Abstract
Cytokines seem to play a crucial role in physiological and pathological conditions of acute myeloid leukemia (AML). The aim of this study was to evaluate the expression levels of interleukins-6 (IL-6) and IL-18 in patients with AML and its correlation with response to therapy and graft versus host disease (GvHD) after bone marrow transplantation. The expression levels of IL-6 and IL-18 genes were done in all patients and compared with matched control. Complete remission (CR) was used for evaluation of the effects of these cytokines on response to treatment in patients group. The expression level of these cytokines was also evaluated in patients who underwent bone marrow transplantation and experienced acute GvHD in compare with patients without aGvHD. Il-6 gene expression level was significantly higher in these patients in comparison with control but Il-18 gene expression level was not statistically significant compared to control group. Il-6 and also Il-18 expression levels were significantly higher in patients without a response to treatment according to CR compared to patient’s whit response to treatment as well as patients experienced aGvHD after bone marrow transplantation. IL-6 and Il-18 are important markers in the progression of the disease and could be considered as a prognostic marker in acute leukemia. It is recommended that more studies with larger study groups and more involved cytokines are needed for more evaluation of the cytokine roles in pathophysiology and progression of acute leukemia.
Keywords: Acute myeloid leukemia, Expression levels, Interleukine-6, Interleukine-18, Response to treatment
Introduction
Acute myeloid leukemia (AML) is a most common acute leukemia in adults which characterized by myeloblast accumulation (> 20%) in blood or bone marrow, due to defects in myelopoiesis maturation [1]. It might be cured in 35–40% of the patients younger than the age of 60 and in 5–15% of the patients older than the age of 60 years old [2]. Prognostic markers are of importance in risk assessment and treatment strategy, which affect the clinical outcomes. The understanding of the genetic basis and molecular aspects of AML has provided new insights into prognosis and therapy [3]. Considering the underlying mechanisms of AML, cytokine deregulation has been found to contribute to disease progression [4]. Cytokines are released to control the induced cellular stresses in cancer, infection, and inflammation. On the other hand, tumor cells can either release cytokines [5] or recruit the host cytokines for division and invasion [6]. Interleukin-6 (IL-6) represents a major cytokine, involved in versatile regulatory pathways of inflammation, immune regulation [7], and cancer (proliferation promotion, apoptosis prevention, metastasis facilitation, and metabolism of cancer cells) [8–10]. Signal transducer and activator of transcription (STATs) and Janus kinase (JAKs) are of IL-6-induced pathways [11]. Since the elevated concentrations of IL-6 are associated with poor prognosis, it might be considered as a predictor of survival in patients with cancer [12]. IL-18 represents a cytokine with pro-inflammatory and immune regulatory functions. The expression of IL-18 in inflammation, cancers, autoimmune diseases, and serious infections has been observed [13]. Considering cancer, a dual role has been observed: it can either provoke the immune system to fight against cancer or contribute to tumor development, angiogenesis, and prometastatic processes. Other studies have reported a correlation between the levels of IL-18 and the prognosis of AML. Higher levels of IL-18 have been correlated with worse prognosis of AML [14]. Graft versus host disease (GvHD) is an immune-mediated reaction which frequently occurred after bone marrow transplantation in AML patients. T cell-mediated immunity and cytokine release are main mechanisms involved in GvHD reaction. The aim of this study was to evaluate the expression levels of IL-6 and IL-18 in Iranian patients with AML and its correlation with response to therapy and GvHD after bone marrow transplantation.
Methods
Patients and Samples
In a cross-sectional study, 70 newly diagnosed AML patients were enrolled as a study group during 2015 and 2016. Fifty age-sex matched healthy subjects were also evaluated as normal controls. The patients were diagnosed based on clinical manifestations, bone marrow examination, and immunophenotyping. All patients received standard chemotherapy, which consisted of daunorubicin and cytarabine, also, for M3 patients, arsenic trioxide and ATRA in 2 divided doses in addition to standard induction chemotherapy regiment according to the manufacturer’s instructions as previously described briefly [15–18]. A total of 29 patients received HSCT from related HLA-matched donors and sub-grouped to aGvHD experienced and not-experienced (non-aGvHD) HSCT patients. Consequently, 9 patients experienced aGvHD, while 20 did not experience aGvHD. aGvHD was graded according to the classic Glucksberg–Seattle criteria and the International Bone Marrow Transplant Registry [19]. From all aGvHD experienced patients, 4 developed low grade (grade I + II) while 5 developed high grade (grade III + IV) of aGvHD. The study was approved by the Ethical Committee of Shiraz University of Medical Sciences, and the informed consent was obtained from all patients. For determination of the mRNA expression level of IL-6 and IL-18 genes, an in-house- SYBR Green Real-Time PCR was used and GAPDH also considered as internal control.
RNA Isolation and cDNA Synthesis
Total RNA was extracted by RNX-Plus solution (CinnaGen, Tehran, Iran). The quantity of extracted RNA was evaluated by NanoDrop spectrophotometer at 260/280 nm, and the quality of extracted RNA was assessed by running 3 μL on 1% agarose gel. The first-strand cDNA was synthesized using Prime Script RT Reagent Kit (Takara, Japan) according to the manufacturer’s guidelines [15–17].
SYBR Green Real-Time Polymerase Chain Reaction (Real-Time PCR)
For the quantitative analysis of IL-6 and IL-18 mRNAs expression profile in the understudied groups, SYBR Green Real-Time PCR method was performed. Glyceraldehyde 3-phosphate dehydrogenase gene was used as internal control for minor fluctuations. Polymerase chain reaction program and primer sequences are summarized in Table 1. A melt curve was analyzed to confirm the specificity of reaction at the end of the program. To check the specificity of the amplification reaction, a melting-curve analysis was evaluated. The results for the target genes were measured as fluorescent signal intensity and normalized to the internal standard gene GAPDH. Relative quantification was measured using the comparative Ct (2−ΔΔCt) method [20].
Table 1.
The primers and thermocycling condition for the IL-6, IL-18, and GAPDH transcripts
| Gene | Primer sequences (5'- > 3') | PCR Product length | Thermocycling condition |
|---|---|---|---|
|
IL-6: F IL-6: R |
ACCCCCAATAAATATAGGACTGGA GCTTCTCTTTCGTTCCCGGT |
101 bp | 95 °C/2 min, 40 cycles of 95 °C/30 s, 60 °C/20 s, and 70 °C/30 s |
|
IL-18: F IL-18: R |
AGCTTGTGAAAAAGAGAGAGACCT GCTAGTCTTCGTTTTGAACAGTGA |
75 bp | 95 °C/2 min, 40 cycles of 95 °C/30 s, 60 °C/20 s, and 70 °C/30 s |
|
GAPDH:F GAPDH:R |
GGACTCATGACCACAGTCCA CCAGTAGAGGCAGGGATGAT |
119 bp | 95 °C/2 min, 40 cycles of 95 °C/30 s, 57.5 °C/20 s, and 70 °C/30 s |
Statistical Analyses
Statistical analyses were performed with SPSS software (SPSS: An IBM Company, version 19.0, IBM Corporation, Armonk, NY, USA). The statistical differences in expression level of IL-6 and IL-18 and the fold changes in patients and controls were compared via independent t-test. The expression level of IL-6 and IL-18 was compared between patients according to the presence of aGvHD and response to chemotherapy treatment by independent t test. P values less than 0.05 were considered significant.
Results
From 70 studied recipients, 37 were male (52.8%) and 33 (47.2%) were female. The mean age of patients was 49 ± 2.4 ranged from 20 to 86 years, and the mean age of the control group was 48 ± 3.5 ranged 19–84 years. The laboratory characteristics of patients with AML patients showed the mean white blood cells (WBC) counts (49,514 ± 9672 /cmm), platelet count (55,136 ± 8312 /mL), Hb level (9.6 ± 0.23 g/dL), and lactate dehydrogenase (LDH) level (1313 ± 258 U/L).
Interleukin-6 Gene Expression in AML Patients and Controls
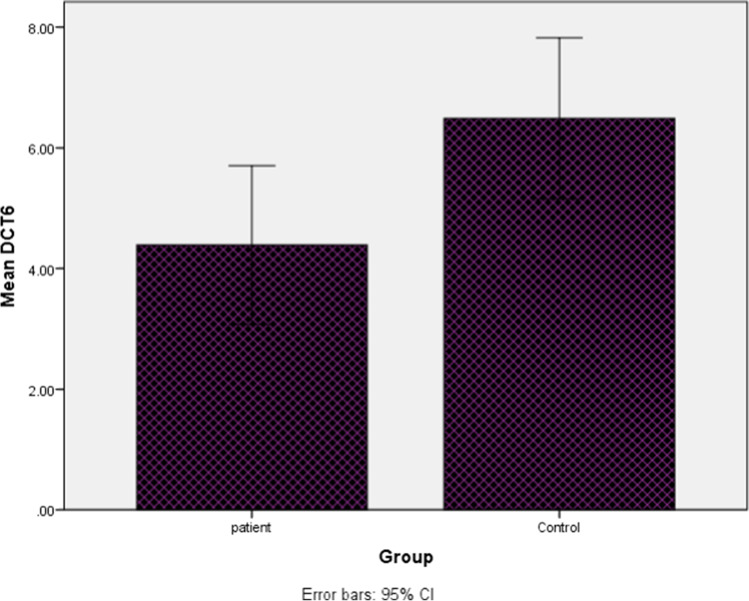
The mRNA expression analysis of IL-6 gene measured as cycle threshold (Ct) and ∆Ct values (Fig. 1). After the statistical analysis, our results revealed that the expression level of IL-6 was significantly (4.92 times) higher in the PBMCs of AML patients compared to healthy controls (P < 0.03).
Fig. 1.
Boxplot of mean of interleukin-6 ∆CT in (AML) patients and controls
Interleukin-18 Gene Expression in AML Patients and Controls
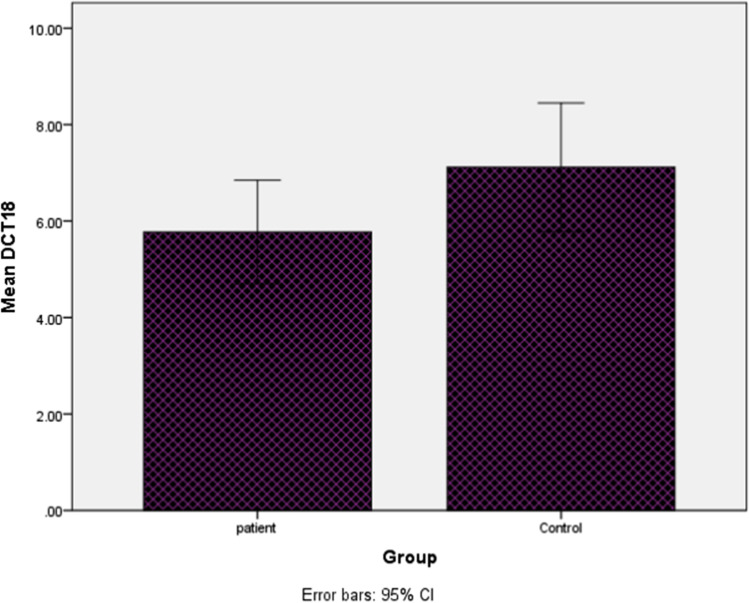
The mRNA expression analysis of IL-18 gene measured as cycle threshold (Ct) and ∆Ct values (Fig. 2). After the statistical analysis, our results revealed that the expression level of IL-18 was not significantly (2.63 times) higher in the PBMCs of AML patients compared to healthy controls (P = 0.1).
Fig. 2.
Boxplot of mean of interleukin-18 ∆CT in (AML) patients and controls
IL-6 and IL-18 Expression in HSCT Patients and Development of the aGvHD
The mean expression of IL-6 and IL-18 was compared between patients who underwent bone marrow transplantation with and without aGvHD. The results demonstrated that IL-6 and IL-18 were upregulated in patients developed aGvHD compared to those without aGvHD, although the difference was not statistically significant (1.2 ± 1.1 vs. 2.9 ± 0.7; P = 0.2 and 3.6 ± 1.08 vs. 3.8 ± 0.93; P = 0.8 respectively). IL-6 and IL-18 were overexpressed in patients experienced high grade (grades III–IV) aGvHD compared to those patients who developed low grade (grades 0–II) aGvHD, although the difference was not statistically significant too (3.2 ± 1.7 vs. 1.8 ± 1.3; P = 0.5 and 1.3 ± 1.8 vs. 3.5 ± 1.2; P = 0.3 respectively).
IL-6 and IL-18 Expression in AML Patients and Response to Treatment
The expression levels of IL-6 and IL-18 were evaluated in AML patients according to their response to treatment based on CR. The results showed that both IL-6 and IL-18 gene expression levels were increased in AML patients who did not respond to therapy compared to those patients who respond to therapy. Although the expression level of IL-6 was significantly higher (P = 0.03), the expression level of IL-18 was not statistically significant between two groups (P = 0.5).
IL-6 and IL-18 Expression According to Cytogenetic Risk Factors
Details of cytogenetic information of AML patients are shown in Table 2. Among all AML patients, 33 (30.5%) had FLT3-ITD mutation. Based on the generally genetic risk stratification, AML patients were divided into three groups; favorable, intermediate, and high-risk groups. Accordingly, 34 patients were included in high risk, 21 in intermediate, and the remaining 15 in favorable risk groups. The mean expression level of IL-6 and IL-18 were compared within risk stratification group. The results showed that the mean expression level of IL-18 was significantly decreased in favorable risk groups compared to high-risk groups (6.1 ± 0.6 vs. 2.3 ± 6; P = 0.001), while no significant difference was observed between high risk and favorable risk for the mean expression level of IL-6. The results also showed that the mean expression level of IL-6 and IL-18 was significantly increased in high-risk groups compared to intermediate risk group (3.6 ± 1.02 vs. 10.2 ± 0.64; P = 0.001 and 6.1 ± 66 vs. 10.1 ± 0.92; P = 0.02, respectively).
Table 2.
Acute myeloid leukemia with recurrent cytogenetic abnormalities
| Cytogenetic abnormalities | No. (%) |
|---|---|
| t(8;21)(q22;q22); RUNX1-RUNX1T1 | 17 (17.5%) |
| inv(16)(p13.1q22) or t(16;16)(p13.1;q22); CBFB-MYH11 | 15 (14.3%) |
| t(15;17)(q22;q12); PML-RARA | 4 (5.7.5%) |
| t(9;11)(p22;q23); MLLT3-MLL | 13 (9.1%) |
Association IL-18 and IL-6 Gene Expression and Risk Factors
In this study, a significant association was found between sex and fms-like tyrosine kinase 3 (FLT3) with IL-6 gene expression (P = 0.04 and P = 0.02, respectively) in AML patients. Also, a significant association was found between sex, LDH and FLT3 with IL-18 gene expression (P = 0.006, P = 0.05, and P = 0.03, respectively) of AML patients.
Discussion
Leukemia has been reported as the sixth prevalent cancer type in Iran [21], represented with the higher incidence in males than females [22]. In the present study, the expression profiles of IL-6 and IL-18 were considered as a prognostic marker in AML patients, evaluating 70 patients in Shiraz, Iran. Some prognostic factors have been identified in AML such as clinical features (age and performance status), cytogenetic abnormalities, rearrangements in the chromosome, gene mutations, gene expression profiles [23], WBC count, and CD34 expression [24]. These prognostic markers might help with defining the treatment plan for AML patients.
It has been observed that cytokine signaling pathways are disrupted in AML, which in turn affect various aspects of cell development and proliferation [4]. These deviations might be considered in diagnosis, prognosis, and drug development. For instance, elevated concentrations of tumor necrosis factor alpha determined as an adverse prognostic factor [25].
High levels of IL-6 have been found in most of the cancer types, and its role in several aspects of cancer progression has been elucidated [26, 27]. Additionally, drug therapy was found to be less effective in higher IL-6 levels [11]. IL-6 concentrations are correlated with various stages of tumor growth and consequently, the survival rate. So, it has been identified as a prognostic marker in pancreatic carcinoma [12], diffuse large cell lymphoma [28], advanced Hodgkin’s disease [29], colorectal [30], and metastatic renal carcinoma [31]. In this study, overexpression of IL-6 was observed in AML patients, in agreement with the studies by Sanches-Correa et al. [32] and Heikkilä et al. [33]. This overexpression has been correlated to a worse prognosis for AML patients. It can also be used as a marker to validate the efficacy of some medications, as Mitsunaga et al. have applied IL-6 as a predictor for gemcitabine efficacy in pancreatic carcinoma [34]. Moreover, anti-IL-6 monoclonal antibodies are in clinical trials for some of the cancer types [26]. Additionally, IL-18 levels might be considered as an indicator of worse prognostic effects, which have been reported in various types of cancers such as AML [14] pancreatic [35], gastric [36], and breast cancer [37]. The higher expression levels of IL-18 and its receptor have been also reported to induce drug resistance in AML, proposing a target for drug therapy [38]. Although elevated expression levels of IL-18 were observed in this study, a significant difference was not detected in AML patients compared to the control group. As mentioned in the study by Zhang et al., the expression of IL-18 might be associated with other known risk factors and prognostic indicators of AML such as age, WBC and CD34 titers, and high-risk karyotype (unfavorable cytogenetic abnormalities, FAB subtype) [14]. On the other hand, different levels of IL-18 have been associated to different stages of cancers [37]; as in the study by Kawabata et al., no significant elevation was noted in the stages Ι and IV of gastric carcinoma, while the expression level of IL-18 was significantly increased in stages ΙΙ and ΙΙΙ [36]. According to the results of the present study, the highest expression of IL-6 and IL-18 genes was seen in patients who had a high genetic risk; this increase was statistically significant only in the IL-18 level of high-risk group when compared with the favorable groups. Furthermore, the lowest expression of these genes was seen in patients with favorable genetic risk. The observed results in the present work might be affected by other risk factors and inter-individual differences, as well. Mutations in FLT3 and nucleophosmin-1 are known as adverse prognostic markers in AML [39]. Also, overexpression of FLT3 in AML has been reported previously, contributing to apoptosis inhibition or proliferation enhancement in primary AML blasts [40]. In the studies by Ozeki et al. [41] and Kuchenbauer et al. [42], FLT3 overexpression was mentioned as a prognostic marker for AML which is associated with lower survival rate. In this study, the correlation between the expression profiles of IL-6 and IL-18 with FLT3 was determined. A positive correlation was found between IL-6 and IL-18 expressions with FLT3 expression. In some of the studies, higher concentration levels of LDH have been mentioned as another prognostic marker in AML [3, 43]. Our investigations implicated that the expression of IL-18 is positively correlated to LDH levels. It has been reported that the incidence of AML is affected by sex and age. Females of 30–54 years of age and males over 50 years of age are more affected [44]. In the present study, a correlation between the expression of IL-6 and IL-18 with sex is reported, as well.
Based on our results, the expression levels of Il-6 and Il-18 were higher in patients experiencing aGvHD after bone marrow transplantation although it was not statically significant in comparison with patients without aGvHD. It is demonstrated that higher levels of cytokines including Il-6 and Il-18 are important mediators in pathophysiology and aGvHD [45, 46]. So, regulation of these cytokines is effective prophylaxis and control of aGvHD.
Conclusion
In conclusion, cytokine profiles including Il-6 and Il-18 are important markers in the progression of the disease and could be considered as a prognostic marker in acute leukemia. So, the control of the expression and regulation of these cytokines are a good indicator for the management of the disease. It is recommended that more studies with larger study groups and more involved cytokines are needed for more evaluation of the cytokine roles in pathophysiology and progression of acute leukemia.
Declarations
Conflict of Interest
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Maryam Owjfard, Email: Maryam.owjfard@yahoo.com.
Abdolhossein Zare, Email: zarea854@yahoo.com.
References
- 1.Hasserjian RP. Acute myeloid leukemia: advances in diagnosis and classification. Int J Lab Hematol. 2013;35(3):358–366. doi: 10.1111/ijlh.12081. [DOI] [PubMed] [Google Scholar]
- 2.Döhner H, Weisdorf DJ, Bloomfield CD. Acute myeloid leukemia. N Engl J Med. 2015;373(12):1136–1152. doi: 10.1056/NEJMra1406184. [DOI] [PubMed] [Google Scholar]
- 3.Döhner H, Estey EH, Amadori S, Appelbaum FR, Büchner T, Burnett AK, et al. Diagnosis and management of acute myeloid leukemia in adults: recommendations from an international expert panel, on behalf of the European LeukemiaNet. Blood. 2010;115(3):453–474. doi: 10.1182/blood-2009-07-235358. [DOI] [PubMed] [Google Scholar]
- 4.Van Etten RA. Aberrant cytokine signaling in leukemia. Oncogene. 2007;26(47):6738. doi: 10.1038/sj.onc.1210758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang B, Ma X-T, Zheng G-G, Li G, Rao Q, Wu K-F. Expression of IL-18 and its receptor in human leukemia cells. Leuk Res. 2003;27(9):813–822. doi: 10.1016/S0145-2126(03)00005-5. [DOI] [PubMed] [Google Scholar]
- 6.Dranoff G. Cytokines in cancer pathogenesis and cancer therapy. Nat Rev Cancer. 2004;4(1):11–22. doi: 10.1038/nrc1252. [DOI] [PubMed] [Google Scholar]
- 7.Tanaka T, Narazaki M, Kishimoto T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb Perspect Biol. 2014;6(10):a016295. doi: 10.1101/cshperspect.a016295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hodge DR, Hurt EM, Farrar WL. The role of IL-6 and STAT3 in inflammation and cancer. Eur J Cancer. 2005;41(16):2502–2512. doi: 10.1016/j.ejca.2005.08.016. [DOI] [PubMed] [Google Scholar]
- 9.Mauer J, Denson JL, Brüning JC. Versatile functions for IL-6 in metabolism and cancer. Trends Immunol. 2015;36(2):92–101. doi: 10.1016/j.it.2014.12.008. [DOI] [PubMed] [Google Scholar]
- 10.Scheller J, Chalaris A, Schmidt-Arras D. Rose-John S (2011) The pro-and anti-inflammatory properties of the cytokine interleukin-6. Biochim Biophys Acta. 1813;5:878–888. doi: 10.1016/j.bbamcr.2011.01.034. [DOI] [PubMed] [Google Scholar]
- 11.Guo Y, Xu F, Lu T, Duan Z, Zhang Z. Interleukin-6 signaling pathway in targeted therapy for cancer. Cancer Treat Rev. 2012;38(7):904–910. doi: 10.1016/j.ctrv.2012.04.007. [DOI] [PubMed] [Google Scholar]
- 12.Ebrahimi B, Tucker SL, Li D, Abbruzzese JL, Kurzrock R. Cytokines in pancreatic carcinoma. Cancer. 2004;101(12):2727–2736. doi: 10.1002/cncr.20672. [DOI] [PubMed] [Google Scholar]
- 13.Gracie JA, Robertson SE, McInnes IB. Interleukin-18. J Leukoc Biol. 2003;73(2):213–224. doi: 10.1189/jlb.0602313. [DOI] [PubMed] [Google Scholar]
- 14.Zhang B, Wang Y, Zheng G-G, Ma X-T, Li G, Zhang F-K, et al. Clinical significance of IL-18 gene over-expression in AML. Leuk Res. 2002;26(10):887–892. doi: 10.1016/S0145-2126(02)00025-5. [DOI] [PubMed] [Google Scholar]
- 15.IravaniSaadi M, Arandi N, Yaghobi R, Azarpira N, Geramizadeh B, Ramzi M. Aberrant expression of the miR-181b/miR-222 after hematopoietic stem cell transplantation in patients with acute myeloid leukemia. Indian J Hematol Blood Transfus. 2019;35(3):446–450. doi: 10.1007/s12288-018-01066-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.SAADI MI, Arandi N, Yaghobi R, Azarpira N, Geramizadeh B, Ramzi M Up-regulation of the miR-92a and miR-181a in patients with acute myeloid leukemia and their inhibition with locked nucleic acid (LNA)-antimiRNA; introducing c-Kit as a new target gene. Int J Hematol Oncol 28(3):001–009
- 17.Saadi MI, Beigi MAB, Ghavipishe M, Tahamtan M, Geramizadeh B, Zare A, et al. The circulating level of interleukins 6 and 18 in ischemic and idiopathic dilated cardiomyopathy. Journal of cardiovascular and thoracic research. 2019;11(2):132. doi: 10.15171/jcvtr.2019.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ramzi M, Saadi MI, Yaghobi R, Arandi N (2019) Dysregulated expression of CD28 and CTLA-4 molecules in patients with acute myeloid leukemia and possible association with development of graft versus host disease after hematopoietic stem cell transplantation. Int J Organ Transplant Med (IJOTM) 10(2) [PMC free article] [PubMed]
- 19.Glucksberg H, Storb R, Fefer A, Buckner CD, Neiman PE, Clift RA, et al. Clinical manifestations of graft-versus-host disease in human recipients of marrow from HL-A-matched sibling donors. Transplantation. 1974;18(4):295–304. doi: 10.1097/00007890-197410000-00001. [DOI] [PubMed] [Google Scholar]
- 20.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods (San Diego, Calif) 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 21.Sadjadi A, Malekzadeh R, Derakhshan MH, Sepehr A, Nouraie M, Sotoudeh M, et al. Cancer occurrence in Ardabil: results of a population-based cancer registry from Iran. Int J Cancer. 2003;107(1):113–118. doi: 10.1002/ijc.11359. [DOI] [PubMed] [Google Scholar]
- 22.Dastgiri S, Fozounkhah S, Shokrgozar S, Taghavinia M, Kermani AA. Incidence of leukemia in the Northwest of Iran. Health Promot Perspect. 2011;1(1):50. doi: 10.5681/hpp.2011.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.De Kouchkovsky I, Abdul-Hay M. Acute myeloid leukemia: a comprehensive review and 2016 update. Blood Cancer J. 2016;6(7):e441. doi: 10.1038/bcj.2016.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Röllig C, Thiede C, Gramatzki M, Aulitzky W, Bodenstein H, Bornhäuser M, et al. A novel prognostic model in elderly patients with acute myeloid leukemia: results of 909 patients entered into the prospective AML96 trial. Blood. 2010;116(6):971–978. doi: 10.1182/blood-2010-01-267302. [DOI] [PubMed] [Google Scholar]
- 25.Tsimberidou AM, Estey E, Wen S, Pierce S, Kantarjian H, Albitar M, et al. The prognostic significance of cytokine levels in newly diagnosed acute myeloid leukemia and high-risk myelodysplastic syndromes. Cancer. 2008;113(7):1605–1613. doi: 10.1002/cncr.23785. [DOI] [PubMed] [Google Scholar]
- 26.Kumari N, Dwarakanath B, Das A, Bhatt AN. Role of interleukin-6 in cancer progression and therapeutic resistance. Tumour Biol. 2016;37(9):11553–11572. doi: 10.1007/s13277-016-5098-7. [DOI] [PubMed] [Google Scholar]
- 27.Tian G, Mi J, Wei X, Zhao D, Qiao L, Yang C, et al (2015) Circulating interleukin-6 and cancer: a meta-analysis using Mendelian randomization. Sci Rep 5 [DOI] [PMC free article] [PubMed]
- 28.Seymour JF, Talpaz M, Cabanillas F, Wetzler M, Kurzrock R. Serum interleukin-6 levels correlate with prognosis in diffuse large-cell lymphoma. J Clin Oncol. 1995;13(3):575–582. doi: 10.1200/JCO.1995.13.3.575. [DOI] [PubMed] [Google Scholar]
- 29.Kurzrock R, Redman J, Cabanillas F, Jones D, Rothberg J, Talpaz M. Serum interleukin 6 levels are elevated in lymphoma patients and correlate with survival in advanced Hodgkin's disease and with B symptoms. Can Res. 1993;53(9):2118–2122. [PubMed] [Google Scholar]
- 30.Chung YC, Chang YF. Serum interleukin-6 levels reflect the disease status of colorectal cancer. J Surg Oncol. 2003;83(4):222–226. doi: 10.1002/jso.10269. [DOI] [PubMed] [Google Scholar]
- 31.Blay J-Y, Negrier S, Combaret V, Attali S, Goillot E, Merrouche Y, et al. Serum level of interleukin 6 as a prognosis factor in metastatic renal cell carcinoma. Can Res. 1992;52(12):3317–3322. [PubMed] [Google Scholar]
- 32.Sanchez-Correa B, Bergua JM, Campos C, Gayoso I, Arcos MJ, Bañas H, et al. Cytokine profiles in acute myeloid leukemia patients at diagnosis: survival is inversely correlated with IL-6 and directly correlated with IL-10 levels. Cytokine. 2013;61(3):885–891. doi: 10.1016/j.cyto.2012.12.023. [DOI] [PubMed] [Google Scholar]
- 33.Heikkilä K, Ebrahim S, Lawlor DA. Systematic review of the association between circulating interleukin-6 (IL-6) and cancer. Eur J Cancer. 2008;44(7):937–945. doi: 10.1016/j.ejca.2008.02.047. [DOI] [PubMed] [Google Scholar]
- 34.Mitsunaga S, Ikeda M, Shimizu S, Ohno I, Furuse J, Inagaki M, et al. Serum levels of IL-6 and IL-1β can predict the efficacy of gemcitabine in patients with advanced pancreatic cancer. Br J Cancer. 2013;108(10):2063. doi: 10.1038/bjc.2013.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bellone G, Smirne C, Mauri FA, Tonel E, Carbone A, Buffolino A, et al. Cytokine expression profile in human pancreatic carcinoma cells and in surgical specimens: implications for survival. Cancer Immunol Immunother. 2006;55(6):684–698. doi: 10.1007/s00262-005-0047-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kawabata T, Ichikura T, Majima T, Seki S, Chochi K, Takayama E, et al. Preoperative serum interleukin-18 level as a postoperative prognostic marker in patients with gastric carcinoma. Cancer. 2001;92(8):2050–2055. doi: 10.1002/1097-0142(20011015)92:8<2050::AID-CNCR1544>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 37.Günel N, Coskun U, Sancak B, Hasdemir O, Sare M, Bayram O, et al. Prognostic value of serum IL-18 and nitric oxide activity in breast cancer patients at operable stage. Am J Clin Oncol. 2003;26(4):416–421. doi: 10.1097/01.COC.0000027416.15170.53. [DOI] [PubMed] [Google Scholar]
- 38.Ko C-Y, Wang W-L, Li C-F, Jeng Y-M, Chu Y-Y, Wang H-Y, et al. IL-18-induced interaction between IMP3 and HuR contributes to COX-2 mRNA stabilization in acute myeloid leukemia. J Leukoc Biol. 2016;99(1):131–141. doi: 10.1189/jlb.2A0414-228RR. [DOI] [PubMed] [Google Scholar]
- 39.Saultz JN, Garzon R. Acute myeloid leukemia: a concise review. J Clin Med. 2016;5(3):33. doi: 10.3390/jcm5030033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Levis M, Small D. FLT3: It does matter in leukemia. Leukemia. 2003;17(9):1738–1752. doi: 10.1038/sj.leu.2403099. [DOI] [PubMed] [Google Scholar]
- 41.Ozeki K, Kiyoi H, Hirose Y, Iwai M, Ninomiya M, Kodera Y, et al. Biologic and clinical significance of the FLT3 transcript level in acute myeloid leukemia. Blood. 2004;103(5):1901–1908. doi: 10.1182/blood-2003-06-1845. [DOI] [PubMed] [Google Scholar]
- 42.Kuchenbauer F, Kern W, Schoch C, Kohlmann A, Hiddemann W, Haferlach T, et al. Detailed analysis of FLT3 expression levels in acute myeloid leukemia. Haematologica. 2005;90(12):1617–1625. [PubMed] [Google Scholar]
- 43.Pui C-H, Dodge RK, Dahl GV, Rivera G, Look AT, Kalwinsky D, et al. Serum lactic dehydrogenase level has prognostic value in childhood acute lymphoblastic leukemia. Blood. 1985;66(4):778–782. doi: 10.1182/blood.V66.4.778.778. [DOI] [PubMed] [Google Scholar]
- 44.Cartwright RA, Gurney KA, Moorman AV. Sex ratios and the risks of haematological malignancies. Br J Haematol. 2002;118(4):1071–1077. doi: 10.1046/j.1365-2141.2002.03750.x. [DOI] [PubMed] [Google Scholar]
- 45.Tvedt THA, Ersvaer E, Tveita AA, Bruserud O. Interleukin-6 in Allogeneic stem cell transplantation: its possible importance for immunoregulation and as a therapeutic target. Front Immunol. 2017;8:667. doi: 10.3389/fimmu.2017.00667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Reddy P, Ferrara JL. Role of interleukin-18 in acute graft-vs-host disease. J Lab Clin Med. 2003;141(6):365–371. doi: 10.1016/S0022-2143(03)00028-3. [DOI] [PubMed] [Google Scholar]