Abstract
Background
To investigate the thyroid function changes during controlled ovarian hyperstimulation (COH) and ascertain its impact on reproductive outcomes.
Methods
We conducted meta-analysis in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. A comprehensive literature search was performed to identify studies reported changes in thyroid parameters during COH. We analyzed thyroid-stimulating hormone (TSH) levels, free thyroxin (fT4) levels, changes in estrogens (E2), thyroxine-binding globulin (TBG), relative risks (RRs) of clinical pregnancy rate (CPR), live birth rate (LBR), and mean difference (MD) of TSH increment between the miscarriage group and ongoing pregnancy group.
Results
This meta-analysis included fifteen individual studies (n = 1665 subjects). At the end of COH, the mean TSH (2.53 mIU/L; 95% CI, 2.19 to 2.88; I2 = 92.9%) exceeded the upper limit (2.5 mIU/L) and remained above the threshold until one month following embryo transfer (ET). Thyroxin decreased from baseline to the end of COH (−0.18 ng/l; 95% CI, −0.35 to 0.00; I2 = 92.2%). The CPR and LBR of patients with TSH exceeding the cutoff after COH were significantly lower than those of patients with TSH below the threshold (CPR: RR, 0.62; 95% CI, 0.47 to 0.82; I2 = 0.0% and LBR: RR, 0.64; 95% CI, 0.44 to 0.92; I2 = 0.0%). The MD of the increment in TSH levels between the miscarriage and ongoing pregnancy groups was 0.40 mIU/L (95% CI, 0.15 to 0.65; I2 = 0.0%).
Conclusions
This meta-analysis shows that TSH increases and fT4 decreases during COH. COH-induced thyroid disorder impairs reproductive outcomes.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10815-021-02206-0.
Keywords: Reproductive endocrine, Assisted reproduction technology, Controlled ovarian hyperstimulation, Thyroid function
Introduction
Placental and fetal development depend on the supply of maternal thyroid hormone; thus, maternal thyroid diseases are associated with adverse pregnancy outcomes such as miscarriage and preterm delivery [1] as well as adverse outcomes for the child [2], so that maintaining normal thyroid function during conception is essential for pregnancy and fetal development. In recent decades, there is a trend towards delaying child birth, meanwhile females’ fertility potential declines with age. Considering that the incidence of thyroid diseases and the prevalence of thyroid autoimmune antibody positivity increase with age [3], it is unsurprising that a remarkable proportion of women who seek assisted reproduction technology (ART) have concomitant thyroid diseases [4, 5]. Women with basal thyroid diseases suffer from more pronounced thyroxin insufficiency during controlled ovarian hyperstimulation (COH) due to the inability of a compromised thyroid to face the increased demand for thyroid hormones [6, 7]; thus, the potential risk of thyroid dysfunction is high among women in ART consultations.
Controlled ovarian hyperstimulation (COH) is an important part of assisted reproductive technology (ART), which combines treatment of pituitary-gonadal axis regulation and ovarian stimulation to obtain multiple cumulus-oocyte complexes [8]. Some evidence suggests that COH strains the hypothalamic-pituitary-thyroid axis and can induce an increase in thyroid-stimulating hormone (TSH) levels, especially in women with limited thyroid function [9, 10]. TSH levels have been reported to increase in 63.3–77.1% of women undergoing COH [11, 12], and in some, even exceeded the 2.5 mU/L threshold which was recommended by the American Thyroid Association for the first trimester [13], albeit with initial TSH < 2.5 mU/L. The reported incidences of serum TSH above the limit after COH range from 16–44% in euthyroid cases and 51–64% in hypothyroid-treated cases (deemed eligible if they had a certified diagnosis of clinical or subclinical hypothyroidism) [12, 14–16]. Notably, the period after COH is the “implantation window,” which encompasses the early stages of embryo development. Thyroid hormone plays a crucial role in endometrial preparation for pregnancy and initial trophoblast development [17]; even slightly abnormal TSH levels may be associated with ART failure [7]. Therefore, the COH-induced elevated TSH levels would be associated with poor reproductive outcomes, despite few previous studies reported on it.
A major concern is whether women with COH-induced elevations in TSH above the threshold have a worse reproductive outcome and a lower success ratio for embryo transfer (ET) than those whose TSH levels are always maintained below the cutoff. In our review, we aimed to investigate the changes in thyroid parameters during COH, factors that associated with it, and their adverse impacts on pregnancy chances and reproductive outcomes.
Materials and methods
This systematic review was conducted and reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [18] and checklist (Supplementary Checklist).
Registration of review protocol
This systematic review was registered with the International Prospective Register of Systematic Reviews (PROSPERO) in August 2019 (registration number CRD42019134906).
Data sources and searches
We searched PubMed, EMBASE, and Web of Science systematically from inception to March 2020. The search terms and strategy are available in the supplement (Supplementary Table 1).
Study selection
To ensure comprehensive retrieval, any clinical studies were eligible if investigated women received ART treatment and reported changes in thyroid parameters during COH. There were no exclusion criteria for language or publication year. Reviews, editorials, comments, and letters were excluded.
Data extraction and assessment of quality
The following information was extracted from each study independently by two reviewers (D. Li and S. Hu): the name of the first author, year of publication, study type, location, number of participants, age, basal thyroid function, presence of thyroid autoimmunity (TAI), COH protocol, changes in thyroid parameters (TSH and fT4), changes in estrogens (E2) and thyroxine-binding globulin (TBG), and reproductive outcomes. Assessment of risk of bias was performed according to the modified Newcastle-Ottawa scale (NOS). Eight studies (53%) were assessed as having a low risk of bias (Supplementary Table 2).
Data synthesis and analysis
Most outcomes are summarized as mean differences with 95% confidence intervals (CIs). The clinical pregnancy rate (CPR) and live birth rate (LBR) are summarized as relative risks (RRs). Statistical heterogeneity was assessed with the I2 statistic, and I2 values greater than 50% suggested substantial heterogeneity. The changes in TSH and fT4 were pooled using random-effects models, while measures regarding reproductive outcomes were synthesized using fixed-effects models defined a priori given a small heterogeneity. Funnel plots were conducted to examine publication bias [19]. We ran two prespecified additional analyses: (1) subgroup analyses to explore the implications of basal thyroid disease, different types of protocols, ages of the participants, location, and reproductive outcomes and (2) sensitivity analyses to determine if excluding any individual study altered our results. All analyses were performed using Stata statistical software, version 15.1 (Stata Corp LLC), and plots were generated by R Studio, version 1.1.442 (RStudio, Inc.).
Results
Literature search
A literature search was conducted according to the PRISMA statement. The flow diagram for literature selection is presented in Supplementary Figure 1.
Included studies and characteristics
Fifteen cohort studies with 1665 women undergoing ART with information on thyroid function changes during COH [6, 7, 9, 11, 12, 14–16, 20–26] were included (Supplementary Table 3). These studies were published from 2000 to 2019. The COH protocols included the gonadotropin-releasing hormone (GnRH) agonist long protocol [6, 9, 11, 20, 21, 23, 24] or the GnRH antagonist protocol [7, 15, 24, 26] or were determined on an individual basis [12, 14, 16, 22, 25]. Thirteen studies with 1143 participants investigated thyroid function changes in euthyroid patients [6, 7, 9, 11, 12, 14, 15, 20–22, 24–26] and 3 studies with 118 participants investigated women with a certified diagnosis of clinical or subclinical hypothyroidism [14–16]. Six studies with 242 participants were conducted with women with TAI [6, 7, 14, 16, 20, 26] and 10 studies with 1354 participants without TAI [6, 7, 9, 11, 12, 21–25]. Nine studies contained data on the association between thyroid function during COH and reproductive outcomes (Supplementary Table 4) [6, 7, 11, 12, 15, 16, 21, 25, 27].
Process of assisted reproductive technology treatment
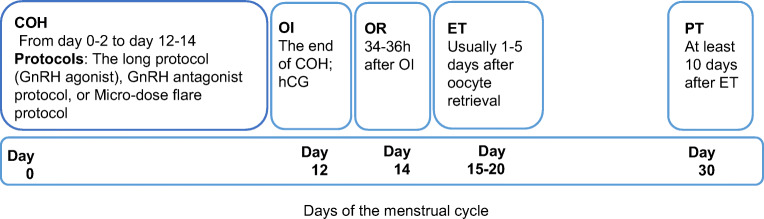
The ART process is shown in Fig. 1. In all studies, female partners received COH from days 0–2 to days 12–14 of the menstrual cycle, received ovulation induction (OI) using 10,000 IU human chorionic gonadotropin (hCG) at the end of COH, and then received follicle puncture 34–36 h after OI. ET was performed 1 day [6, 7, 9], 2–3 days [12, 16, 24], 3–5 days [15], or 5–7 days [21] after oocyte retrieval. Pregnancy was diagnosed at least 10 days after ET.
Fig. 1.
The first-month procedure of ART. COH, controlled ovarian hyperstimulation; ET, embryo transfer; GnRH, gonadotropin-releasing hormone; OI, ovulation induction (the end of COH); OR, oocyte retrieval; and PT, pregnancy tests. Subgroup “Hypothyroid” was defined as with a certified diagnosis of clinical or subclinical hypothyroidism
Changes in serum thyroid-stimulating hormone (TSH)
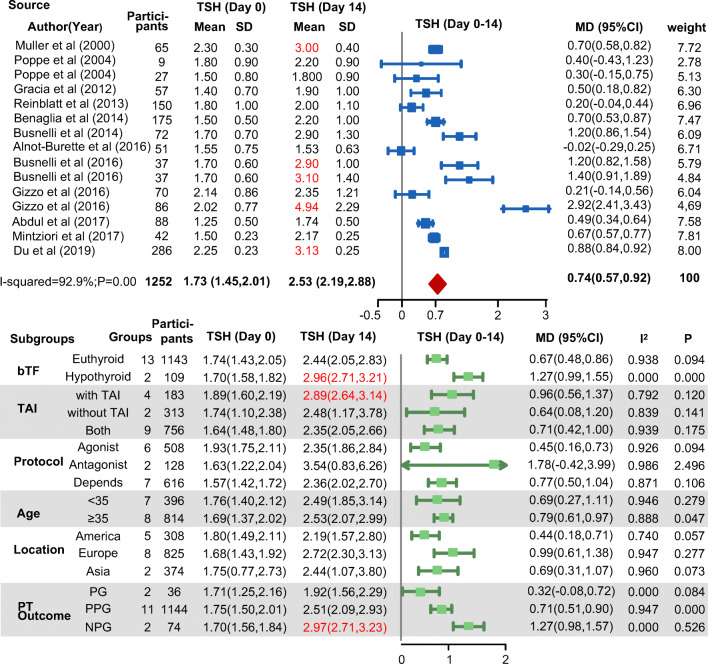
Serum TSH increased throughout the first month of ART treatment, peaking at the time of the pregnancy test (Fig. 2). At the end of COH, the mean TSH (2.53 mIU/L; 95% CI, 2.19 to 2.88; I2 = 92.9%) exceeded the upper limit (2.5 mIU/L) acceptable for the first trimester. Then, at the pregnancy test, it increased to 2.67 mIU/L (95% CI, 2.37 to 2.97; I2 = 70.3%) and remained above the threshold until 1 month following ET (Fig. 3 and Supplementary Figure 2). At later time points, serum TSH levels progressively decreased to the basal levels.
Fig. 2.
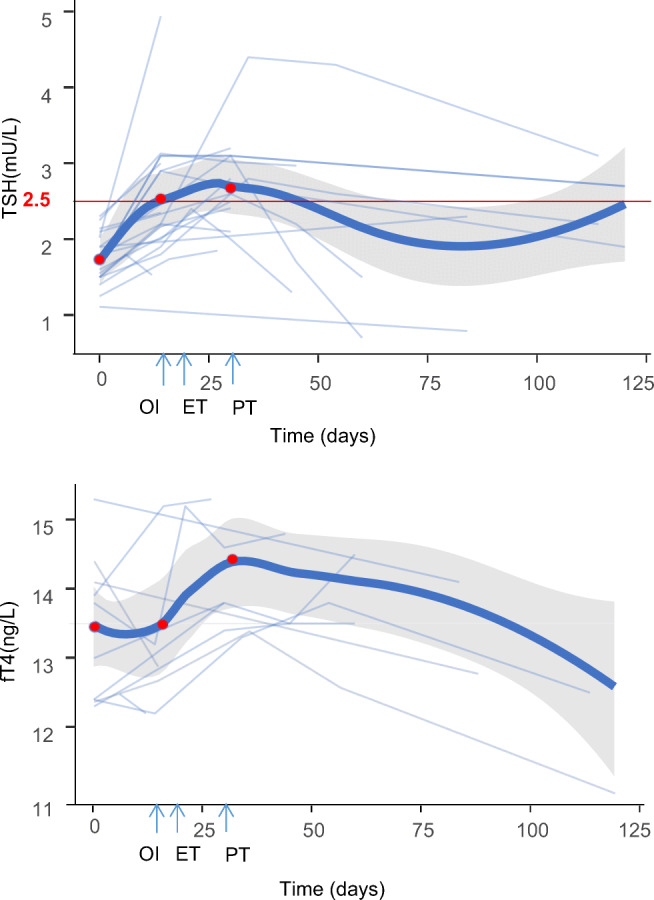
The first-trimester changes in thyroid parameters of women undergoing COH. COH, controlled ovarian hyperstimulation; OI, ovulation induction (the end of COH); ET, embryo transplantation; PT, pregnancy tests; and TSH, thyroid-stimulating hormone. The bold lines indicate the pooled changes. The light blue lines indicate changes in each study. The three red points represent the beginning of COH, the end of COH, and the pregnancy test
Fig. 3.
Forest plot for changes in TSH (from baseline to the end of COH). bTF, basal thyroid function; MD, mean difference; NPG, not pregnant later (all patients); PGO, ongoing pregnancy later (all patients); PGP, pregnancy in part of patient group; TAI, thyroid autoimmunity; TSH, thyroid-stimulating hormone. The upper panel shows the changes in TSH levels from baseline to the pregnancy test, and the panel below shows the subgroup analysis of the upper panel
Subgroup analysis revealed that the serum TSH increments were higher in the subgroups with hypothyroidism, with TAI, and without pregnancy later (Fig. 3). At the time of the pregnancy test, serum TSH levels were much higher than the cutoff level in these subgroups (hypothyroidism: 3.16 mIU/L, 95% CI 2.86 to 3.45, I2 = 0.0%; TAI: 3.18 mIU/L, 95% CI 2.89 to 3.47, I2 = 0.0%; not pregnant: 2.87 mIU/L, 95% CI 2.50 to 3.25, I2 = 37.8%) (Supplementary Figure 2), indicating that the deterioration of the thyroidal axis induced by COH was more severe in women with basal thyroid disease. This corroborated the assumptions of previous studies that patients with anti-thyroid antibodies would be more likely to experience a significant change in TSH after COH because they are naturally predisposed to developing hypothyroidism [6, 7, 22], and this was consistent with the conclusion that TSH curves of hypothyroid-treated patients were significantly higher than those of euthyroid patients [15, 16].
Changes in serum free thyroxine (fT4)
Serum free thyroxine (fT4) decreased from baseline to the end of COH (−0.18 ng/l; 95% CI, −0.35 to 0.00; I2 = 92.2%) (Supplementary Figure 3). After a pituitary feedback response to the decreasing fT4 levels and the thyrotrophic action of hCG injected at OI, fT4 gradually increased after OI (1.17 ng/l; 95% CI 0.92 to 1.43; I2 = 0.0%).
Thyroxine-binding globulin (TBG) and estrogens (E2)
Five studies [15, 20, 22–24] reported E2 changes during COH, among which only two investigated TBG levels [15, 20]. Mean E2 levels increased from 0.23 nmol/l (95% CI 0.17 to 0.28; I2 = 96.6%) at baseline to 6.24 nmol/l (95% CI 4.58 to 7.89; I2 = 98.9%) at the end of COH. Additionally, TBG levels increased by 6.39 mg/l (95% CI 1.78 to 10.99; I2 = 86.7%) after COH.
Reproductive outcomes
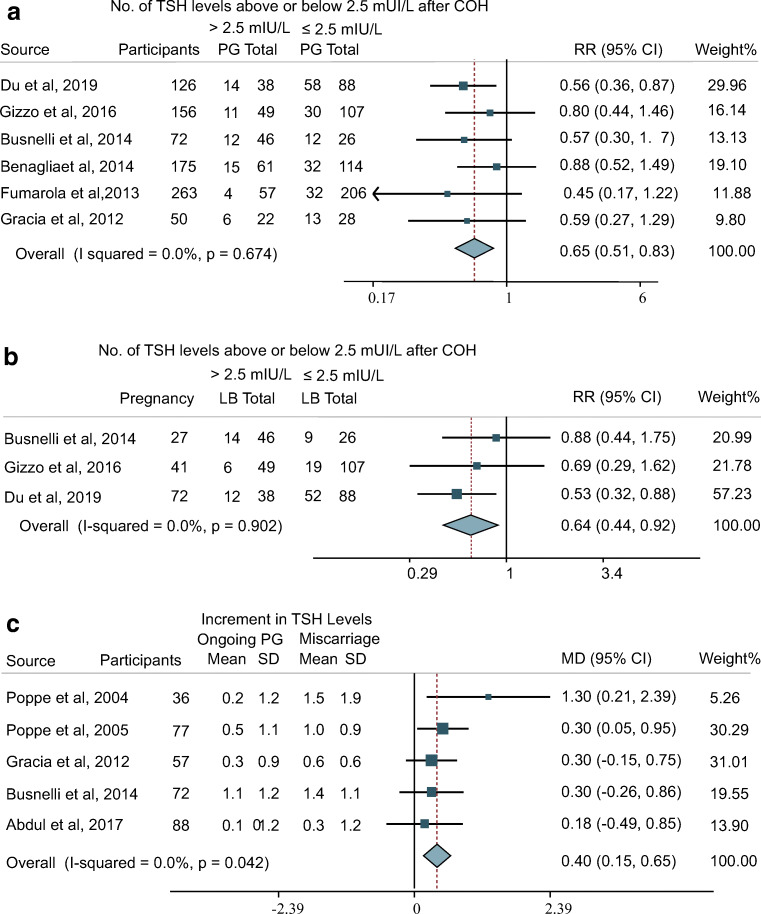
Our meta-analysis found that the CPR and LBR of patients with TSH exceeding the cutoff level after COH were significantly lower than those of patients with TSH below the threshold (CPR: RR, 0.62; 95% CI, 0.47 to 0.82; I2 = 0.0% and LBR: RR, 0.64; 95% CI, 0.44 to 0.92; I2 = 0.0%) (Fig. 4a and b). This result suggested that even with initial TSH levels within the normal range, COH-induced elevations in TSH would still impair the CPR and LBR. However, the LBRs of the groups of patients with successful pregnancies with TSH levels (after COH) above and below 2.5 mIU/L were comparable (RR, 1.00; 95% CI, 0.82 to 1.23; I2 = 0%), indicating that COH-induced thyroid dysfunction mainly influences the early stage of pregnancy, namely, implantation and initial trophoblast development, rather than later fetal development (Supplementary Figure 4).
Fig. 4.
Forest plot for reproductive outcomes a CPR, b LBR, and c MD of TSH increment between miscarriage and ongoing pregnancy group. CPR, clinical pregnancy rate; fT4, free thyroxine; LB, live birth; LBR, live birth rate; MD, mean difference; PG, pregnancy; RR, relative risk; TSH, thyroid-stimulating hormone
The mean difference in the increment in TSH levels between the miscarriage and ongoing pregnancy groups was 0.40 mIU/L (95% CI, 0.15 to 0.65; I2 = 0.0%) (Fig. 4c). The subgroup analysis of TSH changes also showed that the increment of serum TSH was higher in the subgroup not pregnant later (Fig. 3). This result indicated that there was a trend for a higher TSH increment in the miscarriage group compared with the ongoing pregnancy group.
Publication bias and sensitivity analysis
Begg-Mazumdar regression tests indicated no publication bias (Supplementary Figure 5). In the sensitivity analysis, the results were robust after exclusion of any single study from the analysis (Supplementary Figure 6).
Discussion
During the first trimester, the upper limit for TSH recommended by American Thyroid Association is 2.5 mIU/L, which predominantly based on six pregnancy studies comprising a total cohort of approximately 5500 subjects [13]. The Endocrine Society also recommends that TSH should not exceed 2.5 mIU/L before pregnancy for better reproductive outcomes [28]. In our systematic review and meta-analysis of 15 studies, we found that mean TSH levels increased throughout COH and exceeded the threshold of 2.5 mU/L until 1 month after ET, especially in women with basal thyroid diseases. ET usually occurs within 1 week after OI, but at that time, patients may suffer from relative thyroid insufficiency induced by COH. Accordingly, we conducted an analysis of reproductive outcomes and found that the CPR and LBR of women with elevated TSH above the threshold during COH were impaired. We also revealed that the TSH increment of the miscarriage group was higher than that of the ongoing pregnancy group. This indicated that a large TSH increment during COH would be an indicator of failure to achieve a subsequent pregnancy.
The pathophysiological mechanisms of thyroid dysfunction during COH have not been fully elucidated, even though its central role seems to be the most credible hypothesis. The rapid increase in serum estrogen concentrations induced by COH leads to a rise in circulating T4 binding sites (TBG) and results in a consequent reduction in fT4, which induces an increase in TSH levels via pituitary feedback [29]. However, recent evidence suggests that estrogens may not play an exclusive role, as previously hypothesized. The E2 levels were significantly higher in the OHSS group than in the no-OHSS group, but serum TSH and fT4 levels in both groups remained comparable during COH [9]. The antagonist protocol group showed lower E2 levels but a higher increase in TSH levels than the agonist protocol group [24]. Considering this evidence, there must be other mechanisms underlying these modifications.
Another hypothesis was that these modifications may represent a mere physiological response to treatment. Evidence reporting that gonadotropin-releasing hormone receptors (GnRH-Rs) were present in the thyrotrophic cells of the normal human adenohypophysis suggests that the GnRH agonist or antagonist may directly act as a trigger that is responsible for the serum TSH increase [12, 30]. If the hypothesis was true, the agonist or antagonist would show opposing effects on TSH changes, but the TSH levels increased during COH regardless of which protocol was chosen [24]; thus, this hypothesis was not supported.
In our meta-analysis, we found that there was a subtle reduction in fT4 during COH. Accordingly, the increase in TSH levels was more likely due to peripheral insufficiency than pituitary upregulation. The increased demand for TH would result from a combination of factors, not merely the effect of the increased secretion of TBG. Both T3 and T4 have been found in human follicular fluid and are involved in the maturation of the preovulatory follicle and oocyte cumulus cell (CC) complex [31]. A recent study demonstrated the correlation between thyroid hormones and the number of oocytes retrieved [32]. Moreover, thyrotropin receptors (TSHRs) and TH receptors (TRs) are expressed by oocytes, granulosa cells (GCs), and ovarian stromal cells at different stages of follicular development, representing a potential target for TH and TSH. Administration of COH allowed multiple follicular growth events to achieve the optimal number (8–15 oocytes), much more above physiological conditions [33]. Considering that the development and maturation of the CC complex require TH, the multiple follicular growth events seem to consume more TH than physiological conditions, causing relative thyroid insufficiency. Further study is needed to investigate whether there is an association between the oocyte number and TSH increase during COH.
TRs and TSHRs are widely expressed in the feto-maternal unit during implantation, and TH might influence both the endometrium and trophoblasts either directly or indirectly through TH effects on the synthesis and activity of implantation-mediating molecules, indicating the crucial role of TH in the implantation process and early blastocyst development [17, 34]. Follicular fluid TH (ffTH) levels were significantly higher in the successful pregnancy group than in the implantation failure group [35]. Therefore, the TH insufficiency induced by COH may disrupt the normal process of implantation and results in an impaired CPR. Our analysis confirmed that a COH-induced increase in TSH to the threshold was a risk factor for a low CPR.
One question raised is whether continuous monitoring of thyroid function during COH should be recommended for women receiving COH. Although the Practice Committee of the American Society for Reproductive Medicine (ASRM) and the Endocrine Society (ES) suggest screening for thyroid disorders in all infertile women before ART [13, 36, 37], we recognize that currently, there is no routine testing of thyroid function during and especially after COH. Given the likely transient nature of elevations in TSH with COH and the negative effects of these elevations on reproductive outcomes, we recommended that all patients, especially TAI patients, be retested at the end of COH to determine subsequent treatment [22]. If the TSH levels are above 2.5 mIU/L, levothyroxine supplementation should be considered. Considering that hypothyroid-treated women had a higher risk of COH-induced TSH elevation [12, 14–16], we recommend three-times thyroid function tests, respectively, at the beginning of COH, ovulation induction, and pregnancy test.
Another recommended strategy is to postpone ET. A study of endometrial biopsy samples obtained on day 3 after ET showed that COH may alter the signaling pathways for thyroid hormones in the endometrium, wherein the mRNA expression levels of TSHRs, THR-β1, and deiodinase 2 are decreased in donors undergoing COH compared to controls [38]. This indicated that the post-COH endometrium would be insensitive to TH so that a compensatory increase in TSH occurs. Therefore, supplement therapy may not be effective for the insensitive endometrium. This was corroborated by an elegant study of 600 participants with TAI in 2018, which showed that the CPR was not increased in euthyroid patients who received levothyroxine during ART [39]. There was some evidence that the LBR and CPR were significantly higher for frozen-thawed ET than for fresh ET [40, 41], probably due to maternal thyroid function recovery from the influence of COH. Previous studies found that COH induced delayed endometrial maturation and suboptimal endometrial receptivity, which could lead to the observed decreased implantation rates in ART with fresh cycles [42]. In conclusion, we propose that the embryo transfer be postponed until a more suitable endometrium can be developed and that embryos be frozen until the patient recovers from COH-induced thyroid disorder.
Limitations
One limitation of our study is that the total number of participants involved in the investigation of reproductive outcomes was not large enough, especially for the LBR, because the follow-up of most studies was too short to provide reproductive outcomes. Second, we cannot distinguish the possible difference of clinical and subclinical cases for limited information. Another limitation is that this was a meta-analysis of the results of observational studies; thus, we cannot form conclusions on the cause-effect relationship between elevated TSH during COH and impaired reproductive outcomes. We look forward to further randomized controlled trials to characterize the effect of thyroxin replacement therapy on reproductive outcomes of patients with abnormally elevated TSH during COH.
Conclusion
TSH may increase throughout the first month of ART treatment, peaking at the pregnancy test and exceeding the recommended limit during the whole implantation window, even during early embryo development. The COH-induced elevation in TSH was much higher in patients with basal thyroid diseases. This thyroid dysfunction induced by COH may have a negative impact on the reproductive outcomes.
Supplementary Information
(DOCX 1259 kb).
Acknowledgements
We would like to thank all investigators and participants in this study, especially the guidance of professor Yu. We acknowledge that Danpei Li provided data sources and technical support during the analysis of this study. We also acknowledge the support from Endocrinology Division of Tongji Hospital.
Author contribution
Dr. Yu and D. Li had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Li.
Acquisition, analysis, or interpretation of data: Li and Hu.
Drafting of the manuscript: Li.
Critical revision of the manuscript for important intellectual content: All authors. Obtained funding: the National Key R&D Program of China (2016YFC0901203) and the National Natural Science Foundation of China (81974109, 81570740).
Statistical analysis: Li and Hu.
Administrative, technical, or material support: Yu.
Study supervision: Yu.
Funding
This study was supported by grants from the National Key R&D Program of China (2016YFC0901203) and the National Natural Science Foundation of China (81974109, 81570740).
Data availability
The data that supports the findings of this study are available in the supplementary material of this article.
Declarations
Conflicts of interest
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Danpei Li and Sitao Hu contributed equally to this work.
Contributor Information
Danpei Li, Email: ldp19940730@163.com.
Xuefeng Yu, Email: xfyu188@163.com.
References
- 1.De Leo S, Pearce EN. Autoimmune thyroid disease during pregnancy. Lancet Diabetes Endocrinol. 2018;6:575–586. doi: 10.1016/S2213-8587(17)30402-3. [DOI] [PubMed] [Google Scholar]
- 2.Korevaar TIM, Medici M, Visser TJ, Peeters RP. Thyroid disease in pregnancy: new insights in diagnosis and clinical management. Nat Rev Endocrinol. 2017;13:610–622. doi: 10.1038/nrendo.2017.93. [DOI] [PubMed] [Google Scholar]
- 3.Hollowell JG, Staehling NW, Flanders WD, Hannon WH, Gunter EW, Spencer CA, Braverman LE. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III) J Clin Endocrinol Metab. 2002;87:489–499. doi: 10.1210/jcem.87.2.8182. [DOI] [PubMed] [Google Scholar]
- 4.Negro R. Thyroid and assisted reproduction technologies: a brief clinical update with recommendations for practice. Endocr Metab Immune Disord Drug Targets. 2018;18:194–200. doi: 10.2174/1871530318666180131103029. [DOI] [PubMed] [Google Scholar]
- 5.Poppe K, Velkeniers B, Glinoer D. The role of thyroid autoimmunity in fertility and pregnancy. Nat Clin Pract Endocrinol Metab. 2008;4:394–405. doi: 10.1038/ncpendmet0846. [DOI] [PubMed] [Google Scholar]
- 6.Poppe K, Glinoer D, Tournaye H, Schiettecatte J, Devroey P, van Steirteghem A, Haentjens P, Velkeniers B. Impact of ovarian hyperstimulation on thyroid function in women with and without thyroid autoimmunity. J Clin Endocrinol Metab. 2004;89:3808–3812. doi: 10.1210/jc.2004-0105. [DOI] [PubMed] [Google Scholar]
- 7.Negro R, Formoso G, Coppola L, Presicce G, Mangieri T, Pezzarossa A, Dazzi D. Euthyroid women with autoimmune disease undergoing assisted reproduction technologies: the role of autoimmunity and thyroid function. J Endocrinol Investig. 2007;30:3–8. doi: 10.1007/BF03347388. [DOI] [PubMed] [Google Scholar]
- 8.Krassas GE, Poppe K, Glinoer D. Thyroid function and human reproductive health. Endocr Rev. 2010;31:702–755. doi: 10.1210/er.2009-0041. [DOI] [PubMed] [Google Scholar]
- 9.Poppe K, Unuane D, D'Haeseleer M, Tournaye H, Schiettecatte J, Haentjens P, Velkeniers B. Thyroid function after controlled ovarian hyperstimulation in women with and without the hyperstimulation syndrome. Fertil Steril. 2011;96:241–245. doi: 10.1016/j.fertnstert.2011.04.039. [DOI] [PubMed] [Google Scholar]
- 10.Mintziori G, Goulis DG, Toulis KA, Venetis CA, Kolibianakis EM, Tarlatzis BC. Thyroid function during ovarian stimulation: a systematic review. Fertil Steril. 2011;96:780–785. doi: 10.1016/j.fertnstert.2011.06.020. [DOI] [PubMed] [Google Scholar]
- 11.Du YJ, Xin X, Cui N, Jiang L, Yang AM, Hao GM, Gao BL. Effects of controlled ovarian stimulation on thyroid stimulating hormone in infertile women. Eur J Obstet Gynecol Reprod Biol. 2019;234:207–212. doi: 10.1016/j.ejogrb.2019.01.025. [DOI] [PubMed] [Google Scholar]
- 12.Benaglia L, Busnelli A, Somigliana E, Leonardi M, Vannucchi G, De Leo S, Fugazzola L, Ragni G, Fedele L. Incidence of elevation of serum thyroid-stimulating hormone during controlled ovarian hyperstimulation for in vitro fertilization. Eur J Obstet Gynecol Reprod Biol. 2014;173:53–57. doi: 10.1016/j.ejogrb.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 13.Alexander EK, Pearce EN, Brent GA, Brown RS, Chen H, Dosiou C, Grobman WA, Laurberg P, Lazarus JH, Mandel SJ, Peeters RP, Sullivan S. 2017 Guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and the postpartum. Thyroid. 2017;27:315–389. doi: 10.1089/thy.2016.0457. [DOI] [PubMed] [Google Scholar]
- 14.Busnelli A, Somigliana E, Ferrari S, Filippi F, Vannucchi G, Fugazzola L, Fedele L. The long-term impact of controlled ovarian hyperstimulation on thyroid function. Endocr Pract. 2016;22:389–395. doi: 10.4158/EP15933.OR. [DOI] [PubMed] [Google Scholar]
- 15.Gracia CR, Morse CB, Chan G, Schilling S, Prewitt M, Sammel MD, Mandel SJ. Thyroid function during controlled ovarian hyperstimulation as part of in vitro fertilization. Fertil Steril. 2012;97:585–591. doi: 10.1016/j.fertnstert.2011.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Busnelli A, Somigliana E, Benaglia L, Sarais V, Ragni G, Fedele L. Thyroid axis dysregulation during in vitro fertilization in hypothyroid-treated patients. Thyroid. 2014;24:1650–1655. doi: 10.1089/thy.2014.0088. [DOI] [PubMed] [Google Scholar]
- 17.Colicchia M, Campagnolo L, Baldini E, Ulisse S, Valensise H, Moretti C. Molecular basis of thyrotropin and thyroid hormone action during implantation and early development. Hum Reprod Update. 2014;20:884–904. doi: 10.1093/humupd/dmu028. [DOI] [PubMed] [Google Scholar]
- 18.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62:1006–1012. doi: 10.1016/j.jclinepi.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 19.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Muller AF, Verhoeff A, Mantel MJ, De Jong FH, Berghout A. Decrease of free thyroxine levels after controlled ovarian hyperstimulation. J Clin Endocrinol Metab. 2000;85:545–548. doi: 10.1210/jcem.85.2.6373. [DOI] [PubMed] [Google Scholar]
- 21.Poppe K, Glinoer D, Tournaye H, Schiettecatte J, Haentjens P, Velkeniers B. Thyroid function after assisted reproductive technology in women free of thyroid disease. Fertil Steril. 2005;83:1753–1757. doi: 10.1016/j.fertnstert.2004.12.036. [DOI] [PubMed] [Google Scholar]
- 22.Reinblatt S, Herrero B, Correa JA, Shalom-Paz E, Ata B, Wiser A, Morris D, Holzer H. Thyroid stimulating hormone levels rise after assisted reproductive technology. J Assist Reprod Genet. 2013;30:1347–1352. doi: 10.1007/s10815-013-0081-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alnot-Burette J, Nakib I, Lipere A, Delemer B, Graesslin O. Thyroid function for infertile women during ovarian hyperstimulation as part of IVF. Gynecol Obstet Fertil. 2016;44:156–162. doi: 10.1016/j.gyobfe.2016.01.007. [DOI] [PubMed] [Google Scholar]
- 24.Gizzo S, Noventa M, Quaranta M, Vitagliano A, Esposito F, Andrisani A, Venturella R, Alviggi C, Plebani M, Gangemi M, Nardelli GB, D'Antona D. The potential role of GnRH agonists and antagonists in inducing thyroid physiopathological changes during IVF. Reprod Sci. 2016;23:515–523. doi: 10.1177/1933719115608000. [DOI] [PubMed] [Google Scholar]
- 25.Abdul Karim AK, Azrai Abu M, Chelliah B, Mohd Razi ZR, Omar MH, Othman H, Man ZC. Maternal thyroid function in women undergoing controlled ovarian hyperstimulation during in-vitro fertilization and its relation to reproductive outcome. Minerva Ginecol. 2017;69:431–437. doi: 10.23736/S0026-4784.17.04069-2. [DOI] [PubMed] [Google Scholar]
- 26.Mintziori G, Goulis DG, Kolibianakis EM, Slavakis A, Bosdou J, Grimbizis G, Tarlatzis BC. Thyroid function and autoimmunity during ovarian stimulation for intracytoplasmic sperm injection. Reprod Fertil Dev. 2017;29:603–608. doi: 10.1071/RD15172. [DOI] [PubMed] [Google Scholar]
- 27.Fumarola A, Grani G, Romanzi D, Del Sordo M, Bianchini M, Aragona A, Tranquilli D, Aragona C. Thyroid function in infertile patients undergoing assisted reproduction. Am J Reprod Immunol. 2013;70:336–341. doi: 10.1111/aji.12113. [DOI] [PubMed] [Google Scholar]
- 28.De Groot L, Abalovich M, Alexander EK, Amino N, Barbour L, Cobin RH, Eastman CJ, Lazarus JH, Luton D, Mandel SJ, Mestman J, Rovet J, Sullivan S. Management of thyroid dysfunction during pregnancy and postpartum: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2012;97:2543–2565. doi: 10.1210/jc.2011-2803. [DOI] [PubMed] [Google Scholar]
- 29.Bartalena L. Recent achievements in studies on thyroid hormone-binding proteins. Endocr Rev. 1990;11:47–64. doi: 10.1210/edrv-11-1-47. [DOI] [PubMed] [Google Scholar]
- 30.La Rosa S, Celato N, Uccella S, Capella C. Detection of gonadotropin-releasing hormone receptor in normal human pituitary cells and pituitary adenomas using immunohistochemistry. Virchows Arch. 2000;437:264–269. doi: 10.1007/s004280000247. [DOI] [PubMed] [Google Scholar]
- 31.Wakim AN, Polizotto SL, Buffo MJ, Marrero MA, Burholt DR. Thyroid hormones in human follicular fluid and thyroid hormone receptors in human granulosa cells. Fertil Steril. 1993;59:1187–1190. doi: 10.1016/S0015-0282(16)55974-3. [DOI] [PubMed] [Google Scholar]
- 32.Rosales M, Nunez M, Abdala A, Mesch V, Mendeluk G. Thyroid hormones in ovarian follicular fluid: association with oocyte retrieval in women undergoing assisted fertilization procedures. JBRA Assist Reprod. 2020;24:245–249. [DOI] [PMC free article] [PubMed]
- 33.Ji J, Liu Y, Tong XH, Luo L, Ma J, Chen Z. The optimum number of oocytes in IVF treatment: an analysis of 2455 cycles in China. Hum Reprod. 2013;28:2728–2734. doi: 10.1093/humrep/det303. [DOI] [PubMed] [Google Scholar]
- 34.Aghajanova L, Stavreus-Evers A, Lindeberg M, Landgren BM, Sparre LS, Hovatta O. Thyroid-stimulating hormone receptor and thyroid hormone receptors are involved in human endometrial physiology. Fertil Steril. 2011;95:230–237. doi: 10.1016/j.fertnstert.2010.06.079. [DOI] [PubMed] [Google Scholar]
- 35.Cai YY, Lin N, Zhong LP, Duan HJ, Dong YH, Wu Z, Su H. Serum and follicular fluid thyroid hormone levels and assisted reproductive technology outcomes. Reprod Biol Endocrinol. 2019;17:90. doi: 10.1186/s12958-019-0529-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Practice Committee of the American Society for Reproductive M Subclinical hypothyroidism in the infertile female population: a guideline. Fertil Steril. 2015;104:545–553. doi: 10.1016/j.fertnstert.2015.05.028. [DOI] [PubMed] [Google Scholar]
- 37.American College of O, Gynecology ACOG practice bulletin. Thyroid disease in pregnancy. Number 37, August 2002. American College of Obstetrics and Gynecology. Int J Gynaecol Obstet. 2002;79:171–180. doi: 10.1016/S0020-7292(02)00327-2. [DOI] [PubMed] [Google Scholar]
- 38.Detti L, Uhlmann RA, Fletcher NM, Diamond MP, Saed GM. Endometrial signaling pathways during ovarian stimulation for assisted reproduction technology. Fertil Steril. 2013;100:889–894. doi: 10.1016/j.fertnstert.2013.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang H, Gao H, Chi H, Zeng L, Xiao W, Wang Y, Li R, Liu P, Wang C, Tian Q, Zhou Z, Yang J, Liu Y, Wei R, Mol BWJ, Hong T, Qiao J. Effect of levothyroxine on miscarriage among women with normal thyroid function and thyroid autoimmunity undergoing in vitro fertilization and embryo transfer: a randomized clinical trial. JAMA. 2017;318:2190–2198. doi: 10.1001/jama.2017.18249. [DOI] [PubMed] [Google Scholar]
- 40.Zhang W, Xiao X, Zhang J, Wang W, Wu J, Peng L, Wang X. Clinical outcomes of frozen embryo versus fresh embryo transfer following in vitro fertilization: a meta-analysis of randomized controlled trials. Arch Gynecol Obstet. 2018;298:259–272. doi: 10.1007/s00404-018-4786-5. [DOI] [PubMed] [Google Scholar]
- 41.Wong KM, Mastenbroek S, Repping S. Cryopreservation of human embryos and its contribution to in vitro fertilization success rates. Fertil Steril. 2014;102:19–26. doi: 10.1016/j.fertnstert.2014.05.027. [DOI] [PubMed] [Google Scholar]
- 42.Horcajadas JA, Minguez P, Dopazo J, Esteban FJ, Dominguez F, Giudice LC, Pellicer A, Simon C. Controlled ovarian stimulation induces a functional genomic delay of the endometrium with potential clinical implications. J Clin Endocrinol Metab. 2008;93:4500–4510. doi: 10.1210/jc.2008-0588. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 1259 kb).
Data Availability Statement
The data that supports the findings of this study are available in the supplementary material of this article.