Abstract
Homeobox (hox) proteins have been shown to regulate cell fate and segment identity by promoting the expression of specific genetic programs. In contrast to their restricted biological action in vivo, however, most homeodomain factors exhibit promiscuous DNA binding properties in vitro, suggesting a requirement for additional cofactors that enhance target site selectivity. In this regard, the pbx family of homeobox genes has been found to heterodimerize with and thereby augment the DNA binding activity of certain hox proteins on a subset of potential target sites. Here we examine the transcriptional properties of a forced hox-pbx heterodimer containing the pancreas-specific orphan homeobox factor pdx fused to pbx-1a. Compared to the pdx monomer, the forced pdx-pbx1a dimer, displayed 10- to 20-fold-higher affinity for a consensus hox-pbx binding site but was completely unable to bind a hox monomer recognition site. The pdx-pbx dimer stimulated target gene expression via an N-terminal trans-activation domain in pdx that interacts with the coactivator CREB binding protein. The pdx-pbx dimer was also found to repress transcription via a C-terminal domain in pbx-1a that associates with the corepressors SMRT and NCoR. The transcriptional properties of the pdx-pbx1 complex appear to be regulated at the level of alternative splicing; a pdx-pbx polypeptide containing the pbx1b isoform, which lacks the C-terminal extension in pbx1a, was unable to repress target gene expression via NCoR-SMRT. Since pbx1a and pbx1b are differentially expressed in endocrine versus exocrine compartments of the adult pancreas, our results illustrate a novel mechanism by which pbx proteins may modulate the expression of specific genetic programs, either positively or negatively, during development.
Homeobox proteins have been shown to regulate cell fate and segment identity by promoting the expression of specific genetic programs. Initially characterized as an endoderm-specific homeobox protein (35) and later as a transcription factor for the somatostatin (15, 17) and insulin genes (23), the orphan homeobox protein pdx, for example, performs a critical role in pancreatic development; targeted disruption of the pdx gene leads to a null pancreas phenotype with pancreatic morphogenesis arrested at the early bud stage (11, 21).
Predating the appearance of visible pancreatic rudiments, pdx expression is first detected histologically at embryonic day 8.5 (E8.5) (8). Although initially produced in both exocrine and endocrine compartments of the developing pancreas, pdx expression shifts to β cells, where it regulates insulin gene expression and functions importantly in glucose homeostasis (8, 17, 25, 28). Heterozygous pdx7+/− mice develop glucose intolerance in adulthood (7), and mutations in the human pdx gene are associated with maturity onset diabetes (33).
Like other Hox proteins, Pdx binds promiscuously to target promoters containing a consensus CTAATG recognition site, but its affinity for certain sites is strongly potentiated by heterodimerization with pbx (26). The importance of pbx in modulating hox activity is perhaps best illustrated by studies in Drosophila melanogaster showing that the pbx homologue exd strongly influences segmentation (27). Complex formation with pbx and exd requires a conserved YPWMK pentapeptide motif located upstream of the homeodomain in pdx and other hox proteins (3, 4, 6, 27, 30). The crystal structures of ubx-exd and hox1b-pbx1 complexes reveal that the pentapeptide motif functions primarily in protein-protein interactions, articulating with a hydrophobic pocket in the homeodomains of both pbx and exd (24, 29).
Although exd potentiates hox activity in most cases, repressive effects have also been described. pbx has been found to inhibit target gene, for example, in transfected cells, although the mechanism underlying this function is unclear (16). To evaluate the mechanism by which pbx-hox complexes activate or repress target gene expression without potential interference from other nuclear factors, we have employed a forced pdx-pbx heterodimer in which pdx sequences are fused in frame to pbx1. Our results illustrate a novel mechanism by which pbx proteins may influence the expression of genetic programs during development.
MATERIALS AND METHODS
Plasmid and transfections.
Wild-type (SP) and mutant (SμP) pbx-pdx forced heterodimers were constructed by three-way ligation into a PstI/XbaI-cut pBK cytomegalovirus (CMV) vector (Stratagene). The rat pdx1 cDNAs (wild-type and mutant lacking the pbx interaction motif) were amplified by PCR by using the primers 5′-TTACTACTGCAGATTATGGTATACCCATACGATGTTCCAGATTACGCTGGGCCCATGAATAGTGAGCAG-3′ (sense strand containing Kozak consensus and HA tag at the N terminus) and 5′-GGGCTCGAGCCGGGGTTCCTGCGG-3′ (antisense strand lacking stop codon). The amplified fragments were then digested with PstI and XhoI. The human Pbx1 cDNA was amplified by PCR by using the primers 5′-GCACTCGAGGGCATGGACGAGCAGCCCAGG-3′ (sense strand) and 5′-CTTCTTTCTAGATCACTTGTCGTCGTCGTCTTTGTAGTCGTTGGAGGTATCAGAGTG-3′ (antisense strand, containing stop codon and FLAG tag). The PCR-amplified Pbx1 cDNA fragment was then digested with XhoI and XbaI. Pbx1 and Pdx1 fragments were then ligated into the pBK-CMV vector to generate the chimeric expression constructs that encoded polypeptides of 105 kDa. The pbx interaction-defective heterodimer, referred to as SμP, contains point mutations in the pentapeptide motif (FPWMK/AAGGQ) at amino acids (aa) 119 to 123. Somatostatin TSEI and TSEII constructs and NcoR-SMRT plasmids have been described elsewhere (25, 26). Transfection assays were performed in 293T cells as previously described (20), and reporter activities were normalized to activity from cotransfected Rous sarcoma virus–β-galactosidase (RSV–β-Gal) expression plasmid.
DNA-binding studies.
Gel mobility shift assays were performed with 32P-labeled double-stranded somatostatin TSEII or TSEI oligonucleotides plus in vitro-translated pdx and pbx polypeptides as described previously (25, 26).
Western blotting and pull-down assays.
Western blot and co-immunoprecipitation assays were performed as previously described (20). Pbx1-specific antiserum was obtained from Santa Cruz Biotech. Pdx1 antiserum has been reported elsewhere (8, 15). For glutathione S-transferase (GST) pull-down assays, 35S-labeled polypeptides were incubated with GST resins in binding buffer (20 mM HEPES, pH 7.0; 2 mM MgCl2; 20% glycerol; 0.2 mM EDTA; 0.05% NP-40; 1 mM β mercaptoethanol) for 30 min at room temperature. Binding reactions were then washed four times with binding buffer, and the bound fractions were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis.
RESULTS
A forced pdx-pbx heterodimer displays selective DNA binding and trans-activation properties.
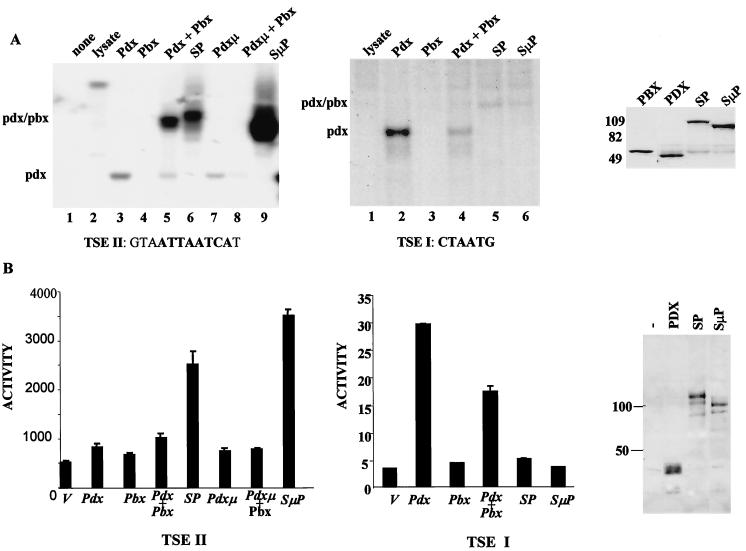
To characterize pdx-pbx activity, we constructed a forced heterodimer, referred to as SP, which contains the full-length pdx polypeptide fused at its C terminus to pbx1a. Compared with monomeric pdx, the SP dimer displayed 10 to 20 times higher affinity for a pbx-hox binding site on the somatostatin promoter (TSEII) (26) in gel mobility shift assays (Fig. 1A, left, compare lanes 3, 5, and 6). Mutagenesis of the pbx interaction motif in pdx (FPWMK/AAGGQ) actually enhanced binding of the mutant SμP construct to the TSEII site, suggesting that the pentapeptide motif in pdx functions exclusively in protein-protein interactions and that fusion of pdx with pbx is sufficient to promote cooperative DNA binding (Fig. 1A, left, compare lanes 6 and 9).
FIG. 1.
A forced pdx-pbx heterodimer, referred to as SP, binds selectively to and stimulates target gene expression from a consensus hox-pbx (TSEII) but not a monomeric hox (TSEI) binding site on the somatostatin promoter. (A) Gel mobility shift assay of SP binding activity compared with monomeric pdx on somatostatin TSEII (left) and TSEI (middle) elements. pbx interaction-defective pdx polypeptides containing (FPWMK/AAGGQ) substitution in the pbx interaction motif (aa 119 to 123) were examined either in the context of the pdx monomer (pdxμ) or a forced heterodimer (SμP). Gel mobility shift assays were performed with 32P-labeled somatostatin oligonucleotides plus in vitro-translated pdx and pbx polypeptides. (Right) Western blot assay of in vitro-translated pbx, pbx, SP, and SμP constructs show equivalent levels of expression. (B) Transient assay of pdx and SP polypeptides after cotransfection with somatostatin TSEII (left) and TSEI (middle) reporter constructs. In this and all subsequent assays, reporter activity is shown after normalizing to β-Gal activity from co-transfected RSV–β-Gal construct. (Right) Western blot assay of pdx, SP, and SμP polypeptides in nuclear extracts of transfected 293T cells with pdx-specific antiserum.
By contrast with their activities on the somatostatin TSEII element, SP and SμP polypeptides showed far lower affinity for a consensus hox monomer binding site on the somatostatin promoter (TSEI) compared to pdx alone (Fig. 1A, middle, compare lanes 2, 5, and 6). Consistent with the notion that pbx may destabilize the binding of heterodimerized hox proteins to certain sites, the addition of pbx also inhibited binding of monomeric pdx to the TSEI site in vitro (Fig. 1A, middle, compare lanes 2 and 4). These results indicate that two-site binding (pbx and pdx) is required to stabilize interaction of the pbx-pdx dimer with DNA and that single-site interaction with DNA (pdx) in this context is not sufficient for stable occupancy.
To evaluate the transcriptional properties of the pdx-pbx dimer, we performed transfection assays in 293T cells. pdx alone induced a TSEII reporter construct 1.5-fold, and cotransfection with pbx potentiated pdx activity somewhat in 293T cells (Fig. 1B, bottom left). Reflecting their enhanced DNA binding activities relative to pdx in gel shift assays, wild-type SP and mutant (SμP) dimers stimulated TSEII reporter activity five- and sevenfold, respectively (Fig. 1B, left).
Confirming the ability of pbx to block recruitment of pdx to promoters containing monomer binding sites, SP and SμP constructs were completely inactive on a TSEI reporter construct (Fig. 1B, middle). The pdx monomer stimulated TSEI reporter activity 15-fold, but overexpression of pbx inhibited TSEI-dependent reporter expression by ca. 50% (Fig. 1B, middle). These results suggest that, in addition to augmenting binding to consensus hox-pbx recognition sites, pbx family members may repress transcription from promoters containing monomer binding sites, depending on their affinities for hox partners in solution.
The pdx-pbx dimer stimulates transcription by associating with the C/H3 domain of the coactivator CBP.
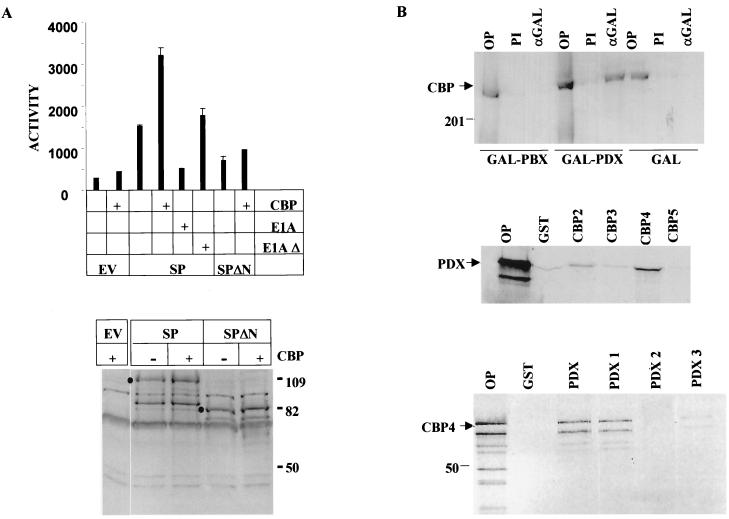
pdx has been shown to regulate both somatostatin and insulin gene expression via an N-terminal trans-activation domain (aa 1 to 140) (25). The ability of E1A to block insulin gene expression (32), potentially by sequestering the coactivator CREB binding protein (CBP) and its paralog P300, prompted us to examine whether pdx stimulates target gene expression, in the context of the pdx-pbx dimer, by associating with this coactivator. In transient-transfection assays, wild-type but not CBP interaction-defective E1A oncoprotein (E1AΔ) potently inhibited activation of the TSEII reporter via the SP polypeptide in 293T cells (Fig. 2A, top). Conversely, overexpression of CBP stimulated target gene activation two- to threefold by SP (Fig. 2A, left). CBP potentiation required the pdx trans-activation domain; CBP had no effect on TSEII reporter induction via a mutant SP dimer (SPΔN) lacking that region (Fig. 2A, left).
FIG. 2.
The pdx-pbx heterodimer stimulates target gene expression via an N-terminal trans-activation domain in pdx that associates with the coactivator CBP. (A) The top panel shows the transcriptional activity of wild-type (SP) or mutant (SPΔN) pdx-pbx forced heterodimer lacking N-terminal Pdx trans-activation domain (aa 1 to 135) in 293T cells. SP and SPΔN activities were evaluated on a somatostatin TSEII luciferase reporter plasmid. Cells cotransfected with CBP and E1A expression vectors (wild type and CBP/P300 interaction defective [E1AΔ]) indicated. In the bottom panel, the expression levels of SP and SPΔN in transfected 293T cells by Western blot assay with anti-pdx antiserum are shown. Cells co-transfected with CBP expression vector or empty vector indicated over each lane. (B) CBP associates with the N-terminal trans-activation domain of pdx in vivo. (Top) Western blot assay of CBP recovered from immunoprecipitates of GAL4 (αGAL) in 293T cells cotransfected with expression plasmids for CBP plus either GAL4 DNA-binding domain (aa 1 to 147), GAL4-PDX, or GAL4-PBX, as shown. OP, 10% of total input extract; PI, preimmune serum. (Middle) GST pull-down assay of 35S-labeled CBP fragments with the following amino acid endpoints: CBP2 (1 to 737), CBP3 (737 to 1626), CBP4 (1626 to 2260), and CBP5 (2260 to 2389). Assays were performed with GST or GST-pdx polypeptides bound to glutathione-Sepharose beads as indicated. OP, 10% of total input. (Bottom) GST pull-down assay of 35S-labeled CBP4 (aa 1626 to 2260) with GST-pdx polypeptides with the following amino acid endpoints: PDX, full-length pdx (1 to 283). PDX1 (1 to 140), PDX2 (141 to 215), and PDX3 (210 to 283).
To determine whether pdx interacts physically with CBP in vivo, we performed coimmunoprecipitation studies. CBP was efficiently recovered from immunoprecipitates of GAL4 pdx in transfected 293T cells (Fig. 2B, top). By contrast, no CBP was detected in anti-GAL4 immunoprecipitates from cells transfected with GAL4-pbx or GAL4 DNA binding domain expression vectors, indicating that the SP dimer associates with CBP via residues in pdx (Fig. 2B, top). To assign relevant interaction domains that mediate complex formation between pdx and CBP, we performed affinity interaction assays. Using fragments of CBP fused to GST, we observed selective binding of 35S-labeled pdx to the C/H3 region, which coincides with the E1A binding domain of CBP, but not to other regions (Fig. 2B, middle). In reciprocal pull-down assays with a series of GST pdx polypeptides, the 35S-labeled C/H3 region of CBP was found to bind specifically to the N-terminal trans-activation domain (aa 1 to 140) but not to the homeodomain (aa 140 to 215) or the carboxy-terminal region (aa 210 to 283) of pdx (Fig. 2B, bottom).
Pbx1 represses transcription via a C-terminal domain that interacts with the corepressors NCoR and SMRT.
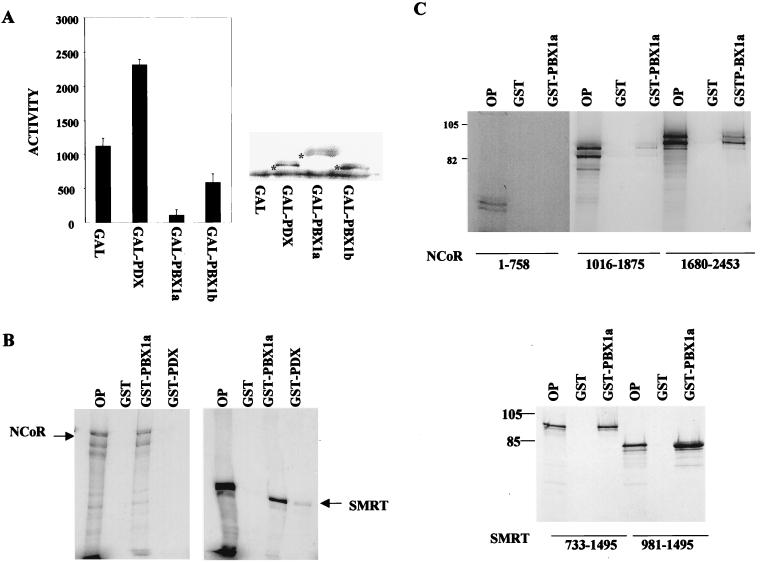
To evaluate regulatory contributions from pbx towards target gene expression, we fused pbx1a to the GAL4 DNA binding domain. After transfection into 293T cells, GAL4-pbx1a potently repressed transcription from a GAL4 thymidine kinase luciferase reporter (Fig. 3A). Remarkably, a GAL4 fusion construct containing the alternatively spliced pbx1b polypeptide, which lacks the carboxy-terminal 83 aa in pbx1a, was fivefold less active in repressing target gene expression (Fig. 3A). Expression levels of GAL4 pbx1a and GAL4 pbx1b were comparable in transfected cells, however, suggesting that the carboxy-terminal region in pbx1a is required for target gene repression in 293T cells (Fig. 3A, right).
FIG. 3.
pbx1a associates with the corepressors NCoR and SMRT. (A) Transient-transfection assay of GAL4-pbx1a and GAL4-pbx1b polypeptides on a GAL4 thymidine kinase luciferase reporter plasmid in 293T cells. The activities of GAL4 DNA-binding domain and GAL4-pdx polypeptides are shown for comparison. A Western blot assay of nuclear extracts from transfected 293T cells with αGAL4 antiserum was done to show comparable expression levels of each GAL4 fusion construct. Asterisks indicate immunoreactive bands corresponding to each polypeptide. (B) GST pull-down assays of 35S-labeled NCoR and SMRT after incubation with GST, GST-pbx1a, or GST-pdx polypeptides in vitro. OP, 10% of input. (C) Carboxy-terminal domains of NCoR and SMRT associate with pbx1a. GST pull-down assay of 35S-labeled NCoR (top) and SMRT (bottom) polypeptides with GST or GST-pbx1a glutathione-Sepharose resins. Inclusive amino acid endpoints for each NCoR and SMRT polypeptide are shown.
Previous studies, demonstrating that the corepressors NCoR and SMRT (5, 10) interact functionally with the homeodomain proteins Pit-1 and Rpx-1 (36), prompted us to examine whether pbx inhibits transcription via a similar mechanism. In GST pull-down experiments, 35S-labeled NCoR and SMRT polypeptides were found to bind efficiently to glutathione-Sepharose beads containing GST-pbx1a but not GST-pdx (Fig. 3B). The carboxy-terminal region corresponding to the nuclear hormone receptor binding domain of each corepressor (3, 7) appeared to mediate interaction with pbx1a; other regions did not bind detectably to GST-pbx1a resin in affinity interaction assays (Fig. 3C).
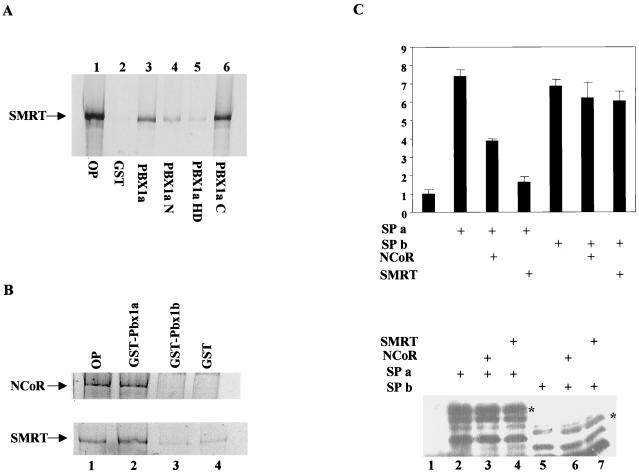
In reciprocal binding assays, SMRT (Fig. 4A) and NCoR (not shown) were found to bind efficiently to the alternatively spliced carboxy-terminal region of pbx1 (Fig. 4A, lane 6). A weaker secondary interaction of SMRT with the N-terminal 240 aa of pbx1a was also noted, however (Fig. 4A, compare lanes 2 and 4). In agreement with these truncation studies, pbx1b, which lacks the C-terminal domain in pbx1a, did not interact detectably with either NCoR or SMRT (Fig. 4B, compare lanes 2 and 3). The ability of the alternatively spliced carboxy-terminal region in pbx1 to associate with NCoR and SMRT prompted us to test whether pbx1a and pbx1b display different regulatory properties in the context of a pbx-pdx heterodimer. In transient-transfection assays, forced dimers containing either pbx1a (SPa) or pbx1b (SPb) induced TSEII reporter activity seven- to eightfold in 293T cells (Fig. 4C). Overexpression of either NCoR or SMRT strongly inhibited SPa activity on the TSEII reporter without altering the expression levels of the SPa polypeptide in transfected cells, suggesting that the repression we observed with these constructs reflects functional recruitment of NCoR and SMRT to the promoter (Fig. 4C). By contrast with SPa, NCoR and SMRT had no repressive effect on target gene induction via the SPb dimer, which lacks the NCoR-SMRT interaction domain (Fig. 4C). Taken together, these results suggest that pbx-hox dimers may either repress or activate target gene expression depending on relative cellular levels of pbx1a and pbx1b isoforms.
FIG. 4.
NCoR and SMRT are selectively recruited to pdx-pbx complexes containing pbx1a but not the alternatively spliced pbx1b polypeptide, which lacks the carboxy-terminal NcoR-SMRT interaction domain. (A) GST pull-down assay of 35S-labeled NCoR and SMRT polypeptides to GST-pdx1a and GST-pdx1b resins or GST only. OP, 10% of total input. (B) Pull-down assay of 35S-labeled SMRT with GST-pbx polypeptides. OP, 10% of total SMRT input; pull-downs were performed with glutathione-Sepharose resins containing either GST alone (GST), full-length GST-pbx1a (Pbx1a), or pbx fragments including N-terminal aa 1 to 240 (Pbx1aN), residues 233 to 320 containing the homeodomain (PBX1aHD), or carboxy-terminal residues 287 to 430 (PBX1aC). (C) In the upper panel, the results of a transient-transfection assay of SPa and SPb expression constructs containing full-length pdx fused to pbx1a and pbx1b, respectively, are shown. SPa and SPb activities were examined on a somatostatin TSEII luciferase reporter. Cotransfection with NCoR or SMRT expression vectors is as indicated. The lower panel shows a Western blot assay of nuclear extracts from transfected 293T cells with anti-pdx antiserum to show comparable expression of SPa (lanes 2 to 4) and SPb (lanes 5 to 7). Lane 1 shows extracts from cells transfected with empty vector.
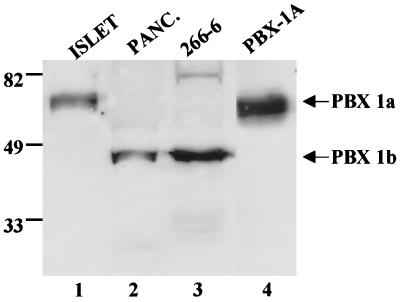
To determine whether pbx1a and pbx1b levels are differentially regulated in the pancreas, we performed Western blot assays with pbx1-specific antiserum. In agreement with a previous report (34), only pbx1b was detected in the acinar cell line 266-6 as well as in extracts from whole pancreas, which consist predominantly of exocrine tissue (Fig. 5, lanes 2 and 3). Pbx1b was not present in extracts from freshly isolated pancreatic islets, but a prominent pbx1a immunoreactive band was readily apparent (Fig. 5, lane 1). These results indicate that pbx1 isoforms are produced at different levels in exocrine versus endocrine cells of the pancreas where they may contribute to alternate activation of pancreatic genes via pdx.
FIG. 5.
pbx1a and pbx1b isoforms are differentially expressed in pancreatic islets and acinar cells. A Western blot assay of whole-cell extracts from freshly isolated islets (ISLET), whole pancreas (PANC.), and the acinar cell line 266-6 was done with α pbx-1-specific antiserum. The migration of in vitro-translated pbx1a polypeptide is shown. The relative positions of pbx1b and pbx1a polypeptides are indicated.
DISCUSSION
The pbx family of homeobox genes has been shown to modulate hox target gene activity by forming heterodimeric complexes that have high binding activity on a subset of potential DNA recognition sites. Our results demonstrate that pbx proteins may additionally inhibit hox target gene expression via two distinct mechanisms: by blocking binding of hox partners to monomer binding sites and by recruiting corepressor complexes to hox-pbx dimer binding sites.
When evaluated in the context of a forced pdx-pbx dimer, pbx strongly potentiated binding of pdx to a consensus hox-pbx binding site (TSEII) on the somatostatin promoter, but it also repressed binding of pdx to a monomer site (TSEI). Consistently, the addition of pbx to gel mobility shift reactions reduced binding of pdx to the TSEI site. pbx has been found to heterodimerize with hox partners in solution (31), suggesting that pbx may repress transcription from promoters containing hox monomer binding sites, depending on the nuclear levels of pbx.
Using a forced heterodimer approach to evaluate the activity of the pdx-pbx complex, we observed that target gene activation via pdx-pbx relies on an N-terminal trans-activation domain that interacts functionally with the coactivator CBP. CBP, in turn, has been proposed to mediate target gene expression, in part, via an association with general factors in the transcriptional apparatus (14, 19) and by intrinsic histone acetyltransferase activities that may disrupt promoter bound nucleosomes (2, 22). The ability of CBP to mediate target gene activation via pdx thus suggests a chromatin-dependent mechanism by which the pancreas-specific genetic program is activated during development.
By contrast with pdx, pbx was found to repress target gene expression via a C-terminal domain that associates with the corepressors NCoR and SMRT. Remarkably, no recruitment of NCoR or SMRT to the promoter was observed in the context of a pdx-pbx1 heterodimer containing the alternatively spliced pbx1b polypeptide that lacks the C-terminal trans-repression domain. These observations point to a potential function for alternative splice products of pbx: target gene expression may be alternately inhibited or activated by pbx-hox heterodimers depending on the relative expression levels of the two splice products.
The mechanism by which NCoR and SMRT repress target gene expression in this context is unclear. Previously shown to associate with histone deacetylases via Sin3 complexes (1, 9, 18), NCoR and SMRT may consequently block pdx activity by opposing CBP-mediated nucleosome acetylation. In this regard, pbx has been found to induce leukemic transformation in pre-B cells after fusion to the helix-loop-helix protein E2A (13). Importantly, the pbx1b alternative splice product lacking the C-terminal SMRT-NCoR interaction domain displays higher transforming activity compared to E2A-pbx1a fusion (12). Our results suggest that recruitment of SMRT-NCoR to the promoter via pbx1a may interfere with leukemogenesis, to some extent, by interfering with E2A-mediated target gene activation. In this regard, it will be of interest to determine whether B-cell transformation via E2a-pbx1a is potentiated by histone deacetylase inhibitors.
ACKNOWLEDGMENT
H.A. and S.D. contributed equally to this work.
This work was supported by the Foundation for Medical Research and by NIH grants ROI-DK 49777 and POI-54418. H.A. is supported by JSPS postdoctoral fellowships for research abroad (FY1999) and a grant from the Japan Orthopedics and Traumatology Foundation, Inc. (no. 0086).
REFERENCES
- 1.Alland L, Muhle R, Hou H, Potes J, Chin L, Schreiber-Agus N, DePinho R. A Role for N-CoR and histone deacetylase in Sin3-mediated transcriptional repression. Nature. 1997;387:49–55. doi: 10.1038/387049a0. [DOI] [PubMed] [Google Scholar]
- 2.Bannister A J, Kouzarides T. The CBP co-activator is a histone acetyltransferase. Nature. 1996;384:641–643. doi: 10.1038/384641a0. [DOI] [PubMed] [Google Scholar]
- 3.Chan S, Jaffe L, Capovilla M, Botas J, Mann R. The DNA binding specificity of ultrabithorax is modulated by cooperative interactions with extradenticle, another homeoprotein. Cell. 1994;78:603–615. doi: 10.1016/0092-8674(94)90525-8. [DOI] [PubMed] [Google Scholar]
- 4.Chang C, Shen W, Rozenfeld S, Lawrence H, Largman C, Cleary M. Pbx proteins display hexapeptide-dependent cooperative DNA binding with a subset of Hox proteins. Genes Dev. 1995;9:663–674. doi: 10.1101/gad.9.6.663. [DOI] [PubMed] [Google Scholar]
- 5.Chen J, Evans R. A transcriptional co-repressor that interacts with nuclear hormone receptors. Nature. 1995;377:454–457. doi: 10.1038/377454a0. [DOI] [PubMed] [Google Scholar]
- 6.Dijk M V, Murre C. Extradenticle raises the DNA binding specificity of homeotic selector gene products. Cell. 1994;78:616–624. doi: 10.1016/0092-8674(94)90526-6. [DOI] [PubMed] [Google Scholar]
- 7.Dutta S, Bonner-Wier S, Wright C, Montminy M. Regulatory factor linked to late-onset diabetes. Nature. 1998;392:560. doi: 10.1038/33311. [DOI] [PubMed] [Google Scholar]
- 8.Guz Y, Montminy M, Stein R, Leonard J, Gamer L, Wright C, Teitelman G. Expression of murine STF-1, a putative insulin gene transcription factor, in β cells of pancreas, duodenal epithelium, and pancreatic exocrine and endocrine progenitors during ontogeny. Development. 1995;121:11–18. doi: 10.1242/dev.121.1.11. [DOI] [PubMed] [Google Scholar]
- 9.Heinzel T, Lavinsky R, Mullen T, Soderstrom M, Laherty C, Torchia J, Yang W, Brad G, Ngo S, Davie J, Seto E, Eisenman R, Rose D, Glass C, Rosenfeld M. A Complex containing N-CoR, mSin3 and histone deacetylase mediates transcriptional repression. Nature. 1997;387:43–48. doi: 10.1038/387043a0. [DOI] [PubMed] [Google Scholar]
- 10.Horlein A, Naar A, Heinzel T, Torchia J, Gloss B, Kurokawa R, Ryan A, Kamei Y, Soderstrom M, Glass C, Rosenfeld M. Ligand-independent repression by the thyroid hormone receptor mediated by a nuclear receptor co-repressor. Nature. 1995;377:397–404. doi: 10.1038/377397a0. [DOI] [PubMed] [Google Scholar]
- 11.Jonsson J, Carlsson L, Edlund T, Edlund H. Insulin-promoter-factor 1 is required for pancreas development in mice. Nature. 1994;13:606–609. doi: 10.1038/371606a0. [DOI] [PubMed] [Google Scholar]
- 12.Kamps M, Look A, Baltimore D. The human t(1;19) translocation in pre-B ALL produces multiple nuclear E2A-Pbx1 fusion proteins with differing transforming potentials. Genes Dev. 1991;5:358–368. doi: 10.1101/gad.5.3.358. [DOI] [PubMed] [Google Scholar]
- 13.Kamps M, Murre C, Sun X, Baltimore D. A new homeobox gene contributes the DNA binding domain of the t(1;19) translocation protein in pre-B ALL. Cell. 1990;60:547–555. doi: 10.1016/0092-8674(90)90658-2. [DOI] [PubMed] [Google Scholar]
- 14.Kee B, Arias J, Montminy M. Adaptor mediated recruitment of RNA polymerase II to a signal dependent activator. J Biol Chem. 1996;271:2373–2375. doi: 10.1074/jbc.271.5.2373. [DOI] [PubMed] [Google Scholar]
- 15.Leonard J, Peers B, Johnson T, Ferreri K, Lee S, Montminy M. Characterization of somatostatin transactivating factor-1, a novel homeobox factor which stimulates somatostatin expression in pancreatic islet cells. Mol Endocrinol. 1993;7:1275–1283. doi: 10.1210/mend.7.10.7505393. [DOI] [PubMed] [Google Scholar]
- 16.Lu Q, Kamps M. Selective repression of transcriptional activators by Pbx1 does not require the homeodomain. Proc Natl Acad Sci USA. 1996;93:470–474. doi: 10.1073/pnas.93.1.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miller C, McGehee R, Habener J. IDX-1: a new homeodomain transcription factor expressed in rat pancreatic islets and duodenum that transactivates the somatostatin gene. EMBO J. 1994;13:1145–1156. doi: 10.1002/j.1460-2075.1994.tb06363.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nagy L, Kao H, Chakravarti D, Lin R, Hassig C, Ayer D, Schreiber S, Evans R. Nuclear receptor repression mediated by a complex containing SMRT, mSin3A, and histone deacetylase. Cell. 1997;89:373–380. doi: 10.1016/s0092-8674(00)80218-4. [DOI] [PubMed] [Google Scholar]
- 19.Nakajima T, Uchida C, Anderson S F, Parvin J D, Montminy M R. Analysis of a cAMP-responsive activator reveals a two-component mechanism for transcriptional induction via signal-dependent factors. Genes Dev. 1997;11:738–747. doi: 10.1101/gad.11.6.738. [DOI] [PubMed] [Google Scholar]
- 20.Nakajima T, Uchida C, Anderson S F, Lee C G, Hurwitz J, Parvin J, Montminy M. RNA helicase A mediates association of CBP with RNA polymerase II. Cell. 1997;90:1107–1112. doi: 10.1016/s0092-8674(00)80376-1. [DOI] [PubMed] [Google Scholar]
- 21.Offield M, Jetton T, Labosky P, Ray M, Stein R, Magnuson M, Hogan B, Wright C. PDX-1 is required for pancreatic outgrowth and differentiation of the rostral duodenum. Development. 1996;122:983–995. doi: 10.1242/dev.122.3.983. [DOI] [PubMed] [Google Scholar]
- 22.Ogryzko V V, Russanova V, Howard B H, Nakatani M. The transcriptional coactivators p300 and CBP are histone acetytransferases. Cell. 1996;87:953–959. doi: 10.1016/s0092-8674(00)82001-2. [DOI] [PubMed] [Google Scholar]
- 23.Ohlsson H, Karlsson K, Edlund T. IPF1, a homeodomain-containing transactivator of the insulin gene. EMBO J. 1993;12:4251–4259. doi: 10.1002/j.1460-2075.1993.tb06109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Passner J, Ryoo H, Shen L, Mann R, Aggarwal A. Structure of a DNA bound ultrabithorax-extradenticle homeodomain complex. Nature. 1999;397:714–719. doi: 10.1038/17833. [DOI] [PubMed] [Google Scholar]
- 25.Peers B, Leonard J, Sharma S, Teitelman G, Montminy M R. Insulin expression in pancreatic islet cells relies on cooperative interactions between the helix loop helix factor E47 and the homeobox factor STF-1. Mol Endocrinol. 1994;8:1798–1806. doi: 10.1210/mend.8.12.7708065. [DOI] [PubMed] [Google Scholar]
- 26.Peers B, Sharma S, Johnson T, Kamps M, Montminy M. The pancreatic islet factor STF-1 binds cooperatively with Pbx to a regulatory element in the somatostatin promoter: importance of the FPWMK motif and of the homeodomain. Mol Cell Biol. 1995;15:7091–7097. doi: 10.1128/mcb.15.12.7091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peifer M, Wieschaus E. Mutations in the Drosophila gene extradenticle affect the way specific homeodomain proteins regulate segmental identity. Genes Dev. 1990;4:1209–1223. doi: 10.1101/gad.4.7.1209. [DOI] [PubMed] [Google Scholar]
- 28.Peshavaria M, Gamer L, Henderson E, Teitelman G, Wright C, Stein R. X1Hbox 8, an endoderm-specific Xenopus homeodomain protein, is closely related to a mammalian insulin gene transcription factor. Mol Endocrinol. 1994;8:806–816. doi: 10.1210/mend.8.6.7935494. [DOI] [PubMed] [Google Scholar]
- 29.Piper D, Batchelor A, Chang C, Cleary M, Wolberger C. Structure of a HoxB1-Pbx1 heterodimer bound to DNA: role of the hexapeptide and a fourth homeodomain helix in complex formation. Cell. 1999;96:587–597. doi: 10.1016/s0092-8674(00)80662-5. [DOI] [PubMed] [Google Scholar]
- 30.Rauskolb C, Wieschaus E. Extradenticle, a regulator of homeotic gene activity, is a homolog of the homeobox-containing human proto-oncogene pbx1. Cell. 1993;74:1101–1112. doi: 10.1016/0092-8674(93)90731-5. [DOI] [PubMed] [Google Scholar]
- 31.Sanchez M, Jennings P, Murre C. Conformational changes induced in Hoxb-8/Pbx-1 heterodimers in solution and upon interaction with specific DNA. Mol Cell Biol. 1997;17:5369–5376. doi: 10.1128/mcb.17.9.5369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stein R, Corrigan M, Yaciuk P, Whelan J, Moran E. Analysis of E1A-mediated growth regulation functions: binding of the 300-kilodalton cellular product correlates with E1A enhancer repression function and DNA synthesis-inducing activity. J Virol. 1990;64:4421–4427. doi: 10.1128/jvi.64.9.4421-4427.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stoffers D, Ferrer J, Clarke W, Habener J. Early onset type II diabetes mellitus linked to IPF1. Nat Genet. 1997;17:138–139. doi: 10.1038/ng1097-138. [DOI] [PubMed] [Google Scholar]
- 34.Swift G, Liu Y, Rose S, Bischof L, Steelman S, Buchberg A, Wright C, MacDonald R. An endocrine-exocrine switch in the activity of the pancreatic homeodomain protein PDX1 through formation of a trimeric complex with PBX1b and MRG1. Mol Cell Biol. 1998;18:5109–5120. doi: 10.1128/mcb.18.9.5109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wright C, Schnegelsberg P, DeRobertis E M. XIHbox 8: a novel Xenopus homeo protein restricted to a narrow band of endoderm. Development. 1988;104:787–794. doi: 10.1242/dev.105.4.787. [DOI] [PubMed] [Google Scholar]
- 36.Xu L, Lavinsky R, Dasen J, Flynn S, McInerney E, Mullen T, Heinzel T, Szeto D, Korzus E, Kurokawa R, Aggarwal A, Rosenfeld M. Signal-specific co-activator domain requirements for Pit-1 activation. Nature. 1998;395:301–306. doi: 10.1038/26270. [DOI] [PubMed] [Google Scholar]