mean ± standard error of the mean (SEM) from at least three independent experiments. One-way ANOVA followed by the non-parametric Kruskal-Wallis test was used to perform comparisons among experimental groups. P-values < 0.05 were considered to indicate statistically significant differences.
Results
Primary human fetal Sertoli cells express ERβ
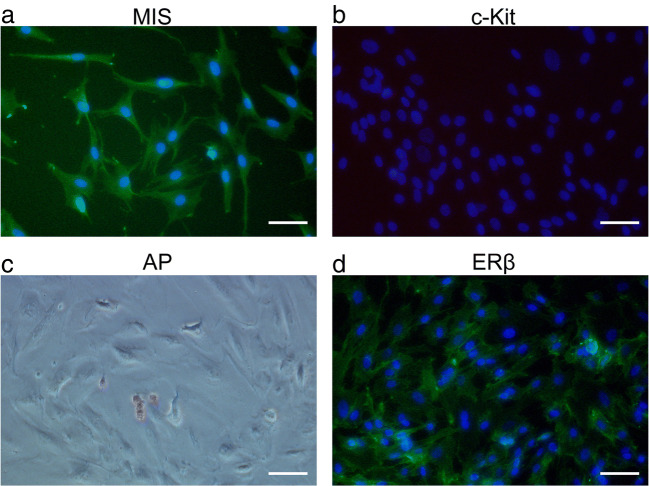
We performed immunocytochemical staining to validate the isolation and culture of Sertoli cells from human fetal testes. Isolated Sertoli cells were attached to the bottom of the culture dish and showed high expression of MIS, a specific embryonic stage marker (Fig. 1a). In addition, these cells were largely negative for c-Kit, a specific germ cell marker (Fig. 1b), and AP, a stem cell marker (Fig. 1c). The cell marker expression profiles were unchanged after 12 passages. Notably, ERβ expression was observed in the nucleus, cytoplasm, and cytoplastic membrane of isolated human fetal Sertoli cells (Fig. 1d), consistent with the expression pattern in fetal testicular tissues described in a previous report [20].
Fig. 1.
Identification of primary Sertoli cells isolated from human fetal testes. Primary human Sertoli cells were isolated from 16 human fetal testes. Immunocytochemical staining was performed on cells passaged no more than 12 times and prior to confluency. Representative images are shown. The nuclei are stained with DAPI (blue). Scale bar: 50 μm. a Immunostaining for Mullerian inhibiting substance (MIS) (green), a specific Sertoli cell marker; b immunostaining for c-Kit (green), a specific germ cell marker; c immunostaining for alkaline phosphatase (AP) (red), a stem cell marker; and d immunostaining for estrogen-responsive (ER)β (green)
E2 promotes SCF production in primary human fetal Sertoli cells
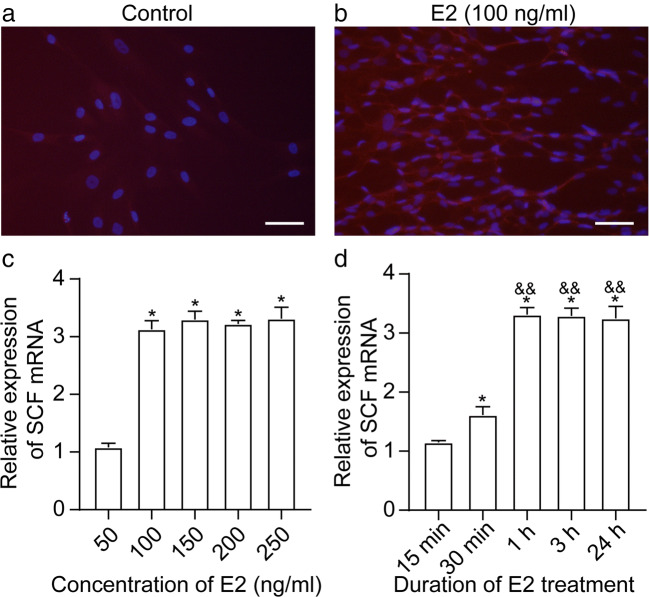
Next, we evaluated the ability of cultured primary human Sertoli cells to produce SCF in response to E2 (β-estradiol) stimulation, an important biological function of Sertoli cells [25]. Due to the limited number of specimens, the number of cultured Sertoli cells was insufficient; thus, we did not acquire reliable experimental results of SCF expression in Sertoli cells detected by western blot. Therefore, we tested our hypothesis by adding E2 to Sertoli cells to enhance the IF signal intensity of SCF expression in Sertoli cells. As shown in Fig. 2a, in the presence of 100-ng/ml E2, a strong fluorescence signal of SCF was generated in Sertoli cells, compared with minimal background fluorescence of SCF in the absence of E2 stimulation. To determine whether E2-induced SCF is dose- or time-dependent, we subjected Sertoli cells to 0-, 50-, 100-, 150-, 200-, or 500-ng/ml E2 for 1 h, or to 100-ng/ml E2 for 0, 15, or 30 min, or 1, 3, or 24 h, and subsequently performed quantitative RT-PCR for relative SCF mRNA expression. Compared to the vehicle control, 50-ng/ml E2 had no effect on the SCF level, 100-ng/ml E2 significantly induced the production of SCF (P < 0.05), but higher doses of E2 (150, 200, or 500 ng/ml) did not further increase SCF mRNA in Sertoli cells (Fig. 2b). Compared to the 0-h control group, SCF expression was markedly increased 30 min after E2 induction (P < 0.01 vs. 0 h) and peaked at 1-h post E2 stimulation (P < 0.01 vs. 0 h; P < 0.01 vs. 30 min). Induced SCF mRNA expression was not significantly different between 1 h and longer E2 treatment (3 or 24 h) (P > 0.05) (Fig. 2c).
Fig. 2.
E2 upregulates SCF in primary Sertoli cells. Primary human Sertoli cells were treated with vehicle control (a) or 100-ng/ml E2 (b). Immunofluorescence staining of SCF (red) was performed after 48 h. The nuclei are stained with DAPI (blue). Scale bar: 50 μm. c Primary human Sertoli cells were treated with 0-, 50-, 100-, 150-, 200-, or 500-ng/ml E2 for 1 h, and the relative expression of SCF mRNA was measured by qRT-PCR. β-actin was used as a housekeeping gene. *P < 0.05 vs. vehicle control. d Primary human Sertoli cells were treated with 100-ng/ml E2 for 0, 15, or 30 min, or 1, 3, or 24 h, and the relative expression of SCF mRNA was measured by qRT-PCR. β-actin was used as a housekeeping gene. *P < 0.01 vs. control group (0 h). &&P < 0.01 vs. 100-ng/ml E2 treatment for 30 min
Co-culture of fetal SSCs and Sertoli cells isolated from human fetal testes
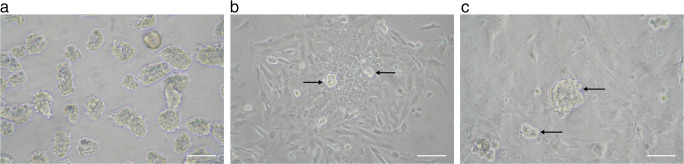
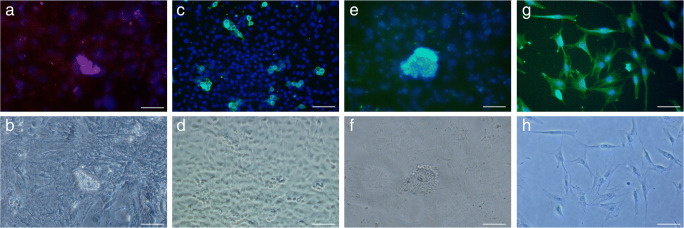
Immunofluorescence analysis was performed on a cryosection of a 20-week-old human fetal testis prior to the isolation of Sertoli cells and SSCs (Fig. S1). To obtain a purified co-culture of fetal SSCs and Sertoli cells, human fetal testicular tissue was processed through a two-step enzymatic digestion followed by differential adherence. The cell suspension was further cultured in gelatin-coated tissue culture plates (Fig. 3a). Over a 3–4-day period, Sertoli cells started to grow and attach to the bottom of the plates, displaying a columnar-shaped, fibroblast-like morphology. The round-shaped fetal SSCs, existing as single cells or in pairs, were anchored on top of the Sertoli cell layer (Fig. 3b). After 6–7 days, the fetal SSCs formed colonies consisting of 2–32 cells (Fig. 3c). We further clarified the phenotypic characteristics of these fetal SSC-derived colonies using immunofluorescence staining. SSC colonies clearly expressed c-Kit (Fig. 4a–b) and several pluripotent stem cell markers, including SSEA-4 (Fig. 4c–d) and Oct-4 (Fig. 4e–f), suggesting that the primary fetal SSCs isolated from human fetal testes were pluripotent. Additionally, the expression of MIS was found in the Sertoli cells growing in the bottom layer (Fig. 4g–h).
Fig. 3.
In vitro culture of seminiferous tubule fragments isolated from human fetal testes. a Human seminiferous tubule fragments were isolated from fetal testes via two-step enzymatic digestion followed by differential adherence. Scale bar: 100 μm. b Human seminiferous tubule fragments after 3 days of culture. c Human seminiferous tubule fragments after 7 days of culture. For b and c, the columnar-shaped Sertoli cells were attached to the bottom of the culture dish, and the round-shaped fetal SSCs grew on top of the Sertoli cells (black arrow). Scale bar: 50 μm
Fig. 4.
Identification of fetal SSCs and Sertoli cells isolated from human fetal testes. Immunocytochemical staining was performed in a unique co-culture system including human fetal SSCs and Sertoli cells. a Immunostaining of c-Kit (green); b phase-contrast microscopic image of a; c immunostaining of SSEA-4 (green); d phase-contrast microscopic image of c; e immunostaining of Oct-4 (green); f phase-contrast microscopic image of e; g immunostaining of MIS (green); and h phase-contrast microscopic image of g. The nuclei are stained with DAPI (blue). Scale bar: 50 μm
E2 enhances the proliferation of primary human fetal SSCs
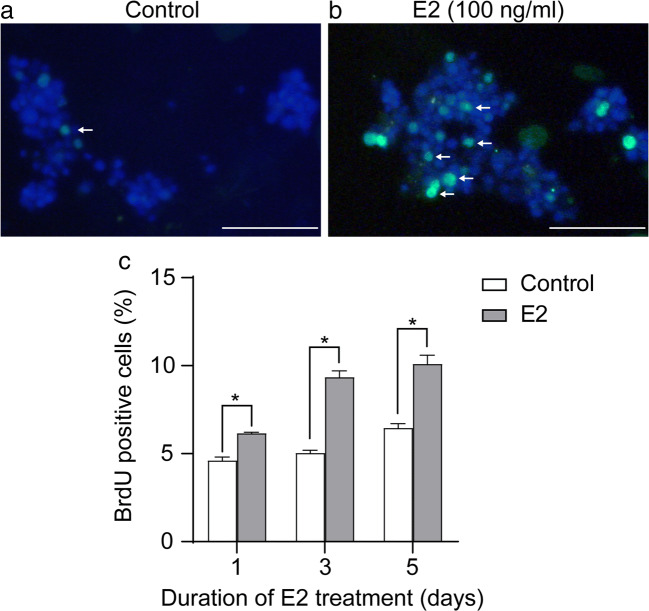
It is well known that SCF/c-Kit is essential for the differentiation and proliferation of spermatogonia [26]. Here, using a unique co-culture system of primary fetal SSCs and Sertoli cells, we investigated whether E2 could regulate the proliferation of human fetal SSCs by inducing the production of SCF from Sertoli cells. The cells were treated with 100-ng/ml E2 or left untreated, and the proliferation of SSCs was analyzed after 7 days. Based on their size, SSC colonies were briefly categorized into groups of 2–4 cells, 4–8 cells, 8–16 cells, and > 16 cells. We found that in the control group, the average size of the SSC colonies was 4–8 cells, while the majority of E2-induced SSC colonies consisted of 8–16 cells and > 16 cells (Fig. 5), suggesting that E2 increased the proliferative ability of SSCs. However, we have not statistically analyzed the clone numbers of 2–4 cells, 4–8 cells, 8–16 cells, and > 16 cells due to the limited number of specimens and the difficulties related to SSCs culture. We further performed a BrdU incorporation test to determine the DNA replication activity and verify the above findings. The cells were treated with or without 100-ng/ml E2 for 5 days. Because Sertoli cells are adherent in the co-culture system, and since SSCs are clones protruding above Sertoli cells, we distinguished them according to their positions when counting BrdU-positive cells. The results demonstrated that the percentage of BrdU-positive SSCs was significantly increased in the E2-treated group compared to the control group at all time points tested (day 1 6.16% ± 0.06% vs. 4.61% ± 0.19%, P < 0.01; day 3 9.35% ± 0.36% vs. 5.03% ± 0.16%, P < 0.01; day 5 10.10% ± 0.49% vs. 6.45% ± 0.26%, P < 0.01) (Fig. 6a–b).
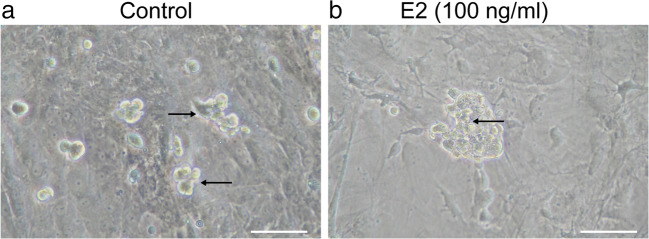
Fig. 5.
E2 stimulates the colony formation capability of human fetal SSCs. Co-cultured fetal SSCs and Sertoli cells isolated from human fetal testes were treated with vehicle control (a) or 100-ng/ml E2 (b). Representative image showing colony formation (black arrow) 7 days after treatment. Scale bar: 50 μm
Fig. 6.
E2 stimulates DNA replication in human fetal SSCs. Analysis of DNA replication in the absence or presence of 100-ng/ml E2 was measured by BrdU incorporation in human fetal SSCs co-cultured with Sertoli cells. Representative image of BrdU fluorescence (green) (white arrow) and DAPI (blue) in control (a) and E2-treated (b) groups. Scale bar: 50 μm. c Percentage of BrdU-positive cells relative to the total cell count (200 random cells) after 1, 3, or 5 days of vehicle control or E2 treatment. Data are shown as mean ± SEM (n = 3 in each group). *P < 0.01 vs. control
E2 suppresses apoptosis of primary human fetal SSCs
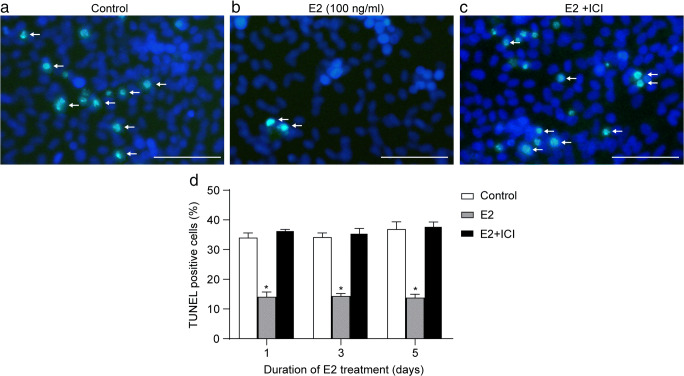
We also explored the impact of E2 on the apoptosis of human fetal SSCs using our co-culture system of primary fetal SSCs and Sertoli cells. Under the microscope, we found that at day 5, the SSC colonies in the control group dissociated into single cells with typical apoptotic features as confirmed by TUNEL staining. SSC colonies remained intact and integrated in the E2-treated group, and the combination of E2 and ICI 182780 almost completely abolished this effect (Fig. 7a). Quantification of TUNEL staining demonstrated that the percentage of TUNEL-positive apoptotic cells was significantly reduced in the E2 group compared to the control group at all time points tested (days 1, 3, and 5) (P < 0.01 vs. control). The addition of ICI 182780, which blocked the effect of E2 in co-cultured Sertoli cells, completely reversed the reduction in apoptosis in fetal SSCs (P > 0.05 vs. control) (Fig. 7b). We note that the identification of apoptotic cells and SSCs markers in the co-culture system would make our findings more convincing.
Fig. 7.
E2 suppresses apoptosis of human fetal SSCs. Human fetal SSCs co-cultured with Sertoli cells were treated with vehicle control, 100-ng/ml E2, or 100-ng/ml E2 + 10−7 M ICI 182780 for 1, 3, or 5 days, stained for TUNEL, and examined by fluorescence microscopy. Representative image of TUNEL staining (green) (white arrow) and DAPI (blue) in control (a), E2-treated (b), and E2 + ICI treated (c). Scale bar: 50 μm. d The percentage of apoptotic nuclei in human fetal SSCs relative to the total cell count (200 random cells) under different treatments. Values are presented as the mean ± SEM of three separate experiments. *P < 0.01 vs. control
Discussion
Idiopathic male infertility, a condition in which defective fertility spontaneously arises, is a global reproductive health issue [27]. Over 500 genes are correlated to this disease [28], among which stem cell factor (SCF)/c-Kit signaling, which regulates the proliferation and apoptosis of spermatogonial stem cells [29], is particularly important. However, little is known of the gene-environment interactive regulation of SCF/c-Kit in spermatogenesis.
In the fetal testes, Sertoli cells form the testis cords, which play an important role in the regulation of fetal SSCs development [8]. Here, we report that E2 (β-estradiol), via binding to ERβ, induced the expression of SCF in primary Sertoli cells isolated from human fetal testes between 16 and 28 weeks of gestation. Interestingly, we found that E2 induced SCF in a time-dependent manner. After E2 treatment, a significant increase in SCF mRNA was observed after 30 min, peaked at 1 h, and persisted until 24 h. This finding may be partly explained by different mechanisms of ligand-dependent ER activation. In the absence of estrogens, the majority of ER is located in the nucleus and forms a Hsp90-based chaperon complex, which inactivates the transcriptional activity of ER. The binding of estrogens dissociates ER from this chaperon complex, which in turn dimerizes and binds to estrogen-response elements (EREs) in estrogen-responsive genes [30]. This nuclear ER-mediated signal transduction is relatively slow and may be responsible for the induction of SCF up to 24 h. Furthermore, it has been shown that estrogens can bind to plasma membrane ER and rapidly induce the production of second messengers, including Ca2+ and cAMP, which activate various kinases, such as Src, PI3K, or ERK, leading to the phosphorylation of the proteins responsible for cell migration, growth, and survival [31]. This membrane ER-mediated signaling likely contributes to the early induction of SCF within minutes. Consistent with this explanation, we showed that ERβ was widely expressed in the nucleus, cytoplasm, and cytoplasmic membrane of fetal Sertoli cells. These findings suggest that estrogens affect the development of SSCs through regulating SCF/c-Kit signaling.
Although the expression of ERβ in primordial germ cells has been reported in certain non-human animal models [32], it is not considered to be able to initiate the proliferation of spermatogonial stem cells. Our previous work confirmed this speculation by showing that the addition of E2 had no significant effect on the growth of human fetal SSCs in the absence of Sertoli cells. Extensive studies have confirmed the pivotal functions of somatic cells within the stem cell niche, including Sertoli cells, in spermatogenesis through both endocrine and paracrine signaling pathways [33]. For example, Moe-Behrens’ group demonstrated that estrogens promoted the proliferation of mouse primordial germ cells through stimulating the transcription of several growth factors and the subsequent activation of the Akt/PTEN signaling pathway in somatic cells within the testicular stem cell niche [34]. Similarly, SCF induction in human fetal Sertoli cells might be a key hub connecting estrogens and the development of SSCs.
To test the above hypothesis, we established a unique co-culture system of fetal SSCs and Sertoli cells isolated from human fetal testes to mimic the microenvironment of the testicular stem cell niche. Phase-contrast microscopy and immunocytochemical staining of cellular markers were used to validate the morphology and biological activity of fetal SSCs and Sertoli cells. Our data further showed that estrogens significantly increased the proliferation and decreased the apoptosis of human fetal SSCs via transcriptionally inducing SCF/c-Kit signaling in Sertoli cells. These findings suggest that the 100-ng/ml dose of E2 used in our study is sufficient to positively regulate the development of SSCs in human fetal testes.
A recent study in adult rat seminiferous tubules demonstrated that E2 increased SCF expression, which was consistent with our results. However, this previous study also showed that E2 inhibited c-Kit expression, increased apoptosis, and decreased proliferation in rat seminiferous tubules [35]. Despite the differences in the study subjects and the dose of E2, this discrepancy may be partially due to differences in the expression of the SCF/c-Kit system between fetal and adult testes. Indeed, an early study showed abundant expression of c-Kit in differentiating spermatogonia and Leydig cells and minimal expression in undifferentiated spermatogonia and Sertoli cells [36]. Although c-Kit is not directly correlated with the self-renewal capacity of SSCs [37], it might be involved in the phenotypic transitions of SSCs [38]. Therefore, fetal SSCs with low c-Kit expression and adult seminiferous tubules with high c-Kit expression might respond differently to E2 treatment. In addition, the SCF/c-Kit system plays different roles at different stages of spermatogenesis, which further complicates the potential cellular response to SCF/c-Kit and its upstream regulators. For example, in germline stem cells at an early stage of development, disruption of c-Kit or SCF has been shown to reduce the adhesiveness of primordial germ cells to nearby somatic cells [39]. Moreover, directional migration in response to SCF treatment was shown in primordial germ cells, but not in c-Kit–absent or c-Kit–inhibited cells, suggesting the involvement of the SCF/c-Kit system in the chemotactic migration of primordial germ cells [40].
The regulation of cell proliferation in SSCs is complex and multifactorial. Due to the limitations of our study, we were unable to perform rescue assays to confirm whether SCF is the only mediator of E2-regulated SSC proliferation. Nevertheless, we found that the stimulatory effects of E2 on SCF and SSC proliferation were limited to 2 weeks, indicating that E2 alone was unable to promote continuous growth of SSCs. Therefore, we speculate that other key factors and regulatory mechanisms are necessary for the self-renewal and differentiation of SSCs and are likely to form a tight interactive network to modulate the growth and survival of SSCs. These possibilities will be investigated in future studies.
In summary, this study shows, for the first time, that exogenous E2 regulates the proliferation and apoptosis of fetal SSCs through regulation of Sertoli cells. These findings provide some valuable information in the context of fetal testes development. In addition, our co-culture system of fetal SSCs and Sertoli cells isolated from human fetal testes accurately reflects the testicular stem cell niche and could be further optimized and used as a platform to study the gene-environment interactive regulations of SCF/c-Kit in spermatogenesis.
Supplementary information
Identification of fetal SSCs and Sertoli cells in 20-week-old human fetal testes. Immunofluorescence analysis was performed on a cryosection of a 20-week-old human fetal testis. (a) Immunostaining of Oct4 (green); (b) immunostaining of MIS (green). The nuclei are stained with DAPI (blue). Scale bar: 50 μm. (PNG 267 kb)
Author contribution
LQF and KT designed the study. YCC, YS, LX, and DZ supervised sample collection. KT, YS, and WBZ performed the experiments and data collection. LZL revised data analysis and figures preparation. All authors have participated in the manuscript preparation.
Funding
This work was supported by the National Natural Science Foundation of China (No. 31472054), the National Key Research and Development Program of China (2016YFC1000600), the Natural Science Foundation of Hunan Province (No. 2018JJ3367), and the Scientific Research Foundation of Hunan Provincial Education Department (No. 18C0067).
Declarations
Ethics approval
This study was approved by the Ethics Committee of the Reproductive & Genetic Hospital of CITIC-Xiangya, Basic Medical Science School, Central South University, Changsha, China.
Consent to participate
Oral and written informed consent was obtained from each participant.
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Phillips BT, Gassei K, Orwig KE. Spermatogonial stem cell regulation and spermatogenesis. Philos Trans R Soc Lond Ser B Biol Sci. 2010;365(1546):1663–1678. doi: 10.1098/rstb.2010.0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Rooij DG. The spermatogonial stem cell niche. Microsc Res Tech. 2009;72(8):580–585. doi: 10.1002/jemt.20699. [DOI] [PubMed] [Google Scholar]
- 3.Dadoune JP. New insights into male gametogenesis: what about the spermatogonial stem cell niche. Folia Histochem Cytobiol. 2007;45(3):141–147. [PubMed] [Google Scholar]
- 4.Kanatsu-Shinohara M, Miki H, Inoue K, Ogonuki N, Toyokuni S, Ogura A, Shinohara T. Germline niche transplantation restores fertility in infertile mice. Hum Reprod. 2005;20(9):2376–2382. doi: 10.1093/humrep/dei096. [DOI] [PubMed] [Google Scholar]
- 5.Griswold MD. The central role of Sertoli cells in spermatogenesis. Semin Cell Dev Biol. 1998;9(4):411–416. doi: 10.1006/scdb.1998.0203. [DOI] [PubMed] [Google Scholar]
- 6.Cardoso HJ, Figueira MI, Correia S, Vaz CV, Socorro S. The SCF/c-KIT system in the male: survival strategies in fertility and cancer. Mol Reprod Dev. 2014;81(12):1064–1079. doi: 10.1002/mrd.22430. [DOI] [PubMed] [Google Scholar]
- 7.Allard EK, Blanchard KT, Boekelheide K. Exogenous stem cell factor (SCF) compensates for altered endogenous SCF expression in 2,5-hexanedione-induced testicular atrophy in rats. Biol Reprod. 1996;55(1):185–193. doi: 10.1095/biolreprod55.1.185. [DOI] [PubMed] [Google Scholar]
- 8.Chojnacka K, Zarzycka M, Mruk DD. Biology of the Sertoli cell in the fetal, pubertal, and adult mammalian testis. Results Probl Cell Differ. 2016;58:225–251. doi: 10.1007/978-3-319-31973-5_9. [DOI] [PubMed] [Google Scholar]
- 9.Altman E, Yango P, Moustafa R, Smith JF, Klatsky PC, Tran ND. Characterization of human spermatogonial stem cell markers in fetal, pediatric, and adult testicular tissues. Reproduction. 2014;148(4):417–427. doi: 10.1530/REP-14-0123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baroni T, Arato I, Mancuso F, Calafiore R, Luca G. On the origin of testicular germ cell tumors: from gonocytes to testicular cancer. Front Endocrinol (Lausanne) 2019;10:343. doi: 10.3389/fendo.2019.00343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mäkelä JA, Koskenniemi JJ, Virtanen HE, Toppari J. Testis development. Endocr Rev. 2019;40(4):857–905. doi: 10.1210/er.2018-00140. [DOI] [PubMed] [Google Scholar]
- 12.Carreau S, Bouraima-Lelong H, Delalande C. Estrogen, a female hormone involved in spermatogenesis. Adv Med Sci. 2012;57(1):31–36. doi: 10.2478/v10039-012-0005-y. [DOI] [PubMed] [Google Scholar]
- 13.Robertson KM, O'Donnell L, Jones MEE, Meachem SJ, Boon WC, Fisher CR, Graves KH, McLachlan RI, Simpson ER. Impairment of spermatogenesis in mice lacking a functional aromatase (cyp 19) gene. Proc Natl Acad Sci U S A. 1999;96(14):7986–7991. doi: 10.1073/pnas.96.14.7986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eddy EM, Washburn TF, Bunch DO, Goulding EH, Gladen BC, Lubahn DB, Korach KS. Targeted disruption of the estrogen receptor gene in male mice causes alteration of spermatogenesis and infertility. Endocrinology. 1996;137(11):4796–4805. doi: 10.1210/endo.137.11.8895349. [DOI] [PubMed] [Google Scholar]
- 15.Hess RA. Estrogen in the adult male reproductive tract: a review. Reprod Biol Endocrinol. 2003;1:52. doi: 10.1186/1477-7827-1-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heldring N, Pike A, Andersson S, Matthews J, Cheng G, Hartman J, Tujague M, Ström A, Treuter E, Warner M, Gustafsson JÅ. Estrogen receptors: how do they signal and what are their targets. Physiol Rev. 2007;87(3):905–931. doi: 10.1152/physrev.00026.2006. [DOI] [PubMed] [Google Scholar]
- 17.Rago V, et al. Differential expression of estrogen receptors (ERalpha/ERbeta) in testis of mature and immature pigs. Anat Rec A Discov Mol Cell Evol Biol. 2004;281(2):1234–1239. doi: 10.1002/ar.a.20131. [DOI] [PubMed] [Google Scholar]
- 18.Saunders PT, Sharpe RM, Williams K, Macpherson S, Urquart H, Irvine DS, Millar MR. Differential expression of oestrogen receptor alpha and beta proteins in the testes and male reproductive system of human and non-human primates. Mol Hum Reprod. 2001;7(3):227–236. doi: 10.1093/molehr/7.3.227. [DOI] [PubMed] [Google Scholar]
- 19.Boukari K, Ciampi ML, Guiochon-Mantel A, Young J, Lombes M, Meduri G. Human fetal testis: source of estrogen and target of estrogen action. Hum Reprod. 2007;22(7):1885–1892. doi: 10.1093/humrep/dem091. [DOI] [PubMed] [Google Scholar]
- 20.Gaskell TL, Robinson LLL, Groome NP, Anderson RA, Saunders PTK. Differential expression of two estrogen receptor beta isoforms in the human fetal testis during the second trimester of pregnancy. J Clin Endocrinol Metab. 2003;88(1):424–432. doi: 10.1210/jc.2002-020811. [DOI] [PubMed] [Google Scholar]
- 21.Tu J, Fan L, Tao K, Zhu W, Li J, Lu G. Stem cell factor affects fate determination of human gonocytes in vitro. Reproduction. 2007;134(6):757–765. doi: 10.1530/REP-07-0161. [DOI] [PubMed] [Google Scholar]
- 22.Lakpour MR, Aghajanpour S, Koruji M, Shahverdi A, Sadighi-Gilani MA, Sabbaghian M, Aflatoonian R, Rajabian-Naghandar M. Isolation, culture and characterization of human Sertoli cells by flow cytometry: development of procedure. J Reprod Infertil. 2017;18(2):213–217. [PMC free article] [PubMed] [Google Scholar]
- 23.Guo Y, Hai Y, Gong Y, Li Z, He Z. Characterization, isolation, and culture of mouse and human spermatogonial stem cells. J Cell Physiol. 2014;229(4):407–413. doi: 10.1002/jcp.24471. [DOI] [PubMed] [Google Scholar]
- 24.Liu S, Tang Z, Xiong T, Tang W. Isolation and characterization of human spermatogonial stem cells. Reprod Biol Endocrinol. 2011;9:141. doi: 10.1186/1477-7827-9-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chui K, Trivedi A, Cheng CY, Cherbavaz DB, Dazin PF, Huynh ALT, Mitchell JB, Rabinovich GA, Noble-Haeusslein LJ, John CM. Characterization and functionality of proliferative human Sertoli cells. Cell Transplant. 2011;20(5):619–635. doi: 10.3727/096368910X536563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mei XX, Wang J, Wu J. Extrinsic and intrinsic factors controlling spermatogonial stem cell self-renewal and differentiation. Asian J Androl. 2015;17(3):347–354. doi: 10.4103/1008-682X.148080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gunes S, Arslan MA, Hekim GNT, Asci R. The role of epigenetics in idiopathic male infertility. J Assist Reprod Genet. 2016;33(5):553–569. doi: 10.1007/s10815-016-0682-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Krausz C, Escamilla AR, Chianese C. Genetics of male infertility: from research to clinic. Reproduction. 2015;150(5):R159–R174. doi: 10.1530/REP-15-0261. [DOI] [PubMed] [Google Scholar]
- 29.Mauduit C, Hamamah S, Benahmed M. Stem cell factor/c-kit system in spermatogenesis. Hum Reprod Update. 1999;5(5):535–545. doi: 10.1093/humupd/5.5.535. [DOI] [PubMed] [Google Scholar]
- 30.Fliss AE, Benzeno S, Rao J, Caplan AJ. Control of estrogen receptor ligand binding by Hsp90. J Steroid Biochem Mol Biol. 2000;72(5):223–230. doi: 10.1016/S0960-0760(00)00037-6. [DOI] [PubMed] [Google Scholar]
- 31.Levin ER. Plasma membrane estrogen receptors. Trends Endocrinol Metab. 2009;20(10):477–482. doi: 10.1016/j.tem.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Caneguim BH, Beltrame FL, da Luz JS, Valentini SR, Cerri PS, Sasso-Cerri E. Primordial germ cells (spermatogonial stem cells) of bullfrogs express sex hormone-binding globulin and steroid receptors during seasonal spermatogenesis. Cells Tissues Organs. 2013;197(2):136–144. doi: 10.1159/000341517. [DOI] [PubMed] [Google Scholar]
- 33.Oatley JM, Brinster RL. The germline stem cell niche unit in mammalian testes. Physiol Rev. 2012;92(2):577–595. doi: 10.1152/physrev.00025.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moe-Behrens GH, et al. Akt/PTEN signaling mediates estrogen-dependent proliferation of primordial germ cells in vitro. Mol Endocrinol. 2003;17(12):2630–2638. doi: 10.1210/me.2003-0006. [DOI] [PubMed] [Google Scholar]
- 35.Correia S, Alves MR, Cavaco JE, Oliveira PF, Socorro S. Estrogenic regulation of testicular expression of stem cell factor and c-kit: implications in germ cell survival and male fertility. Fertil Steril. 2014;102(1):299–306. doi: 10.1016/j.fertnstert.2014.04.009. [DOI] [PubMed] [Google Scholar]
- 36.Schrans-Stassen BH, et al. Differential expression of c-kit in mouse undifferentiated and differentiating type A spermatogonia. Endocrinology. 1999;140(12):5894–5900. doi: 10.1210/endo.140.12.7172. [DOI] [PubMed] [Google Scholar]
- 37.Morimoto H, Kanatsu-Shinohara M, Takashima S, Chuma S, Nakatsuji N, Takehashi M, Shinohara T. Phenotypic plasticity of mouse spermatogonial stem cells. PLoS One. 2009;4(11):e7909. doi: 10.1371/journal.pone.0007909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang L, Tang J, Haines CJ, Feng H, Lai L, Teng X, Han Y. c-kit and its related genes in spermatogonial differentiation. Spermatogenesis. 2011;1(3):186–194. doi: 10.4161/spmg.1.3.17760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pesce M, Di Carlo A, De Felici M. The c-kit receptor is involved in the adhesion of mouse primordial germ cells to somatic cells in culture. Mech Dev. 1997;68(1-2):37–44. doi: 10.1016/S0925-4773(97)00120-2. [DOI] [PubMed] [Google Scholar]
- 40.Srihawong T, Kuwana T, Siripattarapravat K, Tirawattanawanich C. Chicken primordial germ cell motility in response to stem cell factor sensing. Int J Dev Biol. 2015;59(10-12):453–460. doi: 10.1387/ijdb.140287ct. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Identification of fetal SSCs and Sertoli cells in 20-week-old human fetal testes. Immunofluorescence analysis was performed on a cryosection of a 20-week-old human fetal testis. (a) Immunostaining of Oct4 (green); (b) immunostaining of MIS (green). The nuclei are stained with DAPI (blue). Scale bar: 50 μm. (PNG 267 kb)