Abstract
Purpose
We studied the quality differences between the different hypo-osmotic swelling test (HOST) classes, as measured by criteria of DNA fragmentation, DNA decondensation, and nuclear architecture. The aim was to find particular HOST classes associated with good-quality metrics, which may be potentially used in ICSI (intra-cytoplasmic sperm injection).
Methods
Ten patients from the Department of Reproductive Medicine at Tenon Hospital (Paris, France) were included. Their semen samples were collected and divided into two fractions: one was incubated in a hypo-osmotic solution as per HOST protocol and sorted by sperm morphology, and a second was incubated without undergoing the HOST protocol to serve as an unsorted baseline. Three parameters were assessed: DNA fragmentation (TUNEL assay), DNA decondensation (chromomycin A3 assay), and nuclear architecture (FISH, with telomeric and whole chromosome painting probes). The different HOST classes were evaluated for these three parameters, and statistical analysis was performed for each class versus the unsorted non-HOST-treated sperm. Results with p<0.05 were considered statistically significant.
Results
For each of the parameters evaluated, we found significant differences between HOST-selected spermatozoa and non-selected spermatozoa. Overall, spermatozoa of HOST classes B and B+ exhibited the highest quality based on four metrics (low DNA fragmentation, low DNA decondensation, short inter-telomeric distance, and small chromosome 1 territory area), while spermatozoa of HOST classes A and G exhibited the poorest quality by these metrics.
Conclusion
In addition to their pathophysiological interest, our results open possibilities of sperm selection prior to ICSI, which may allow for optimization of reproductive outcomes in heretofore unstudied patient populations.
Keywords: Sperm selection, Hypo-osmotic swelling test, Host, ICSI, DNA fragmentation, DNA decondensation, Nuclear architecture
Introduction
The hypo-osmotic swelling test was first introduced in 1984 [1] to assess the functional integrity of the sperm membrane. It has since been included in the WHO manual since its 3rd edition in 1992 [2]. In this technique, spermatozoa are incubated in a hypo-osmotic solution (prepared from sodium chloride or sodium citrate and fructose) which induces flagellar swelling in cells with an intact cell membrane. This swelling is caused by an influx of fluids occurring across a morphologically and physiologically sound membrane.
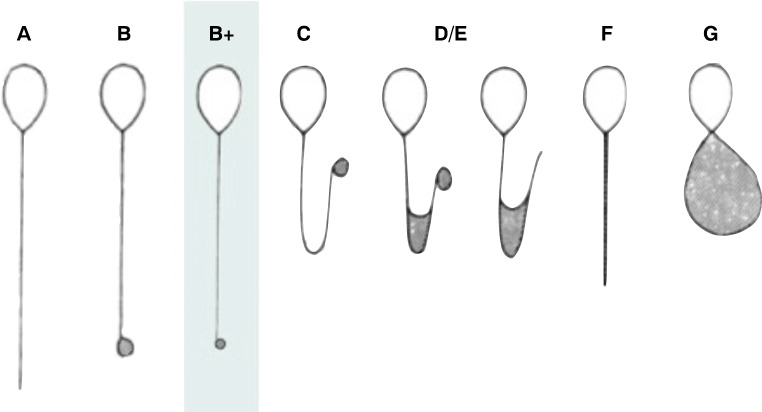
While HOST sperm were initially analyzed in a binary manner (i.e., “HOST-positive” sperm with any type of flagellar swelling, versus “HOST-negative” with no swelling at all), a classification scheme has since been proposed based on the type of flagellar swelling (Fig. 1). Previous studies have suggested a difference in quality between the different morphological classes. Examining each HOST class individually, Bassiri et al. and Stanger et al. found discordant results based on DNA fragmentation, DNA decondensation, and phosphatidyl-serine externalization [4, 5]. Other authors have compared HOST classes individually and assessed aneuploidy rates in patients with a normal karyotype [6] as well as segregation modes in chromosomal rearrangement carriers [3]. The latter study proposed the addition of a new morphological class, termed B+, which we accordingly included as a separate class here.
Fig. 1.
The HOST classification. The HOST classification, as proposed in the WHO Laboratory Manual for Examination and Processing of Human Sperm (starting from the 3rd edition in 1992), with the additional B+ class, described by our team [3]
In the present study, we evaluated spermatozoa from each of the 7 HOST morphological classes based on three parameters, i.e., DNA fragmentation, DNA decondensation, and nuclear architecture, which was assessed through the evaluation of the inter-telomeric distance and chromosomal territory area for a given chromosome, similar to our previous work [7].
Materials and methods
Ten male subjects were recruited from the Department of Reproductive Medicine at Tenon Hospital, AP-HP, Paris, France. They initially presented with their partners for fertility consultation of various etiologies. The ethics commission CECOS (Centre d’Etude et de Conservation des Oeufs et du Sperm humains) considered this project to be exempted from IRB approval since it did not involve any additional medical intervention and solely used remnants from sperm samples obtained as part of the routine clinical care. Informed consent was nevertheless obtained from each subject. In this study, each subject served as its own control: each subject’s sample was divided into a HOST-treated and a HOST-untreated fraction, and sperm quality characteristics were compared between the identified HOST classes and the untreated fraction.
Subject semen samples were obtained by masturbation after 1–5 days of abstinence, as per the WHO guidelines (ref WHO 2010). Samples were treated in triplicate. After incubation for 30 min at 37 °C, routine sperm analysis was performed. All the subjects had sperm parameters within the normal range (data not shown). Next, the samples underwent discontinuous gradient centrifugation (15 min at 2G, 2 layers of 40 and 80%, PureSperm, JCD, France) before being separated into two fractions. The first fraction was fixed in a methanol and acetic acid solution (3:1) and was used as the unsorted (non-HOST) control, for each subject. The second fraction was incubated for 10 min at 37 °C, in a hypo-osmotic 150 mOsm solution (25 mM sodium citrate, 75 mM fructose, sterile water). This incubation time, considered a compromise between the two recommendations of the WHO manual (5 min for therapeutic use, and 30 min for diagnostic purposes), was used for our previous study regarding HOST use in chromosomal rearrangement carriers [3]. The cells were then fixed in a methanol and acetic acid solution (3:1) for 30 min at room temperature, and were then spread on microscope slides.
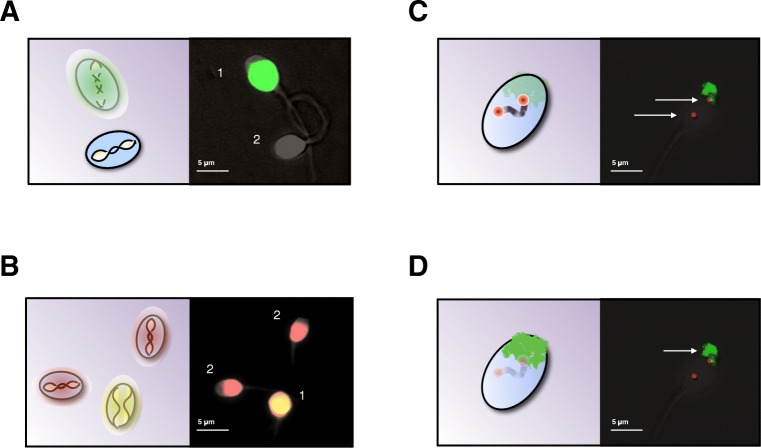
Three sperm quality parameters were evaluated for each subject, both on the control slides and on the HOST slides (Fig. 2): DNA fragmentation, DNA decondensation, and nuclear architecture (inter-telomeric distance, and chromosomal territory area).
Fig. 2.
Nuclear quality analysis was performed with four different assays. The spermatozoa from the different HOST classes, as well as unsorted spermatozoa, were evaluated based on 4 different parameters: A DNA fragmentation with a TUNEL assay, causing the sperm nuclei with significant DNA breaks to exhibit a green fluorescence (1), absent in spermatozoa with intact DNA (2). B DNA decondensation, with a chromomycin A3 assay, with which spermatozoa with decondensed DNA exhibit bright yellow fluorescence (1), as opposed to their condensed nuclei associated with dull orange fluorescence. C Inter-telomeric distance, using telomeric probes for the two arms of chromosomes 1. D Chromosomal territory area for chromosome 1, using a whole chromosome painting probe specific of that chromosome
DNA fragmentation was assessed through a TUNEL (terminal deoxynucleotidyl-transferase dUTP nick end labeling) assay, using the In Situ Cell Death Detection kit (Roche, Indiana, USA). After being washed twice in a phosphate buffer solution (PBS, Eurabio, France), the slides were permeabilized (0.1% sodium citrate, 0.1% Triton) for 5 min at −20 °C. Next the TdT enzyme was diluted at 1/10 in the buffer solution provided in the kit, and was placed on the slides which were incubated for 15 min at 37 °C in a moist chamber. The slides were then washed twice for 5 min in PBS and examined on an Olympus BX-UCB fluorescence microscope. The same procedure without the TdT enzyme was used as a negative control (data not shown). An intense green fluorescence over the sperm head was interpreted as DNA fragmentation. The proportion of such cells was evaluated for both the unsorted (non-HOST) spermatozoa (300 spermatozoa per subject), as well as for the HOST-treated spermatozoa (20–50 spermatozoa for each individual class, per subject).
DNA decondensation was assessed through the Chromomycin A3 (CMA3) assay (Sigma Chemicals, MO, USA). Each slide was treated for 20 min with 100 μL CMA3 solution (0.25 mg/ml in McIlvane’s buffer), washed with in McIlvane’s buffer, and was subsequently analyzed on a fluorescence microscope. Dull yellow/orange staining was associated with a normal chromatin packaging, and bright yellow fluorescence was associated with an abnormal chromatin packaging (DNA decondensation).
Nuclear architecture was evaluated by two separate FISH (fluorescent in situ hybridization) procedures, as previously described by our team [7]: evaluation of the inter-telomeric distance (i.e., the distance between the extremities of the two telomeric ends) for chromosome 1, and the area of the chromosomal territory occupied by chromosome 1 within the nucleus. For both procedures, FISH was conducted as described previously. Spermatozoa underwent a decondensation step, with 2 min 30 s in NaOH, followed by hybridization with the probes at 70 °C for 2 min and 30 s. Image acquisition was performed on a fluorescence microscope (×100 oil immersion objective) with a COHU 4912-5000 CCIR camera. With this configuration, 12 pixels on the images were equivalent to 1 μm. The images were analyzed on open-source software Fiji [8] along with the Shape Filter plug-in [9].
For inter-telomeric distance evaluation, two sets of in-house contiguous probes for the two telomeric ends (p and q) of chromosome 1 were selected. On Fiji, the distance between the two signals was measured, as well as at the length of the sperm head, from the implantation base of the intermediary piece to the tip of the head. As a way to eliminate the effect of a possible decondensation of the head, for each cell, the ratio between the crude inter-telomeric distance and the length of the head was calculated, and was recorded as the “inter-telomeric distance.”
For the chromosomal territory area evaluation, we used whole chromosome painting probes for chromosome 1 resulting in a fluorescence of the whole chromosome. The area of the fluorescent signal and the overall area of the sperm head were measured on Fiji. For each cell, the ratio between the crude area of chromosome and the overall area of the head was calculated, and recorded as the “chromosomal territory area.”
For each parameter and each subject, 300 spermatozoa were evaluated in the unsorted group (except for decondensation: 200), 50 in the A class, 30 in the B class (except for decondensation: 20), 30 in the B+ class (except for decondensation: 20), and 50 in the C, D/E, F, and G classes. Those classes are described in the WHO laboratory manual, from the 3rd edition onward, with addition of the B+ class, as shown by our team [3]. For each parameter, each HOST class was compared to the unsorted spermatozoa (chi-square and t student tests, GraphPad software), and results with p<0.05 were considered statistically significant.
Results
Spermatozoa belonging to each of the different 7 HOST classes were analyzed based on the aforementioned parameters, and compared to unsorted spermatozoa (i.e., spermatozoa not treated by HOST). Results from all 10 subjects were collected and pooled. This was done in order to obtain exploitable data considering the very small number of spermatozoa belonging to certain classes, such as B and B+ which each comprise approximately 2% of any given sample [3].
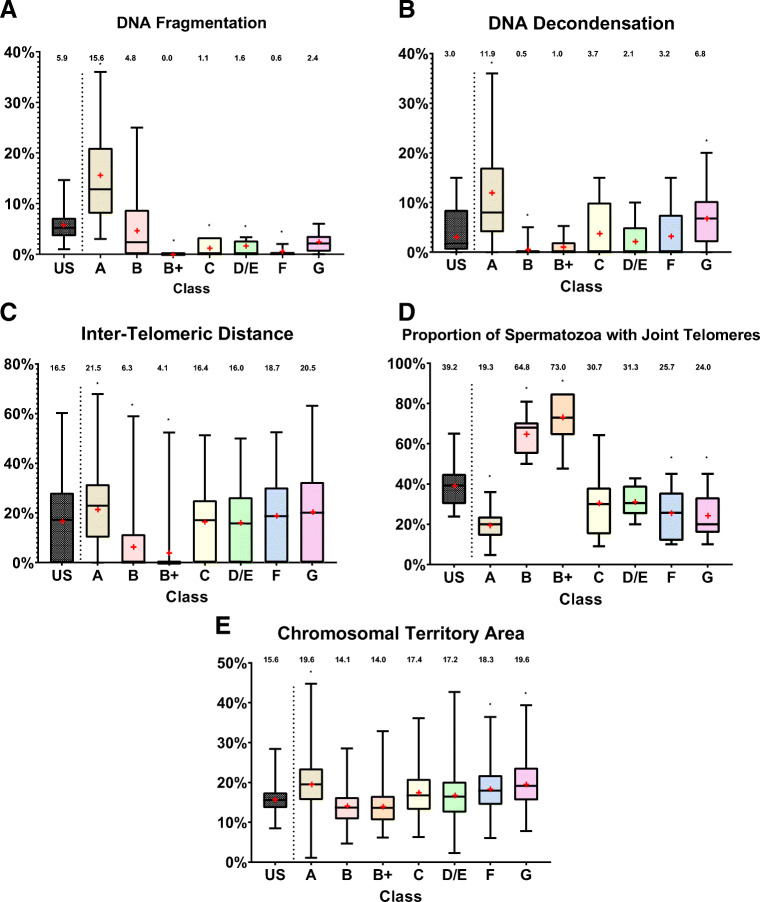
DNA fragmentation (Fig. 3A)
Fig. 3.
Evaluation of the different HOST classes, compared to that of unsorted, pre-HOST, spermatozoa, based on four nuclear quality parameters. Data shown corresponds to all the subjects together. Results with p<0.05 were considered statistically significant. A DNA fragmentation: Compared to unsorted spermatozoa (US), classes B, C, D/E, and F exhibit lower DNA fragmentation rates, and class A exhibits higher DNA fragmentation rates. B DNA decondensation: Compared to US, classes B and B+ exhibit lower decondensation rates, and classes A and G exhibit higher decondensation rates. C Inter-telomeric distance: The y axis corresponds to the mean inter-telomeric distance for chromosome 1 in relationship to the head’s length. Compared to US, classes B and B+ exhibit shorter inter-telomeric distances, and classes A, D/E, and G exhibit longer inter-telomeric distances. D Proportion of spermatozoa with joint telomeres: Compared to US, classes B and B+ exhibit a higher proportion of joint telomeres, and classes A, F, and G exhibit a lower proportion. E Chromosomal territory area: The y-axis corresponds to the mean chromosome 1 area, in relation to the total nuclear area. Compared to US, classes A, F, and G exhibit a larger chromosomal territory area for chromosome 1
The global DNA fragmentation proportion among unsorted spermatozoa was 5.9% (177 out of 3000 spermatozoa). Class A was associated with a significantly higher proportion of DNA-fragmented spermatozoa (15.6%, 78/500 spermatozoa, p<0.00001), while classes B+, C, D/E, and F were associated with a significantly lower DNA fragmentation rate (respectively 1% or 3/300, p<0.01; 1.6% or 8/500, p<0.01; 1.6% or 8/500, p<0.01; and 0.6% or 3/500, p<0.001). Classes B and G did not show statistically significant differences in DNA fragmentation rate as compared to the unsorted spermatozoa.
DNA decondensation (Fig. 3B)
The global proportion of unsorted spermatozoa exhibiting DNA decondensation was 4.4% (88/2000 spermatozoa). The proportion of spermatozoa with DNA decondensation was significantly lower among the B and B+ HOST classes (respectively 0.05%, 1/200, p<0.05; and 1.5%, 3/200, p<0.05), and significantly higher in A and G HOST classes (respectively 11.4%, 57/500, p<0.00001; and 7%, 35/500, p<0.01). Classes C, D/E, and F showed no significant differences from the unsorted group.
Nuclear architecture, inter-telomeric distance (Fig. 3C)
The inter-telomeric distance for chromosome 1 in unsorted spermatozoa was 16.5% (compared to the average head length). It was significantly higher among HOST class A, D/E, and G spermatozoa (21.5%, p<0.01; 21.5%, p<0.01; 27.5%, p<0.001), and significantly lower among B and B+ spermatozoa (respectively 6.3%, p<0.0001; and 4.1%, p<0.0001).
Nuclear architecture, proportion of spermatozoa with joint telomeric ends (Fig. 3D)
An alternate method of examining the phenomenon of inter-telomeric distance increase in low quality spermatozoa is to evaluate the proportion of cells with joint telomeric ends (inter-telomeric distance = 0). In the unsorted spermatozoa, the global proportion of spermatozoa with joint telomeric ends for chromosome 1 was 39.2%. HOST classes A, F, and G had a significantly lower proportion of cells with joint telomeric ends (respectively 19.3%, p<0.01; 25.7%, p<0.05; and 24.0%, p<0.05). Conversely, HOST classes B and B+ were associated with a significantly higher proportion of cells with joint telomeric ends (respectively 64.8%, p<0.01; and 73.0%, p<0.001). Classes C and D/E showed no significant differences from the unsorted group.
Nuclear architecture, chromosome 1 territory area (Fig. 3E)
Among the unsorted spermatozoa, the average chromosome 1 territory area (over the total head area) was 15.6%. This parameter was increased among HOST classes A, F, and G (respectively 19.6%, p<0.05; 18.3%, p<0.05; 19.5%, p<0.02). There was a trend toward decreased territory area among HOST classes B and B+ (both 14.1%), but this was not statistically significant.
Discussion
The hypo-osmotic swelling test (HOST), developed in 1984, was initially used solely as a diagnostic test [1]. Initially, treated spermatozoa were characterized simply as “HOST-positive” if they exhibited flagellar swelling, which would correspond to present-day classes B through G [10]. Samples were considered normal if they contained 60% or more “HOST-positive” spermatozoa, and abnormal if they contained less than 50% [11]. Associations between the percentage of “HOST-positive” spermatozoa and reproductive outcomes (both for natural and ART-related conception) have been previously evidenced [12, 13].
Subsequently, different types of tail swelling were identified and grouped into classes (A, B, B+, C, D/E, F, G) (Fig. 1). The previous characterization of “HOST-positive” effectively included all the classes except A (i.e., B, B+, C, D/E, F, and G) [14]. Stanger et al. were the first to examine individual sperm classes with the aim of finding a correlation between HOST and DNA fragmentation as evidenced by TUNEL [5]. They found that the classes with the highest DNA fragmentation rates were A (34% TUNEL positive) and G (15%), while the ones associated with the lowest DNA fragmentation rates were D/E (4%) and F (8%). Two years later, Bassiri et al. conducted a similar study with additional parameters [15]. Concerning DNA fragmentation, they found conflicting results to those of Stanger et al., with A, F, and G being the HOST classes associated with the highest levels of DNA fragmentation, and B, C, and D/E being associated with the lowest levels. Examining DNA decondensation and phosphatidyl-serine externalization, they found that the D class showed the lowest levels of both.
Additional studies evaluated other parameters in each HOST class individually. Pang et al. compared the rate of aneuploidy between HOST classes and found that spermatozoa belonging to the B, C, and D/E classes carried 17 times less aneuploidy than unselected spermatozoa [6]. Similarly, our team [3] showed that, in chromosomal rearrangement carriers, HOST could be used to select chromosomally balanced spermatozoa. We described a new HOST class, termed B+, that can be identified by having a ratio of the length of the flagellum to the diameter of the flagellar distal loop that is greater than 20. B+ spermatozoa were associated with an 84% decreased proportion of unbalanced spermatozoa.
We hypothesized that treatment by HOST could aid in discriminating between high and low fertility spermatozoa. The purpose of the present study was therefore threefold: (1) to address the discordant results of Bassiri and Stanger concerning DNA fragmentation and decondensation, (2) to evaluate the aforementioned B+ HOST class on parameters other than chromosomal content in rearrangement carriers, and (3) to evaluate different HOST classes by their nuclear architecture, which may represent a reflection of sperm quality [7].
The pathophysiological model that we suggest is as follows: any disturbance in the sperm architecture (DNA breaks, an abnormal chromosomal content, etc.) may trigger an apoptotic process, an early sign of which is an alteration of the cell membrane. Only an intact membrane will be able to adequately respond to a change in osmolarity in the extracellular space and thereby lead to characteristic flagellar shapes. HOST, based on this hypothetic model, would therefore represent a functional evaluation assay for early apoptosis. This could potentially be formally proven in further studies.
DNA fragmentation
DNA fragmentation corresponds to the accumulation of single- and double-strand DNA breaks and is thought to negatively influence reproductive potential [16]. During spermatogenesis, the sperm DNA molecule adopts a unique structure: the histones are replaced by protamines and the sperm loses its cytoplasm, resulting in a tightly packed DNA molecule. The removal of the cytoplasm leaves the sperm nucleus vulnerable to a variety of potential insults: free radicals, radiation, infectious agents, drugs, and heat, among others [17]. This leads to the accumulation of DNA breaks, or DNA fragmentation [18]. DNA fragmentation is irreversible, since spermatozoa lack machinery for DNA repair. Many studies have suggested a relationship between DNA fragmentation and male infertility [19, 20], including in men with idiopathic or unexplained infertility [21]. Some authors even advise that DNA fragmentation evaluation be included in routine clinical fertility evaluation [22]. A recent study evaluated DNA fragmentation, using the Halosperm assay, in relationship to HOST morphology [23]. The authors of this study showed that the spermatozoa with the lowest DNA fragmentation rates were the ones belonging to classes D, F, and G. However, unlike in the present study, the control group was made of spermatozoa which did not undergo sperm processing by discontinuous gradient centrifugation (DGC). It is therefore difficult to know if those differences are related to DGC or HOST, since in that study the two parameters are evaluated simultaneously.
We show here a correlation between HOST morphology and DNA fragmentation rate. The most striking result is the high DNA fragmentation rate among A spermatozoa, almost three times as much as in unselected non-HOST-treated spermatozoa. This is however not surprising since the majority—although not all—of HOST-A spermatozoa are dead [24]. These spermatozoa are therefore likely to have undergone apoptosis, which may be either the cause or the consequence of DNA breaks. It has been shown that DNA fragmentation has a greater influence on IVF reproductive outcome compared to that of ICSI [16]. This is thought to be related to the fact that during ICSI spermatozoa are selected based on their motility and morphology. Most A spermatozoa would not be motile in non-HOST conditions since they are dead. It is unclear why the G class, which is associated with poorer quality when evaluated with other parameters, had less DNA fragmentation than the unselected spermatozoa.
DNA decondensation
As stated above, nuclear condensation is a critical event of spermatogenesis, allowing the male genome to reach the female gamete safely. The condensation of the nucleus in spermatozoa is striking: while the nucleus of a lymphocyte has a volume of roughly 180 μm3, that of a mature spermatozoon approximates 16 μm3, for only half as much DNA content. Decondensation is evaluated in the laboratory by using a chromomycin (CMA3)-based assay. CMA3 is an anti-tumor agent derived from the Streptomyces cerevisiae bacterium. CMA3 competes with protamine for binding to DNA regions rich in guanine and cytosine. Bonding of CMA3, in case of protamine deficiency, leads to a bright yellow color, indicative of nuclear decondensation and protamine deficiency [25, 26]. Sperm decondensation has been shown to be a predictor of failure in spontaneous procreation [27] as well as in ART, with a negative correlation between decondensation and fertilization rate in IVF and ICSI [28]. Moreover, sperm samples with a decondensation rate of greater than 30% were shown to be correlated with ICSI failure (Sakkas et al., 1998)
We show here that compared to pre-HOST spermatozoa, A and G spermatozoa were associated with higher decondensation rates, while B and B+ spermatozoa were associated with lower decondensation rates. We propose that, regardless of cause, nuclear decondensation like DNA fragmentation can trigger an apoptotic process which will hinder the membrane’s ability to react to hypo-osmolarity. It would be of interest to conduct similar studies in men with high global decondensation rates (> 30%) so as to examine the potential interest of selection of B or B+ spermatozoa in these men.
Nuclear architecture
In addition to replacement of histones by protamine and to the removal of cytoplasm, the high degree of nuclear condensation in spermatozoa is achieved through the establishment of a specific nuclear tridimensional architecture during spermatogenesis. It has been previously evidenced that chromosomes have a specific conformation within the sperm nucleus, with all centromeres gathered in the center forming a structure called the chromocenter, and the telomere ends of a given chromosome covalently bound to each other near the periphery [29–31]. This hairpin conformation, along with a preferential positioning for each chromosome, allows for a high degree of nuclear condensation [32]. Our team showed in a recent study [7] that the presence of a chromosomal translocation hindered the achievement of such nuclear architecture, even for chromosomes not included in the translocation. We hypothesized that multiple pathologic processes might interfere with the establishment of this architecture, and therefore that the evaluation of nuclear architecture could be used as a way to assess overall sperm quality. We suggested two parameters that could be analyzed for such a purpose: the inter-telomeric distance (ITD) for chromosome 1 (chosen because of its large size), and the measured area of the chromosome 1 territory. In the aforementioned study, those two parameters were significantly increased in chromosomal rearrangement carriers compared to controls with a normal karyotype.
In order to neutralize the effect of nuclear decondensation, which results in larger nuclei, the ratio between the ITD and the length of the sperm nucleus (from the insertion spot of the intermediary piece to the tip of nucleus) was calculated for each cell. We showed here an increased ITD in HOST classes A, F, and G, and a decreased ITD in HOST classes B and B+. This is in accordance with the hypothesis that B and B+ are the spermatozoa with the healthiest physiology. In order to confirm those results, we assessed the proportion of spermatozoa with joint telomeric ends (ITD = 0%) in each class. Similarly, we found that A, F, and G classes were associated with a significantly lower proportion of joint telomeres, while B and B+ were associated with a higher one. Again, B and B+ spermatozoa seem to exhibit the most canonical nuclear architecture, while conversely it appeared disrupted in A and G spermatozoa.
As described, each chromosome has a preferential location within the nucleus, with a spatially delimited territory. While we did not assess the exact location of all chromosomes here, as opposed to our procedure in our previous study in chromosomal rearrangement carriers [7], we studied here the area occupied by chromosome 1 by using chromosome probes that allow for the identification of a whole chromosome (whole chromosome painting). We found a chromosomal territory area (CMT) of 15.6% among non-sorted spermatozoa. We found this area to be significantly larger in A, F, and G spermatozoa, and smaller in B and B+ spermatozoa. Again, it is believed that a small chromosomal area is associated with healthy spermatozoa of relatively normal physiology.
Our team initially categorized the B+ HOST class for a specific patient population of chromosomal rearrangement carriers [3]. In that study, we compared the proportion of chromosomally unbalanced spermatozoa, which can range from 0 to 75% in each HOST class, to that of unsorted spermatozoa. Since we found a 55% decrease among B spermatozoa, we described a new class, termed B+, with a longer flagellum and smaller distal flagellar loop than those of the B class. Among those, the decrease in the proportion of unbalanced spermatozoa was 83%. We therefore included this newly described class in the present study. However, we did not find any statistically significant difference between B and B+ spermatozoa for the parameters evaluated here. While the utility of specifically selecting B+ spermatozoa in chromosomal rearrangement carriers appears justified based on prior studies, it remains unclear whether this would be advantageous for patients with normal karyotypes. This hypothesis should be evaluated in future studies with a larger number of subjects and spermatozoa.
Taken together, those results suggest that HOST allows for a broad evaluation of sperm quality which is reflected by significant differences in DNA fragmentation, DNA decondensation, and nuclear architecture. These results suggest that B and B+ are “higher quality” spermatozoa, while A and G may be considered “poorer quality.” We hypothesize that the structural basis for these findings is a disruption in the spermatic physiology triggering an apoptotic process which would render the membrane differentially responsive to changes in extracellular osmolarity, leading to variable flagellar morphology in compromised spermatozoa (A, G).
Further studies may examine these findings using a larger number of subjects, and possibly using different sub-groups (normal/abnormal semen parameters, elevated DNA fragmentation, levels, etc.). The strengths of this study, however, include that the controls in the present study are the unsorted spermatozoa, and a large number of spermatozoa were analyzed.
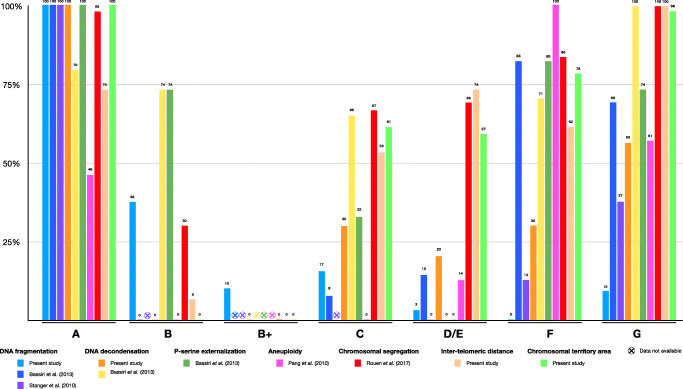
Figure 4 maps our results against those of prior analyses of the characteristics of different HOST classes. For each parameter, the results were normalized: 100% means that this class had the highest/worst score for that parameter in that particular study. Conversely, 0% means that this class had the lowest/best score. This allows for meaningful comparison of respective quality of the different HOST-classes across multiple studies. This comparison underscores the lower quality of A, F, and G spermatozoa, and the higher quality of B and B+ spermatozoa.
Fig. 4.
Review of the past and current studies assessing sperm quality based on HOST class. This figure summarizes the results of the present study as well as of others on the respective quality of the HOST classes, based on the following parameters: DNA fragmentation, DNA decondensation, phosphatidyl-serine externalization, aneuploidy, chromosomal segregation in rearrangement carriers, inter-telomeric distance, and chromosomal territory area. For each parameter and study, the results were normalized: 100% means that the HOST class showed the highest score for a given parameter, and 0% means it showed the lowest score. We highlight the relatively higher quality of spermatozoa belonging to the B and B+ HOST classes, and the relatively poorer quality of those belonging to the A, G, and possibly C, D/E, and F classes
The results presented here may have implications both in male fertility evaluation and assisted reproduction.
As a diagnostic test, HOST could allow a functional evaluation of sperm physiology. We show a correlation between HOST morphology and other sperm evaluation parameters used clinically, such as DNA fragmentation and DNA decondensation, as well as with parameters related to sperm physiology (nuclear architecture). We suggest that the respective proportion of the different HOST classes (which we suggest terming “HOST patterns”) may be reflective of a sample’s reproductive potential.
Additionally, HOST could be used for sperm selection prior to ICSI. Traditionally, during ICSI, sperm are first selected based on their density via gradient centrifugation, and then based on their microscopically assessed morphology. HOST could add another layer of selection with the ultimate goal of optimizing ART outcome. Based on the results presented here, as well as from other authors, it appears that HOST morphology encompasses many aspects of sperm morphology, such as DNA architecture, DNA condensation, chromosomal content, and membrane function. It has therefore been hypothesized that HOST could reflect a spermatozoon’s ability to fertilize [33]. Furthermore, as opposed to other potential advanced sperm selection methods (such as magnetic-activated cell sorting or flow cytometry), it does not alter the sperm and is safe to use in ART. The precise indication of HOST-based sperm selection remains to be fully elucidated. In addition to chromosomal rearrangements (reciprocal and Robertsonian translocations, pericentric inversion) and elevated aneuploidy rates, we suggest that patients with high DNA fragmentation rates, high DNA decondensation rates, or abnormal HOST score could benefit from HOST-based sperm selection. The present study, along with past studies on the subject, highlight the interest of selecting B and B+ spermatozoa, and avoiding the ones belonging to the A and G classes. The next step would be to conduct randomized controlled studies, comparing HOST-selected spermatozoa versus traditionally selected spermatozoa prior to ICSI, as was previously performed for HOST and immotile spermatozoa [34].
Author contribution
Adrien Bloch, Eli Rogers, and Cynthia Nicolas performed microscope analysis, and participated in recruiting subjects and in writing the manuscript.
Tanguy Martin-Denavit performed the chromomycin A3 technique.
Miguel Monteiro and Daniel Thomas provided expertise on the figures and on sperm selection possibilities.
Hélène Morel participated in microscope analysis.
Rachel Levy and Jean-Pierre Siffroi participated in conceiving the study.
Charlotte Dupont participated in recruiting the subjects and performed the initial routine sperm analysis.
Alexandre Rouen conceived and coordinated the study.
Funding
No specific funding was sought for this study, and departmental funds were used to support the authors (Sorbonne Université, INSERM, Maladies génétiques d’expression pédiatrique, APHP, Hôpital d’Enfants Armand Trousseau, Département de Génétique Médicale, F-75012 Paris, France).
Availability of data and material
Data and material are available.
Declarations
Ethics approval
The ethics commission CECOS (Centre d’Etude et de Conservation des Oeufs et du Sperm humains) considered this project to be exempted from IRB approval since it did not involve any additional medical intervention and solely used remnants from sperm samples obtained as part of the routine clinical care
Consent to participate
Informed consent was obtained from each subject.
Consent for publication
All authors haven consented for publication of this manuscript.
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Adrien Bloch, Cynthia Nicolas, and Eli Rogers equally contributed to this work as first authors.
References
- 1.Jeyendran RS, Van der Ven HH, Perez-Pelaez M, Crabo BG, Zaneveld LJ. Development of an assay to assess the functional integrity of the human sperm membrane and its relationship to other semen characteristics. J Reprod Fertil. 1984;70:219–228. doi: 10.1530/jrf.0.0700219. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization, editor. WHO laboratory manual for the examination and processing of human semen. 5. Geneva: World Health Organization; 2010. [Google Scholar]
- 3.Rouen A, Carlier L, Heide S, Egloff M, Marzin P, Ader F, et al. Potential selection of genetically balanced spermatozoa based on the hypo-osmotic swelling test in chromosomal rearrangement carriers. Reprod Biomed Online; 2017. [DOI] [PubMed]
- 4.Bassiri F, Tavalaee M, Nasr Esfahani MH. Correlation between different patterns of hypo-osmotic swelling and sperm functional tests. Int J Fertil Steril. 2013;7:193–198. [PMC free article] [PubMed] [Google Scholar]
- 5.Stanger JD, Vo L, Yovich JL, Almahbobi G. Hypo-osmotic swelling test identifies individual spermatozoa with minimal DNA fragmentation. Reprod BioMed Online. 2010;21:474–484. doi: 10.1016/j.rbmo.2010.06.026. [DOI] [PubMed] [Google Scholar]
- 6.Pang M-G, You Y-A, Park Y-J, Oh S-A, Kim D-S, Kim Y-J. Numerical chromosome abnormalities are associated with sperm tail swelling patterns. Fertil Steril. 2010;94:1012–1020. doi: 10.1016/j.fertnstert.2009.04.043. [DOI] [PubMed] [Google Scholar]
- 7.Mebrek ML, Clède S, de Chalus A, Heide S, Ruoso L, Rogers E, Lédée N, Prat-Ellenberg L, Cassuto NG, Siffroi JP, Rouen A. Simple FISH-based evaluation of spermatic nuclear architecture shows an abnormal chromosomal organization in balanced chromosomal rearrangement carriers. J Assist Reprod Genet. 2020;37:803–809. doi: 10.1007/s10815-020-01736-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, Tinevez JY, White DJ, Hartenstein V, Eliceiri K, Tomancak P, Cardona A. Fiji: an open-source platform for biological-image analysis. Nat Methods. 2012;9:676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wagner T, Lipinski H-G. IJBlob: An ImageJ library for connected component analysis and shape analysis. J Open Res Softw. 2013;1:e6. doi: 10.5334/jors.ae. [DOI] [Google Scholar]
- 10.Ramu S, Jeyendran RS. The hypo-osmotic swelling test for evaluation of sperm membrane integrity. Methods Mol Biol. 2013;927:21–25. doi: 10.1007/978-1-62703-038-0_3. [DOI] [PubMed] [Google Scholar]
- 11.Van der Ven HH, Jeyendran RS, Al-Hasani S, Perez-Pelaez M, Diedrich K, Zaneveld LJ. Correlation between human sperm swelling in hypoosmotic medium (hypoosmotic swelling test) and in vitro fertilization. J Androl. 1986;7:190–196. doi: 10.1002/j.1939-4640.1986.tb00909.x. [DOI] [PubMed] [Google Scholar]
- 12.Check JH, Epstein R, Nowroozi K, Shanis BS, Wu CH, Bollendorf A. The hypoosmotic swelling test as a useful adjunct to the semen analysis to predict fertility potential. Fertil Steril. 1989;52:159–161. doi: 10.1016/S0015-0282(16)60808-7. [DOI] [PubMed] [Google Scholar]
- 13.Jedrzejczak P, Pawelczyk L, Taszarek-Hauke G, Kotwicka M, Warchoł W, Kurpisz M. Predictive value of selected sperm parameters for classical in vitro fertilization procedure of oocyte fertilization. Andrologia. 2005;37:72–82. doi: 10.1111/j.1439-0272.2005.00657.x. [DOI] [PubMed] [Google Scholar]
- 14.Hossain AM, Rizk B, Barik S, Huff C, Thorneycroft IH. Time course of hypo-osmotic swellings of human spermatozoa: evidence of ordered transition between swelling subtypes. Hum Reprod. 1998;13:1578–1583. doi: 10.1093/humrep/13.6.1578. [DOI] [PubMed] [Google Scholar]
- 15.Bassiri F, Tavalaee M, Shiravi AH, Mansouri S, Nasr-Esfahani MH. Is there an association between HOST grades and sperm quality? Hum Reprod. 2012;27:2277–2284. doi: 10.1093/humrep/des155. [DOI] [PubMed] [Google Scholar]
- 16.Simon L, Emery B, Carrell DT. Sperm DNA Fragmentation: consequences for reproduction. Adv Exp Med Biol. 2019;1166:87–105. doi: 10.1007/978-3-030-21664-1_6. [DOI] [PubMed] [Google Scholar]
- 17.Aitken RJ, Jones KT, Robertson SA. Reactive oxygen species and sperm function--in sickness and in health. J Androl. 2012;33:1096–1106. doi: 10.2164/jandrol.112.016535. [DOI] [PubMed] [Google Scholar]
- 18.Saleh RA, Agarwal A, Nelson DR, Nada EA, El-Tonsy MH, Alvarez JG, et al. Increased sperm nuclear DNA damage in normozoospermic infertile men: a prospective study. Fertil Steril. 2002;78:313–318. doi: 10.1016/S0015-0282(02)03219-3. [DOI] [PubMed] [Google Scholar]
- 19.Castillo J, Simon L, de Mateo S, Lewis S, Oliva R. Protamine/DNA ratios and DNA damage in native and density gradient centrifuged sperm from infertile patients. J Androl. 2011;32:324–332. doi: 10.2164/jandrol.110.011015. [DOI] [PubMed] [Google Scholar]
- 20.Simon L, Castillo J, Oliva R, Lewis SEM. Relationships between human sperm protamines, DNA damage and assisted reproduction outcomes. Reprod BioMed Online. 2011;23:724–734. doi: 10.1016/j.rbmo.2011.08.010. [DOI] [PubMed] [Google Scholar]
- 21.Saleh RA, Agarwal A, Nada EA, El-Tonsy MH, Sharma RK, Meyer A, et al. Negative effects of increased sperm DNA damage in relation to seminal oxidative stress in men with idiopathic and male factor infertility. Fertil Steril. 2003;79 Suppl 3:1597–1605. doi: 10.1016/S0015-0282(03)00337-6. [DOI] [PubMed] [Google Scholar]
- 22.Simon L, Emery BR, Carrell DT. Review: Diagnosis and impact of sperm DNA alterations in assisted reproduction. Best Pract Res Clin Obstet Gynaecol. 2017;44:38–56. doi: 10.1016/j.bpobgyn.2017.07.003. [DOI] [PubMed] [Google Scholar]
- 23.Kim SW, Nho EJ, Lee JY, Jee BC. Specific tail swelling pattern in hypo-osmotic solution as a predictor of DNA fragmentation status in human spermatozoa. Clin Exp Reprod Med. 2019;46:147–151. doi: 10.5653/cerm.2019.00458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bollendorf A, Check JH, Kramer D. The majority of males with subnormal hypoosmotic test scores have normal vitality. Clin Exp Obstet Gynecol. 2012;39:25–26. doi: 10.1016/j.ogc.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 25.Nasr-Esfahani MH, Razavi S, Mardani M. Relation between different human sperm nuclear maturity tests and in vitro fertilization. J Assist Reprod Genet. 2001;18:219–225. doi: 10.1023/A:1009412130417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sabeti P, Amidi F, Kalantar SM, Sedighi Gilani MA, Pourmasumi S, Najafi A, Talebi AR. Evaluation of intracellular anion superoxide level, heat shock protein A2 and protamine positive spermatozoa percentages in teratoasthenozoospermia. Int J Reprod Biomed. 2017;15:279–286. [PMC free article] [PubMed] [Google Scholar]
- 27.Lolis D, Georgiou I, Syrrou M, Zikopoulos K, Konstantelli M, Messinis I. Chromomycin A3-staining as an indicator of protamine deficiency and fertilization. Int J Androl. 1996;19:23–27. doi: 10.1111/j.1365-2605.1996.tb00429.x. [DOI] [PubMed] [Google Scholar]
- 28.Iranpour FG, Nasr-Esfahani MH, Valojerdi MR. al-Taraihi TM. Chromomycin A3 staining as a useful tool for evaluation of male fertility. J Assist Reprod Genet. 2000;17:60–66. doi: 10.1023/A:1009406231811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meyer-Ficca M, Müller-Navia J, Scherthan H. Clustering of pericentromeres initiates in step 9 of spermiogenesis of the rat (Rattus norvegicus) and contributes to a well defined genome architecture in the sperm nucleus. J Cell Sci. 1998;111(Pt 10):1363–1370. doi: 10.1242/jcs.111.10.1363. [DOI] [PubMed] [Google Scholar]
- 30.Schmid M, Krone W. The relationship of a specific chromosomal region to the development of the acrosome. Chromosoma. 1976;56:327–347. doi: 10.1007/BF00292954. [DOI] [PubMed] [Google Scholar]
- 31.Zalensky AO, Allen MJ, Kobayashi A, Zalenskaya IA, Balhórn R, Bradbury EM. Well-defined genome architecture in the human sperm nucleus. Chromosoma. 1995;103:577–590. doi: 10.1007/BF00357684. [DOI] [PubMed] [Google Scholar]
- 32.Zalenskaya IA, Zalensky AO. Non-random positioning of chromosomes in human sperm nuclei. Chromosom Res. 2004;12:163–173. doi: 10.1023/B:CHRO.0000013166.04629.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jeyendran RS, Caroppo E, Rouen A, Anderson A, Puscheck E. Selecting the most competent sperm for assisted reproductive technologies. Fertil Steril. 2019;111:851–863. doi: 10.1016/j.fertnstert.2019.03.024. [DOI] [PubMed] [Google Scholar]
- 34.Sallam HN, Farrag A, Agameya A-F, El-Garem Y, Ezzeldin F. The use of the modified hypo-osmotic swelling test for the selection of immotile testicular spermatozoa in patients treated with ICSI: a randomized controlled study. Hum Reprod. 2005;20:3435–3440. doi: 10.1093/humrep/dei249. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data and material are available.