Abstract
Purpose
TUBB8 is a gene that is frequently analysed in the genetic diagnosis of female infertility; 102 variants of this gene have been identified. However, the evaluation of its pathogenicity and the resulting phenotypes vary. Here, we aimed to identify novel TUBB8 variants as well as to summarize the reported variants and phenotypes in order for them to be included in genetic counselling analyses.
Methods
We performed whole exome sequencing to screen for candidate variants in 100 infertile female subjects and 100 controls who were able to conceive naturally. All variants were confirmed by Sanger sequencing. The effects of the variants in oocytes/arrested embryos were assessed by morphological observations, polar body biopsies, and chromosome analysis. A molecular modelling analysis was used to evaluate the possible effects of variants on protein secondary structure.
Results
We identified 29 TUBB8 variants, of which 20 were novel and five were maternally inherited. We identified three of a total of six recurrent variants that were specific for complete cleavage failure. Moreover, we obtained evidence that TUBB8 variants with large polar bodies had chromosome segregation errors.
Conclusions
Our study expands the spectrum of TUBB8 variants, particularly for embryonic arrest. Together with the extant knowledge of TUBB8 variants, this study provides a foundation for the genetic counselling of female infertility.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10815-021-02219-9.
Keywords: TUBB8 variants, Phenotype, Embryonic arrest, Female infertility, Genetic counselling
Introduction
Female infertility is a worldwide health problem that affects an estimated 48 million women [1]. With the help of in vitro fertilization (IVF) and intracytoplasmic sperm injection (ICSI) techniques, many otherwise infertile couples have had babies successfully. However, there are still women who cannot establish a pregnancy, presenting with recurrent IVF/ICSI failure due to oocyte meiotic arrest, fertilization failure, or embryonic arrest [2–4].
It is known that human oocyte maturation is the first and key process for the acquisition of accurate cleavage competence, including nuclear maturation and cytoplasmic maturation [5]. Nuclear maturation can be easily verified by the extrusion of the first polar body (PB1), whereas cytoplasmic maturation involves various invisible events such as spindle assembly, organelle distribution, and maternal mRNA metabolism [6].
Tubulin beta 8 class VII (TUBB8) is a highly conserved primate-specific β-tubulin isotype, which specifically participates in spindle assembly in oocytes and early embryos [7]. In 2016, Feng et al. identified TUBB8 as the first disease-causing gene involved in oocyte meiotic arrest, and their two consecutive studies revealed a 37.2% prevalence of TUBB8 variants in a total of 43 infertile women experienced IVF/ICSI failure [7, 8]. Totally, 102 different TUBB8 variants have been reported that are related to abnormalities in oocyte maturation, fertilization, and embryonic development [7–18]. As a result, TUBB8 is now a gene that is commonly analysed in the genetic diagnosis of infertile women. However, it should be noted that the evidence for the pathogenicity of these TUBB8 variant has been obtained using a variety of different means, including in vitro functional verification, morphological observation of the spindle, pedigree segregation analysis, and the genetic detection of probands. The causal relationship between these TUBB8 variants and the various observed phenotypes thus requires further investigation, as does the spectrum of potential pathogenic TUBB8 variants.
In the present study, using whole exome sequencing (WES), we identified 29 variants in TUBB8 from 32 independent families, of which 20 were novel, and summarized the reported 102 variants and phenotypes. We demonstrated various abnormalities in the oocytes, zygotes, and embryos from patients with these TUBB8 variants. Our findings significantly extend the spectrum of TUBB8 variants and, together with the available knowledge on TUBB8 variants and female infertility, will potentially impact the genetic counselling of infertile women in future.
Materials and methods
Clinical samples
In this study, a total of 100 infertile females experienced IVF/ICSI failure due to oocyte meiotic arrest, fertilization failure, or embryonic arrest, and 100 fertile control females who had naturally conceived were recruited from the Reproductive and Genetic Hospital of CITIC-XIANGYA, China. All blood samples were donated for research after informed consent was obtained. This study was approved by the Ethics Committee of the Reproductive and Genetic Hospital of CITIC-XIANGYA (reference LL-SC-2017-009).
WES and variant analysis
Genomic DNA samples were extracted from peripheral blood using standard protocol (QIAamp DNA Blood Kit, Qiagen, 51106, Germany), and exome capture and sequencing of the probands and fertile controls was performed using xGen Exome Research Panel v1.0 and Illumina HiSeq 2500 platform. The raw sequencing data is with a read depth reached 100X, and more than 95% of the area covered more than 20X. The single nucleotide variants (SNVs) and small insertions and deletions (indels) were generated with Genome Analysis Toolkit (GATK) pipeline and annotated with ANNOVAR. Variants were filtered as previously described [19], using the following criteria: (1) variants with minor allele frequencies (less than 1% in the Genome AD database); (2) exonic nonsynonymous or splice site variants, or coding insertions or deletions (indels); (3) mRNA and/or proteins that were highly or specifically expressed in oocytes; and (4) coexistence in both probands but absence in controls.
Sanger sequencing
All potential TUBB8 pathogenic variants were validated using Sanger sequencing. Specific primers flanking the variants in the TUBB8 gene were used for polymerase chain reaction (PCR) amplification with an ABI 3100 DNA analyser (Applied Biosystems, Foster City, CA, USA). The 29 families have been performed the segregation analysis of human pedigree.
Molecular modelling and evolutionary conservation analysis
Wild-type (WT) and TUBB8 variants were assessed using the SWISS-MODEL software (https://swissmodel.expasy.org) based on the 6e88.B.pdb template. TUBB8 mutants were mapped to the atomic model using PyMol (http://www. pymol.org). Evolutionary conservation analysis was performed with MultiAlin (http://multalin.toulouse.inra.fr/multalin/multalin.html) software.
Polar body biopsy and chromosome analysis
The PB1 was removed from the perivitelline space of the oocyte with a biopsy needle after laser dissection opening of the zona pellucida (ZP) and then placed into a 0.2-mL microtube. Whole genome amplification was then performed using the PicoPLEX whole genome amplification kit (Rubicon Genomics, Ann Arbor, USA) to produce the DNA required for next-generation sequencing by an Illumina NextSeq 550 platform (Illumina, San Diego, USA) [20].
Results
Identification of TUBB8 pathogenic variants
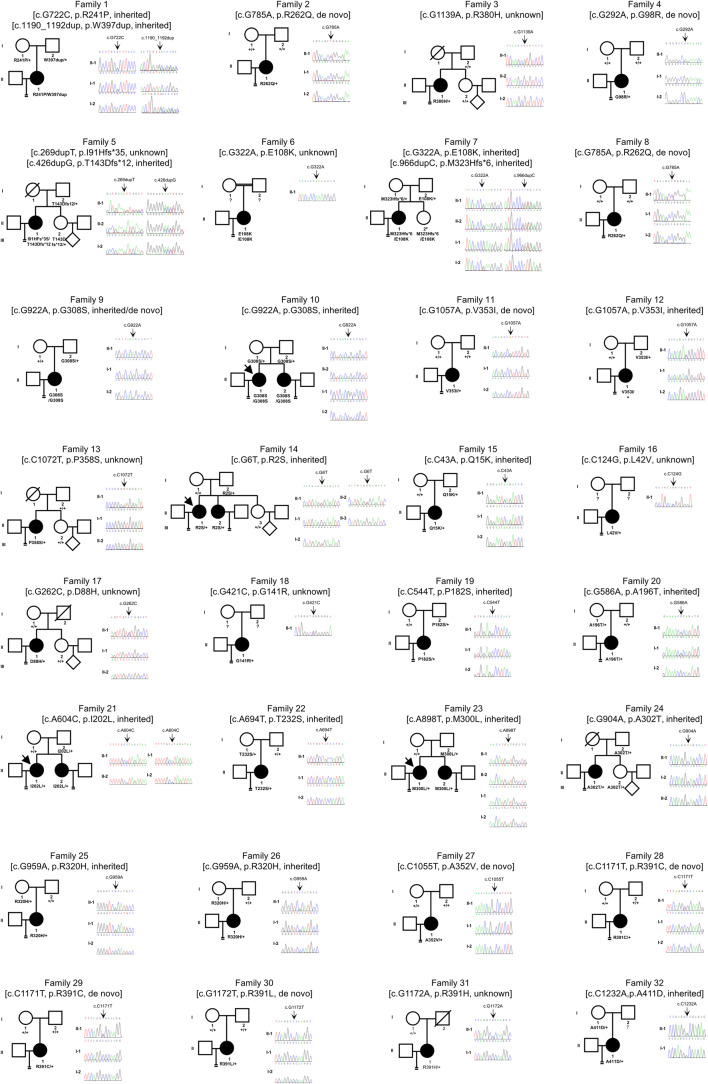
WES and Sanger sequencing were used to identify pathogenic variants. After filtering the data according to the criteria described in the “Materials and methods” section, we identified TUBB8 (MIM: 616768; GenBank: NM_177987) variants from 32 of a total of 100 independent individuals. We identified 29 variants in total, of which 20 were novel, including 25 missense variants, three frameshift insertion variants, and one non-frameshift insertion variant (Fig. 1). All of these were extremely rare (frequencies < 10−4) or absent in the gnomAD v2.1 database and were also not found in 100 fertile controls (Table 1). Most of the variants (22/29) were heterozygous; however, some of the inheritance patterns were unknown due to the absence of DNA samples from parents. Five families showed a recessive inheritance pattern, including families 6, 9, and 10 that were identified with a homozygous variant and families 1 and 7 that were identified with compound heterozygous variant (Fig. 1 and Table 1). We found some variants such as p.E108K, p.R262Q, p.G308S, p.R320H, p.V353I, and p.R391C where each of these variants was identified in two patients. p.E108K, p.R262Q, and p.R391C have previously been reported to have a causal relationship with complete cleavage failure [12], oocyte meiotic arrest [7], and embryonic arrest [13], respectively. These aspects were consistent with the phenotypes observed for the same variants in our study. However, this study is the first to show that p.G308S and p.V353I are first presented as other two recurrent variant sites related to complete cleavage failure. Among the variants identified, we found five (p.A196T, p.T232S, p.A302T, p.R320H, and p.A411D) in six embryonic arrest families (20, 22, 24, 25, 26, and 32) that were maternally inherited or were also detected in a fertile sister in the proband (Fig. 1).
Fig. 1.
Identification of variants in TUBB8. Pedigree analysis of 32 families with TUBB8 variants. The black circles with arrows indicate affected individuals. Sanger sequencing traces as confirmation are shown on the right. Unavailable parental information is indicated by question marks. “#” labelling in the II-2 of family 7 represents unclarity in the fertility because she is virgin despite carrying the same mutations as proband
Table 1.
Overview of the TUBB8 variants observed in 32 families
| Probands in families | cDNA change | Protein change | Mutation type | Genotype | gnomAD AFa | SIFTb | Mutation Tasterb | Main phenotypec | Met criteria codesd | Classificationd |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | c.G722C | p.R241P | Missense | com-het | 0 | D | D | MA | PM2+ PP3 | VUS |
| c.1190_1192dup | p.W397dup | Non-frameshift insertion | com-het | 0 | NA | NA | MA | PM2+PM4 | VUS | |
| 2/8 | c.G785A | p.R262Q | Missense | het | 0 | D | D | MA/CCF | PS2+PS3_Moderate+PM2 | LP |
| 3 | c.G1139A | p.R380H | Missense | het | 0 | D | D | MA | PM2, PP3 | VUS |
| 4 | c.G292A | p.G98R | Missense | het | 0 | D | D | PF | PS2, PM2, PP3 | LP |
| 5 | c.269dupT | p.I91Hfs*35 | Frameshift insertion | het | 0 | NA | NA | CCF | PVS1_Strong+PM2+PM3 | LP |
| c.426dupG | p.T143Dfs*12 | Frameshift insertion | het | 0.0001 | NA | NA | CCF | PVS1_Strong+PS3_Moderate+PM2 | LP | |
| 6/7 | c.G322A | p.E108K | Missense | homo/com-het | 0.0003 | D | D | CCF | PM2, PM3, PP3 | VUS |
| 7 | c.966dupC | p.M323Hfs*6 | Frameshift insertion | com-het | 0 | NA | NA | CCF | PVS1_Strong, PM2 | LP |
| 9/10 | c.G922A | p.G308S | Missense | homo | 5.44E-05 | D | D | CCF | PM2, PP3 | VUS |
| 11/12 | c.G1057A | p.V353I | Missense | het | 0 | D | N | CCF | PM2+PS4_Moderate | LP |
| 13 | c.C1072T | p.P358S | Missense | het | 0 | D | D | CCF | PM2 | VUS |
| 14 | c.G6T | p.R2S | Missense | het | 0 | D | D | EA | PM2, PP3 | VUS |
| 15 | c.C43A | p.Q15K | Missense | het | 0 | D | D | EA | PM2, PP3 | VUS |
| 16 | c.C124G | p.L42V | Missense | het | 0.0011 | D | D | EA | PM2, PP3 | VUS |
| 17 | c.G262C | p.D88H | Missense | het | 0 | D | D | EA | PM2 | VUS |
| 18 | c.G421C | p.G141R | Missense | het | 0 | D | D | EA | PM2, PP3 | VUS |
| 19 | c.C544T | p.P182S | Missense | het | 0 | D | D | EA | PM2+PP3 | VUS |
| 20 | c.G586A | p.A196T | Missense | het | 0 | T | D | EA | PM2 | VUS |
| 21 | c.A604C | p.I202L | Missense | het | 0 | D | D | EA | PM2, PP3 | VUS |
| 22 | c.A694T | p.T232S | Missense | het | 0 | D | D | EA | PM2 | VUS |
| 23 | c.A898T | p.M300L | Missense | het | 0 | D | D | EA | PM2, PP3 | VUS |
| 24 | c.G904A | p.A302T | Missense | het | 0 | D | D | EA | PM2 | VUS |
| 25/26 | c.G959A | p.R320H | Missense | het | 0 | D | D | EA | PM2 | VUS |
| 27 | c.C1055T | p.A352V | Missense | het | 0 | D | D | EA | PM2, PP3 | VUS |
| 28/29 | c.C1171T | p.R391C | Missense | het | 0 | D | D | EA | PS2_Moderate+PM2+PP3 | VUS |
| 30 | c.G1172T | p.R391L | Missense | het | 0 | D | D | EA | PM2+PP3 | VUS |
| 31 | c.G1172A | p.R391H | Missense | het | 0 | D | D | EA | PM2, PP3 | VUS |
| 32 | c.C1232A | p.A411D | Missense | het | 0 | D | D | EA | PM2 | VUS |
aAllele frequency of corresponding variants in gnomAD database (Version 2.1)
bVariant assessment by SIFT and Mutation Taster. D damaging, T tolerated, N neutral, NA not available
cPhenotypes of our study and reported studies. MA oocyte meiotic arrest, PF poor fertilization, CCF complete cleavage failure, EA embryonic arrest
dAssessment the variants according to the American College of Medical Genetics and Genomics (ACMG) guideline. PM pathogenic moderate, PP pathogenic supporting, PS pathogenic strong, PVS pathogenic very strong, LP likely pathogenic, VUS variant of uncertain significance
Clinical characteristics and phenotypes of patients
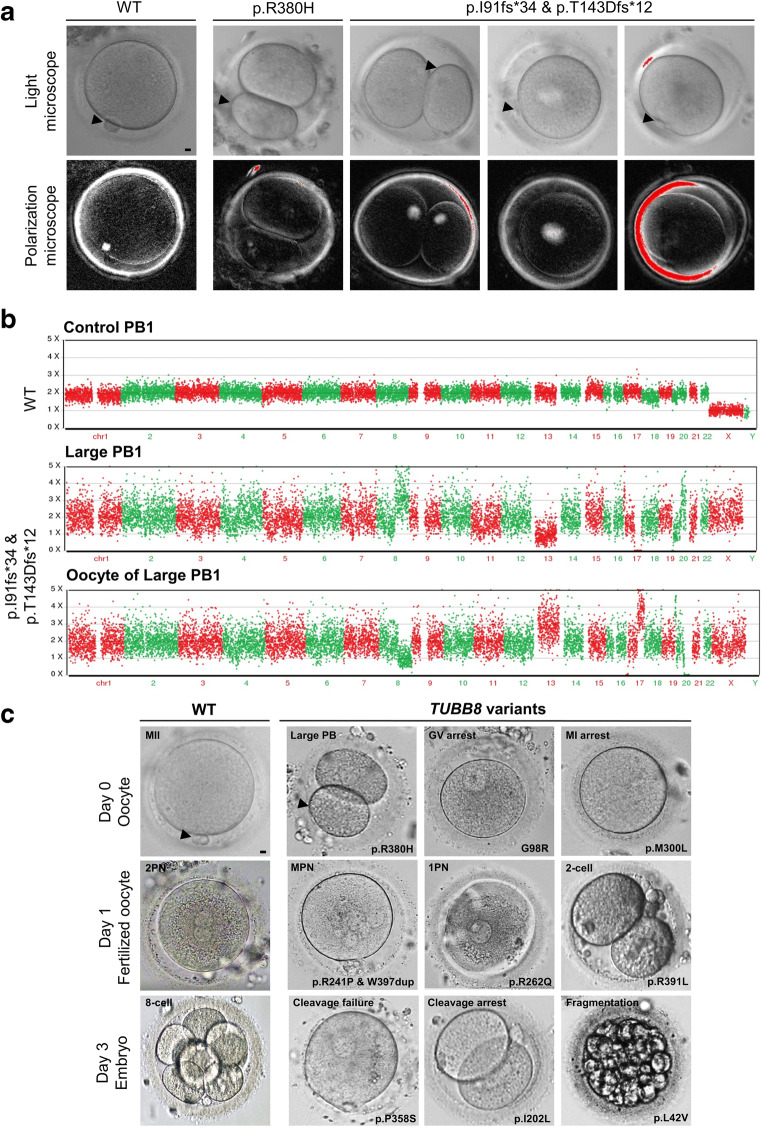
All of the 32 individuals from independent and hitherto uncharacterized families with primary female infertility underwent two to three failed IVF/ICSI attempts in our centre or at other hospitals despite a normal menstrual cycle, endocrine level, and karyotype. Detailed patient clinical information obtained from our centre including the controlled ovarian hyperstimulation (COH) protocol, oocyte maturity and morphology, as well as fertilization and embryo development outcomes are shown in Table 2, as the results present the sum of the attempts (if any). We observed that some oocytes extruded a large polar body (PB), indicating a mitotic-like cleavage event producing a PB of equal size to the oocyte, in affected individuals from families 3 and 5 (26.7% and 41.7%, respectively) (Fig. 2a and Table 2). Both the PB and the oocyte exhibited abnormal chromosome compositions, including partial chromosome trisomy or monosomy (Fig. 2b). Since the phenotypes varied, we categorized them as oocyte meiotic arrest (maturation frequency ≤0.2), poor fertilization (maturation frequency>0.2 yet fertilization frequency ≤0.2), complete cleavage failure (both maturation and fertilization frequency>0.2, yet cleavage frequency ≤0.2), and embryonic arrest before embryonic genome activation (all the maturation, fertilization, and cleavage frequency>0.2). The retrieved oocytes had been through germinal vesicle (GV)/metaphase I (MI) mixed arrest in families 1–3 and poor fertilization in family 4; and nearly all the 2PN zygotes were arrested before the first cleavage, even after two extra days of cultivation (families 5–13). All the other families presented with embryonic arrest, and although 1–2 usable embryos were produced, these embryos failed to form blastocysts during subsequent culture or establish pregnancy after transfer. We also observed various abnormalities in the fertilized zygotes or embryos; there were problems such as high proportion of abnormal fertilization including 1PN (single pronucleus formation in zygotes) and MPN (multi-pronucleus formation in zygotes) (frequency>0.5, in families 1, 2, 8, 10, 12, 17, and 20), or embryonic arrest, with or without severe fragmentation (Fig. 2c and Table 2).
Table 2.
Clinical characteristics of patients in our centre
| Family NO. | Member ID | Female age (years) | Duration of infertility (years) | COH protocol | IVF/ICSI attempt | Retrieved oocytes | Immature oocytes (GV+MI) | PB1 oocytes | Oocyte maturation rate | Oocytes with abnormal morphology |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | II-1 | 34 | 9 | 1 LE protocol + 1 ultra-long | 2 ICSI | 36 | 16+15 | 4 | 11.1% | 1 large PBs |
| 2 | II-1 | 32 | 4 | 2 GnRH-ant | 1 IVF + 1 ICSI | 12 | 0+7 | 2 | 16.7% | 1 large PBs, 1 degeneration |
| 3 | II-1 | 34 | 11 | 2 GnRH-ant | 2 ICSI | 12 | 4+0 | 2 | 16.7% | 5 large PBs and 1 degeneration |
| 4 | II-1 | 31 | 8 | 1 long + 1 short + 1 GnRH-ant | 1 IVF + 2 ICSI | 60 (2 frozen) | 6+31 | 20 | 33.3% | 1 degeneration |
| 5 | II-1 | 24 | 4 | Long | ICSI | 30 | 0+9 | 9 | 30.0% | 8 large PBs, 3 degeneration, 1 empty ZP |
| 6 | II-1 | 35 | 7 | 1 ultra-long + 1 GnRH-ant | 1 IVF + 1 ICSI | 13 | 0+1 | 12 | 92.3% | / |
| 7 | II-1 | 28 | 4 | Long | half ICSI | 15 | 2+0 | 13 | 86.7% | / |
| 8 | II-1 | 29 | 4 | 1 long + 1 GnRH-ant | 1 1VF + 1 half ICSI | 41 | 0+15 | 24 | 58.5% | 2 degeneration |
| 9 | II-1 | 29 | 10 | 1 long and 1 microstimulation | 1 IVF + 1 ICSI/AOA | 30 | 5+1 | 24 | 80.0% | / |
| 10 | II-1 | 31 | 5 | Unknown | 1 IVF + 1 ICSI | 18 | unknown | unknown | NA | / |
| II-2 | 30 | 3 | Long | ICSI | 15 | 2+7 | 6 (2 frozen) | 40.0% | / | |
| 11 | II-1 | 31 | 8 | Long | half ICSI | 10 | 2+2 | 6 | 60.0% | / |
| 12 | II-1 | 27 | 5 | 1 ultra-long + 1 GnRH-ant | 1 half ICSI + 1 ICSI | 18 | 2+2 | 13 | 72.2% | 1 degeneration |
| 13 | II-1 | 36 | 5 | Long | IVF | 16 | 0+2 | 14 | 87.5% | / |
| 14 | II-1 | 34 | 10 | 1 GnRH-ant | 1 ICSI | 4 | 0+1 | 3 | 75.0% | / |
| 15 | II-1 | 30 | 4 | 2 long | 1 IVF + 1 half ICSI | 42 | 3+3 | 35 | 83.3% | 1 degeneration |
| 16 | II-1 | 25 | 4 | 1 long and 1 GnRH-ant | 1 IVF + 1 ICSI | 15 | 1+0 | 13 | 86.7% | 1 empty ZP |
| 17 | II-1 | 22 | 4 | 2 long | 1 IVF + 1 ICSI | 42 | 4+8 | 30 | 71.4% | / |
| 18 | II-1 | 32 | 4 | 2 long | 1 IVF + 1 ICSI | 28 | 4+8 | 16 | 57.1% | / |
| 19 | II-1 | 31 | 5 | 1 ultra-long + 1 GnRH-ant | 1 IVF + 1 ICSI | 16 | 3+1 | 12 | 75.0% | / |
| 20 | II-1 | 30 | 8 | 1 ultra-long + 1 GnRH-ant | 1 half ICSI + 1 IVF | 42 (12 frozen) | 2+3 | 23 | 54.8% | 1 degeneration and 1 empty ZP |
| 21 | II-1 | 32 | 8 | 1 ultra-Long | 1 ICSI | 14 | 3+2 | 9 | 64.3% | / |
| II-2 | 30 | 6 | 1 ultra-Long | 1 ICSI | 17 | 2+0 | 15 | 88.2% | / | |
| 22 | II-1 | 34 | 10 | 1 short + 1 GnRH-ant | 1 IVF+1 half ICSI | 37 | 6+2 | 29 (6 frozen) | 78.4% | / |
| 23 | II-1 | 33 | 12 | 1 long and 1 GnRH-ant | 1 IVF + 1 ICSI | 46 | 4+12 | 30 | 65.2% | / |
| 24 | II-1 | 36 | 11 | 1 PPOS + 1 GnRH-ant | 2 ICSI | 16 | 6+4 | 4 | 25.0% | 2 degeneration |
| 25 | II-1 | 29 | 5 | 1 long + 1 GnRH-ant | 2 ICSI | 28 | 7+0 | 21 | 75.0% | / |
| 26 | II-1 | 32 | 7 | 1 long + 1 GnRH-ant | 1 IVF + 1 ICSI | 14 | 3+0 | 11 | 78.6% | / |
| 27 | II-1 | 37 | 11 | 1 long + 1 short + 1 GnRH-ant | 1 IVF + 2 ICSI | 36 | 4+5 | 25 | 69.4% | 2 degeneration |
| 28 | II-1 | 30 | 6 | 1 long + 1 short | 1 IVF + 1 ICSI | 29 | 3+0 | 26 | 89.7% | / |
| 29 | II-1 | 35 | 9 | 1 long | 1 ICSI | 21 | 2+3 | 16 | 76.2% | / |
| 30 | II-1 | 28 | 7 | 1 short + 1 long | 2 ICSI | 40 | 0+26 | 12 | 30.0% | 2 degeneration |
| 31 | II-1 | 31 | 10 | 1 long | 1 half ICSI | 14 | 3+5 | 6 | 42.9% | / |
| 32 | II-1 | 34 | 9 | 2 GnRH-ant + 1 long | 1 IVF + 2 ICSI | 10 | 0+3 | 7 | 70.0% | / |
| Family NO. | Member ID | Fertilization outcomes (2PN+1PN+MPN+0PN) | Fertilization rate | Cleaved embryos | Cleavage rate | Embryo outcomes | ||||
| 1 | II-1 | 0+1+3+0 | 100.0% | 3 | 75.0% | 2*3-cell, 1*2-cell, arrested | ||||
| 2 | II-1 | 0+0+1+1 | 33.3% | / | 0 | / | ||||
| 3 | II-1 | 0+0+1+0, 1 degeneration | 50.0% | 0 | 0.0% | / | ||||
| 4 | II-1 | 0+0+0+1 | 0.0% | / | NA | / | ||||
| 5 | II-1 | 1+1+0+7 | 22.2% | / | 0.0% | / | ||||
| 6 | II-1 | 7+0+4+1 | 91.7% | / | 0.0% | / | ||||
| 7 | II-1 | 7+2+2+2 | 84.6% | / | 0.0% | / | ||||
| 8 | II-1 | 3+1+13+6, 1 degeneration | 70.8% | / | 0 | / | ||||
| 9 | II-1 | 10+1+1+12 | 50.0% | 1 | 8.3% | 1*3-cell, arrested | ||||
| 10 | II-1 | 11+0+0+0 | NA | / | 0.0% | / | ||||
| II-2 | 0+3+0+0, 1 degeneration | 50.0% | / | 0.0% | / | |||||
| 11 | II-1 | 2+0+0+4 | 33.3% | / | 0.0% | / | ||||
| 12 | II-1 | 2+3+0+8 | 38.5% | 1 | 20.0% | 1*3-cell, arrested | ||||
| 13 | II-1 | 8+5+1+0 | 100.0% | 2 | 14.3% | 1*4-cell, 1*2-cell, arrested | ||||
| 14 | II-1 | 3+0+0+0 | 100.0% | 3 | 100.0% | 1*7-cell, 2*3-cell, arrested | ||||
| 15 | II-1 | 13+1+2+19 | 45.7% | 19 | 100.0% | 5*4-cell, 8*3-cell, 6*2-cell, arrested | ||||
| 16 | II-1 | 6+0+0+7 | 46.2% | 6 | 100.0% | 1*6-cell, 2*4-cell, 1*3-cell, 2*2-cell, arrested | ||||
| 17 | II-1 | 6+8+2+14 | 53.3% | 13 | 81.3% | 1*5-cell, 4*4-cell, 4*3-cell, 4*2-cell, arrested | ||||
| 18 | II-1 | 11+3+0+2 | 87.5% | 7 | 50.0% | 1*5-cell, 1*4-cell, 2*3-cell, 3*2-cell, arrested | ||||
| 19 | II-1 | 4+1+2+5 | 58.3% | 6 | 85.7% | 1*4-cell, 5*2-cell, arrested | ||||
| 20 | II-1 | 5+2+7+3, 1 degeneration, 3 3-cell and 2 2-cell | 60.9% | 20 | 100.0% | 3*10-cell, 2*8-cell, 1*7-cell, 2*6-cell, 4*5-cell, 6*4-cell, 1*3-cell, 1*2-cell, no pregnancy after 8-cell and 5-cell transfer, others arrested | ||||
| 21 | II-1 | 3+0+0+6 | 33.3% | 2 | 66.7% | 2*2-cell, arrested | ||||
| II-2 | 8+7+0+0 | 100.0% | 9 | 60.0% | 1*8-cell, 3*4-cell, 2*3-cell, 3*2-cell, no pregnancy after 8 cell transfer, and others arrested | |||||
| 22 | II-1 | 14+0+2+7 | 69.6% | 19 | 100.0% | 6*5-cell, 10*4-cell, 3*3-cell, arrested | ||||
| 23 | II-1 | 5+1+3+20, 1 degeneration | 30.0% | 12 | 100.0% | 3*5-cell, 3*4-cell, 4*3-cell, 2*2-cell, arrested | ||||
| 24 | II-1 | 0+3+0+0, 1 degeneration | 75.0% | 1 | 25.0% | 1*2-cell, arrested | ||||
| 25 | II-1 | 17+0+2+2 | 90.5% | 20 | 100.0% | 1*8-cell, 1*5-cell, 4*4-cell, 6*3-cell, 8*2-cell, no pregnancy after 8-cell and 4-cell embryos transfer, others arrested | ||||
| 26 | II-1 | 8+0+1+2 | 81.8% | 9 | 100.0% | 1*11-cell, 1*7-cell, 6*4-cell, 1*2-cell, arrested | ||||
| 27 | II-1 | 12+3+0+9, 1 degeneration | 60.0% | 11 | 73.3% | 2*6-cell, 2*5-cell, 5*4-cell, 1*3-cell, 1*2-cell, arrested | ||||
| 28 | II-1 | 13+5+5+3 | 88.5% | 21 | 91.3% | 1*8-cell, 1*7-cell, 4*5-cell, 13*4-cell, 2*2-cell, no pregnancy after 8-cell and 7-cell transfer and others arrested | ||||
| 29 | II-1 | 7+3+1+4, 1 degeneration | 68.8% | 12 | 100.0% | 4*4-cell,8*2-cell, arrested | ||||
| 30 | II-1 | 2+1+0+3, 1 2-cell | 25.0% | 5 | 100.0% | 1*4-cell, 2*3-cell, 2*2-cell, arrested | ||||
| 31 | II-1 | 1+2+0+3 | 50.0% | 6 | 100.0% | 6*4-cell, arrested | ||||
| 32 | II-1 | 5+0+0+2 | 71.4% | 5 | 100.0% | 1*5-cell, 1*4-cell, 1*3-cell, 2*2-cell and no pregnancy after 5-cell and 4-cell transfer | ||||
PB polar body, COH controlled ovarian hyperstimulation, GnRH-ant GnRH antagonist, LE protocol letrozole protocol, PPOS progestin-primed ovarian stimulation, AOA assisted oocyte activation; arrested means embryo is proceeded to be cultivated but failed to form blastocyst; NA not available
Fig. 2.
Morphology of oocytes, zygotes, and embryos from control individuals and patients. a PB1 oocytes from a control and patients in families 1 and 2 were examined by light and polarization microscopy. The control oocyte has a visible spindle located near PB1, while it is only very weakly visible in the R380H variant. No detectable spindle can be observed in the p.I91Hfs*34 and p.T143Dfs*12 compound heterozygous variants. Scale bar = 10 μm. b Control PB1 has a diploid chromosome copy number. The large PB and oocyte had abnormal chromosome compositions, including partial chromosome trisomy and partial chromosome monosomy. c Morphology of a control and an affected individuals’ oocytes on day 0, zygotes on day 1, and embryos on day 3 after fertilization presenting various morphological abnormalities. Arrow indicated the PB1. Scale bar = 10 μm
Impact of TUBB8 variants on protein structure
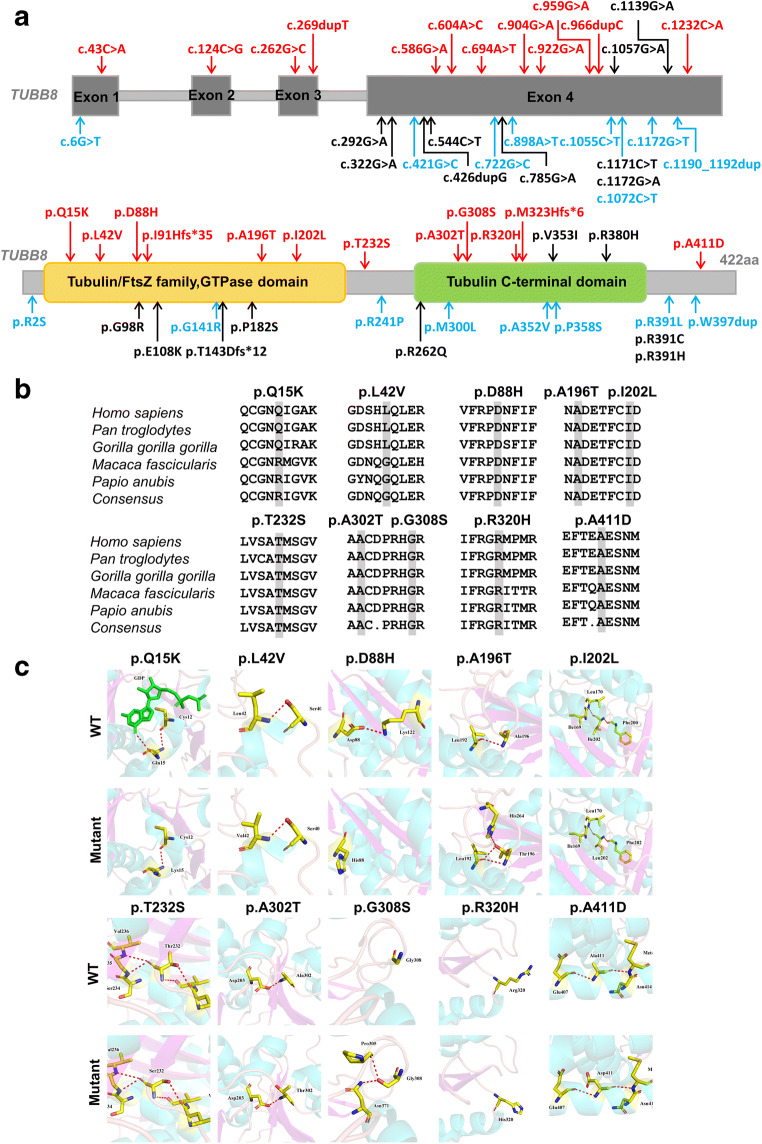
Among the 10 of total 29 novel missense variants (a novel missense amino acid change occurring at the same position as another previously reported missense change was excluded) (Fig. 3a), the amino acids at variant positions were highly conserved across different primate species except for p.Q15K and p.L42V (Fig. 3b). Based on the three-dimensional (3D) structure of the TUBB8 proteins used to assess the effect of missense variants, the p.Q15K variant is predicted to lead to the loss of binding of the guanosine diphosphate (GDP) ligand, whereas other variants had no obvious effects on the protein structure but resulted in changes in hydrogen bonding: some hydrogen bonds were absent (p.Q15K and p.D88H) and some new hydrogen bonds were produced (p.A196T and p.R308S). The remaining variants (p.L42V, p.I202L, p.T232S, p.A302T, p.R320H, and p.A411D) did not display any changes in hydrogen bonds (Fig. 3c).
Fig. 3.
Bioinformatic analysis of TUBB8 variants. a Summary of our TUBB8 variants. The red font represents 12 novel variants, the blue font represents the 8 novel missense amino acid change occurring at the same position as another previously reported missense change, and black font represents the previously reported variants. b Conservation analysis of mutated amino acids in five different primate species. All the variant sites are highly conserved except for Q15K and L42V. c Protein conformation predictions of variants in TUBB8. Red dashed lines represent hydrogen bonds, indicated by the black arrowhead
Discussion
TUBB8 encodes a primate-specific beta-tubulin subunit that is expressed in the oocyte and early embryo. An increasing number of studies indicate that TUBB8 heterozygous variants have dominant negative effects and that homozygous/compound heterozygous variants disrupt spindle assembly and cause abnormalities in oocyte maturation, fertilization, and embryonic development [7–14]. In this study, we reported 29 TUBB8 variants (20 of which are novel) that further expand the variant spectrum of TUBB8, extremely involved in complete cleavage failure and embryonic arrest.
So far, 16 of the reported 102 TUBB8 variants have been examined in in vitro functional assays [7–9, 16, 21] (Supplemental Table 1) and can be classified as “likely pathogenic” (LP) using the American College of Medical Genetics and Genomics (ACMG) guidelines, implying that they can be used as genetic markers for genetic counselling [21]. For these patients with the LP variants, donor eggs may be the most feasible method at present. Some of the variants conformed to pedigrees in a segregation analysis; however, some were only observed in the proband due to the unavailability of DNA samples from their parents [13, 22]; therefore, the pathogenicity of these TUBB8 variants could not be completely evaluated (Fig. 3a). Since TUBB8 is a primate-specific subunit with a high prevalence of variants, building animal models for each of these variants is not trivial, and neither is their in vitro functional verification. The most effective method includes summarizing the reported variants and the phenotype of patients, which revealed that there are 34 TUBB8 variant hotspots (recurrent variants summarized in Supplemental Table 1). These variants should receive more attention even in the absence of powerful functional verification.
We identified variant hotspots that were specific for complete cleavage failure, such as p.G308S (from families 9 and 10), p.E108K (from families 6 and 7, and previously reported in one patient with the same phenotype [12]), and p.V353I (from families 11 and 12, and previously reported in two patients with a similar phenotype [9, 13]). We suggest that TUBB8 variant screening should be conducted the first time a patient experiences complete cleavage failure. In future work, we will explore the mechanism of complete cleavage failure by examining the effects of the three TUBB8 variant hotspots mentioned above by overexpression in transgenic mice. Apart from some paternally inherited or de novo TUBB8 variants, we also found five TUBB8 variants in six families that were maternally inherited. This type of maternal inheritance has been previously explained by the possibility of an incomplete penetrance pattern or a modifier gene [13].
Human oocyte maturation includes nuclear maturation and cytoplasmic maturation. Nuclear maturation is verified by PB1 extrusion. Normally at MI, the spindle is first centrally positioned in the oocyte but then migrates to the cortex. This is a prerequisite for the asymmetric cleavage that produces the small PB and the remaining larger competent secondary oocyte. However, in our patients (families 3 and 5), the spindle presented with various abnormalities such as spindle absence, altered shapes, or localization errors. At the end of metaphase I, we observed that 26.7% (8/30) and 41.7% (5/12) of the oocytes extruded abnormally large polar bodies, some even achieving mitotic-like cleavage and producing cells of equal size (Fig. 2a and Table 2). Choi et al. also observed a similar phenotype in maturing oocytes of Mos knockout mice oocytes, accompanied by the abnormal spindle, and they hypothesized that increased genetic instability may exist in Mos knockout mice oocytes [23]. An abnormal spindle may easily cause chromosome segregation error, and here we were able to demonstrate chromosome separation errors in the large PBs (Fig. 2b). Additionally, the p.R380H variant has been reported in three independent individuals with larger PBs [22]. We suggest that this recurrent variant might be analysed the first time a patient experiences a larger PB.
In the reported 102 TUBB8 variants, we found that 50% of the variants were associated with meiotic arrest; however, 27 variants were reported to cause embryonic arrest (Supplemental Table 1). We further analysed the correlation between phenotype and genotype, and we found 17 variants that caused different phenotypes under the same variant, such as p.V255M variant that could cause meiotic arrest or embryonic arrest in different patients, which should be further explored in order for them to be included in genetic counselling.
In addition to TUBB8, other maternal effect genes have been implicated in oocyte maturation, fertilization, and embryonic development defects, such as PATL2, WEE2, BTG4 and subcortical maternal complex (SCMC) genes [19, 24–26]. In our study, we showed that the 68% of patients are negative for TUBB8 as a cause of infertility, and 10% of patients carry biallelic variants in the SCMC genes [27]. The statistics of this incidence may be biased in the patient recruitment or limited by the number of patients, which should be further investigated.
In conclusion, the 20 novel (a total of 29) TUBB8 variants encountered here may contribute to female infertility and further expand the variant spectrum of TUBB8, especially with respect to embryonic arrest. Our findings identify variant hotspots that are specific for complete cleavage failure. Moreover, we found that large polar bodies in TUBB8 variants might lead to chromosome segregation errors. Our findings, together with extant knowledge of TUBB8 variants, might benefit the genetic counselling of female infertility in the future.
Supplementary information
(DOCX 36 kb)
Acknowledgements
We would like to thank the patients and their families for participating in this work and Editage (www.editage.cn) for English language editing.
Author contribution
W.Z. and G.L. performed study design. W.Z. performed study execution and statistical analysis and wrote the manuscript. H.H., X.X., and Y.G performed the variant arrangement. S.Z., F.G., and G.L. performed the patient recruitment.
Funding
This work was supported by the National Natural Science Foundation of China (grant number 82001633), China Postdoctoral Science Foundation (grant number 2020M682575), the Changsha Municipal Natural Science Foundation (grant number kq2007022), the Research Grant of CITIC-XIANGYA (grant number YNXM-201913), and the Hunan Provincial Grant for Innovative Province Construction (2019SK4012).
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Wei Zheng and Huiling Hu contributed equally to this work.
References
- 1.Mascarenhas MN, Flaxman SR, Boerma T, Vanderpoel S, Stevens GA. National, regional, and global trends in infertility prevalence since 1990: a systematic analysis of 277 health surveys. PLoS Med. 2012;9(12):e1001356. doi: 10.1371/journal.pmed.1001356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hart RJ. Physiological aspects of female fertility: role of the environment, modern lifestyle, and genetics. Physiol Rev. 2016;96(3):873–909. doi: 10.1152/physrev.00023.2015. [DOI] [PubMed] [Google Scholar]
- 3.Matzuk MM, Lamb DJ. The biology of infertility: research advances and clinical challenges. Nat Med. 2008;14(11):1197–1213. doi: 10.1038/nm.f.1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yatsenko SA, Rajkovic A. Genetics of human female infertilitydagger. Biol Reprod. 2019;101(3):549–566. doi: 10.1093/biolre/ioz084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moor RM, Dai Y, Lee C, Fulka J., Jr Oocyte maturation and embryonic failure. Hum Reprod Update. 1998;4(3):223–236. doi: 10.1093/humupd/4.3.223. [DOI] [PubMed] [Google Scholar]
- 6.Sha QQ, Zhang J, Fan HY. A story of birth and death: mRNA translation and clearance at the onset of maternal-to-zygotic transition in mammalsdagger. Biol Reprod. 2019;101(3):579–590. doi: 10.1093/biolre/ioz012. [DOI] [PubMed] [Google Scholar]
- 7.Feng R, Sang Q, Kuang Y, Sun X, Yan Z, Zhang S, Shi J, Tian G, Luchniak A, Fukuda Y, Li B, Yu M, Chen J, Xu Y, Guo L, Qu R, Wang X, Sun Z, Liu M, Shi H, Wang H, Feng Y, Shao R, Chai R, Li Q, Xing Q, Zhang R, Nogales E, Jin L, He L, Gupta ML, Jr, Cowan NJ, Wang L. Mutations in TUBB8 and human oocyte meiotic arrest. New Engl J Med. 2016;374(3):223–232. doi: 10.1056/NEJMoa1510791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Feng R, Yan Z, Li B, Yu M, Sang Q, Tian G, Xu Y, Chen B, Qu R, Sun Z, Sun X, Jin L, He L, Kuang Y, Cowan NJ, Wang L. Mutations in TUBB8 cause a multiplicity of phenotypes in human oocytes and early embryos. J Med Genet. 2016;53(10):662–671. doi: 10.1136/jmedgenet-2016-103891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen B, Li B, Li D, Yan Z, Mao X, Xu Y, Mu J, Li Q, Jin L, He L, Kuang Y, Sang Q, Wang L. Novel mutations and structural deletions in TUBB8: expanding mutational and phenotypic spectrum of patients with arrest in oocyte maturation, fertilization or early embryonic development. Hum Reprod. 2017;32(2):457–464. doi: 10.1093/humrep/dew322. [DOI] [PubMed] [Google Scholar]
- 10.Huang L, Tong X, Luo L, Zheng S, Jin R, Fu Y, et al. Mutation analysis of the TUBB8 gene in nine infertile women with oocyte maturation arrest. Reprod Biomed Online. 2017;35(3):305–10. doi: 10.1016/j.rbmo.2017.05.017. [DOI] [PubMed] [Google Scholar]
- 11.Wang AC, Zhang YS, Wang BS, Zhao XY, Wu FX, Zhai XH, Sun JX, Mei SY. Mutation analysis of the TUBB8 gene in primary infertile women with arrest in oocyte maturation. Gynecol Endocrinol. 2018;34(10):900–904. doi: 10.1080/09513590.2018.1464138. [DOI] [PubMed] [Google Scholar]
- 12.Yuan P, Zheng L, Liang H, Li Y, Zhao H, Li R, Lai L, Zhang Q, Wang W. A novel mutation in the TUBB8 gene is associated with complete cleavage failure in fertilized eggs. J Assist Reprod Genet. 2018;35(7):1349–1356. doi: 10.1007/s10815-018-1188-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen B, Wang W, Peng X, Jiang H, Zhang S, Li D, Li B, Fu J, Kuang Y, Sun X, Wang X, Zhang Z, Wu L, Zhou Z, Lyu Q, Yan Z, Mao X, Xu Y, Mu J, Li Q, Jin L, He L, Sang Q, Wang L. The comprehensive mutational and phenotypic spectrum of TUBB8 in female infertility. European journal of human genetics: EJHG. 2019;27(2):300–307. doi: 10.1038/s41431-018-0283-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lanuza-Lopez MC, Martinez-Garza SG, Solorzano-Vazquez JF, Paz-Cervantes D, Gonzalez-Ortega C, Maldonado-Rosas I, et al. Oocyte maturation arrest produced by TUBB8 mutations: impact of genetic disorders in infertility treatment. Gynecological endocrinology : the official journal of the International Society of Gynecological Endocrinology. 2020;36:1–6. doi: 10.1080/09513590.2020.1725968. [DOI] [PubMed] [Google Scholar]
- 15.Xing Q, Wang R, Chen B, Li L, Pan H, Li T, Ma X, Cao Y, Wang B. Rare homozygous mutation in TUBB8 associated with oocyte maturation defect-2 in a consanguineous mating family. J Ovarian Res. 2020;13(1):42. doi: 10.1186/s13048-020-00637-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jia Y, Li K, Zheng C, Tang Y, Bai D, Yin J, Chi F, Zhang Y, Li Y, Tu Z, Wang Y, Pan J, Liang S, Guo Y, Ruan J, Kong P, Wu B, Hu Y, Wang H, Liu W, Teng X, Gao S. Identification and rescue of a novel TUBB8 mutation that causes the first mitotic division defects and infertility. J Assist Reprod Genet. 2020;37:2713–2722. doi: 10.1007/s10815-020-01945-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sha Q, Zheng W, Feng X, Yuan R, Hu H, Gong F, Hu L, Lin G, Ou X. Novel mutations in TUBB8 expand the mutational and phenotypic spectrum of patients with zygotes containing multiple pronuclei. Gene. 2021;769:145227. doi: 10.1016/j.gene.2020.145227. [DOI] [PubMed] [Google Scholar]
- 18.Yang P, Yin C, Li M, Ma S, Cao Y, Zhang C, Chen T, Zhao H. Mutation analysis of tubulin beta 8 class VIII in infertile females with oocyte or embryonic defects. Clin Genet. 2021;99(1):208–214. doi: 10.1111/cge.13855. [DOI] [PubMed] [Google Scholar]
- 19.Zheng W, Zhou Z, Sha Q, Niu X, Sun X, Shi J, Zhao L, Zhang S, Dai J, Cai S, Meng F, Hu L, Gong F, Li X, Fu J, Shi R, Lu G, Chen B, Fan H, Wang L, Lin G, Sang Q. Homozygous mutations in BTG4 cause zygotic cleavage failure and female infertility. Am J Hum Genet. 2020;107(1):24–33. doi: 10.1016/j.ajhg.2020.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xie P, Hu L, Tan Y, Gong F, Zhang S, Xiong B, Peng Y, Lu GX, Lin G. Retrospective analysis of meiotic segregation pattern and interchromosomal effects in blastocysts from inversion preimplantation genetic testing cycles. Fertil Steril. 2019;112(2):336–342. doi: 10.1016/j.fertnstert.2019.03.041. [DOI] [PubMed] [Google Scholar]
- 21.Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17(5):405–424. doi: 10.1038/gim.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhao L, Guan Y, Wang W, Chen B, Xu S, Wu L, Yan Z, Li B, Fu J, Shi R, Shi J, du J, Li Q, Zhang Z, Mu J, Zhou Z, Dong J, Jin L, He L, Sun X, Kuang Y, Wang L, Sang Q. Identification novel mutations in TUBB8 in female infertility and a novel phenotype of large polar body in oocytes with TUBB8 mutations. J Assist Reprod Genet. 2020;37(8):1837–1847. doi: 10.1007/s10815-020-01830-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Choi T, Fukasawa K, Zhou R, Tessarollo L, Borror K, Resau J, Vande Woude GF. The Mos/mitogen-activated protein kinase (MAPK) pathway regulates the size and degradation of the first polar body in maturing mouse oocytes. Proc Natl Acad Sci U S A. 1996;93(14):7032–7035. doi: 10.1073/pnas.93.14.7032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu Y, Shi Y, Fu J, Yu M, Feng R, Sang Q, Liang B, Chen B, Qu R, Li B, Yan Z, Mao X, Kuang Y, Jin L, He L, Sun X, Wang L. Mutations in PADI6 cause female infertility characterized by early embryonic arrest. Am J Hum Genet. 2016;99(3):744–752. doi: 10.1016/j.ajhg.2016.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen B, Zhang Z, Sun X, Kuang Y, Mao X, Wang X, Yan Z, Li B, Xu Y, Yu M, Fu J, Mu J, Zhou Z, Li Q, Jin L, He L, Sang Q, Wang L. Biallelic mutations in PATL2 cause female infertility characterized by oocyte maturation arrest. Am J Hum Genet. 2017;101(4):609–615. doi: 10.1016/j.ajhg.2017.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sang Q, Li B, Kuang Y, Wang X, Zhang Z, Chen B, Wu L, Lyu Q, Fu Y, Yan Z, Mao X, Xu Y, Mu J, Li Q, Jin L, He L, Wang L. Homozygous mutations in WEE2 cause fertilization failure and female infertility. Am J Hum Genet. 2018;102(4):649–657. doi: 10.1016/j.ajhg.2018.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zheng W, Hu H, Dai J, Zhang S, Gu Y, Dai C, Guo J, Xu X, Li Y, Zhang S, Hu L, Gong F, Lu G, Lin G. Expanding the genetic and phenotypic spectrum of the subcortical maternal complex genes in recurrent preimplantation embryonic arrest. Clin Genet. 2021;99(2):286–291. doi: 10.1111/cge.13858. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 36 kb)
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.